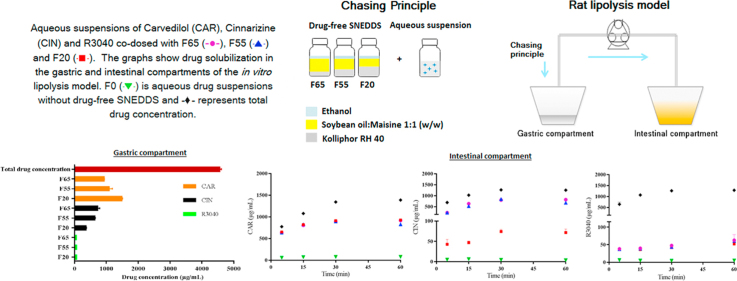
Abstract
This study assessed the influence of the composition of drug-free SNEDDS co-dosed with aqueous suspensions of carvedilol (CAR), cinnarizine (CIN) or R3040 on drug solubilization in a two-compartment in vitro lipolysis model. Correlation of drug logP or solubility in SNEDDS with drug solubilization during in vitro lipolysis in the presence of drug-free SNEDDS was assessed. SNEDDS with varying ratios of soybean oil:Maisine 35-1 (1:1, w/w) and Kolliphor RH40, with ethanol at 10% (w/w) were used. SNEDDS were named F65, F55 and F20 (numbers refer to the percentage of lipids) and aqueous suspensions without drug-free SNEDDS (F0) were also analyzed. While the ranking order of drug solubilization was F65=F55=F20>F0 for CAR; F65=F55>F20>F0 for CIN and F65=F55=F20>F0 for R3040 - with higher CAR solubilization than for R3040 and CIN - the ranking of Seq of CAR, CIN and R3040 in SNEDDS was F65<F55<F20, F65=F55>F20 and F65>F55>F20, respectively. Therefore, the composition of SNEDDS influenced the solubilization of CIN, but not CAR and R3040. Furthermore, high Seq in SNEDDS did not reflect high drug solubilization. As CAR (logP 3.8) showed higher solubilization than CIN (logP 5.8) and R3040 (logP 10.4), a correlation between drug logP and drug solubilization was observed.
KEY WORDS: Self-nanoemulsiying drug delivery system (SNEDDS), Chasing principle, Two-compartment in vitro lipolysis, Rat gastrointestinal conditions, Drug solubilization
Graphical abstract
This study assessed the influence of the composition of drug-free SNEDDS co-dosed with aqueous suspensions of carvedilol, cinnarizine or R3040 on drug solubilization in a two-compartment in vitro lipolysis model.
1. Introduction
Oral administration remains the preferred route for drug administration due to ease of administration, easy patient compliance and established manufacturing processes1. However, the increasing number of poorly water-soluble compounds developed by the pharmaceutical industry, leading to limited oral drug absorption, calls for advanced drug delivery systems2. Self-nanoemulsifying drug delivery systems (SNEDDS) have shown to improve the oral bioavailability of many poorly water-soluble drugs2., 3., 4., 5., 6., 7., 8.. Usually, SNEDDS contain the drug dissolved in a lipid based water-free preconcentrate, thereby avoiding the dissolution step of the solid drug prior to absorption, which is believed to be one of the advantages of SNEDDS9., 10., 11.. However, there are two main problems concerning the application of SNEDDS for broader use; firstly, many drugs show low solubility in pharmaceutically relevant lipids, which can result in low drug loads in SNEDDS and secondly, a number of drugs have low chemical stability in the presence of lipids12. Application of SNEDDS with a low drug load result in the need to dose a higher dose of excipients, which can be a point of concern especially when high drug doses are needed, e.g., in toxicological studies. However, it has been demonstrated that having the drug dissolved in SNEDDS is not a strict condition for improved drug absorption9. In fact, the successful use of lipid suspensions to improve the bioavailability of griseofulvin, phenytoin, cinnarizine (CIN), danazol and fenofibrate has previously been described9., 13., 14., 15.. One of the reasons for this is likely to be related to the presence of lipids and their digestion products in the gastrointestinal tract, upon administration of the drug lipid suspension. These digestion products, e.g., monoacylglycerides and free fatty acids, play an important role in solubilization and absorption of poorly water-soluble compounds16., 17.. In support of this, it has been demonstrated that a number of poorly water-soluble drugs, dosed in tablets, show a positive food effect, i.e., drug absorption is enhanced when ingested with a (high fat) meal12. This is partly due to the postprandial increase in the concentrations of bile salts, phospholipids and the products of lipid digestion in the intestine, which facilitates the dissolution and solubilization of some drugs because of the presence of mixed micelles and uni- and multi-lamellar vesicles16., 18., 19., 20., 21.. In this context, Christiansen et al.12 have shown that under fasted state conditions, the co-administration of 0.5 g of drug-free SNEDDS (sesame oil, Cremophor RH40, oleic acid, Brij and ethanol) with a CIN tablet (Sepan®) induced an increased extent of CIN absorption in humans compared to the administration of a CIN tablet without drug-free SNEDDS.
Recently, the co-administration of a drug-free SNEDDS and a CIN aqueous suspension (so called Chasing Principle) displayed a comparable absorption profile in rats to that of CIN dosed in a SNEDDS solution5. This has been explained by an enhanced solubilization of the drug in the presence of lipids, the lipid digestion products and the formed colloidal structures4., 5.. Similarly, Larsen et al.22 found no significant differences in the absorption of CIN, danazol or halofantrine when the drugs were dosed either as the Chasing Principle (co-dosed with drug-free Labrafil M2125CS) or pre-dissolved in Labrafil M2125CS. The main advantages of the Chasing Principle are the ease of preparation, the fact that the drug dose is not limited by the drug solubility in the SNEDDS preconcentrate, and its suitability for drugs that are chemically unstable in the presence of lipids5.
