Abstract
Osteoarthritis (OA) is an inflammatory condition still lacking effective treatments. Mesenchymal stem/stromal cells (MSCs) have been successfully employed in pre-clinical models aiming to resurface the degenerated cartilage. In early-phase clinical trials, intra-articular (IA) administration of MSCs leads to pain reduction and cartilage protection or healing. However, the consistent lack of engraftment indicates that the observed effect is delivered through a “hit-and-run” mechanism, by a temporal release of paracrine molecules. MSCs express a variety of chemokines and cytokines that aid in repair of degraded tissue, restoration of normal tissue metabolism and, most importantly, counteracting inflammation. Secretion of therapeutic factors is increased upon licensing by inflammatory signals or apoptosis, induced by the host immune system. Trophic effectors are released as soluble molecules or carried by extracellular vesicles (ECVs). This review provides an overview of the functions and mechanisms of MSC-secreted molecules found to be upregulated in models of OA, whether using in vitro or in vivo models.
Keywords: osteoarthristis, mesenchymal stem cells, immunomodulation, secretome, paracrine action, chondroprotection
Mesenchymal Stromal Cells
Since described by Friedenstein (Friedenstein et al., 1966), mesenchymal stem/stromal cells (MSCs) have been the focus of research efforts to exploit their therapeutic potential. Due to their immune-evasive nature, MSCs release immunomodulatory factors which allow them to escape rejection mechanisms for sufficient time to exert their therapeutic action (Ankrum et al., 2014). MSCs also express a variety of cytokine and chemokine receptors, such as CXCR4, CXCR7, and CCR7, enabling migration to sites of injury and inflammation (Sasaki et al., 2008; Liu et al., 2012).
As a paradigm for tissue regeneration, MSCs have been used for many orthopedic conditions, including osteoarthritis (OA). The first successful treatment used a caprine model of OA involving anterior cruciate ligament transection combined with total medial meniscectomy (Murphy et al., 2003). Direct intra-articular (IA) delivery of autologous bone marrow (BM)-MSCs, 6 weeks after injury, led to meniscal repair and chondro-protection. The green fluorescent protein (GFP)-transduced cells were detectable in the synovial capsule, fat pad and newly-formed meniscus, but not in articular cartilage. This work led to the hypothesis that MSCs act via alternate mechanisms to cell replacement i.e., trophic mechanisms to promote tissue regeneration through modulation of the host environment and/or stimulation of endogenous progenitors (Jeong et al., 2013). The study was subsequently validated in other pre-clinical models of OA (Barry and Murphy, 2013). In general, this phenomenon transfers to other disease scenarios, as reviewed comprehensively by Prockop et al. (Prockop, 2009). More recently, phase I trials have provided evidence that MSCs also have clinical utility in modulating OA (Jo et al., 2014; Pers et al., 2016); a number of unpublished Phase 2 trials are ongoing assessing adipose-derived MSCs in OA including ADIPOA2 (http://adipoa2.eu/).
MSCs disappear from the target tissue quickly after administration, but are still able to deliver chondroprotective and immunomodulatory effects (ter Huurne et al., 2012). Since their therapeutic efficacy seems to be independent of their engraftment, it is now considered to be mainly paracrine-mediated. The increasingly accepted model is that MSCs are found dormant in vivo as pericytes (Crisan et al., 2008). These participate in the development of tissues, including synovium, and are involved in tissue repair during adult life (Roelofs et al., 2017). Once activated in response to signals associated with the injured environment, such as pro-inflammatory cytokines, a phenomenon generally referred to as “licensing,” they secrete factors, including chemokines and cytokines, to establish a regenerative environment. Depending on the environment of the specific disease, anti-apoptotic and anti-fibrotic factors may limit the extent of damage to improve tissue healing (Ryan et al., 2017). Tissue-intrinsic progenitors are prompted to proliferate and differentiate, while chemoattractants recruit endogenous progenitors to the site of injury. Concurrently, activated MSCs are capable of modulating the immune response locally by selectively inhibiting the proliferation of immune cells (Aggarwal and Pittenger, 2005) (Figure 1). This paper will review the evidence for these therapeutic effects in models relevant to OA, either in vivo or in vitro (summarized in Table 1). It will be critical in the future to validate those findings using freshly isolated stromal cells.
Figure 1.
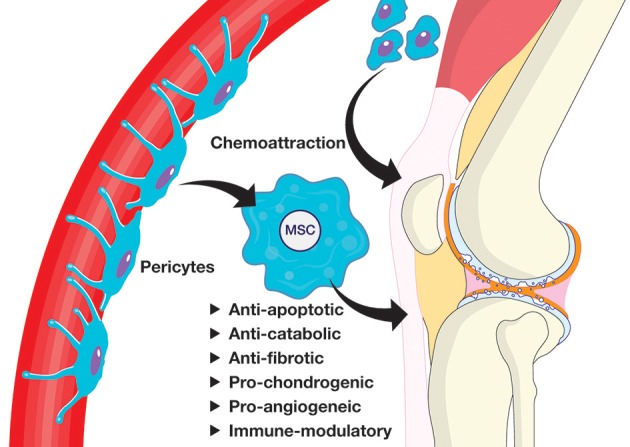
Proposed mechanism of action for tissue repair by endogenous MSCs.
Table 1.
The MSC secretome and OA/cartilage protection.
| Activity | Factor | References |
|---|---|---|
| Anti-apoptosis | STC-1, | Rehman et al., 2004; Block et al., 2009 |
| Anti-fibrosis | bFGF, AMD, HGF | Li et al., 2009; Suga et al., 2009; Maumus et al., 2013 |
| Tissue metabolism | TIMP-1, TIMP-2 | Lozito and Tuan, 2011 |
| Chondrogenesis | TSP2 | Jeong et al., 2013, 2015 |
| Immunosuppression | PGE2 | Aggarwal and Pittenger, 2005; Sotiropoulou et al., 2006; Martinet et al., 2009 |
| Immunosuppression | TSG-6 | Mindrescu et al., 2000; Bárdos et al., 2001; Lee et al., 2009 |
| Anti-apoptosis | ECVs | Liu et al., 2018a,b |
| Immunosuppression | ECVs | Mokarizadeh et al., 2012; Budoni et al., 2013; Zhang et al., 2014 |
| Chondrogenesis | ECVs | Zhu et al., 2017 |
| Chondroprotective/ anti-inflammatory effects | ECVs | Cosenza et al., 2017 |
The MSC Secretome
MSCs display a rich secretory profile which is enhanced by exposure to inflammatory signals. A proteomics approach identified 118 proteins differentially expressed by human adipose-derived stem cells (ASCs) upon tumor necrosis factor (TNF)-α stimulation (Lee et al., 2010). These included many cytokines and chemokines [interleukin (IL)-6, 8; chemokine (C-X-C motif) ligand or CXCL2, 5, 6, and 10 and monocyte chemoattractant protein 1 (MCP1)], proteases and protease inhibitors [matrix metalloproteinases (MMP)-1 and 2, tissue inhibitors of metalloproteinases (TIMP)-1 and 2], extracellular matrix (ECM) molecules and factors involved in immune regulation and cell signaling.
