Abstract
Over the years Pavlovian fear conditioning has proved to be a powerful model to investigate the neural underpinnings of aversive associative memory formation. Although it is well appreciated that plasticity occurring at excitatory synapses within the basolateral complex of the amygdala (BLA) plays a critical role in associative memory formation, recent evidence suggests that plasticity within the amygdala is more distributed than previously appreciated. In particular, studies demonstrate that plasticity in the central nucleus (CeA) is critical for the acquisition of conditioned fear. In addition, a variety of interneuron populations within the amygdala, defined by unique neurochemical markers, contribute to distinct aspects of stimulus processing and memory formation during fear conditioning. Here, we will review and summarize recent advances in our understanding of amygdala networks and how unique players within this network contribute to synaptic plasticity associated with the acquisition of conditioned fear.
Keywords: synaptic plasticity, amygdala, fear conditioning
Introduction
The ability to associate aversive events with environmental stimuli that predict them is critical for an organism to organize defensive behaviors and adapt in a dynamic environment. In the laboratory, Pavlovian fear conditioning in rodents (and humans) has been used widely as an experimental model to investigate the neural underpinnings of aversive associative learning and memory. In a standard fear conditioning procedure in rodents, for example, animals learn to associate a neutral sensory stimulus (conditioned stimulus, CS) such as a tone, with an aversive event (unconditioned stimulus, US) such as a foot shock. After several conditioning trials, presentation of the CS alone comes to elicit both a behavioral (e.g., freezing) and autonomic (e.g., heart rate) conditioned response (CR).
Decades of work have revealed the essential neural circuit mediating the acquisition of Pavlovian fear conditioning. This circuit includes a distributed network of brain structures including the medial prefrontal cortex (mPFC), amygdala, and hippocampus [1]. In turn, the expression of conditioned fear memories is mediated by projections from the amygdala to the hypothalamus, midbrain periaqueductal gray (PAG), and medullary cardioregulatory centers, among others. While the brain circuits involved in the acquisition and expression of conditioned fear responses are well documented, how synaptic plasticity maps onto specific neural circuits and contributes to associative learning processes at both a cellular and behavioral level has been the focus of more recent work[2–5].
Synaptic encoding of fear memory in the lateral amygdala
With respect to the neurobiological underpinnings of associative fear learning, the majority of research has focused on plasticity between excitatory sensory afferents and glutamatergic projections neurons within the amygdala. In particular, CS-US convergence within the lateral nucleus of the amygdala (LA) is a putative mechanism by which stimuli are associated during learning. Studies employing optogenetics, a technique that permits precise temporal control of neural activity, have confirmed a role for coincident pre-and postsynaptic activity (i.e., Hebbian plasticity) in the establishment of long-term potentiation (LTP) within the LA [6,7]. Moreover, other work has shown that expression of fear memories can be controlled by manipulating synaptic strength in thalamo-amygdala synapses [8].
Despite clear evidence implicating amygdaloid synaptic plasticity in the acquisition of conditioned fear, it is not known whether LTP is induced in an input specific manner as would be required to support associative learning. This question was recently addressed in an elegant study that employed activity-dependent neuronal labeling techniques along with a discriminative fear conditioning paradigm to functionally tag CS auditory afferents within the LA[9]. Using Fos-CreERT2 knockin mice, the authors first exposed animals to a discrete auditory tone following tamoxifen administration which permitted the selective labeling of sensory afferents in the LA transmitting information about that specific auditory stimulus. Following behavioral labeling, animals were subject to a discriminative fear conditioning procedure in which they were presented with two auditory tones, a CS+ (i.e. the CS paired with the US) to which they had previously been exposed to during behavioral labeling, as well as a novel CS- (i.e. the CS not paired with US) and sacrificed for electrophysiological recordings. Recordings from behaviorally labeled neurons revealed that LTP was not induced globally at synapses within the LA, but rather was restricted to synapses carrying information about the CS+. Moreover, LTP was preferentially induced at postsynaptic neurons within the LA that were both contacted by CS+ afferents and activated by the US during training, consistent with a Hebbian mechanism of plasticity.
