Summary
This study aims to examine the effect of preoperative inspiratory muscle training (IMT) on pre- and postoperative functional exercise performance in patients undergoing esophagectomy. A subcohort of patients recruited to the PREPARE randomized control trial were studied. Following evaluation of respiratory muscle function (spirometry, maximum inspiratory pressure (MIP), and inspiratory muscle endurance), postoperative mobilization (accelerometry) and postoperative physical functioning (6-minute walk test (6MWT)), participants scheduled for esophagectomy were randomly assigned to either 2 weeks of preoperative IMT or a control group. Measures were repeated on the day before surgery and postoperatively. Sixty participants (mean (standard deviation) age 64.13 (7.8) years; n = 42 male; n = 43 transthoracic esophagectomy; n = 17 transhiatial esophagectomy) were included in the final analysis (n = 28 IMT; n = 32 control). There was a significant improvement in preoperative MIP (P = 0.03) and inspiratory muscle endurance (P = 0.04); however preoperative 6MWT distance did not change. Postoperatively, control participants were more active on postoperative day (POD)1, and from POD1–POD5 (P = 0.04). Predischarge, 6MWT distance was significantly lower in the IMT group (305.61 (116.3) m) compared to controls (380.2 (47.1) m, P = 0.03). Despite an increase in preoperative respiratory muscle function, preoperative IMT does not improve pre- or postoperative physical functioning or postoperative mobilization following esophagectomy.
Keywords: early ambulation, esophagectomy, exercise therapy, physiotherapy techniques, postoperative care
INTRODUCTION
Despite recent advances, esophagectomy remains one of the most complex oncologic resections with a relatively high postoperative risk of mortality and major morbidity.1 Care is increasingly centralized to high-volume centers with an enhanced recovery after surgery (ERAS) approach, aiming to attenuate postoperative risk and expedite recovery through preoperative preparation and collaborative postoperative care.2 Early postoperative mobilization is an integral component of ERAS and an important strategy in the management of postoperative pulmonary complications,3 including pneumonia and adult respiratory distress syndrome (ARDS), the main causes of in-hospital mortality.4 Despite the reported benefits of early mobilization, activity level postsurgery are suboptimal,3,5 and strategies to increase postoperative mobilization are required.
Cardiopulmonary fitness and physical functioning are key determinants of fitness for major surgery.2,6 Consequently, interventions that optimize preoperative fitness have considerable therapeutic potential.7,8 In comparison to control/usual care interventions, preoperative exercise reduces postoperative complications following abdominal surgery by over 40%.7 With increasing use of multimodal treatment regimens in esophageal cancer, the attritional impact of neoadjuvant chemo(radio)therapy on cardiopulmonary fitness9 and muscle mass10 may act as a barrier to effective exercise participation pre-esophagectomy. Inspiratory muscle training (IMT) has considerable potential as a prehabilitative intervention.11,12 IMT aims to strengthen the inspiratory muscles using a hand-held breathing device that adds resistance to inspiratory efforts.13 IMT is hypothesized to improve physical functioning through alterations in inspiratory muscle structure and function, and attenuating muscle fatigue and work of breathing.14 As an unimodal intervention, IMT improves physical functioning in numerous pathologies including chronic obstructive pulmonary disease (COPD),15 obesity,16 hematopoietic stem cell transplantation,17 and heart failure.18
Practiced preoperatively, IMT increases inspiratory muscle function,12,19,20 thus attenuating inspiratory muscle impairment postoperatively, potentially expediting recovery of postoperative lung function.21 In patients post coronary artery bypass graft, postoperative inspiratory muscle strength correlates with postoperative peak exercise capacity22 and is considered an important determinant of functional exercise performance.12,22 Therefore, it stands to reason that gains in inspiratory muscle strength may influence pre- and postoperative physical functioning and consequently postoperative mobilization; however, this has not been evaluated. This study aimed to examine the impact of preoperative IMT on physical functioning, namely pre- and postoperative walking capacity and postoperative mobilization, in patients undergoing esophagectomy.
MATERIALS AND METHODS
Study design
This study was embedded in the preoperative inspiratory muscle training to prevent postoperative pulmonary complications in patients undergoing esophageal resection (PREPARE), single-blind randomized controlled trial (RCT).13 PREPARE recruited 241 participants from 9 hospitals across Europe, including the Oesophageal and Gastric Centre at St James's Hospital (SJH), Dublin, Ireland.20
Patients with esophageal carcinoma were recruited from the preoperative gastrointestinal review clinic. Eligibility criteria included cognitive ability to perform IMT, esophagectomy scheduled ≥2 weeks following consent, ability to communicate in English, >18 years old, and not participating in a conflicting trial evaluating post esophagectomy outcome.13
Randomization, coordinated by the University Medical Center Utrecht (UMCU), was performed on a 1:1 ratio using a flexible web-based randomized system, stratified by recruitment center and surgical approach (transthoracic vs. transhiatial vs. minimally invasive). Patients were randomly assigned to either IMT or usual care, following baseline assessments, completed by a physiotherapist, blinded to the participants’ intervention assignment.
