Abstract
This study was conducted to assess whether systemic arterial stiffness, indicated by cardio-ankle vascular index (CAVI), is related to changes in the microvasculature of the retina and choroid in diabetes mellitus (DM). This study included 113 patients with a confirmed diagnosis of type-2 DM. Among them, 18 patients did not have diabetic retinopathy (DR), 71 had non-proliferative DR (NPDR), and 24 had proliferative DR (PDR). The mean CAVI was 7.58 ± 1.41 in no DR, 8.72 ± 1.47 in NPDR, and 8.43 ± 1.25 in PDR group. Of the 113 eyes, 42 (37.2%) were classified as abnormal CAVI group (CAVI ≥ 9). This group had significantly higher cardiac autonomic neuropathy risk index score, decreased central choroidal thickness, and decreased choroidal vascularity index (CVI). Deep foveal avascular zone area was higher in the abnormal CAVI group. After adjustment for possible confounding factors, CAVI showed negative correlation with the CVI (r = −0.247, P = 0.013). In conclusion, there was a significant correlation between arteriosclerosis and choroidal vascular changes in DR. We suggest prompt ophthalmic evaluation in patients with systemic arteriosclerosis. If the ophthalmologist notes advanced DR, the patient should be referred to a cardiovascular clinic for detailed evaluation of systemic arteriosclerosis.
Introduction
Arterial stiffness is a term used to describe the degree of vascular rigidity due to reduced elasticity of the artery. Many studies have demonstrated the predictive value of arterial stiffness on systemic cardiovascular diseases1–5. The most important factor determining arterial stiffness is age; the elasticity of the arterial wall decreases with age and arterial stiffness increases. In addition, increased arterial stiffness can occur as a result of elevated blood pressure, smoking, obesity, and other systemic vascular diseases including diabetes mellitus (DM)6.
Arterial stiffness, termed arteriosclerosis, can be assessed noninvasively by measuring the pulse wave velocity (PWV)1, central blood pressure (CBP)7, or cardio-ankle vascular index (CAVI)8. Among these, CAVI is superior to PWV because it is not affected by blood pressure at the time of measurement8,9. CAVI increases with age. It is high in many arteriosclerotic diseases, such as cerebrovascular diseases, chronic kidney disease, carotid arteriosclerosis, and coronary artery disease; it is also related to many coronary risk factors including smoking, DM, hypertension, and dyslipidemia8.
Atherosclerosis is a narrowing of the arteries caused by a buildup of plaque, and the term is sometimes used as a specific subtype of arteriosclerosis. Atherosclerosis can be assessed by measuring the ankle-brachial index (ABI) or intima-media thickness of the common carotid artery. Studies on the association between arterial stiffness and atherosclerosis showed conflicting results10,11.
Recent advances in multimodal imaging have facilitated many studies on microvascular changes in diabetic retinopathy12–15. Further, attempts have been made to quantitatively analyze diabetic microvascular changes of the retina and choroid. In patients with DM, increased foveal avascular zone area12, decreased retinal perfusion density12,14, and decreased choroidal vascularity index (CVI)15 were associated with worsening diabetic retinopathy (DR). Moreover, in patients with DM, these microvascular changes were evident even without DR.
Generally, DR is a metabolic disorder caused by hyperglycemia, and it shares common risk factors with other diabetic microvascular complications including diabetic nephropathy and neuropathy. Some previous studies identified a relationship between diabetic retinopathy and systemic endothelial dysfunction with peripheral arterial stiffness16–18. However, these studies evaluated only the presence of DR18 or the grade of severity of DR16,17. To date, the association between arterial stiffness and diabetic ocular microvascular changes using quantitative vascular parameters has not been explored. Further, the underlying mechanism and the association between large-artery stiffness and diabetic ocular microvascular changes remain unclear.
In this study, we evaluated the CAVI and the quantitative vascular parameters of the retina and choroid in patients with type 2 DM. The aim of the current study was to assess whether systemic arterial stiffness, as indicated by CAVI, has an association with changes in the microvasculature of the retina and choroid in type 2 DM.
Results
In total, 113 eyes of patients with diabetes were included in this study. We have summarized the demographic, ocular, and systemic characteristics of the subjects according to the severity of diabetic retinopathy in Table 1. Of the 113 eyes, 18 (15.9%) were classified as no DR, 71 (62.8%) as non-proliferative DR (NPDR), and 24 (21.2%) as proliferative diabetic retinopathy (PDR). There were no significant differences with regard to age, sex, blood pressure, visual acuity, intraocular pressure, or refractive error among the 3 groups. Disease duration (P < 0.001), glycated hemoglobin (HbA1c) (P < 0.001), and glycoalbumin level (P = 0.030) differed significantly between each DR group. The mean CAVI was 7.58 ± 1.41 in the no DR, 8.72 ± 1.47 in NPDR, and 8.43 ± 1.25 in the PDR group (P = 0.012). If the ABI was set into ordinal variables (as normal versus abnormal ABI), advanced DR severity and abnormal ABI showed a significant correlation (P = 0.042, linear-by-linear association test). However, the mean ABI was 1.06 ± 0.14 in the no DR, 1.05 ± 0.10 in NPDR, and 1.10 ± 0.10 in the PDR group; the differences were not statistically significant (P = 0.162).
Table 1.