Considering the advantages of the Chasing Principle as an approach to improve the in vivo exposure of poorly water-soluble compounds, the primary aim of this work was to assess if the composition of (drug-free) SNEDDS would influence the solubilization of carvedilol (CAR), CIN or R3040 in an in vitro lipolysis model simulating rat gastrointestinal conditions. The in vitro lipolysis model contained a gastric and intestinal compartment, where drug dispersion was assessed in the gastric compartment and digestion and drug solubilization were assessed in the intestinal compartment. A second aim was to investigate if a high drug solubility (Seq) in the SNEDDS would reflect high drug dispersion and solubilization in the in vitro lipolysis model, upon co-dosing of an aqueous suspension of a drug with drug-free SNEDDS. Furthermore, the correlation between the logP of the drug and the ability of the drug to be dispersed and solubilized in the presence of SNEDDS was assessed. The model drugs were selected based on their different physicochemical properties to represent a range of poorly water-soluble compounds. CAR is a β-adrenergic antagonist used in cardiovascular diseases23, CIN is a calcium antagonist used in the treatment of motion sickness12 and R3040 is a research compound from F. Hoffmann - La Roche, Switzerland. Three SNEDDS with increasing lipophilicity were used (Table 1). The distribution of CAR, CIN and R3040 in the aqueous phase was determined during gastric dispersion and intestinal digestion using a two-compartment in vitro lipolysis model simulating rat gastrointestinal conditions.
Table 1.
Composition of the drug-free SNEDDS.
| Formulationsa | Excipient (%, w/w) |
||
|---|---|---|---|
| Soybean oil:Maisineb | Kolliphor RH40 | Ethanol | |
| F65 | 65 | 25 | 10 |
| F55 | 55 | 35 | 10 |
| F20 | 20 | 70 | 10 |
F65, F55 and F20 mean SNEDDS containing 65%, 55% and 20% of lipid mixture.
soybean oil:Maisine=1:1, w/w, respectively.
2. Materials and methods
2.1. Materials
CIN (C-5270), soybean oil (long-chain glycerides), bile extract (bovine) (B-3883), and porcine pancreatic lipase extract (P-1625) were purchased from Sigma–Aldrich (Saint Louis, MO, USA). R3040 was donated by F. Hoffmann-La Roche (Basel, Switzerland). CAR was donated by Cipla Ltd. (Pune, India). Maisine 35-1 (a mixture of long chain mono-, di-, and triglycerides) was donated by Gattefossé (St. Priest, France), Kolliphor RH 40 was donated by BASF (Ludwigshafen, Germany), and soy phospholipid (S-PC) was purchased from Lipoid (Ludwigshafen, Germany). Sodium hydroxide and calcium chloride dihydrate were purchased from Merck (Darmstadt, Germany). Sodium chloride and 4-bromobenzeneboronic acid (4-BBBA) was obtained from Fluka Chemie AG (Buchs, Switzerland). Ethanol (Ph. Eur. grade) and acetonitrile (HPLC grade) were purchased from VWR (Herlev, Denmark).
2.2. Preparation of formulations
The composition of the three SNEDDS used in this study is shown in Table 1. SNEDDS were prepared as previously described24. Briefly, Maisine 35-1 (heated to 50 °C) was mixed with soybean oil followed by the addition of Kolliphor RH 40 (heated to 50 °C) and ethanol.
Aqueous suspensions (F0) of CAR, CIN and R3040 at 25 mg/mL, suspended in 0.5% (w/v) methylcellulose solution containing 5% propylene glycol were prepared. The droplet sizes of F65, F55 and F20 have been previously determined by Thomas et al.24. The particle size of the aqueous suspensions was determined using a Mastersizer S from Malvern Instruments Ltd. (Worcester, UK). Immediately before the measurements, the suspensions were dispersed in purified water at a dilution of 1:50 (v/v).
2.3. Two-compartment in vitro lipolysis model
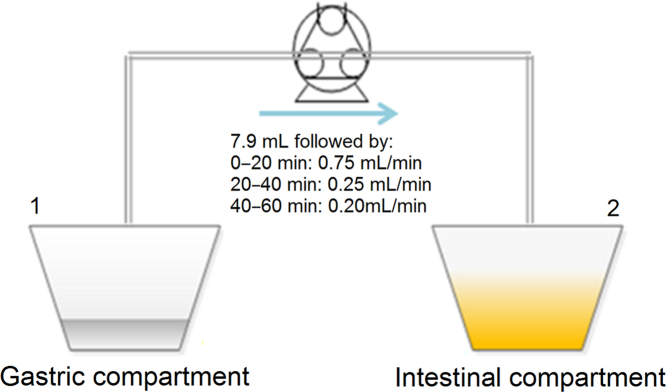
An in vitro lipolysis model simulating rat gastrointestinal conditions was used as previously described. The rat gastrointestinal conditions were simulated in terms of volume of gastrointestinal fluids, pH, enzyme activity and bile salt and phospholipid concentrations, based on a literature review. Briefly, the model was composed of two compartments representing the rat stomach and intestine, which were connected by a peristaltic pump (Fig. 1, Ismatec, NJ, USA).
Figure 1.
Schematic representation of the rat in vitro lipolysis model. Gastric (1) and intestinal compartments (2) are connected by a peristaltic pump. Initially, 7.9 mL of the gastric content were transferred manually followed by the flows described above.
Even though bile salts and phospholipids are secreted into the duodenum together with bile, there is reflux from the duodenal fluids into the stomach of the rat, as described by Tanaka et al.25. Therefore, low concentrations of bile salts and phospholipids were added in the gastric medium used in the current in vitro lipolysis model. The compositions of the gastric and the initial intestinal media, the enzyme activity in the intestinal compartment, the volumes of media used in each compartment and the respective pH values are shown in Table 2.
Table 2.