Ethical approval was granted from the independent ethics committee of the UMCU and all procedures, including this substudy, were approved from the SJH/Tallaght Hospital Joint Research Ethics Committee. Written informed consent was obtained prior to study commencement. The study was conducted in accordance with the 1964 Helsinki declaration and its later amendments.
Clinical treatment
Participants were treated according to standardized care pathways involving either multimodal therapy (pre- and/or postoperative chemotherapy (MAGIC regimen) or neoadjuvant chemoradiation (CROSS protocol)),1 or surgery only. Surgical resection, either transthoracic en-bloc esophagectomy (two stage or three stage) or transhiatial esophagectomy, was performed at least 6 weeks following neoadjuvant therapy. Postoperatively, patients were immediately extubated and admitted to a monitored bed, normally the high dependency unit (HDU). Patients were transferred to the ward on postoperative day (POD)3 or when medically suitable. The institutional ERAS protocol included early enteral feeding via jejunostomy, removal of chest drains on POD2, and contrast study for anastomotic integrity on POD4. Postoperative analgesia was managed using thoracic epidural analgesia (TEA). Physiotherapy interventions, commencing from POD1, included airway clearance techniques and early mobilization. Early mobilization goals were determined individually for each patient by the treating physiotherapist following assessment. Suitability to mobilize was determined on the basis of the standard assessment of medical status, cardiovascular reserve, and respiratory reserve. Patients did not receive preoperative physiotherapy, however were advised by the surgical team to be physically active in preparation for surgery.
Prepare intervention
The PREPARE intervention is described elsewhere.13 Participants assigned to the intervention arm completed IMT for ≥2 weeks preoperatively, performing 30 breaths, twice daily, using a tapered flow resistive inspiratory loading device (K3, POWERbreathe®). Training commenced at 60% of baseline maximal inspiratory pressure (MIP) and progressed by 5% when participant-reported rate of perceived exertion (RPE) <7 (RPE scale 1–10).
At program commencement, participants received one face-to-face IMT instructional session. All subsequent training sessions were performed at home with weekly telephone calls from the guiding physiotherapist. Additionally, participants received an instructional training diary and video. Participants reported weekly training statistics, recorded in an exercise diary, to the guiding physiotherapist and performed several inspiratory manoeuvres using the device while on the telephone to monitor adherence and technique, respectively. Additional training appointments were scheduled if required. The control group prepared for surgery according to the standard pathway.
Clinical data
Demographic and clinicopathologic data were gathered from medical charts and the institutional upper gastrointestinal cancer database. Postoperative data included in-hospital mortality, hospital and critical care length of stay (LOS), mechanical ventilation data, and postoperative complications.
Physical functioning
Walking capacity was measured preoperatively at baseline, on the day before surgery and postoperatively on POD9, using the 6-minute walk test (6MWT), a valid measure of functional capacity in patients with cancer23 and in postoperative recovery.24 Participants walked at their fastest pace for 6 minutes along a 30-m walkway with the aim of achieving the furthest distance possible with standardized verbal encouragement.25
Habitual physical activity was measured at baseline using the ActiGraph GT3X + triaxial accelerometer (Actigraph Pensacola, FL), worn at the right hip, for 7 days during waking hours. Data were analyzed using the Actilife software for time in each activity domain26 and moderate-to-vigorous physical activity (MVPA) bout analysis27 to determine compliance with activity recommendations (≥150 weekly minutes of MVPA accumulated in bouts ≥10 minutes).28
Postoperatively, the ActiGraph GT3X + measured daily activity and, using the pedometer functionality, quantified daily step count, from POD1 to POD5. The Actigraph was attached on the lateral aspect of the right hip using adhesive tape prior to first physiotherapy intervention on POD1 and was removed on the morning of POD6. Data were examined from 12:00 on POD1 until 08:00 on POD6. Physical activity data were downloaded to the Actilife software and categorized vector magnitude counts per minute (CPM) into sedentary (0–99 CPM), light (100–2019 CPM), moderate (2020–5998 CPM), and vigorous (≥5999 CPM) intensity activity domains.
Respiratory function
Measures of pulmonary and respiratory muscle function were completed at baseline, before surgery, and on POD3, POD6, and POD9.13 Inspiratory muscle endurance was measured at baseline and before surgery only. Forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) were assessed using a portable spirometer (Micro I, CareFusion). MIP and inspiratory muscle endurance were measured using a modified KH1 POWERbreathe handheld device.13
Statistical analysis
Data were analyzed using IBM SPSS Statistics version 22 (SPSS Inc., Chicago, IL). Data normality was checked using the Shapiro-Wilk test. Normally distributed data were presented as mean (standard deviation (SD)) and non-normally distributed data as median (interquartile range (IQR)). Categorical variables were presented as frequency (percentage). Differences between groups were compared using independent sample t-tests, independent samples Mann-Whitney U test, and chi-squared test as appropriate.
