Abstract
Posttraumatic stress disorder (PTSD) is associated with fear response system dysregulation. Research has shown that the anterior cingulate cortex (ACC) may modulate the fear response and that individuals with PTSD have abnormalities in ACC structure and functioning. Our objective was to assess whether ACC volume moderates the relationship between PTSD and fear-potentiated psychophysiological response in a sample of Gulf War Veterans. 142 Veteran participants who were associated with a larger study associated with Gulf War Illness were exposed to no threat, ambiguous threat, and high threat conditions in a fear conditioned startle response paradigm and also provided MRI imaging data. PTSD was assessed using the Clinician Administered PTSD Scale (CAPS). Decreased caudal ACC volume predicted greater psychophysiological responses with a slower habituation of psychophysiological magnitudes across trials (p < 0.001). PTSD diagnosis interacted significantly with both caudal and rostral ACC volumes on psychophysiological response magnitudes, where participants with PTSD and smaller rostral and caudal ACC volumes had greater psychophysiological magnitudes across trials (p < 0.05 and p < 0.001, respectively) and threat conditions (p < 0.05 and p < 0.005). Our results suggest that ACC volume may moderate both threat sensitivity and threat response via impaired habituation in individuals who have been exposed to traumatic events. More research is needed to assess whether ACC size and these associated response patterns are due to neurological processes resulting from trauma exposure or if they are indicative of a premorbid risk for PTSD subsequent to trauma exposure.
Keywords: PTSD/Posttraumatic Stress Disorder, Neuroimaging, Startle, Trauma, Biological markers, Habituation
1. Introduction
Approximately 50–60% of Americans are exposed to traumatic events (Fukuda et al., 1998; Kessler et al., 2005), and 5–20% of these individuals develop Posttraumatic Stress Disorder (PTSD; (Ramchand et al., 2010)). As our understanding of the neurobiology of PTSD continues to develop, research suggests that certain biomarkers may be associated with increased risk for the disorder (Ross et al., 2017) and understanding how these biomarkers are linked to PTSD symptom expression may lead to therapeutically useful findings (Stevens et al., 2017; Yehuda, Neylan, Flory, & McFarlane, 2013). Psychophysiological biomarkers such as exaggerated startle responding have emerged as relatively robust biomarkers of PTSD (Orr, Lasko, Shalev, & Pitman, 1995; Orr, Metzger, & Pitman, 2002). However, the neural under-pinnings of exaggerated startle in PTSD are not clearly understood. While several neural structures such as the hippocampus and amygdala have been implicated in the development and maintenance of PTSD, the anterior cingulate cortex (ACC) has garnered considerable interest as a modulator of fear response in PTSD, to our knowledge, no studies have examined associations of ACC structure with psychophysiological responding in PTSD.
In addition to being a key structure for top-down and bottom-up processing sequences, selective attention, and certain social behaviors, the ACC has both afferent and efferent connections to key emotion regulatory limbic structures, such as the amygdala and hippocampus (Lanius, Bluhm, Lanius, & Pain, 2006). Given its proximity and connections to limbic structures, the ACC may impact PTSD susceptibility through its inhibition and resolution of amygdala activation to threatening stimuli (Etkin, Egner, Peraza, Kandel, & Hirsch, 2006; Shin et al., 2001). Imaging studies of healthy participants have shown that the ACC is activated during the processing of significant but non-threatening stimuli (Bush, Luu, & Posner, 2000; Yamasaki, LaBar, & McCarthy, 2002). Conversely, individuals with PTSD were found to have less ACC activation when exposed to distressing stimuli compared to healthy controls (Bremner et al., 1999; Shin et al., 2001). Moreover, studies by our group and others have shown that smaller ACC volume is associated with current chronic PTSD in Veterans (Chao, Weiner, & Neylan, 2013; Woodward et al., 2006). Recent studies also indicate functional heterogeneity within the ACC where the caudal/dorsal ACC, with its projections to the prefrontal cortex is more so associated with cognitive processes compared to the rostral/ventral ACC, with its functional connectivity to limbic structures such as the hippocampus, amygdala, and other subcortical structures such as the insula is more so associated with emotional function (Etkin, Egner, & Kalisch, 2011; Somerville, Heatherton, & Kelley, 2006).
Earlier studies have shown that smaller ACC volume is associated with both abuse and combat related PTSD diagnoses (Kitayama, Quinn, & Bremner, 2006; Woodward et al., 2006), which suggests that reduced ACC volume may linked to PTSD via impaired ACC activation. Given the ACC plays a major role in areas such areas as threat expectancy and emotional regulation (for a review see Etkin et al., 2011), little attention has been given to the ACC – PTSD relationship within the context of established bio-behavioral markers of threat such as psychophysiological reactivity. A body of literature has shown that individuals diagnosed with PTSD exhibit greater fear-potentiated psychophysiological responses to sudden or threatening stimuli (Ramirez-Moreno & Sejnowski, 2012) compared to those who do not have a PTSD diagnosis (Grillon, Morgan, Davis, & Southwick, 1998; Orr et al., 1995; Pole, Neylan, Best, Orr, & Marmar, 2003). Thus, exploring how ACC might be related to psychophysiological response magnitudes may shed light on brain abnormalities that contribute to altered psychophysiological responding in PTSD. And while previous imaging studies that have investigated the relationship between the ACC and PTSD have focused on paradigms such as the Emotional Stroop task, responses to trauma-related distractors, and engaging in a go/no go task (for a review, see Admon, Milad, & Hendler, 2013), very few studies have focused on exploring the relationship between PTSD, the ACC (either functional or structural), and psychophysiological responses. One study has shown that elevated negative affect reactivity to startle was associated with greater ACC and amygdala activation in individuals with snake and spider phobias (Pissiota et al., 2003). Similarly, a more recent study of traumatized women found that greater activation of the prefrontal cortex/ACC region is associated with greater inhibition of fear-potentiated startle responses (Jovanovic et al., 2013). These studies underscore the importance of examining for the first time if abnormal ACC structure can be linked to exagerrated psychophysiological reactivity in PTSD.
