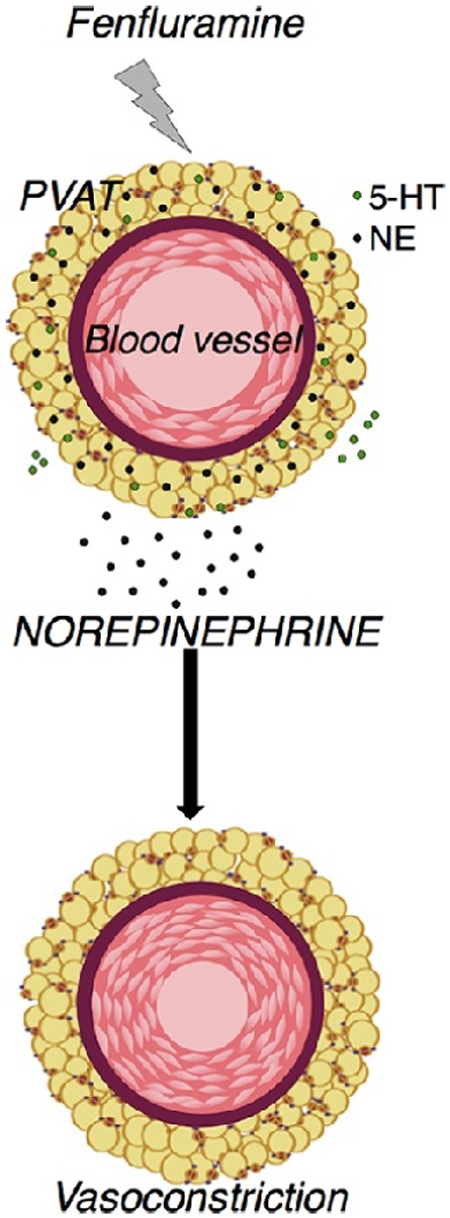
Abstract
Perivascular adipose tissue (PVAT) modulates vascular tone and altered PVAT function is observed in vascular diseases such as hypertension and atherosclerosis. We discovered that the PVAT surrounding rat thoracic aorta (RA) and the superior mesenteric artery (SMA) contain significant amounts of 5-hydroxytryptamine (5-HT). We hypothesized that the 5-HT contained within the PVAT is functional and vasoactive. Isolated tissue baths were used for isometric contractility studies and high performance liquid chromatography was used to quantitatively measure amines in the PVAT and release studies. The 5-HT releaser fenfluramine (10 nM-100 μΜ) was tested for its ability to contract arteries with and without PVAT. Contraction was reported as a percentage of the initial contraction to 10 μΜ phenylephrine. The RA with PVAT contracted to fenfluramine to a greater maximum (98±10%) than RA without PVAT (24±4%), while no difference in contraction of SMA to maximum fenfluramine with (78±2%) and without (75±6%) PVAT was observed. Contradicting our hypothesis, the maximum contraction of RA with PVAT to fenfluramine was diminished by the alpha-1 adrenoreceptor antagonist prazosin (100 nM; vehicle: 71±4%, prazosin: 24±2%) and the norepinephrine transporter (NET) inhibitor nisoxetine (1 μΜ; vehicle: 71±4%, nisoxetine: 25±4%) but not the 5-HT2A/2C receptor antagonist ketanserin (10 nM) or serotonin specific reuptake inhibitor fluoxetine (10 μΜ). To test if fenfluramine caused release of 5-HT or NE from PVAT, PVAT from RA was incubated with vehicle or fenfluramine (10 μΜ−10 mM), and amines released into the incubating buffer were quantified. A pronounced concentration-dependent NE-release (more than 5-HT) was observed. Collectively, this research illustrates the pharmacology of fenfluramine to primarily stimulate NE release (better than 5-HT) in a NET-dependent manner, leading to vasoconstriction. This adds additional support to PVAT as being an important reservoir of amines.
Keywords: Serotonin (5-HT), Norepinephrine (NE), perivascular adipose tissue (PVAT), Fenfluramine
Graphical Abstract
1.0. Introduction
Perivascular adipose tissue (PVAT) is the fat that surrounds almost all blood vessels, with the exception of cerebral vessels. PVAT was first identified for its role in providing structural support to the vessels. In 1991, Soltis and Cassis reported that PVAT significantly reduced norepinephrine (NE)-induced contraction in rat aorta [1]. Since then, PVAT has been recognized for its ability to modulate vascular tone in both paracrine and endocrine manners, by releasing vasoactive substances. Dysfunction of PVAT has been linked to obesity, insulin resistance, diabetes, hypertension and atherosclerosis as well as vascular remodeling [2,3]. Because of PVAT’s intimate relationship with the blood vessel, it is critical to understand its biological composition and functions under normal physiological conditions firsthand.
PVAT is distinct from other adipose tissue depots in structure, anatomical location, secretion profiles and functions. PVAT around human coronary arteries express higher levels of pro-inflammatory cytokines such as interleukin (IL)-6, IL-8, macrophage chemoattractant protein (MCP)-1 and lesser adiponectin, compared to perirenal and subcutaneous depots [4]. PVAT adipocytes express lesser adipocyte differentiation markers vs non-PVAT fats [5]. Also, different PVAT depots have different developmental properties and functions, depending on their location and composition [6]. Thus, findings focusing on adipose depots such as epididymal, subscapular, subcutaneous, retroperitoneal fats may not be necessarily applicable to PVAT.
One of the long standing goals of our lab is to understand the vasoactive functions of 5-hydroxytryptamine (5-HT/serotonin). 5-HT is synthesized from the essential amino acid L-tryptophan. Evidence suggests that various rat PVAT depots express measurable amounts of indolamine 2,3 dioxygenase (IDO) [7]. Most of the L-tryptophan is metabolized by IDO to N-formylkynurenine, followed by conversion to its metabolite kynurenine [8]. Approximately 5–10% of tryptophan ultimately becomes 5-HT by actions of tryptophan hydroxylase (TPH). Though tryptophan to kynurenine conversion is dominant, we could measure 5-HT levels in PVAT and non-PVAT fats. This led to our hypothesis that the PVAT contained a releasable pool of 5-HT that could cause vasoconstriction. Fenfluramine, a molecule with a well-known pharmacology for its ability to release 5-HT from the nerve terminals in the central nervous system, was thus used to understand if 5-HT, a recognized vasoconstrictor, could be released from PVAT to cause a local contraction [9,10].
5-HT is well-documented as a primary neurotransmitter of the central nervous system (CNS), involved in mood and appetite regulation, cognition and memory [11]. However, 5-HT is found in many peripheral tissues that have diverse physiological functions [12]. Close to 90% of the peripheral 5-HT is gut-derived; produced by the enterochromaffin cells and stored in platelets [13]. Peripheral 5-HT is a well-known vasoconstrictor in arteries in vitro [14]. Hence, it is important to understand the sources and functions of peripheral serotonergic systems.