A one-way between-groups analysis of covariance (ANCOVA) was conducted to determine the difference between the IMT and the control group, at each time point, on measures of inspiratory muscle function and functional exercise performance, using baseline values as covariates. Preliminary checks were conducted to ensure the assumptions of normality, linearity, homogeneity of variances, homogeneity of regression slopes, and reliable measurement of the covariate were maintained. Changes in pre- to postoperative measures were examined using paired sample t-tests or Wilcoxen Signed Rank Test. Statistical significance was set at P < 0.05.
RESULTS
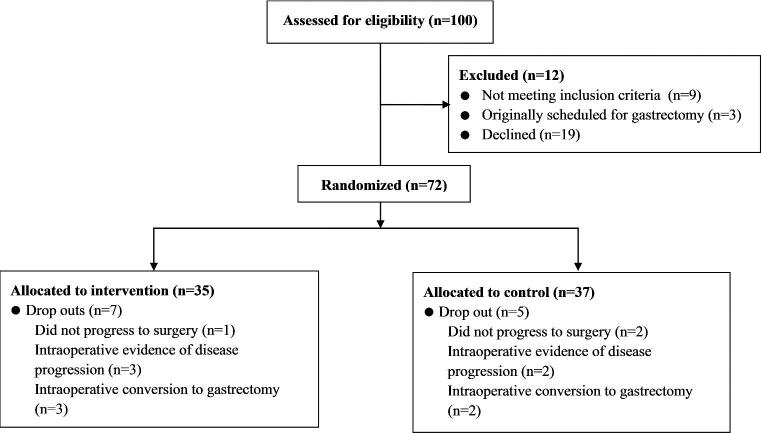
Between March 2014 and May 2016,100 patients were screened for participation in PREPARE at SJH (Fig. 1). At screening, nine patients were ineligible and of the 91 invited to participate, 19 declined. The reasons for refusal were not interested (n = 9), concerned participation would delay surgery (n = 2), heightened presurgery anxiety (n = 3), unwilling to be randomized to a control group (n = 1), and unable to attend additional appointments (n = 4).
Fig. 1.
Flow of participant recruitment.
Seventy-two participants were recruited at SJH, 30% of the total PREPARE cohort (n = 241).20 Following randomization, 12 participants were excluded due to change in eligibility (Fig. 1). Sixty participants were included in the final analysis (IMT n = 28; control n = 32). Demographic and treatment data (Table 1), and pulmonary function, respiratory muscle function, and 6MWT distance (Table 2) were matched at baseline. Preoperatively, participants spent the majority of waking hours sedentary (66.03(9.8)%) and 12 participants (IMT n = 4, control n = 8) exercised to recommended levels.28 The control group completed significantly more moderate-intensity activity (median (IQR) 3.55(4.07)% of waking hours) compared with the IMT group (1.46(3.03)%) (P = 0.04) (Table 2).
Table 1.
Clinicopathological characteristics
| Total (n = 60) | IMT (n = 28) | Control (n = 32) | p-value | |
|---|---|---|---|---|
| Demographics | ||||
| Age (mean (SD) years) | 64.13 (7.8) | 63.07 (8.8) | 65.06 (67.78) | 0.33 |
| Gender (n (%)) | ||||
| Male | 42 (70) | 20 | 22 | 0.82 |
| Female | 18 | 8 | 10 | |
| Height (mean (SD) cm) | 166.22 (16.45) | 167.93 (14.27) | 164.72 (18.22) | 0.46 |
| Weight (mean (SD) kg) | 80.68 (18.62) | 81.91 (16.27) | 79.60 (20.64) | 0.64 |
| Clinical details | ||||
| Tumor histology (n (%)) | ||||
| Adenocarcinoma | 42 (70) | 21 (75) | 21 (66) | 0.43 |
| Squamous cell carcinoma | 18 (30) | 7 (25) | 11 (34) | |
| Neoadjuvant therapy (n (%)) | ||||
| CROSS | 30 (50) | 16 (57) | 14 (44) | 0.56 |
| MAGIC | 15 (25) | 6 (21.5) | 9 (28) | |
| None | 15 (25) | 6 (21.5) | 9 (28) | |
| ASA Score (n (%)) | ||||
| 1 | 7 (12) | 4 (14) | 3 (9) | N/A |
| 2 | 38 (63) | 18 (64) | 20 (63) | |
| 3 | 13 (22) | 4 (14) | 9 (28) | |
| 4 | 2 (3) | 2 (7) | 0 | |
| Operative details | ||||
| Surgical approach (n (%)) | ||||
| Transthoracic | 43 (72) | 22 (79) | 21 (66) | 0.27 |
| Transhiatial | 17 (28) | 6 (21) | 11 (33) | |
| Surgery duration (median (IQR) hours) | 5.0 (1.19) | 5.0 (1.5) | 5.0 (1.4) | 0.32 |
| Blood loss (median (IQR) mLs) | 742.93 (475.03) | 757.59 (493.16) | 729.06 (465.85) | 0.93 |
| Postoperative recovery | ||||
| Hospital LOS (median (IQR) days) | 17.5 (13.0) | 17.0 (8.0) | 18.0 (15) | 0.87 |
| Critical care LOS (median (IQR) days) | 4.0 (3.0) | 4.0 (3.75) | 4.0 (2.75) | 1.00 |