Thus, to expand on previous findings, we investigated whether the interaction between ACC volume and PTSD diagnosis was associated with psychophysiological reactivity to startling sounds over successive trials across three different threat conditions in a sample of Gulf War Veterans. Threat conditions included no threat, ambiguous threat, and high threat. We hypothesized that: (1) smaller ACC volume would be associated with greater psychophysiological response magnitudes across each of the threat conditions and (2) ACC volume would interact with PTSD where individuals who had smaller ACC volumes and were also diagnosed with PTSD would exhibit greater psychophysiological response magnitudes compared to other participants in each of the threat conditions. Based upon prior research that suggests differential caudal and rostral ACC functioning in association to stress response (Admon et al., 2013), we also explored whether or not the caudal and rostral ACC volumes were separately linked to psychophysiological response magnitudes and whether this was moderated by PTSD.
2. Materials and methods
2.1. Participants
We conducted a secondary analysis of data on Veterans from a cross-sectional study that was originally designed to assess the effects of Gulf War deployment on the brain. The original study examined the hypothesis that Gulf War illness was associated with decreased N-acetyl aspartate in the basal ganglia and pons of participants. Gulf War Veterans were recruited between 2002 and 2007 through contacts with physicians at VA clinics in Northern California using methods described elsewhere (Apfel et al., 2011; Weiner et al., 2011). The University of California San Francisco and Committee on Human Research and the Department of Defense Human Research Protection Office approved all research protocols. The sample included both treatment seeking and non-treatment seeking Veterans. Of the 369 Veterans from the original sample, 244 and 172 Veterans engaged in the psychophysiological response task and provided imaging data respectively. Out of those, we had both psychophysiological task and imaging data from 142 Veterans.
Demographic variables including participants’ age, sex, education level, race (white versus minority), and whether the participant had a current diagnosis of PTSD were recorded for use in subsequent analyses based upon prior literature linking them to traumatic stress response (Engelhard, Van Den Hout, & Schouten, 2006; Neylan et al., 2005). Current PTSD symptoms (i.e., within the past month) were evaluated by a Ph.D. level clinical interviewer using the Clinician Administered PTSD Scale (CAPS; Blake et al., 1995). Participants were diagnosed with PTSD based upon frequency and severity of their CAPS scores (e.g. the “1, 2” rule) and the DSM-IV-TR algorithm (for a review, see Weathers, Litz, Herman, Huska, & Keane, 1993). Exposure to child abuse occurring prior to the age of 16 years old was assessed using the last six items of the Trauma History Questionnaire (Green, 1996).
2.2. Psychophysiological response procedure
Three indices of psychophysiological response were collected by trained technicians, who were blind to participants’ psychometric status. The participant’s left eye blink electromyogram (EMG) activity, skin conductance response (SCR) level, and heart rate (HR) were assessed during a two-minute baseline period. Participants were fitted with headphones and told that they would hear potentially startling sounds. They were asked to focus their eyes on a monitor in front of them. A Coulbourn Instruments Lablinc V Modular System binaurally presented 106-dB(A), 40 ms white noise bursts with nominal 0-milli-second rise and fall times separated by inter-trial intervals of between 30 and 50 s in each threat condition. In the “no threat” condition, participants were instructed that they would not be shocked until later in the study. They were then exposed to ten startling sounds. Only their last five responses were retained. In the “ambiguous threat” condition, participants were fitted with a Coulbourn Instruments Transcutaneous Aversive Finger Stimulator but were told that they would not be shocked. Five additional startling sounds were presented. In the “high threat” condition, Veterans wore the finger stimulator and were told that shocks were imminent. Then five additional startling sounds were presented followed by a 2.5 mA shock. Each condition lasted approximately 4 min and was separated by about 1 min. The medium and high threat conditions were counterbalanced to minimize carry-over effects between these conditions. All physiological signals were sampled at 2 Hz during the resting baseline and at 1000 Hz during the acoustic presentations, digitized, and stored for off-line analysis. EMG, measured in microvolts was captured using three, 4-mm (sensor diameter) In Vivo Metrics Ag/AgCl surface electrodes filled with electrolyte paste according to specifications published elsewhere (Blumenthal et al., 2005). SCR was measured in microsiemens by sending a constant 0.5 V through 9-mm (sensor diameter) InVivo Metrics Ag/AgCl electrodes filled with isotonic paste and placed on the hypothenar surface of the medial phalanges of the middle and index fingers of the non-dominant hand. HR was measured in beats per minute and recorded via electrodes attached in a Type-I EKG configuration. Human Startle Software (Coulbourn Instruments, Allentown, PA) automatically calculated mean psychophysiology at baseline and during the one second prior to each stimulus onset. It also calculated the peak post-stimulus levels within 21–200 ms for eyeblink EMG and within 1–4 s for SCR and HR. Data were inspected for artifact and rejected accordingly. No minimum response threshold was designated for any physiological measure. Each measurement of psychophysiological response was recorded prior to and following exposure to the startle stimulus on each of five trials under each threat condition. Participants needed at least four (of five) valid responses for all three psychophysiological measures within each threat condition to be included in the study. Responses were inspected for potential artifact and rejected accordingly.
2.3. Image acquisition and processing
Subjects were scanned on a 1.5Tesla Vision, Siemens MRI scanner (Siemens Medical Systems, Iselin, New Jersey). A T1-weighted 3D volumetric magnetization-prepared rapid gradient echo (MPRAGE) sequence was acquired with the following parameters: repetition time/ spin-echo time/inversion time = 10/4/300 ms, 1 mm × 1 mm in-plane resolution, and 1.5-mm slab thickness, angulated per-pendicular to the long axis of the caudal, rostral, and posterior anterior cingulate gyrus. Freesufer version 4.5 (http://surfer.nmr.mgh.harvard.edu) was used to estimate each subject’s left and right volumes of their rostral and caudal ACC along with their intracranial volume as previously described in (Chao, Mohlenhoff, Weiner, & Neylan, 2014).
2.4. Data analyses
Due to non-normal distribution, the rostral and caudal ACC volumes were natural log transformed and entered in as continuous variables in all models used for analysis. PTSD diagnosis was entered as a dichotomous variable (PTSD vs. no PTSD) for all analyses. Psychophysiological response outcome was assessed by using within trial square root post-minus pre-EMG, SCR, and heart rate responses. Separate rostral and caudal ACC repeated measures linear mixed models were used to assess their relationship on mean psychophysiological response. Models included ACC volume (either caudal or rostral) × PTSD × trial and ACC (either caudal or rostral) × PTSD × threat condition interactions terms to assess whether any ACC on psychophysiological response relationship was moderated by PTSD within each of the five trials and over the three threat (no threat, ambiguous threat, and high threat) conditions respectively. Age, race (white vs. non-white), sex (female vs. male), education (in years), and whether participants had been exposed to both adult trauma and child abuse were also included as covariates in each model. Based on previous findings we also controlled for whether or not these individuals met criteria for the Gulf War Illness case definition (Fukuda et al., 1998). Stata Statistical Software: Release 13.1 was used to conduct all statistical analyses (StataCorp LP, 2013 College Station, TX). Cohen’s f2 was used to assess proportion of model variance explained (Cohen, 1988). f2 was generated using user written code based on previously published methods described elsewhere (Selya, Rose, Dierker, Hedeker, & Mermelstein, 2012).