Although these studies imply that coincident neural activity at glutamatergic synapses in the LA is sufficient to produce an associative fear memory, it is likely that other neuromodulators, such as norepinephrine, adenosine, acetylcholine, and dopamine, modulate synaptic plasticity on unique time scales to optimize learning[10–14]. Interestingly, findings from a recent study suggest that although Hebbian plasticity likely contributes to learning related synaptic changes, this mechanism alone does not explain how stimuli of different modalities (e.g. auditory, olfactory, visual) are associated within the BLA [15]. In this study, the activity of neural ensembles within the BLA were tracked across multiple days of a fear conditioning procedure in mice using in vivo Ca2+ imaging methods. Although many neurons in the BLA showed learning-induced increases in activity to the CS, only 38% of these cells also responded to the US during conditioning. In other words, fear conditioning potentiated CS-evoked responses in the BLA in neurons that did not receive convergent US input. Moreover, around 65% of the neurons that were initially responsive to both the CS and US during conditioning showed a decrease in CSevoked activity following conditioning (relative to CS-evoked activity prior to conditioning). These findings suggest that conditioning-related plasticity in BLA neurons does not adhere to strict Hebbian plasticity rules, which posit that only neurons responding to both the CS and US will exhibit plasticity at CS afferents following conditioning. Instead, the authors argue that the conditioning may depend on a distributed network representation of the CS that comes to mimic the neural ensemble activated by the US. Indeed, their results indicate that the pattern of neural activity supported by the CS late in training (e.g., at a point when there is high behavioral responding to the CS) is more comparable to the neural representation of the US than the pattern of neural activity elicited by the CS early in training (e.g., at a point when behavioral responding to the CS is low). Although previous studies have suggested that US-driven activity acts as a cellular teaching signal (e.g., instructing which CS inputs are potentiated) the results from this study suggest that US-driven activity may instead provide a neuromodulatory network-level teaching signal that serves to shape the activity of neural ensembles activated by a particular CS [15,16].
Intra-amygdalar inhibitory networks controlling associative fear learning
It is well established that the intrinsic excitability of glutamatergic projection neurons within the BLA plays a selective role in determining which synapses will be strengthened [17–20]. Interestingly, recent evidence suggests that inhibitory networks within the BLA play a critical role shaping and magnifying the difference in excitability between glutamatergic projection neurons [21,22]. For instance, perisomatic GABAergic synapses control the excitability and subsequent output of projections neurons [23], and abnormalities associated with the expression of GABA synapses affect the induction of LTP within the BLA[24]. Moreover, distinct inhibitory neurons, classified by unique neurochemical markers, not only control input activity and subsequent output of principal neurons, but they do so in a compartmental specific manner [3,23,25]. According to this model (Figure 1), auditory and nociceptive signals converge within the BLA and are associated via interactions with local inhibitory networks. Specifically, CSprocessing within the BLA is under control of a microcircuit in which parvalbumin (PV)- and somatostatin (SOM)-expressing inhibitory neurons act together to control BLA input by disinhibiting principal neuron dendrites during CS presentation. In contrast, the perisomatic domain of principal neurons receives strong inhibition directly from PV+ neurons and these inputs are well positioned to gate BLA output. Moreover, intrinsic inhibitory mechanisms controlling BLA output were recently demonstrated to occur in a projection-specific manner [26]. This study found that although inhibitory cholecystokinin (CCK)-expressing basket cells inhibit medial prefrontal cortex (mPFC)-projecting BLA principal neurons with similar connectivity and synaptic strength, output from these neurons is differentially regulated. Specifically, these authors found that BLA neurons projecting to the infralimbic (IL) division of the mPFC were more susceptible to endocannabinoid-mediated activity-dependent suppression than neurons in the BLA projecting to the prelimbic (PL) division of the mPFC. This finding is particularly interesting given that the balance in activity between IL and PL projecting neurons within the BLA was recently demonstrated to be important for the regulation of extinction learning[27].
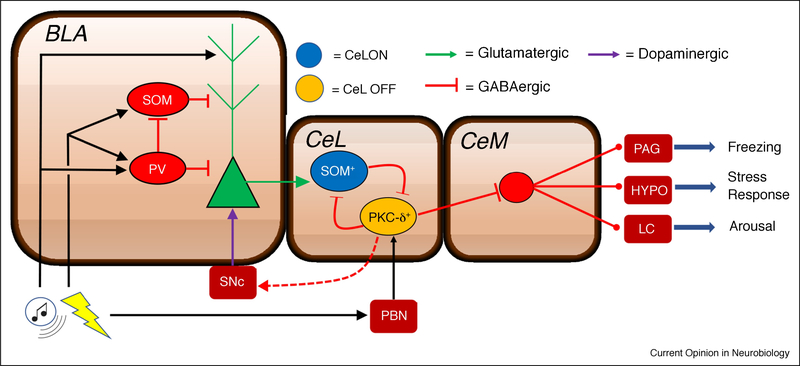
Figure 1.
