Abstract
Background and Purpose:
Given the heterogeneity of mobility outcomes post-stroke, the purpose of this study was to examine how the minimal detectable change (MDC) for gait speed varies based on an individual’s baseline walking speed.
Methods:
Seventy six participants with chronic stroke and able to walk without therapist assistance participated in two visits to record overground self-selected comfortable gait speed (CGS) and fast gait speed (FGS). Based on the CGS at visit one, participants were assigned to one of three speed groups: LOW (<0.4 m/s; N=32), MOD (0.4 m/s to 0.8 m/s; N=29), and HIGH functioning group (>0.8 m/s; N=15). Participants were then reclassified using updated gait speed cutoffs of 0.49 and 0.93 m/s. For each group, we determined test-retest reliability between visits, and the minimal detectable change for CGS and FGS.
Results:
Gait speed significantly increased from visit one to visit two for each group (p<0.001). The reliability for CGS declined with increasing gait speed, and MDC95 values increased with increasing gait speed (LOW: 0.10 m/s; MED: 0.15 m/s; HIGH: 0.18 m/s). Similar findings were observed for FGS, and when participants were recoded using alternative thresholds.
Discussion and Conclusions:
Slower walkers demonstrated greater consistency in walking speed from day to day, which contributed to a smaller MDC95 than faster walkers. These data will help researchers and clinicians adjust their expectations and goals when working with individuals with chronic stroke. Expectations for changing gait speed should be based on baseline gait speed, and will allow for more appropriate assessments of intervention outcomes.
Keywords: stroke, gait, speed, reliability, measurement
INTRODUCTION
Gait speed deficits are prevalent in individuals recovering from stroke and have been associated with limitations in physical activity and participation.1–4 Given the importance of gait speed 5 and the persistent deficits following stroke, rehabilitation interventions are often structured to address reduced gait speed.6 Improvements in gait speed are possible with appropriate interventions, even in the chronic stages of stroke.7, 8 In order to successfully interpret the validity and importance of gait speed changes it is important to know the Minimal Detectable Change (MDC) and Minimum Clinically Important Difference (MCID), respectively. Although there is less data published on MCID’s of gait speed post-stroke,9, 10 there have been numerous references for the MDC of gait speed in both subacute 11, 12 and chronic stroke.13–15 These prior works reveal a strikingly large range of gait speeds, yielding standard deviations of up to 0.30 m/s and suggesting that the computed MDCs may not be appropriate for all individuals. Of note, MDC scores may be influenced by baseline gait speeds.16
Heterogeneity in functional outcomes following stroke has been well documented.17, 18 The large variability in recovery from stroke is due to a variety of factors, including but not limited to, pre-morbid condition, stroke lesion size and location, genetic factors, and rehabilitation. Given this variation in functional recovery, it is likely that a single MDC may be inappropriate for all individuals. In particular, slower walkers tend to have less gait speed reserve,19 and thus may vary their gait speed less on a day to day basis. As a result, a slower walker may not need as large a change in gait speed as a faster walker to constitute a ‘real’ change. If a patient’s baseline gait speed is 0.2 m/s does that individual have to achieve the same increase in gait speed as someone whose baseline gait speed is 1.1 m/s in order to demonstrate a real change?
Previously, speed-based classifications have been developed to denote the importance of gait speed to community mobility.4 Most often cited, the classification by Perry and colleagues4 reported the mean gait speed of groups categorized based on their mobility in the home and the community. These mean gait speeds have since been interpreted as thresholds, and have even been used to imply a leap in functional status.6, 20 Despite a lack of validity for these thresholds, there is clearly value in such a system, as differences in limb mechanics21, 22 and functional outcomes20, 21 have been observed with speed-based classifications. Recently, however, Fulk and colleagues used data from two large randomized controlled trials to recalculate gait speed cut-offs to differentiate between home and community ambulators.23 These authors reported cut-offs which presumably represent a more accurate speed based classification because it is based on actual walking performance in the community.23
Given the variability in gait speed amongst individuals with chronic hemiparesis post-stroke, the primary purpose of this study was to examine how the MDC for gait speed varies according to the individual’s baseline gait speed. We hypothesized that individuals with slower gait speeds would have smaller MDCs, due to the limited ability to vary gait speed compared to individuals with faster gait speeds.19 It is important to present MDC’s for a range of gait speeds, including the thresholds based on walking performance,23 to inform future work.
