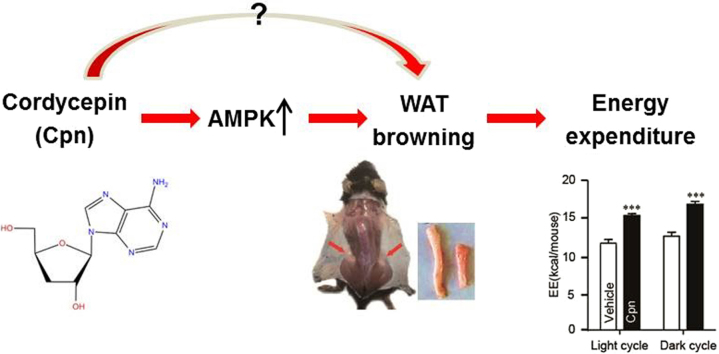
Abstract
Obesity is a worldwide epidemic. Promoting browning of white adipose tissue (WAT) contributes to increased energy expenditure and hence counteracts obesity. Here we show that cordycepin (Cpn), a natural derivative of adenosine, increases energy expenditure, inhibits weight gain, improves metabolic profile and glucose tolerance, decreases WAT mass and adipocyte size, and enhances cold tolerance in normal and high-fat diet-fed mice. Cpn markedly increases the surface temperature around the inguinal WAT and turns the inguinal fat browner. Further investigations show that Cpn induces the development of brown-like adipocytes in inguinal and, to a less degree, epididymal WAT depots. Cpn also increases the expression of uncoupling protein 1 (UCP1) and other thermogenic genes in WAT and 3T3-L1 differentiated adipocytes, in which AMP-activated protein kinase (AMPK) plays an important role. Our results provide novel insights into the function of Cpn in regulating energy balance, and suggest a potential utility of Cpn in the treatment of obesity.
KEY WORDS: Cordycepin, Browning of white adipose tissue (WAT), Thermogenesis, AMP-activated protein kinase (AMPK), Obesity
Graphical Abstract
Cordycepin increases energy expenditure via promoting white adipose tissue browning in mice, in which AMPK activation may play an important role.
1. Introduction
Obesity is becoming a worldwide epidemic, which leads to high incidence of many critical diseases, such as type 2 diabetes and cardiovascular diseases1, 2. The main cause for the development of obesity is the disequilibrium between energy intake and expenditure. Therefore, effective methods for the treatment of obesity usually involve reducing calorie intake, enhancing energy expenditure, or both the same time3. While restricting food intake is an essential way to defense obesity, enhancing the energy expenditure in major metabolic organs, such as fat tissue, represents a critical alternation4.
Mammals have two main types of adipose tissues: the white adipose tissue (WAT) and the brown adipose tissue (BAT). The WAT functions to storage energy by synthesizing and storing triglycerides5, while BAT promotes energy expenditure by oxidizing fatty acids to generate heat6. Recently, a third type of adipocytes, namely beige or brite adipocytes, has been identified, which are uncoupling protein 1 (UCP1)-expressing multilocular adipocytes with thermogenic capacity. Beige adipocytes can be developed in WAT in response to various stimuli including cold exposure and chemical or hormonal stimulations7. Multiple studies have proved that increasing beige adipocyte content in WAT browning will enhance energy expenditure and reduce obesity8, 9
Cordycepin (Cpn), also known as 3′-deoxyadenosine, is a natural derivative of adenosine, possessing multiple pharmacological activities including inhibition of tumor10, modulation of the immunity and suppression of inflammation11. Cellular and animal studies have shown that Cpn is adequate to improve metabolic disorders, such as hyperlipidemia, hyperglycemia and atherosclerosis11, 12, 13. Further studies showed that the lipid-decreasing effects of Cpn may be caused by the activation of AMP-activated protein kinase (AMPK) and inhibition of WAT differentiation14, 15. In this work, we evaluated the effect of Cpn on body weight and energy expenditure in mice and investigated the potential mechanism. We demonstrated that Cpn could stimulate WAT browning, and this effect is AMPK dependent.
2. Materials and methods
2.1. Materials
Cordycepin, with a purity of 99% as determined by high-performance liquid chromatography (HPLC), was prepared by our group as previously reported14. The D12450B normal diet chow (10% fat) and D12492 high-fat diet (60% fat) were purchased from Research Diets Inc. (New Brunswick, USA). Antibodies against AMPKa1 subunit, phosphorylated AMPK (Thr272), UCP1, peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α), cell death-inducing DNA fragmentation factor alpha-like effector A (CIDEA), fibronectin type III domain-containing protein 5 (FNDC5) and β-actin were purchased from Cell Signaling Technology (Beverly, MA, USA). 5-Aminoimidazole-4-carb-oxyamide ribonucleoside (AICAR), compound C, isobutylmethylxanthine (IBMX), indomethacin, dexamethasone, insulin and rosiglitazone were purchased from Sigma--Aldrich Co. Ltd. (St. Louis, MO, USA).
2.2. Animal experiments
All animal experiments were approved by the Medical Ethics Committee of Peking Union Medical College (Beijing, China) and were in accordance with the National Institutes of Health regulations for the care and use of animals in research (Beijing, China).
C57BL/6 male mice, 19–22 g, were purchased from Vital River Laboratories Co., Ltd. (Beijing, China). The animals were divided randomly into different groups with seven mice in each group and fed with D12450B normal diet chow or D12492 high-fat diet (HFD) for 4 weeks. The control group (chow only and HFD only) was given equal volume of distilled water while the test groups (chow+Cpn and HFD+Cpn) were given Cpn (40 mg/kg) by oral gavage. Three or two animals were housed in one cage. Body weight and 24-h food intake were measured every week individually. After treatment for 3 weeks, the 24-h energy expenditure was monitored using CLAMS (Columbus Instruments, Columbus, USA) and cold tolerance was performed at the end of the energy expenditure measurement. Thereafter, oral glucose tolerance test (GTT) and insulin tolerance test (ITT) were performed every other day. At the end of the experiment, after the mice were fasted overnight, blood samples were collected for estimation of plasma levels of lipids by kits (Jian Cheng Biotechnology Company, Nanjing, China). Animals were then euthanized, and the liver, epididymal and subcutaneous fat were collected and weighed. A bulk of the tissue was frozen in liquid nitrogen immediately for molecular and biochemical measures. The rest was fixed in 4% paraformaldehyde for histology and immunofluorescence analysis.