As such, we have investigated the PVAT around the rat thoracic aorta (RA) and superior mesenteric artery (SMA). Using a host of techniques, we tested the hypothesis that the 5-HT in PVAT could be released by fenfluramine and cause local arterial contraction. Contrary to our hypothesis, we discovered that fenfluramine-induced, PVAT-dependent contraction is likely mediated by NE and activation of alpha adrenergic receptors, not by 5-HT.
2.0. Materials and methods
2.1. Chemicals
Dexfenfluramine hydrochloride (Catalog# 2695), ketanserin (cat. # 0908) and fluoxetine hydrochloride (cat. # 0927) were purchased from Tocris Bioscience (Minneapolis, MN). Prazosin (cat. # P7791), nisoxetine (cat. # N151), and semicarbazide hydrochloride (cat. # S2201) were obtained from Sigma-Aldrich (St. Louis, MO). Pargyline hydrochloride (cat. # 10007852) was purchased from Cayman Chemical Company (Ann Arbor, MI). Collagenase type I (cat. # LS004196) was from Worthington Biochemical Corporation (Lakewood, NJ).
2.2. Animals
Animal maintenance and experimental protocols were approved by the MSU Institutional Animal Care and Use Committee, and complied with the National Institutes of Health Guide for Animal Care and Use of Laboratory Animals (2011). Male Sprague-Dawley rats (250–350 g or 8–12 weeks of age, Charles River, Indianapolis, IN USA; RRID: RGD_10395233) were used. Animals were maintained on a 12/12 light/dark cycle at 22–25 °C, fed ad-libitum. Prior to all dissections, the rats were anesthetized with sodium pentobarbital (60–80 mg/kg, i.p.) and death was assured by creating a bilateral pneumothorax.
2.3. Fat handling and dissection
RA, SMA, small mesenteric resistance arteries (MR) and retroperitoneal (RP) fat (fat behind the kidneys) were all dissected from the same rats. Arteries+PVAT were isolated and, where appropriate, cleaned of PVAT by being stabilized on a wire or pinned in a silastic-impregnated dish filled with physiological salt solution [PSS; in mM; 130 NaCl; 4.7 KCl; 1.17 MgSO4 7H2O; 1.18 K2HPO4; 14.8 NaHCO3; 5.5 dextrose; 0.03 CaNa2 ethylenediametetraacetic acid; 1.6 CaCl2 (pH 7.2)]. In other instances, PVAT was dissected from vessels and RP fat was cleaned of small blood vessels and used for HPLC measures. Where necessary, tissues/adipocytes were homogenized in 2.0 mL tubes (Omni, Kennesaw, GA) with 1.4 mm ceramic bead media (cat. #19–645, Omni) using the Omni Bead Ruptor.
2.4. Adipocyte isolation
RA PVAT was added to 1 mL PSS with 1 mg/mL collagenase (type 1) and incubated at 37° C with slow rotation until complete digestion (~ 1 hour). The digested PVAT was centrifuged at 200 g for 10 min. The stromal vascular fraction pellets to the bottom of the tube, while the adipocytes float to the top. The adipocytes were transferred to a new tube with a wide bore pipette tip and washed thrice using PSS. Isolated adipocytes were imaged using Cellometer®. The adipocytes were homogenized, centrifuged for 15 min at 18,000 g and supernatants were saved for HPLC analysis.
2.5. RNA isolation and RT-qPCR
RA PVAT, locus coeruleus, and total brain samples from SD rats were homogenized (as described above). RNA was isolated with the Zymo Quick-RNA Mini Prep Kit (cat. # R1054, Zymo Research, Irvine, CA), then quantified on a NanoDrop 2000c (Thermo Scientific). cDNA was reverse transcribed using the High Capacity cDNA kit (cat. #4368814, Thermo Fisher Scientific). RT-qPCR was performed on a QuantStudio 7 Flex Real-Time PCR system using Fast SYBR Green Master Mix (cat. # 4385612, Thermo Fisher Scientific) with the following parameters: 95°C for 20 s; 95°C for 1 s and 60°C for 20 s for 40 cycles, followed by a melt curve to determine the presence of a single PCR product. Primers were from Integrated DNA Technologies (IDT, Coralville, IA): SERT forward primer (FP) = 5’-GAACTCCTGGAACACTGGCA-3’, reverse primer (RP)= 5’- CAGGACATGGCGCAAGTAGA-3’; NET FP= 5’-AACGCCTACCTGCACATTGA-3’, RP= 5’-GTCAGCAAGGCATCCCTGTA-3’; B2m (reference gene) FP = 5’-CACTGAATTCACACCCACCG-3, RP = 5’-TTACATGTCTCGGTCCCAGG-3’. Data were analyzed with the 2−ΔCq method in which Cq is the threshold cycle.
2.6. Isometric contraction
SMA and RA, cleaned of fat (-PVAT) or with fat intact (+PVAT) and the endothelium intact, were mounted on isometric transducers (FT03; Grass Instruments, Quincy, MA) connected to a PowerLab Data Acquisitions unit (ADInstruments, Colorado Springs, CO, USA). Baths contained warmed, oxygenated PSS. Rings were pulled to optimum passive tension (1.2 g for SMA and 4 g for RA) and equilibrated for 1 h with washes every 20 min. The vessels were exposed to an initial concentration of 10 μΜ phenylephrine (PE) to test viability and to elicit maximum contraction. Tissues were further washed and returned to baseline. Presence of the endothelium was verified with a tissue relaxation caused with addition of acetylcholine (1 μΜ) after inducing contraction with a half-maximal PE concentration. The tissues were washed and returned to baseline. Either vehicle or an inhibitor was added for 1 h without washing. Inhibitors included prazosin (100 nM), nisoxetine (1 μΜ), ketanserin (10 nM) or fluoxetine (10 μM). Fenfluramine was then added in a cumulative fashion (10 nM-300 μM). Concentrations of inhibitors were based on their affinity for their intended target. For example, the 5-HT2A receptor antagonist, at 10 nM, should occupy ~91 % of the 5-HT2A receptors present but should not occupy other 5-HT receptors or alpha adrenergic receptors, as based on binding affinities available from the literature and the PDSP data base. Concentrations of prazosin were similarly determined. For concentrations of SERT and NET inhibitors, these were based on not only their affinity for their targets but on their ability to inhibit ASP+ uptake into PVAT, a transporter use-dependent dye [34].
2.7. Amine content and release studies (HPLC)
Whole rat fats (PVATs and RP fat) were homogenized (using bead ruptor as described above) in 4 times their weights with 0.1 M perchloric acid and run through high performance liquid chromatography (HPLC): a Coulochem III (Thermo Scientific, Waltham, MA) electrochemical detector set at −300 mV, HR-80 reverse-phase column, Cat-A-Phase II mobile phase, 1.1 mL/min flow rate. The separation column was maintained at 35 °C. Quantification of analytes was done by performing a standard curve periodically and the limit of detection was 0.1 ng/mL for catecholamines and 0.5 ng/mL for 5-HT.