Demographic and Clinical Data According to Severity of Diabetic Retinopathy.
| No DR | NPDR | PDR | P value | |
|---|---|---|---|---|
| Number of eyes | 18 | 71 | 24 | — |
| Age, years | 55.94 ± 12.59 | 60.42 ± 7.75 | 56.96 ± 8.21 | 0.073 |
| Sex, male (n) | 7 (38.9%) | 34 (47.9%) | 5 (20.8%) | 0.065 |
| Current smoking (n) | 8 (44.4%) | 34 (47.9%) | 9 (37.5%) | 0.681 |
| Years with diabetes | 5.44 ± 3.97 | 13.63 ± 8.67 | 12.83 ± 6.66 | <0.001 |
| Systolic BP, mmHg | 132.11 ± 11.08 | 135.55 ± 15.23 | 134.75 ± 14.37 | 0.668 |
| Diastolic BP, mmHg | 95.50 ± 10.44 | 83.25 ± 8.77 | 83.33 ± 7.51 | 0.863 |
| Mean arterial BP, mmHg | 100.37 ± 9.74 | 100.69 ± 9.75 | 100.47 ± 9.04 | 0.990 |
| HbA1c, % | 7.13 ± 1.13 | 7.86 ± 1.54 | 9.23 ± 2.48 | <0.001 |
| Fasting blood sugar, mg/dL | 147.72 ± 43.11 | 153.85 ± 72.08 | 140.21 ± 34.93 | 0.640 |
| eGFR, mL/min/1.73 m2 | 93.13 ± 17.79 | 76.86 ± 23.11 | 79.95 ± 29.08 | 0.038 |
| Creatinine, mg/dL | 0.77 ± 0.15 | 0.97 ± 0.45 | 0.90 ± 0.31 | 0.124 |
| Glycoalbumin, % | 18.48 ± 5.15 | 23.98 ± 8.32 | 31.27 ± 7.05 | 0.030 |
| Total cholesterol, mg/dL | 146.56 ± 38.02 | 163.80 ± 53.60 | 155.63 ± 42.73 | 0.385 |
| HDL, mg/dL | 48.56 ± 11.83 | 49.17 ± 12.55 | 50.08 ± 11.38 | 0.917 |
| LDL, mg/dL | 76.72 ± 30.21 | 79.87 ± 28.28 | 82.79 ± 26.16 | 0.787 |
| TG, mg/dL | 126.17 ± 48.88 | 133.37 ± 79.01 | 144.71 ± 68.98 | 0.231 |
| BMI, kg/m2 | 27.87 ± 3.84 | 25.15 ± 3.47 | 25.10 ± 3.01 | 0.010 |
| CAN risk index | 51.60 ± 22.98 | 66.53 ± 16.18 | 74.52 ± 15.81 | <0.001 |
| Mean peripheral neuropathy index | 0.89 ± 0.86 | 1.15 ± 1.21 | 1.81 ± 1.50 | 0.097 |
| CAVI | 7.58 ± 1.41 | 8.72 ± 1.47 | 8.43 ± 1.25 | 0.012 |
| ABI | 1.06 ± 0.14 | 1.05 ± 0.10 | 1.10 ± 0.10 | 0.162 |
| Ophthalmic examinations | ||||
| BCVA (logMAR) | 0.08 ± 0.11 | 0.10 ± 0.11 | 0.11 ± 0.16 | 0.657 |
| IOP, mmHg | 14.83 ± 2.77 | 15.08 ± 2.57 | 14.63 ± 2.68 | 0.746 |
| SE | 0.24 ± 1.85 | −0.23 ± 1.80 | −0.13 ± 1.10 | 0.291 |
Data are expressed as mean ± standard deviation (95% confidence interval).
ABI, ankle-brachial index; BCVA, best-corrected visual acuity; BMI, body mass index; BP, blood pressure; CAN, cardiac autonomic neuropathy; CAVI, cardio-ankle vascular index; DR, diabetic retinopathy; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; IOP, intraocular pressure; LDL, low-density lipoprotein; logMAR, logarithm of the minimum angle of resolution; NPDR, non-proliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; SE, spherical equivalent; TG, triglyceride.
Tables 2 and 3 display comparisons of demographic and clinical data according to CAVI and ABI values. Of the 113 eyes, 42 (37.2%) were classified as abnormal CAVI group (CAVI ≥ 9). cardiac autonomic neuropathy (CAN) risk index score was significantly higher in the abnormal CAVI group (62.54 ± 20.23 and 71.15 ± 14.33, for CAVI < 9 and CAVI ≥ 9, respectively, P = 0.018). We noted decreased central choroidal thickness (P = 0.002) and decreased CVI (P = 0.041) in the abnormal CAVI group. Deep foveal avascular zone (FAZ) area was higher in the abnormal CAVI group (0.62 ± 0.28 and 0.79 ± 0.36, for CAVI < 9 and CAVI ≥ 9, respectively, P = 0.005). However, retinal microvascular parameters including superficial FAZ area, superficial and deep FAZ circulatory index, and superficial and deep capillary vessel density showed no significant differences between the two CAVI groups (all P values > 0.05). Of the 113 eyes, 34 (30.1%) were classified as abnormally low or high ABI (ABI < 0.9 or ≥1.3). There were no significant differences in terms of ocular microvascular parameters between the two ABI groups (all P values > 0.05).
Table 2.