2.3. Metabolic rate and physical activity
Oxygen consumption and physical activity were determined using CLAMS (Columbus Instruments) according to the manufacturer׳s instructions. Mice were acclimated to the system for 24 h, then VO2 and VCO2 were measured in the following 24 h. The animals were kept at 24 °C under a 12-h day/night cycle. Food and water were available ad libitum. Voluntary activity was monitored every 15 min. Heat production and the respiratory exchange ratio (RER) were recorded.
2.4. Histology and immunohistochemistry staining
Liver and adipose tissues were fixed in 4% paraformaldehyde, then embedded in paraffin and cut into 4-μm thick slides. The slides were stained with haematoxylin and eosin (H&E) for histology analysis. For immunohistochemistry staining, slides were antigen retrieved by submerged in 0.01 mol/L sodium citrate (pH 6.0) and heated for 20 min in a microwave. The sections were blocked in blocking buffer containing 5% goat serum, 2% BSA, 0.1% triton X-100 and 0.1% sodium azide in PBS, then incubated overnight with anti-UCP1 (Cell Signalling Danvers, USA) by a dilution of 1:100 at 4 °C. After washed twice in PBS, slices were incubated with secondary antibodies (Cell Signalling, Danvers, MA, USA) for 1 h at room temperature. Slides were counterstained with H&E and digital images were collected with an EVOS X1 microscopy (Thermo Fisher Scientific, Shanghai, China).
2.5. Oral glucose tolerance test (OGTT) and insulin tolerance test (ITT)
OGTT and ITT were performed after a 6-h fast as previously described16 using 2 g/kg of glucose and 0.75 U/kg of human regular insulin (Novo Nordisk, Bagsværd, Denmark), respectively. Blood samples were collected from tail vein for glucose measurement at 0, 30, 60, 90 and 120 min after glucose administration (p.o.) or insulin injection (i.p.). The blood glucose levels were determined by a glucose meter (Roche, ACCU-CHEK Active, Shanghai, China).
2.6. Cold tolerance test
The mice were subjected to a cold room (4 °C) without access to food or water. The rectal temperature was measured at the indicated time after exposure to the cold, using a rectal probe connected to digital thermometer (BAT-12 Microprobe-Thermometer; Physitemp, USA). Skin temperature was recorded with an infrared camera (Fluke TiX660 Infrared Camera, Fluke Corporation, Washington, USA) and analyzed with a specific software package (Smartview3.14.45 Fluke Corporation, Washington, USA).
2.7. Cell differentiation
3T3-L1 preadipocytes were obtained from the Peking Union Medical College and maintained in Dulbecco׳s modified Eagle׳s medium (DMEM) with 10% fetal bovine serum (FBS, Gibco, Grand Island, USA). To initiate differentiation, confluent 3T3-L1 preadipocytes were treated for 48 h in medium containing 10% FBS, 0.5 mmol/L isobutylmethylxanthine (IBMX), 125 μmol/L indomethacin, 1 μmol/L dexamethasone, 850 nmol/L insulin and 1 mmol/L rosiglitazone. Thereafter, cells were switched to differentiation medium (DMEM, 10% FBS, 850 nmol/L insulin, and 1 mmol/L rosiglitazone) with appropriate amount of Cpn. Differentiated adipocytes were analyzed after 48 h of differentiation. DMSO was used as the vehicle for different treatments.
2.8. Adipocyte experiment
3T3-L1 preadipocytes were fully differentiated into mature adipocyte as described above. The adipocytes were treated with Cpn and compound C as the indicated concentration for 24 h. Then the cells were subjected to oil-red O staining, intracellular triglyceride (TG), mtDNA copy number and citrate synthase activity determination and realtime-PCR and western blot analysis as described below.
2.9. Fat pad experiment
The subcutaneous fat pad were rinsed in phosphate-buffered saline, pH 7.40, and cut into fine pieces with average volume about 1 mm3. The pieces were transferred to a 6-well plate containing 30 mL of DMEM medium with 10% FBS, 5.5 mmol/L glucose, with about 100 mg of fat pad in each well. After maintained in DMEM medium for 6 h, the fat pad were supplemented with appropriate amount of Cpn and compound C for 24 h. Then TG and protein contents were determined by specific kits and the expression of key genes were quantified by realtime polymerase chain reaction (realtime PCR).
2.10. Transmission electron microscopy
The inguinal WAT tissues were fixed in 2% (v/v) glutaraldehyde for 24 h at room temperature. The sections were then post-fixed in 1% osmium tetroxide, dehydrated in ascending gradations of ethanol and embedded in fresh epoxy resin. Ultra-thin sections (60–80 nm) were cut and stained with lead citrate before being examined on the Phillip CM-120 transmission electron microscope.
2.11. Mitochondrial DNA (MtDNA) content quantification
The genomic DNA was extracted from inguinal WAT tissue by Genomic DNA Purification Kit (Thermo Fisher Scientific, Cambridge, USA) according to manufacturer׳s instructions. Primers for mitochondrial DNA and nuclear DNA are listed in Supporting Information Table S1. The data were expressed as mtDNA-specific 16 S ribosomal RNA normalized to nuclear specific gene hexokinase.
2.12. Quantitative realtime-PCR analysis
Total RNA extraction, cDNA synthesis and quantitative polymerase chain reaction (PCR) assays were performed as described previously17. At least three independent biological replicates were performed to check the reproducibility of the data. The gene-specific primers used for quantitative PCR are listed in Table S1.