| Intubated (n (%)) | 7 (12) | 3 (11) | 4 (13) | N/A |
| Postoperative complications (n (%)) | ||||
| Pulmonary complications | 20 (33) | 9 (32) | 11 (34) | 0.86 |
| Anastomotic leak | 2 (3) | 1 (3.6) | 1 (3.1) | 1.00 |
| Vocal cord paralysis | 2 (3) | 0 | 2 (6.3) | 0.49 |
| Chyle leak | 3 (5) | 3 (11) | 0 | 0.09 |
| Wound infection | 6 (10) | 3 (11) | 3 (9) | 1.00 |
| Cardiac complications | 9 (15) | 3 (11) | 6 (19) | 0.48 |
| CCI (median (IQR) score) | 8.7 (20.9) | 0.0 (20.9) | 8.7 (26.2) | 0.31 |
| In-hospital mortality (n (%)) | 0 | 0 | 0 | N/A |
P-value for difference between groups at baseline. P-value = N/A data violated assumptions for Chi-Squared analysis. ASA, American Society of Anaesthesiologists; CCI, comprehensive complications index; IQR, interquartile range; LOS, length of stay; SD, standard deviation;
Table 2.
Baseline functional status
| Total (n = 60) | IMT (n = 28) | Control (n = 32) | p-value | |
|---|---|---|---|---|
| Pulmonary function | ||||
| FVC (median (IQR) liters)) | 3.41 (1.26) | 3.44 (1.34) | 3.41 (1.19) | 0.61 |
| FEV1 (mean (SD) liters)) | 2.75 (0.69) | 2.75 (0.79) | 2.75 (0.61) | 0.98 |
| FEV1/FVC (median (IQR) %) | 78.5 (9.75) | 77.00 (10.75) | 79.50 (8.75) | 0.13 |
| Respiratory muscle function | ||||
| MIP (mean (SD) cmH2O) | 51.94 (22.92) | 54.08 (24.43) | 50.10 (21.80) | 0.37 |
| Inspiratory muscle endurance | ||||
| Time sustained (median (IQR) seconds) | 210.0 (208.0) | 245.00 (202.5) | 203.00 (236.0) | 0.97 |
| Functional performance† | ||||
| Six minute walk test (mean (SD) meters) | 486.84 (65.57) | 479.25 (72.89) | 493.85 (58.59) | 0.44 |
| Physical activity‡ | ||||
| Sedentary (mean (SD) %/day) | 66.03 (9.81) | 65.43 (10.26) | 66.54 (9.58) | 0.68 |
| Light intensity (mean (SD) %/day) | 30.85 (9.54) | 31.96 (9.77) | 29.91 (9.42) | 0.43 |
| Moderate intensity (median (IQR) %/day) | 2.27 (3.52) | 1.46 (3.03) | 3.55 (4.07) | 0.04 |
| Vigorous intensity (median (IQR) %/day) | 0 (0) | 0 (0.2) | 0 (0) | 0.42 |
| Physical activity guidelines (n (%)) | 12 | 4 | 8 | 0.34 |
P-value for difference between groups at baseline.
† n = 50; ‡n = 55.
FEV1, forced expiratory volume in one second; FVC, forced vital capacity; IQR, interquartile range; MIP, maximal inspiratory pressure; SD, standard deviation .
Intervention adherence and compliance
The median time between baseline assessment and surgery was 22(12.5) days (IMT 21.5(10.5) days: control 22(14.5) days). The IMT group completed 785/1232 (64%) of prescribed training sessions. Fourteen participants completed at least 80% of training sessions and five completed no training. Training intensity increased by a median RPE score of 8 (range: 0–8) during the intervention. There were no adverse events.
Preoperative respiratory and functional exercise performance
Pulmonary function (FVC, FEV1, or FEV1/FVC) did not change (P > 0.05 for all). Mean MIP increased by 13.9 (95% confidence interval [CI] 6.5 to 21.4) cmH2O in the IMT arm (n = 25) and by 7.8 (95%CI 2.4–13.1) cmH2O in the control arm (n = 29). Median inspiratory muscle endurance (time sustained) increased by 64.0 (186.0) seconds in the IMT group and decreased by −7.5 (132.0) seconds in the control arm (P = 0.04). Postintervention, MIP was significantly higher in the IMT group (adjusted mean 68.37 (95%CI 62.13–74.62) cmH2O) compared to controls (59.20 (95%CI 53.76–64.64) cmH2O, P = 0.03, partial eta squared (ηp2) = 0.09).