Demographic and clinical data according to cardio-ankle vascular index (CAVI) value.
| CAVI < 9 | CAVI ≥ 9 | P value | |
|---|---|---|---|
| Number of eyes | 71 | 42 | — |
| Age, years | 56.08 ± 8.86 | 63.86 ± 6.58 | <0.001 |
| Current smoking (n) | 38 (53.5%) | 13 (31.0%) | 0.018 |
| Years with diabetes | 10.46 ± 7.25 | 15.00 ± 9.11 | 0.004 |
| HbA1c,% | 7.78 ± 1.56 | 8.48 ± 2.20 | 0.061 |
| eGFR, mL/min/1.73 m2 | 86.81 ± 22.31 | 68.78 ± 23.47 | <0.001 |
| Creatinine, mg/dL | 0.85 ± 0.25 | 1.06 ± 0.54 | 0.017 |
| BMI, kg/m2 | 26.15 ± 3.71 | 24.59 ± 3.06 | 0.023 |
| CAN risk index | 62.54 ± 20.23 | 71.15 ± 14.33 | 0.018 |
| CAVI | 7.63 ± 0.91 | 9.90 ± 1.05 | — |
| ABI | 1.06 ± 0.10 | 1.08 ± 0.10 | 0.082 |
| Visual acuity (logMAR) | 0.08 ± 0.12 | 0.13 ± 0.11 | 0.048 |
| GC-IPL thickness, μm | 114.57 ± 13.58 | 113.68 ± 14.64 | 0.744 |
| RNFL thickness, μm | 110.39 ± 18.39 | 106.90 ± 11.71 | 0.221 |
| Central retinal thickness, μm | 236.49 ± 24.87 | 228.36 ± 28.81 | 0.116 |
| Central choroidal thickness, μm | 233.65 ± 72.74 | 188.55 ± 65.95 | 0.002 |
| Superficial FAZ area, mm2 | 0.42 ± 0.21 | 0.44 ± 0.12 | 0.431 |
| Deep FAZ area, mm2 | 0.62 ± 0.28 | 0.79 ± 0.36 | 0.005 |
| Superficial FAZ circulatory index | 0.67 ± 0.11 | 0.67 ± 0.12 | 0.840 |
| Deep FAZ circulatory index | 0.66 ± 0.09 | 0.66 ± 0.08 | 0.655 |
| Superficial capillary vessel density, % | 24.51 ± 3.49 | 24.19 ± 3.58 | 0.417 |
| Deep capillary vessel density, % | 23.13 ± 4.31 | 24.50 ± 3.56 | 0.372 |
| Choroidal vascularity index, % | 35.11 ± 2.20 | 34.25 ± 2.36 | 0.041 |
Data are expressed as mean ± standard deviation (95% confidence interval).
ABI, ankle-brachial index; BMI, body mass index; CAN, cardiac autonomic neuropathy; CAVI, cardio-ankle vascular index; eGFR, estimated glomerular filtration rate; FAZ, foveal avascular zone; GC-IPL, ganglion cell-inner plexiform layer; logMAR, logarithm of the minimum angle of resolution; RNFL, retinal nerve fiber layer.
Table 3.
Demographic and clinical data according to ankle-brachial index (ABI) value.
| Normal ABI (0.9–1.3) | Abnormally Low or High ABI (ABI <0.9 or ≥1.3) | P value | |
|---|---|---|---|
| Number of eyes | 79 | 34 | — |
| Age, years | 58.29 ± 8.24 | 60.56 ± 10.21 | 0.215 |
| Current smoking (n) | 33 (41.8%) | 18 (52.9%) | 0.278 |
| Years with diabetes | 11.56 ± 7.15 | 13.53 ± 10.36 | 0.317 |
| HbA1c,% | 7.99 ± 2.03 | 8.14 ± 1.18 | 0.690 |
| eGFR, mL/min/1.73 m2 | 83.11 ± 25.14 | 68.56 ± 18.12 | 0.003 |
| Creatinine, mg/dL | 0.88 ± 0.40 | 1.04 ± 0.37 | 0.049 |
| BMI, kg/m2 | 25.18 ± 3.69 | 26.48 ± 3.05 | 0.074 |
| CAN risk index | 65.71 ± 18.69 | 66.10 ± 18.71 | 0.820 |
| CAVI | 8.49 ± 1.14 | 8.43 ± 2.04 | 0.866 |
| ABI | 1.08 ± 0.06 | 1.02 ± 0.16 | — |
| Visual acuity (logMAR) | 0.10 ± 0.12 | 0.10 ± 0.99 | 0.947 |
| GC-IPL thickness, μm | 114.07 ± 14.14 | 114.64 ± 13.62 | 0.844 |
| RNFL thickness, μm | 109.54 ± 16.68 | 108.05 ± 15.43 | 0.655 |
| Central retinal thickness, μm | 234.26 ± 28.33 | 231.62 ± 22.22 | 0.629 |
| Central choroidal thickness, μm | 209.67 ± 71.89 | 233.65 ± 75.00 | 0.111 |
| Superficial FAZ area, mm2 | 0.42 ± 0.19 | 0.44 ± 0.16 | 0.486 |
| Deep FAZ area, mm2 | 0.68 ± 0.34 | 0.72 ± 0.28 | 0.560 |
| Superficial FAZ circulatory index | 0.66 ± 0.12 | 0.69 ± 0.09 | 0.195 |
| Deep FAZ circulatory index | 0.65 ± 0.09 | 0.69 ± 0.10 | 0.059 |
| Superficial capillary vessel density, % | 24.33 ± 3.34 | 24.55 ± 3.99 | 0.769 |
| Deep capillary vessel density, % | 23.38 ± 3.44 | 24.25 ± 4.86 | 0.220 |
| Choroidal vascularity index, % | 34.55 ± 2.45 | 34.20 ± 2.28 | 0.488 |
Data are expressed as mean ± standard deviation (95% confidence interval).
ABI, ankle-brachial index; BMI, body mass index; CAN, cardiac autonomic neuropathy; CAVI, cardio-ankle vascular index; eGFR, estimated glomerular filtration rate; FAZ, foveal avascular zone; GC-IPL, ganglion cell-inner plexiform layer; logMAR, logarithm of the minimum angle of resolution; RNFL, retinal nerve fiber layer.