Proposed models of information processing within the amygdala. Auditory and nociceptive signals converge within the BLA and are associated via interactions with local inhibitory networks [25]. Associated information is signaled to the CeL where activation and plasticity at SOM+ (CeL ON) expressing inhibitory neurons provides disinhibition to brainstem projecting CeM neurons [40, 46] via interactions with PKC-δ+ (CeL OFF) expressing neurons. In addition, PKC-δ+ neurons within the CeL also receive nociceptive signals directly from the PBN [49] and plasticity at these synapses is involved in the acquisition of conditioned fear [50·]. BLA, basolateral amygdala; CeL, lateral division of central amygdala; CeM, medial division of central amygdala; SOM, somatostatin; PV, parvalbumin; PKC-δ+, protein kinase C delta; SNc, substantia nigra parscompacta; PBN, parabrachial nucleus; PAG, periaqueductal gray; HYPO, hypothalamus; LC, locus coeruleus.
Although the aforementioned studies provide evidence implicating intra-amygdala inhibitory networks in the regulation of excitatory synaptic plasticity, changes in synaptic transmission between presynaptic excitatory neurons and postsynaptic inhibitory neurons may also play a role in learning [28]. For example, it was recently demonstrated that fear conditioning induces plasticity at glutamatergic synapses between sensory afferents and GABAergic neurons in the medial paracapsular intercalated region (mpITC) of the amygdala [29]. Given that neurons within this region provide feedback and feedforward inhibition to principal neurons within the BLA [29], plastic changes at these synapses may contribute to altered neuronal signaling and plasticity in other regions of the amygdala. Thus, although fear conditioning induces plasticity between excitatory synapses within the BLA that is regulated by interneuron activity, it also produces plasticity among networks of inhibitory interneurons that ultimately control principal neuron output. This indicates that inhibition within amygdaloid networks involved in the acquisition of conditioned fear is not static, but instead is a dynamic process regulated by the learning [30–32]. These findings are particularly intriguing given that irregularities in both GABAergic signaling, as well as increases in BLA principal cell activity, have been associated with the generalization of fear across different types of stimuli, a hallmark symptom of mental psychopathologies such as generalized anxiety disorder (GAD), as well as post-traumatic stress disorder (PTSD) [33–35]. Altogether these findings suggest that amygdala network activity and subsequent plasticity engaged by the learning process is tightly regulated by coordinated activity within intra-BLA inhibitory networks and that irregularities in this network may contribute to stress related psychopathologies [22,35,36].
Synaptic encoding of fear memory in the central amygdala
In contrast to the LA, which is presumed to encode and maintain CS-US associations, the CeA is thought to mediate the behavioral expression of conditioned fear responses [37]. Despite this, recent work suggests that the CeA may also participate in both the acquisition and storage of conditioned fear memories [38]. Early support for this notion comes from studies demonstrating that reversible inactivation of the CeA or NMDA receptor blockade in this region impaired the acquisition of conditioned fear responses [39,40]. In addition, CS-evoked neuronal responses of genetically distinct neural populations within the CeA undergo learning dependent modifications [41–44], similar to what is observed in the LA, with a subpopulation of cells within the CeA showing CS-evoked excitatory responses upon learning [45].
Functionally, the CeA can be divided into a lateral (CeL) and medial (CeM) divisions. The CeM is thought to mediate the expression of fear via projections to brainstem effector systems [37]. Recent evidence suggests that the CeL gates fear expression via inhibitory projections to the CeM. For example, reversible inactivation of the CeL is capable of inducing unconditional freezing [41]. Interestingly, the regulation of CeM output by the CeL is achieved by local recurrent inhibitory networks within the CeL [41,46] and these interactions have also been demonstrated to be critical in mediating behavioral responses of freezing or flight [43]. Moreover, it has been shown plasticity induced by fear learning within the CeL is different in two genetically-identified populations of neurons: CeL neurons that lack protein kinase C δ (PKC δ-) acquire an excitatory response (i.e. increased CS-evoked activity relative to baseline) to an trained CS (termed CeLON), whereas PKC δ+ neurons acquire an inhibitory response (i.e. decrease in CS-evoked activity relative to baseline; termed CeLOFF) [41,46]. Interestingly, CSevoked excitation in CeLON neurons precedes the inhibition of CeLOFF neurons [41]. Genetic dissection of functional connectivity between these two cell populations revealed that CeLON units directly inhibit CeLOFF neurons, which in turn project to and inhibit brainstem projecting neurons in the CeM (Figure 1) [46]. This reveals that the expression of conditioned fear responses may be involve plasticity in CeLON neurons that indirectly disinhibit CeM neurons involved in fear behavior.