2.13. Western blot
Homogenized adipose tissue was lysed in RIPA buffer containing protease and phosphatase inhibitors. After separated on sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE), the proteins were electroblotted to polyvinylidene fluoride (Millipore Billerica, USA). The membranes were blocked and incubated with different antibodies, then incubated with secondary antibodies. The blots were visualized by enhanced chemiluminescence reagents (Amersham Pharmacia Uppsala, Sweden) according to the manufacturer׳s protocol. The immunoreactive bands were quantified using ImageJ 4.1 software (NIH, Bethesda, MD, USA).
2.14. Statistical analyses
All the data are given as mean ± standard error of mean (SEM). Comparisons between the two groups were assessed by Student׳s t-test for independent samples. A P-value < 0.05 was considered statistically significant.
3. Results
3.1. Cordycepin improves metabolic profile
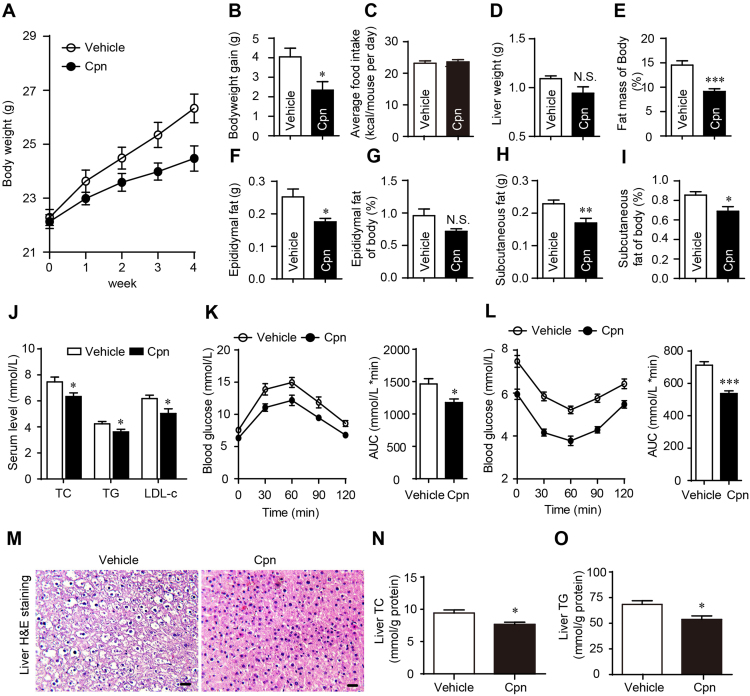
Gavage of Cpn (40 mg/kg) did not decrease appetite (Fig. 1C) but largely inhibited HFD-induced body weight gain (Fig. 1A and B). The liver weight was not significantly changed but the mass of epididymal and subcutaneous fat as well as the percentage of total body fat were dramatically reduced by Cpn (Fig. 1D–F and H). The fat/body weight index showed that the Cpn was more potent to decrease the deposition of subcutaneous fat than epididymal fat, as the decline of percent fat mass of epididymal fat was not as significant as subcutaneous fat (Fig. 1G and I). The serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), urea nitrogen (BUN) and creatinine (CREA), that are associated with liver injury or tissue damage, were not significantly changed, indicating that anti-obesity effects of Cpn were not due to toxic response (Supporting Information Fig. S1).
Figure 1.
Cordycepin reduces high-fat diet (HFD)-induced obesity and improves insulin sensitivity in mice. (A) Body weight curve. (B) Body weight gain. (C) Average food intake per day during 4 weeks of experiment. (D) Liver weight. (E) % fat mass of body. (F) Epididymal fat weight. (G) % epididymal fat of body. (H) Subcutaneous fat weight. (I)% subcutaneous fat of body. (J) Serum level of lipids. (K) Oral glucose tolerance test. (L) Insulin tolerance test. (M) Typical image of liver H&E staining. (N) Liver TC. (O) Liver TG. Data are displayed as mean±SEM, n=7. Significant differences compared with vehicle controls are indicated by *P < 0.05, **P < 0.01 and ***P < 0.001 (assessed by Student׳s t-test). N.S.: nonsignficance. Cpn: cordycepin.
The increased levels of total cholesterol (TC), triglycerides (TG) and low-density lipoprotein cholesterol (LDL-c) of HFD-fed mice were significantly decreased after Cpn treatment (Fig. 1J). Administration of Cpn also resulted in a better tolerance to a glucose load in oral glucose tolerance test (OGTT, Fig. 1K). Combined with the prominent effect in insulin tolerance test (ITT; Fig. 1L), Cpn is adequate to ameliorate glucose tolerance. Simultaneously, Cpn also ameliorated HFD-induced liver steatosis. The hepatic levels of TC and TG as well as vacuolar degeneration were all reduced after gavage of Cpn (Fig. 1M–O). These results demonstrated that Cpn is effective to reduce body weight and improve metabolic profiles.
3.2. Cordycepin enhances energy expenditure
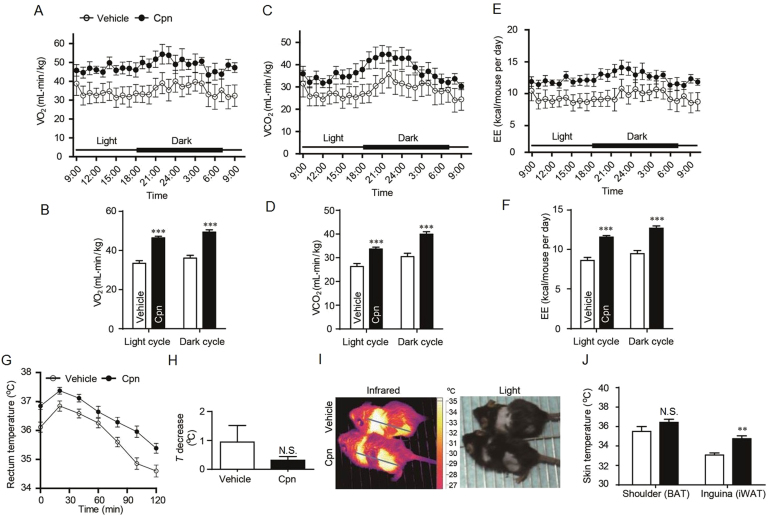
As monitored by lab animal monitoring system (CLAMS), after normalized to bodyweight, Cpn-treated mice displayed higher rates of oxygen consumption (VO2) and carbon dioxide production (VCO2) throughout a 12 h/12 h day/night cycle than the vehicle control mice (Fig. 2A–D). The animals that received Cpn exhibited significant increases in the energy expenditure (Fig. 2E and F). The physical activity was not significantly different between the two groups, indicating that the increased energy expenditure of Cpn-treated animals was not due to enhanced physical activity (Supporting Information Fig. S2a). Simultaneously, the RER of Cpn-treated mice were significantly decreased in the daytime but not at night. As mice are nocturnal animals, the RER data suggest that Cpn can shift the fuel preference towards fatty acids when animals are not doing intensive exercise (Fig. S2b).
