Abstract
1-Aminocyclopropane-1-carboxylate (ACC) deaminase is a plant growth promoting (PGP) trait found in beneficial bacteria including streptomycetes and responsible for stress modulation. The ACC deaminase gene, acdS, of S. venezuelae ATCC 10712 was cloned into an expression plasmid, pIJ86, to generate S. venezuelae/pIJ86-acdS. Expression of acdS and production of ACC deaminase of S. venezuelae/pIJ86-acdS were significantly higher than the unmodified strain. The ACC deaminase-overexpressing mutant and the wild type control were inoculated into Thai jasmine rice (Oryza sativa L. cv. KDML105) under salt stress conditions. S. venezuelae on its own augmented rice growth and significantly increased more tolerance to salinity by reduction of ethylene, reactive oxygen species (ROS) and Na+ contents, while accumulating more proline, total chlorophyll, relative water content (RWC), malondialdehyde (MDA), and K+ than those of uninoculated controls. The overproducer did not alter chlorophyll, RWC, or MDA further–while it did boost more shoot weight and elongation, and significantly regulated salt tolerance of rice by increasing proline and reducing ethylene and Na+ contents further than that of the wild type. This work is the first illustration of the beneficial roles of S. venezuelae to enhance plant fitness endophytically by promotion of growth and salt tolerance of rice.
Introduction
Soil salinity in arid regions is often an important limiting factor for cultivation of agricultural crops such as maize, rice, and sugarcane. Excess of salt affects plant growth by increasing stress factors, such as ethylene production, Na+ accumulation, and reactive oxygen species (ROS) which is detrimental to the plant’s physiology, leading to growth impairment1–3.
Streptomycetes have been recognized recently as plant growth promoting (PGP) bacteria that can protect plants from infectious diseases and enhance plant growth through several PGP-traits, such as siderophore production, plant hormone production, and phosphate solubilization4–6. Furthermore, PGP-bacteria assist plants to grow under severe condition caused by drought, flooding, salinity, and phytopathogens by the action of 1-aminocyclopropane-1-carboxylate (ACC) deaminase7–11. ACC deaminase, encoded by the acdS gene, is responsible for the breakdown of ACC, which is the direct precursor of ethylene in all higher plants, into ammonia and α-ketobutyrate - which bacteria consume as nitrogen and carbon sources12. Overexpression of acdS in endophytic bacteria remarkably improved plant growth and alleviated stresses in plants, when compared to uninoculated plants and those of wild type inoculation. For example, ACC deaminase-overproducing strains of Pseudomonas putida ameliorated flooding stress in tomato13, Sinorhizobium meliloti improved growth and copper tolerance in Medicago lupulina14, and Serratia grimesii enhanced growth and the level of plant protection against seed-borne pathogens in the common bean15.
Streptomyces venezuelae was discovered from soil and, thus far, has been known as a cell factory for the production of diverse natural products including chloramphenicol, watasemycin, and venemycin16–18. Although the genome sequence of S. venezuelae was determined and characterized19, the information was used mainly for investigation of gene clusters involved in antibiotic biosynthesis. The genome sequence has never been inspected for a role of plant-beneficial functions; likewise, S. venezuelae has never been documented as a PGP-endophytic bacterium. Recently, genes contributing to PGP-traits including acdS were not only present in genome sequences of PGP-rhizobacteria (PGPR) but also found in those of non-PGPR20,21.
On this basis, we examined genes related to PGP-function in all genome sequences of members of a genus Streptomyces available in the GenBank database, in particular acdS. Surprisingly, acdS was present in many genomes of non-PGP-endophytic Streptomyces, including S. venezuelae. To address the possible beneficial role of S. venezuelae interacting beneficially with plants and modulating salt stress, S. venezuelae was inoculated into the salt-sensitive Thai jasmine rice KDML105 cultivar. Furthermore, the effects of overexpression of acdS within S. venezuelae towards rice growth and salt tolerance were investigated. The physiology of rice associated with S. venezuelae and its overexpressed mutant under salt stress condition are discussed.
Results
Salt tolerance and PGP-traits of S. venezuelae
Analysis of salt tolerance of S. venezuelae ATCC 10712 revealed that it had tolerated NaCl up to 3% (w/v). During growth in 3% NaCl, proline was accumulated significantly, at 36.66 ± 0.24 µM in cells (Supplementary Table S1). Moreover, S. venezuelae had ACC deaminase activity of 364.21 ± 19.28 nmol α-ketobutyrate mg protein−1 h−1 and produced IAA at 21 ± 1.02 μg mL−1 (Supplementary Table S1).
Characterization of ACC deaminase-overexpressing S. venezuelae
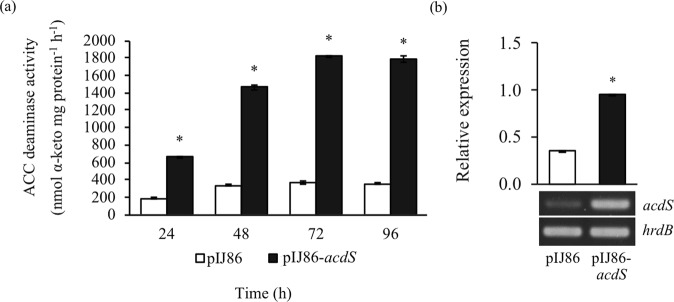
An ACC deaminase-overexpressing mutant, S. venezuelae/pIJ86-acdS, was constructed and verified by resistance to apramycin and thiostrepton. The wild type with empty plasmid, S. venezuelae/pIJ86, was also constructed as a control. In comparison with S. venezuelae/pIJ86, ACC deaminase activity of S. venezuelae/pIJ86-acdS was enhanced 5-fold from 72–96 h of incubation in MM containing 3 mM ACC (Fig. 1a, Supplementary Table S2). This result correlated with the high expression profile of acdS by S. venezuelae/pIJ86-acdS at 72 h (2.7-fold) when compared to that of wild type control (Figs 1b and S1 and Table S3). The ACC deaminase activity of S. venezuelae/pIJ86-acdS were relatively stable when re-streaked for up to 5 generations without antibiotic selection (data not shown).
Figure 1.