Consistent with a serial model of amygdala information processing, changes in the responsiveness of genetically defined cell populations within the CeL appears to be product of LTP induced by excitatory projections originating from the LA [47]. Specifically, fear conditioning strengthens excitatory synapses onto neurons expressing the peptide hormone somatostatin (SOM+)/ PKC δ- neurons, which largely correspond to CeLON units, while weakening those synapsing onto SOM-/ PKC δ+ neurons in the CeL, which largely correspond to CeLOFF units. Accordingly, fear conditioning induces increases in excitatory transmission via the LA onto CeLON units may serve to inhibit CeLOFF units thereby disinhibiting PAG projecting CeM neurons and ultimately promoting the expression of fear [41,47]. It should be noted, however, that PKC δ-/SOM+ (CeLON units) also send projections directly to the PAG and thus may be capable of regulating the behavioral expression of fear independent of the proposed CeL to CeM pathway (Figure 1) [48].
Although these studies demonstrate that plasticity at the level of the CeA is critical for the acquisition of conditioned fear, it has been unclear whether this plasticity was simply a byproduct of upstream plasticity in the LA or whether synaptic plasticity induced in the CeA is critical for learning induced plasticity within the amygdala. This question was recently addressed in a study by Yu and colleagues (2017) who reasoned that if information is transmitted serially from the LA to CeA, then silencing activity in the CeA should leave conditioning-induced synaptic plasticity in the LA intact. Interestingly, they found that inhibiting transmitter release from PKC δ+ expressing neurons within the CeL impaired both conditioned freezing and conditioning-induced increases in LA synaptic strength. Given that PKC δ+ expressing neurons have been shown to be direct postsynaptic targets of the parabrachial nucleus [49], a brainstem structure which provides nociceptive signals, the authors hypothesized that this cell population may play an important role in conveying information about the US to the LA (Figure 1). Indeed, chemogenetic inhibition of CeL PKC δ+ neurons suppressed shock-evoked responses in LA neurons during conditioning and optical inhibition of this cell population specifically during US presentation impaired the acquisition of the fear memory [50]. Given that midbrain dopamine neurons receive input from PKC δ+ cells within the CeL and have been demonstrated to play an important role in fear learning [51], the authors hypothesize that PKC δ+ neurons may drive disinhibition within midbrain dopamine neurons in response to the US, thereby instructing learning in the LA (Figure 1). Altogether the results of this study support a model of parallel information processing in the amygdala, with plasticity in different nuclei mediating distinct incentive processes[52].
Summary
Although synaptic plasticity associated with conditioned fear has been a prominent area of focus for the past several decades, recent advances within the field have yielded new insights into the mechanisms by which amygdala networks encode fear memories. Specifically, these findings suggest that learning-related plasticity is not restricted to any one nucleus within the amygdala. Rather, synaptic encoding of fear occurs in several different amygdaloid nuclei, and this plasticity may represent different features of the CS-US association. In addition, plasticity at different regions within the amygdala appears to be tightly regulated by an intra-amygdala inhibitory network. Given that recent findings suggest irregularities in GABAergic signaling within the amygdala are associated with altered neural plasticity and the subsequent emergence of fear related psychopathologies [24,33,34], understanding how plasticity within and between different nuclei is manifested and regulated is critical to the implementation of future research designs and therapeutic interventions.
Highlights:
Plasticity associated with conditioned fear is distributed across functionally and anatomically segregated nuclei within the amygdala.
Interneuron activity within the basolateral amygdala shapes the excitability and subsequent plasticity associated with conditioned fear.
Neurochemically defined cell types within the central amygdala receive associative information and actively participate in plasticity in other regions of amygdala.