METHODS
Participants
We included participants undergoing baseline testing for clinical trials who exhibited a range of chronic hemiparesis consistent with an ischemic or hemorrhagic unilateral brain lesion for this study. Potential subjects were excluded if they could not walk 10 m without therapist assistance, had a pre-existing cardiovascular, metabolic, or musculoskeletal condition that prohibited strenuous activity, a concurrent neurologic condition that could affect walking ability, a history of balance deficits or unexplained falls that predated the stroke, were receiving concurrent physical therapy, or had impaired cognition that affected the ability to follow directions. All participants signed an informed consent form approved by the University of North Carolina at Chapel Hill prior to participation. Prior to testing, participants completed the lower extremity portion of the Fugl-Meyer 24 to characterize our participant group.
Procedures and Data Management
All individuals participated in two visits separated by 19 ± 13 days (range: 3–57 days) to record overground gait speeds, using techniques previously described.14 Briefly, participants walked across an instrumented walkway (GAITRite, CIR Systems, Franklin, NJ) sampling at 60 Hz. At each visit, participants completed three trials at their self-selected comfortable gait speed (CGS) followed by three trials at the fastest possible walking speed (FGS). For the comfortable speed, participants were instructed to “walk at their normal, comfortable pace”; for the fast speed, participants were instructed to “walk as fast as they safely could”. For each trial, participants began approximately three feet before the mat and continued walking for approximately three feet (1m) beyond the end of the mat to allow for acceleration and deceleration. If necessary, participants used their typical ankle foot orthosis (AFO) or assistive devices, but did not receive therapist assistance. Care was taken to ensure that the same assistive device and/or AFO were used for both visits.
The GAITRite software was used to remove any partial steps (e.g., beginning or end of the walkway) and/or marks from assistive devices. Following the editing process we had an average of 27 ± 10 steps per subject for the CGS condition, and 23 ± 9 steps per subject for the FGS condition. We then used the GAITRite software to determine CGS and FGS for each participant as an average of the CGS and FGS trials, respectively. Based on the CGS at visit one, participants were assigned to one of three speed groups 4 based on walking performance: LOW (<0.40 m/s), MOD (0.40 m/s to 0.80 m/s), and HIGH (>0.80 m/s). Because of the presence of newly presented thresholds, we then re-grouped participants based on the recent work of Fulk and colleagues.23 Here, our LOW, MOD, and HIGH groups were determined by 0.49 m/s and 0.93 m/s thresholds.
Data Analysis
All statistical analyses were performed with SPSS (IBM, Chicago, IL). Gait speed was compared between visits for both CGS and FGS walking conditions using paired samples t-tests. Separate analyses were then performed for CGS and FGS variables. Normality of the difference between test and retest was assessed with the Shapiro-Wilk test (all p-values > 0.515) and confirmed visually with Q-Q plots. Across the entire participant cohort, as well as within each speed-based classification group, we calculated the intraclass correlation coefficient (ICC 2,1) to determine test-retest reliability between visits. Bland-Altman plots were constructed to guide interpretation of the effect of gait speed on reliability between visits. The correlation coefficients were subsequently used in the determination of the standard error of the measurement (SEM):
in which SD is the standard deviation of gait speeds from the first visit, calculated separately for CGS and FGS walking conditions, and r is the correlation coefficient (i.e., ICC(2,1)). The minimal detectable change (MDC95) at the 95%CI, was calculated as:
for both CGS and FGS walking conditions.
