Abstract
Direct and indirect roles of vitamin B6 in leaf acclimation to supplementary UV-B radiation are shown in vitamin B6 deficient Arabidopsis thaliana mutant rsr4-1 and C24 wild type. Responses to 4 days of 3.9 kJ m−2 d−1 biologically effective UV-B dose were compared in terms of leaf photochemistry, vitamer content, and antioxidant enzyme activities; complemented with a comprehensive study of vitamer ROS scavenging capacities. Under UV-B, rsr4-1 leaves lost more (34%) photochemical yield than C24 plants (24%). In the absence of UV-B, rsr4-1 leaves contained markedly less pyridoxal-5′-phosphate (PLP) than C24 ones, but levels increased up to the C24 contents in response to UV-B. Activities of class-III ascorbate and glutathione peroxidases increased in C24 leaves upon the UV-B treatment but not in the rsr4-1 mutant. SOD activities remained the same in C24 but decreased by more than 50% in rsr4-1 under UV-B. Although PLP was shown to be an excellent antioxidant in vitro, our results suggest that the UV-B protective role of B6 vitamers is realized indirectly, via supporting peroxidase defence rather than by direct ROS scavenging. We hypothesize that the two defence pathways are linked through the PLP-dependent biosynthesis of cystein and heme, affecting peroxidases.
Introduction
Vitamin B6 (pyridoxine, PN) and its vitamer derivatives pyridoxal (PL), pyridoxamine (PM) and its phosphorylated analogues) have dual roles in plants. They are important for both development1–4 and stress tolerance1,3,5–9. The role in development is likely to be due to the fact that the pyridoxine vitamer pyridoxal 5′-phosphate (PLP) is a crucial co-factor of a range of enzymes important for biosynthesis of building blocks of biological macromolecules10. On the other hand, one vital mechanism behind the stress tolerance conferred by the pyridoxine vitamers is their ability to function as quenchers of reactive oxygen species7,11–15.
Vitamin B6, primarily PLP, is in plants, fungi and most eubacteria synthesized from ribose 5-phosphate, glyceraldehyde 3-phosphate and glutamine by a large 24 polypeptide multisubunit complex (as revealed from studies of the Bacillus subtilis enzyme) that consists of 12 units of the PDX1 synthase protein and 12 units of the PDX2 glutaminase protein16. In plants, there is one gene encoding PDX2, the mutation of which is lethal, and three PDX1 genes (PDX1.1-PDX1.3). PDX1.1 and PDX1.3 are enzymatically functional proteins. PDX1.2 on the other hand is not catalytically active. Instead, this protein has a regulatory role on vitamer biosynthesis through interaction with the multisubunit enzyme, primarily during stress8,9. Environmental factors that have been shown to cause stress in A. thaliana plants that have been mutated in genes of one of the PDX1 subunits include salt and osmotic stress1,5, high light and photo-oxidative stress3,15, heat9,14, and ultraviolet-B light (UV-B, 280–315 nm)6,7.
UV-B is part of the radiation spectrum of the sun that plants are exposed to and dependent on. UV-B is generally a morphological factor under normal conditions17–19 but can be a stressor in extreme environments or unusual circumstances20,21, or when plants are exposed to multiple stresses at the same time22. UV-B is generally sensed by plants through the UV RESISTANCE LOCUS 8 (UVR8) photoreceptor and its downstream signaling components19 which in turn regulate over 100 genes. The gene encoding the PDX1.3 protein is one of these and is up-regulated by UV-B23–25. Also, UV-B exposure leads to increased levels of PDX1 protein in Arabidopsis6,26,27 and increased levels of vitamin B66. In fact, one of the more non-specific modes of action of UV-B on plants is the formation of ROS28 and the UV-B-induced increase in vitamin B6 content in plants most likely is a result of increased oxidative pressure. This oxidative pressure was shown to increase in an A. thaliana pdx1.3 mutant7 that still had a functional PDX1.1 gene.
In order to further elucidate the roles of the different pyridoxine vitamers in planta, C24 wild type and rsr4-1 mutant A. thaliana were used to draw conclusions about the roles that ROS and the pyridoxine vitamers play during UV-B exposure. Created from C24 using the mutagenic alkylating agent ethyl-methanesulfonate, a glabrous, inbred segregate mutant of A. thaliana, was shown to have a non-functional PDX1.3 protein with respect to vitamin B6 biosynthesis due to a point mutation in one of the PDX1 genes2.
Several publications confirm high ROS reactivities of B6 vitamers. In computational studies Matxain et al.12–14 showed that the basic vitamer pyridoxine (PN) could function as a substrate for attack by a number of different ROS (•OH, hydroxyl radical; •OOH, perhydroxyl radical; and 1O2, singlet oxygen) and thereby as antioxidant for these chemical species. The 1O2 reactivity of PN was shown experimentally by Bilski et al.29, who also found pyridoxamine (PM) and pyridoxal (PL) better 1O2 quenchers than PN or PLP in D2O (deuterium oxide). Although theoretical studies predicted that PN was not reactive toward the superoxide anion radical (O2•−;13), Denslow et al.30 presented experimental evidence for the superoxide neutralizing capacity of PN in vitro, and also showed the reactivity of PL, and to a smaller extent of pyridoxamine PM, to this ROS. In order to present a comprehensive picture of their potential antioxidant functions in planta, we compared reactivities of PN, PL, PM and PLP to all four major ROS (O2•−, H2O2, •OH and 1O2) in vitro.
Based on the UV sensitivity of the pdx1.3 mutant7, we hypothesized that rsr4-1 plants (i) will either be more sensitive to supplementary UV-B doses than the C24 wild type, (ii) or compensate for presumably less efficient non-enzymatic ROS scavenging by higher antioxidant enzyme activities. Our results supported the first scenario and suggested an indirect effect by B6 vitamers in successful stress acclimation via enzymatic defence.
Results and Discussion
Photosynthetic responses of A. thaliana leaves to supplemental UV-B
In the absence of UV treatment, C24 and rsr4-1 leaves displayed similar photochemical efficiencies. At 5 weeks of age, mutant leaves showed no visible signs of previously described leaf yellowing2 (data not shown), and are rather in line with reports on normal development of vitamin B6 deficient plants5,15. Accordingly, we observed no difference between maximum PSII efficiencies of the two genotypes assessed as Fv/Fm (maximum PSII quantum yield). Moreover, the rsr4-1 mutant displayed even slightly higher light acclimated PSII photochemical yield (ϕPSII) than the wild type C24 when tested using 55 μmol m−2 s−1 blue actinic light. This results in 8–9% lower regulated and non-regulated non-photochemical quenching yields, Y(NPQ) and Y(NO), respectively, in rsr4-1 than in C24 leaves (Table 1). Higher non-photochemical quenching was reported for another vitamin B6 deficient mutant pdx1 and was explained by a more efficient photoconversion of violaxanthin to zeaxanthin in the mutant than in the corresponding wild type15, although the authors made this observation at high light conditions only, i.e. at 5–20-times higher photosynthetically active radiation (PAR) than was applied in our experiment. The opposite finding in our experiment, i.e. a lower non-photochemical quenching yields in the rsr4-1 mutant at lower PAR, suggests that the mutant does not sense and respond to the lower PAR as a potential photo-oxidative stress.