Acknowledgements:
Supported by grants from the National Institutes of Health (R01MH065961, R01MH117852), the McKnight Endowment for Neuroscience (Memory and Cognitive Disorders Award), and the Brain & Behavior Research Foundation (Distinguished Investigator Grant).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Sources
- 1.Orsini CA, Maren S: Neural and cellular mechanisms of fear and extinction memory formation. Neurosci. Biobehav. Rev 2012, 36:1773–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tovote P, Fadok JP, Lüthi A: Neuronal circuits for fear and anxiety. Nat. Rev. Neurosci 2015, 16:317–331. [DOI] [PubMed] [Google Scholar]
- 3.Letzkus JJ, Wolff SBE, Lüthi A: Disinhibition, a circuit mechanism for associative learning and memory. Neuron 2015, 88:264–276. [DOI] [PubMed] [Google Scholar]
- 4.Janak PH, Tye KM: From circuits to behaviour in the amygdala. Nature 2015, 517:284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bocchio M, Nabavi S, Capogna M: Synaptic plasticity, engrams, and network oscillations in amygdala circuits for storage and retrieval of emotional memories. Neuron 2017, 94:731–743. [DOI] [PubMed] [Google Scholar]
- 6.Johansen JP, Hamanaka H, Monfils MH, Behnia R, Deisseroth K, Blair HT, LeDoux JE: Optical activation of lateral amygdala pyramidal cells instructs associative fear learning. Proc. Natl. Acad. Sci. USA 2010, 107:12692–12697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwon J-T, Nakajima R, Kim H-S, Jeong Y, Augustine GJ, Han J-H: Optogenetic activation of presynaptic inputs in lateral amygdala forms associative fear memory. Learn. Mem. 2014, 21:627–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nabavi S, Fox R, Proulx CD, Lin JY, Tsien RY, Malinow R: Engineering a memory with LTD and LTP. Nature 2014, 511:348–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •9.Kim WB, Cho J-H: Encoding of Discriminative Fear Memory by Input-Specific LTP in the Amygdala. Neuron 2017, 95:1129–1146.e5. Using a discriminative fear conditioning procedure these authors demonstrated that LTP is induced selectively at CS+ (and not CS-) pathways into the LA. [DOI] [PubMed] [Google Scholar]
- 10.Johansen JP, Diaz-Mataix L, Hamanaka H, Ozawa T, Ycu E, Koivumaa J, Kumar A, Hou M, Deisseroth K, Boyden ES, et al. : Hebbian and neuromodulatory mechanisms interact to trigger associative memory formation. Proc. Natl. Acad. Sci. USA 2014, 111:E5584–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simões AP, Machado NJ, Gonçalves N, Kaster MP, Simões AT, Nunes A, Pereira de Almeida L, Goosens KA, Rial D, Cunha RA: Adenosine A2A receptors in the amygdala control synaptic plasticity and contextual fear memory. Neuropsychopharmacology 2016, 41:2862–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang L, Kundu S, Lederman JD, López-Hernández GY, Ballinger EC, Wang S, Talmage DA, Role LW: Cholinergic Signaling Controls Conditioned Fear Behaviors and Enhances Plasticity of Cortical-Amygdala Circuits. Neuron 2016, 90:1057–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •13.Schiff HC, Johansen JP, Hou M, Bush DEA, Smith EK, Klein JE, LeDoux JE, Sears RM: β-Adrenergic Receptors Regulate the Acquisition and Consolidation Phases of Aversive Memory Formation Through Distinct, Temporally Regulated Signaling Pathways. Neuropsychopharmacology 2017, 42:895–903. This study demonstrates that β-adrenoreceptor activation results in two molecularly and temporally distinct signaling mechanisms that contribute uniquely to the acquisition and consolidation of conditioned fear. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ozawa T, Johansen JP: Learning rules for aversive associative memory formation. Curr. Opin. Neurobiol 2018, 49:148–157. [DOI] [PubMed] [Google Scholar]
- ••15.Grewe BF, Gründemann J, Kitch LJ, Lecoq JA, Parker JG, Marshall JD, Larkin MC, Jercog PE, Grenier F, Li JZ, et al. : Neural ensemble dynamics underlying a long-term associative memory. Nature 2017, 543:670–675. Using Ca2+ imaging, this study demonstrates that over the course of a fear conditioning procedure, CS evoked responses in the amygdala are shaped to resemble the neural ensemble activated by the US. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johansen JP, Cain CK, Ostroff LE, LeDoux JE: Molecular mechanisms of fear learning and memory. Cell 2011, 147:509–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han J-H, Kushner SA, Yiu AP, Cole CJ, Matynia A, Brown RA, Neve RL, Guzowski JF, Silva AJ, Josselyn SA: Neuronal competition and selection during memory formation. Science (80-. ). 2007, 316:457–460. [DOI] [PubMed] [Google Scholar]