3. Results
Demographics and their bivariate relationships to PTSD are described in Table 1. Our sample was predominantly White and male with a mean age of 46. Approximately 70% of participants had been exposed to traumatic events during adulthood and approximately 32% of them met criteria for PTSD at the time of the study. A positive PTSD diagnosis was associated with greater average SCR (t = −2.05, p = 0.041; see Table 1).
Table 1.
Sample descriptive statistics and pairwise comparisons by PTSD diagnosis (N = 142).
| Characteristics | PTSD | No PTSD | Total | |
|---|---|---|---|---|
| N (%) | 46 (32.39) | 96 (67.61) | 142 (100) | |
| Sex | ||||
| Male | 35 (24.65) | 88 (61.97) | 123 (86.62) | |
| Female | 11 (7.75) | 8 (5.63) | 19 (13.38) | |
| Race | ||||
| Asian/PI* | 3 (2.11) | 7 (4.93) | 10 (7.04) | |
| Black | 10 (7.04) | 15 (10.56) | 25 (17.61) | |
| Latino | 4 (2.82) | 6 (4.23) | 10 (7.04) | |
| White | 28 (19.72) | 65 (45.77) | 93 (65.49) | |
| Other | 1 (0.70) | 3 (2.11) | 4 (2.81) | |
| Exposure to trauma | ||||
| Adult trauma | 46 (32.39) | 52 (36.62) | 98 (69.01)*** | |
| Child abuse | 13 (9.15) | 22 (15.49) | 35 (24.65)** | |
| Gulf War Illness Criteria | 11 (7.59) | 5 (4.02) | 16 (11.61)*** | |
| Mean (SD) | ||||
| Age | 44.46 (9.77) | 43.17 (9.98) | 44.83 (9.53)* | |
| Education§ | 14.51 (1.95) | 15.05 (1.96) | 14.63 (2.42)* | |
| Caudal ACC volume+ | 8.27 (0.21) | 8.28 (0.17) | 8.28 (0.18) | |
| Rostral ACC volume+ | 8.66 (0.15) | 8.66 (0.18) | 8.66 (0.16) | |
| EMG | 4.64 (4.82) | 4.50 (4.18) | 4.54 (4.99) | |
| SCR | 0.13 (0.11) | 0.10 (0.08) | 0.11 (0.09)* | |
| Heart rate | 0.65 (0.51) | 0.51 (0.50) | 0.54 (0.52) | |
Note: SD=standard deviation; PI=Pacific Islander.
Education is given in years.
ACC volume was log transformed; EMG, SCR, and heart rate are averaged across trials and threat conditions; N (%) and mean (SD) pairwise statistics were given by the χ2 and t statistic respectfully.
p < 0.05.
p < 0.005.
p < 0.001
3.1. Caudal ACC
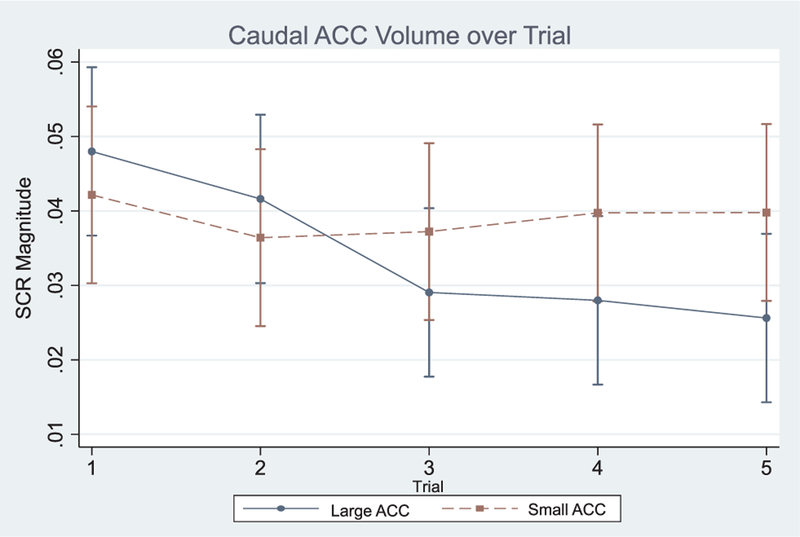
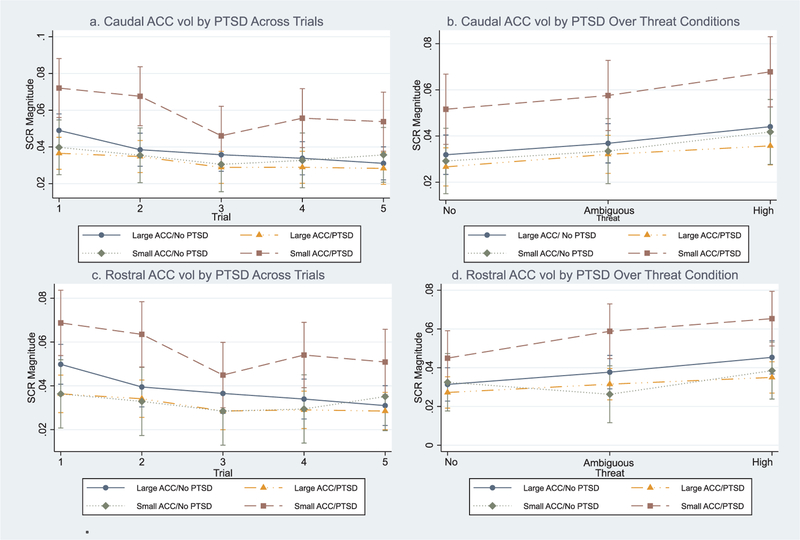
There was a significant overall effect for the SCR repeated measures mixed model (Wald χ2 = 152.88; p < 0.001) but not EMG or heart rate (not shown). Post-hoc analyses revealed a significant caudal ACC × trial interaction suggesting individuals with smaller caudal ACC volume had greater mean SCR magnitudes across the five trials (χ2 = 10.45; f2 = 0.16; p < 0.05; see Fig. 1). To explore this further, we calculated the derivative of SCR magnitude in respect to trial (i.e. comparison of smaller versus larger caudal ACC volume in respect to their between trial changes in slope of SCR magnitude) where SCR magnitude = m and trial (or threat condition and described later) = t; thus in standard notation, m′ (t) ≈ 1/h [m(t + h) − m(t)]. Derivative analyses indicated individuals with smaller ACC volumes exhibited greater between trial changes in SCR magnitudes compared to individuals with larger caudal ACC volume (m′ (t) = 0.04; SE = 0.01; z = 2.41; p = 0.013). A significant three-way caudal ACC × PTSD × trial interaction was also observed suggesting individuals with smaller caudal ACC volumes diagnosed with PTSD had greater mean SCR magnitudes across the five trials (χ2 = 12.70; f2 = 0.32; p < 0.001; see Fig. 2a). Derivative analyses confirmed this where individuals with PTSD and smaller caudal ACC volumes exhibited greater between trial changes in SCR magnitude compared to others in the sample (m′ (t) = 0.13; SE = 0.03; z = 4.17; p < 0.001). A significant caudal ACC × PTSD interaction in respect to threat condition was also observed where participants with smaller caudal ACC volumes with PTSD appeared to exhibited greater mean SCR levels over the three threat conditions compared to other participants (χ2 = 12.91; p < 0.001; f2 = 0.24 see Fig. 2b). Post-hoc derivative analyses confirmed this where individuals with PTSD and smaller caudal ACC volumes exhibited greater between threat condition changes in SCR magnitude compared to others in the sample (m′ (t) = 0.13; SE = 0.04; z = 3.48; p < 0.001). Caudal ACC volume did not interact with PTSD on EMG or heart rate (see Table 2).