Table 1.
Effects of supplemental UV radiation on maximum (Fv/Fm) and 55 μmol m−2 s−1 PAR acclimated effective (ϕPSII) quantum yields, the regulated (Y(NPQ)) and non-regulated (Y(NO)) non-photochemical quenching of PSII (Materials and Methods). Data are presented as means ± SD.
| Fv/Fm | ϕPSII | Y(NPQ) | Y(NO) | |
|---|---|---|---|---|
| C24 C | 0.852 ± 0.005 | 0.655 ± 0.015 | 0.123 ± 0.005 | 0.221 ± 0.019 |
| C24 UV | 0.659 ± 0.054* | 0.499 ± 0.051* | 0.123 ± 0.006 | 0.378 ± 0.051* |
| rsr4-1 C | 0.853 ± 0.003 | 0.658 ± 0.014# | 0.114 ± 0.005# | 0.201 ± 0.011# |
| rsr4-1 UV | 0.574 ± 0.050*# | 0.437 ± 0.050*# | 0.124 ± 0.007* | 0.440 ± 0.047*# |
*UV effect: significant difference between control (C) and UV-exposed (UV) leaves (p < 0.05, n = 8) of the same genotype.
#Genotype effect: significant difference between wild type (C24) and B6 mutant (rsr4-1) control leaves (p < 0.05, n = 8) under the same irradiation conditions.
Supplemental UV imposed a mild stress in both genotypes, as indicated by lower maximum (Fv/Fm) and light acclimated (ϕPSII) photochemical yields than in controls (Table 1). The UV-induced loss was higher (ca 32%) in the rsr4-1 mutant than in the C24 wild type (ca 22%). Yields of photochemical energy conversion in PSII were lowered either entirely (C24 plants) or mostly (rsr4-1 plants) at the expense of increasing energy dissipation via non-regulated non-photochemical quenching Y(NO), signifying suboptimal capacities of photoprotective reactions, that may lead to photodamage31. The UV treatment had no effect on the quantum yield of regulated non-photochemical quenching Y(NPQ) in C24 leaves but resulted in a 9% increase in the rsr4-1 mutant. The latter change brought up Y(NPQ) in UV-treated rsr4-1 to values similar to those in C24. Such an increase indicates elevation of defence against photo-oxidative stress at the PSII level in the mutant that is not occurring in the wild type. This might be explained by the above-mentioned xanthophyll-cycle related changes observed in the pdx1 mutant under high light stress15. However, this pathway is probably inefficient in the rsr4-1, as indicated by the relatively small extent of its increase compared to that of Y(NO). Although the biochemical explanation of non-regulated non-photochemical pathways is still incomplete, it is generally agreed that some of the multiple pathways behind Y(NO), especially those driving this parameter above 0.2–0.25 in light acclimated leaves, reflect the inability of a plant to protect itself against damage caused by excess illumination31,32, presumably via increased ROS production. In the following, we examined how the antioxidant systems of C24 and rsr4-1 leaves met this challenge.
Vitamin B6 content of A. thaliana leaves, and in vitro ROS neutralizing potential of these compounds
The three basic vitamers (PL, PM, PN) and the activated form of vitamin B6 (PLP) were quantified in C24 wild type and rsr4-1 mutant leaves using HPLC. PN contents were low in both genotypes, representing only 1–2% of the total, and showing that PN is the least common B6 vitamer in both genotypes (Table 2). In C24 leaves, the amounts of PL and PLP were the highest, while PLP concentration in the mutant were below the one in the wild type (Table 2). Our data are in agreement with those of Wagner et al.2 that established a PL > PM > PN order of the amounts of these compounds in both genotypes. They also found a higher PL/PM ratio in C24 than in rsr4-1 leaves, although their absolute concentrations were different from ours, most likely due to differences in growth conditions and extraction procedures. Our observations do not agree with Havaux et al.15 who found high PN and PM, and low PL concentrations. However, these authors analysed chloroplasts only (as opposed to whole leaf extracts in our case) and used a different Arabidopsis wild type, Col-0. The UV treatment applied led to elevated concentrations of all vitamers in mutant leaves but did not affect the B6 content in the wild type. The UV treatment brought the PLP levels in rsr4-1 leaves up to those in C24 under UV and increased the PL and PM contents of the mutant to 30% higher than those in the wild type (Table 2).
Table 2.
B6 vitamer profiles of A. thaliana leaves. Means ± SD are expressed as ng vitamer mg−1 leaf FW.
| pyridoxal (PL) | pyridoxamine (PM) | pyridoxine (PN) | pyridoxal 5′-phosphate (PLP) | |
|---|---|---|---|---|
| C24 C | 1.048 ± 0.132 | 0.694 ± 0.060 | 0.064 ± 0.037 | 1.357 ± 0.277 |
| C24 UV | 1.160 ± 0.177 | 0.690 ± 0.138 | 0.052 ± 0.015 | 1.184 ± 0.251 |
| rsr4-1 C | 1.117 ± 0.147 | 0.704 ± 0.070 | 0.030 ± 0.008 | 0.797 ± 0.207# |
| rsr4-1 UV | 1.530 ± 0.141*# | 0.895 ± 0.103*# | 0.062 ± 0.009* | 1.088 ± 0.164* |
*UV effect: significant difference between control (C) and UV-exposed (UV) leaves (p < 0.05, n = 8) of the same genotype.
#Genotype effect: significant difference between wild type (C24) and B6 mutant (rsr4-1) control leaves (p < 0.05, n = 8) under the same irradiation conditions.
In vitro analyses of vitamer reactivities toward ROS provide an excellent tool to study their antioxidant potential. However, the realization of these studies in planta depends on a number of factors, primarily on vitamer localization. Several studies of vitamin B6 quenching of ROS have previously been performed12–14,29,30,33 but these investigations either were limited to one or two ROS only or involved fewer forms of vitamin B6 than the present work. Thus, to our best knowledge, the results in Table 3 provide the first comprehensive data set of antioxidant capacities of four B6 vitamers (the three basic forms PL, PM, PN, and the activated form PLP) against the four principal ROS: singlet oxygen (1O2), superoxide anion (O2•−), hydrogen peroxide (H2O2) and hydroxyl radical (•OH). The only previous study including the antioxidant capacities of the same four vitamers established the following series of total (physical and chemical) 1O2 quenching rates in D2O: PN < PLP < PL = PM, with a 35% difference between the lowest and the highest efficiency (Bilski et al., 2000). Our data confirm the strong reactivity of PM to 1O2 but we found no detectable reactivity for PLP for this particular ROS, and much lower reactivities for PN and PL than for PM. These discrepancies may be due to differences in the solvents and experimental conditions used in the two different studies. In support of the theoretical result of Matxain et al.13, PN showed one or two orders of magnitude lower reactivity to O2•− than to the other ROS in the present study. Furthermore, our comprehensive examination showed that PN is an inefficient O2•− scavenger (Table 3).