Apoptosis
Chondrocyte apoptosis has been associated with degenerative OA for many years (Aigner et al., 2004; Del Carlo and Loeser, 2008). Although there are no reports of direct anti-apoptotic effects of MSCs in the context of OA, indirect evidence suggests that exosomes obtained from human MSCs, and by inference comprised of secreted factors, inhibited IL-1β-induced apoptosis of ex vivo-cultured OA chondrocytes (Liu et al., 2018a). Additionally, MSC-derived exosomes promoted chondrocyte proliferation in a rat model of OA, by blocking miR-206 with lncRNA-KLF3-AS1 (Liu et al., 2018b). Despite soluble factors were not shown in models of OA, MSCs responded to two different apoptotic cell lines in vitro by increased expression and secretion of the anti-apoptotic hormone stanniocalcin (STC)-1 (Block et al., 2009). Future work looking at joint-associated MSC anti-apoptotic effects is likely to identify direct mediators of the process.
Fibrosis
Maumus et al. co-cultured autologous ASCs with chondrocytes derived from OA patients in a transwell system (Maumus et al., 2013). The authors observed marked decreases in expression levels of hypertrophic and fibrotic markers MMP-13, alkaline phosphatase, Runx2, collagens type I, III, VI and vimentin, as well as a 40% increase in TGF-β1 secretion. By using a neutralizing antibody, HGF was identified as the main mediator of the anti-fibrotic effect. This data is of particular relevance as HGF concentration in synovial fluid has a direct correlation with the severity of OA (Dankbar et al., 2007). MSCs also inhibit fibrosis in vivo through bFGF (Suga et al., 2009) and adrenomedullin (Li et al., 2009). In addition, a number of studies proposed that in vivo-administered MSCs secreted TSG-6 and indirectly prevented fibrosis by suppressing the early inflammatory response to various diseases other than OA, including myocardial infarction (Lee et al., 2009), peritonitis (Choi et al., 2011), inflammation of the cornea (Oh et al., 2010), and cornea allograft rejection (Oh et al., 2012).
Tissue Metabolism
Amongst other activities, MMPs break down ECM and are regulated by specific inhibitors called TIMPs. In OA, the balance between anabolic and catabolic factors is disrupted in favor of the latter (Lohmander et al., 1993). MMP-2,-9, and -13 were detected at higher levels in human OA cartilage compared to healthy tissue (Jackson et al., 2014). Furthermore, decreased MMP-13 correlated with improved osteochondral repair in rats treated with doxycycline (Lee H. et al., 2013) and MSCs constitutively secrete high levels of TIMP-2 and -1, which inhibit MMP-2 and MMP-9, respectively. Under pathological stress (IL-1β, TNF-α, hypoxia) TIMP-1 secretion is upregulated to counteract increased catabolic activity (Lozito and Tuan, 2011). In addition, MMP inhibition is not specific; TIMP-1 can inhibit most MMPs (Visse and Nagase, 2003), making MSCs an even more versatile tool for restoring the metabolic balance of degenerating cartilage.
Chondrogenesis
Matricellular proteins, secreted matrix proteins with regulatory roles, bind to ECM and act as receptors for cell-surface molecules, growth factors and MMPs (Bornstein et al., 2000). Thrombospondin (TSP2) for example is a known regulator of cartilage and bone differentiation and is secreted by MSCs to induce proliferation via autocrine mechanisms (Hankenson and Bornstein, 2002). TSP2, secreted by human umbilical cord blood-derived (UCB)-MSCs treated with synovial fluid from OA patients, induced differentiation of chondroprogenitor cells. It promoted cartilage regeneration in a rabbit full-thickness osteochondral-defect model (Jeong et al., 2013). TSP2 was found to have an autocrine action on human UCB-MSCs, BM-MSCs and ASCs, promoting cartilage differentiation and preventing hypertrophy (Jeong et al., 2015). Although data is limited, there is evidence that TSP2 is one of the main paracrine players in MSC-mediated cartilage regeneration.
Immunosuppression
The role of inflammation in the establishment and maintenance of OA is now widely accepted (Ayral et al., 2005) with synovial membrane inflammation a hallmark of OA pathology (Goldring, 1999; Pelletier et al., 2001). Histological studies show that OA patients have variable degrees of synovitis, with higher levels of pro-inflammatory cytokines and infiltration of immune cells, predominantly macrophages (Benito et al., 2005). Biological markers of inflammation positively correlate with knee pain (Baker et al., 2010; Scanzello et al., 2011) and clinical progression of the disease (Krasnokutsky et al., 2011; Roemer et al., 2011). Licensed MSCs secrete an array of anti-inflammatory cytokines which can help re-establish an equilibrium in the inflamed synovium: MSC-conditioned medium (CM) decreased production of inflammatory mediators in OA joint explants (van Buul et al., 2012).
Di Nicola et al. first assessed the potential for allogeneic MSC rejection in a mixed lymphocyte reaction (MLR). Instead of evoking an immune response, the cells suppressed proliferation of T-cells (Di Nicola et al., 2002; Krampera et al., 2003). The relevance of MSC-secreted factors in immunomodulation was shown by the capacity of their supernatant to divert immune cells from injured organs (Parekkadan et al., 2007). Currently, MSCs are under evaluation in numerous clinical trials for many inflammatory conditions (Trounson and McDonald, 2015).
One of the main effectors of MSC-mediated immune-suppression is prostaglandin-E2 (PGE2). PGE2 is constitutively secreted by MSCs and its production is dramatically enhanced via stimulation by interferon (IFN)-γ, TNF-α (English et al., 2007), or IL-1β (Chen et al., 2010). PGE2 negatively affects the proliferation of T- (Martinet et al., 2009) and natural killer (NK) cells (Sotiropoulou et al., 2006), causes an increase in the pool of regulatory T (Treg) cells, stimulates macrophages to produce IL-10 and prevents monocytes from differentiating into dendritic cells (DCs) (Aggarwal and Pittenger, 2005). In OA, PGE2 mediates ASC therapeutic effects and is a regulatory checkpoint in immune-modulation. Manfredini et al. provided evidence that the PGE2/COX2 pathway is responsible for the induction of IL-10 and inhibition of TNFα and IL-6 to induce an M2 switch in human synovial macrophages (Manferdini et al., 2017).
Indoleamine 2,3-dioxygenase (IDO) catalyzes the breakdown of tryptophan, causing suppression of T-cells. It is employed by DCs to modulate immune responses (Mellor and Munn, 2004), but can be secreted by MSCs upon IFN-γ stimulation (Krampera et al., 2006). In a human MLR, IFN-γ-induced expression of IDO in MSCs was responsible for suppression of T-cell proliferation (Meisel et al., 2004). It also drives M2 polarization in macrophages and induces a tolerogenic phenotype in DCs and Tregs (Ge et al., 2010; Sica and Mantovani, 2012). Its' importance in MSC-mediated immunosuppression has been validated using specific inhibitors and knockout MSCs (Krampera et al., 2006; English et al., 2007; Spaggiari et al., 2008).