In another experiment, RA PVAT from each animal was blotted, weighed and equally split into tubes each containing 250 μL fresh acidified PSS (pH 6.0) and the amine metabolism inhibitors semicarbazide hydrochloride (inhibits semicarbazide sensitive amine oxidase; 1 mM) and pargyline (inhibits monoamine oxidases; 10 μΜ). The tissues were minced and equilibrated at 37 °C for 30 min. Each tube then received a different concentration of fenfluramine (10 μΜ−10 mM) or vehicle, and incubated at 37°C for an additional 60 min. The supernatant was filtered through a 30 kDa filter tube, centrifuged (13000 rcf, 15 min at 4°C) and stored in −80 °C for HPLC analysis.
2.8. Data presentation and statistics
Data are reported as means±SEM for number of animals (biological replicates) indicated by N. Statistical analyses were performed with GraphPad Prism 7.0 (GraphPad Software Inc., La Jolla CA; RRID: SCR_002798). Contraction was reported as a percentage of the initial contraction to 10 μΜ PE. Two-way ANOVA with post hoc Tukey test (equal variances validated by Bartlett’s test) was performed for isometric contractility studies. P< 0.05 was considered statistically significant. The vehicles were pooled for contractility studies using inhibitors ketanserin, prazosin, nisoxetine and fluoxetine. Thus, this vehicle curve is replicated in Fig 3A-B and Fig 4A-B. Potencies (-log EC50) were calculated using GraphPad Prism 7.0. If a plateau was not obtained even at the highest fenfluramine concentrations, potencies were not determined. All HPLC studies were reported as ng amine/g tissue. For the amine release study, 95% confidence intervals excluding the baseline averages were considered statistically significant. For other HPLC studies, t-test was performed and P< 0.05 was considered statistically significant.
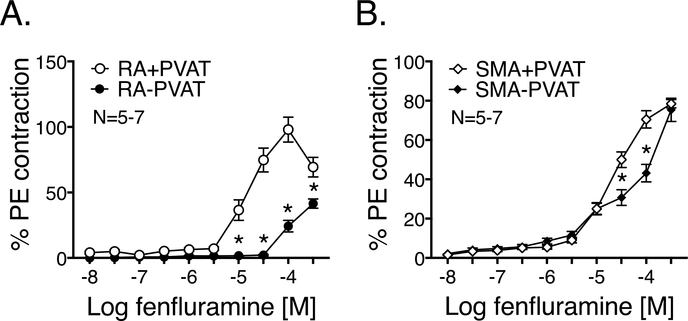
Figure 3: Fenfluramine causes a PVAT-dependent contraction in RA.
Fenfluramine-induced contraction (10 nM- 300 μM) with or without PVAT in the isolated RA (Fig 3A) and SMA (Fig 3B). Observed maximum PE contraction was (mg): 1852± 81.1 (RA+PVAT), 2061.3± 87.8 (RA-PVAT), 1196.4± 51.7 (SMA+PVAT) and 1270.5± 38.4 (SMA-PVAT). Points represent means±SEM for the number of animals indicated by N. * denotes significant difference (P<0.05 by 2-way ANOVA) between the vessel with and without PVAT at concentrations marked.
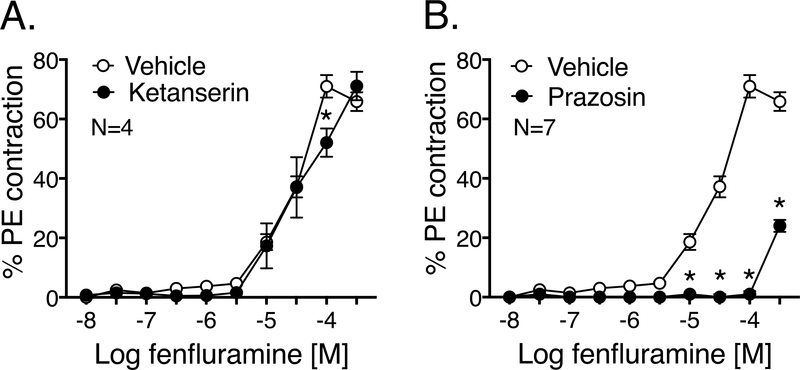
Figure 4: Fenfluramine-induced PVAT-dependent contraction is mediated by norepinephrine.
Fenfluramine-induced contraction (10 nM- 300 μΜ) of isolated RA with PVAT and with/without the following inhibitors: ketanserin 10 nM (Fig 4A) and prazosin 100 nM (Fig 4B). Both the inhibitors were paired in comparison to the same vehicle. Maximum PE contraction was (mg): 1301 ± 85.3 (vehicle), 1625.3± 128 (ketanserin) and 1886.3± 69.4 (prazosin). Points represent means± SEM for the number of animals indicated by N. * denotes significant difference (P<0.05 by 2-way ANOVA) between the vessels with and without the inhibitor.
3.0. Results
3.1. 5-HT and NE are present in PVAT
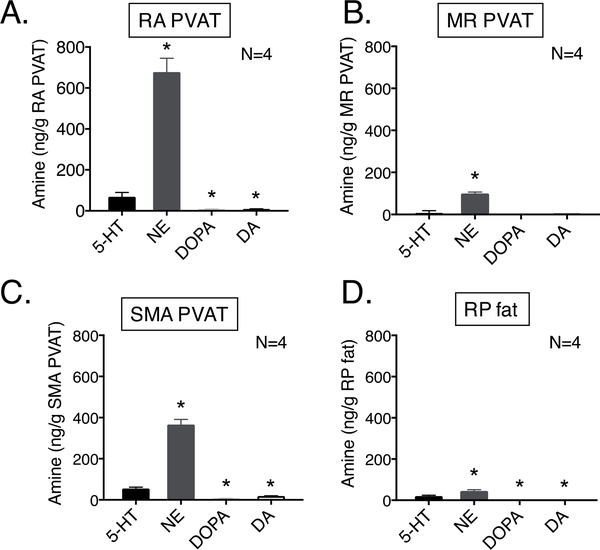
Using HPLC, 5-HT and catecholamines such as NE, epinephrine, DOPA (dihydroxyphenylalanine) and dopamine contents were measured in four different fats (all tissues taken from the same animal): fat around the thoracic aorta (RA PVAT), superior mesenteric artery (SMA PVAT), small mesenteric resistance arteries (MR PVAT) and non-PVAT retroperitoneal fat (RP-fat directly behind the kidneys). Figure 1A-D demonstrates that all the different fat depots, importantly the RA PVAT and SMA PVAT, contain measurable amounts of 5-HT and catecholamines. Epinephrine was not detectable in any of the tissues considered here. NE was more concentrated than 5-HT in all these fats measured.
Figure 1: 5-HT and catecholamines are present in PVAT.
HPLC measures of amines as ng amine/g tissue in fats: rat aortic (RA) PVAT (Fig 1A), mesenteric resistance (MR) PVAT (Fig 1B), superior mesenteric (SMA) PVAT (Fig 1C), non-PVAT retroperitoneal (RP) fat (Fig 1D). All fats were taken from the same rats. Bars represent means±SEM for the number of animals indicated by N. * indicates significant difference (P<0.05 by t-test) from 5-HT in the given fat.