RESULTS
We analyzed data from a total of 76 participants (34 female/42 male; mean age of 58 ± 11 years; mean of 52 ± 62 months post-stroke (range: 5 – 324 months); 42L/34R paretic side). The mean lower extremity portion of the Fugl-Meyer score was 22 ± 6 (out of a possible 34). The CGS for all participants during visit one was 0.49 ± 0.28 m/s and 0.54 ± 0.32 m/s during the second visit (p<0.001). Fast gait speed was not available for four subjects (LOW: 3 and MOD: 1; all with CGS < 0.49 m/s). For the remaining 72 participants, the overall FGS was slower during visit one (0.70 ± 0.43 m/s) compared to visit two (0.74 ± 0.45; p<0.001).
Comfortable Gait Speed
Using previously established speed-based classification,4 we placed 32 participants into the LOW group (i.e., < 0.40 m/s), 29 participants in the MOD group (i.e., between 0.40 and 0.80 m/s), and 15 into the HIGH group (i.e., > 0.80 m/s). The comfortable gait speed was significantly faster during the second visit compared to the first visit for the LOW (p=0.027), MOD (p<0.001), and HIGH (p=0.019) groups (Figure 1). Although the overall reliability was excellent for all participants together (0.955), the ICC (2,1) values declined with increasing gait speed groups (LOW: 0.879; MOD: 0.677; HIGH: 0.522, Table 1). Greater variability can be observed in the faster walkers in the Bland-Altman plots presented in Figure 2. The minimum detectable change for CGS was smaller for slower speed-based groups (LOW: 0.10 m/s; MOD: 0.15 m/s; HIGH: 0.18 m/s).
Figure 1.
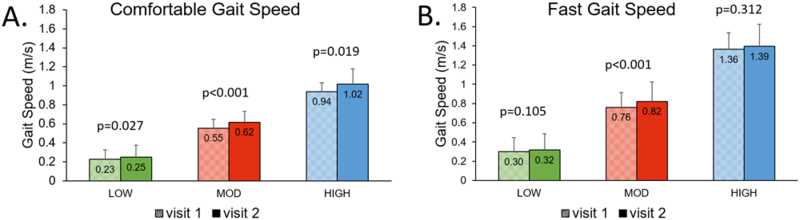
Gait speeds for visits 1 and 2 separated by LOW, MOD, and HIGH groups for both CGS (A) and FGS (B). Shaded bars indicate visit 1 and solid bars represent visit 2 for each group. The subject groupings were chosen using 0.40 and 0.80 m/s thresholds. Values represent mean and SD.
Table 1:
Minimum Detectable Change and Reliability for Repeated Gait Speed Measurements Using Speed-Based Classification
| Comfortable Gait Speed | Fast Gait Speed | |||||
|---|---|---|---|---|---|---|
| N | MDC (m/s) | ICC (95%CI) | N | MDC (m/s) | ICC (95%CI) | |
| Overall | 76 | 0.17 | 0.955 (0.874 – 0.979) | 72 | 0.17 | 0.979 (0.957 – 0.988) |
| LOW (<0.40 m/s) | 32 | 0.10 | 0.879 (0.751 – 0.941) | 29 | 0.11 | 0.930 (0.855 – 0.967) |
| MOD (0.40 – 0.80 m/s) | 29 | 0.15 | 0.677 (0.103 – 0.874) | 28 | 0.17 | 0.845 (0.496 – 0.941) |
| HIGH (> 0.80 m/s) | 15 | 0.18 | 0.522 (0.041 – 0.809) | 15 | 0.19 | 0.842 (0.604 – 0.944) |
| LOW (<0.49 m/s) | 41 | 0.11 | 0.910 (0.788 – 0.957) | 37 | 0.11 | 0.954 (0.902 – 0.977) |
| MOD (0.49 – 0.93 m/s) | 27 | 0.18 | 0.771 (0.378 – 0.907) | 27 | 0.20 | 0.891 (0.713 – 0.954) |
| HIGH (> 0.93 m/s) | 8 | 0.17 | 0.176 (−0.224 – 0.687) | 8 | 0.23 | 0.750 (0.221 – 0.943) |
Figure 2.