Composition of the lipolysis media before initiation of the gastric transfer.
| Component | Initial concentration |
|
|---|---|---|
| Gastric compartment | Intestinal compartment | |
| Bile salts (mmol/L) | 0.08 | 50 |
| Phospholipids (mmol/L) | 0.02 | 3.7 |
| Sodium chloride (mmol/L) | 34.2 | 70 |
| Maleic acid (mmol/L) | 2 | 2 |
| Tris (mmol/L) | – | 2 |
| Enzyme activity (USP/mL) | – | 17926 |
| Gastric volume (mL) | 20 | – |
| Initial intestinal volume (mL) | – | 65+5 (pancreatin solution) |
| pH | 4 | 6.5 |
In the gastric compartment, drug solubilizationi in the presence of the drug-free SNEDDS was assessed in order to investigate how much drug was solubilized prior to the transfer to the intestinal compartment. Briefly, 1.5 mL of drug-free SNEDDS and 6 mL of aqueous suspensions of CAR, CIN or R3040, at 25 mg/mL were added into 20 mL of gastric medium at 37 °C (composition in Table 2) and stirred for 30 min. Samples were collected after 30 min of stirring and centrifuged at 19,000× g for 15 min. The drug concentration in the supernatant was determined by HPLC as described below.
In vitro digestion in the intestinal compartment was initiated after 3 min of dispersion of the formulations in the gastric compartment; then, 7.9 mL of the gastric content were manually transferred to the intestinal compartment followed by the pump flows described in Fig. 1. At the same time of the initiation of the gastric transfer, 5 mL of pancreatic enzyme solution were added into 65 mL of intestinal medium in the intestinal compartment (Compartment 2 in Fig. 1) generating a lipase activity of 179 units/mL in the intestinal medium (Table 226), where one unit corresponds to 1 µmol of fatty acid released per minute27., 28.. The pancreatic enzyme solution was prepared by weighing the pancreatic extract in a polypropylene tube followed by the addition of purified water2. The blend was vortexed until homogeneous and subsequently centrifuged at 6500×g for 7 min. The supernatant was isolated and used as the pancreatic enzyme solution. The pH in the intestinal compartment was maintained at 6.5 by adding 0.4 mol/L NaOH to correct for the pH changes caused by lipid digestion and the constant addition of the gastric content at pH 4. A continuous addition of calcium (0.5 mol/L CaCl2) at a rate of 0.01 mL/min was used to control the lipolysis rate29. In the intestinal compartment, the lipolysis was run for 60 min at 37 °C and 1.05 mL samples were collected at 5, 15, 30 and 60 min. The collected volumes were replaced by fresh drug-free intestinal medium and the enzyme activity in the collected samples was inhibited by the addition of 7 µL of 4-BBBA (1 mol/L in methanol). Subsequently, the samples were centrifuged at 19,000×g for 15 min and the drug content in the supernatant was determined by HPLC as described below. To correct for the lipolysis of the plain medium and the pH shift from the transfer of the gastric content to the intestinal compartment, a lipolysis without lipid formulation was performed.
2.4. Solubility of CAR, CIN and R3040 in SNEDDS and gastric and intestinal lipolysis media
The saturation solubility (Seq) of the drugs was determined by adding an excess of CAR, CIN or R3040 to the SNEDDS (Table 1) or lipolysis media (gastric and intestinal media, Table 2). The suspensions were kept in a rotator (HETO, Birkerød, Denmark) at 25 °C for SNEDDS, and at 37 °C for the lipolysis media. To determine the Seq in SNEDDS, at each 24 h interval, a clear supernatant was obtained by centrifugation at 19,000×g for 15 min. To determine the Seq in the lipolysis media without SNEDDS, the drug concentration in the supernatant was analyzed after 24 h. After centrifugation, the supernatants were appropriately diluted with acetonitrile and the drug content was determined by HPLC as described below. The drug solubility in SNEDDS was considered to be the Seq when the values of drug concentration in the supernatants varied less than 5%. The solubility determinations were performed in triplicate.
2.5. HPLC analysis
The drug concentrations in the samples from the solubility studies and in vitro lipolysis were determined using a HPLC system composed of a Dionex ASI 100 automated sample injector, a P680 HPLC pump and a PDA-100 photo diode array detector (Thermo Fisher Scientific, Waltham, MA, USA). For each compound, a Phenomenex C18 (4.60 mm×150 mm, 5 µm) column (Torrance, CA, USA) was used. CAR was analyzed at a wavelength of 240 nm with a mobile phase consisting of acetonitrile:10 mmol/L potassium phosphate buffer at pH 2 (52:48, v/v). CIN was analyzed at a wavelength of 253 nm with a mobile phase consisting of acetonitrile: 20 mmol/L ammonium dihydrogen phosphate monobasic buffer at pH 4.5 (50:50, v/v) and R3040 was analyzed at a wavelength of 225 nm with a mobile phase consisting of acetonitrile and water (90:10, v/v). All model drugs and mobile phases were eluted at a flow rate of 1 mL/min and an injection volume of 10 µL.
2.6. Statistical analysis
The data sets are expressed as mean ± standard deviation of the mean (SD) and one-way ANOVA followed by Tukey׳s test was used to analyze statistical differences between groups. The statistical analyses were performed using GraphPad Prism 7 (GraphPad Software, San Diego, CA, USA).
3. Results and discussion
The current study assessed if the composition of drug-free SNEDDS co-dosed with aqueous suspensions of CAR, CIN or R3040 (Chasing Principle) would affect the solubilization of the model drugs using an in vitro lipolysis model simulating rat gastrointestinal conditions. In addition, it was investigated if there is a correlation between the solubilization profiles of a drug during in vitro lipolysis, the Seq of the drug in SNEDDS and the logP of the drug. The three drug-free SNEDDS where designed by changing the ratio between lipid and surfactant, keeping the ethanol concentration constant (Table 1).
3.1. Physicochemical properties of the model drugs
Table 330., 31., 32. summarizes the physicochemical properties of CAR, CIN and R3040. Probably due to its comparatively low logP (3.8), the Seq of CAR increased from 25±1 to 73±5 mg/g when the lipid content of the drug-free SNEDDS was decreased from 65% (F65) to 20% (F20) (Table 3).
Table 3.