ACC deaminase activity (a) and semi-quantitative RT-PCR analysis of expression of acdS (b) of S. venezuelae/pIJ86 (pIJ86) and S. venezuelae/pIJ86-acdS (pIJ86-acdS). The values represent the mean ± S.E. of three replicates and an asterisk (*) indicate statistically significant changes in expression (t test, p < 0.05).
Plant colonization and growth promotion by S. venezuelae
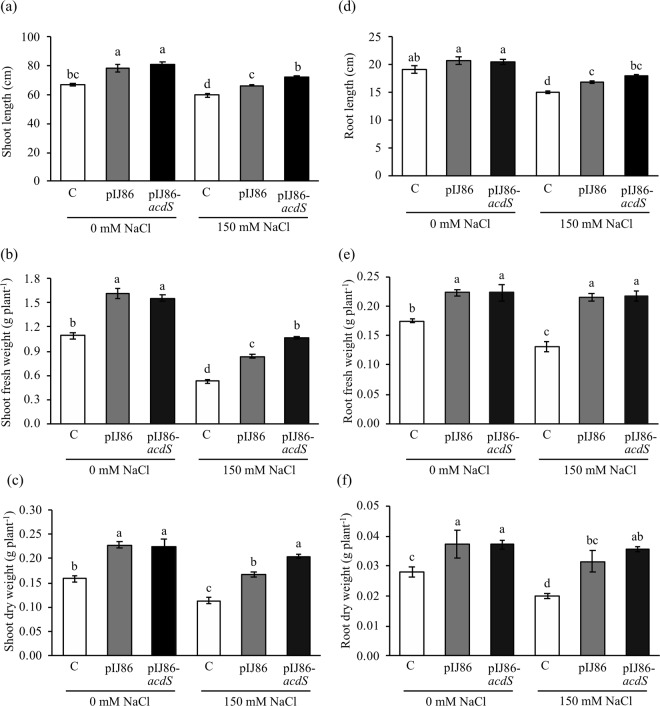
S. venezuelae/pIJ86 was successfully inoculated into Thai jasmine rice cv. KDML105 grown under hydroponic condition with and without salt treatment. S. venezuelae/pIJ86 was re-isolated from rice under both treatments at about 104 CFU g root fresh weight−1 (Supplementary Table S4) indicating that S. venezuelae had the ability to colonize inside plants. In addition, un-inoculated plants did not harbor any streptomycete (data not shown) showing that rice seeds were surface sterilized effectively, and the hydroponic conditions used in this study were free from contamination. Growth parameters of inoculated and uninoculated rice KDML105 were evaluated at 7 days after being treated with and without 150 mM NaCl. In comparison to uninoculated plants, rice associated with S. venezuelae/pIJ86 had significant increases of shoot/root lengths and shoot/root fresh/dry weights in both non-salt and salt treatments (Fig. 2a–f).
Figure 2.
Effect of ACC deaminase-producing Streptomyces venezuelae on shoot length (a), shoot fresh weight (b), shoot dry weight (c), root length (d), root fresh weight (e), and root dry weight (f) of rice plants under non-salt (0 mM NaCl) and salt stress (150 mM NaCl) conditions. The values show the mean ± S.E. of twelve replicates and bars carrying different letters are significantly different (Tukey’s test, p < 0.05). C, uninoculated rice control; pIJ86, rice inoculated with S. venezuelae/pIJ86; pIJ86-acdS, rice inoculated with S. venezuelae/pIJ86-acdS.
Effect of overexpression of ACC deaminase on plant growth parameters
Growth parameters including shoot/root length and shoot/root fresh/dry weights of rice KDML105 inoculated with S. venezuelae/pIJ86 were enhanced significantly, when compared with uninoculated plants in both non-salt and salt treatments (Figs 2a–f and S2). Similar to the original strain, its overexpressing mutant, S. venezuelae/pIJ86-acdS, greatly promoted growth of rice in both non-salt and salt stress conditions (Figs 2a–f and S2), but highly increased shoot length and biomass in particular more than those inoculated with wild type control under salt stress conditions (Fig. 2a–c).
Effect of overexpression of ACC deaminase on plant ethylene
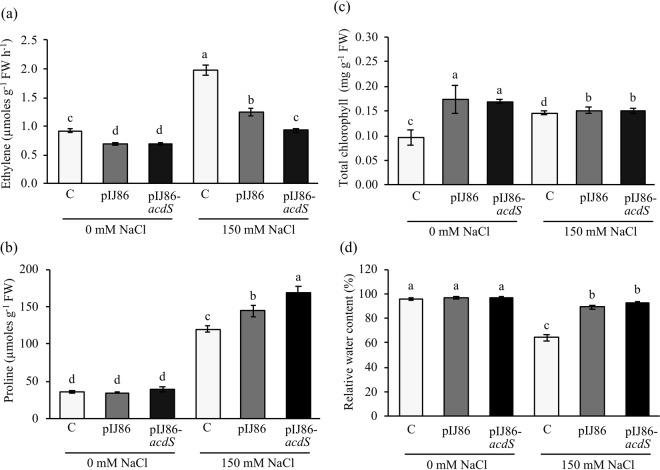
At 7 days after irrigation with 150 mM NaCl, the ethylene level of uninoculated plants was increased about 2-fold when compared with those grown without salt (Fig. 3a, Supplementary Table S4). When rice was associated with S. venezuelae/pIJ86, the ethylene level was reduced 1.6-fold when compared to uninoculated plants (Fig. 3a, Supplementary Table S4). When rice was inoculated with S. venezuelae/pIJ86-acdS, the ethylene level was decreased 2-fold when compared to uninoculated plants (Fig. 3a, Supplementary Table S4). The results indicated that overexpression of ACC deaminase facilitated salt tolerance in plants by reduction of ethylene to the same level as that of the non-salt treatment control.
Figure 3.
Effect of ACC deaminase-producing Streptomyces venezuelae on ethylene (a), proline (b), total chlorophyll (c), and relative water contents (RWC) (d) of rice plants under non-salt (0 mM NaCl) and salt stress (150 mM NaCl) conditions. The values show the mean ± S.E. of twelve replicates and bars carrying different letters are significantly different (Tukey’s test, p < 0.05). C, uninoculated rice control; pIJ86, rice inoculated with S. venezuelae/pIJ86; pIJ86-acdS, rice inoculated with S. venezuelae/pIJ86-acdS.