Figure 2.
Cordycepin increases energy expenditure and adaptive thermogenesis. Energy expenditure was evaluated by measurement of oxygen consumption (VO2) (A) and carbon dioxide release (VCO2) (C) over a 24 h period after 4 weeks of cordycepin treatment. Energy expenditure was expressed as kcal/day per animal (E). The adjacent bar graphs represent the average for each group (B for A, D for C and F for E). (G) Body temperature during cold exposure was recorded in 30 min intervals. After food deprivation, rectal temperatures were measured at the indicated time points for mice placed in a cold room. (H) Average decrease of body temperature. (I) The skin temperature photos taken by infrared camera. (J) The skin temperature around the shoulder (BAT) and inguina (iWAT) measured by infrared camera. Data are displayed as mean±SEM, n=7. Significant differences compared with vehicle controls are indicated by *P < 0.05, **P < 0.01 and **P < 0.001 (assessed by Student׳s t-test). N.S.: nonsignficance. Cpn: cordycepin.
To further examine the effects of Cpn on energy expenditure, cold tolerance test was performed to evaluate the adaptive thermogenesis. During the two hours of cold exposure (4 °C), the body temperatures of control mice declined markedly while Cpn-treated animals showed less drop in body temperatures (Fig. 2G and H). This result suggests that Cpn is capable of enhancing heat generation, thus promoting adaptation to cold exposure.
Infrared camera analysis showed that the skin temperature surrounding BAT was moderately increased by Cpn but not reach significance (Fig. 2I, J). In contrast, the skin temperature surrounding inguinal WAT (iWAT) was obviously increased by Cpn (Fig. 2I, J), implying that Cpn might stimulate subcutaneous WAT browning to enhance heat generation.
3.3. Cordycepin stimulates adipose browning in WAT
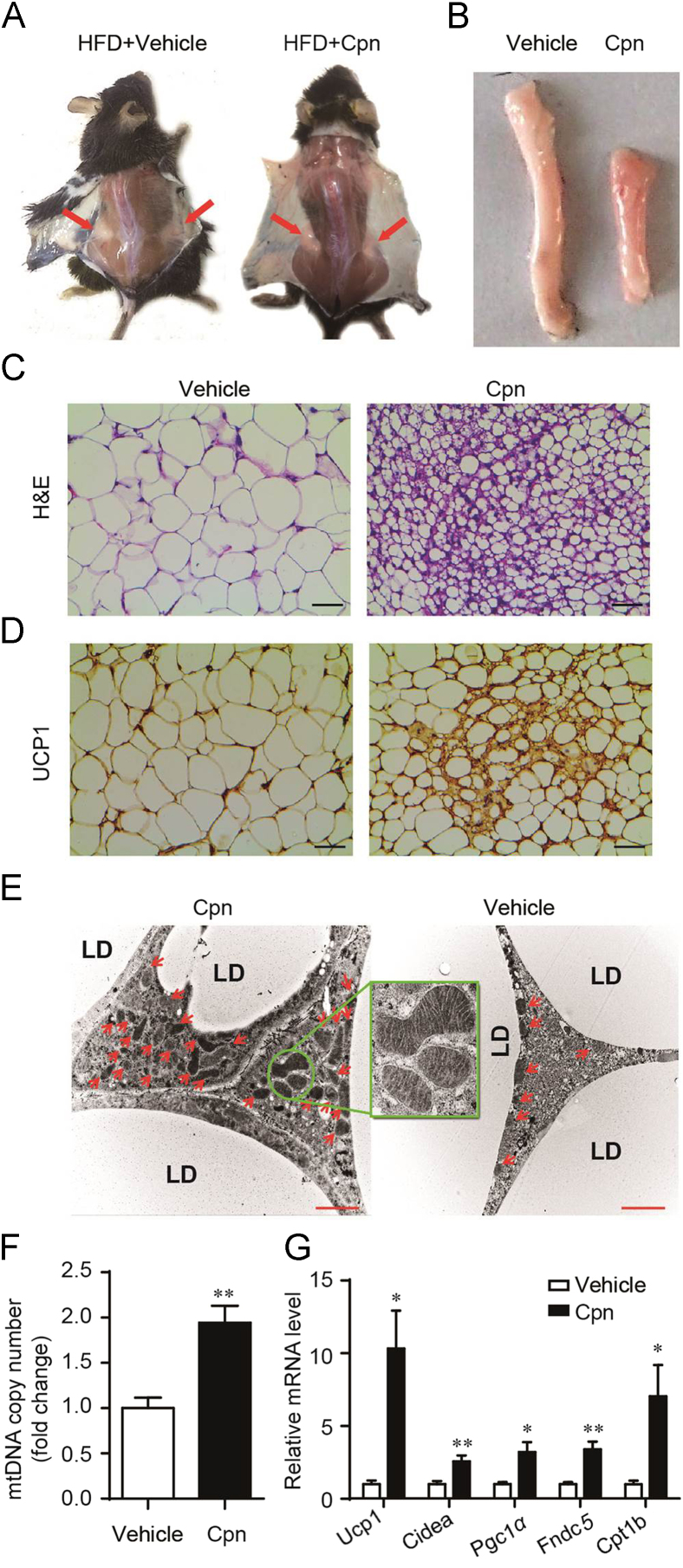
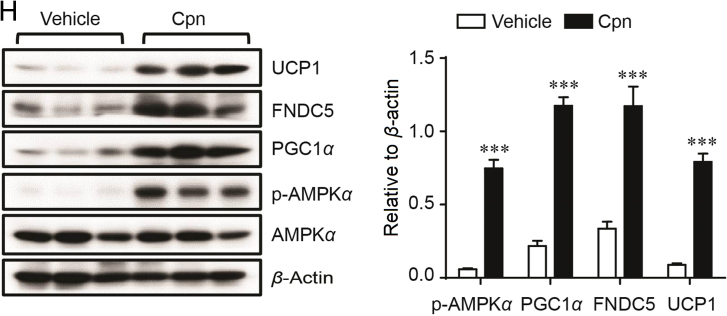
To address whether Cpn promotes WAT browning, we first checked the effects of Cpn on the color, adipocyte morphology, mitochondrial copies and expression levels of fat browning markers in inguinal WAT (iWAT). Cpn administration turned iWAT much browner (Fig. 3A and B), made the adipocytes smaller and filled with multilocular small lipid droplets (Fig. 3C), and enhanced UCP1 immunohistochemistry signals (Fig. 3D). Transmission electronic microscopy and mtDNA copy number quantification displayed that Cpn significantly increased mitochondrial biogenesis (Fig. 3E and F). The markers of brown adipocytes, such as PGC1α, CIDEA, FNDC5 and carnitine palmitoyltransferase 1b (CPT1B), were also significantly increased in iWAT of Cpn group (Fig. 3G and H).