Physicochemical properties of the model drugs.
| Physicochemical property | CAR | CIN | R3040 |
|---|---|---|---|
| Solubility in F65 (mg/g) | 25±1 | 26±1 | 216±13 |
| Solubility in F55 (mg/g) | 42±3 | 25±15 | 205±7 |
| Solubility in F20 (mg/g) | 73±5 | 20±0 | 74±4 |
| Solubility in gastric medium (µg/mL) | 333±18 | 57±1 | 8±2 |
| Solubility in initial intestinal medium (µg/mL) | 669±29 | 84±3 | 34±4 |
| Aqueous solubility (µg/mL) | 3730 | 2031 | <132 |
| pKa | 7.930 | 1.9 and 7.4731 | Neutral32 |
| LogP | 3.830 | 5.831 | 10.4 |
| Particle size (diameter, µm)a | 23±0 | 50±1 | 10±0 |
Particle size of aqueous suspensions of CAR, CIN and R3040. Data are presented as mean±SD (n=3).
In contrast, CIN (logP 5.8) had a higher saturation solubility in F65 (26±1 mg/g) and F55 (25±1 mg/g) than in F20 (20±0 mg/g) (P<0.05), possibly due to the low glycerides content of F20 (Table 3). The low Seq of CIN in F20 is supported by the findings of Larsen et al.33 who showed that CIN had higher Seq in SNEDDS containing higher concentrations of glycerides than in SNEDDS containing higher concentrations of the surfactant Kolliphor RH40. For R3040, which has a high clogP (10.4), higher Seq values were found in F65 (216±13 mg/g) and F55 (205±7 mg/g) than in F20 (74±4 mg/g) (P<0.05) (Table 3).
All model drugs had a higher solubility in the intestinal medium than in the gastric medium. Although CAR and CIN are weak bases (Table 3), the high bile salt level in the initial intestinal medium (50 mmol/L) resulted in a significantly higher drug solubility in this medium (Table 3).
3.2. Solubilization of CAR, CIN and R3040 in the gastric compartment in the presence of drug-free SNEDDS
After 30 min of dispersion of drug-free SNEDDS co-dosed with the drug aqueous suspensions (Chasing Principle) in the gastric compartment, the concentration of drug in the aqueous phase was determined to assess drug solubilization in the gastric compartment. The differences between the gastric and the intestinal compartments are that in the gastric compartment there is no enzyme activity and the pH, bile salts and phospholipids levels are lower than in the intestinal medium (Table 2). In addition, as the drug-free SNEDDS and aqueous suspensions are dosed into the gastric compartment and subsequently slowly transferred to the intestinal compartment (Fig. 1), the concentrations of lipid and drug in the gastric compartment are higher than in the intestinal compartment.
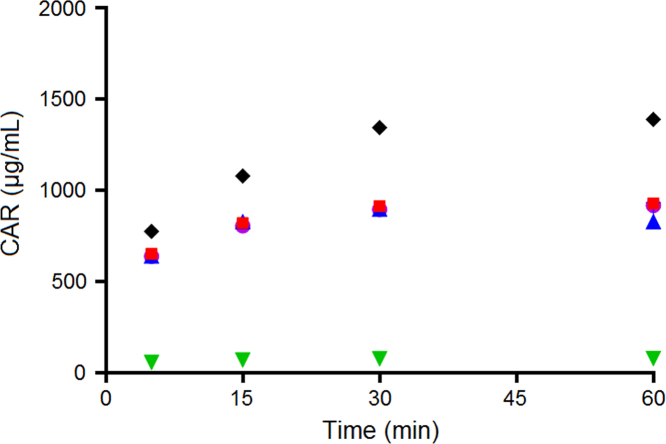
Fig. 2 shows that none of the model drugs were completely solubilized before the transfer to the intestinal compartment. F65 and F55 could not solubilize CAR in the gastric compartment to the same extent as F20 (P<0.05), possibly due to the lower Seq of CAR in F65 and F55 compared to F20 (Table 3). For CIN, however, the formulation containing the lowest concentration of lipids (F20) showed the lowest ability to solubilize CIN (P<0.05), which correlates with the low CIN Seq in F20 (Table 3). Therefore, an increase in the concentration of lipids from 20% in F20 to 55% and 65% in F55 and F65, had a significant influence on CIN solubilization (P<0.05). R3040 showed the lowest solubilization when co-dosed with F65, F55 or F20, in comparison to the solubilization of CAR and CIN (Fig. 2). This may be related to the high logP of R3040 (10.4) and its low solubility in the gastric medium (Table 3).
Figure 2.
Distribution of CAR, CIN and R3040 in the aqueous phase after 30 min of dispersion in the gastric compartment (mean±SD, n=3). CAR, CIN and R3040 aqueous suspensions were co-dosed with F65, F55 and F20.
In Fig. 2, it is observed that CAR showed higher solubilization than CIN and R3040. Considering that CAR has the lowest logP, compared to the logP of CIN and R3040 (Table 3), there seems to be a relation between drug logP and drug solubilization in the gastric compartment.
3.3. Solubilization of CAR, CIN and R3040 in the intestinal compartment
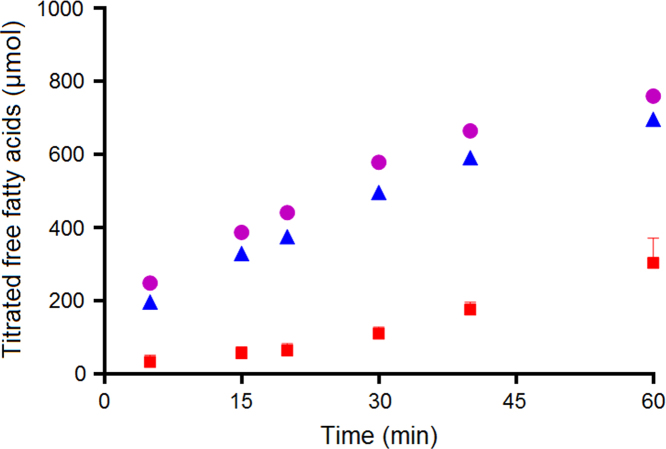
The concentration of fatty acids formed during in vitro lipolysis of the drug-free SNEDDS, corrected for the lipolysis of the plain intestinal medium and the pH shift from the gastric compartment, is shown in Fig. 3. Lipolysis of the plain medium is mainly a result of the enzymatic conversion of phospholipids to lysophospholipids and to some degree impurities in the pancreatin and the crude bile extract34. As can be seen in Fig. 3, the higher concentrations of lipids in F65 and F55 led to a higher formation of free fatty acids compared to F20, which contained the lowest concentration of lipids.