Effect of overexpression of ACC deaminase on proline content
Under non-salt conditions, proline content was unaffected in rice KDML105 inoculated with either S. venezuelae/pIJ86 or S. venezuelae/pIJ86-acdS (Fig. 3b). Under salt-stress conditions, the proline content was 3.4-fold higher in uninoculated plants compared to those grown in non-salt conditions (Fig. 3b, Supplementary Table S4). Nonetheless, the proline content of plants associated with S. venezuelae/ pIJ86 was increased significantly compared to the uninoculated control and even higher in plants inoculated with S. venezuelae/pIJ86-acdS (Fig. 3b). The results demonstrated that overexpression of ACC deaminase facilitated salt tolerance in rice by escalation of proline content.
Effect of overexpression of ACC deaminase on total chlorophyll and RWC
The total chlorophyll content of rice KDML105 inoculated with either S. venezuelae/pIJ86 or S. venezuelae/pIJ86-acdS (1.8-fold) was augmented significantly when compared to uninoculated plants under non-salt conditions (Fig. 3c, Supplementary Table S4). Under salt stress conditions, rice associated with either the wild-type control or the ACC deaminase-overexpressing mutant maintained a higher chlorophyll content compared to that of uninoculated rice (Fig. 3c). RWC of uninoculated plants under salt treatment was 1.5-fold decreased when compared to untreated controls (Fig. 3d, Supplementary Table S4). Significantly, RWC in rice associated with either S. venezuelae/pIJ86 or S. venezuelae/pIJ86-acdS was 1.4-fold higher when compared to the uninoculated control. The results suggested that S. venezuelae induced salt tolerance in rice by elevation of chlorophyll content and RWC.
Effect of overexpression of ACC deaminase on Na+ and K+ contents
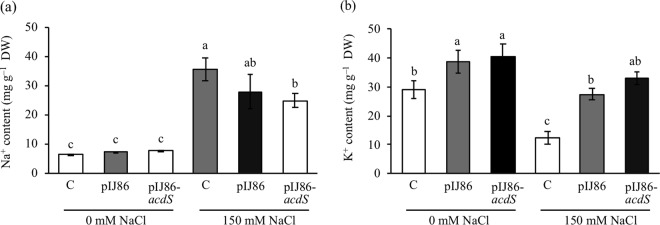
When rice was grown under salt stress conditions, Na+ was accumulated up to 56-fold compared to that of non-salt treatment (Fig. 4a, Supplementary Table S4). Significantly, the Na+ content in rice inoculated with either S. venezuelae/pIJ86 or S. venezuelae/pIJ86-acdS decreased about 1.4-fold when compared to uninoculated rice (Fig. 4a, Supplementary Table S4). On the contrary, the K+ content decreased (2.3-fold) when rice was grown under salt stress conditions (Fig. 4b). However, rice inoculated with either S. venezuelae/pIJ86 or S. venezuelae/pIJ86-acdS had significantly increased K+ content under both non-salt and salt treatments (Fig. 4b, Supplementary Table S4). Markedly, rice inoculated with the ACC deaminase-overexpressing mutant had the highest significant increase in K+ content by 2.6-fold when compared to the uninoculated control under salt stress conditions (Fig. 4b, Supplementary Table S4). The results demonstrated that overexpression of ACC deaminase helped salt tolerance in rice by reduction of Na+ content, and increase in K+ content.
Figure 4.
Effect of ACC deaminase-producing Streptomyces venezuelae on Na+ (a) and K+ (b) contents of rice plants under non-salt (0 mM NaCl) and salt stress (150 mM NaCl) conditions. The values show the mean ± S.E. of twelve replicates and bars carrying different letters are significantly different (Tukey’s test, p < 0.05). C, uninoculated rice control; pIJ86, rice inoculated with S. venezuelae/pIJ86; pIJ86-acdS, rice inoculated with S. venezuelae/pIJ86-acdS.
Effect of overexpression of ACC deaminase on ROS
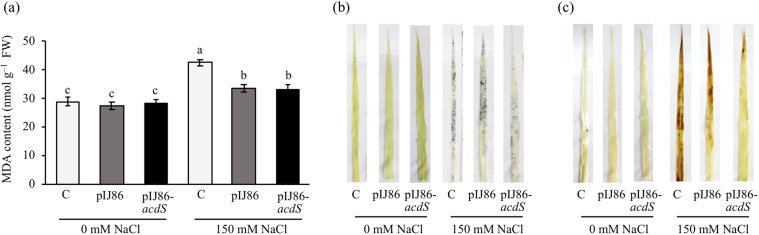
Salt stress drastically induced lipid peroxidation. The MDA content was increased up to 1.5-fold in rice grown under salt stress conditions (Fig. 5a, Supplementary Table S4). However, rice KDML105 inoculated with either S. venezuelae/pIJ86 or S. venezuelae/pIJ86-acdS had a significant reduction in MDA content - about 1.3-fold when compared to the uninoculated control (Fig. 5a, Supplementary Table S4). ROS in leaves were detected by the presence of superoxide and hydrogen peroxide by staining with nitrobluetrazolium (NBT) (Fig. 5b) and 3,3′-diaminobenzidine (DAB) (Fig. 5c), respectively. In the presence of salt, both ROS species were present, shown by the intense staining of leaves; however rice inoculated with S. venezuelae/pIJ86 or S. venezuelae/pIJ86-acdS showed fainter staining than those of the uninoculated control (Fig. 5b,c). The results indicated that S. venezuelae helped salt tolerance in rice by reduction of MDA content and ROS species. However, under salt stress conditions, the overexpression of ACC deaminase did not induce those characteristics more than those of the wild type control.
Figure 5.
Effect of ACC deaminase-producing Streptomyces venezuelae on MDA content (a), histochemical NBT staining (b), and DAB staining (c) of rice plants under non-salt (0 mM NaCl) and salt stress (150 mM NaCl) conditions. The values indicate the mean ± S.E. of twelve replicates and bars carrying different letters are significantly different (Tukey’s test, p < 0.05). C, uninoculated rice control; pIJ86, rice inoculated with S. venezuelae/pIJ86; pIJ86-acdS, rice inoculated with S. venezuelae/pIJ86-acdS.