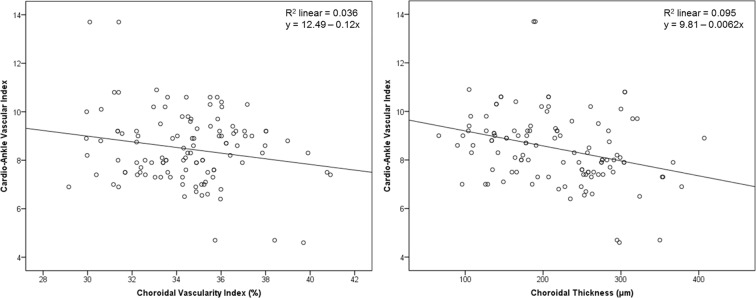
The results of Pearson’s correlation analysis are shown in Table 4 and Fig. 1. In this analysis, we adjusted for possible confounding factors including age, sex, smoking status, duration of diabetes, glycated hemoglobin, mean arterial blood pressure, fasting blood sugar, estimated glomerular filtration rate (eGFR), creatinine, total cholesterol, body mass index (BMI), intraocular pressure, and spherical equivalent. CAVI was negatively correlated with superficial capillary vessel density (r = −0.276, P = 0.045), and CVI (r = −0.247, P = 0.013). ABI exhibited no correlation with any ocular microvascular parameters as per Pearson’s correlation analysis (all P values > 0.05).
Table 4.
Correlation analysis of cardio-ankle vascular index (CAVI) and retinal and choroidal vascular parameters.
| Variables | Unadjusted | Adjusted* | ||
|---|---|---|---|---|
| r | P value | r | P value | |
| GC-IPL thickness, μm | 0.016 | 0.863 | 0.078 | 0.437 |
| RNFL thickness, μm | −0.102 | 0.282 | 0.087 | 0.389 |
| Central retinal thickness, μm | −0.089 | 0.347 | −0.115 | 0.253 |
| Central choroidal thickness, μm | −0.309 | 0.001 | −0.125 | 0.115 |
| Superficial FAZ area, mm2 | 0.084 | 0.376 | 0.201 | 0.054 |
| Deep FAZ area, mm2 | 0.162 | 0.086 | 0.149 | 0.137 |
| Superficial FAZ circulatory index | 0.095 | 0.317 | 0.013 | 0.899 |
| Deep FAZ circulatory index | −0.026 | 0.788 | −0.004 | 0.966 |
| Superficial capillary vessel density, % | −0.238 | 0.011 | −0.276 | 0.045 |
| Deep capillary vessel density, % | −0.070 | 0.459 | −0.054 | 0.593 |
| Choroidal vascularity index, % | −0.191 | 0.043 | −0.247 | 0.013 |
*Adjusted for variables including age, sex, smoking status, duration of diabetes, glycated hemoglobin, mean arterial blood pressure, fasting blood sugar, eGFR, creatinine, total cholesterol, BMI, intraocular pressure, and spherical equivalent.
BMI, body mass index; CAVI, cardio-ankle vascular index; eGFR, estimated glomerular filtration rate; FAZ, foveal avascular zone; GC-IPL, ganglion cell-inner plexiform layer; logMAR, logarithm of the minimum angle of resolution; RNFL, retinal nerve fiber layer.
Figure 1.
Pearson correlation between the cardio-ankle vascular index (CAVI) and the choroidal vascularity index (Left) and the choroidal thickness (Right). In this analysis, we adjusted for possible confounding factors including age, sex, smoking status, duration of diabetes, glycated hemoglobin, mean arterial blood pressure, fasting blood sugar, estimated glomerular filtration rate, creatinine, total cholesterol, body mass index, intraocular pressure, and spherical equivalent. CAVI was negatively correlated with choroidal vascularity index (r = −0.247, P = 0.013). However, CAVI exhibited no significant correlation with choroidal thickness (P = 0.115).
Among the 113 study participants, 6 (5.3%) patients showed asymmetric DR. The degrees of asymmetry were as follows; 3 patients had severe NPDR and PDR, 2 patients had moderate NPDR and severe NPDR, and 1 patient had moderate NPDR and PDR in each of the eyes. Three of these 6 patients were classified as abnormal CAVI group (CAVI ≥ 9). To validate our data, we conducted additional analysis only including 107 participants with symmetric DR; it showed the same results.
Discussion
In this study, we aimed to assess whether systemic arterial stiffness was associated with the condition of the microvasculature of the retina and choroid in type 2 DM. Higher CAVI (which reflects stiffness of the aorta, femoral artery, and tibial artery) was associated with decreased CVI, and this correlation was statistically significant. CAVI could be used as a surrogate marker for DR progression, given that CVI exhibited some linear correlation with the progression of DR severity15. DR remains one of the most important diabetic microvascular complications and is a leading cause of irreversible blindness, highlighting the importance of early detection of progression and close monitoring.
There have been several reports of relationships between systemic arteriosclerosis and ocular diseases, such as branch retinal vein occlusion and age-related macular degeneration19–21. To the best of our knowledge, this is the first study to investigate the association between CAVI and microvascular changes in diabetes. Our findings revealed that systemic arterial stiffness, as measured by CAVI showed correlation ocular microvasculature changes in type 2 DM. The measurement protocol of CAVI is easy and the reproducibility of CAVI is also acceptable as per a previous report22. Furthermore, CAVI has the advantage that it is not affected by blood pressure (BP) at the time of measurement8. CAVI can be used as a physiologic surrogate marker for monitoring systemic arterial stiffness and the risk of DR progression in type 2 DM.