Fig. 1.
Note: ACC = anterior cingulate cortex; while ACC volume was a continuous variable in all models, for the purposes of visual illustration only, top and bottom ACC volume quartiles were compared in this figure; SCR magnitude is given in √μV; included model covariates: age, race, years of education, adult trauma exposure, GW illness, and high threat condition exposure order.
Fig. 2.
Note: ACC = anterior cingulate cortex; while ACC volume was a continuous variable in all models, for the purposes of visual illustration only, the top and bottom ACC volume quartiles were compared in this figure; SCR magnitude is given in √μV; included model covariates: age, race, years of education, adult trauma exposure, GW illness, and high threat condition exposure order.
Table 2.
Mixed models on psychophysiological response.
| Measure | Predictors by trial | χ2 | f2 | Predictors by threat | χ2 | f2 |
|---|---|---|---|---|---|---|
| EMG | Trial | 23.23*** | 0.24 | Threat | 50.93** | 0.50 |
| PTSD × Trial | 1.05 | 0.00 | PTSD × Threat | 7.66* | 0.14 | |
| Caudal ACC × Trial | 6.34 | 0.00 | Caudal ACC × Threat | 3.39 | 0.01 | |
| Caudal ACC × PTSD | 1.89 | 0.00 | Caudal ACC × PTSD | 1.90 | 0.00 | |
| Caudal ACC × PTSD × Trial | 1.04 | 0.00 | Caudal ACC × PTSD × Threat | 4.66+ | 0.02 | |
| Rostral ACC × Trial | 2.07 | 0.00 | Rostral ACC × Threat | 0.22 | 0.00 | |
| Rostral ACC × PTSD | 1.96 | 0.00 | Rostral ACC × PTSD | 1.78 | 0.00 | |
| Rostral ACC × PTSD × Trial | 0.20 | 0.00 | Rostral ACC × PTSD × Threat | 6.33+ | 0.02 | |
| SCR | Trial | 54.63*** | 0.45 | Threat | 84.43*** | 0.46 |
| PTSD × Trial | 15.40*** | 0.20 | PTSD × Threat | 1.50 | 0.00 | |
| Caudal ACC × Trial | 10.45* | 0.16 | Caudal ACC × Threat | 0.13 | 0.00 | |
| Caudal ACC × PTSD | 29.61*** | 0.40 | Caudal ACC × PTSD | 17.50*** | 0.24 | |
| Caudal ACC × PTSD × Trial | 12.70*** | 0.32 | Caudal ACC × PTSD × Threat | 1.55 | 0.00 | |
| Rostral ACC × Trial | 6.80 | 0.00 | Rostral ACC × Threat | 10.08** | 0.06 | |
| Rostral ACC × PTSD | 31.55*** | 0.30 | Rostral ACC × PTSD | 17.24*** | 0.20 | |
| Rostral ACC × PTSD × Trial | 15.64*** | 0.28 | Rostral ACC × PTSD × Threat | 19.41*** | 0.28 | |
| Heart rate | Trial | 3.71 | 0.00 | Threat | 11.56*** | 0.20 |
| PTSD × Trial | 1.42 | 0.00 | PTSD × Threat | 4.26 | 0.01 | |
| Caudal ACC × Trial | 2.65 | 0.00 | Caudal ACC × Threat | 5.53+ | 0.08 | |
| Caudal ACC × PTSD | 0.13 | 0.00 | Caudal ACC × PTSD | 0.12 | 0.00 | |
| Caudal ACC × PTSD × Trial | 3.91 | 0.01 | Caudal ACC × PTSD × Threat | 1.71 | 0.00 | |
| Rostral ACC × Trial | 0.86 | 0.00 | Rostral ACC × Threat | 1.63 | 0.00 | |
| Rostral ACC × PTSD | 0.83 | 0.00 | Rostral ACC × PTSD | 1.06 | 0.00 | |
| Rostral ACC × PTSD × Trial | 0.20 | 0.00 | Rostral ACC × PTSD × Threat | 0.07 | 0.00 |
Note: All models included the following covariates: age, race, years of education, adult trauma exposure, and the order that participants were exposed to the high threat condition. f2 ≥ 0.02, f2 ≥ 0.15, and f2 ≥ 0.35 represent small, medium, and large effect sizes, respectively.
p < 0.10.
p < 0.05.
p < 0.01.
p < 0.001.