- 18.Zhou Y, Won J, Karlsson MG, Zhou M, Rogerson T, Balaji J, Neve R, Poirazi P, Silva AJ: CREB regulates excitability and the allocation of memory to subsets of neurons in the amygdala. Nat. Neurosci 2009, 12:1438–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gouty-Colomer LA, Hosseini B, Marcelo IM, Schreiber J, Slump DE, Yamaguchi S, Houweling AR, Jaarsma D, Elgersma Y, Kushner SA: Arc expression identifies the lateral amygdala fear memory trace. Mol. Psychiatry 2016, 21:364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yiu AP, Mercaldo V, Yan C, Richards B, Rashid AJ, Hsiang H-LL, Pressey J, Mahadevan V, Tran MM, Kushner SA, et al. : Neurons are recruited to a memory trace based on relative neuronal excitability immediately before training. Neuron 2014, 83:722–735. [DOI] [PubMed] [Google Scholar]
- 21.Feng F, Samarth P, Paré D, Nair SS: Mechanisms underlying the formation of the amygdalar fear memory trace: A computational perspective. Neuroscience 2016, 322:370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krabbe S, Gründemann J, Lüthi A: Amygdala inhibitory circuits regulate associative fear conditioning. Biol. Psychiatry 2018, 83:800–809. [DOI] [PubMed] [Google Scholar]
- 23.Veres JM, Nagy GA, Hájos N: Perisomatic GABAergic synapses of basket cells effectively control principal neuron activity in amygdala networks. Elife 2017, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saha R, Knapp S, Chakraborty D, Horovitz O, Albrecht A, Kriebel M, Kaphzan H, Ehrlich I, Volkmer H, Richter-Levin G: GABAergic Synapses at the Axon Initial Segment of Basolateral Amygdala Projection Neurons Modulate Fear Extinction. Neuropsychopharmacology 2016, doi: 10.1038/npp.2016.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolff SBE, Gründemann J, Tovote P, Krabbe S, Jacobson GA, Müller C, Herry C, Ehrlich I, Friedrich RW, Letzkus JJ, et al. : Amygdala interneuron subtypes control fear learning through disinhibition. Nature 2014, 509:453–458. [DOI] [PubMed] [Google Scholar]
- •26.Vogel E, Krabbe S, Gründemann J, Wamsteeker Cusulin JI, Lüthi A: Projection-Specific Dynamic Regulation of Inhibition in Amygdala Micro-Circuits. Neuron 2016, 91:644–651. This study demonstrates that inhibitory synaptic transmission onto principal neurons within the basal amygdala via retrograde endocannabinoid signaling is regulated in a projection specific manner. [DOI] [PubMed] [Google Scholar]
- 27.Senn V, Wolff SBE, Herry C, Grenier F, Ehrlich I, Gründemann J, Fadok JP, Müller C, Letzkus JJ, Lüthi A: Long-range connectivity defines behavioral specificity of amygdala neurons. Neuron 2014, 81:428–437. [DOI] [PubMed] [Google Scholar]
- 28.Lucas EK, Jegarl AM, Morishita H, Clem RL: Multimodal and Site-Specific Plasticity of Amygdala Parvalbumin Interneurons after Fear Learning. Neuron 2016, 91:629–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •29.Asede D, Bosch D, Lüthi A, Ferraguti F, Ehrlich I: Sensory inputs to intercalated cells provide fear-learning modulated inhibition to the basolateral amygdala. Neuron 2015, 86:541–554. This study provides evidence that inhibitory neurons within the medial paracapsular division (mpITC)of the amygdala receive sensory information and undergo learning dependent modifications. [DOI] [PubMed] [Google Scholar]
- 30.Trouche S, Sasaki JM, Tu T, Reijmers LG: Fear extinction causes target-specific remodeling of perisomatic inhibitory synapses. Neuron 2013, 80:1054–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lucas EK, Clem RL: GABAergic interneurons: The orchestra or the conductor in fear learning and memory? Brain Res. Bull 2017, doi: 10.1016/j.brainresbull.2017.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis P, Zaki Y, Maguire J, Reijmers LG: Cellular and oscillatory substrates of fear extinction learning. Nat. Neurosci 2017, 20:1624–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghosh S, Chattarji S: Neuronal encoding of the switch from specific to generalized fear. Nat. Neurosci 2015, 18:112–120. [DOI] [PubMed] [Google Scholar]