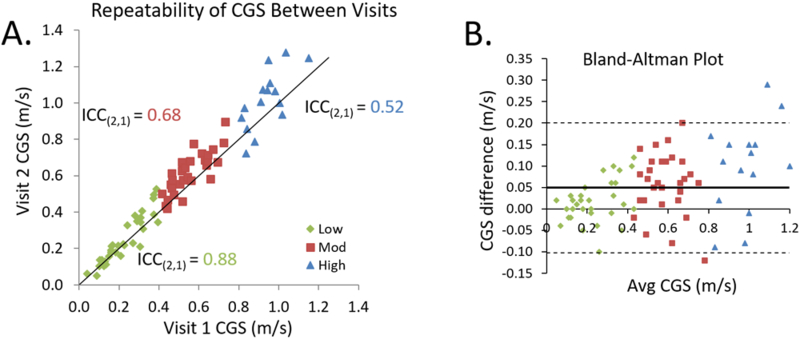
A) Reliability of comfortable gait speed between visits one and two. Individual participants are plotted against a unity line and are divided into speed-based classification groups (LOW: diamonds; MOD: squares; HIGH: triangles). The subject groupings were chosen using 0.40 and 0.80 m/s thresholds. B) Bland-Altman plot shows greater variability in faster walkers compared to slower walkers. The thick solid line represents the mean difference between sessions and the dashed lines represent the limits of agreement.
Fast Gait Speed
Although speed-based groups were based on CGS, we calculated the respective FGS for each of the CGS-based groups. Within the speed-based groups,4 we observed that only the MOD group demonstrated a significant increase in FGS from session one to session two (Figure 1). The ICC (2,1) values for FGS were high across groups (LOW: 0.930; MOD: 0.845; HIGH: 0.842, Figure 3). MDC95s for FGS increased substantially for individuals with faster baseline CGS (Table 1).
Figure 3.
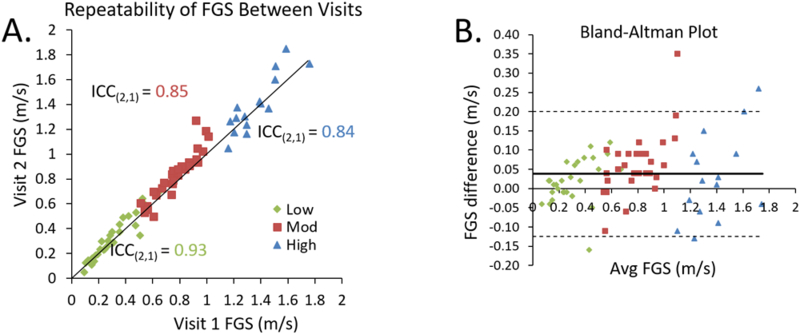
A) Reliability of fast gait speed between visits one and two. Individual participants are plotted against a unity line and are divided into speed-based classification groups (LOW: diamonds; MOD: squares; HIGH: triangles). The subject groupings were chosen using 0.40 and 0.80 m/s thresholds. B) Bland-Altman plot shows greater variability in faster walkers compared to slower walkers. The thick solid line represents the mean difference between sessions and the dashed lines represent the limits of agreement.
Walking activity-based gait speed thresholds
Participants were subsequently re-classified using the walking activity-based gait speed cutoffs,23 which yielded 41 participants into the LOW group (i.e., < 0.49 m/s), 27 participants in the MOD group (i.e., between 0.49 and 0.93 m/s), and 8 participants in the HIGH group (i.e., > 0.93 m/s). Using this alternative classification scheme, the comfortable gait speed was also significantly faster during the second visit compared to the first visit for each group (LOW: p=0.001; MOD: p<0.001; and HIGH: p=0.034). The reliability of CGS declined with increasing gait speed groups (LOW: 0.910; MOD: 0.771; HIGH: 0.176). The minimum detectable change for CGS within each of the groups are presented in Table 1. When recoded for the alternative speed-based classification, we observed that both the LOW (p=0.014) and MOD groups (p=0.006) walked significantly faster during the second visit compared to the first visit. Similar to CGS, the reliability of FGS declined with increasing gait speed and MDC95 values increased substantially between groups.