Figure 3.
Free fatty acids generated in the intestinal compartment during in vitro lipolysis (mean±SD, n=3). F65 ( ), F55 (
), F55 ( ) and F20 (
) and F20 ( ).
).
In vitro lipolysis of plain aqueous suspensions (F0) of CAR, CIN and R3040 was carried out to exclude the possibility that a high bile salt concentration in the intestinal step (Table 2) would have similar solubilization capacity as F65, F55 and F20 and therefore hamper the interpretation of the data. Furthermore, the advantage of dosing drug aqueous suspensions with drug-free SNEDDS was assessed by comparing the solubilization of CAR, CIN and R3040 dosed as aqueous suspensions without drug-free SNEDDS.
As CAR, CIN and R3040 dosed as aqueous suspensions without the drug-free SNEDDS showed lower drug concentrations in solution than the drugs co-dosed with drug-free SNEDDS (Figure 4, Figure 5, Figure 6), the high bile salt concentration in the intestinal step did not solubilize the model drugs to the same extent as the drug-free SNEDDS. Furthermore, the solubilization potential of the drug-free SNEDDS on the CAR, CIN and R3040 solubilization has been demonstrated. The key factors influencing the solubilization ability of SNEDDS during in vitro digestion are associated with the digestibility of the excipients and the resulting digestion products formed35. In addition, the kinetics of drug partitioning between the colloidal structures and SNEDDS droplets, also play a role in the solubilization of poorly water-soluble drugs dosed in SNEDDS during lipolysis36.
Figure 4.
Distribution of CAR in the aqueous phase of the intestinal compartment during in vitro lipolysis (mean±SD, n=3). F65 ( ), F55 (
), F55 ( ), F20 (
), F20 ( ), F0 (
), F0 ( ) and total drug concentration (-♦-). The SD values were smaller than the symbols.
) and total drug concentration (-♦-). The SD values were smaller than the symbols.
Figure 5.
Distribution of CIN in the aqueous phase of the intestinal compartment during in vitro lipolysis (mean±SD, n=3). F65 ( ), F55 (
), F55 ( ), F20 (
), F20 ( ), F0 (
), F0 ( ) and total drug concentration (-♦-).
) and total drug concentration (-♦-).
Figure 6.
Distribution of R3040 in the aqueous phase of the intestinal compartment during in vitro lipolysis (mean±SD, n=3). F65 ( ), F55 (
), F55 ( ), F20 (
), F20 ( ), F0 (
), F0 ( ) and total drug concentration (-♦-).
) and total drug concentration (-♦-).
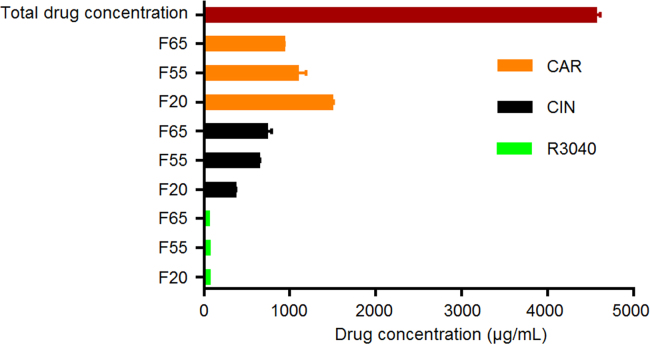
Due to the gradual transfer of the gastric content to the intestinal compartment during in vitro lipolysis, the total final drug concentrations (black diamonds in Figure 4, Figure 5, Figure 6) increased from approximately 700 µg/mL, at the beginning of in vitro lipolysis, to 1300 µg/mL at 60 min of in vitro lipolysis. Fig. 4 shows that while less than 100 µg/mL of CAR were solubilized when dosed in aqueous suspension without drug-free SNEDDS, F65, F55 and F20 were able to keep more than 500 µg/mL of CAR in solution during the course of the in vitro lipolysis. Therefore, the co-dosing of drug-free SNEDDS played an important role in the solubilization of CAR. In addition, the lower solubility of CAR in F65 and F55 compared to the CAR solubility in F20 (Table 3) was not reflected in lower solubilization capacity of F65 and F55 for CAR during the in vitro lipolysis (Fig. 4). This may be due to the low logP of CAR and the subsequently high solubility in the intestinal medium (Table 3).
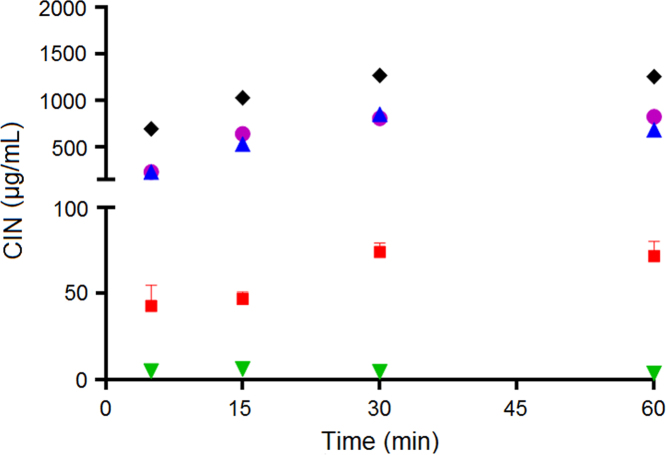
When CIN was co-dosed with F65 and F55, increasing and superimposable profiles of drug solubilization were obtained (Fig. 5). However, when CIN was co-dosed with F20, the concentration of CIN in solution was lower than when co-dosed with F65 and F55, but still significantly higher than for the aqueous suspension without drug-free SNEDDS (P<0.05) (Fig. 5). The low ability of F20 to solubilize CIN correlates with the lower CIN Seq in F20 compared to the CIN Seq in F65 and F55 (Table 3). Therefore, the concentrations of mono-, di- and triglycerides in the drug-free SNEDDS and the generation of free fatty acids upon lipid digestion were important for the solubilization of CIN. Previous studies have shown that free fatty acids have a profound impact on CIN solubilization16., 37., 38.; e.g., Larsen et al.37 showed that the solubility of CIN in lipolysis medium increased as a function of increasing levels of fatty acids.