3.2. 5-HT and NE are present in RA PVAT adipocytes
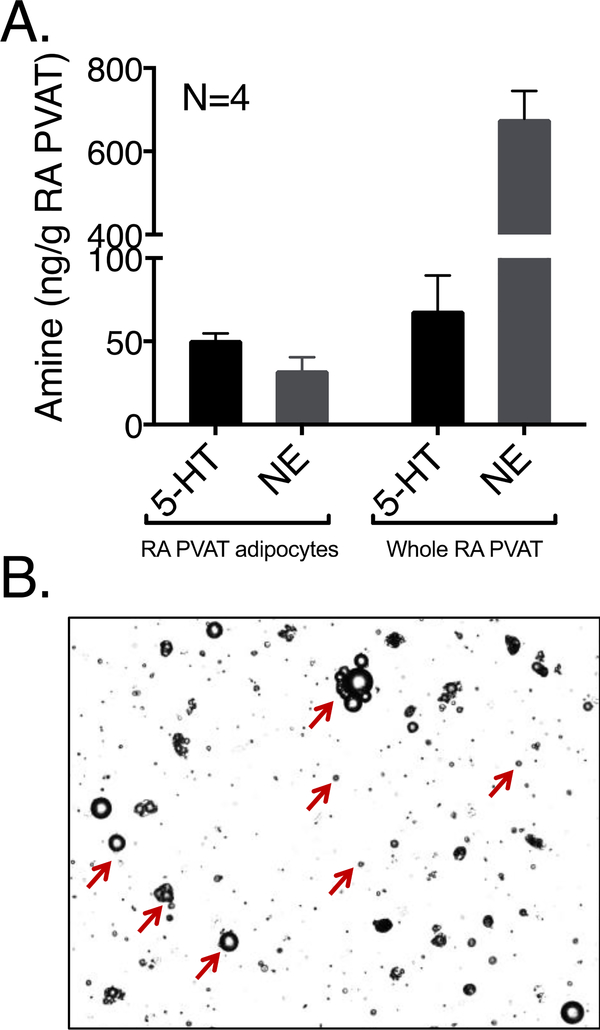
5-HT and catecholamines including NE, epinephrine, DOPA and DA were measured by HPLC in adipocytes isolated from RA PVAT (Figure 2A) as compared to those from whole RA PVAT (repeated from Figure 1A). Epinephrine, DOPA and DA were not present in detectable quantities in the adipocytes. The purity of adipocyte isolation was captured using Cellometer® Vision (Nexcelcom Bioscience LLC, Lawrence, MA) with every isolation and a representative image is shown in Figure 2B. We conclude that the concentration of NE and 5-HT in the adipocytes themselves is similar.
Figure 2: RA PVAT adipocytes contain 5-HT and NE.
HPLC measures of amines as ng amine/g tissue in RA PVAT (Fig 2A). Amine measures in whole RA PVAT is adopted from Fig 1A for reference. Pictorial representation of isolated adipocytes (marked by red arrows) from RA PVAT after collagenase digestion (Fig 2B). Bars represent means±SEM for the number of animals indicated by N.
3.3. Fenfluramine induces a PVAT-dependent contraction in RA
Fenfluramine (10 nM- 300 μΜ) caused a concentration-dependent contraction of isolated RA, in the presence of intact PVAT. Contraction was significantly reduced when PVAT was removed (Figure 3A). Such a PVAT-dependent contraction, when stimulated with fenfluramine, was not prominent in SMA (Figure 3B). The potencies (-log EC50 [M]) of fenfluramine-induced contraction were 4.69±0.46 for RA+PVAT and 4.49±0.54 for SMA+PVAT. If a plateau was not obtained even at the highest fenfluramine concentrations, potencies were not determined.
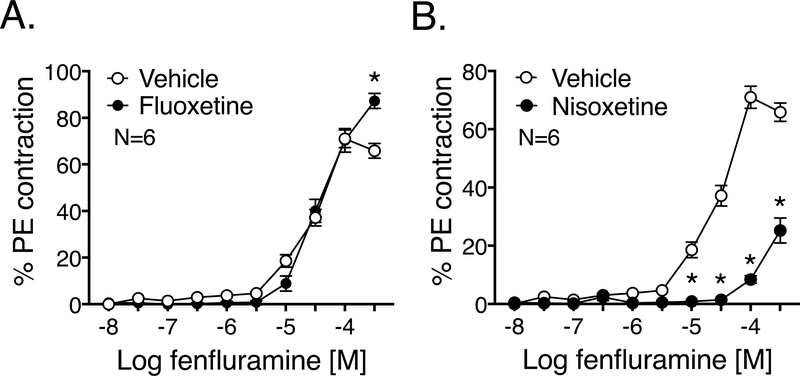
3.4. The PVAT-dependent fenfluramine-induced contraction is NE-dependent
Because there was a PVAT-dependent fenfluramine-induced contraction in RA, this artery+PVAT was used as the primary model to study the mechanism of fenfluramine-induced contraction. Surprisingly, the 5-HT2A/2C receptor antagonist ketanserin (10 nM) did not change the vessel’s ability to contract to fenfluramine (Figure 4A). By contrast, the α-adrenoreceptor antagonist prazosin (100 nM) caused a significant shift and reduction in fenfluramine-induced maximum contraction of RA with intact PVAT- vehicle: 71± 4%, prazosin: 24±2% (Figure 4B). The potency (-log EC50 [M]) of fenfluramine-induced contraction with vehicle was 4.34±0.11 (same vehicle curve for Figures 4A-B, 5A-B).
Figure 5: Fenfluramine-induced PVAT-dependent contraction is also NET-dependent.
Fenfluramine-induced contraction (10 nM-300 μΜ) of isolated RA with PVAT and with/without the following inhibitors: fluoxetine 10 μM (Fig 5A) and nisoxetine 1 μM (Fig 5B). Both the inhibitors were paired in comparison to the same vehicle as in Fig 3A-B. Maximum PE contraction was (mg): 2032.5± 63.5 (vehicle) and 2272.7± 132 (fluoxetine); 1301 ± 85.3 (vehicle) and 1618.3± 121.7 (nisoxetine). Points represent means± SEM for the number of animals indicated by N. * denotes significant difference (P<0.05 by 2-way ANOVA) between the vessel with and without the inhibitor.
3.5. The PVAT-dependent fenfluramine-induced contraction is NET-mediated
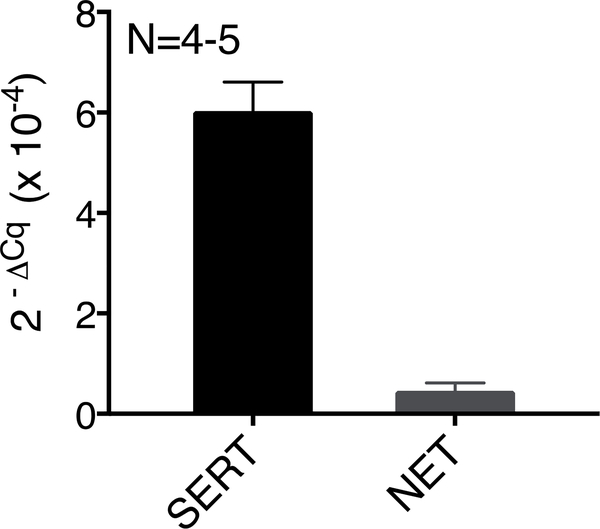
Isolated RA+PVAT was subject to fluoxetine incubation to investigate the involvement of SERT in fenfluramine-stimulated contraction. Fluoxetine (10 μM) did not shift the fenfluramine-induced contraction (Figure 5A). Conversely, the NET inhibitor nisoxetine (1 μM) caused a significant shift and attenuated the fenfluramine-induced maximum contraction of RA+PVAT- vehicle: 71±4%, nisoxetine: 25±4% (Figure 5B). RA PVAT contains SERT and NET mRNA, as measured by quantitative RT-PCR (Figure 6).