Preoperative 6MWT distance increased by 16.4 (95%CI −10.9–43.6) m in the IMT group (n = 17) and by 15.6 (95%CI −7.9–39.1) m in the control group (n = 23). There was no difference in postintervention 6MWT distances (adjusted mean IMT 516.93 (95%CI 490.32 to 543.54) m versus control 503.30 (95%CI 482.56–524.05) m, P = 0.42, ηp2 = 0.02).
Postoperative respiratory, physical activity, and functional exercise performance
Oxygen saturation was comparable on each postoperative day; however the IMT group required significantly more supplemental oxygen therapy on POD1 (Table 3). Consistent with the impact of major surgery, MIP reduced significant from presurgery to POD3 in both the IMT (−37.6 (95%CI −27.2–−47.9) cmH2O, P < 0.001)) and control group (−31.5 (95%CI −19.9–−43.1) cmH2O, P < 0.001). There was no difference in MIP between groups on POD3 (P = 0.63, ηp2 = 0.01); POD6 (P = 0.94, ηp2 = 0.00) or POD9 (P = 0.26, ηp2 = 0.03).
Table 3.
Postoperative respiratory status and activity
| Total (n = 60) | IMT (n = 28) | Control (n = 32) | p-value | |
|---|---|---|---|---|
| Respiratory status | ||||
| Oxygen saturation | ||||
| POD1 (%) | 96.12 (2.0) | 95.93 (2.18) | 96.28 (1.85) | 0.50 |
| POD2 (%) | 96.43 (1.93) | 96.39 (1.97) | 96.47 (1.93) | 0.88 |
| POD3 (%) | 96.12 (2.76) | 95.5 (3.67) | 96.95 (1.45) | 0.13 |
| POD4 (%) | 96.48 (1.99 | 96.64 (1.99) | 96.34 (2.01) | 0.57 |
| Oxygen therapy | ||||
| POD1 (L/min) | 32.0 (12.0) | 40.0 (15.8) | 30.0 (7.0) | 0.005 |
| POD2 (L/min) | 36.0 (12.0) | 36.0 (9.5) | 34.0 (12.0) | 0.08 |
| POD3 (L/min) | 32.0 (13.3) | 33.5 (9.5) | 28.0 (36.0) | 0.008 |
| POD 4 (L/min) | 28.0 (36.0) | 28.0 (36.0) | 28.0 (28.0) | 0.17 |
| Postoperative activity† | ||||
| Light intensity activity | ||||
| POD1 (minutes/day) | 10.0 (14.75) | 4.5 (13.75) | 14.5 (13.0) | 0.03 |
| POD2 (minutes/day) | 13.0 (22.5) | 9.5 (23.75) | 17.0 (23.0) | 0.16 |
| POD3 (minutes/day) | 17.0 (28.5) | 15.5 (21.5) | 17.0 (40.0) | 0.46 |
| POD4 (minutes/day) | 37.0 (44.0) | 34.0 (47.8) | 39.0 (48.0) | 0.42 |
| POD5 (minutes/day) | 41.0 (59.0) | 39.0 (46.0) | 67.0 (46.0) | 0.22 |
| Total (minutes/day) | 135.0 (166.5) | 115.0 (116.75) | 166.0 (154.0) | 0.24 |
| Total active minutes | ||||
| POD1 (minutes/day) | 12.0 (16.50) | 5.0 (16.0) | 15.5 (14.0) | 0.03 |
| POD2 (minutes/day) | 13.0 (22.5) | 9.5 (23.75) | 17.0 (23.0) | 0.16 |
| POD3 (minutes/day) | 17.0 (28.0) | 15.5 (21.25) | 17.0 (41.0) | 0.73 |
| POD4 (minutes/day) | 37.0 (43.0) | 34.0 (47.75) | 39.0 (47.0) | 0.37 |
| POD5 (minutes/day) | 41.0 (56.0) | 39.0 (46.0) | 67.0 (56.0) | 0.17 |
| Total (minutes/day) | 140.0 (159.0) | 118.0 (114.5) | 166.0 (143.0) | 0.15 |
| Step count | ||||
| POD1 (steps/day) | 89.0 (153.0) | 43.5 (143.5) | 115.0 (299.3) | 0.04 |
| POD2 (steps/day) | 113.0 (124.5) | 96.5 (104.0) | 113.0 (124.0) | 0.54 |
| POD3 (steps/day) | 132.0 (226.0) | 128.0 (233.25) | 141.0 (214.0) | 0.83 |
| POD4 (steps/day) | 218.0 (294.5) | 200.5 (263.8) | 279.0 (299.0) | 0.21 |
| POD5 (steps/day) | 332.0 (462.0) | 240.0 (373.0) | 412.0 (509.5) | 0.11 |
| Total (steps/day) | 879.0 (873.5) | 723.0 (575.8) | 1170.0 (974.0) | 0.04 |
Data presented as median (interquartile range). P-values for independent samples Mann–Whitney U test.
† n = 40.
POD, postoperative day; L/min, liters per minute.