TNF-inducible gene (TSG)-6 is known for its multiple and diverse anti-inflammatory mechanisms (Wisniewski and Vilcek, 2004). Produced in response to inflammatory signals, it has a pivotal role in MSC-mediated immunosuppression (Lee et al., 2009). On the other hand, it was identified as one of the most significantly up-regulated genes in human OA articular cartilage (Chou et al., 2015) and proposed as a disease biomarker, as its activity in synovial fluid predicted OA progression (Wisniewski et al., 2014). TSG-6 has a complex role in cartilage pathology, as it is involved in matrix assembly during synthesis of new tissue (Chou et al., 2018).
Other molecules have been shown to mediate MSC immunosuppression, such as C-C motif ligand 2 (CCL2) (Rafei et al., 2009), galectins (Sioud et al., 2011), IL-6 (Scheller et al., 2011), and TGF-β (Di Nicola et al., 2002). None of these factors has an exclusive role; their functions may be redundant and/or synergistic. To fully express an anti-inflammatory phenotype, MSCs need to be licensed. This can be achieved in response to IFN-γ alone (Krampera et al., 2006) or in combination with TNF-α, IL-1α, or IL-1β (Ren et al., 2008). Additionally, IL-1β, granulocyte-colony stimulating factor (G-CSF), stromal cell-derived factor 1 (SDF1) and stem cell factor (SCF) induced differential expression of numerous cytokines in MSCs after only 2 h of treatment (Czekanska et al., 2014). Licensed MSCs have an improved regenerative capacity in pre-clinical models, with better homing potential (Duijvestein et al., 2011) and recruitment of host immune cells (Lee S. et al., 2013).
The Role of Apoptotic MSCs
Once administered, MSCs can undergo biological changes more radical than differentiation or licensing. Toupet et al. observed that most MSCs disappear 10 days post-IA injection in a murine model of OA, with similar results obtained with syngeneic and xenogenic human ASCs (Toupet et al., 2013, 2015). Despite death and clearance of administered cells, significant therapeutic effects are observed in response to IA injection of mouse ASCs (ter Huurne et al., 2012; Schelbergen et al., 2014).
Apoptotic cells communicate with immune cells through two different mechanisms: direct effects associated with apoptotic cells themselves and indirect effects triggered in phagocytizing cells (Figure 2). Direct effects include secretion of IL-10 and TGF-β, generating an immunosuppressive microenvironment (Chen et al., 2001; Korns et al., 2011). This milieu inhibits lipopolysaccharide (LPS)-stimulated macrophages from secreting IL-1β and TNF-α (McDonald et al., 1999). Indirect effects are associated with elimination of apoptotic cells by phagocytes, resulting in reduced responsiveness to LPS (Perruche et al., 2009) and a switch to an anti-inflammatory profile (Fadok et al., 1998). Immune cells that internalize apoptotic cells also fail to induce CD4+ T helper cells, leaving the effector lymphocytes in a “helpless” state (Griffith et al., 2007) and induce clonal expansion of Foxp3+ Treg cells (Xia et al., 2007; Perruche et al., 2008).
Figure 2.
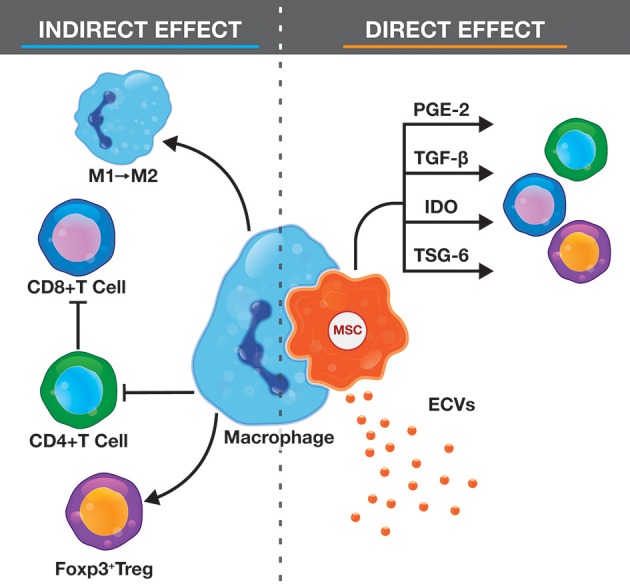
Representation of the immunomodulatory effects of apoptotic MSCs.
Using a murine model of graft-vs.-host disease (GvHD), researchers demonstrated that infused MSC apoptosis is induced by recipient T cells through cell-to-cell contact with release of perforin- and granzyme B-containing granules (Galleu et al., 2017). Phagocytes were also shown to have a key role producing IDO upon engulfing apoptotic MSCs. When these components were knocked down or inhibited, the therapeutic efficacy of MSCs was lost. Most importantly, infusion of MSCs rendered apoptotic ex vivo restored therapeutic effects. Interestingly, patient responsiveness to MSCs correlated with their cytotoxic capacity. These findings provide evidence that apoptosis is one of the driving mechanism of MSC-mediated immunosuppression.
TGF-β-mediated tolerance induction is the most commonly reported mechanism in pre-clinical studies of extracorporeal photopheresis, the administration of leukocytes rendered apoptotic ex vivo. A strong immunomodulatory effect was observed in inflammatory arthritis (Michlewska et al., 2009; Perruche et al., 2009) and photopheresis is an approved therapy for cutaneous T cell lymphoma and GvHD (Weitz et al., 2015). Apoptosis may also represent an important component of MSC therapy in OA. Unpublished data in our laboratory shows as low as 1.6% MSC engraftment 3 days after IA administration of GFP+ MSCs in murine OA knees. Fluorescent cells were not detected in any adjacent tissue, including local lymph nodes. This reinforces the hypothesis that implanted cells could undergo apoptosis and modulate inflammation with subsequent protection from OA development. Whereas, apoptosis post-infusion is a transient event, Galleu et al. showed that the subsequent response might represent a reprogramming of certain aspects the host immune system (Galleu et al., 2017).
Looking Further: Extra-Cellular Vesicles
The paracrine action of MSCs is not limited to soluble factors. MSCs, like many other cells, have been shown to produce extracellular vesicles (ECVs) (Lai et al., 2010), small structures enclosed in a phospholipid bilayer, carrying many cytoplasmic components. ECVs are involved in intercellular communication through horizontal transfer of mRNA and protein and are grouped based on size, with different composition and biogenesis. Exosomes range between 40 and 100 nm in diameter. They are constitutively released from the late endosomal compartment by fusion of multivesicular bodies with the plasma membrane, but their production can increase upon cytoskeleton activation. Exosomes are characterized by proteins required for their formation and transport, such as tetraspanins, Alix and tumor susceptibility gene 101. Microvesicles are a heterogeneous population of ECVs between 100 and 1,000 nm generated via direct budding upon activation by a stress signal, which alters the phospholipid balance of the membrane, forming lipid rafts. Microvesicles are characterized by membrane markers specific to the parent cell type. In pre-clinical models, ECVs were observed to have anti-apoptotic (Bruno et al., 2012), anti-fibrotic (Li et al., 2013), pro-angiogenic (Bian et al., 2014), and anti-inflammatory effects (Lee et al., 2012). MSC-derived ECVs induce generation of Tregs, inhibit proliferation of lymphocytes (Mokarizadeh et al., 2012), macrophages (Zhang et al., 2014), and B cells (Budoni et al., 2013). However, ECVs alone may fail to deliver the same immunomodulatory effects of parental cells, with cell-cell contact still required to modulate lymphocyte proliferation and function (Conforti et al., 2014).