DISCUSSION
The overall purpose of this study was to examine how the MDC for gait speed varies according to an individual’s baseline gait speed. These data support our hypothesis that gait speed is more consistent for individuals with chronic stroke who are slower walkers, resulting in smaller MDC95 values for individuals with slower initial walking speeds. This work has important implications for determining responders to various treatments and was motivated by the observation that individuals may have very different baseline gait speeds, yet a single MDC may be indiscriminately applied to all participants to determine success.26–28 Here, we suggest that MDCs should be differentiated based on baseline gait speed.
Although the MDC95 for the entire cohort is consistent with previous literature for higher functioning individuals with chronic hemiparesis post-stroke,13, 14 we observed a clear difference in MDC95 when participants were stratified based on a speed-based classification system. In particular, the MDC95 values seen in the lowest functioning group are considerably smaller than the MDC95s for the entire cohort. This finding suggests that expectations should be different for individuals with different baseline gait speeds. Nair and colleagues noted a similar observation in individuals with incomplete spinal cord injury.16 Baseline gait speed also appears to influence the MCID of the six-minute walk test in individuals in the sub-acute phase of stroke.29 Importantly, we observed comparable results whether we used the values proposed by Perry and colleagues,4 or the more recent values recommended by Fulk.23
The observed difference in MDC95 between speed-based classification groups is likely due to the greater consistency of gait speed demonstrated by the slower walkers. In particular, individuals who walk at a slower speed following stroke do not have a large gait speed reserve to exploit, potentially due to greater balance deficits.19 As these individuals are more restricted in their speed range, their gait speed is more stable across visits. Previously, Fulk and Echternach12 reported that MDC values for gait speed are different when participants were stratified by physical assistance required. Our work extends this previous work to report that the difference in baseline gait speed appears to contribute to the different MDC values.
Study Limitations
A potential limitation of our work was the apparent difference in gait speed between visits. Stratford and Riddle 30 note that MDCs should be assessed from a group of ‘stable’ individuals presenting with difference scores between tests that exhibits a normal distribution and is close to zero. We confirmed that our distribution of difference scores follows a normal distribution and the mean difference between visits was considerably less than our calculated MDC scores. Given the chronicity of stroke in our group and the lack of an imposed intervention, there was no reason to expect a systematic change in gait speed between visits. Nevertheless, we did observe a small systematic increase in gait speed from visit one to visit two (overall 0.05 m/s increase without any intervention). This change in gait speed may indicate that individuals were not comfortable with testing during the first visit. From a research perspective, multiple initial testing visits are therefore critical to avoid overestimating the effect of training.
Another limitation of our work was the chronicity of the stroke. These MDC95 and reliability values are established for individuals in the chronic stage post-stroke, when presumably mobility deficits are more stable and less likely to respond to spontaneous recovery. Future work would need to be done to determine the effect of baseline gait speed on MDCs in individuals recovering in the (sub)acute phase of recovery where baseline scores would presumably be more variable. Furthermore, we allowed individuals to maintain their typical bracing and assistive device if necessary. Although we were careful to ensure that these devices were kept constant from visit one to visit two, we recognize that our results may have changed if we had restricted access to participant’s typical devices. Our goal was to replicate gait as it typically occurs in the participant’s home or community. Restricting the use of assistive devices and bracing would have limited our subject population and would not have been an accurate indication of how patients present to a clinical setting. A final limitation of the study was that the small sample size for the HIGH functioning group based on walking performance thresholds (i.e., > 0.93 m/s) yielded a low ICC. As these data came from clinical research, our goal with recruitment was to target individuals who needed the most help with increasing gait speed, and so there were few individuals who were ‘fast’ walkers. As a result, the small number of subjects may have influenced the MDC95 value of this subset. Regardless, the MDC95 values between classification scales were quite similar suggesting that our estimates are reasonable.
CONCLUSIONS
These data suggest that it may be necessary to adjust expectations based on the individual’s baseline functional level. In particular, a small change (i.e. 0.10 m/s) for a slower walker suggests that the random day-to-day fluctuation in gait speed was exceeded, whereas the same change would not be considered to exceed the normal day-to-day variation for a faster walker. Whether it is in the clinical or research setting, it is important to consider the individual’s baseline status, as the same MDC should not be applied to all participants. Finally, whereas we calculated MDCs, future work is needed to determine MCIDs for individuals with chronic stroke based on baseline gait speeds. These values will be critical for determining whether observed changes in gait speed are clinically meaningful.