Table 3.
ROS-specific neutralizing capacities of B6 vitamers.
| anti-1O2 | anti-O2•− | anti-H2O2 | anti-•OH | |
|---|---|---|---|---|
| pyridoxal (PL) | 69.81 ± 6.84 | 14.47 ± 5.30 | 25.04 ± 2.78 | 42.71 ± 0.54 |
| pyridoxamine (PM) | 459.7 ± 52.19 | 0.5 ± 0.09 | 39.69 ± 7.20 | 16.21 ± 0.58 |
| pyridoxine (PN) | 5.69 ± 0.06 | 0.47 ± 0.06 | 48.17 ± 10.09 | 8.29 ± 0.65 |
| pyridoxal 5′-phosphate (PLP) | ND | 1.04 ± 0.02 | 287.90 ± 64.81 | 66.71 ± 4.45 |
Singlet oxygen (1O2), superoxide anion (O2•−), hydrogen peroxide (H2O2) and hydroxyl radical (•OH) antioxidant abilities were expressed as µM vitamer/µM Trolox equivalents. ND, non-detectable. Data represent means ± SD (n = 6–8).
Based on a comparison of leaf B6 vitamer profiles (Table 2) and in vitro vitamer ROS reactivities (Table 3), the observed UV-induced increase in PM, PL and PLP contents in rsr4-1 leaves may support defence against H2O2, •OH, and 1O2. Singlet oxygen in leaves is mainly produced in photodynamic energy transfer from excited PSII chlorophylls to oxygen34, and defence against this ROS is realized non-enzymatically only. There was no significant change in the highly 1O2-reactive PM content in C24 plants in response to UV, which is in line with the earlier observation that UV-B had little effect on energy transfer derived 1O2 concentrations in spinach leaves35. In contrast, the higher PM content in rsr4-1 leaves under PAR plus UV-B than under PAR only suggests a response to photo-oxidative stress and argues against the role of UV-inducible high Y(NPQ) (Table 1) in preventing 1O2 production. Interestingly, the amount of PLP, the most capable vitamer of providing H2O2 and •OH neutralization (Table 3) did not increase in the C24 wild type upon UV-exposure (Table 2), although the defence against these two ROS was found pivotal in the UV acclimation of tobacco leaves36,37.
Leaf antioxidant responses to supplemental UV-B
In the following, we compare the activities of enzymes neutralizing electron transfer derived ROS (H2O2 and O2•−) in leaves of wild type and mutant plants and discuss their UV-B-induced changes. Because •OH neutralization is only supported non-enzymatically, this was also included in the analysis.
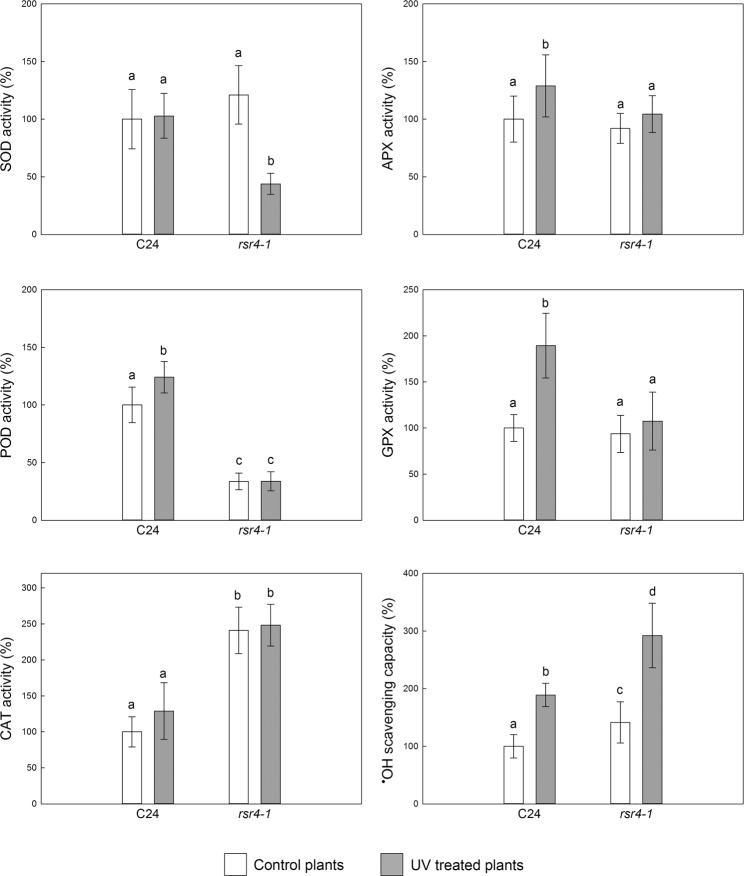
Under growth light conditions, in the absence of UV, the rsr4-1 mutant controlled cellular H2O2 concentrations by significantly higher catalase (CAT, EC 1.11.1.6) activity, while keeping class III peroxidase (POD, EC 1.11.1.7) activity lower than the wild type C24 plants. There were no significant differences between the two genotypes in superoxide dismutase (SOD, EC 1.15.1.1), ascorbate peroxidase (APX, EC 1.11.1.11), or glutathione peroxidase (GPX, EC 1.11.1.9) activities (Fig. 1). In response to the UV treatment, POD, APX and GPX activities increased in wild type C24 leaves whereas SOD and CAT remained unchanged. Unchanged SOD and increased peroxidase activities under UV are in line with our observation of higher activation of APX and POD than of SOD in tobacco leaves36,37. This strategy keeps cellular H2O2 concentrations low in order to decrease the risk of oxidative damage38, potentially aggravated by the UV-B photo-conversion of H2O2 to •OH7. The increased •OH scavenging capacity in C24 leaves under UV-B (Fig. 1), that was also found in tobacco leaves36,37, may serve as a second line of defence. In contrast, rsr4-1 leaves were unable to up-regulate any peroxidase (GPX, APX or POD) activities upon the UV treatment. The observed strong decrease in SOD activity of UV-treated rsr4-1 plants lowers the production of H2O2 via O2•− dismutation and highlights the lack of efficient direct H2O2 scavenging in the mutant.