The underlying mechanisms of the association between arterial stiffness and ocular microvasculature changes are unclear. Our hypothesis is that, increased pulsatility due to the arterial stiffening affects the retinal and choroidal microvasculatures23. This increased pressure transmission to the microvessels may be the underlying pathophysiology of the increased risk of DR progression with increased CAVI. Another possible explanation is that, systemic arteriosclerosis and ocular microvasculature changes are both caused by the same underlying pathologic process of DM.
These associations were evident in choroidal vascular parameters rather than retinal vascular parameters. One possible mechanism of the lack of correlation between higher CAVI and disturbed retinal microvasculature may be due to the auto-regulation of retinal vessels. As is widely known, there is no neuronal innervation in the retinal vascular beds, and retinal blood flow is regulated by local mechanisms24. Due to the intrinsic autoregulatory mechanisms, retinal microvessels might be better protected from hypoxic damage compared to the choroidal vessels. Considering autoregulatory disturbances of retinal vessels in diabetic patients25, further studies are required with large-scale data with central retinal artery and vein equivalents, which could provide supportive evidence for our results.
In this study, arteriosclerosis was significantly correlated with ocular microvascular changes in diabetes, but atherosclerosis was not. Studies exploring the link between atherosclerosis, as measured by ABI, and DR have produced mixed results. In some reports, DR was associated with an abnormally low ABI (ABI < 0.9), but not with an abnormally high ABI (ABI ≥ 1.3)26,27. In contrast, in a recent study, abnormally low or high ABI was associated with PDR28. Previous studies evaluated only the grade of severity of DR. In our study population, the ABI did not show a correlation with the ocular microvascular parameters; quantitative analysis of the microvascular parameters in our study might provide stronger supportive evidence for our work.
Subjects with an excessively low ABI (<0.9) may have a severely arteriosclerotic femoral artery22. The data from these individuals should be cautiously analyzed while using CAVI due to the possibility of a false low CAVI score. In our study, 5 subjects with low ABI (<0.9) were included. The ABI of these 5 subjects ranged from 0.76 to 0.87, and the CAVI score ranged from 8.3 to 10.2. There was no statistical difference even after excluding these low ABI subjects (data not shown).
Our study had some limitations. First, systemic arteriosclerosis and DR might share common risk factors and both might be caused by the same underlying pathologic process of DM. This might cause underestimation of the associations between CAVI and ocular microvascular changes. And we did not collect data on the presence of carotid artery disease. Second, due to its retrospective design based on medical records, this study does not show changes of CAVI and ocular microvascular parameters over time, and if unrecognized variables, such as the use of statins or antihypertensive medications within each group, could have affected the results. Therefore, a longitudinal study is needed to confirm the pathophysiological mechanisms linking systemic arterial stiffness and ocular microvascular changes. Third, we did not evaluate the ocular blood flow. The effect of ocular blood flow regulation on DR remains unclear, and retinal capillary vessel density or CVI cannot accurately reflect the ocular blood flow. Fourth, FAZ area and retinal vessel density can be influenced by the spherical equivalent or axial length. We adjusted the spherical equivalent to exclude the confounding effect, but we did not analyze the axial length. Future studies should address these limitations.
In summary, systemic arteriosclerosis had a significant correlation with choroidal vascular changes in DR. Further longitudinal studies are necessary to support these findings and correlate them with structural and functional parameters. We suggest prompt ophthalmic evaluation in patients with systemic arteriosclerosis in DR. If the ophthalmologist notes advanced DR, the patient should be referred to a cardiovascular clinic for a detailed evaluation of systemic arteriosclerosis.
Methods
Study population
The study included patients with a confirmed diagnosis of type 2 DM. We recruited all participants between December 2016 and March 2018 at Seoul St. Mary’s Hospital in Korea and conducted a retrospective chart review. This retrospective study adhered to the tenets of the Declaration of Helsinki. Institutional Review Board (IRB)/Ethics Committee approval was obtained from the Catholic University of Korea, which waived the requirement for obtaining informed patient consent because of the retrospective nature of the study. In patients with symmetric DR, only the right eye was included in the analysis. In patients with asymmetric DR, the data of the worse eye was included.
Exclusion criteria for the study were as follows: (1) age over 75 years, (2) medication for dyslipidemia started prior to CAVI measurement, (3) diagnosis of cardiovascular disease besides DM, (4) presence of other retinal diseases, including glaucoma, age-related macular degeneration, epiretinal membrane, uveitis, or retinal vein occlusion, (5) clinically significant diabetic macular edema, and (6) eyes with a history of laser treatment, intravitreal injection, or intraocular surgery. Previous studies have demonstrated retinal and choroidal thinning even during the normal aging process29,30. To minimize the confounding effect of age-related retinal and choroidal thinning, we excluded patients aged over 75 years.
General examination and laboratory tests
Patients were referred to the specialized endocrinology center, and a thorough evaluation for diabetes was performed. In the clinic, all patients were evaluated standardly for cardiovascular diseases (pulse-wave velocity using vascular screening system), peripheral neuropathy test (sensory nerve conduction threshold evaluation) and autonomic nervous system test. We retrospectively collected these medical records. We recorded the height, body weight, and BMI data. The systolic and diastolic BPs were measured at rest, and the mean arterial pressure was calculated as diastolic BP +1/3 (systolic BP − diastolic BP). Laboratory test data including glycated hemoglobin (HbA1c), fasting blood sugar, eGFR, creatinine, glycoalbumin and lipid profile [total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and triglycerides (TG)] were obtained.
Ophthalmic examination
All subjects underwent ocular examinations including best-corrected visual acuity (BCVA) evaluation (logarithm of the minimum angle of resolution [logMAR] scale), non-contact pneumatic tonometry, slit-lamp biomicroscopy, dilated fundus examination, and optical coherence tomography (OCT). OCT imaging was performed with a swept-source OCT device (DRI Triton, Topcon, Tokyo, Japan).