Supplementary Material
Acknowledgments
This work was supported in part by the NIH (R21-HD068805), American Heart Association (09BGIA2210015), and the Foundation for Physical Therapy, Inc. Geriatric Endowment Fund. A portion of these data were presented at the 2017 Combined Sections Meeting of the American Physical Therapy Association in San Antonio, TX.
Footnotes
The authors have no conflicts of interest to report.
Video Abstract available for more insights from the authors (see Video, Supplemental Digital Content 1)
References:
- 1.Faria-Fortini I, Basilio ML, Scianni AA, Faria C, Teixeira-Salmela LF. Performance and capacity-based measures of locomotion, compared to impairment-based measures, best predicted participation in individuals with hemiparesis due to stroke. Disabil Rehabil 2017:1–8. [DOI] [PubMed] [Google Scholar]
- 2.Robinson CA, Shumway-Cook A, Matsuda PN, Ciol MA. Understanding physical factors associated with participation in community ambulation following stroke. Disabil Rehabil 2011;33(12):1033–42. [DOI] [PubMed] [Google Scholar]
- 3.Flansbjer UB, Downham D, Lexell J. Knee muscle strength, gait performance, and perceived participation after stroke. Archives of physical medicine and rehabilitation 2006;87(7):974–80. [DOI] [PubMed] [Google Scholar]
- 4.Perry J, Garrett M, Gronley JK, Mulroy SJ. Classification of walking handicap in the stroke population. Stroke; a journal of cerebral circulation 1995;26(6):982–9. [DOI] [PubMed] [Google Scholar]
- 5.Fritz S, Lusardi M. White paper: “walking speed: the sixth vital sign”. J Geriatr Phys Ther 2009;32(2):46–9. [PubMed] [Google Scholar]
- 6.Duncan PW, Sullivan KJ, Behrman AL, Azen SP, Wu SS, Nadeau SE et al. Body-weight-supported treadmill rehabilitation after stroke. The New England journal of medicine 2011;364(21):2026–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore JL, Roth EJ, Killian C, Hornby TG. Locomotor training improves daily stepping activity and gait efficiency in individuals poststroke who have reached a “plateau” in recovery. Stroke; a journal of cerebral circulation 2010;41(1):129–35. [DOI] [PubMed] [Google Scholar]
- 8.Lewek MD, Braun CH, Wutzke C, Giuliani C. The role of movement errors in modifying spatiotemporal gait asymmetry post stroke: a randomized controlled trial. Clin Rehabil 2018;32(2):161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tilson JK, Sullivan KJ, Cen SY, Rose DK, Koradia CH, Azen SP et al. Meaningful gait speed improvement during the first 60 days poststroke: minimal clinically important difference. Physical therapy 2010;90(2):196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fulk GD, Ludwig M, Dunning K, Golden S, Boyne P, West T. Estimating clinically important change in gait speed in people with stroke undergoing outpatient rehabilitation. J Neurol Phys Ther 2011;35(2):82–9. [DOI] [PubMed] [Google Scholar]
- 11.Evans MD, Goldie PA, Hill KD. Systematic and random error in repeated measurements of temporal and distance parameters of gait after stroke. Archives of physical medicine and rehabilitation 1997;78(7):725–9. [DOI] [PubMed] [Google Scholar]
- 12.Fulk GD, Echternach JL. Test-retest reliability and minimal detectable change of gait speed in individuals undergoing rehabilitation after stroke. J Neurol Phys Ther 2008;32(1):8–13. [DOI] [PubMed] [Google Scholar]
- 13.Flansbjer UB, Holmback AM, Downham D, Patten C, Lexell J. Reliability of gait performance tests in men and women with hemiparesis after stroke. J Rehabil Med 2005;37(2):75–82. [DOI] [PubMed] [Google Scholar]
- 14.Lewek MD, Randall EP. Reliability of spatiotemporal asymmetry during overground walking for individuals following chronic stroke. J Neurol Phys Ther 2011;35(3):116–21. [DOI] [PubMed] [Google Scholar]