MSC-derived ECVs produced promising results in rat models of osteoporosis (Qi et al., 2016) and osteochondral defect repair (Zhang et al., 2016). More recently, MSC-ECVs were tested in OA models. Exosomes derived from synovium MSCs and induced pluripotent stem cells attenuated disease scores in a collagenase-induced OA (CIOA) mouse model, by promoting chondrocyte proliferation and migration (Zhu et al., 2017). Notably, exosomes derived from synovial MSCs overexpressing miR-140-5p induced proliferation of chondrocytes in vitro. When administered in a rat model of OA disease progression and cartilage degeneration were significantly delayed (Tao et al., 2017). Cosenza et al. delivered MSC-ECVs in a CIOA model and reported reduced joint damage (Cosenza et al., 2017). The use of MSC-ECVs as a therapy for OA would bring many advantages compared to cell-derived products, avoiding concerns of possible malignant transformations. However, issues may arise with ECV production as they may need to be specifically tailored for the indication to be treated. Additionally, their manufacture is not as yet standardized for clinical production, as is the case for cellular products.
Conclusions
Reports summarized here suggest significant potential for the use of MSCs or MSC-CM in OA. In vitro, co-culture of OA chondrocytes with ASC-CM resulted in NF-κB-mediated cytoprotective effects via enhanced production of collagen II, inhibition of IL-6, TNF and various MMPs, as well as upregulation of IL-10 (Platas et al., 2013). Similarly, using OA cartilage explants, MSC-CM was shown to interfere with the NF-κB pathway to mediate anti-inflammatory and anti-catabolic effects (van Buul et al., 2012). MSCs have already proved to be a valuable tool for many conditions, including acute GvHD (Le Blanc et al., 2008) and multiple sclerosis (Karussis et al., 2008).
Phase I clinical trials have demonstrated the safety of direct IA administration of MSCs in OA patients (Centeno et al., 2008; Davatchi et al., 2011). In 2012, pain reduction was reported up to 6 months after injection of 20–24 million MSCs, with increased cartilage thickness and reduction of edematous subchondral patches in three out of six patients (Emadedin et al., 2012). Jo et al. injected higher doses of ASCs (up to 10 × 108), obtaining significantly improved WOMAC score with a clinically meaningful pain reduction and, most importantly, regenerated hyaline articular cartilage in the most severely degenerated site in the knee (Jo et al., 2014). In the ADIPOA trial, a single dose of 2 million ASCs significantly improved pain levels and function (Pers et al., 2016).
In summary, MSCs may act through a hit-and-run mechanism rather than stably engrafting in the tissue. Autopsies of patients that received MSC IV infusions for different conditions within a year before death confirm that donor MSCs are not normally retained in the host tissue. Detection of donor DNA did not correlate with the degree of HLA mismatch or the clinical response, suggesting that clearance is not immune-mediated (von Bahr et al., 2012). However, the role of cell death in mediating the therapeutic effects of MSCs needs further investigation and the phenotype and activity of cells that survive even for a short time at the site of implantation elucidated.
Author Contributions
PM: contributed to the development of the concept underpinning the review, researched the relevant literature, and wrote the body of the review; SR: also contributed to the concept of the submission and helped with the writing; AG: researched the literature about apoptosis; JMM: participated in manuscript preparation, on-going and final review of the submission; FB: also participated in manuscript conception and review.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Maciek Doczyk for designing the figures.
Footnotes
Funding. PM is funded by Science Foundation Ireland through Cúram (Grant no. 13/RC/2073) and the Irish Blood Transfusion Service. SR is funded by a NUI Galway Hardiman scholarship. JMM and FB by the European Union (H2020 PHC16-2015, grant no. 667932; H2020-PHC-2014-2015, grant no. 643809) and by Science Foundation Ireland through Cúram (Grant no. 13/RC/2073).
References
- Aggarwal S., Pittenger M. F. (2005). Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105, 1815–1822. 10.1182/blood-2004-04-1559 [DOI] [PubMed] [Google Scholar]
- Aigner T., Kim H. A., Roach H. I. (2004). Apoptosis in osteoarthritis. Rheum Dis Clin North Am. 30, 639–653, xi. 10.1016/j.rdc.2004.04.002 [DOI] [PubMed] [Google Scholar]
- Ankrum J. A., Ong J. F., Karp J. M. (2014). Mesenchymal stem cells: immune evasive, not immune privileged. Nat. Biotechnol. 32, 252–260. 10.1038/nbt.2816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayral X., Pickering E. H., Woodworth T. G., Mackillop N., Dougados M. (2005). Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis – results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthr. Cartil. 13, 361–367. 10.1016/j.joca.2005.01.005 [DOI] [PubMed] [Google Scholar]
- Baker K., Grainger A., Niu J., Clancy M., Guermazi A., Crema M., et al. (2010). Relation of synovitis to knee pain using contrast-enhanced MRIs. Ann. Rheum. Dis. 69, 1779–1783. 10.1136/ard.2009.121426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bárdos T., Kamath R. V., Mikecz K., Glant T. T. (2001). Anti-inflammatory and chondroprotective effect of TSG-6 (tumor necrosis factor-alpha-stimulated gene-6) in murine models of experimental arthritis. Am. J. Pathol. 159, 1711–1721. 10.1016/S0002-9440(10)63018-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry F., Murphy M. (2013). Mesenchymal stem cells in joint disease and repair. Nat. Rev. Rheumatol. 9, 584–594. 10.1038/nrrheum.2013.109 [DOI] [PubMed] [Google Scholar]
- Benito M. J., Veale D. J., FitzGerald O., van den Berg W. B., Bresnihan B. (2005). Synovial tissue inflammation in early and late osteoarthritis. Ann. Rheum. Dis. 64, 1263–1267. 10.1136/ard.2004.025270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian S., Zhang L., Duan L., Wang X., Min Y., Yu H. (2014). Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J. Mol. Med. 92, 387–397. 10.1007/s00109-013-1110-5 [DOI] [PubMed] [Google Scholar]