Figure 6: RA PVAT expresses SERT mRNA.
SERT and NET mRNA, quantified by qPCR in RA PVAT. Beta-2-microglobulin was used as the reference gene. Rat brain and locus coeruleus were used as positive controls for SERT and NET respectively (data not shown). Points represent means± SEM for the number of animals indicated by N.
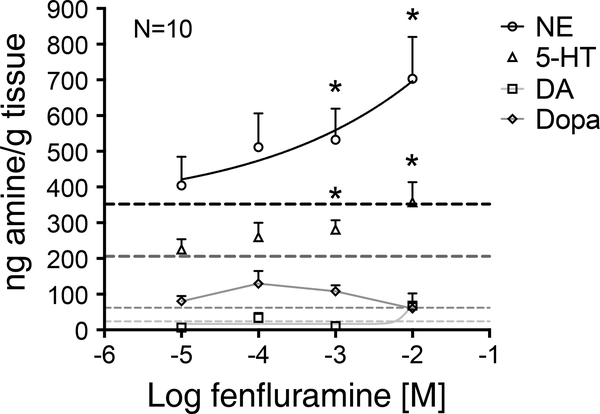
3.6. Fenfluramine predominantly promotes NE (more than 5-HT) release from the RA PVAT
In separate experiments, fenfluramine (10 μM-10 mM) stimulated a significant concentration-dependent release of both NE and 5-HT from PVAT isolated from RA. Reflecting the greater content of NE vs 5-HT in PVAT, the magnitude of NE released from the PVAT into the surrounding buffer was greater than the 5-HT released (Figure 7).
Figure 7: Fenfluramine predominantly promotes NE release (more than 5-HT) from PVAT.
Fenfluramine-induced (10 μΜ−10 mM) amine release from PVAT isolated from RA: NE, 5-HT, DOPA and dopamine (DA), as measured in ng amine/g tissue by HPLC. Dotted lines represent respective basal amine release without fenfluramine induction. Points represent means±SEM for the number of animals indicated by N. * denotes significant difference between basal and stimulated release, as determined by 95% confidence intervals, excluding their respective basal release values.
4.0. Discussion
The current study investigated the presence and ability of 5-HT in PVAT to cause a vascular contraction with paracrine release by fenfluramine. The results of this research disproved our original hypothesis. Although 5-HT from PVAT could be released by fenfluramine, the released 5-HT was not responsible for vasoconstriction. This work is important because (1) it is the first time presence of 5-HT has been reported in any kind of PVAT; (2) the study highlights the ability of fenfluramine to trigger NE release to a greater magnitude than 5-HT from PVAT; and (3) the work demonstrates fenfluramine-induced contraction in RA+PVAT to be NET-dependent and SERT-independent. These confirm an early but not well remembered study that fenfluramine is not highly selective for SERT and is also a potent NET substrate [15].
PVAT is a reservoir of biogenic amines:
PVAT is composed of adipocytes, pre-adipocytes, immune cells, endothelial cells, fibroblasts and red blood cells [2]. HPLC measures support that significant concentrations of NE, 5-HT, DOPA and DA are present in different PVATs as well as a non-PVAT visceral fat. The ability to synthesize and/or take up 5-HT by any specific cell type in PVAT is unestablished, though such actions have been observed in the blood vessels that PVAT surrounds [16]. SERT-mediated accumulation of 5-HT occurs in different tissues of cardiovascular, endocrine, gastrointestinal and genitourinary systems [12]. Importantly, adipocytes from rat visceral adipose tissue can synthesize and uptake 5-HT [17]. HPLC measures in our study also support that 5-HT and NE are present in isolated primary adipocytes from RA PVAT and interestingly, a majority of the 5-HT in RA PVAT is contained in the adipocytes. This differed for NE, where a significant amount of NE was lost when isolating adipocytes from whole PVAT. This suggests there are cells in the stromal vascular fraction that could contain NE, and these could include progenitor cells, neurons, fibroblasts and immune cells. Formal innervation of PVAT by branches of the autonomic nervous system has not been established. However, our lab has published that in a different fat, the mesenteric PVAT, adipocytes contain NE and 5-HT independent of the sympathetic nerves [18]. Whether a PVAT adipocyte contains a functional serotonergic system needs further evaluation. Our data confirms findings from other studies that PVAT and non-PVAT fats are similar in containing catecholamines and suggest that fats may be a reservoir of bioactive amines [18,19]. PVAT can be added to the list of existing peripheral 5-HT reserves.
Regional differences between PVATs around different vascular beds:
PVAT-dependent fenfluramine-induced contraction was evident with RA and not so with SMA. Such regional differences of PVAT have been recognized. Different potassium channels are activated in PVAT around mesenteric arteries (Kv) vs aorta (Katp) in effect of ADRF (adipocyte derived relaxing factor) [20]. In another study, RA exhibited sluggish responses to nerve stimulation and ineffective single impulses in comparison to SMA [21]. By contrast, both RA and SMA PVATs responded similarly to tyramine by releasing 5-HT [18]. These observed differences between PVATs from different vascular beds could arise due to several inherent differences between the tissues. First, RA PVAT is more brown adipose tissue (BAT)-like while SMA PVAT is a mix of brown and white adipose tissue (WAT) [6]. Second, the innervation status of PVATs itself is unknown, although RA has poorer adrenergic innervation than SMA [22]. Third, RA PVAT has higher NE and 5-HT content than SMA PVAT. The regional differences reported in this study are not surprising and could be explained by the differences in anatomical location as well as composition of RA and SMA PVATs. Regional differences are also reflected in lack of a PVAT-dependent contraction to fenfluramine in SMA vs RA. However, like the RA+PVAT, the 5-HT2A receptor antagonist ketanserin (10 nM) did not shift fenfluramine-induced contraction in isolated SMA+PVAT (not shown). Thus, the mechanism by which fenfluramine causes contraction is still not clear, though this is independent of 5-HT2A/2C receptor activation in both arteries.