We defined and graded the severity of the DR according to the modified Early Treatment Diabetic Retinopathy Study (ETDRS) retinopathy severity scale31, and classified patients into 3 groups according to the DR severity as follows: no DR, NPDR, or PDR.
We obtained retinal and choroidal thicknesses using in-built OCT software. We created thickness maps in accordance with the standard ETDRS subfield, consisting of 3 concentric circular areas with 9 independent sectors, and used the central retinal and choroidal thicknesses of the innermost 1-mm circular area for analysis. The automated segmentation software of the OCT device can identify the outer boundary of the retinal nerve fiber layer (RNFL) and the inner plexiform layer (IPL). For every OCT scan, each segmented layer line can be manually adjusted to avoid possible segmentation errors. The difference between the RNFL and the IPL outer boundary segmentation yields the ganglion cell-IPL (GC-IPL) thickness. In this study, we measured the average macular GC-IPL thickness within an annulus with inner and outer diameters of 1-mm and 3-mm, respectively. We then used the average RNFL thickness at the peripapillary area for analysis.
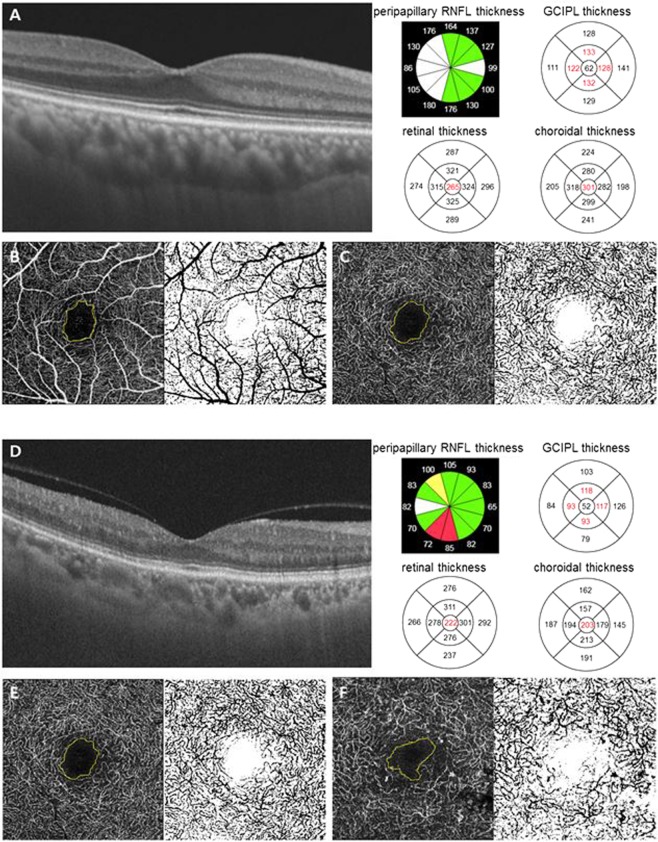
We measured the FAZ area, FAZ circulatory index, and vessel density at the superficial and deep retinal capillary plexuses manually using ImageJ software (version 1.51; National Institutes of Health, Bethesda, MD, USA). The 3 × 3 mm sized en-face OCT angiography images at the superficial and deep capillary plexuses were used for analysis. Representative OCT images are shown in Fig. 2.
Figure 2.
Representative optical coherence tomography (OCT) and OCT angiography images. Images from a patient with mild non-proliferative diabetic retinopathy (A–C) and from a patient with proliferative diabetic retinopathy (D–F). (A,D) We obtained retinal and choroidal thicknesses using in-built OCT software. Average RNFL thickness at peripapillary area, GC-IPL thickness within an annulus with inner and outer diameters of 1-mm and 3-mm, and central retinal and choroidal thicknesses of the innermost 1-mm circular area were used for analysis. (B,C,E,F) Representative 3 × 3 mm sized en face OCT angiography images demonstrate visualization of the superficial retinal capillary plexus and the deep retinal capillary plexus. We measured the FAZ area, FAZ circulatory index, and vessel density at the superficial and deep retinal capillary plexuses manually using ImageJ software. Notes: RNFL, retinal nerve fiber layer; GC-IPL, ganglion cell-inner plexiform layer.
For quantitative analysis of the choroidal vasculature, we calculated the CVI in the total macular area. We used the segmentation protocol described by Agrawal et al.32. CVI, which represents the choroidal vascular component, was defined as the ratio of the vascular luminal area to the total choroidal area.
Measurement of systemic arterial stiffness
We assessed arterial stiffness by measuring CAVI. Measurements of CAVI, ABI, and blood pressure were performed using the program embedded in the vascular screening system (VaSera VS-1000, Fukuda Denshi Co. Ltd, Tokyo, Japan). Electrocardiography (ECG) and phonocardiography (PCG) were performed, pressure and waveforms of the brachial and ankle arteries were evaluated, and brachial-ankle PWV (baPWV) and subsequently CAVI were calculated automatically22,33.
Ocular examination, measurement of CAVI, and the laboratory tests were performed within a 1-month time frame.
Evaluation of cardiac autonomic neuropathy and peripheral neuropathy
We evaluated CAN using Ewing’s traditional five simple tests, namely, changes in the R-R with standing, deep breathing, and the Valsalva maneuver as well as changes in blood pressure in response to sustained handgrip and standing up34. Spectral analysis of heart rate variability (HRV) was performed, and the standard deviation of all normal R-R intervals (SDNN) and the root-mean square of the difference of successive R-R intervals (rMSSD) were evaluated. CAN risk index was assessed with a CAN analyzer (DiCAN, Medicore, Seoul, Korea). The CAN risk index value was classified as follows: normal <38.4; borderline 38.4–53.8; and abnormal >53.8.