- Block G. J., Ohkouchi S., Fung F., Frenkel J., Gregory C., Pochampally R., et al. (2009). Multipotent stromal cells are activated to reduce apoptosis in part by upregulation and secretion of stanniocalcin-1. Stem Cells 27, 670–681. 10.1002/stem.20080742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P., Armstrong L. C., Hankenson K. D., Kyriakides T. R., Yang Z. (2000). Thrombospondin 2, a matricellular protein with diverse functions. Matrix Biol. 19, 557–568. 10.1016/S0945-053X(00)00104-9 [DOI] [PubMed] [Google Scholar]
- Bruno S., Grange C., Collino F., Deregibus M. C., Cantaluppi V., Biancone L., et al. (2012). Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS ONE 7:e33115. 10.1371/journal.pone.0033115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budoni M., Fierabracci A., Luciano R., Petrini S., Di Ciommo V., Muraca M. (2013). The immunosuppressive effect of mesenchymal stromal cells on B lymphocytes is mediated by membrane vesicles. Cell Transplant. 22, 369–379. 10.3727/096368911X582769b [DOI] [PubMed] [Google Scholar]
- Centeno C. J., Busse D., Kisiday J., Keohan C., Freeman M., Karli D. (2008). Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Physician 11, 343–353. [PubMed] [Google Scholar]
- Chen K., Wang D., Du W. T., Han Z. B., Ren H., Chi Y., et al. (2010). Human umbilical cord mesenchymal stem cells hUC-MSCs exert immunosuppressive activities through a PGE2-dependent mechanism. Clin. Immunol. 135, 448–458. 10.1016/j.clim.2010.01.015 [DOI] [PubMed] [Google Scholar]
- Chen W., Frank M. E., Jin W., Wahl S. M. (2001). TGF-beta released by apoptotic T cells contributes to an immunosuppressive milieu. Immunity 14, 715–725. 10.1016/S1074-7613(01)00147-9 [DOI] [PubMed] [Google Scholar]
- Choi H., Lee R. H., Bazhanov N., Oh J. Y., Prockop D. J. (2011). Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-kappaB signaling in resident macrophages. Blood 118, 330–338. 10.1182/blood-2010-12-327353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou C. H., Attarian D. E., Wisniewski H. G., Band P. A., Kraus V. B. (2018). TSG-6 - a double-edged sword for osteoarthritis (OA). Osteoarthr. Cartil. 26, 245–254. 10.1016/j.joca.2017.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou C. H., Lee M. T., Song I. W., Lu L. S., Shen H. C., Lee C. H., et al. (2015). Insights into osteoarthritis progression revealed by analyses of both knee tibiofemoral compartments. Osteoarthr. Cartil. 23, 571–580. 10.1016/j.joca.2014.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforti A., Scarsella M., Starc N., Giorda E., Biagini S., Proia A., et al. (2014). Microvescicles derived from mesenchymal stromal cells are not as effective as their cellular counterpart in the ability to modulate immune responses in vitro. Stem Cells Dev. 23, 2591–2599. 10.1089/scd.2014.0091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosenza S., Ruiz M., Toupet K., Jorgensen C., Noël D. (2017). Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci. Rep. 7:16214. 10.1038/s41598-017-15376-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisan M., Yap S., Casteilla L., Chen C. W., Corselli M., Park T. S., et al. (2008). A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3, 301–313. 10.1016/j.stem.2008.07.003 [DOI] [PubMed] [Google Scholar]
- Czekanska E. M., Ralphs J. R., Alini M., Stoddart M. J. (2014). Enhancing inflammatory and chemotactic signals to regulate bone regeneration. Eur. Cell. Mater. 28, 320–334. 10.22203/eCM.v028a22 [DOI] [PubMed] [Google Scholar]
- Dankbar B., Neugebauer K., Wunrau C., Tibesku C. O., Skwara A., Pap T., et al. (2007). Hepatocyte growth factor induction of macrophage chemoattractant protein-1 and osteophyte-inducing factors in osteoarthritis. J. Orthop. Res. 25, 569–577. 10.1002/jor.20338 [DOI] [PubMed] [Google Scholar]
- Davatchi F., Abdollahi B. S., Mohyeddin M., Shahram F., Nikbin B. (2011). Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int. J. Rheum. Dis. 14, 211–215. 10.1111/j.1756-185X.2011.01599.x [DOI] [PubMed] [Google Scholar]
- Del Carlo M., Jr., Loeser R. F. (2008). Cell death in osteoarthritis. Curr. Rheumatol. Rep. 10, 37–42. 10.1007/s11926-008-0007-8 [DOI] [PubMed] [Google Scholar]
- Di Nicola M., Carlo-Stella C., Magni M., Milanesi M., Longoni P. D., Matteucci P., et al. (2002). Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 99, 3838–3843. 10.1182/blood.V99.10.3838 [DOI] [PubMed] [Google Scholar]
- Duijvestein M., Wildenberg M. E., Welling M. M., Hennink S., Molendijk I., van Zuylen V. L., et al. (2011). Pretreatment with interferon-gamma enhances the therapeutic activity of mesenchymal stromal cells in animal models of colitis. Stem Cells 29, 1549–1558. 10.1002/stem.698 [DOI] [PubMed] [Google Scholar]
- Emadedin M., Aghdami N., Taghiyar L., Fazeli R., Moghadasali R., Jahangir S., et al. (2012). Intra-articular injection of autologous mesenchymal stem cells in six patients with knee osteoarthritis. Arch. Iran. Med. 15, 422–428. [PubMed] [Google Scholar]
- English K., Barry F. P., Field-Corbett C. P., Mahon B. P. (2007). IFN-gamma and TNF-alpha differentially regulate immunomodulation by murine mesenchymal stem cells. Immunol. Lett. 110, 91–100. 10.1016/j.imlet.2007.04.001 [DOI] [PubMed] [Google Scholar]
- Fadok V. A., Bratton D. L., Konowal A., Freed P. W., Westcott J. Y., Henson P. M. (1998). Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J. Clin. Invest. 101, 890–898. 10.1172/JCI1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedenstein A. J., Piatetzky-Shapiro II., Petrakova K. V. (1966). Osteogenesis in transplants of bone marrow cells. J. Embryol. Exp. Morphol. 16, 381–390. [PubMed] [Google Scholar]
- Galleu A., Riffo-Vasquez Y., Trento C., Lomas C., Dolcetti L., Cheung T. S., et al. (2017). Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci. Transl. Med. 9:eaam7828. 10.1126/scitranslmed.aam7828 [DOI] [PubMed] [Google Scholar]
- Ge W., Jiang J., Arp J., Liu W., Garcia B., Wang H. (2010). Regulatory T-cell generation and kidney allograft tolerance induced by mesenchymal stem cells associated with indoleamine 2,3-dioxygenase expression. Transplantation 90, 1312–1320. 10.1097/TP.0b013e3181fed001 [DOI] [PubMed] [Google Scholar]
- Goldring M. B. (1999). The role of cytokines as inflammatory mediators in osteoarthritis: lessons from animal models. Connect. Tissue Res. 40, 1–11. [DOI] [PubMed] [Google Scholar]
- Griffith T. S., Kazama H., VanOosten R. L., Earle J. K., Jr., Herndon J. M., Green D. R., et al. (2007). Apoptotic cells induce tolerance by generating helpless CD8+ T cells that produce TRAIL. J. Immunol. 178, 2679–2687. 10.4049/jimmunol.178.5.2679 [DOI] [PubMed] [Google Scholar]