Noradrenergic actions of fenfluramine:
Fenfluramine was used to test the hypothesis that PVAT 5-HT is vasoactive. As a potent (< 1 μΜ) 5-HT SERT substrate, fenfluramine has been widely used to study the dependence of 5-HT functions in the CNS [23]. Fenfluramine gets into the nerve terminal and facilitates reverse transport of 5-HT from neurons in the brain by an exchange-diffusion process as mediated by SERT [23]. If 5-HT was releasable from PVAT, then fenfluramine could use SERT to cause a PVAT-dependent contraction. Most findings indicate that fenfluramine uses SERT to cause an elevated extracellular 5-HT [24]. However, the SERT inhibitor fluoxetine did not attenuate fenfluramine-induced PVAT-dependent contraction. By contrast, the NET inhibitor nisoxetine caused a significant reduction in contraction. This supports an earlier study in which the ability of nisoxetine to attenuate a tyramine-induced PVAT-dependent contraction suggested that functional NET was present in PVAT [18]. SERT mRNA was 12-times more concentrated than NET in RA PVAT. We have not been able to use NET or SERT antibodies, most important to the present study, with any level of confidence in either IHC or Western analyses to determine if more SERT protein vs NET protein exists. These data suggest that SERT does not participate in fenfluramine-induced contraction and that the contraction is facilitated by NET. Our results are in agreement with a critical study by Rothman et al, that fenfluramine and its metabolite norfenfluramine are potent NET substrates in brain tissues [15].
5-HT caused vasoconstriction of RA primarily via 5-HT2 receptor activation [26]. A reduction in fenfluramine-induced contraction was expected with blockade of this receptor if this PVAT-dependent contraction was 5-HT-dependent. Ketanserin used here at 10 nM concentration should be considered as a 5-HT2A antagonist according to published Ki values for cloned rat 5-HT2A (1 nM), rat 5-HT2B (4 μM) and rat 5-HT2C (60 nM) [34]. However, ketanserin did not shift or attenuate the PVAT-dependent contraction. In contrast, the alpha-1 adrenoreceptor antagonist prazosin caused a significant reduction in fenfluramine-induced contraction. According to PDSP database, fenfluramine has low affinity for 5-HT receptors (Ki; rat 5-HT2A 5 μM, rat 5- HT2C 3 μM, human 5-HT2B 6 μM) but has fairly high affinity for human α1-adrenergic receptor (200 nM). It is unlikely fenfluramine directly caused contraction through adrenergic receptor stimulation given the ability of nisoxetine to reduce contraction. In addition, our findings suggest that basal NE content is higher than 5-HT in PVAT and release of NE by fenfluramine into the external buffer is higher than 5-HT. Qualitatively, these observations line up with one another.
Rothman et al published that norfenfluramine was more potent than fenfuramine in releasing NE than 5-HT [15]. It is possible that fenfluramine was metabolized (de-ethylated) to norfenfluramine within our experimental setup. Regardless, NE was the mediator of contraction, because it was that which could be released by fenfluramine and/or because more of it than 5-HT was released. The question remains as to whether this mechanism can be tapped into, physiologically. Are there endogenous hormones that could function like fenfluramine in promoting amine release from PVAT to influence blood pressure? We simply do not know at this time. Nevertheless, these results give pause to attributing all the pathological effects of fenfluramine (for example, pulmonary hypertension and valvular dysfunction) to 5-HT release and suggest NE might also be considered [27]. Together, the data presented indicate that fenfluramine-induced PVAT-dependent contraction in RA occurs through NE and not 5-HT.
The many colors of 5-HT:
What other functions could PVAT 5-HT likely be involved in? Although the released 5-HT could not be ascribed to fenfluramine-induced contraction, the 5-HT released might be sufficient to produce other local biological effects. Specifically, adipocyte-specific inhibition of 5-HT synthesis (in mice) leads to inhibition of lipogenesis in epididymal WAT, induction of browning in inguinal WAT and activation of adaptive thermogenesis in BAT [28]. This suggests that 5-HT is needed for fatty acid synthesis in an adipocyte. 5-HT accelerates adipocyte differentiation in 3T3-L1 mouse adipocyte cells via 5-HT2A/2C receptors [29]. In a different study, 5-HT stimulated WAT lipolysis and suppressed adiponectin (an adipokine) release from WAT [30]. 5-HT can also potentiate contraction induced by other agonists as well as heighten the mitogenic effects of some mitogenic compounds [31–33]. Thus, understanding PVAT-specific functions of 5-HT could facilitate new diagnostic/therapeutic targets to modulate specific serotonergic systems in hypertension and metabolic dysregulation.
5.0. Limitations:
The mouse adipocyte model 3T3-L1 cells as well as adipocytes from rat visceral adipose tissue express the rate limiting enzyme TPH1 for 5-HT synthesis [29]. But every adipose tissue depot is relatively unique in location, form and function. Hence, application of findings in non-PVAT adipose tissues to PVAT is limited. It remains unknown whether 5-HT can be synthesized in primary PVAT adipocytes or if the 5- HT content we measured in the whole fats and adipocytes is the result of 5-HT taken up into tissue from external sources (e.g. circulating 5-HT, nerve endings or platelets in PVAT). Though our data suggest that most of the 5-HT in RA PVAT comes from the adipocytes (and not platelets or nerves), we cannot exclude that platelets/nerves remaining in PVAT after washing could be a minor source of 5-HT. SERT and NET protein expression in PVAT is yet to be evaluated. From our experimental results, we cannot say whether NE and 5-HT are stored together, released together or if what is released reflects a percentage of what is stored in the tissue relative to each amine. Finally, we have used fenfluramine, a prototypical 5-HT releaser, which also is a potent NET substrate, to understand the vasoactivity of 5-HT. To understand the serotonergic system in PVAT, identifying a more specific 5-HT releaser is important.
6.0. Conclusion
In conclusion, this study has discovered PVAT as a peripheral 5-HT reservoir, and confirms PVAT as a repository of bioamines. Though 5-HT is present in PVAT, fenfluramine caused a PVAT-dependent, NE-mediated, NET-dependent vascular contraction of RA. This raises the question of whether there is a functional PVAT serotonergic system and if so, can this be specifically modulated in pathological states, for therapeutic benefits.