Figure 1.
UV-B-induced alteration of antioxidant enzyme activities and hydroxyl radical scavenging capacities of A. thaliana leaves. ‘C24’, wild type A. thaliana; rsr4-1, A. thaliana mutant reduced in vitamin B6 synthesis. Control plants were exposed to PAR only and UV-treated plants were exposed to PAR and supplemental UV radiation. White bars represent data from plants kept under PAR only and grey bars correspond to data measured in UV-treated plants. Data are expressed as % of control C24 averages. Bar lengths correspond to means and error bars represent standard deviations (n = 8). For each antioxidant, the four means were compared pair wise and significantly (p < 0.05) different means are marked with different letters. 100% SOD = 37.17 ± 9.2 U mg−1 protein, 100% APX = 6.09 ± 1.19 U mg−1 protein, 100% CAT = 5.41 ± 1.14 U mg−1 protein, 100%, GPX = 80.3 ± 11.6 U g−1 protein, 100% POD = 231.79 ± 35.9 U mg−1 protein, 100% hydroxyl radical (•OH) scavenging = 17.45 ± 3.53 µM Trolox equivalent g−1 fresh leaf weight.
The lack of change in CAT activity in UV-irradiated C24 plants (Fig. 1) suggests that peroxisomal H2O2 production is either low or well regulated already and does not initiate oxidative stress. Although UV irradiation of wheat under different experimental conditions upregulated CAT39,40, we found no significant change in CAT activity in UV experiments with tobacco leaves either (Czégény et al., unpublished). Our results may be explained by assuming (i) a low photorespiratory activity in wild type plants grown under relatively low PAR in our growth chambers, and/or (ii) catalase reactions being unaffected by the applied UV treatment. Either hypothesis is in line with the lack of CAT UV response in the rsr4-1 mutant (Fig. 1).
A weak line of enzymatic H2O2 neutralization explains the need for more efficient •OH scavenging in the vitamin B6 mutant. Accordingly, non-treated rsr4-1 plants had 40% more efficient •OH neutralizing capacity than the wild type and both genotypes were capable of upregulating this activity under UV. Although B6 vitamers, especially PLP, are efficient •OH antioxidants in vitro (Table 3), differences in leaf •OH antioxidant capacity do not match the vitamin B6 content, and thus a strong contribution of other antioxidants has to be assumed. Potential other •OH antioxidants include α-tocopherol, phenolic compounds, ascorbate and reduced glutathione (GSH)41–44, each shown to increase under mild UV-B exposure45. Among the above candidates, both the plant phenol chlorogenic acid and α-tocopherol were found to be more reactive to •OH than ascorbate or GSH43. Chlorogenic acid is synthesized in A. thaliana46 and, similarly to other phenolic compounds, its synthesis is regulated by the UVR8 photoreceptor47.
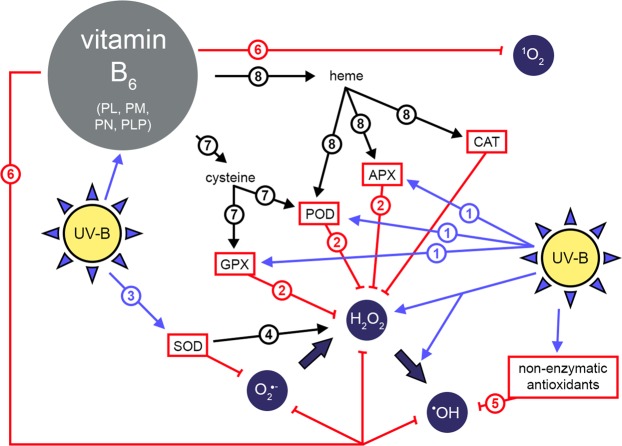
Figure 2 is a schematic representation of a hypothesis on possible roles of B6 vitamers in UV-B responses. Experiments with the C24 wild type in this work, just as our earlier studies using tobacco plants36,37, showed that supplemental UV-B enhanced the peroxidase defence (route-1 in Fig. 2), decreasing cellular H2O2 concentrations (route-2). Depending on the applied UV dose, SOD activities may also increase (route-3), although generally to a smaller extent than peroxidases38 in order to avoid excessive H2O2 production (route-4). Hydroxyl radical concentrations are kept low by the non-enzymatic antioxidants discussed above, including GSH (route-5). The B6 vitamers, PLP and PL, are also reactive toward a variety of ROS (Table 3, route-6 in Fig. 2).
Figure 2.
A schematic representation of pathways discussed in the present work. UV-B inducible and antioxidative routes are shown in blue and red, respectively. Routes are discussed in the text according to the encircled identifying numbers.
Being a coenzyme, PLP takes part in several biosynthetic pathways, for example in cysteine biosynthesis. The PLP dependence of this pathway is in the conversion of O-acetylserine into cysteine48. Since both POD and GPX contain cysteine residues in the active site49,50, a nearly 50% lower PLP content in the rsr4-1 mutant (Table 2) may contribute to the limited UV-B inducibility of these peroxidases (Fig. 1, route-7 in Fig. 2). Heme biosynthesis is also PLP dependent51 and thus the observed lower PLP content in the rsr4-1 mutant may affect heme peroxidases, too (route-8 in Fig. 2). We hypothesise that the limited availability of both heme and cysteine in the rsr4-1 mutant contributes to the low base level of POD and the lack of UV-B response of this enzyme. Although leaf PLP contents increased upon UV-B treatment in the rsr4-1 mutant, additional levels do not seem to cover the multiplicity of pathways that are involved in acclimative responses to UV-B. Lower availability of cysteine implies lower GSH levels in vitamin B6 deficient mutants than in the wild type and may also limit the availability of phytochelatin in rsr4-1 leaves. The latter increases the probability of H2O2 → •OH conversion catalyzed by free iron52, a condition towards which the rsr4-1 mutant responds with higher •OH scavenging capacity than the wild type even in the absence of UV-B (Fig. 1). Elements of the above hypothesis, such as routes-7 and -8 are yet to be proven experimentally.
In summary, a comprehensive study of antioxidant responses to supplemental UV-B radiation in the rsr4-1 mutant and the C24 wild type point to a multiple role of B6 vitamers in UV-B tolerance. Moreover, our results suggest a link between the UV-B sensitivity of vitamin B6 deficient plants that we identified in the pdx1.3 mutant6,7 and the pivotal role of efficient H2O2 neutralizing in acclimation to UV-B37,38.