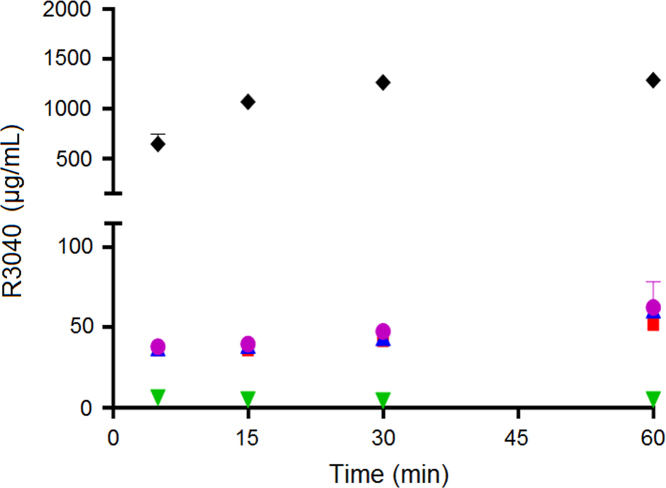
The Seq of R3040 in F65 and F55 (Table 3) would suggest that the capacity of F65 and F55 to solubilize R3040 in the in vitro lipolysis model would be higher than the capacity of F20. However, independent of which formulation was co-dosed with R3040 aqueous suspension, no significant improvement on the R3040 solubilization was obtained (Fig. 6).
The droplet sizes of F65, F55 and F20 are 110±2, 44±1 and 26±2 nm, respectively. Thus, decreasing the concentrations of the lipid mixture soybean oil:Maisine and increasing Kolliphor RH40 concentrations in F65, F55 and F20 (Table 1) resulted in decreased SNEDDS droplet sizes. Correspondingly, Tran et al.39 have shown that increasing the concentration of Kolliphor RH40 from 30% to 50% (w/w) in SNEDDS containing soybean oil:Maisine as lipid mixture, significantly decreased SNEDDS droplet size. Considering the differences in droplet size of F65, F55 and F20, and the similar solubilization abilities towards CAR, CIN and R3040 (Figure 2, Figure 3, Figure 4, Figure 5, Figure 6), it is observed that SNEDDS droplet size did not affect the solubilization ability of SNEDDS during in vitro lipolysis.
Comparing the solubilization of CIN and R3040, it is observed that the solubilization of CIN was significantly higher than the solubilization of R3040 (Figure 5, Figure 6). This finding would suggest that in vivo, the Chasing Principle would provide higher absorption of CIN than for R3040. This hypothesis is supported by two previous pharmacokinetic studies in rats where R304026 or CIN5 were dosed in SNEDDS (same composition as F55) using different drug loads and physical states of the drug, i.e., dissolved in SNEDDS with a drug load of 80% of Seq (SNEDDS 80%), supersaturated in SNEDDS at 200% Seq (Super-SNEDDS solution), and suspended in SNEDDS containing R3040 or CIN at 200% Seq (Super-SNEDDS suspension), as the Chasing Principle and aqueous suspension5. The rank order of absorption for CIN dosed in the dosing regimens was SNEDDS 80%=Chasing Principle>Super-SNEDDS solution=Super-SNEDDS suspension>aqueous suspension. For R3040, the ranking of drug absorption changed to SNEDDS 80%=Super-SNEDDS solution>Super-SNEDDS suspension>Chasing Principle>aqueous suspension. Thus, CIN dosed in the Chasing Principle produced similar absorptions profiles to CIN dosed dissolved in SNEDDS, while R3040 did not show significant absorption improvement upon dosing in the Chasing Principle5., 26..
The higher capacity of F65, F55 and F20 to solubilize CAR (Fig. 4) compared to the ability of F65, F55 and F20 to solubilize CIN (Fig. 5) and R3040 (Fig. 6) may be related to the lower logP of CAR and consequently higher Seq in the gastric and intestinal medium as compared to CIN (logP 5.8) and R3040 (logP 10.4) (Table 3). The high capacity of the drug-free SNEDDS to solubilize CAR in this work indicates that the in vivo dosing of CAR in the Chasing Principle could provide improved CAR absorption and/or similar absorptions profiles to CAR dosed dissolved in SNEDDS, as shown above for CIN. However, a pharmacokinetic study needs to be performed to confirm this hypothesis.
4. Conclusions
The purpose of this study was to evaluate if the composition of SNEDDS used in the Chasing Principle would affect drug solubilization and if high drug Seq in SNEDDS would result in a high drug solubilization during in vitro lipolysis. In addition, the correlation between logP and the solubilization of the drug during in vitro lipolysis when dosed in the Chasing Principle was assessed. The data obtained in this study established that the composition of the drug-free SNEDDS influenced the solubilization of CIN, but not R3040 and CAR, implying that the effect of the SNEDDS composition is compound specific. It is important to attempt, though, that the levels of solubilization of CAR and CIN dosed in the Chasing Principle were higher than for R3040. Furthermore, even the high bile salt concentration (50 mmol/L) used in the intestinal step of the in vitro lipolysis was not able to induce drug solubilization at the same level as when adding drug-free SNEDDS. While there was no correlation between drug Seq in SNEDDS and drug solubilization during in vitro lipolysis, CAR, which had the lowest logP, showed a higher solubilization during in vitro lipolysis, compared to CIN (logP 5.8) and R3040 (logP 10.4), suggesting that there is a relation between drug logP and drug solubilization. For a better understanding of the utility of the Chasing Principle, however, further studies with different drug-free SNEDDS and model drugs are desirable.