We evaluated peripheral neuropathy by measuring the current perception threshold (CPT) using a neurometer (Neurotron Inc., Baltimore, MD, USA). The standard method of CPT testing has been described previously35. The neurometer generates a constant alternating current stimulus, which is applied to two different test sites, the median + ulnar nerves on the ring finger (C7, C8 level), and the deep and superficial peroneal nerves on toe 1 (L4, L5 level). We performed the data analysis using the Neuval® software provided with the device, and used the mean CPT grade for analysis.
Statistical analysis
We expressed categorical data as an absolute number, and continuous data as mean ± standard deviation (95% confidence interval). We performed statistical analysis using the Statistical Package for the Social Sciences for Windows version 23.0 (SPSS Inc., Chicago, IL, USA). The normality of data distribution was confirmed via the Kolmogorov–Smirnov test. Demographic and clinical data of patients were compared by one-way analysis of variance (ANOVA), chi-square, and Fisher’s exact tests. In cases of significant results (P < 0.05), the ANOVA was followed by a post-hoc Bonferroni correction. We used the linear-by-linear association test to examine the linear correlation between the advanced DR stage and abnormal ABI. We used Pearson’s correlation test to investigate the associations between CAVI and retinal and choroidal vascular parameters. Statistical significance was assumed if P < 0.05.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (2016R1A6A1A03010528).
Author Contributions
Mirinae Kim (M.K.), Rae-Young Kim (R.Y.K.), Joo-Young Kim (J.Y.K.), Young-Hoon Park (Y.H.P.). Drafting/revising the manuscript for content, including medical writing for content: M.K., R.Y.K., J.Y.K., Y.H.P. Study concept or design: M.K., Y.H.P. Analysis or interpretation of data: M.K., J.Y.K., Y.H.P. Acquisition of data: M.K., R.Y.K. Statistical analysis: M.K., J.Y.K. Study supervision or coordination: M.K., Y.H.P.
Availability of Data and Materials
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Boutouyrie P, et al. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension (Dallas. Tex.: 1979) 2002;39:10–15. doi: 10.1161/hy0102.099031. [DOI] [PubMed] [Google Scholar]
- 2.Mattace-Raso FU, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–663. doi: 10.1161/circulationaha.105.555235. [DOI] [PubMed] [Google Scholar]
- 3.Meaume S, Benetos A, Henry OF, Rudnichi A, Safar ME. Aortic pulse wave velocity predicts cardiovascular mortality in subjects >70 years of age. Arteriosclerosis, thrombosis, and vascular biology. 2001;21:2046–2050. doi: 10.1161/hq1201.100226. [DOI] [PubMed] [Google Scholar]
- 4.Willum-Hansen T, et al. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–670. doi: 10.1161/circulationaha.105.579342. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell GF, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. doi: 10.1161/circulationaha.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benetos A, et al. Influence of age, risk factors, and cardiovascular and renal disease on arterial stiffness: clinical applications. American journal of hypertension. 2002;15:1101–1108. doi: 10.1016/S0895-7061(02)03029-7. [DOI] [PubMed] [Google Scholar]
- 7.Roman MJ, et al. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension (Dallas, Tex.: 1979) 2007;50:197–203. doi: 10.1161/hypertensionaha.107.089078. [DOI] [PubMed] [Google Scholar]
- 8.Shirai K, et al. Cardio-ankle vascular index (CAVI) as a novel indicator of arterial stiffness: theory, evidence and perspectives. Journal of atherosclerosis and thrombosis. 2011;18:924–938. doi: 10.5551/jat.7716. [DOI] [PubMed] [Google Scholar]
- 9.Shirai K, et al. Contradictory effects of beta1- and alpha1- aderenergic receptor blockers on cardio-ankle vascular stiffness index (CAVI)–CAVI independent of blood pressure. Journal of atherosclerosis and thrombosis. 2011;18:49–55. doi: 10.5551/jat.3582. [DOI] [PubMed] [Google Scholar]
- 10.van Popele NM, et al. Association between arterial stiffness and atherosclerosis: the Rotterdam Study. Stroke. 2001;32:454–460. doi: 10.1161/01.STR.32.2.454. [DOI] [PubMed] [Google Scholar]
- 11.Megnien JL, et al. Aortic stiffening does not predict coronary and extracoronary atherosclerosis in asymptomatic men at risk for cardiovascular disease. American journal of hypertension. 1998;11:293–301. doi: 10.1016/S0895-7061(97)00477-9. [DOI] [PubMed] [Google Scholar]
- 12.Bhanushali D, et al. Linking Retinal Microvasculature Features With Severity of Diabetic Retinopathy Using Optical Coherence Tomography Angiography. Investigative ophthalmology & visual science. 2016;57:Oct519–525. doi: 10.1167/iovs.15-18901. [DOI] [PubMed] [Google Scholar]
- 13.Kim, K., Kim, E. S. & Yu, S. Y. Optical coherence tomography angiography analysis of foveal microvascular changes and inner retinal layer thinning in patients with diabetes. The British journal of ophthalmology, 10.1136/bjophthalmol-2017-311149 (2017). [DOI] [PubMed]
- 14.Ting DSW, et al. Optical Coherence Tomographic Angiography in Type 2 Diabetes and Diabetic Retinopathy. JAMA ophthalmology. 2017;135:306–312. doi: 10.1001/jamaophthalmol.2016.5877. [DOI] [PubMed] [Google Scholar]