From presurgery to POD9, 6MWT distance reduced by 194.6 (95%CI 107.8–281.4) m, P = 0.001) in the IMT group (n = 18) and by 134.7 (95%CI 102.1–167.4) m, P < 0.001) in the control group (n = 19). On POD9, mean 6MWT distance was significantly lower in the IMT group (305.61 (116.3) m) compared to controls (380.2 (47.1) m) (mean difference 74.89 (95%CI (9.9–139.3) m, P = 0.03).
Control participants were significantly more active on POD1 in comparison to the IMT group (Table 3). Following POD1, there were no differences in daily activity levels or step counts between the groups however total step count accumulated from POD1–POD5 was significantly higher in the control group versus the intervention group (median (IQR) 1170.0 (974) steps vs.723.0 (575.8) steps, P = 0.04).
DISCUSSION
Preoperative IMT led to improvements in pre-esophagectomy respiratory muscle function, however preoperative physical functioning did not change. Surprisingly, postoperatively, the control group had higher mobilization scores on both on POD1 and cumulatively from POD1–POD5, and greater 6MWT distance predischarge.
IMT is advocated for exercise prehabilitation due to the considerable feasibility advantages over traditional exercise training.29 Importantly, the benefits of IMT occur relatively quickly (2–3weeks),11,12 consistent with the timelines for oncologic resection. In agreement with the main PREPARE trial,20 this sub-study observed a significant increase in preoperative MIP with ≥2 weeks of IMT. PREPARE recruited 241 participants, with the aim of reducing postoperative pneumonia by 50%.13 Accordingly, PREPARE was the first adequately powered trial of preoperative IMT in esophagectomy, and reported no effect on postoperative outcome.20 Similarly, in this subanalysis, postoperative recovery (hospital LOS, critical care LOS, and postoperative pulmonary complications) was comparable for both groups.
Despite gains in MIP, preoperative IMT did not influence physical functioning in this analysis. Interventions eliciting improvements in physical functioning tend to prescribe IMT for periods of 4–12 weeks.16–18 In a study of similar training intensity to PREPARE, 4 weeks IMT in 15 overweight and obese adults led to significant gains in 6MWT distance compared to controls (P = 0.05).16 In contrast, lower intensity training protocols (30–40% MIP) require 6–12 weeks training to demonstrate effects.16–18 The median training period in PREPARE (21.5(10.5) days) may have been insufficient for therapeutic response, despite a suitable training intensity (60% MIP). Longer interventions are not appropriate in this setting due to the limited preoperative timeframe and therefore novel strategies of maximizing gains in physical functioning over short training periods need to be explored. With increasing use of multimodal treatment regimens in esophageal cancer, the opportunity may exist to commence training programs during neoadjuvant therapy and continue into the preoperative period in order to maximize therapeutic gains.
Surprisingly, in this subcohort, postoperative mobilization was higher in the control group, with patients completing more activity on POD1 and cumulatively from POD1–POD5, and a greater distance on predischarge 6MWT. Furthermore, the intervention arm had higher supplemental oxygen requirements on POD1. The reason for this is unknown, however, adequate respiratory reserve is a key determinant of suitability to mobilize and therefore may have influence results observed.30 Of note, preoperatively, control participants completed more moderate-intensity activity and twice as many participants exercised to recommended levels.28 Preoperative moderate-intensity activity is associated with a lower risk of postoperative complications following oesophagectomy31,32 and therefore may have potential as a prehabilitative intervention. In one RCT in colorectal surgery, more participants experienced gains in 6MWT distance following unsupervised walking and breathing exercises (n = 54) compared to supervised, higher intensity aerobic and resistance exercise prehabilitation (n = 58).33 In the present study, preoperative activity was advised to all patients as standard care and not prescribed as a control intervention, and therefore we are unable to determine if the greater volume of preoperative activity completed by the control arm had a causal impact on results, however results provide the basis for future investigation to elucidate the value of moderate-to-vigorous intensity exercise pre-esophagectomy.
In PREPARE, the IMT intervention was unsupervised.13 At SJH, participants completed 64% of prescribed training sessions, with five participants completing no training (n = 2 received no IMT device due to logistical restraints; n = 3 feeling too overwhelmed). Intervention compliance was monitored using training diaries and weekly telephone calls. This training model overcomes barriers commonly cited with supervised training,29 however does compromise ability to monitor adherence, address training issues, and progress intensity.34 Interestingly, the IMT group both engaged in less preoperative activity and had poor IMT compliance, while in contrast, the control group, who did not receive a breathing device, engaged in more habitual preoperative exercise. Possibly, participants in the IMT group were unable to both exercise optimally and participate fully with preoperative IMT, and the reasons for this warrant exploration. While multimodal programs are often advocated for surgical prehabilitation, the ability of patients with cancer, overwhelmed and unwell preoperatively, to participate effectively in multiple interventions requires consideration.