Methods
Plant growth and UV treatment
A. thaliana plants (C24 wild type and rsr4-1 mutant) were grown using 90 μmol m−2 s−1 photosynthetically active radiation (PAR) in growth chambers with constant 70% relative humidity and 6 h/18 h, 22 °C/18 °C day/night conditions. 5-week old plants were divided into two groups, each containing eight plants from each genotype. The first group (UV plants) was exposed to supplemental UV radiation from Q-Panel UVB-313EL tubes (Q-Lab Ltd., Bolton, UK) through a cellulose diacetate filter (Courtaulds Chemicals, Derby, UK) between 10.00 and 14.00 daily for 4 days. The UV spectrum was centred around 318 nm. UV-B irradiation corresponded to 3.9 kJ m−2 d−1 biologically effective dose53. The second group (control plants) were kept under PAR only. Photosynthesis measurements were carried out at the end of the 4-day treatments, and then leaves were frozen in liquid N2 and stored at −80 °C for analytical measurements.
Chlorophyll fluorescence measurements
Photosynthesis was characterized by chlorophyll fluorescence measurements using the MAXI version of Walz Imaging PAM. Maximum quantum yield of photosystem II (Fv/Fm) was measured after 30 min dark adaptation. Light acclimated effective PSII quantum yield (ϕPSII), regulated non-photochemical quenching (Y(NPQ)) and non-regulated non-photochemical quenching (Y(NO)) were determined under 55 μmol m−2 s−1 blue actinic light according to Klughammer & Schreiber31. In this model, the three PSII quantum yields are complementary as ϕPSII + Y(NPQ) + Y(NO) = 1, representing three possible pathways of disposing quanta.
HPLC analysis
Thirty mg of leaves were ground in liquid nitrogen, placed into a plastic tube and extracted with 1.0 ml of 50 mM H3PO4 solution using ultrasonic bath for 15 min. The resulting suspension was centrifuged at 20,660 × g. Supernatants were filtered using a 0.22 μm PTFE syringe filter and analysed by high-performance liquid chromatography (HPLC). HPLC-FLD analysis was performed using a PerkinElmer Series 200 HPLC system consisting of a vacuum degassing unit, quaternary pump, autosampler, column thermostat and a fluorescence detector (FLD). Separations were achieved by using a Phenomenex SynergiTM 4 µm Hydro-RP 80 Å, 250 × 4.6 mm column. Column temperature was maintained at 25 °C. For elution, 50 mM H3PO4 (eluent A) and a 1:1 mixture of 100 mM phosphoric acid and acetonitrile (eluent B) were used at a flow rate of 1 ml min−1. The elution program started at 100% A and after 10 min of isocratic run, eluent B was increased linearly up to 100% in 5 min. Finally, the column was re-equilibrated with 100% A for 15 min. For fluorometric detection, the excitation and emission wavelengths were 290 nm and 395 nm, respectively54. Pure PL, PLP, PM and PN were purchased from Sigma-Aldrich and used as standards. The quantification was based on the measurements of these compounds with known concentrations.
Sample preparation for antioxidant measurements
Leaves were ground in liquid nitrogen and then homogenized in ice cold Na-phosphate buffer (50 mM, pH 7.0) containing 1 mM EDTA. Samples for the ascorbate peroxidase measurements were made separately with the above grinding buffer containing 5 mM ascorbate as well. Leaf extracts were centrifuged (24,400 × g, 30 min, 4 °C) and supernatants were used for antioxidant capacity measurements. Protein contents were determined as described in Bradford’s protocol55.
Antioxidant measurements on leaf samples
Superoxide dismutase (SOD, EC 1.15.1.1) activity measurements were carried out as described earlier36, based on the inhibition of nitroblue tetrazolium (NBT) reduction by xanthine - xanthine-oxidase generated superoxide anions, and results were expressed as U SOD mg−1 protein.
Class III peroxidase (POD, EC 1.11.1.7) activity was measured via the oxidation of ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid))56 that was found to be the most effective general POD substrate for UV-treated samples57. The colour change was detected by Multiskan FC plate reader (Thermo Fischer Scientific, Shanghai, China) at 651 nm and POD activities were given as U POD mg−1 protein.
Ascorbate peroxidase (APX, EC 1.11.1.11) activities were measured according to Nakano & Asada58, following ascorbate oxidation as decrease in absorbance at 295 nm with a spectrophotometer (Shimadzu UV-1800, Shimadzu Corporation, Tokyo, Japan). The reagent solution contained 0.5 mM ascorbic acid, 1 mM H2O2 and 1 mM EDTA in a Na-phosphate buffer (50 mM, pH 7.0) plus leaf samples. Values were corrected for the APX independent, direct oxidation of H2O2. Enzyme activities were described as U APX mg−1 protein.
Catalase (CAT, EC 1.11.1.6) activity was measured as described in Aebi et al.59, by following the decrease in H2O2 concentration as 240 nm absorbance for 70 seconds (measured at every second). The assay contained 18.6 mM H2O2 and 1 mM EDTA in 50 mM Na-phosphate buffer (pH 7.0) and the reaction was started by adding the leaf sample. Activity was expressed as U CAT mg−1 protein.
Glutathione peroxidase (GPX, EC 1.11.1.9) activity was determined by following NADPH oxidation at 340 nm according to Lawrence & Burk60. The reaction mixture contained 1 mM EDTA, 0.2 mM NADPH, 1 mM NaN3, 1 mM reduced glutathione and 1 U mL−1 glutathione reductase in 50 mM potassium phosphate buffer (pH 7.0) and the reaction was started by adding 0.25 mM H2O2. Following this, absorbance at 340 nm was measured (every second) for 4 min. Enzyme activity was determined as U GPX g−1 protein.
Hydroxyl radical (•OH) scavenging capacity was assessed via measuring the inhibition of the oxidation of terephthalic acid (TPA) to fluorescent hydroxyterephthalate (HTPA) by •OH from the Fenton reaction61 in a Hitachi F-7000 spectrofluorimeter (Hitachi High-Technologies, Tokyo, Japan) with excitation at 315 nm and emission at 420 nm. The method is based on the fact that the antioxidant containing leaf samples can delay the •OH-driven formation of HTPA. Hydroxyl radical antioxidant capacities were characterized by the amounts of plant samples needed to decrease HTPA fluorescence by 50%62 and were given as µM Trolox (6-Hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) equivalent g−1 leaf fresh weight.
ROS specific antioxidant capacities of vitamin B6
Singlet oxygen (1O2) scavenging was determined by the ability of B6 vitamers to decrease the oxidation of DPBF (1,3-diphenylisobenzofuran) by 1O263. As the 1O2 source we used methylene blue (MB) that was irradiated with 50 μmol m−2 s−1 red light (600–650 nm) for 1 minute. 1 mL of reaction mixture contained 20 μM MB and 100 μM DPBF in 60:40 v/v methanol/water. Oxidation of DPBF caused a decrease in absorbance which was followed at 410 nm using a Shimadzu UV-1800 spectrophotometer (Shimadzu Corporation, Kyoto, Japan). 1O2 scavenging abilities of B6 vitamers were assessed based on their ability to lessen the decrease of 410 nm absorbance and were presented as μM Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) equivalents.