3.2. Rostral ACC
There was a significant overall effect for the SCR repeated measures mixed model (Wald χ2 = 79.01; p < 0.001) but not EMG or heart rate (not shown). Post-hoc analyses revealed a significant rostral ACC × PTSD × trial interaction where individuals with smaller rostral ACC volumes and PTSD appeared to have greater mean SCR magnitudes across the five trials (χ2 = 15.64; f2 = 0.28; p < 0.001; see Fig. 2c). Post hoc derivative analyses confirmed this where individuals with PTSD and smaller rostral ACC volumes exhibited greater between trial changes in SCR magnitude compared to others in the sample (m′(t) = 0.15; SE = 0.05; z = 3.65; p < 0.001). A significant rostral ACC volume × PTSD × threat condition interaction was observed where participants with smaller rostral ACC volumes and PTSD appeared to exhibit greater mean SCR levels over the three threat conditions compared to other participants (χ2 = 19.41; f2 = 0.28; p = 0.002; see Fig. 2d). Post-hoc derivative analyses confirmed this where individuals with PTSD and smaller caudal ACC volumes exhibited greater between threat condition changes in SCR magnitude compared to others in the sample (m′ (t) = 0.15; SE = 0.04; z = 3.66; p < 0.001). While post-hoc analyses revealed a significant rostral ACC × threat condition interaction (χ2 = 10.08; f2 = 0.06; p = 0.007), the differences between the slopes of individuals with smaller and larger rostral ACC volumes were not significant (m′ (t) = 0.02; SE = 0.01; z = 1.18; p < 0.240). Rostral ACC volume did not interact with PTSD on EMG or heart rate (see Table 2).
4. Discussion
Our primary finding is that only smaller caudal ACC volume appears to be associated with greater psychophysiological response magnitudes in a fear-potentiated startle paradigm; participants who had smaller caudal ACC volumes exhibited greater within trial mean SCR response magnitudes with a delayed decrease in these magnitudes across trials. We also found that individuals with PTSD who had smaller caudal and rostral volumes exhibited greater within trial SCR response magnitudes and exhibited greater overall arousal as evidenced by larger mean SCR magnitudes over the three threat conditions even after controlling for factors such as age, sex, and adult trauma exposure. Our results are consistent with others’ that suggest PTSD etiology may stem from ACC hypofunction, which may be linked to impaired fear response inhibition (Jovanovic et al., 2013; Milad et al., 2009; Stevens et al., 2017). Moreover, ACC hypofunction may stem from ACC structural abnormalities (Chao et al., 2013; Kasai et al., 2008; Woodward et al., 2006). More broadly, while it remains unclear whether these structural differences are indicative of a premorbid risk for PTSD or stem from etiological processes subsequent to trauma exposure, our results suggest that fear response inhibition may involve both caudal and rostral ACC recruitment both of which appear to be impaired in PTSD diagnosed individuals (Etkin & Wager, 2007).
The pattern of psychophysiological response magnitudes that participants with smaller caudal ACC volumes and participants with both smaller ACC volumes and PTSD displayed within trials may indicative a deficit in habituation. Under normal circumstances, organisms habituate to novel stimuli subsequent to successive presentations due to the stimulus losing its threat value because it is not being paired with any aversive stimulus (Rankin et al., 2009). However, participants with smaller ACC volumes with a PTSD diagnosis, continued to respond with elevated psychophysiological magnitudes across all five of the trials, which would imply a decrement in habituation. One of the key aspects of PTSD is the inability to inhibit the fear response within the context of safety cues, particularly for the no threat condition (e.g. the no shock possible cue) (Jovanovic, Kazama, Bachevalier, & Davis, 2012) and animal models suggest the inability to habituate to a stressor may be associated with neurobehavioral changes linked to PTSD (Servatius, Ottenweller, & Natelson, 1995).
On the other hand, our observation that PTSD diagnosed participants with smaller ACC volumes also exhibited greater overall arousal, particularly in the ambiguous and high threat conditions may indicate a more acute sensitivity to threat and an overactive threat response system in these individuals. As the SCR response is one of the putative biomarkers of anxiety (Orr et al., 2002; Pole et al., 2003, 2009), smaller caudal ACC volume may reffect general ACC hypofunction, which may be associated with a greater threat sensitivity and a stronger threat response. There is some evidence that suggests ACC dysfunction in individuals with PTSD is linked to a compromised arousal network (Felmingham et al., 2009). Thus, smaller ACC volume may interact with trauma exposure to inffuence resting arousal, which could in turn lead to greater threat sensitivity (e.g. hypervigilance) and exaggerated threat responses (e.g. anger, emotional outbursts) when exposed to potentially threatening stimuli (Chemtob, Novaco, Hamada, Gross, & Smith, 1997; Orth & Wieland, 2006).
The finding that caudal ACC volume was associated with greater SCR magnitudes within trials was interesting. However, it remains to be seen how caudal ACC morphometry and function are related. Although this study was not equipped to assess how these structural differences impacted caudal ACC signaling, it has been suggested that the dorsal/ caudal ACC with its functional connectivity to the amygdala is associated with the inhibition of threat responses to neutral stimuli and the rostral/ventral ACC with its connections to the hippocampus is more associated with the inhibition of emotional responses (Admon et al., 2013; Aupperle, Melrose, Stein, & Paulus, 2012). Furthermore, recent findings suggest a relationship between greater dorsal/caudal ACC recruitment during conditions of uncertain threat (Gorka, Lieberman, Shankman, & Phan, 2017). Thus, reduced caudal ACC volume may be linked to a caudal ACC functioning deficit where individuals with less caudal ACC volume also have a limited ability to engage in threat response inhibition and attend to safety cues when exposed to novel but non-threatening stimuli even after successive presentations. However, we also observed that participants who had smaller caudal and rostral ACC volumes with PTSD exhibited greater mean SCR magnitudes within trials and over threat conditions. Therefore, smaller caudal ACC volume may confer increased risk for threat sensitivity but trauma exposure appears to have toxic effects on both rostral and caudal ACC structural integrity and possibly functionality, which may manifest in PTSD-related threat sensitivity (Garfinkel, & Liberzon, 2009; O’Donovan et al., 2017).