Figure 3.
Cordycepin induces browning of WAT. (A) Dorsal view of vehicle- and cordycepin-treated mice. Red arrows indicate the inguinal WAT (iWAT). (B) Representative image of WAT. (C) H&E staining of iWAT from control and cordycepin-treated mice. (D) UCP1 immunoreactivity signal (brown) (E) Representative electron microscopy micrographs determined in iWAT of vehicle and cordycepin-treated mice. Red arrows indicate the mitochondria. The enlarged image displays the cristae of mitochondria. Scale bar = 2 μm. (F) MtDNA copy number of iWAT from vehicle- or cordycepin-treated mice. (G) Real-time PCR analysis of thermogenic gene expression. (H) Representative western blots showing the key protein changes in iWAT. Data are displayed as mean±SEM, n=7. Significant differences compared with vehicle controls are indicated by *P < 0.05 and **P < 0.01 (assessed by Student׳s t-test). LD: lipid droplet, Cpn: cordycepin.
Similar to iWAT, the adipocytes of epididymal WAT (eWAT) in Cpn-treated mice exhibited much smaller size (Supporting Information Fig. S3a). The mRNA levels of thermogenic genes such as Ucp1,Cidea, Pgc1α and Cpt1b were increased by Cpn, but the difference did not reach significance. The UCP1-positive adipocyte could be seen in the eWAT from Cpn-treated mice but the intensity was not as high as in iWAT (Fig. S3B and C). These results indicated that Cpn can induce browning in both iWAT and, to a less extent, eWAT.
As BAT is a main tissue responsible for energy expenditure and thermogenesis in rodents, we also checked the effect of Cpn on the expression of genes controlling energy expenditure and thermogenic programme in BAT (Supporting Information Fig. S4). The transcription of transcription factor such as PGC1α, classical BAT marker genes such as Ucp1,Cidea, and genes controlling fatty acid oxidation including Cpt1b, fatty acid transporter protein1 (Fatp1) and cytochrome c (Cyto-c) were all strongly enhanced. These results suggest that Cpn may function as an activator of thermogenic programming thus increasing the activity of the BAT.
3.4. Cordycepin may induce adipocyte browning through activation of AMPK
AMPK is well-known to play a role in promoting WAT browning and Cpn has been proved to be a potent AMPK activator. So we suppose that Cpn may stimulate WAT browning through activation of AMPK. To explore the potential mechanism of Cpn-induced browning in WAT and UCP1 upregulation, three sets of experiments were performed. First, 3T3-L1 pre-adipocytes were differentiated in the presence or absence of Cpn. Cpn significantly inhibited differentiation of pre-adipocytes to lipid-laden adipocytes (Supporting Information Fig. S5a and b) and decreased the percentage of adipocytes and cellular lipid accumulation (Fig. S5c and d). The browning markers such as Ucp1,Cidea, Pgc1α and Fndc5 were all significantly increased by Cpn (Fig. S5e). When blocked the AMPK activity with compound C, the effects of Cpn on the differentiation of 3T3-L1 pre-adipocyte and on the expression of browning markers were substantially abolished (Fig. S5f–h).