Table 1:
Author Checklist for Original Articles to be submitted to Pharmacological Research
| Questions | reply | ||
|---|---|---|---|
| Yes | No | Not Applicable | |
| Formatting - The submission will automatically be rejected if the first six questions are not marked “yes” (questions 1–6) or “not applicable” (limited to questions 3–6) | |||
| 1. Are all tables and figures numbered and appropriately titled with descriptive legends that permit stand-alone interpretation? | yes | ||
| 2. Are all data shown on figures and tables mentioned in the text of the Results section? | yes | ||
| 3. Are the whole un-cropped images of the original western blots from which figures have been derived shown as supplemental figures? | n.a. | ||
| 4. In case of human studies, has specific mention been made of the study compliance with the regulations of the country(ies) in which the study was carried out ? | n.a. | ||
| 5. In case of human studies, has the study been registered on an accessible international registry/database of clinical trials (e.g. EudraCT, ClinicalTrials.gov, ChiCTR, ANZCTR, JPRN and the like) | n.a. | ||
| 6. In case of studies on animals, is there a statement indicating compliance with regulations on the ethical treatment of animals including identification of the institutional committee that approved the experiments? | yes | ||
| Introduction | |||
| 7. Is there a clear statement with background describing the hypothesis being tested by this study? | yes | ||
| 8. Are the primary endpoints clearly described? | yes | ||
| Materials and Methods | |||
| 9. Are the sources of all materials clearly indicated? | yes | ||
| 10. Is(are) the chemical structure(s) of any new compound(s) presented as a figure in the manuscript or referenced in the literature? | n.a. | ||
| 11. Are the source(s) and passage number of cell lines indicated? | n.a. | ||
| 12. Are the source, catalogue number and lot for commercial antibodies indicated? | n.a. | ||
| 13. Are the species, strain, sex, weight and source of the animal subjects provided? | yes | ||
| 14. Is the rationale provided for the selection of concentrations, doses, route and frequency of compound administration? | yes | ||
| 15. Are quantified results (e.g. IC50 and/or EC50 values) of concentration- and dose-response experiments included in the report? | yes | ||
| 16. Is the method of anaesthesia described? | yes | ||
| 17. Are all group sizes approximately the same? | yes | ||
| 18. Were the criteria used for excluding any data from analysis determined prospectively and clearly stated? | n.a. | ||
| 19. Was the investigator responsible for data analysis blinded to which samples/animals represent treatments and controls? | no | ||
| 20. Is the exact sample size (n) for each experimental group/condition clearly indicated in the text and/or on the tables and figures? | yes | ||
| 21. Are the reported data displayed as the mean +/− an estimate of variability (SD, SEM) of three or more independent experimental replications? | yes | ||
| 22. Is the number of replications used to generate an individual data point in each of the independent experiments clearly indicated? | yes | ||
| 23. Were the statistical tests employed to analyse the primary endpoints predetermined as part of the experimental design? | no | ||
| 24. Is the threshold for statistical significance (P value) clearly indicated? | yes | ||
| 25. Were the data normalised? | yes | ||
| 26. Were post-hoc tests used to assess the statistical significance among means? | yes | ||
| 27. Was the study exploratory rather than hypothesis-driven? | no | ||
| 28. Were human tissues or fluids used in this study? | no | ||
| Results | |||
| 29. If western blots are shown, are the following included: i) appropriate loading controls for each western blot, ii) replication data, iii) quantification, and iv) the results of a statistical analysis? | n.a. | ||
| 30. Were MIQE guidelines followed in the quantitative analysis and presentation of PCR and RT-PCR findings? | yes | ||
| 31. Was a reference standard (positive or negative controls) included in the study to validate the experiment? | yes | ||
| Discussion | |||
| 32. Are all the findings considered within the context of the hypothesis presented in the Introduction? | yes | ||
| 33. Are the primary conclusions and their implications clearly stated? | yes | ||
| 34. Are any secondary endpoints reported and are these sufficiently powered for appropriate statistical analysis? | n.a. | ||
| 35. Are the limitations of the current study or alternative interpretations of the findings clearly stated? | yes | ||
| Conflict of Interest/Financial Support | |||
| 36. Is the conflict of interest statement is included in the manuscript? | yes | ||
| 37. Are all organisations providing funding for this work listed in the Acknowledgments? | yes | ||
Feedback/suggestions on the checklist by the author
We really liked the idea of having an author checklist. It was very helpful.
7.0 Acknowledgement
This work was funded by the National Institutes of Health P01 HL70687.
Footnotes
Conflict of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
8.0 References:
- [1].Soltis EE, Cassis LA (1991) Influence of perivascular adipose tissue on rat aortic smooth muscle responsiveness. Clin Exp hypertens A. 13(2): 277–96. PMID: . [DOI] [PubMed] [Google Scholar]
- [2].Szaz T, Webb RC (2012) Perivascular adipose tissue: more than just structural support. Clin Sci (Lond). 122(1):1–12. PMID: ; doi: 10.1042/CS20110151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Takaoka M, Nagata D, Kihara S, Shimomura I, Kimura Y, Tabata Y, Saito Y, Nagai R, Sata M (2009) Periadventital adipose tissue plays a critical role in vascular remodeling. Circ Res. 105(9): 906–11. PMID: ; doi: 10.1161/CIRCRESAHA.109.199653. [DOI] [PubMed] [Google Scholar]
- [4].Omar A, Chatterjee TK, Tang Y, Hui DY, Weintraub NL (2014) Proinflammatory phenotype of perivascular adipocytes. Arterioscler Thromb Vasc Biol. 34(8):1631–1636. PMID: ; doi: 10.1161/ATVBAHA.114.303030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chatterjee TK, Stoll LL, Denning GM, Harrelson A, Blomkalns AL, Idelman G, Rothenberg FG, Neltner B, Romig-Martin SA, Dickson EW, Rudich S, Weintraub NL (2009) Proinflammatory phenotype of perivascular adipocytes: influence of high-fat feeding. Circ Res. 104(4):541–9. PMID: ; doi: 10.1161/CIRCRESAHA.108.182998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fernández-Alfonso MS, Gil-Ortega M, Somoza B (2009) Role of Perivascular Adipose Tissue in Vascular Function In: Abraham D, Clive H, Dashwood M, Coghlan G (eds) Advances in Vascular Medicine. Springer, London: Print ISBN: 978–1-84882–636-6; Online ISBN: 978–1-84882–637-3; doi: 10.1007/978-1-84882-637-3_10. [DOI] [Google Scholar]