Discussion
ACC deaminase is a bacterial enzyme found in several PGP-bacteria including Bacillus, Enterobacter, Pseudomonas and Streptomyces. Its improved stress tolerance of plants to drought, flooding, salinity and phytopathogens7–11. Interestingly, increasing the ACC deaminase activity by overexpression of the corresponding gene in PGP-bacteria remarkably facilitated growth and alleviated environmental stresses of host plants more than those of wild type strains13–15.
In this work acdS, encoding ACC deaminase located in the genome of S. venezuelae ATCC 10712, was cloned and expressed in this strain. The overexpressing mutant, S. venezuelae/pIJ86-acdS, had higher ACC deaminase activity, compared to S. venezuelae/pIJ86. The results were in agreement with previous reports that overexpression of ACC deaminase in Mesorhizobium cicero, Serratia grimesii and Sinorhizobium meliloti resulted in higher ACC deaminase activity, compared to the corresponding wild type strains14,15,22. In addition, acdS expression under the ermE promoter of multi-copy plasmid pIJ86 in S. venezuelae without antibiotic selection was maintained up to 5 generations, consistent with the previous report23. Interestingly, S. venezuelae showed endophytic ability in rice plants; which was proven by re-isolation of the bacterium responsible for promotion of rice growth from plant tissues; a trait that is herein shown for the first time for this bacterium. Soil actinomycetes, therefore, potentially act as endophytes, supporting the hypothesis that bacterial communities in the rhizosphere, rhizoplane, and endosphere of rice root microbiomes were overlapping24. In this work, it was demonstrated for the first time that S. venezuelae behaves as a PGP-endophytic bacterium.
Under normal conditions, rice inoculated with S. venezuelae/pIJ86 significantly increased biomass of shoot and root, and elongation. This might be due to an action of IAA produced by this strain that would encourage plant growth and elongation. Moreover, the results were in agreement with previous work showing that ACC deaminase-producing Streptomyces have the ability to enhance growth of Jatropha curcas, mung bean, sugarcane, and rice6,8,10,25. Apart from Streptomyces, IAA and ACC deaminase-producing species from the genera Agromyces, Bacillus, Enterobacter, Methylophaga, Microbacterium, Paenibacillus, and Pseudomonas were also reported to promote growth of canola, rice, sugarcane, and tomato26,27.
Under salt stress conditions, rice plants inoculated with either S. venezuelae/pIJ86 or its overexpressing mutant, S. venezuelae/pIJ86-acdS, showed enhanced growth parameters compared to those of uninoculated controls. However, shoot length and biomass of rice associated with the ACC deaminase-overexpressing mutant were significantly greater than plants inoculated with the unmodified strain. Our results were in congruence with other studies in which ACC deaminase overexpressing strains of Pseudomonas putida and Serratia grimesii promoted growth of tomato and common bean, respectively compared to wild type strains13,15. Therefore, the results unambiguously demonstrated that ACC deaminase-overexpressing S. venezuelae facilitated rice growth better than the original strain under salt stress conditions.
It is generally known that ethylene production is a main response in plants exposed to environmental stress. Salinity induced a high level of ethylene via the actions of ACC synthase and ACC oxidase towards ACC, an ethylene precursor. Whereas, ACC deaminase of bacteria assists plants in responding by conversion of ACC into ammonia and α-ketobutyrate and, thus, reducing ethylene as a consequence28. In this work, the ethylene levels were significantly lower when rice was associated with either S. venezuelae/pIJ86 or S. venezuelae/pIJ86-acdS compared to that of the uninoculated control. Our results were similar to previous reports that ethylene levels in rice and sugarcane were reduced by ACC deaminase-producing Streptomyces sp. GMKU 33610 and Enterobacter sp. EN-219 respectively, under salt stress conditions. The ethylene level was lowest in rice inoculated with the ACC deaminase-overexpressing mutant, correlating with the high ACC deaminase activity of this strain. The results were consistent with another report that overexpression of ACC deaminase in endophytic Pseudomonas spp. enhanced salt tolerance in tomato by reducing ethylene production29. In addition, a lower amount of ACC was observed in tomatoes inoculated with ACC deaminase-overexpressing psychrotolerant bacteria under chilling stress30.
Proline accumulation is one of the adaptation mechanisms of plants under salt stress. At 7 days after irrigation with salt, the proline content of rice associated with S. venezuelae was high and particularly higher in rice inoculated with the ACC deaminase-overexpressing mutant. The results agreed with data on ACC-deaminase producing Dietzia natronolimnaea and Streptomyces sp. GMKU 336, associated with wheat and rice respectively – which induced elevated proline content31,32. Accumulation of higher levels of proline stabilized proteins, cell structures, and osmotic balance33 in rice associated with S. venezuelae/pIJ86-acdS and, thus, accelerated salt tolerance.
Reduction of total chlorophyll and RWC of plants are generally the first notable effects of salt stress such as those reported in black gram and rice10,34,35. In this work, the total chlorophyll and RWC of rice plants were increased significantly in plants under salt treatment, when inoculated with either S. venezuelae/pIJ86 or S. venezuelae/pIJ86-acdS. The results were in congruence with other studies in which ACC deaminase-producing Enterobacter sp. SBP-6 in wheat32, Enterobacter cloacae HSNJ4 in canola36, Bacillus subtilis RJ46, Ochrobactrum pseudogrignonense RJ12, and Pseudomonas sp. RJ15 in black gram and pea37, and bacterial consortia in avocado38 increased chlorophyll level more than those of non-inoculated plants, when under salt stress. Moreover, the results were in agreement with other for the ACC deaminase-overexpressing endophytic Pseudomonas spp., which improved photosynthetic performance and water content in tomato29. The results suggested that S. venezuelae facilitates rice growth in saline environments by increasing total chlorophyll and RWC. However, as the ACC deaminase-overproducing S. venezuelae enhanced chlorophyll content and RWC equally to those of the wild type control, it can be concluded that the overexpression of ACC deaminase did not influence those characters.