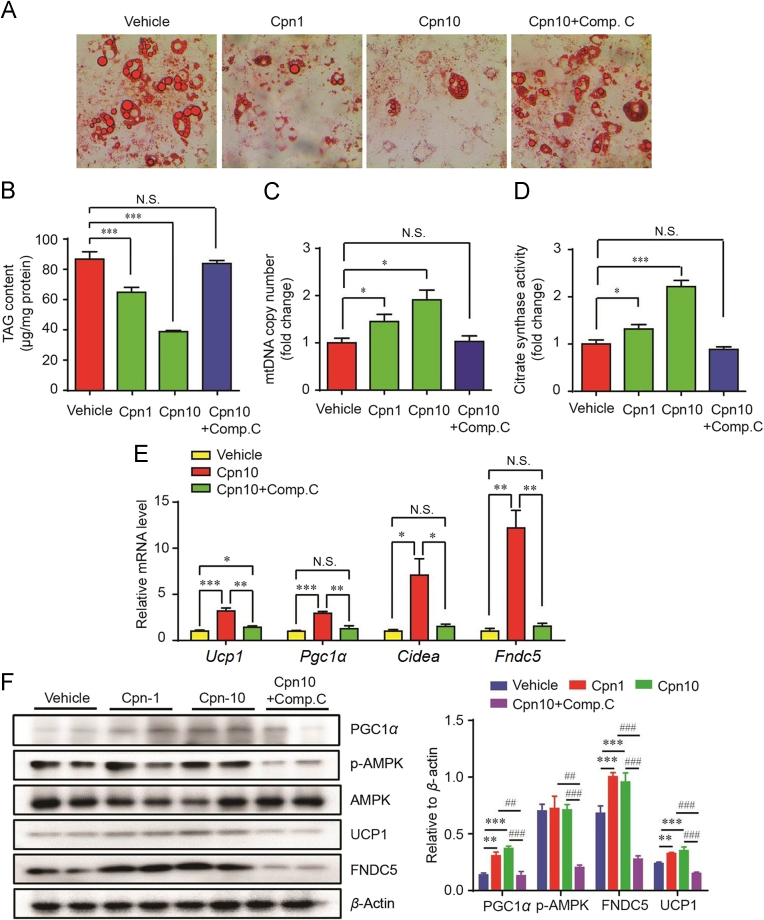
To distinguish the effect of Cpn on adipocyte differentiation and browning, adipocytes were treated after fully differentiated. Cpn significantly decreased lipid load of mature adipocytes (Fig. 4A and B). The mtDNA copy number and citrate synthase activity were significantly increased by Cpn (Fig. 4C and D). Simultaneously, the browning markers including Ucp1,Cidea, Pgc1α and Fndc5 were all significantly increased (Fig. 4E), indicating adipocyte browning. When blocked the AMPK activity with compound C, the effects of Cpn on the lipid accumulation, mitochondriopoiesis and the expression of browning markers were substantially abolished (Fig. 4A–E). Western blot showed that Cpn enhanced the phosphorylation of AMPK and increased the protein levels of FNDC5 and UCP1 (Fig. 4F). The blockage of AMPK activity by compound C almost prevented the total increased expression in FNDC5 and UCP1 that were originally induced by Cpn (Fig. 4F). These data suggested that Cpn could activate AMPK, thus inducing FNDC5 and UCP1 expression.
Figure 4.
Cordycepin-regulated UCP1 expression is AMPK-dependent. 3T3-L1 pre-adipocytes were differentiated into mature adipocytes in IDI medium then treated with or without cordycepin (10 μmol/L) for 24 h and subjected to analyses. (A) Oil red O staining. (B) Quantitative analysis of lipid content. (C) MtDNA copy number. (D) Citrate synthase activity. (E) Realtime PCR analysis of thermogenic gene expression. (F) Western blots for key proteins involved in thermogenesis. Data are displayed as mean±SEM, n=6. Significant differences compared with vehicle controls are indicated by *P < 0.05, **P < 0.01 and ***P < 0.001 (assessed by Student׳s t-test). N.S.: nonsignficance. Cpn: cordycepin; Comp.C: compound C.
We also checked the effects Cpn on TG level and the expression of adipose browning genes in the subcutaneous fat pad. As shown in Supporting Information Fig. S6, treatment with Cpn for 24 h significantly decreased the TG content and increased the expression of UCP1, CIDEA, PGC1α, FNDC5 and CPT1B. Co-treatment with compound C totally abolished these promoting effects of Cpn. These data were in accordance with that obtained in 3T3-L1 cell experiments, suggesting that Cpn might stimulate adipocyte browning through activation of AMPK pathway. All these results indicated that activation of AMPK may participate, at least partially, in the WAT-browning effect of Cpn.
4. Discussion
White adipose browning, a process that transforms energy-storing white adipocytes into heat-producing beige adipocytes in WAT, represents an attractive strategy to increase energy expenditure and treat obesity and diabetes18, 19. Discovering compounds with WAT browning effects is therefore of great importance to provide alternative approaches for the treatment of these common diseases. Previous studies demonstrated that Cpn is adequate to ameliorate hyperlipidemia and hyperglycemia12, 13 but the mechanism is remained to be elucidated. In this work, we present clear data supporting a stimulatory function of Cpn in raising energy expenditure by promoting WAT browning, and provide profound insights into the therapeutic effects of this compound on obesity and related complications.
Typical features that indicate the transform of white fat into beige adipose include brown appearance, multilocular lipid droplets, highly abundant mitochondria, unique staining of UCP1 and increased expression of thermogenic proteins such as PGC1α, CIDEA and CPT1B7, 19. Based on the five aspects characterizing beige adipocytes, administration of Cpn for 4 weeks potently promoted adipocyte browning in the inguinal WAT. First, the color of iWAT pad appears much browner in Cpn-treated mice. Second, Cpn not only reduced the total mass of iWAT but also transformed large adipocytes into small cells filled with multilocular lipid droplets. Third, the number of mitochondria and mtDNA copies in iWAT adipocytes were significantly increased after Cpn treatment. Fourth, the immune-signals of UCP1 in iWAT were remarkably enhanced. And last, the browning markers, such as PGC1α, CIDEA and CPT1B, were all significantly enhanced by Cpn. These results clearly demonstrated that oral administration of Cpn is adequate to promote WAT browning.
WAT browning is responsible for an increase in total energy expenditure20. Treatment with Cpn did not change the physical activity but significantly increased VO2, VCO2 and energy expenditure (EE), suggesting that the increased energy expenditure by Cpn should be mainly due to the enhancement of basal metabolic rate. Accordingly, the body temperature (rectal temperature) was largely increased by Cpn. The Cpn-treated mice were more tolerance to cold exposure. These indicate that Cpn is capable of enhancing energy expenditure by promoting heat generation. As increased energy expenditure represents an effective way to reduce body weight and alleviate the dysregulation of lipids and glucose21, the promotive effect of Cpn on basic energy expenditure provided a new insight into the anti-obesity and anti-diabetic action.
The RER value reflects the preference for fuel utilization. An RER of 0.70 indicates that fat is the predominant fuel source, and a value of 1.00 or above is indicative of carbohydrate being the predominant fuel source. RER analysis showed that treatment with Cpn significantly reduced RER towards 0.7 in the daytime but not at night. As mice are nocturnal animals, they mainly exercise at night and rest in the daytime. The RER data suggest that Cpn might promote fat motivation and utilize fatty acids as preferred fuel substance when animals take rest.