- Hankenson K. D., Bornstein P. (2002). The secreted protein thrombospondin 2 is an autocrine inhibitor of marrow stromal cell proliferation. J. Bone Miner. Res. 17, 415–425. 10.1359/jbmr.2002.17.3.415 [DOI] [PubMed] [Google Scholar]
- Jackson M. T., Moradi B., Smith M. M., Jackson C. J., Little C. B. (2014). Activation of matrix metalloproteinases 2, 9, and 13 by activated protein C in human osteoarthritic cartilage chondrocytes. Arthritis Rheumatol. 66, 1525–1536. 10.1002/art.38401 [DOI] [PubMed] [Google Scholar]
- Jeong S. Y., Ha J., Lee M., Jin H. J., Kim D. H., Choi S. J., et al. (2015). Autocrine action of thrombospondin-2 determines the chondrogenic differentiation potential and suppresses hypertrophic maturation of human umbilical cord blood-derived mesenchymal stem cells. Stem Cells 33, 3291–3303. 10.1002/stem.2120 [DOI] [PubMed] [Google Scholar]
- Jeong S. Y., Kim D. H., Ha J., Jin H. J., Kwon S. J., Chang J. W., et al. (2013). Thrombospondin-2 secreted by human umbilical cord blood-derived mesenchymal stem cells promotes chondrogenic differentiation. Stem Cells 31, 2136–2148. 10.1002/stem.1471 [DOI] [PubMed] [Google Scholar]
- Jo C. H., Lee Y. G., Shin W. H., Kim H., Chai J. W., Jeong E. C., et al. (2014). Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells 32, 1254–1266. 10.1002/stem.1634 [DOI] [PubMed] [Google Scholar]
- Karussis D., Kassis I., Kurkalli B. G., Slavin S. (2008). Immunomodulation and neuroprotection with mesenchymal bone marrow stem cells (MSCs): a proposed treatment for multiple sclerosis and other neuroimmunological/neurodegenerative diseases. J. Neurol. Sci. 265, 131–135. 10.1016/j.jns.2007.05.005 [DOI] [PubMed] [Google Scholar]
- Korns D., Frasch S. C., Fernandez-Boyanapalli R., Henson P. M., Bratton D. L. (2011). Modulation of macrophage efferocytosis in inflammation. Front. Immunol. 2:57. 10.3389/fimmu.2011.00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krampera M., Cosmi L., Angeli R., Pasini A., Liotta F., Andreini A., et al. (2006). Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells 24, 386–398. 10.1634/stemcells.2005-0008 [DOI] [PubMed] [Google Scholar]
- Krampera M., Glennie S., Dyson J., Scott D., Laylor R., Simpson E., et al. (2003). Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 101, 3722–3729. 10.1182/blood-2002-07-2104 [DOI] [PubMed] [Google Scholar]
- Krasnokutsky S., Belitskaya-Levy I., Bencardino J., Samuels J., Attur M., Regatte R., et al. (2011). Quantitative magnetic resonance imaging evidence of synovial proliferation is associated with radiographic severity of knee osteoarthritis. Arthritis Rheum. 63, 2983–2991. 10.1002/art.30471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai R. C., Arslan F., Lee M. M., Sze N. S., Choo A., Chen T. S., et al. (2010). Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 4, 214–222. 10.1016/j.scr.2009.12.003 [DOI] [PubMed] [Google Scholar]
- Le Blanc K., Frassoni F., Ball L., Locatelli F., Roelofs H., Lewis I., et al. (2008). Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 371, 1579–1586. 10.1016/S0140-6736(08)60690-X [DOI] [PubMed] [Google Scholar]
- Lee C., Mitsialis S. A., Aslam M., Vitali S. H., Vergadi E., Konstantinou G., et al. (2012). Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation 126, 2601–2611. 10.1161/CIRCULATIONAHA.112.114173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H., O'Malley M. J., Friel N. A., Chu C. R. (2013). Effects of doxycycline on mesenchymal stem cell chondrogenesis and cartilage repair. Osteoarthr. Cartil. 21, 385–393. 10.1016/j.joca.2012.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. J., Kim J., Kim M. Y., Bae Y. S., Ryu S. H., Lee T. G., et al. (2010). Proteomic analysis of tumor necrosis factor-alpha-induced secretome of human adipose tissue-derived mesenchymal stem cells. J. Proteome Res. 9, 1754–1762. 10.1021/pr900898n [DOI] [PubMed] [Google Scholar]
- Lee R. H., Pulin A. A., Seo M. J., Kota D. J., Ylostalo J., Larson B. L., et al. (2009). Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 5, 54–63. 10.1016/j.stem.2009.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Szilagyi E., Chen L., Premanand K., DiPietro L. A., Ennis W., et al. (2013). Activated mesenchymal stem cells increase wound tensile strength in aged mouse model via macrophages. J. Surg. Res. 181, 20–24. 10.1016/j.jss.2012.05.040 [DOI] [PubMed] [Google Scholar]
- Li L., Zhang S., Zhang Y., Yu B., Xu Y., Guan Z. (2009). Paracrine action mediate the antifibrotic effect of transplanted mesenchymal stem cells in a rat model of global heart failure. Mol. Biol. Rep. 36, 725–731. 10.1007/s11033-008-9235-2 [DOI] [PubMed] [Google Scholar]
- Li T., Yan Y., Wang B., Qian H., Zhang X., Shen L., et al. (2013). Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 22, 845–854. 10.1089/scd.2012.0395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Liu S., Li Y., Wang X., Xue W., Ge G., et al. (2012). The role of SDF-1-CXCR4/CXCR7 axis in the therapeutic effects of hypoxia-preconditioned mesenchymal stem cells for renal ischemia/reperfusion injury. PLoS ONE 7:e34608. 10.1371/journal.pone.0034608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Lin L., Zou R., Wen C., Wang Z., Lin F. (2018a). MSC-derived exosomes promote proliferation and inhibit apoptosis of chondrocytes via lncRNA-KLF3-AS1/miR-206/GIT1 axis in osteoarthritis. Cell Cycle 17, 2411–2422. 10.1080/15384101.2018.1526603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Zou R., Wang Z., Wen C., Zhang F., Lin F. (2018b). Exosomal KLF3-AS1 from hMSCs promoted cartilage repair and chondrocyte proliferation in osteoarthritis. Biochem. J. 475, 3629–3638. 10.1042/BCJ20180675 [DOI] [PubMed] [Google Scholar]
- Lohmander L. S., Neame P. J., Sandy J. D. (1993). The structure of aggrecan fragments in human synovial fluid. Evidence that aggrecanase mediates cartilage degradation in inflammatory joint disease, joint injury, and osteoarthritis. Arthritis Rheum. 36, 1214–1222. 10.1002/art.1780360906 [DOI] [PubMed] [Google Scholar]
- Lozito T. P., Tuan R. S. (2011). Mesenchymal stem cells inhibit both endogenous and exogenous MMPs via secreted TIMPs. J. Cell. Physiol. 226, 385–396. 10.1002/jcp.22344 [DOI] [PubMed] [Google Scholar]
- Manferdini C., Paolella F., Gabusi E., Gambari L., Piacentini A., Filardo G., et al. (2017). Adipose stromal cells mediated switching of the pro-inflammatory profile of M1-like macrophages is facilitated by PGE2: in vitro evaluation. Osteoarthr. Cartil. 25, 1161–1171. 10.1016/j.joca.2017.01.011 [DOI] [PubMed] [Google Scholar]