This work has some limitations. First, results are a subset of a larger trial and therefore secondary outcomes of PREPARE. The limitations of secondary analysis, particularly in relation to statistical power, apply. Randomization was stratified by recruiting site and therefore examining outcomes in the context of randomization assigned is possible. Second, full data are not available at all time points. This is a limitation of pragmatic clinical research in a complex cohort. Accordingly, statistical analysis was completed using ANCOVA, comparing available data between groups at each time point, correcting for baseline values. Third, the PREPARE intervention was unsupervised and while fidelity was monitored, the ability to tightly control intervention adherence was compromised.
In conclusion, despite an improvement in preoperative MIP, preoperative IMT does not improve either pre- or postoperative physical functioning or postoperative mobilization following esophagectomy. Given the limited timeframe for exercise prehabilitation in oncologic resection, further work is needed to identify effective exercise prehabilitation strategies.
Acknowledgements
We acknowledge the support and assistance of the Wellcome Trust-HRB Clinical Research Facility, which provides a dedicated environment for the conduct of clinical research activities in St. James’ Hospital, and the Upper Gastrointestinal Surgical team at St James's Hospital.
References
- 1. Reynolds J V, Preston S R, O’Neill B et al. ICORG 10-14: NEOadjuvant trial in adenocarcinoma of the oEsophagus and oesophagoGastric junction international study (Neo-AEGIS). BMC Cancer 2017; 17: 401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guinan E M, Dowds J, Donohoe C L, Reynolds J V, Hussey J. The physiotherapist and the oesophageal cancer patient: from prehabilitation to rehabilitation. Dis Esophagus 2016; 30: 1–12. [DOI] [PubMed] [Google Scholar]
- 3. Hussey J, Yang T-Y, Dowds J, O’Connor L, Reynolds J V, Guinan E M. Quantifying Postoperative Mobilisation Following Oesophagectomy. Physiotherapy (United Kingdom) 10.1016/j.physio.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 4. Atkins B Z, Shah A S, Hutcheson K A et al. Reducing hospital morbidity and mortality following esophagectomy. Ann Thorac Surg 2004; 78: 1170–6; discussion -6. [DOI] [PubMed] [Google Scholar]
- 5. Agostini P, Cieslik H, Rathinam S et al. Postoperative pulmonary complications following thoracic surgery: are there any modifiable risk factors? Thorax 2010; 65: 815–8. [DOI] [PubMed] [Google Scholar]
- 6. Moran J, Wilson F, Guinan E, McCormick P, Hussey J, Moriarty J. Role of cardiopulmonary exercise testing as a risk-assessment method in patients undergoing intra-abdominal surgery: a systematic review. Br J Anaesth 2016; 116: 177–91. [DOI] [PubMed] [Google Scholar]
- 7. Moran J, Guinan E, McCormick P et al. The ability of prehabilitation to influence postoperative outcome after intra-abdominal operation: a systematic review and meta-analysis. Surgery 2016; 160: 1189–201. [DOI] [PubMed] [Google Scholar]
- 8. Valkenet K, van de Port I G, Dronkers J J, de Vries W R, Lindeman E, Backx F J. The effects of preoperative exercise therapy on postoperative outcome: a systematic review. Clin Rehabil 2011; 25: 99–111. [DOI] [PubMed] [Google Scholar]
- 9. Jack S, West M A, Raw D et al. The effect of neoadjuvant chemotherapy on physical fitness and survival in patients undergoing oesophagogastric cancer surgery. Eur J Surg Oncol 2014; 40: 1313–20. [DOI] [PubMed] [Google Scholar]
- 10. Elliott J A, Doyle S L, Murphy C F et al. Sarcopenia. Ann Surg 2017; 266: 822–30. [DOI] [PubMed] [Google Scholar]
- 11. van Adrichem E J, Meulenbroek R L, Plukker J T, Groen H, van Weert E. Comparison of two preoperative inspiratory muscle training programs to prevent pulmonary complications in patients undergoing esophagectomy: a randomized controlled pilot study. Ann Surg Oncol 2014; 21: 2353–60. [DOI] [PubMed] [Google Scholar]
- 12. Hulzebos E H, Helders P J, Favie N J, De Bie R A, Brutel de la Riviere A, Van Meeteren N L. Preoperative intensive inspiratory muscle training to prevent postoperative pulmonary complications in High-Risk patients undergoing CABG surgery. JAMA 2006; 296: 1851–7. [DOI] [PubMed] [Google Scholar]
- 13. Valkenet K, Trappenburg J C, Gosselink R et al. Preoperative inspiratory muscle training to prevent postoperative pulmonary complications in patients undergoing esophageal resection (PREPARE study): study protocol for a randomized controlled trial. Trials 2014; 15: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dempsey J A, Miller J D, Romer L, Amann M, Smith C A. Exercise-induced respiratory muscle work: effects on blood flow, fatigue and performance. Adv Exp Med Biol 2008; 605: 209–12. [DOI] [PubMed] [Google Scholar]