BAT is a major thermogenic organ and the skin temperature surrounding BAT is usually much higher than that around inguinal WAT. Treatment with Cpn moderately increased the skin temperature surrounding BAT but not reached significance, indicating that Cpn may slightly enhance the activity and thermogenesis of BAT. In accordance, the mRNA levels of BAT markers and genes controlling thermogenic programming such as Pgc1α, Ucp1, Cidea, Cpt1b, Fatp1, Cyto-c were all strongly increased after Cpn treatment. These results suggest that Cpn may function as an activator of thermogenic programming thus increasing the activity of the BAT. AMPK is a key regulator of energy metabolism and mitochondrial biogenesis22. The promotive effects of AMPK on WAT browning and BAT activity have been extensively investigated. It was reported that the modulatory functions of myostatin23, adiponectin24, and irisin25 on WAT browning involve AMPK activation. On the other hand, activation of AMPK by 5-aminoimidazole-4-carboxamide 1-β-d-ribofuranoside (AICAR)26, liraglutide27, curcumin28 and berberine29, 30 is adequate to induce accumulation of beige adipocytes in WAT of mice. Recent studies have demonstrated that AMPK is a target for Cpn13, 14, 15, 31. We therefore supposed that Cpn may promote fat browning through, at least in part, activation of AMPK. Experiments on preadipocyte, differentiated adipocyte and mouse adipose pad showed that the browning-promotive effects of Cpn on adipocytes and fat pad were substantially abolished by the treatment with AMPK inhibitors compound C, indicating that AMPK played a key role in Cpn-induced thermogenic programme in vitro. Although further investigations such as animal experiments using systemic or adipose-specific AMPK knockout mice are still needed to prove that AMPK is the cause of fat browning in vivo, the current data provide an important hint that AMPK activation may involve the Cpn-induced WAT browning at least in vitro.
It should also be noted that Cpn may stimulate browned fat formation through other routes other than AMPK activation. A recent report showed that adenosine can activate brown adipose tissue and recruit beige adipocytes via A2A receptors32. As Cpn is an analog of adenosine and previous studies have proved that Cpn may exert its pharmacological effect through A2A receptor33, 34. Therefore, Cpn may also stimulate beige WAT formation through A2A receptor-related and other pathways, which could be explored in the future.
Combined with our work, cordycepin could activate and expand the thermogenic machinery to provide a robust defence against obesity. We reveal the molecular target and mechanism by which cordycepin potently regulated the transcription of UCP1 in white adipocytes through, at least in part, AMPK activation. These findings establish an important role for cordycepin in regulating adipose tissue thermogenesis and white adipose plasticity towards BAT, and we identify cordycepin as a new potential drug for treating patients with obesity.
Acknowledgements
We thank Prof. Ping Zhu at Institute of Materia Medica, Chinese Academy of Medical Sciences & Peking Union Medical College for kindly providing high quality cordycepin. This work was supported financially by the National Natural Science Foundation of China (81402983, 81573436), CAMS Innovation Fund for Medical Sciences (CIFMS) 2016-I2M-3–015, and the National Major Scientific and Technological Special Project for “Significant New Drugs Development” (2015ZX09501005, China).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.apsb.2018.10.004.
Contributor Information
Chongming Wu, Email: cmwu@implad.ac.cn.
Peng Guo, Email: pguo@implad.ac.cn.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Bhupathiraju S.N., Hu F.B. Epidemiology of obesity and diabetes and their cardiovascular complications. Circ Res. 2016;118:1723–1735. doi: 10.1161/CIRCRESAHA.115.306825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang S., Wu C., Li X., Zhou Y., Zhang Q., Ma F. Syringaresinol-4-O-β-d-glucoside alters lipid and glucose metabolism in HepG2 cells and C2C12 myotubes. Acta Pharm Sin B. 2017;7:453–460. doi: 10.1016/j.apsb.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crowley V.E., Yeo G.S., O׳Rahilly S. Obesity therapy: altering the energy intake-and-expenditure balance sheet. Nat Rev Drug Discov. 2002;1:276–286. doi: 10.1038/nrd770. [DOI] [PubMed] [Google Scholar]
- 4.Tseng Y.H., Cypess A.M., Kahn C.R. Cellular bioenergetics as a target for obesity therapy. Nat Rev Drug Discov. 2010;9:465–482. doi: 10.1038/nrd3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuo T., Chen T.C., Lee R.A., Thao Nguyen N.H., Broughton A.E., Zhang D. Pik3r1 is required for glucocorticoid-induced perilipin 1 phosphorylation in lipid droplet for adipocyte lipolysis. Diabetes. 2017;66:1601–1610. doi: 10.2337/db16-0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu J., Zhang S., Cui L., Wang W., Na H., Zhu X. Lipid droplet remodeling and interaction with mitochondria in mouse brown adipose tissue during cold treatment. Biochim Biophys Acta. 2015;1853:918–928. doi: 10.1016/j.bbamcr.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 7.Wu J., Bostrom P., Sparks L.M., Ye L., Choi J.H., Giang A.H. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dodd G.T., Decherf S., Loh K., Simonds S.E., Wiede F., Balland E. Leptin and insulin act on POMC neurons to promote the browning of white fat. Cell. 2015;160:88–104. doi: 10.1016/j.cell.2014.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang H., Liu L., Lin J.Z., Aprahamian T.R., Farmer S.R. Browning of white adipose tissue with roscovitine induces a distinct population of UCP1+ adipocytes. Cell Metab. 2016;24:835–847. doi: 10.1016/j.cmet.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang P., Huang C., Fu C., Tian Y., Hu Y., Wang B. Cordycepin (3׳-deoxyadenosine) suppressed HMGA2, Twist1 and ZEB1-dependent melanoma invasion and metastasis by targeting miR-33b. Oncotarget. 2015;6:9834–9853. doi: 10.18632/oncotarget.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X., Zhou Y., Zhang X., Cao X., Wu C., Guo P. Cordycepin stimulates autophagy in macrophages and prevents atherosclerotic plaque formation in ApoE--/-- mice. Oncotarget. 2017;8:94726–94737. doi: 10.18632/oncotarget.21886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma L., Zhang S., Du M. Cordycepin from Cordyceps militaris prevents hyperglycemia in alloxan-induced diabetic mice. Nutr Res. 2015;35:431–439. doi: 10.1016/j.nutres.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Guo P., Kai Q., Gao J., Lian Z.Q., Wu C.M., Wu C.A. Cordycepin prevents hyperlipidemia in hamsters fed a high-fat diet via activation of AMP-activated protein kinase. J Pharmacol Sci. 2010;113:395–403. doi: 10.1254/jphs.10041fp. [DOI] [PubMed] [Google Scholar]