- Martinet L., Fleury-Cappellesso S., Gadelorge M., Dietrich G., Bourin P., Fournie J. J., et al. (2009). A regulatory cross-talk between Vgamma9Vdelta2 T lymphocytes and mesenchymal stem cells. Eur. J. Immunol. 39, 752–762. 10.1002/eji.200838812 [DOI] [PubMed] [Google Scholar]
- Maumus M., Manferdini C., Toupet K., Peyrafitte J. A., Ferreira R., Facchini A., et al. (2013). Adipose mesenchymal stem cells protect chondrocytes from degeneration associated with osteoarthritis. Stem Cell Res. 11, 834–844. 10.1016/j.scr.2013.05.008 [DOI] [PubMed] [Google Scholar]
- McDonald P. P., Fadok V. A., Bratton D., Henson P. M. (1999). Transcriptional and translational regulation of inflammatory mediator production by endogenous TGF-beta in macrophages that have ingested apoptotic cells. J. Immunol. 163, 6164–6172. [PubMed] [Google Scholar]
- Meisel R., Zibert A., Laryea M., Göbel U., Däubener W., Dilloo D. (2004). Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood 103, 4619–4621. 10.1182/blood-2003-11-3909 [DOI] [PubMed] [Google Scholar]
- Mellor A. L., Munn D. H. (2004). IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat. Rev. Immunol. 4, 762–774. 10.1038/nri1457 [DOI] [PubMed] [Google Scholar]
- Michlewska S., Dransfield I., Megson I. L., Rossi A. G. (2009). Macrophage phagocytosis of apoptotic neutrophils is critically regulated by the opposing actions of pro-inflammatory and anti-inflammatory agents: key role for TNF-alpha. FASEB J. 23, 844–854. 10.1096/fj.08-121228 [DOI] [PubMed] [Google Scholar]
- Mindrescu C., Thorbecke G. J., Klein M. J., Vilcek J., Wisniewski H. G. (2000). Amelioration of collagen-induced arthritis in DBA/1J mice by recombinant TSG-6, a tumor necrosis factor/interleukin-1-inducible protein. Arthritis Rheum. 43, 2668–2677. [DOI] [PubMed] [Google Scholar]
- Mokarizadeh A., Delirezh N., Morshedi A., Mosayebi G., Farshid A. A., Mardani K. (2012). Microvesicles derived from mesenchymal stem cells: potent organelles for induction of tolerogenic signaling. Immunol. Lett. 147, 47–54. 10.1016/j.imlet.2012.06.001 [DOI] [PubMed] [Google Scholar]
- Murphy J. M., Fink D. J., Hunziker E. B., Barry F. P. (2003). Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 48, 3464–3474. 10.1002/art.11365 [DOI] [PubMed] [Google Scholar]
- Oh J. Y., Lee R. H., Yu J. M., Ko J. H., Lee H. J., Ko A. Y., et al. (2012). Intravenous mesenchymal stem cells prevented rejection of allogeneic corneal transplants by aborting the early inflammatory response. Mol. Ther. 20, 2143–2152. 10.1038/mt.2012.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J. Y., Roddy G. W., Choi H., Lee R. H., Ylostalo J. H., Rosa R. H., Jr., et al. (2010). Anti-inflammatory protein TSG-6 reduces inflammatory damage to the cornea following chemical and mechanical injury. Proc. Natl. Acad. Sci. U.S.A. 107, 16875–16880. 10.1073/pnas.1012451107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekkadan B., van Poll D., Suganuma K., Carter E. A., Berthiaume F., Tilles A. W., et al. (2007). Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS ONE 2:e941. 10.1371/journal.pone.0000941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J. P., Martel-Pelletier J., Abramson S. B. (2001). Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum. 44, 1237–1247. [DOI] [PubMed] [Google Scholar]
- Perruche S., Saas P., Chen W. (2009). Apoptotic cell-mediated suppression of streptococcal cell wall-induced arthritis is associated with alteration of macrophage function and local regulatory T-cell increase: a potential cell-based therapy? Arthritis Res. Ther. 11:R104. 10.1186/ar2750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perruche S., Zhang P., Liu Y., Saas P., Bluestone J. A., Chen W. (2008). CD3-specific antibody-induced immune tolerance involves transforming growth factor-beta from phagocytes digesting apoptotic T cells. Nat. Med. 14, 528–535. 10.1038/nm1749 [DOI] [PubMed] [Google Scholar]
- Pers Y. M., Rackwitz L., Ferreira R., Pullig O., Delfour C., Barry F., et al. (2016). Adipose mesenchymal stromal cell-based therapy for severe osteoarthritis of the knee: a phase i dose-escalation trial. Stem Cells Transl. Med. 5, 847–856. 10.5966/sctm.2015-0245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platas J., Guillen M. I., del Caz M. D., Gomar F., Mirabet V., Alcaraz M. J. (2013). Conditioned media from adipose-tissue-derived mesenchymal stem cells downregulate degradative mediators induced by interleukin-1beta in osteoarthritic chondrocytes. Mediators Inflamm. 2013:357014. 10.1155/2013/357014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop D. J. (2009). Repair of tissues by adult stem/progenitor cells (MSCs): controversies, myths, and changing paradigms. Mol. Ther. 17, 939–946. 10.1038/mt.2009.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X., Zhang J., Yuan H., Xu Z., Li Q., Niu X., et al. (2016). Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells repair critical-sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats. Int. J. Biol. Sci. 12, 836–849. 10.7150/ijbs.14809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafei M., Campeau P. M., Aguilar-Mahecha A., Buchanan M., Williams P., Birman E., et al. (2009). Mesenchymal stromal cells ameliorate experimental autoimmune encephalomyelitis by inhibiting CD4 Th17 T cells in a CC chemokine ligand 2-dependent manner. J. Immunol. 182, 5994–6002. 10.4049/jimmunol.0803962 [DOI] [PubMed] [Google Scholar]
- Rehman J., Traktuev D., Li J., Merfeld-Clauss S., Temm-Grove C. J., Bovenkerk J. E., et al. (2004). Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 109, 1292–1298. 10.1161/01.CIR.0000121425.42966.F1 [DOI] [PubMed] [Google Scholar]
- Ren G., Zhang L., Zhao X., Xu G., Zhang Y., Roberts A. I., et al. (2008). Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2, 141–150. 10.1016/j.stem.2007.11.014 [DOI] [PubMed] [Google Scholar]
- Roelofs A. J., Zupan J., Riemen A. H. K., Kania K., Ansboro S., White N., et al. (2017). Joint morphogenetic cells in the adult mammalian synovium. Nat. Commun. 8:15040. 10.1038/ncomms15040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roemer F. W., Guermazi A., Felson D. T., Niu J., Nevitt M. C., Crema M. D., et al. (2011). Presence of MRI-detected joint effusion and synovitis increases the risk of cartilage loss in knees without osteoarthritis at 30-month follow-up: the MOST study. Ann. Rheum. Dis. 70, 1804–1809. 10.1136/ard.2011.150243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan A., Murphy M., Barry F. (2017). Mesenchymal stem/stromal cell therapy in The Biology and Therapeutic Application of Mesenchymal Cells, ed Atkinson K. (Hoboken, NJ: Wiley-Blackwell; ), 426–440. [Google Scholar]