Superoxide anion (O2•−) antioxidant ability of vitamin B6 was characterized via a slightly modified assay described by Majer et al.36. B6 vitamers can inhibit the superoxide-induced reduction of NBT (nitro blue tetrazolium) to formazan. The reaction mixture contained 0.3 mM xanthine, 0.3 mM EDTA in 50 mM K-phosphate buffer (pH 7.2) and the reaction was started by adding 0.015 U xanthine oxidase. Formazan production was measured as absorbance change at 540 nm in a plate reader. Results were expressed as μM Trolox equivalents.
Hydrogen peroxide (H2O2) neutralization were measured using 3–3′-diaminobenzidine (DAB)64. 280 μM DAB and 400 μM H2O2 were dissolved in 50 mM Na-phosphate buffer (pH 7.0) and the reaction was started by adding 100 μU horseradish peroxidase and DAB oxidation was measured at 500 nm in a spectrophotometer. The H2O2 scavenging ability of B6 vitamers were defined as μM Trolox equivalents.
Hydroxyl radical (•OH) scavenging capacities were measured using a modification of the TBARS (thiobarbituric acid reactive substances) assay65. Such an indirect approach was necessary, because all B6 vitamers fluoresce upon UV excitation preventing the use of the more direct, terephthalate based method61 that was applied for characterizing leaf extracts. The assay contained 0.1 mM FeSO4, 1 mM EDTA, 0.25 mM ascorbate, 1 mM H2O2 and 3 mM deoxyribose in 20 mM potassium-phosphate buffer (pH 7.4). Hydroxyl radicals generated in a Fenton reaction oxidize deoxyribose yielding products that form a pink chromogen upon incubation with 0.1 w/v % thiobarbituric acid at 40 °C for 30 min in an 8% TCA solution. Added B6 vitamers competed with deoxyribose for the hydroxyl radicals and thus decreased chromogen formation that was followed as 540 nm absorbance using a plate reader. Results were expressed as μM Trolox equivalents.
Statistics
All treatment groups contained 8 plants of each genotype as biological replicates that were assayed separately to form one (n = 8) data set. ROS reactivity measurements of pure vitamers, where no leaf material was used, were carried out with 6–8 technical repetitions to calculate one mean value. For each variable, differences between means were compared with two-sample Student’s t-tests and significantly different (p < 0.05) means are marked with either different letters (in graphs) or different symbols (in tables).
Acknowledgements
The authors thank Dr. Hanjo Hellmann for the kind gift of rsr4-1 seeds. Gy. Cz. acknowledges the support of the ÚNKP-17-3-III-PTE-229 New National Excellence Program of the Ministry of Human Capacities. Å.S. was supported by grants from the Knowledge Foundation, the Swedish Research Council FORMAS, and Örebro University’s Faculty for Business, Science and Technology. L.K. was supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences. The project has been supported by the European Union, co-financed by the European Social Fund Grant no.: EFOP-3.6.1.-16-2016-00004 entitled by Comprehensive Development for Implementing Smart Specialization Strategies at the University of Pécs.
Author Contributions
É.H. and Å.S. conceived the research plan with contributions of Gy.Cz.; Gy.Cz. and É.H. designed the experiments; Gy.Cz. performed most of the experiments with the exception of HPLC analysis that was done by L.K.; the article was written with contributions of all the authors.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chen H, Xiong L. Pyridoxine is required for postembryonic root development and tolerance to osmotic and oxidative stresses. Plant Journal. 2005;44:396–408. doi: 10.1111/j.1365-313X.2005.02538.x. [DOI] [PubMed] [Google Scholar]
- 2.Wagner S, et al. Analysis of the Arabidopsis rsr4-1/pdx1-3 mutant reveals critical function of the PDX1 protein family in metabolism, development, and Vitamin B6 biosynthesis. Plant Cell. 2006;18:1722–1735. doi: 10.1105/tpc.105.036269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raschke M, et al. Enhanced levels of vitamin B6 increase aerial organ size and positively affect stress tolerance in Arabidopsis. The Plant Journal. 2011;66:414–432. doi: 10.1111/j.1365-313X.2011.04499.x. [DOI] [PubMed] [Google Scholar]
- 4.Boycheva S, Dominguez A, Rolcik J, Boller T, Fitzpatrick TB. Consequences of a deficit in vitamin B6 biosynthesis de novo for hormone homeostasis and root development in Arabidopsis. Plant Physiology. 2015;167:102–117. doi: 10.1104/pp.114.247767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Titiz O, et al. PDX1 is essential for vitamin B6 biosynthesis, development and stress tolerance in Arabidopsis. The Plant Journal. 2006;48:933–946. doi: 10.1111/j.1365-313X.2006.02928.x. [DOI] [PubMed] [Google Scholar]
- 6.Ristilä M, Strid H, Eriksson LA, Strid Å, Sävenstrand H. The role of the pyridoxine (vitamin B6) biosynthesis enzyme PDX1 in ultraviolet-B radiation responses in plants. Plant Physiology and Biochemistry. 2011;49:284–292. doi: 10.1016/j.plaphy.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Czégény G, et al. Hydrogen peroxide contributes to the ultraviolet-B (280-315 nm) induced oxidative stress of plant leaves through multiple pathways. FEBS Letters. 2014;588:2255–2261. doi: 10.1016/j.febslet.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Moccand C, et al. The pseudoenzyme PDX1.2 boosts vitamin B6 biosynthesis under heat and oxidative stress in Arabidopsis. Journal of Biological Chemistry. 2014;289:8203–8216. doi: 10.1074/jbc.M113.540526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dell’Aglio E, Boycheva S, Fitzpatrick TB. The pseudoenzyme PDX1.2 sustains vitamin B6 biosynthesis as a function of heat stress. Plant Physiology. 2017;174:2098–2112. doi: 10.1104/pp.17.00531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Percudani R, Peracchi A. A genomic overview of pyridoxal-phosphate-dependent enzymes. EMBO Reports. 2003;4:850–854. doi: 10.1038/sj.embor.embor914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matxain JM, Ristilä M, Strid Å, Eriksson LA. Theoretical Study of the Antioxidant Properties of Pyridoxine. The Journal of Physical Chemistry A. 2006;110:13068–13072. doi: 10.1021/jp065115p. [DOI] [PubMed] [Google Scholar]
- 12.Matxain JM, Ristilä M, Strid Å, Eriksson LA. Theoretical study of the reaction of vitamin B6 with 1O2. Chemistry – A European Journal. 2007;13:4636–4642. doi: 10.1002/chem.200700002. [DOI] [PubMed] [Google Scholar]
- 13.Matxain JM, Padro D, Ristilä M, Strid Å, Eriksson LA. Evidence of High •OH Radical Quenching Efficiency by Vitamin B6. The Journal of Physical Chemistry B. 2009;113:9629–9632. doi: 10.1021/jp903023c. [DOI] [PubMed] [Google Scholar]
- 14.Havaux M, et al. Vitamin B6 deficient plants display increased sensitivity to high light and photo-oxidative stress. BMC Plant Biology. 2009;9:130. doi: 10.1186/1471-2229-9-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moccand C, Kaufmann M, Fitzpatrick TB. It takes two to tango: Defining an essential second active site in pyridoxal 5′-phosphate synthase. PLoS One. 2011;6:e16042. doi: 10.1371/journal.pone.0016042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jordan, B. R., Strid, Å. & Wargent, J. J. What role does UV-B play in determining photosynthesis? in: Handbook of Photosynthesis (ed. Pessarakli, M.) 275–286 (3rd edition. CRC Press 2016).