- 14.Wu C., Guo Y., Su Y., Zhang X., Luan H., Zhang X. Cordycepin activates AMP-activated protein kinase (AMPK) via interaction with the gamma1 subunit. J Cell Mol Med. 2014;18:293–304. doi: 10.1111/jcmm.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takahashi S., Tamai M., Nakajima S., Kato H., Johno H., Nakamura T. Blockade of adipocyte differentiation by cordycepin. Br J Pharmacol. 2012;167:561–575. doi: 10.1111/j.1476-5381.2012.02005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu C., Zhang X., Zhang X., Luan H., Sun G., Sun X. The caffeoylquinic acid-rich Pandanus tectorius fruit extract increases insulin sensitivity and regulates hepatic glucose and lipid metabolism in diabetic db/db mice. J Nutr Biochem. 2014;25:412–419. doi: 10.1016/j.jnutbio.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Wu C., Feng J., Wang R., Liu H., Yang H., Rodriguez P.L. HRS1 acts as a negative regulator of abscisic acid signaling to promote timely germination of Arabidopsis seeds. PLoS One. 2012;7:e35764. doi: 10.1371/journal.pone.0035764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shan T., Xiong Y., Zhang P., Li Z., Jiang Q., Bi P. Lkb1 controls brown adipose tissue growth and thermogenesis by regulating the intracellular localization of CRTC3. Nat Commun. 2016;7:12205. doi: 10.1038/ncomms12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bi P., Shan T., Liu W., Yue F., Yang X., Liang X.R. Inhibition of Notch signaling promotes browning of white adipose tissue and ameliorates obesity. Nat Med. 2014;20:911–918. doi: 10.1038/nm.3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petruzzelli M., Schweiger M., Schreiber R., Campos-Olivas R., Tsoli M., Allen J. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab. 2014;20:433–447. doi: 10.1016/j.cmet.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 21.Tomas E., Stanojevic V., McManus K., Khatri A., Everill P., Bachovchin W.W. GLP-1(32-36)amide pentapeptide increases basal energy expenditure and inhibits weight gain in obese mice. Diabetes. 2015;64:2409–2419. doi: 10.2337/db14-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J., Zhong L., Wang F., Zhu H. Dissecting the role of AMP-activated protein kinase in human diseases. Acta Pharm Sin B. 2017;7:249–259. doi: 10.1016/j.apsb.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shan T., Liang X., Bi P., Kuang S. Myostatin knockout drives browning of white adipose tissue through activating the AMPK-PGC1a-Fndc5 pathway in muscle. FASEB J. 2013;27:1981–1989. doi: 10.1096/fj.12-225755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hui X., Gu P., Zhang J., Nie T., Pan Y., Wu D. Adiponectin enhances cold-induced browning of subcutaneous adipose tissue via promoting M2 macrophage proliferation. Cell Metab. 2015;22:279–290. doi: 10.1016/j.cmet.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Lee H.J., Lee J.O., Kim N., Kim J.K., Kim H.I., Lee Y.W. Irisin, a novel nyokine, regulates glucose uptake in skeletal muscle cells via AMPK. Mol Endocrinol. 2015;29:873–881. doi: 10.1210/me.2014-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaidhu M.P., Frontini A., Hung S., Pistor K., Cinti S., Ceddia R.B. Chronic AMP-kinase activation with AICAR reduces adiposity by remodeling adipocyte metabolism and increasing leptin sensitivity. J Lipid Res. 2011;52:1702–1711. doi: 10.1194/jlr.M015354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beiroa D., Imbernon M., Gallego R., Senra A., Herranz D., Villarroya F. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes. 2014;63:3346–3358. doi: 10.2337/db14-0302. [DOI] [PubMed] [Google Scholar]
- 28.Lone J., Choi J.H., Kim S.W., Yun J.W. Curcumin induces brown fat-like phenotype in 3T3-L1 and primary white adipocytes. J Nutr Biochem. 2016;27:193–202. doi: 10.1016/j.jnutbio.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Z., Zhang H., Li B., Meng X., Wang J., Zhang Y. Berberine activates thermogenesis in white and brown adipose tissue. Nat Commun. 2014;5:5493. doi: 10.1038/ncomms6493. [DOI] [PubMed] [Google Scholar]
- 30.Hardie GD. Regulation of AMP-activated protein kinase by natural and synthetic activators. Acta Pharm Sin B. 2016;6:1–19. doi: 10.1016/j.apsb.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong Y.Y., Moon A., Duffin R., Barthet-Barateig A., Meijer H.A., Clemens M.J. Cordycepin inhibits protein synthesis and cell adhesion through effects on signal transduction. J Biol Chem. 2010;285:2610–2621. doi: 10.1074/jbc.M109.071159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gnad T., Scheibler S., von Kugelgen I., Scheele C., Kilic A., Glode A. Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature. 2014;516:395–399. doi: 10.1038/nature13816. [DOI] [PubMed] [Google Scholar]
- 33.Leu S.F., Poon S.L., Pao H.Y., Huang B.M. The in vivo and in vitro stimulatory effects of cordycepin on mouse leydig cell steroidogenesis. Biosci Biotechnol Biochem. 2011;75:723–731. doi: 10.1271/bbb.100853. [DOI] [PubMed] [Google Scholar]
- 34.Cao Z.P., Dai D., Wei P.J., Han Y.Y., Guan Y.Q., Li H.H. Effects of cordycepin on spontaneous alternation behavior and adenosine receptors expression in hippocampus. Physiol Behav. 2018;184:135–142. doi: 10.1016/j.physbeh.2017.11.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material