- Sasaki M., Abe R., Fujita Y., Ando S., Inokuma D., Shimizu H. (2008). Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J. Immunol. 180, 2581–2587. 10.4049/jimmunol.180.4.2581 [DOI] [PubMed] [Google Scholar]
- Scanzello C. R., McKeon B., Swaim B. H., DiCarlo E., Asomugha E. U., Kanda V., et al. (2011). Synovial inflammation in patients undergoing arthroscopic meniscectomy: molecular characterization and relationship to symptoms. Arthritis Rheum. 63, 391–400. 10.1002/art.30137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schelbergen R. F., van Dalen S., ter Huurne M., Roth J., Vogl T., Noel D., et al. (2014). Treatment efficacy of adipose-derived stem cells in experimental osteoarthritis is driven by high synovial activation and reflected by S100A8/A9 serum levels. Osteoarthr. Cartil. 22, 1158–1166. 10.1016/j.joca.2014.05.022 [DOI] [PubMed] [Google Scholar]
- Scheller J., Chalaris A., Schmidt-Arras D., Rose-John S. (2011). The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 1813, 878–888. 10.1016/j.bbamcr.2011.01.034 [DOI] [PubMed] [Google Scholar]
- Sica A., Mantovani A. (2012). Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 122, 787–795. 10.1172/JCI59643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sioud M., Mobergslien A., Boudabous A., Floisand Y. (2011). Mesenchymal stem cell-mediated T cell suppression occurs through secreted galectins. Int. J. Oncol. 38, 385–390. 10.3892/ijo.2010.869 [DOI] [PubMed] [Google Scholar]
- Sotiropoulou P. A., Perez S. A., Gritzapis A. D., Baxevanis C. N., Papamichail M. (2006). Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells 24, 74–85. 10.1634/stemcells.2004-0359 [DOI] [PubMed] [Google Scholar]
- Spaggiari G. M., Capobianco A., Abdelrazik H., Becchetti F., Mingari M. C., Moretta L. (2008). Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood 111, 1327–1333. 10.1182/blood-2007-02-074997 [DOI] [PubMed] [Google Scholar]
- Suga H., Eto H., Shigeura T., Inoue K., Aoi N., Kato H., et al. (2009). IFATS collection: fibroblast growth factor-2-induced hepatocyte growth factor secretion by adipose-derived stromal cells inhibits postinjury fibrogenesis through a c-Jun N-terminal kinase-dependent mechanism. Stem Cells 27, 238–249. 10.1634/stemcells.2008-0261 [DOI] [PubMed] [Google Scholar]
- Tao S. C., Yuan T., Zhang Y. L., Yin W. J., Guo S. C., Zhang C. Q. (2017). Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics 7, 180–195. 10.7150/thno.17133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Huurne M., Schelbergen R., Blattes R., Blom A., de Munter W., Grevers L. C., et al. (2012). Antiinflammatory and chondroprotective effects of intraarticular injection of adipose-derived stem cells in experimental osteoarthritis. Arthritis Rheum. 64, 3604–3613. 10.1002/art.34626 [DOI] [PubMed] [Google Scholar]
- Toupet K., Maumus M., Luz-Crawford P., Lombardo E., Lopez-Belmonte J., van Lent P., et al. (2015). Survival and biodistribution of xenogenic adipose mesenchymal stem cells is not affected by the degree of inflammation in arthritis. PLoS ONE 10:e0114962 10.1371/journal.pone.0114962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toupet K., Maumus M., Peyrafitte J. A., Bourin P., van Lent P. L., Ferreira R., et al. (2013). Long-term detection of human adipose-derived mesenchymal stem cells after intraarticular injection in SCID mice. Arthritis Rheum. 65, 1786–1794. 10.1002/art.37960 [DOI] [PubMed] [Google Scholar]
- Trounson A., McDonald C. (2015). Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell 17, 11–22. 10.1016/j.stem.2015.06.007 [DOI] [PubMed] [Google Scholar]
- van Buul G. M., Villafuertes E., Bos P. K., Waarsing J. H., Kops N., Narcisi R., et al. (2012). Mesenchymal stem cells secrete factors that inhibit inflammatory processes in short-term osteoarthritic synovium and cartilage explant culture. Osteoarthr. Cartil. 20, 1186–1196. 10.1016/j.joca.2012.06.003 [DOI] [PubMed] [Google Scholar]
- Visse R., Nagase H. (2003). Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ. Res. 92, 827–839. 10.1161/01.RES.0000070112.80711.3D [DOI] [PubMed] [Google Scholar]
- von Bahr L., Batsis I., Moll G., Hagg M., Szakos A., Sundberg B., et al. (2012). Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells 30, 1575–1578. 10.1002/stem.1118 [DOI] [PubMed] [Google Scholar]
- Weitz M., Strahm B., Meerpohl J. J., Schmidt M., Bassler D. (2015). Extracorporeal photopheresis versus standard treatment for acute graft-versus-host disease after haematopoietic stem cell transplantation in paediatric patients. Cochrane Database Syst. Rev. CD009759 10.1002/14651858.CD009759.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski H. G., Colon E., Liublinska V., Karia R. J., Stabler T. V., Attur M., et al. (2014). TSG-6 activity as a novel biomarker of progression in knee osteoarthritis. Osteoarthr. Cartil. 22, 235–241. 10.1016/j.joca.2013.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski H. G., Vilcek J. (2004). Cytokine-induced gene expression at the crossroads of innate immunity, inflammation and fertility: TSG-6 and PTX3/TSG-14. Cytokine Growth Factor Rev. 15, 129–146. 10.1016/j.cytogfr.2004.01.005 [DOI] [PubMed] [Google Scholar]
- Xia C. Q., Peng R., Qiu Y., Annamalai M., Gordon D., Clare-Salzler M. J. (2007). Transfusion of apoptotic beta-cells induces immune tolerance to beta-cell antigens and prevents type 1 diabetes in NOD mice. Diabetes 56, 2116–2123. 10.2337/db06-0825 [DOI] [PubMed] [Google Scholar]
- Zhang B., Yin Y., Lai R. C., Tan S. S., Choo A. B., Lim S. K. (2014). Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev. 23, 1233–1244. 10.1089/scd.2013.0479 [DOI] [PubMed] [Google Scholar]
- Zhang S., Chu W. C., Lai R. C., Lim S. K., Hui J. H., Toh W. S. (2016). Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthr. Cartil. 24, 2135–2140. 10.1016/j.joca.2016.06.022 [DOI] [PubMed] [Google Scholar]
- Zhu Y., Wang Y., Zhao B., Niu X., Hu B., Li Q., et al. (2017). Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res. Ther. 8:64. 10.1186/s13287-017-0510-9 [DOI] [PMC free article] [PubMed] [Google Scholar]