- 15.Kim M, Ha MJ, Choi SY, Park YH. Choroidal vascularity index in type-2 diabetes analyzed by swept-source optical coherence tomography. Scientific reports. 2018;8:70. doi: 10.1038/s41598-017-18511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim LS, et al. Relationship of systemic endothelial function and peripheral arterial stiffness with diabetic retinopathy. British Journal of Ophthalmology. 2015;99:837–841. doi: 10.1136/bjophthalmol-2014-306075. [DOI] [PubMed] [Google Scholar]
- 17.Siasos G, et al. Role of endothelial dysfunction and arterial stiffness in the development of diabetic retinopathy. Diabetes care. 2015;38:e9–e10. doi: 10.2337/dc14-1741. [DOI] [PubMed] [Google Scholar]
- 18.Kim WJ, et al. The association between regional arterial stiffness and diabetic retinopathy in type 2 diabetes. Atherosclerosis. 2012;225:237–241. doi: 10.1016/j.atherosclerosis.2012.08.034. [DOI] [PubMed] [Google Scholar]
- 19.Kaderli AA, Kaderli B, Gullulu S, Avci R. Impaired aortic stiffness and pulse wave velocity in patients with branch retinal vein occlusion. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2010;248:369–374. doi: 10.1007/s00417-009-1271-7. [DOI] [PubMed] [Google Scholar]
- 20.Taniguchi H, Shiba T, Maeno T, Takahashi M. Evaluation of Carotid Atherosclerosis, Peripheral Arterial Disease, and Chronic Kidney Disease in Patients with Exudative Age-Related Macular Degeneration without Coronary Artery Disease or Stroke. Ophthalmologica. Journal international d’ophtalmologie. International journal of ophthalmology. Zeitschrift fur Augenheilkunde. 2015;233:128–133. doi: 10.1159/000371716. [DOI] [PubMed] [Google Scholar]
- 21.Wong TY, et al. Cardiovascular risk factors for retinal vein occlusion and arteriolar emboli: the Atherosclerosis Risk in Communities & Cardiovascular Health studies. Ophthalmology. 2005;112:540–547. doi: 10.1016/j.ophtha.2004.10.039. [DOI] [PubMed] [Google Scholar]
- 22.Shirai K, Utino J, Otsuka K, Takata M. A novel blood pressure-independent arterial wall stiffness parameter; cardio-ankle vascular index (CAVI) Journal of atherosclerosis and thrombosis. 2006;13:101–107. doi: 10.5551/jat.13.101. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. Journal of applied physiology (Bethesda, Md.: 1985) 2008;105:1652–1660. doi: 10.1152/japplphysiol.90549.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo X, Shen YM, Jiang MN, Lou XF, Shen Y. Ocular Blood Flow Autoregulation Mechanisms and Methods. Journal of ophthalmology. 2015;2015:864871. doi: 10.1155/2015/864871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Hanno T, Bertelsen G, Sjolie AK, Mathiesen EB. Retinal vascular calibres are significantly associated with cardiovascular risk factors: the Tromso Eye Study. Acta ophthalmologica. 2014;92:40–46. doi: 10.1111/aos.12102. [DOI] [PubMed] [Google Scholar]
- 26.Li X, Wang YZ, Yang XP, Xu ZR. Prevalence of and risk factors for abnormal ankle-brachial index in patients with type 2 diabetes. Journal of diabetes. 2012;4:140–146. doi: 10.1111/j.1753-0407.2011.00171.x. [DOI] [PubMed] [Google Scholar]
- 27.Yu JH, et al. The prevalence of peripheral arterial disease in korean patients with type 2 diabetes mellitus attending a university hospital. Diabetes & metabolism journal. 2011;35:543–550. doi: 10.4093/dmj.2011.35.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen SC, et al. Abnormally Low or High Ankle-Brachial Index Is Associated with Proliferative Diabetic Retinopathy in Type 2 Diabetic Mellitus Patients. PloS one. 2015;10:e0134718. doi: 10.1371/journal.pone.0134718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruiz-Medrano J, et al. Analysis of age-related choroidal layers thinning in healthy eyes using swept-source optical coherence tomography. Retina (Philadelphia, Pa.) 2017;37:1305–1313. doi: 10.1097/iae.0000000000001347. [DOI] [PubMed] [Google Scholar]
- 30.Alasil T, et al. Analysis of normal retinal nerve fiber layer thickness by age, sex, and race using spectral domain optical coherence tomography. Journal of glaucoma. 2013;22:532–541. doi: 10.1097/IJG.0b013e318255bb4a. [DOI] [PubMed] [Google Scholar]
- 31.Grading diabetic retinopathy from stereoscopic color fundus photographs–an extension of the modified Airlie House classification ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98:786–806. doi: 10.1016/S0161-6420(13)38012-9. [DOI] [PubMed] [Google Scholar]
- 32.Agrawal R, et al. Choroidal Vascularity Index in Central Serous Chorioretinopathy. Retina (Philadelphia, Pa.) 2016;36:1646–1651. doi: 10.1097/iae.0000000000001040. [DOI] [PubMed] [Google Scholar]
- 33.Kim KJ, et al. Associations between cardio-ankle vascular index and microvascular complications in type 2 diabetes mellitus patients. Journal of atherosclerosis and thrombosis. 2011;18:328–336. doi: 10.5551/jat.5983. [DOI] [PubMed] [Google Scholar]
- 34.Boulton AJ, et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes care. 2005;28:956–962. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- 35.Matsutomo R, Takebayashi K, Aso Y. Assessment of peripheral neuropathy using measurement of the current perception threshold with the neurometer in patients with type 2 diabetes mellitus. The Journal of international medical research. 2005;33:442–453. doi: 10.1177/147323000503300410. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.