It is currently unclear whether ACC structural differences represent a preexisting vulnerability to threat sensitivity and impaired fear response inhibition or if they stem from the adverse effects of trauma exposure. As we described previously, there is controversy associated with whether smaller regional brain volumes suggest a premorbid PTSD risk or whether they are associated with the underlying neurological process of PTSD after trauma exposure (Chao et al., 2013). Twin studies indicate that genetics may have considerable inffuence over individuals’ habituation patterns (Kotchoubei, 1987; Lykken, Iacono, Haroian, Mc Gue, & Bouchard, 1988) and earlier theories have posited that individual differences in ACC structure and/or functioning may indicate preexisting PTSD vulnerability via enhancements in fear conditioning to threatening stimuli and impaired habituation to novel but non-threatening stimuli (Hamner, Lorberbaum, & George, 1999). More recently, it has postulated that neuronal abnormalities in the rostral/ ventral ACC may be reffective of etiological processes associated with PTSD and acquired as a result of trauma exposure whereas caudal/ dorsal ACC neuronal abnormalities may be reffective of premorbid risk factors (Admon et al., 2013). There may be some evidence for neuronal changes as a result of PTSD (Admon et al., 2009; Sekiguchi et al., 2013) but it is unclear whether these changes in of themselves are associated with some predisposed risk factor. Similarly, while we previously reported in this sample that participants with current PTSD had smaller ACC volume, we noted that there was no significant difference in ACC volume between participants whose PTSD symptoms remitted, participants who were exposed to trauma but never developed PTSD, and participants who were never exposed to trauma, which may indicate a neurological vulnerability to the adverse effects of trauma exposure, possibly through ACC volume (we also found no relationship between trauma exposure and ACC volume in this study) (Chao et al., 2013). Although findings from some genetic imaging studies that suggest certain genetic polymorphisms may be associated with abnormal ACC functioning (Gerritsen et al., 2012; Outhred et al., 2012), more studies are needed that consider how potential genetic factors impact neural structures such as the ACC.
It is important to discuss these results within the context of treatment. Our findings would suggest that individuals with smaller ACC volume who have been exposed to trauma may exhibit limited response to extinction training, possibly through a decrement in habituation and a decreased ability to tolerate distress related to therapeutic re-exposure. Indeed, evidence suggests that individuals with smaller ACC volumes and individuals with reduced dorsal ACC activity exhibit less treatment gains in psychotherapy compared to individuals with larger ACC volume and greater dorsal ACC activity (Aupperle et al., 2013; Roy et al., 2010), which further suggests smaller ACC volume may be a premorbid risk factor for PTSD. On a similar note and based upon our results and others, it is tempting to suggest specific therapy modalities, such as prolonged exposure and eye movement desensitization and reprocessing (EMDR) therapy that purport to focus on habituation and extinction learning might be well suited to reduce PTSD symptoms through improved ACC functioning compared to other empirically validated therapy modalities. However, a recent review of the literature regarding the neural correlates of psychotherapeutic treatment of PTSD indicated that no one treatment in particular (i.e. CBT versus exposure therapy versus EMDR) appeared to be associated with greater symptom resolution (Malejko, Abler, Plener, & Straub, 2017). Rather the predominant modalities used to treat PTSD appear to all use elements of habituation, fear extinction learning, reduction of threat sensitivity, among other cognitive and behavioral concepts that appear to target ACC functioning.
These findings should be considered within the context of several limitations. First, our study was cross-sectional in nature and therefore no causality can be drawn from our results. On a similar note, out of the 142 participants in our sample, only 46 participants had PTSD and out of those only 20 participants had smaller ACC volumes. Given that the magnitude of impact of many of these observed ACC × PTSD interactions on SCR response were modest to moderate (Cohen, 1988), future studies could benefit from a larger sample size. Secondly, our sample was made up of mostly male white Veterans, which limits the generalizability of our findings to the broader non-white mixed gender civilian population. Thirdly, although we can speculate through other findings, we cannot implicate psychophysiological response inhibition as being impacted by any neural structures outside of the ACC. Future studies should explore possible links between different neural structures and other biomarkers related to PTSD. While we were able to assess ACC volume in this study, we were not equipped to explore ACC functioning as it relates to PTSD and psychophysiological response. Similarly, we only focused on individuals with current PTSD in this study. Another important caveat is that these ACC × PTSD relationships were only observed on SCR and not EMG or heart rate. While it is presently unclear why these interactions were restricted to SCR, this may due to a number of reasons including but not limited to SCR being particularly sensitive to the ACC – psychophysiological response relationship or some otherwise unknown methodological issues associated with the other two psychophysiological response measures. Other studies will be needed to explore this further. Finally, we were not able to assess the impact that treatment may have had on ACC volume within the context of PTSD and psychophysiological response magnitude. Although logistically complex, a study of ACC volume and/ or functioning and fear potentiated startle response within the context of treatment for PTSD would be particularly illuminating.
5. Conclusion
In conclusion, we found evidence that caudal ACC volume was associated with greater mean within trial SCR magnitudes in a fear conditioning paradigm and that individuals with PTSD and smaller caudal and rostral ACC volume exhibit greater mean SCR response magnitudes within trials and greater levels of arousal over threat conditions. Further, based upon our observations of the SCR response patterns, smaller ACC volume may impair stimulus habituation and be associated with enhanced threat sensitivity and possibly an exaggerated threat response, particularly for those with PTSD. Although our results suggest that small caudal ACC size indicates a detriment in functioning, it is unclear whether this detriment is driven by some premorbid risk factor or if it is reffective of the neurological etiology of PTSD after trauma exposure. Future studies that assess whether certain genetic markers interact with ACC volume and other brain structures on psychophysiological outcome variables may be able to elucidate this.
Acknowledgements
The authors wish to thank the Gulf War Veteran study participants and the Stress and Health Research Group for all of their help in making this manuscript possible. All authors have approved the final version of this manuscript.
Role of Funding Source
This work was supported by Department of Defense Grant DAMD17-01-1-0764, entitled “Magnetic Resonance and Spectroscopy of the Human Brain in Gulf War Illness,” awarded to the Northern California Institute for Research and Education from the Department of Defense Gulf War Illnesses Research Program, U.S. Army Medical Research and Materiel Command. This study was also supported by National Institutes of Health/National Institute of Environmental Health Sciences Grant ES09883. This material is the result of work supported with resources and the use of facilities at the San Francisco VA Medical Center. This work was also supported by the VA Advanced Fellowship Program in Women’s Health at the San Francisco Veterans Affairs Medical Center.
Footnotes
Declaration of interest
None.