- 17.Hideg, É. & Strid, Å. The effects of UV-B on the biochemistry and metabolism in plants. in UV-B radiation and plant life. Molecular biology to ecology. (ed. Jordan, B. R.) 90–110 (Oxford UK, CABI press 2017).
- 18.Jenkins GI. Photomorphogenic responses to ultraviolet-B light. Plant, Cell & Environment. 2017;40:2544–2557. doi: 10.1111/pce.12934. [DOI] [PubMed] [Google Scholar]
- 19.Kataria S, Guruprasad KN, Ahuja S, Singh B. Enhancement of growth, photosynthetic performance and yield by exclusion of ambient UV components in C3 and C4 plants. Journal of Photochemistry and Photobiology B: Biology. 2013;127:140–152. doi: 10.1016/j.jphotobiol.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 20.Hideg É, Jansen MAK, Strid Å. UV-B, ROS and stress; inseparable companions or loosely linked associates? Trends in Plant Science. 2013;18:107–115. doi: 10.1016/j.tplants.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Brosché M, Schuler MA, Kalbina I, Connor L, Strid Å. Gene regulation by low level UV-B radiation: identification by DNA array analysis. Photochemical and Photobiological Sciences. 2002;1:656–664. doi: 10.1039/B202659G. [DOI] [PubMed] [Google Scholar]
- 22.Kalbina I, Li S, Kalbin G, Björn L-O, Strid Å. Two separate UV-B radiation wavelength regions control expression of different molecular markers in Arabidopsis thaliana. Functional Plant Biology. 2008;35:222–227. doi: 10.1071/FP07197. [DOI] [PubMed] [Google Scholar]
- 23.Favory JJ, et al. Interaction of COP1 and UVR8 regulates UV-B-induced photomorphogenesis and stress acclimation in Arabidopsis. EMBO Journal. 2009;28:591–601. doi: 10.1038/emboj.2009.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morales LO, et al. Multiple roles for the UV RESISTANT LOCUS 8 in regulating gene expression and metabolite accumulation in Arabidopsis under solar UV radiation. Plant Physiology. 2013;161:744–759. doi: 10.1104/pp.112.211375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morales LO, et al. Are solar UV-B- and UV-A-dependent gene expression and metabolite accumulation in Arabidopsis mediated by the stress response regulator RADICAL CELL DEATH1? Plant, Cell & Environment. 2015;38:878–891. doi: 10.1111/pce.12341. [DOI] [PubMed] [Google Scholar]
- 26.Hideg É, Vass I. UV-B induced free radical production in plant leaves and isolated thylakoid membranes. Plant Science. 1996;115:251–260. doi: 10.1016/0168-9452(96)04364-6. [DOI] [Google Scholar]
- 27.Bilski P, Li MY, Ehrenshaft M, Daub ME, Chignell CF. Vitamin B6 (pyridoxine) and its derivatives are efficient singlet oxygen quenchers and potential fungal antioxidants. Photochemistry and Photobiology. 2000;71:129–134. doi: 10.1562/0031-8655(2000)071<0129:SIPVBP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 28.Denslow SA, Walls AA, Daub ME. Regulation of biosynthetic genes and antioxidant properties of vitamin B6 vitamers during plant defense responses. Physiological and Molecular Plant Pathology. 2005;66:244–255. doi: 10.1016/j.pmpp.2005.09.004. [DOI] [Google Scholar]
- 29.Klughammer C, Schreiber U. Complementary PS II quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the Saturation Pulse method. PAM Application Notes. 2008;1:27–35. [Google Scholar]
- 30.Kramer DM, Johnson G, Kiirats O, Edwards GE. New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynthesis Research. 2004;79:209–218. doi: 10.1023/B:PRES.0000015391.99477.0d. [DOI] [PubMed] [Google Scholar]
- 31.Ehrenshaft M, Bilski P, Li MY, Chignell CF, Daub ME. A highly conserved sequence is a novel gene involved in de novo vitamin B6 biosynthesis. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:9374–9378. doi: 10.1073/pnas.96.16.9374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fischer BB, Hideg É, Krieger-Liszkay A. Production, detection and signaling of singlet oxygen in photosynthetic organisms. Antioxidants & Redox Signaling. 2013;18:2145–2162. doi: 10.1089/ars.2012.5124. [DOI] [PubMed] [Google Scholar]
- 33.Barta C, Kálai T, Hideg K, Vass I, Hideg É. Differences in the ROS-generating efficacy of various ultraviolet wavelengths in detached spinach leaves. Functional Plant Biology. 2004;31:23–28. doi: 10.1071/FP03170. [DOI] [PubMed] [Google Scholar]
- 34.Majer P, Czégény G, Sándor G, Dix PJ, Hideg É. Antioxidant defence in UV-irradiated tobacco leaves is centred on hydrogen-peroxide neutralization. Plant Physiology and Biochemistry. 2014;82:239–243. doi: 10.1016/j.plaphy.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 35.Czégény G, et al. Elevated ROS-scavenging enzymes contribute to acclimation to UV-B exposure in transplastomic tobacco plants, reducing the role of plastid peroxidases. Journal of Plant Physiology. 2016;201:95–100. doi: 10.1016/j.jplph.2016.05.026. [DOI] [PubMed] [Google Scholar]
- 36.Czégény G, Mátai A, Hideg É. UV-B effects on leaves – oxidative stress and acclimation in controlled environments. Plant Science. 2016;248:57–63. doi: 10.1016/j.plantsci.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 37.Alexieva V, Sergiev I, Mapelli S, Karanov E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant, Cell & Environment. 2001;24:1337–1344. doi: 10.1046/j.1365-3040.2001.00778.x. [DOI] [Google Scholar]
- 38.Kovács V, et al. UV-B radiation modifies the acclimation processes to drought or cadmium in wheat. Environmental and Experimental Botany. 2014;100:122–131. doi: 10.1016/j.envexpbot.2013.12.019. [DOI] [Google Scholar]
- 39.Larson RA. The antioxidants of higher plants. Phytochemistry. 1988;27:969–978. doi: 10.1016/0031-9422(88)80254-1. [DOI] [Google Scholar]