- 15.Hiengkaew V, Jitaree K, Chaiyawat P. Minimal detectable changes of the Berg Balance Scale, Fugl-Meyer Assessment Scale, Timed “Up & Go” Test, gait speeds, and 2-minute walk test in individuals with chronic stroke with different degrees of ankle plantarflexor tone. Archives of physical medicine and rehabilitation 2012;93(7):1201–8. [DOI] [PubMed] [Google Scholar]
- 16.Nair PM, Hornby TG, Behrman AL. Minimal detectable change for spatial and temporal measurements of gait after incomplete spinal cord injury. Topics in spinal cord injury rehabilitation 2012;18(3):273–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jorgensen HS, Nakayama H, Raaschou HO, Olsen TS. Recovery of walking function in stroke patients: the Copenhagen Stroke Study. Archives of physical medicine and rehabilitation 1995;76(1):27–32. [DOI] [PubMed] [Google Scholar]
- 18.Kwakkel G, Kollen B, Lindeman E. Understanding the pattern of functional recovery after stroke: facts and theories. Restorative neurology and neuroscience 2004;22(3–5):281–99. [PubMed] [Google Scholar]
- 19.Middleton A, Braun CH, Lewek MD, Fritz SL. Balance impairment limits ability to increase walking speed in individuals with chronic stroke. Disabil Rehabil 2017;39(5):497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmid A, Duncan PW, Studenski S, Lai SM, Richards L, Perera S et al. Improvements in speed-based gait classifications are meaningful. Stroke; a journal of cerebral circulation 2007;38(7):2096–100. [DOI] [PubMed] [Google Scholar]
- 21.Bowden MG, Balasubramanian CK, Behrman AL, Kautz SA. Validation of a speed-based classification system using quantitative measures of walking performance poststroke. Neurorehabilitation and neural repair 2008;22(6):672–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahon CE, Farris DJ, Sawicki GS, Lewek MD. Individual limb mechanical analysis of gait following stroke. J Biomech 2015;48(6):984–9. [DOI] [PubMed] [Google Scholar]
- 23.Fulk GD, He Y, Boyne P, Dunning K. Predicting Home and Community Walking Activity Poststroke. Stroke; a journal of cerebral circulation 2017;48(2):406–11. [DOI] [PubMed] [Google Scholar]
- 24.Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med 1975;7(1):13–31. [PubMed] [Google Scholar]
- 25.Beckerman H, Roebroeck ME, Lankhorst GJ, Becher JG, Bezemer PD, Verbeek AL. Smallest real difference, a link between reproducibility and responsiveness. Qual Life Res 2001;10(7):571–8. [DOI] [PubMed] [Google Scholar]
- 26.Lewek MD, Feasel J, Wentz E, Brooks FP Jr., Whitton MC. Use of visual and proprioceptive feedback to improve gait speed and spatiotemporal symmetry following chronic stroke: a case series. Physical therapy 2012;92(5):748–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patterson KK, Mansfield A, Biasin L, Brunton K, Inness EL, McIlroy WE. Longitudinal Changes in Poststroke Spatiotemporal Gait Asymmetry Over Inpatient Rehabilitation. Neurorehabilitation and neural repair 2014. [DOI] [PubMed] [Google Scholar]
- 28.Westlake KP, Patten C. Pilot study of Lokomat versus manual-assisted treadmill training for locomotor recovery post-stroke. J Neuroeng Rehabil 2009;6:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fulk GD, He Y. Minimal clinically important difference of the six-minute walk test in people with stroke. J Neurol Phys Ther 2018;42(4):235–240. [DOI] [PubMed] [Google Scholar]
- 30.Stratford PW, Riddle DL. When minimal detectable change exceeds a diagnostic test-based threshold change value for an outcome measure: resolving the conflict. Physical therapy 2012;92(10):1338–47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