References
- Admon R, Lubin G, Stern O, Rosenberg K, Sela L, Ben-Ami H, & Hendler T (2009). Human vulnerability to stress depends on amygdala’s predisposition and hippocampal plasticity. Proceedings of the National Academy of Sciences, 106(33), 14120–14125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Admon R, Milad MR, & Hendler T (2013). A causal model of post-traumatic stress disorder: Disentangling predisposed from acquired neural abnormalities. Trends in Cognitive Sciences, 17(7), 337–347. [DOI] [PubMed] [Google Scholar]
- Apfel BA, Ross J, Hlavin J, Meyerhoff DJ, Metzler TJ, Marmar CR, … Neylan TC (2011). Hippocampal volume differences in Gulf War veterans with current versus lifetime posttraumatic stress disorder symptoms. Biological Psychiatry, 69(6), 541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aupperle RL, Allard CB, Simmons AN, Flagan T, Thorp SR, Norman SB, … Stein MB (2013). Neural responses during emotional processing before and after cognitive trauma therapy for battered women. Psychiatry Research: Neuroimaging, 214(1), 48–55. [DOI] [PubMed] [Google Scholar]
- Aupperle RL, Melrose AJ, Stein MB, & Paulus MP (2012). Executive function and PTSD: Disengaging from trauma. Neuropharmacology, 62(2), 686–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Charney DS, & Keane TM (1995). Clinician-Administered PTSD Scale for DSM-IV (CAPS-DX). Boston, MA: National Center for Posttraumatic Stress Disorder, Behavioral Science Division, Boston VA Medical Center. [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, & Van Boxtel A (2005). Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology, 42(1), 1–15. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Staib LH, Kaloupek D, Southwick SM, Soufer R, & Charney DS (1999). Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: A positron emission tomography study. Biological Psychiatry, 45(7), 806–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, & Posner MI (2000). Cognitive and emotional inffuences in anterior cingulate cortex. Trends in Cognitive Sciences, 4(6), 215–222. [DOI] [PubMed] [Google Scholar]
- Chao LL, Mohlenhoff BS, Weiner MW, & Neylan TC (2014). Associations between subjective sleep quality and brain volume in Gulf War veterans. Sleep, 37(3), 445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao L, Weiner M, & Neylan T (2013). Regional cerebral volumes in veterans with current versus remitted posttraumatic stress disorder. Psychiatry Research: Neuroimaging, 213(3), 193–201. [DOI] [PubMed] [Google Scholar]
- Chemtob CM, Novaco RW, Hamada RS, Gross DM, & Smith G (1997). Anger regulation deficits in combat-related posttraumatic stress disorder. Journal of Traumatic Stress, 10(1), 17–36. [DOI] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Engelhard IM, Van Den Hout MA, & Schouten EG (2006). Neuroticism and low educational level predict the risk of posttraumatic stress disorder in women after miscarriage or stillbirth. General Hospital Psychiatry, 28(5), 414–417. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, & Kalisch R (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences, 15(2), 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, & Hirsch J (2006). Resolving emotional conffict: A role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron, 51(6), 871–882. [DOI] [PubMed] [Google Scholar]
- Etkin A, & Wager TD (2007). Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry, 164(10), 1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmingham KL, Williams LM, Kemp AH, Rennie C, Gordon E, & Bryant RA (2009). Anterior cingulate activity to salient stimuli is modulated by autonomic arousal in posttraumatic stress disorder. Psychiatry Research: Neuroimaging, 173(1), 59–62. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Nisenbaum R, Stewart G, Thompson WW, Robin L, Washko RM, … Herwaldt BL (1998). Chronic multisymptom illness affecting Air Force veterans of the Gulf War. JAMA, 280(11), 981–988. [DOI] [PubMed] [Google Scholar]
- Garfinkel SN, & Liberzon I (2009). Neurobiology of PTSD: A review of neuroimagingc findings. Psychiatric Annals, 39(6), 370. [Google Scholar]
- Gerritsen L, Tendolkar I, Franke B, Vasquez AA, Kooijman S, Buitelaar J, … Rijpkema M (2012). BDNF Val66Met genotype modulates the effect of childhood adversity on subgenual anterior cingulate cortex volume in healthy subjects. Molecular Psychiatry, 17(6), 597–603. [DOI] [PubMed] [Google Scholar]
- Gorka SM, Lieberman L, Shankman SA, & Phan KL (2017). Association between neural reactivity and startle reactivity to uncertain threat in two independent samples. Psychophysiology, 54(5), 652–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green B (1996). Trauma history questionnaire. Measurement of Stress, Trauma, and Adaptation, 1, 366–369. [Google Scholar]
- Grillon C, Morgan CA, Davis M, & Southwick SM (1998). Effects of experimental context and explicit threat cues on acoustic startle in Vietnam veterans with posttraumatic stress disorder. Biological Psychiatry, 44(10), 1027–1036. [DOI] [PubMed] [Google Scholar]
- Hamner MB, Lorberbaum JP, & George MS (1999). Potential role of the anterior cingulate cortex in PTSD: Review and hypothesis. Depression and Anxiety, 9(1), 1–14. [PubMed] [Google Scholar]
- Jovanovic T, Ely T, Fani N, Glover EM, Gutman D, Tone EB, … Ressler KJ (2013). Reduced neural activation during an inhibition task is associated with impaired fear inhibition in a traumatized civilian sample. Cortex, 49(7), 1884–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Kazama A, Bachevalier J, & Davis M (2012). Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology, 62(2), 695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai K, Yamasue H, Gilbertson MW, Shenton ME, Rauch SL, & Pitman RK (2008). Evidence for acquired pregenual anterior cingulate gray matter loss from a twin study of combat-related posttraumatic stress disorder. Biological Psychiatry, 63(6), 550–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, & Walters EE (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62(6), 593–602. [DOI] [PubMed] [Google Scholar]
- Kitayama N, Quinn S, & Bremner JD (2006). Smaller volume of anterior cingulate cortex in abuse-related posttraumatic stress disorder. Journal of Affective Disorders, 90(2), 171–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotchoubei B (1987). Human orienting reaction: The role of genetic and environmental factors in the variability of evoked potentials and autonomic components. Activitas Nervosa Superior, 29(2), 103–108. [PubMed] [Google Scholar]
- Lanius R, Bluhm R, Lanius U, & Pain C (2006). A review of neuroimaging studies in PTSD: Heterogeneity of response to symptom provocation. Journal of Psychiatric Research, 40(8), 709–729. [DOI] [PubMed] [Google Scholar]