Acknowledgments
The authors would like to acknowledge F. Hoffmann-La Roche Ltd., Basel (1073861001), Switzerland for the financial support and the CAPES Foundation, Ministry of Education of Brazil, Brasília (009416/2013-07) for the financial support of Ph.D student Scheyla Siqueira.
Footnotes
Invited for Special Column.Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
The solubilization assessed in the gastric compartment was determined in an independent experiment, where the gastric compartment was not connected to the intestinal compartment.
References
- 1.Kuentz M., Holm R., Elder D.P. Methodology of oral formulation selection in the pharmaceutical industry. Eur J Pharm Sci. 2016;87:136–163. doi: 10.1016/j.ejps.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Michaelsen M.H., Wasan K.M., Sivak O., Müllertz A., Rades T. The effect of digestion and drug load on halofantrine absorption from self-nanoemulsifying drug delivery system (SNEDDS) AAPS J. 2015;18:180–186. doi: 10.1208/s12248-015-9832-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacGregor K.J., Embleton J.K., Lacy J.E., Perry E.A., Solomon L.J., Seager H. Influence of lipolysis on drug absorption from the gastro-intestinal tract. Adv Drug Deliv Rev. 1997;25:33–46. [Google Scholar]
- 4.Humberstone A.J., Charman W.N. Lipid-based vehicles for the oral delivery of poorly water soluble drugs. Adv Drug Deliv Rev. 1997;25:103–128. [Google Scholar]
- 5.Siqueira S.D., Müllertz A., Gräeser K., Kasten G., Mu H., Rades T. Influence of drug load and physical form of cinnarizine in new SNEDDS dosing regimens: in vivo and in vitro evaluations. AAPS J. 2017;19:587–594. doi: 10.1208/s12248-016-0038-4. [DOI] [PubMed] [Google Scholar]
- 6.Larsen A.T., Sassene P., Mullertz A. In vitro lipolysis models as a tool for the characterization of oral lipid and surfactant based drug delivery systems. Int J Pharm. 2011;417:245–255. doi: 10.1016/j.ijpharm.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Mahmoud E.A., Bendas E.R., Mohamed M.I. Preparation and evaluation of self-nanoemulsifying tablets of carvedilol. AAPS PharmSciTech. 2009;10:183–192. doi: 10.1208/s12249-009-9192-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shekhawat P.B., Pokharkar V.B. Understanding peroral absorption: regulatory aspects and contemporary approaches to tackling solubility and permeability hurdles. Acta Pharm Sin B. 2017;7:260–280. doi: 10.1016/j.apsb.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas N., Richter K., Pedersen T.B., Holm R., Müllertz A., Rades T. In vitro lipolysis data does not adequately predict the in vivo performance of lipid-based drug delivery systems containing fenofibrate. AAPS J. 2014;16:539–549. doi: 10.1208/s12248-014-9589-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan J., Rades T., Boyd B. The precipitation behavior of poorly water-soluble drugs with an emphasis on the digestion of lipid based formulations. Pharm Res. 2016;33:548–562. doi: 10.1007/s11095-015-1829-5. [DOI] [PubMed] [Google Scholar]
- 11.Kollipara S., Gandhi R.K. Pharmacokinetic aspects and in vitro–in vivo correlation potential for lipid-based formulations. Acta Pharm Sin B. 2014;4:333–349. doi: 10.1016/j.apsb.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christiansen M.L., Holm R., Abrahamsson B., Jacobsen J., Kristensen J., Andersen J.R. Effect of food intake and co-administration of placebo self-nanoemulsifying drug delivery systems on the absorption of cinnarizine in healthy human volunteers. Eur J Pharm Sci. 2016;84:77–82. doi: 10.1016/j.ejps.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Carrigan P.J., Bates T.R. Biopharmaceutics of drugs administered in lipid-containing dosage forms I: GI absorption of griseofulvin from an oil-in-water emulsion in the rat. J Pharm Sci. 1973;62:1476–1479. doi: 10.1002/jps.2600620918. [DOI] [PubMed] [Google Scholar]
- 14.Chakrabarti S., Belpaire F.M. Bioavailability of phenytoin in lipid containing dosage forms in rats. J Pharm Pharmacol. 1978;30:330–331. doi: 10.1111/j.2042-7158.1978.tb13247.x. [DOI] [PubMed] [Google Scholar]
- 15.Larsen A., Holm R., Pedersen M.L., Müllertz A. Lipid-based formulations for danazol containing a digestible surfactant, labrafil M2125CS: in vivo bioavailability and dynamic in vitro lipolysis. Pharm Res. 2008;25:2769–2777. doi: 10.1007/s11095-008-9641-0. [DOI] [PubMed] [Google Scholar]
- 16.Kleberg K., Jacobsen F., Fatouros D.G., Müllertz A. Biorelevant media simulating fed state intestinal fluids: colloid phase characterization and impact on solubilization capacity. J Pharm Sci. 2010;99:3522–3532. doi: 10.1002/jps.22122. [DOI] [PubMed] [Google Scholar]
- 17.Kostewicz E.S., Brauns U., Becker R., Dressman J.B. Forecasting the oral absorption behavior of poorly soluble weak bases using solubility and dissolution studies in biorelevant media. Pharm Res. 2002;19:345–349. doi: 10.1023/a:1014407421366. [DOI] [PubMed] [Google Scholar]
- 18.Hernell O., Staggers J.E., Carey M.C. Physical-chemical behavior of dietary and biliary lipids during intestinal digestion and absorption. 2. Phase analysis and aggregation states of luminal lipids during duodenal fat digestion in healthy adult human beings. Biochemistry. 1990;29:2041–2056. doi: 10.1021/bi00460a012. [DOI] [PubMed] [Google Scholar]
- 19.Fatouros D.G., Walrand I., Bergenstahl B., Müllertz A. Colloidal structures in media simulating intestinal fed state conditions with and without lipolysis products. Pharm Res. 2009;26:361–374. doi: 10.1007/s11095-008-9750-9. [DOI] [PubMed] [Google Scholar]