Excess accumulation of Na+ and inhibition of K+ uptake under salt stress are very harmful for plant cells, leading to growth impairment3. Several reports have indicated that increasing the K+/Na+ ratio is crucial for salt tolerance in plants39–41. In this work, the Na+ content was significantly enhanced, while the K+ content was decreased drastically in salt-stressed uninoculated rice. On the contrary, rice inoculated with either S. venezuelae/pIJ86 or S. venezuelae/pIJ86-acdS had markedly reduced Na+ content and enhanced K+ content. The results were similar to recent reports that ACC deaminase-producing Dietzia natronolimnaea and Streptomyces sp. GMKU 336 enhanced salt tolerance in plants by increasing the K+/Na+ ratio via up-regulation of the Na+/H+ antiporter gene (NHX1) involved in maintenance of the Na+ level in the cytoplasm10,42. Besides, the increment in K+/Na+ ratio was observed in maize, pea, and sugarcane associated respectively with ACC deaminase-producing Pseudomonas fluorescens, Variovorax paradoxus 5C-2, and Enterobacter sp. EN-21, under salinity stress9,43,44.
ROS production plays a crucial role as signalling molecules involved in stress conditions including attack by pathogens, drought, and salinity which leads to high accumulation of MDA, a product of membrane lipid peroxidation1,2. In this study, rice KDML105 inoculated with either S. venezuelae/pIJ86 or S. venezuelae/pIJ86-acdS had significantly decreased MDA content under salt stress conditions. Moreover, histochemical staining with NBT and DAB indicated that levels of H2O2 and O2− were reduced in the corresponding leaves. The results were in agreement with previous reports that ACC deaminase-producing endophytes caused a reduction in MDA content, including Bacillus subtilis GB03 in white clover31, Enterobacter sp. EN-21 in sugarcane9, Streptomyces sp. GMKU 336 in Thai jasmine rice10, and Dietzia natronolimnaea in wheat42. Moreover, higher accumulation of proline in rice associated with S. venezuelae might help stabilize ROS33 and, thus, alleviate salt stress by modulation of the antioxidant system.
This work is the first demonstration that S. venezuelae carries PGP-traits and promotes growth of rice KDML105 endophytically under normal and salinity conditions. Moreover, the ACC deaminase-overexpressing mutant, S. venezuelae/pIJ86-acdS, enhanced rice growth and salt tolerance more than the original strain. The physiology of the rice benefitted remarkably from the ACC-deaminase trait. Overproduction of ACC deaminase of S. venezuelae is an important model to investigate how excessive ACC deaminase-producing inocula can be effective for crop health improvement under severe conditions.
Methods
Bacterial salt tolerance and plant growth promoting (PGP) traits
Streptomyces venezuelae ATCC 10712 was grown and maintained on mannitol soybean agar (MS)45. Salt tolerance was determined by growth of colonies on ISP 2 (Difco™) supplemented with 1–4% NaCl (w/v) at 28 °C for 7 days.
Proline accumulation was determined by growing S. venezuelae in 10 mL tryptic soy broth (TSB) supplemented with 1–3% NaCl at 28 °C for 3 days. Cells were treated with 2 mL 20% trichloroacetic acid, mixed and centrifuged. The aqueous solution was mixed with 2 mL ninhydrin solution (1.25 g ninhydrin in 30 mL glacial acetic acid and 20 mL 6 M phosphoric acid) and 2 mL glacial acetic acid, and incubated at 95 °C for 1 h, then cooled on ice. The reaction mixture was extracted and mixed vigorously with 4 mL toluene for 15–20 sec. The absorbance of the red-colored organic layer of the ninhydrin-proline complex was measured at 520 nm by spectrophotometry. Proline concentration was determined from a standard curve of commercial proline and calculated as described by Bates, et al.46.
Indole-3-acetic acid (IAA) was determined by a colorimetric method47. S. venezuelae was grown in the dark in glucose-beef extract broth supplemented with 10 mM L-tryptophan at 28 °C for 7 days. The culture was then centrifuged and 2 mL of supernatant was mixed with 1 mL of Salkowski’s reagent48. The mixture was left at room temperature for 30 min in the dark. IAA production was indicated by development of a pink-red color.
ACC deaminase activity was monitored by the amount of α-ketobutyrate generated from ACC cleavage as described by Penrose and Glick49. S. venezuelae was cultured in TSB and washed twice before transferring onto minimal medium (MM) containing 3 mM ACC as a sole source of nitrogen and incubated on a rotary shaker in the dark for 0, 24, 48, 72 and 98 h. The amount of α-ketobutyrate was determined by measuring absorbance at 540 nm and comparing to a standard curve of α-ketobutyrate. Protein content was performed according to Bradford50. ACC deaminase activity was expressed as α-ketobutyrate production in nmol mg−1 protein h−1.
Construction of ACC deaminase-overexpressing mutant
The ACC deaminase gene (acdS) (SVEN_RS07535) was retrieved from the genome sequence of S. venezuelae ATCC 10712 (Accession no. NC_018750). Specific primers for amplification of acdS were designed as ATT151F (5′-TTTTTTAAGCTTGAGATGACGGCGATGGGCGAGTT-3′) and ATT151R (5′-TTTTTTCATATGCCGACCAGCAGCCGTCACTCAAC-3′) including respectively HindIII and NdeI sites (underlined). PCR conditions were initially 98 °C, 30 sec; and 30 cycles of 98 °C, 10 sec; 69 °C, 30 sec; 72 °C, 1 min; and finally at 72 °C, 10 min. The PCR product was then cloned into the pJET cloning vector (Fermentas, USA) and subcloned into constitutive multi-copy expression plasmid pIJ86 under ermE* promoter51 to obtain pIJ86-acdS. Next, pIJ86-acdS was transformed into E. coli ET12567/pUZ800252 and intergeneric conjugation was performed using 24-h mycelium of S. venezuelae as described by Vitayakritsirikul, et al.23. Exconjugants (S. venezuelae/pIJ86-acdS) were selected by apramycin (100 μg mL−1) and thiostrepton (50 μg mL−1) resistance, and verified by (i) PCR amplification of the thiostrepton resistance gene using primers and conditions as described previously by Rungin, et al.5 and (ii) ACC deaminase activity. S. venezuelae/pIJ86 was also constructed as a control.