- Lykken DT, Iacono WG, Haroian K, Mc Gue M, & Bouchard T (1988). Habituation of the skin conductance response to strong stimuli: A twin study. Psychophysiology, 25(1), 4–15. [DOI] [PubMed] [Google Scholar]
- Malejko K, Abler B, Plener PL, & Straub J (2017). neural correlates of Psychotherapeutic Treatment of Post-traumatic stress Disorder: A systematic literature review. Frontiers in Psychiatry, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, … Rauch SL (2009). Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biological Psychiatry, 66(12), 1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neylan TC, Brunet A, Pole N, Best SR, Metzler TJ, Yehuda R, & Marmar CR (2005). PTSD symptoms predict waking salivary cortisol levels in police officers. Psychoneuroendocrinology, 30(4), 373–381. [DOI] [PubMed] [Google Scholar]
- O’Donovan A, Ahmadian AJ, Neylan TC, Pacult MA, Edmondson D, & Cohen BE (2017). Current posttraumatic stress disorder and exaggerated threat sensitivity associated with elevated inffammation in the Mind Your Heart Study. Brain, Behavior, and Immunity, 60, 198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr SP, Lasko NB, Shalev AY, & Pitman RK (1995). Physiologic responses to loud tones in Vietnam veterans with posttraumatic stress disorder. Journal of Abnormal Psychology, 104(1), 75. [DOI] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, & Pitman RK (2002). Psychophysiology of post-traumatic stress disorder. Psychiatric Clinics, 25(2), 271–293. [DOI] [PubMed] [Google Scholar]
- Orth U, & Wieland E (2006). Anger, hostility, and posttraumatic stress disorder in trauma-exposed adults: A meta-analysis. Journal of Consulting and Clinical Psychology, 74(4), 698–706. [DOI] [PubMed] [Google Scholar]
- Outhred T, Das P, Dobson-Stone C, Griffiths K, Felmingham KL, Bryant RA, … Kemp AH (2012). The functional epistasis of 5-HTTLPR and BDNF Val66Met on emotion processing: A preliminary study. Brain and Behavior, 2(6), 778–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pissiota A, Frans Ö, Michelgård Å, Appel L, Långström B, Flaten MA, & Fredrikson M (2003). Amygdala and anterior cingulate cortex activation during affective startle modulation: A PET study of fear. European Journal of Neuroscience, 18(5), 1325–1331. [DOI] [PubMed] [Google Scholar]
- Pole N, Neylan TC, Best SR, Orr SP, & Marmar CR (2003). Fear-potentiated startle and posttraumatic stress symptoms in urban police officers. Journal of Traumatic Stress, 16(5), 471–479. [DOI] [PubMed] [Google Scholar]
- Pole N, Neylan TC, Otte C, Henn-Hasse C, Metzler TJ, & Marmar CR (2009). Prospective prediction of posttraumatic stress disorder symptoms using fear potentiated auditory startle responses. Biological Psychiatry, 65(3), 235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramchand R, Schell TL, Karney BR, Osilla KC, Burns RM, & Caldarone LB (2010). Disparate prevalence estimates of PTSD among service members who served in Iraq and Afghanistan: Possible explanations. Journal of Traumatic Stress, 23(1), 59–68. [DOI] [PubMed] [Google Scholar]
- Ramirez-Moreno DF, & Sejnowski TJ (2012). A computational model for the modulation of the prepulse inhibition of the acoustic startle reffex. Biological Cybernetics, 106(3), 169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin CH, Abrams T, Barry RJ, Bhatnagar S, Clayton DF, Colombo J, … Marsland S (2009). Habituation revisited: An updated and revised description of the behavioral characteristics of habituation. Neurobiology of Learning and Memory, 92(2), 135–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross DA, Arbuckle MR, Travis MJ, Dwyer JB, van Schalkwyk GI, & Ressler KJ (2017). An integrated neuroscience perspective on formulation and treatment planning for posttraumatic stress disorder: An educational review. JAMA Psychiatry, 74(4), 407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy MJ, Francis J, Friedlander J, Banks-Williams L, Lande RG, Taylor P, … Tarpley V (2010). Improvement in cerebral function with treatment of posttraumatic stress disorder. Annals of the New York Academy of Sciences, 1208(1), 142–149. [DOI] [PubMed] [Google Scholar]
- Sekiguchi A, Sugiura M, Taki Y, Kotozaki Y, Nouchi R, Takeuchi H, … Miyauchi C (2013). Brain structural changes as vulnerability factors and acquired signs of post-earthquake stress. Molecular Psychiatry, 18(5), 618–623. [DOI] [PubMed] [Google Scholar]
- Selya AS, Rose JS, Dierker LC, Hedeker D, & Mermelstein RJ (2012). A practical guide to calculating Cohen’s f2, a measure of local effect size, from PROC MIXED. Frontiers in Psychology, 3(APR), 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servatius RJ, Ottenweller JE, & Natelson BH (1995). Delayed startle sensitization distinguishes rats exposed to one or three stress sessions: Further evidence toward an animal model of PTSD. Biological Psychiatry, 38(8), 539–546. [DOI] [PubMed] [Google Scholar]
- Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB, … Rauch SL (2001). An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biological Psychiatry, 50(12), 932–942. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Heatherton TF, & Kelley WM (2006). Anterior cingulate cortex responds differentially to expectancy violation and social rejection. Nature Neuroscience, 9(8), 1007–1008. [DOI] [PubMed] [Google Scholar]
- Stevens JS, Kim YJ, Galatzer-Levy IR, Reddy R, Ely TD, Nemeroff CB, … Ressler KJ (2017). Amygdala reactivity and anterior cingulate habituation predict posttraumatic stress disorder symptom maintenance after acute civilian trauma. Biological Psychiatry, 81(12), 1023–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers F, Litz B, Herman D, Huska J, & Keane T (1993). The PTSD Checklist (PCL): Reliability, validity, and diagnostic utility. Paper presented at the International Society for Traumatic Stress, San Antonio, TX. [Google Scholar]
- Weiner MW, Meyerhoff DJ, Neylan TC, Hlavin J, Ramage ER, McCoy D, … Truran D (2011). The relationship between Gulf War illness, brain N-acet-ylaspartate, and post-traumatic stress disorder. Military Medicine, 176(8), 896–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward SH, Kaloupek DG, Streeter CC, Martinez C, Schaer M, & Eliez S (2006). Decreased anterior cingulate volume in combat-related PTSD. Biological Psychiatry, 59(7), 582–587. [DOI] [PubMed] [Google Scholar]
- Yamasaki H, LaBar KS, & McCarthy G (2002). Dissociable prefrontal brain systems for attention and emotion. Proceedings of the National Academy of Sciences, 99(17), 11447–11451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Neylan TC, Flory JD, & McFarlane AC (2013). The use of biomarkers in the military: From theory to practice. Psychoneuroendocrinology, 38(9), 1912–1922. [DOI] [PubMed] [Google Scholar]