- 34.Bender CL, Otamendi A, Calfa GD, Molina VA: Prior stress promotes the generalization of contextual fear memories: Involvement of the gabaergic signaling within the basolateral amygdala complex. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2018, 83:18–26. [DOI] [PubMed] [Google Scholar]
- 35.Mahan AL, Ressler KJ: Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends Neurosci 2012, 35:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Lüthi A: Amygdala inhibitory circuits and the control of fear memory. Neuron 2009, 62:757–771. [DOI] [PubMed] [Google Scholar]
- 37.LeDoux JE, Iwata J, Cicchetti P, Reis DJ: Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J. Neurosci 1988, 8:2517–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fadok JP, Markovic M, Tovote P, Lüthi A: New perspectives on central amygdala function. Curr. Opin. Neurobiol 2018, 49:141–147. [DOI] [PubMed] [Google Scholar]
- 39.Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE: Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J. Neurosci 2006, 26:12387–12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goosens KA, Maren S: Pretraining NMDA receptor blockade in the basolateral complex, but not the central nucleus, of the amygdala prevents savings of conditional fear. Behav. Neurosci 2003, 117:738–750. [DOI] [PubMed] [Google Scholar]
- 41.Ciocchi S, Herry C, Grenier F, Wolff SBE, Letzkus JJ, Vlachos I, Ehrlich I, Sprengel R, Deisseroth K, Stadler MB, et al. : Encoding of conditioned fear in central amygdala inhibitory circuits. Nature 2010, 468:277–282. [DOI] [PubMed] [Google Scholar]
- 42.Duvarci S, Popa D, Paré D: Central amygdala activity during fear conditioning. J. Neurosci 2011, 31:289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••43.Fadok JP, Krabbe S, Markovic M, Courtin J, Xu C, Massi L, Botta P, Bylund K, Müller C, Kovacevic A, et al. : A competitive inhibitory circuit for selection of active and passive fear responses. Nature 2017, 542:96–100. This paper provides evidence that active (e.g. flight) and passive (e.g. freezing) fear responses are mediated by distinct mutually competitive inhibitory circuits within the CeA. [DOI] [PubMed] [Google Scholar]
- 44.Sanford CA, Soden ME, Baird MA, Miller SM, Schulkin J, Palmiter RD, Clark M, Zweifel LS: A Central Amygdala CRF Circuit Facilitates Learning about Weak Threats. Neuron 2017, 93:164–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maren S, Quirk GJ: Neuronal signalling of fear memory. Nat. Rev. Neurosci 2004, 5:844–852. [DOI] [PubMed] [Google Scholar]
- 46.Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, Biag J, Dong H-W, Deisseroth K, Callaway EM, et al. : Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature 2010, 468:270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li H, Penzo MA, Taniguchi H, Kopec CD, Huang ZJ, Li B: Experience-dependent modification of a central amygdala fear circuit. Nat. Neurosci 2013, 16:332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Penzo MA, Robert V, Li B: Fear conditioning potentiates synaptic transmission onto long-range projection neurons in the lateral subdivision of central amygdala. J. Neurosci 2014, 34:2432–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han S, Soleiman MT, Soden ME, Zweifel LS, Palmiter RD: Elucidating an Affective Pain Circuit that Creates a Threat Memory. Cell 2015, 162:363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •50.Yu K, Ahrens S, Zhang X, Schiff H, Ramakrishnan C, Fenno L, Deisseroth K, Zhao F, Luo M-H, Gong L, et al. : The central amygdala controls learning in the lateral amygdala. Nat. Neurosci 2017, 20:1680–1685. This study provides evidence that activity of protein kinase C δ (PKC δ+) expressing cells in the lateral division of the central nucleus (CeL) is necessary for fear conditioning induced synaptic strengthening in the LA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bromberg-Martin ES, Matsumoto M, Hikosaka O: Dopamine in motivational control: rewarding, aversive, and alerting. Neuron 2010, 68:815–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Balleine BW, Killcross S: Parallel incentive processing: an integrated view of amygdala function. Trends Neurosci 2006, 29:272–279. [DOI] [PubMed] [Google Scholar]