RNA purification and semi-quantitative RT-PCR
S. venezuelae/pIJ86 and S. venezuelae/pIJ86-acdS were grown in TSB for 24 h, then harvested by centrifugation, washed twice with 0.1 M Tris-HCl (pH 8.5) and inoculated onto MM medium containing 3 mM ACC and incubated for 72 h. Total RNA was isolated using TRIzol (Ambion, USA) and treated with RNase-free DNase I according to the manufacturer’s protocol (Thermo Fisher Scientific, USA). cDNA was synthesized using a RevertAid™ First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, USA). Semi-quantitative RT-PCR analysis of acdS gene was performed using cDNA and primers, ATT165F (5′-CGGGTGATCTGCTCGTGGGTCGGTA-3′) and ATT165R (5′-GCGGGCTTCGGCATCGGCTT-3′), using Phusion Hot Start II-High Fidelity DNA polymerase (Thermo Fisher Scientific, USA). PCR conditions started with 98 °C, 30 sec; and 30 cycles of 98 °C, 10 sec; 58 °C, 30 sec; 72 °C, 1 min; and finally at 72 °C, 10 min. The expression level of acdS was quantified by Gel DocTM XR + with Image LabTM Software (Biorad, USA) and normalized against the expression of a housekeeping gene, hrdB53.
Analysis of rice growth parameters
Thai jasmine rice seeds (Oryza sativa L. cv. KDML105) were surface sterilized by 70% (v/v) ethanol for 1 min followed by 15 min in 5% (w/v) sodium hypochlorite and thoroughly rinsed with sterile distilled water before transferring into a sterile moist chamber and incubated at room temperature in the dark for 7 days. Roots of seedlings were cut into the same length and individually immersed into sterile glass beakers containing 108 spores mL−1 of either S. venezuelae/pIJ86 or S. venezuelae/pIJ86-acdS and incubated for 24 h. Seedlings were then re-located to a moist sponge support for 1 day before transferring to a 20-L container filled with half-Yoshida solution (YS)54 for 14 days. Next, salt stress was introduced by replacing the nutrient solution with YS supplemented with 150 mM NaCl and further incubated for 7 days. The pH of nutrient solution was maintained between 5.0–5.5 throughout the growth period. A positive control of non-salt stressed rice was grown under the same conditions without NaCl treatment. Growth parameters of non-salt and salt-stressed rice plants at 7 days were determined for root and shoot lengths, fresh (FW) and dry (DW) weights.
Analysis of ethylene level and proline accumulation
Ethylene emission was analyzed by the method of Cristescu, et al.55 7-day rice plants were placed in a 550 mL bottle tightly sealed with a rubber septum and left for 1 h. Fifty millilitres of headspace air was sampled and analyzed for ethylene by gas chromatography (GC 7890A, Agilent Technologies, USA) packed with a Poropak-N column at 60 °C, equipped with a flame ionization detector. The amount of ethylene emission was calculated as nmol of ethylene g−1 FW h−1 by comparison to a standard curve generated with pure ethylene.
For proline content, fresh leaf samples (50 mg) were immediately homogenized with liquid nitrogen. The powder was mixed with 3% (v/v) sulfosalicylic acid and centrifuged. The aqueous solution was mixed with ninhydrin solution and glacial acetic acid following the protocol described above.
Analysis of total chlorophyll and relative water content (RWC)
Total chlorophyll was measured according to the method of Porra, et al.56. Fresh leaf samples (50 mg) were immediately homogenized with liquid nitrogen. The powder was dissolved in DMSO and centrifuged at 4 °C for 10 min. Absorbance was measured at 645 and 663 nm by spectrophotometry. Total chlorophyll content was calculated based on chlorophyll equations of Arnon57.
RWC was determined according to the method of Mostofa and Fujita58. Leaf fresh weight was measured and soaked in distilled water for 6 h to determine a turgid weight. The leaves were then dried at 60 °C for 72 h to determine a dry weight. RWC was calculated from each weigh according to Smart and Bingham59.
Determination of Na+ and K+ contents
Na+ and K+ contents were analyzed using an atomic absorption spectrophotometer according to the method of Johnson and Ulrich60 at The Soil-Fertilizer-Environment Scientific Development Project, Department of Soil Science, Faculty of Agriculture, Kasetsart University. The concentrations of Na+ and K+ were quantified and calculated as mg g−1 DW.
Analysis of lipid peroxidation and reactive oxygen species (ROS) staining
Lipid peroxidation of leaf samples was estimated by measuring the amount of malondialdehyde (MDA) by a colorimetric method61. Fresh leaf samples (50 mg) were immediately homogenized with liquid nitrogen and mixed with 80% ethanol followed by centrifugation. The aqueous solution was mixed with either (i) −TBA solution [20% (w/v) trichloroacetic acid and 0.01% butylated hydroxytoluene], or (ii) +TBA solution (0.65% TBA in −TBA solution). Samples were mixed vigorously and heated at 95 °C for 1 h, cooled on ice and centrifuged. The TBA-MDA complex absorbance was measured at 400, 523 and 600 nm by spectrophotometry. The MDA level was calculated as described by Hodges, et al.61.
ROS staining of leaf samples was detected using nitrotetrazolium blue chloride (NBT) and 3,3′-diaminobenzidine (DAB) for superoxide and hydrogen peroxide, respectively following the protocol described by Kumar, et al.62. Leaf samples were separately immersed in 25 mL 2.5 mM NBT staining solution (pH 7.5) and 5 mM DAB staining solution (pH 3.8) for 24 h at room temperature in the dark. The leaves were then decolorized by boiling in 95% (v/v) ethanol for 30 min and further immersed in 60% glycerol for 16 h before color detection.
Statistical analysis
Data were subjected to statistical analysis using standard ANOVA and Tukey’s multiple range tests of SPSS (version 18.0). Data were presented as mean ± S.E. calculated from four plants per treatment in three different replicates, with a different letter indicating statistical significance at p < 0.05. ACC deaminase activity and gene expression ratio data were analysed statistically using a t test at p < 0.05. The values represented the mean ± S.E. of three replicates and an asterisk represents a statistically-significant change in expression.