- 20.Persson E.M., Gustafsson A.S., Carlsson A.S., Nilsson R.G., Knutson L., Forsell P. The effects of food on the dissolution of poorly soluble drugs in human and in model small intestinal fluids. Pharm Res. 2005;22:2141–2151. doi: 10.1007/s11095-005-8192-x. [DOI] [PubMed] [Google Scholar]
- 21.Tran T., Siqueira S.D., Amenitsch H., Rades T., Müllertz A. Monoacyl phosphatidylcholine inhibits the formation of lipid multilamellar structures during in vitro lipolysis of self-emulsifying drug delivery systems. Eur J Pharm Sci. 2017;108:62–70. doi: 10.1016/j.ejps.2016.11.022. [DOI] [PubMed] [Google Scholar]
- 22.Larsen A.T., Holm R., Müllertz A. Solution or suspension–does it matter for lipid based systems? In vivo studies of chase dosing lipid vehicles with aqueous suspensions of a poorly soluble drug. Eur J Pharm Biopharm. 2017;117:308–314. doi: 10.1016/j.ejpb.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 23.Doughty R.N., White H.D. Carvedilol: use in chronic heart failure. Expert Rev Cardiovasc Ther. 2007;5:21–31. doi: 10.1586/14779072.5.1.21. [DOI] [PubMed] [Google Scholar]
- 24.Thomas N., Müllertz A., Graf A., Rades T. Influence of lipid composition and drug load on the in vitro performance of self-nanoemulsifying drug delivery systems. J Pharm Sci. 2012;101:1721–1731. doi: 10.1002/jps.23054. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka Y., Hara T., Waki R., Nagata S. Regional differences in the components of luminal water from rat gastrointestinal tract and comparison with other species. J Pharm Pharm Sci. 2012;15:510–518. doi: 10.18433/j3f602. [DOI] [PubMed] [Google Scholar]
- 26.Siqueira Jørgensen S.D., Al Sawaf M., Graeser K., Mu H., Müllertz A., Rades T. The ability of two in vitro lipolysis models reflecting the human and rat gastro-intestinal conditions to predict the in vivo performance of SNEDDS dosing regimens. Eur J Pharm Biopharm. 2018;124:116–124. doi: 10.1016/j.ejpb.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 27.Carrière F., Renou C., Lopez V., De Caro J., Ferrato F., Lengsfeld H. The specific activities of human digestive lipases measured from the in vivo and in vitro lipolysis of test meals. Gastroenterology. 2000;119:949–960. doi: 10.1053/gast.2000.18140. [DOI] [PubMed] [Google Scholar]
- 28.Christophersen P.C., Christiansen M.L., Holm R., Kristensen J., Jacobsen J., Abrahamsson B. Fed and fasted state gastro-intestinal in vitro lipolysis: in vitro in vivo relations of a conventional tablet, a SNEDDS and a solidified SNEDDS. Eur J Pharm Sci. 2014;57:232–239. doi: 10.1016/j.ejps.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 29.Thomas N., Holm R., Müllertz A., Rades T. In vitro and in vivo performance of novel supersaturated self-nanoemulsifying drug delivery systems (super-SNEDDS) J Control Release. 2012;160:25–32. doi: 10.1016/j.jconrel.2012.02.027. [DOI] [PubMed] [Google Scholar]
- 30.Brittain H.G. Profiles of drug substances, excipients and related methodology. 1st ed. Elsevier Academic Press; San Diego, CA: 2013. [DOI] [PubMed] [Google Scholar]
- 31.Budavar S, O'Neil MJ, Smith A, Heckelman PE. Cinnarizine. In: The merck index. 11th ed. Rahway: Merck and Co., Inc.; 1989. p. 359.
- 32.F. Hoffmann-La Roche. Datasheet of compound CSE-3040, 2014.
- 33.Larsen A.T., Ogbonna A., Abu-Rmaileh R., Abrahamsson B., Østergaard J., Müllertz A. SNEDDS containing poorly water soluble cinnarizine; development and in vitro characterization of dispersion, digestion and solubilization. Pharmaceutics. 2012;4:641–665. doi: 10.3390/pharmaceutics4040641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larsen A.T., Ohlsson A.G., Polentarutti B., Barker R.A., Phillips A.R., Abu-Rmaileh R. Oral bioavailability of cinnarizine in dogs: relation to SNEDDS droplet size, drug solubility and in vitro precipitation. Eur J Pharm Sci. 2013;48:339–350. doi: 10.1016/j.ejps.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Phan S., Salentinig S., Prestidge C.A., Boyd B.J. Self-assembled structures formed during lipid digestion: characterization and implications for oral lipid-based drug delivery systems. Drug Deliv Transl Res. 2014;4:275–294. doi: 10.1007/s13346-013-0168-5. [DOI] [PubMed] [Google Scholar]
- 36.Kaukonen A.M., Boyd B.J., Porter C.J.H., Charman W.N. Drug solubilization behavior during in vitro digestion of simple triglyceride lipid solution formulations. Pharm Res. 2004;21:245–253. doi: 10.1023/b:pham.0000016282.77887.1f. [DOI] [PubMed] [Google Scholar]
- 37.Larsen A.T., Åkesson P., Juréus A., Saaby L., Abu-Rmaileh R., Abrahamsson B. Bioavailability of cinnarizine in dogs: effect of SNEDDS loading level and correlation with cinnarizine solubilization during in vitro lipolysis. Pharm Res. 2013;30:3101–3113. doi: 10.1007/s11095-013-1145-x. [DOI] [PubMed] [Google Scholar]
- 38.Sassene P.J., Knopp M.M., Hesselkilde J.Z., Koradia V., Larsen A., Rades T. Precipitation of a poorly soluble model drug during in vitro lipolysis: characterization and dissolution of the precipitate. J Pharm Sci. 2010;99:4982–4991. doi: 10.1002/jps.22226. [DOI] [PubMed] [Google Scholar]
- 39.Tran T, Rades T, Müllertz A. Formulation of self-nanoemulsifying drug delivery systems containing monoacyl phosphatidylcholine and Kolliphor® RH40 using experimental design. Asian J Pharm Sci 2017;13:536-45. [DOI] [PMC free article] [PubMed]