- 15. Gosselink R, De Vos J, van den Heuvel S P, Segers J, Decramer M, Kwakkel G. Impact of inspiratory muscle training in patients with COPD: what is the evidence? Eur Respir J 2011; 37: 416–25. [DOI] [PubMed] [Google Scholar]
- 16. Edwards A M, Maguire G P, Graham D, Boland V, Richardson G. Four weeks of inspiratory muscle training improves self-paced walking performance in overweight and obese adults: a randomised controlled trial. J Obes 2012; 2012: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bargi G, Guclu M B, Aribas Z, Aki S Z, Sucak G T. Inspiratory muscle training in allogeneic hematopoietic stem cell transplantation recipients: a randomized controlled trial. Support Care Cancer 2016; 24: 647–59. [DOI] [PubMed] [Google Scholar]
- 18. Bosnak-Guclu M, Arikan H, Savci S et al. Effects of inspiratory muscle training in patients with heart failure. Respir Med 2011; 105: 1671–81. [DOI] [PubMed] [Google Scholar]
- 19. Dettling D S, van der Schaaf M, Blom R L, Nollet F, Busch O R, van Berge Henegouwen M I. Feasibility and effectiveness of pre-operative inspiratory muscle training in patients undergoing oesophagectomy: a pilot study. Physiother Res Int 2013; 18: 16–26. [DOI] [PubMed] [Google Scholar]
- 20. Valkenet K, Trappenburg J C A, Ruurda J P et al. Multicentre randomized clinical trial of inspiratory muscle training versus usual care before surgery for oesophageal cancer. Br J Surg 2018; 105: 502–11. [DOI] [PubMed] [Google Scholar]
- 21. Brocki B C, Andreasen J J, Langer D, Souza D S, Westerdahl E. Postoperative inspiratory muscle training in addition to breathing exercises and early mobilization improves oxygenation in high-risk patients after lung cancer surgery: a randomized controlled trial. Eur J Cardiothorac Surg 2016; 49: 1483–91. [DOI] [PubMed] [Google Scholar]
- 22. Stein R, Maia C P, Silveira A D, Chiappa G R, Myers J, Ribeiro J P. Inspiratory muscle strength as a determinant of functional capacity early after coronary artery bypass graft surgery. Arch Phys Med Rehabil 2009; 90: 1685–91. [DOI] [PubMed] [Google Scholar]
- 23. Schmidt K, Vogt L, Thiel C, Jager E, Banzer W. Validity of the six-minute walk test in cancer patients. Int J Sports Med 2013; 34: 631–6. [DOI] [PubMed] [Google Scholar]
- 24. Pecorelli N, Fiore J F Jr, Gillis C et al. The six-minute walk test as a measure of postoperative recovery after colorectal resection: further examination of its measurement properties. Surg Endosc 2016; 30: 2199–206. [DOI] [PubMed] [Google Scholar]
- 25. ATS statement: ATS statement. Am J Respir Crit Care Med 2002; 166: 111–7. [DOI] [PubMed] [Google Scholar]
- 26. Troiano R P, Berrigan D, Dodd K W, Masse L C, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc 2008; 40: 181–8. [DOI] [PubMed] [Google Scholar]
- 27. Freedson P S, Melanson E, Sirard J. Calibration of the computer science and applications, Inc. accelerometer. Med Sci Sports Exerc 1998; 30: 777–81. [DOI] [PubMed] [Google Scholar]
- 28. Schmitz K H, Courneya K S, Matthews C et al. American college of sports medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc 2010; 42: 1409–26. [DOI] [PubMed] [Google Scholar]
- 29. Benzo R, Wigle D, Novotny P et al. Preoperative pulmonary rehabilitation before lung cancer resection: results from two randomized studies. Lung Cancer 2011; 74: 441–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stiller K, Phillips A. Safety aspects of mobilising acutely ill inpatients. Phyiother Theory Pract 2003; 19: 239–57. [Google Scholar]
- 31. Feeney C, Reynolds J V, Hussey J. Preoperative physical activity levels and postoperative pulmonary complications post-esophagectomy. Dis Esophagus 2011; 24: 489–94. [DOI] [PubMed] [Google Scholar]
- 32. Tatematsu N, Park M, Tanaka E, Sakai Y, Tsuboyama T. Association between physical activity and postoperative complications after esophagectomy for cancer: a prospective observational study. Asian Pac J Cancer Prev 2013; 14: 47–51. [DOI] [PubMed] [Google Scholar]
- 33. Carli F, Charlebois P, Stein B et al. Randomized clinical trial of prehabilitation in colorectal surgery. Br J Surg 2010; 97: 1187–97. [DOI] [PubMed] [Google Scholar]
- 34. Bollen J C, Dean S G, Siegert R J, Howe T E, Goodwin V A. A systematic review of measures of self-reported adherence to unsupervised home-based rehabilitation exercise programmes, and their psychometric properties. BMJ Open 2014; 4: e005044. [DOI] [PMC free article] [PubMed] [Google Scholar]