Supplementary information
Acknowledgements
S.Y. was granted a MSc scholarship by Kasetsart University 72th Year Anniversary Graduate Scholarship, The Graduate School, Kasetsart University. Special thanks to the Rice Department for providing rice seeds; and Dr. Kunlayakorn Prongjunteuak and her staff in the Soil Microbiology Research Group, Department of Agriculture for ethylene analysis. This work was financially supported by Thailand Research Fund (TRF) under grant no. BRG5880004, and Thailand Toray Science Foundation (TTSF).
Author Contributions
S.Y. and A.T. conceived and designed the experiments. S.Y., H.P. performed the experiments. S.Y., W.K., R.J., C.J. and A.T. analyzed the data. S.Y., W.K., R.J. and A.T. wrote the manuscript. All authors have reviewed the manuscript and have given approval to the final version.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-37987-5.
References
- 1.You J, Chan Z. ROS regulation during abiotic stress responses in crop plants. Front. Plant Sci. 2015;6:1092. doi: 10.3389/fpls.2015.01092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bournonville CF, Díaz-Ricci JC. Quantitative determination of superoxide in plant leaves using a modified NBT staining method. Phytochem Anal. 2011;22:268–271. doi: 10.1002/pca.1275. [DOI] [PubMed] [Google Scholar]
- 3.Assaha DVM, Ueda A, Saneoka H, Al-Yahyai R, Yaish MW. The role of Na+ and K+ transporters in salt stress adaptation in glycophytes. Front. Physiol. 2017;8:509. doi: 10.3389/fphys.2017.00509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Misk A, Franco C. Biocontrol of chickpea root rot using endophytic actinobacteria. BioControl. 2011;56:811–822. doi: 10.1007/s10526-011-9352-z. [DOI] [Google Scholar]
- 5.Rungin S, et al. Plant growth enhancing effects by a siderophore-producing endophytic streptomycete isolated from a Thai jasmine rice plant (Oryza sativa L. cv. KDML105) Antonie Van Leeuwenhoek. 2012;102:463–472. doi: 10.1007/s10482-012-9778-z. [DOI] [PubMed] [Google Scholar]
- 6.Kruasuwan W, Thamchaipenet A. Diversity of culturable plant growth-promoting bacterial endophytes associated with sugarcane roots and their effect of growth by co-inoculation of diazotrophs and actinomycetes. J. Plant Growth Regul. 2016;35:1074–1087. doi: 10.1007/s00344-016-9604-3. [DOI] [Google Scholar]
- 7.Saleem AR, et al. Drought response of Mucuna pruriens (L.) DC. inoculated with ACC deaminase and IAA producing rhizobacteria. PLoS One. 2018;13:e0191218. doi: 10.1371/journal.pone.0191218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaemsaeng R, Jantasuriyarat C, Thamchaipenet A. Positive role of 1-aminocyclopropane-1-carboxylate deaminase-producing endophytic Streptomyces sp. GMKU 336 on flooding resistance of mung bean. Agri. Nat. Resour. 2018;52:330–334. doi: 10.1038/s41598-018-19799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kruasuwan W, Thamchaipenet A. 1-aminocyclopropane-1-carboxylate (ACC) deaminase-producing endophytic diazotrophic Enterobacter sp. EN-21 modulates salt–stress response in sugarcane. J. Plant Growth Regul. 2018;37:849–858. doi: 10.1007/s00344-018-9780-4. [DOI] [Google Scholar]
- 10.Jaemsaeng R, Jantasuriyarat C, Thamchaipenet A. Molecular interaction of 1-aminocyclopropane-1-carboxylate deaminase (ACCD)-producing endophytic Streptomyces sp. GMKU 336 towards salt-stress resistance of Oryza sativa L. cv. KDML105. Sci. Rep. 2018;8:1950. doi: 10.1038/s41598-018-19799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toklikishvili N, et al. Inhibitory effect of ACC deaminase-producing bacteria on crown gall formation in tomato plants infected by Agrobacterium tumefaciens or A. vitis. Plant Pathol. 2010;59:1023–1030. doi: 10.1111/j.1365-3059.2010.02326.x. [DOI] [Google Scholar]
- 12.Honma M, Shimomura T. Metabolism of 1-aminocyclopropane-1-carboxylic acid. Agric. Biol. Chem. 1978;42:1825–1831. [Google Scholar]
- 13.Grichko VP, Glick BR. Amelioration of flooding stress by ACC deaminase-containingplant growth-promoting bacteria. Plant Physiol Biochem. 2001;39:11–17. doi: 10.1016/S0981-9428(00)01212-2. [DOI] [Google Scholar]
- 14.Kong Z, et al. Effects of 1-aminocyclopropane-1-carboxylate (ACC) deaminase-overproducing Sinorhizobium meliloti on plant growth and copper tolerance of Medicago lupulina. Plant Soil. 2015;391:383–398. doi: 10.1007/s11104-015-2434-4. [DOI] [Google Scholar]
- 15.Tavares MJ, Nascimento FX, Glick BR, Rossi MJ. The expression of an exogenous ACC deaminase by the endophyte Serratia grimesii BXF1 promotes the early nodulation and growth of common bean. Lett. Appl. Microbiol. 2018;66:252–259. doi: 10.1111/lam.12847. [DOI] [PubMed] [Google Scholar]
- 16.Fernández-Martínez LT, et al. New insights into chloramphenicol biosynthesis in Streptomyces venezuelae ATCC 10712. Antimicrob. Agents Chemother. 2014;58:7441–7450. doi: 10.1128/AAC.04272-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inahashi Y, et al. Watasemycin biosynthesis in Streptomyces venezuelae: thiazoline C-methylation by a type B radical-SAM methylase homologue. Chem. Sci. 2017;8:2823–2831. doi: 10.1039/C6SC03533G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ehrlich J, Gottlieb D, Burkholder PR, Anderson LE, Pridham TG. Streptomyces venezuelae, N. sp., the source of chloromycetin. J. Bacteriol. 1948;56:467–477. doi: 10.1128/jb.56.4.467-477.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pullan ST, Chandra G, Bibb MJ, Merrick M. Genome-wide analysis of the role of GlnR in Streptomyces venezuelae provides new insights into global nitrogen regulation in actinomycetes. BMC Genomics. 