- 40.Noctor G, Foyer C. Ascorbate and glutathione: Keeping Active Oxygen Under Control. Annual Review of Plant Physiology and Plant Molecular Biology. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- 41.Wang SY, Jiao H. Scavenging capacity of berry crops on superoxide radicals, hydrogen peroxide, hydroxyl radicals, and singlet oxygen. Journal of Agricultural and Food Chemistry. 2000;48:5677–5684. doi: 10.1021/jf000766i. [DOI] [PubMed] [Google Scholar]
- 42.Zang L-Y, Cosma G, Gardner H, Castranova V, Vallyathan V. Effect of chlorogenic acid on hydroxyl radical. Molecular and Cellular Biochemistry. 2003;247:205–210. doi: 10.1023/A:1024103428348. [DOI] [PubMed] [Google Scholar]
- 43.Jansen MAK, Hectors K, O’Brien NM, Guisez Y, Potters G. Plant stress and human health: Do human consumers benefit from UV-B acclimated crops? Plant Science. 2008;175:449–458. doi: 10.1016/j.plantsci.2008.04.010. [DOI] [Google Scholar]
- 44.Schoch G, et al. CYP98A3 from Arabidopsis thaliana is a 3′-hydroxylase of phenolic esters, a missing link in the phenylpropanoid pathway. Journal of Biological Chemistry. 2001;276:36566–36574. doi: 10.1074/jbc.M104047200. [DOI] [PubMed] [Google Scholar]
- 45.Brown BA, et al. A UV-B-specific signaling component orchestrates plant UV protection. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:18225–18230. doi: 10.1073/pnas.0507187102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bonner ER, Cahoon RE, Knapke SM, Jez JM. Molecular Basis of Cysteine Biosynthesis in Plants. Structural and functional analysis of O-acetylserine sulfhydrylase from A. thaliana. The Journal of Biological Chemistry. 2005;280:38803–38813. doi: 10.1074/jbc.M505313200. [DOI] [PubMed] [Google Scholar]
- 47.Hiraga S, Sasaki K, Ito H, Ohashi Y, Matsui HA. Large Family of Class III Plant Peroxidases. Plant and Cell Physiology. 2001;42:462–468. doi: 10.1093/pcp/pce061. [DOI] [PubMed] [Google Scholar]
- 48.Welinder KG, et al. Structural diversity and transcription of class III peroxidases from Arabidopsis thaliana. European Journal of Biochemistry. 2002;269:6063–6081. doi: 10.1046/j.1432-1033.2002.03311.x. [DOI] [PubMed] [Google Scholar]
- 49.Noctor G, et al. Glutathione in plants: an integrated overview. Plant, Cell & Environment. 2012;35:454–484. doi: 10.1111/j.1365-3040.2011.02400.x. [DOI] [PubMed] [Google Scholar]
- 50.Bela K, et al. Plant glutathione peroxidases: Emerging role of the antioxidant enzymes in plant development and stress responses. Journal of Plant Physiology. 2015;176:192–201. doi: 10.1016/j.jplph.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 51.Heinemann IU, Jahn M, Jahn D. The biochemistry of heme biosynthesis. Archives of Biochemistry and Biophysics. 2008;474:238–251. doi: 10.1016/j.abb.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 52.Halliwell, B. & Gutteridge, J. M. C. Free Radicals in Biology and Medicine. (Ed 4. Oxford University Press, Oxford 2007).
- 53.Flint SD, Caldwell MM. A biological spectral weighting function for ozone depletion research with higher plants. Physiologia Plantarum. 2003;117:137–144. doi: 10.1034/j.1399-3054.2003.1170117.x. [DOI] [Google Scholar]
- 54.González E, Danehower D, Daub ME. Vitamer levels, stress response, enzyme activity, and gene regulation of Arabidopsis lines mutant in the pyridoxine/pyridoxamine 5-phosphate oxidase (PDX3) and the pyridoxal kinase (SOS4) genes involved in the Vitamin B6 salvage pathway. Plant Physiology. 2007;145:985–996. doi: 10.1104/pp.107.105189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 56.Childs RE, Bardsley WG. The steady-state kinetics of peroxidase with 2,2′-azinodi-(3-ethyl-benzthiazoline-6-sulphonic acid) as chromogen. Biochemical Journal. 1975;145:93–103. doi: 10.1042/bj1450093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rácz A, Hideg É, Czégény G. Selective responses of class III plant peroxidase isoforms to environmentally relevant UV-B doses. Journal of Plant Physiology. 2018;221:101–106. doi: 10.1016/j.jplph.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 58.Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant and Cell Physiology. 1981;22:867–880. [Google Scholar]
- 59.Aebi H. Catalase in vitro. Methods in Enzymology. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 60.Lawrence RA, Burk RF. Glutathione peroxidase activity in selenium-deficient rat liver. Biochemical and Biophysical Research Communications. 1976;71:952–958. doi: 10.1016/0006-291X(76)90747-6. [DOI] [PubMed] [Google Scholar]
- 61.Šnyrychová I, Hideg É. The first application of terephthalate fluorescence for highly selective detection of hydroxyl radicals in thylakoid membranes. Functional Plant Biology. 2007;34:1105–1111. doi: 10.1071/FP07150. [DOI] [PubMed] [Google Scholar]
- 62.Stoyanova S, Geuns J, Hideg É, Van den Ende W. The food additives inulin and stevioside counteract oxidative stress. International Journal of Food Sciences and Nutrition. 2011;62:207–214. doi: 10.3109/09637486.2010.523416. [DOI] [PubMed] [Google Scholar]
- 63.DeRosa MC, Crutchley RJ. Photosensitized singlet oxygen and its applications. Coordination Chemistry Reviews. 2002;233-234:351–371. doi: 10.1016/S0010-8545(02)00034-6. [DOI] [Google Scholar]
- 64.Thordal-Christensen H, Zhang Z, Wei YD, Collinge DB. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. The Plant Journal. 1997;11:1187–1194. doi: 10.1046/j.1365-313X.1997.11061187.x. [DOI] [Google Scholar]
- 65.Halliwell B, Gutteridge JMC, Aruoma OI. The deoxyribose method: A simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Analytical Biochemistry. 1987;165:215–219. doi: 10.1016/0003-2697(87)90222-3. [DOI] [PubMed] [Google Scholar]