- [7].Watts SW, Shaw S, Burnett R, Dorrance AM (2011) Indoleamine 2,3-diooxygenase in periaortic fat: mechanisms of inhibition of contraction. Am J Physiol Heart Circ Physiol. 301(4): H1236–47. PMID: ; doi: 10.1152/ajpheart.00384.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ball HJ, Yuasa HJ, Austin CJD, Weiser S, Hunt NH (2009) Indoleamine 2,3-dioxygenase-2; a new enzyme in the kynurenine pathway. Int J Biochem Cell Biol. 41(3) 467–71. doi: 10.1016/j.biocel.2008.01.005. [DOI] [PubMed] [Google Scholar]
- [9].Garattini S, Buczko W, Jori A, Samanin R (1975) The mechanism of action of fenfluramine. Postgraduate Medical Journal, 51(Sup.1), 27–35. [PubMed] [Google Scholar]
- [10].Raiteri M, Bonanno G, Vallebuona F (1995) In vitro and in vivo effects of d-fenfluramine: no apparent relation between 5-hydroxytryptamine release and hypophagia. J Pharmacol Exp Ther. 273(2):643–9. PMID: . [PubMed] [Google Scholar]
- [11].Meneses A (1999) 5-HT system and cognition. Neurosci Biobehav Rev. 23(8):1111–25. PMID: . [DOI] [PubMed] [Google Scholar]
- [12].Linder AE, Beggs KM, Burnett RJ, Watts SW (2009) Body distribution of infused serotonin in rats. Clin Exp Pharm Physiol. 36(5–6):599–601. PMID: ; doi: 10.1111/j.1440-1681.2009.05147.x. [DOI] [PubMed] [Google Scholar]
- [13].Keszthelyi D, Troost FJ, Masclee AA (2009) Understanding the role of tryptophan and serotonin metabolism in gastrointestinal function. Neurogastroenterol Motil. 21(12):1239–49. PMID: ; doi: 10.1111/j.1365-2982.2009.01370.x. [DOI] [PubMed] [Google Scholar]
- [14].Watts SW, Davis RP (2011) 5-hydroxytryptamine receptors in systemic hypertension: an arterial focus. Cardiovasc Ther. 29(1):54–67. PMID: ; doi: 10.1111/j.1755-5922.2010.00173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rothman RB, Clark RD, Partilla JS, Baumann MH (2003) (+)-Fenfluramine and its major metabolite, (+)-norfenfluramine, are potent substrates for norepinephrine transporters. J Pharmacol Exp Ther. 305(3):1191–9. PMID: ; doi: 10.1124/jpet.103.049684. [DOI] [PubMed] [Google Scholar]
- [16].Ni W, Geddes TJ, Priestley JR, Szasz T, Kuhn DM, Watts SW (2008) The existence of a local 5-hydroxytryptaminergic system in peripheral arteries. Br J Pharmacol. 154(3):663–74. PMID: ; doi: 10.1038/bjp.2008.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Stunes AK, Reseland JE, Hauso O, Kidd M, Tommeras K, Waldum HL, Syversen U, Gustafsson BI (2011) Adipocytes express a functional system for serotonin synthesis, reuptake and receptor activation. Diabetes Obes Metab. 13(6):551–8. PMID: ; doi: 10.1111/j.1463-1326.2011.01378.x. [DOI] [PubMed] [Google Scholar]
- [18].Ayala-Lopez N, Martini M, Jackson WF, Darios E, Burnett R, Seitz B, Fink GD, Watts SW (2014) Perivascular adipose tissue contains functional catecholamines. Phar Res Perspect. 2(3):e00041 PMID: ; doi: 10.1002/prp2.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Vargovic P, Ukropec J, Laukova M, Cleary S, Manz B, Pacak K, Kvetnansky R (2011) Adipocytes as a new source of catecholamine production. FEBS Lett. 585(14):2279–84. PMID: ; doi: 10.1016/j.febslet.2011.06.001. [DOI] [PubMed] [Google Scholar]
- [20].Verlohren S, Dubrovska G, Tsang SY, Essin K, Luft FC, Huang Y, Gollasch M. (2004) Visceral periadventitial adipose tissue regulates arterial tone of mesenteric arteries. Hypertension. 44(3):271–6. PMID: ; doi: 10.1161/01.HYP.0000140058.28994.ec. [DOI] [PubMed] [Google Scholar]
- [21].Nilsson H (1984) Different nerve responses in consecutive sections of the arterial system. Acta Physiol Scand. 121:353–36. doi: 10.1111/j.1748-1716.1984.tb07466.x. [DOI] [PubMed] [Google Scholar]
- [22].Kuchii M, Shibata S, Mori J (1973) Pharmacological nature of adrenergic innervation in rat aorta. Comp Gen Pharmac. 4:131–138. doi: 10.1016/0010-4035(73)90031-1. [DOI] [PubMed] [Google Scholar]
- [23].Rothman RB, Ayestas MA, Dersch CM, Baumann MH (1999) Aminorex, fenfluramine, and chlorphentermine are serotonin transporter substrates. Implications for primary pulmonary hypertension. Circ Res. 100(8):869–75. PMID: . [DOI] [PubMed] [Google Scholar]
- [24].Garattini S (1995) Biological actions of drugs affecting serotonin and eating. Obesity Res. 3(Suppl. 4), pp 463S–470S. doi: 10.1002/j.1550-8528.1995.tb00213.x. [DOI] [PubMed] [Google Scholar]
- [25].Caccia S, Conforti I, Duchier J, Garattini S (1985) Pharmacokinetics of fenfluramine and norfenfluramine in volunteers given D- and DL-fenfluramine for 15 days. Eur J Clin Pharmacol. 29(2):221–4. PMID: . [DOI] [PubMed] [Google Scholar]
- [26].Cohen ML, Fuller RW, Wiley KS (1981) Evidence for 5-HT2 receptors mediating contraction in vascular smooth muscle. J Pharmacol Exp Ther. 218(2):421–5. PMID: . [PubMed] [Google Scholar]
- [27].Teramae CY, Connolly HM, Grogan M, Miller FA Jr (2000) Diet drug-related cardiac valve disease: the Mayo Clinic echocardiographic laboratory experience. Mayo Clin Proc. 75(5):456–61. PMID: ; doi: 10.4065/75.5.456. [DOI] [PubMed] [Google Scholar]
- [28].Namkung J, Kim H, Park S (2015) Peripheral serotonin: a new player in systemic energy homeostasis. Mol Cells. 38(12):1023–1028. PMID: ; doi: 10.14348/molcells.2015.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kinoshita M, Ono K, Horie T, Nagao K, Nishi H, Kuwabara Y, Takanabe-Mori R, Hasegawa K, Kita T, Kimura T (2010) Regulation of Adipocyte Differentiation by Activation of Serotonin (5-HT) Receptors 5-HT2AR and 5-HT2CR and Involvement of MicroRNA-448-Mediated Repression of KLF5. Mol Endocrinol; 24(10): 1978–87. PMID: ; doi: 10.1210/me.2010-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Arreola R, Becerril-Villanueva E2, Cruz-Fuentes C1, Velasco-Velazquez MA3, Garces-Alvarez ME2, Hurtado-Alvarado G4, Quintero-Fabian S5, Pavon L2 (2015) Immunomodulatory effects mediated by serotonin. J Immunol Res. 2015:354957 PMID: ; doi: 10.1155/2015/354957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Van Nuetena JM, Janssensa WJ, Vanhoutte PM (1985) Serotonin and vascular reactivity. Pharm Res Comm. (Vol 17, No 7) pp 585–608. doi: 10.1016/0031-6989(85)90067-0. [DOI] [PubMed] [Google Scholar]
- [32].Nemecek GM, Coughlin SR, Handley DA, Moskowitz MA (1986) Stimulation of aortic smooth muscle cell mitogenesis by serotonin. Proc Natl Acad Sci USA. 83(3):674–678. PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Pakala R, Willerson JT, Benedict CR. (1994) Mitogenic effect of serotonin on vascular endothelial cells. Circ Res. 90(4):1919–26. PMID: . [DOI] [PubMed] [Google Scholar]
- [34].Bonhaus DW, Bach C, Desouza A, Salazar FHR, Matsouka BD, Zuppan P, Chan HW, Eglen RM (1995) The pharmacology and distribution of human 5- hydroxytryptamine2B (5-HT2B) receptor gene products: comparison with 5-HT2A and 5-HT2C receptors. Br J Pharm. 115,622–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ayala-Lopez N, Jackson WF, Burnett R, Wilson JN, Thompson JM, Watts SW (2015) Organic cation transporter 3 contributes to norepinephrine uptake into perivascular adipose tissue. Am J Physiol Heart Circ Physiol. PMID: ; doi: 10.1152/ajpheart.00308.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]