2011;12:175. doi: 10.1186/1471-2164-12-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruto M, Prigent-Combaret C, Muller D, Moënne-Loccoz Y. Analysis of genes contributing to plant-beneficial functions in plant growth-promoting rhizobacteria and related Proteobacteria. Sci. Rep. 2014;4:6261. doi: 10.1038/srep06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nascimento FX, Rossi MJ, Soares CRFS, McConkey BJ, Glick BR. New insights into 1-aminocyclopropane-1-carboxylate (ACC) deaminase phylogeny, evolution and ecological significance. PLoS ONE. 2014;9:e99168. doi: 10.1371/journal.pone.0099168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nascimento FX, Brígido C, Glick BR, Oliveira S, Alho L. Mesorhizobium ciceri LMS-1 expressing an exogenous 1-aminocyclopropane-1-carboxylate (ACC) deaminase increases its nodulation abilities and chickpea plant resistance to soil constraints. Lett. Appl. Microbiol. 2012;55:15–21. doi: 10.1111/j.1472-765X.2012.03251.x. [DOI] [PubMed] [Google Scholar]
- 23.Vitayakritsirikul V, et al. Improvement of chloramphenicol production in Streptomyces venezuelae ATCC 10712 by overexpression of the aroB and aroK genes catalysing steps in the shikimate pathway. Antonie Van Leeuwenhoek. 2016;109:379–388. doi: 10.1007/s10482-015-0640-y. [DOI] [PubMed] [Google Scholar]
- 24.Edwards J, et al. Structure, variation, and assembly of the root-associated microbiomes of rice. Proc. Natl. Acad. Sci. USA. 2015;112:E911–E920. doi: 10.1073/pnas.1414592112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qin S, et al. Biodiversity and plant growth promoting traits of culturable endophytic actinobacteria associated with Jatropha curcas L. growing in Panxi dry-hot valley soil. Appl. Soil Ecol. 2015;93:47–55. doi: 10.1016/j.apsoil.2015.04.004. [DOI] [Google Scholar]
- 26.Glick BR, Jacobson CB, Schwarze MMK, Pasternak JJ. 1-Aminocyclopropane-1-carboxylic acid deaminase mutants of the plant growth promoting rhizobacterium Pseudomonas putida GR12-2 do not stimulate canola root elongation. Can. J. Microbiol. 1994;40:911–915. doi: 10.1139/m94-146. [DOI] [Google Scholar]
- 27.Bal HB, Das S, Dangar TK, Adhya TK. ACC deaminase and IAA producing growth promoting bacteria from the rhizosphere soil of tropical rice plants. J. Basic Microbiol. 2013;53:972–984. doi: 10.1002/jobm.201200445. [DOI] [PubMed] [Google Scholar]
- 28.Glick BR. Bacterial ACC deaminase and the alleviation of plant stress. Adv. Appl. Microbiol. 2004;56:291–312. doi: 10.1016/S0065-2164(04)56009-4. [DOI] [PubMed] [Google Scholar]
- 29.Win K, Fukuyo T, Keiki O, Ohwaki Y. The ACC deaminase expressing endophyte Pseudomonas spp. enhances NaCl stress tolerance by reducing stress-related ethylene production, resulting in improved growth, photosynthetic performance, and ionic balance in tomato plants. Plant Physiol. Biochem. 2018;127:599–607. doi: 10.1016/j.plaphy.2018.04.038. [DOI] [PubMed] [Google Scholar]
- 30.Subramanian P, Krishnamoorthy R, Chanratana M, Kim K, Sa T. Expression of an exogenous 1-aminocyclopropane-1-carboxylate deaminase gene in psychrotolerant bacteria modulates ethylene metabolism and cold induced genes in tomato under chilling stress. Plant Physiol. Biochem. 2015;89:18–23. doi: 10.1016/j.plaphy.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 31.Han Q-Q, et al. Beneficial soil bacterium Bacillus subtilis (GB03) augments salt tolerance of white clover. Front. Plant Sci. 2014;5:1–8. doi: 10.3389/fpls.2014.00525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh RP, Jha PN. Mitigation of salt stress in wheat plant (Triticum aestivum) by ACC deaminase bacterium Enterobacter sp. SBP-6 isolated from Sorghum bicolor. Acta Physiol. Plant. 2016;38:110. doi: 10.1007/s11738-016-2123-9. [DOI] [Google Scholar]
- 33.Munns R, Tester M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- 34.Turan S, Tripathy BC. Salt‐stress induced modulation of chlorophyll biosynthesis during de‐etiolation of rice seedlings. Physiol. Plant. 2015;153:477–491. doi: 10.1111/ppl.12250. [DOI] [PubMed] [Google Scholar]
- 35.Win KT, et al. Varietal differences in growth and Cs allocation of blackgram (Vigna mungo) under water stress. Environ. Exp. Bot. 2015;109:244–253. doi: 10.1016/j.envexpbot.2014.06.019. [DOI] [Google Scholar]
- 36.Li H, et al. Enhanced tolerance to salt stress in canola (iL.) seedlings inoculated with the halotolerant Enterobacter cloacae HSNJ4. Appl. Soil Ecol. 2017;119:26–34. doi: 10.1016/j.apsoil.2017.05.033. [DOI] [Google Scholar]
- 37.Saikia J, et al. Alleviation of drought stress in pulse crops with ACC deaminase producing rhizobacteria isolated from acidic soil of Northeast India. Sci. Rep. 2018;8:3560. doi: 10.1038/s41598-018-21921-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barra PJ, Inostroza NG, Mora ML, Crowley DE, Jorquera MA. Bacterial consortia inoculation mitigates the water shortage and salt stress in an avocado (Persea americana Mill.) nursery. Appl Soil Ecol. 2017;111:39–47. doi: 10.1016/j.apsoil.2016.11.012. [DOI] [Google Scholar]
- 39.Shi H, Quintero FJ, Pardo JM, Zhu J-K. The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transport in plants. Plant Cell. 2002;14:465–477. doi: 10.1105/tpc.010371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ren Z-H, et al. A rice quantitative trait locus for salt tolerance encodes a sodium transporter. Nature Genet. 2005;37:1141. doi: 10.1038/ng1643. [DOI] [PubMed] [Google Scholar]
- 41.Sunarpi, et al. Enhanced salt tolerance mediated by AtHKT1 transporter-induced Na+ unloading from xylem vessels to xylem parenchyma cells. Plant J. 2005;44:928–938. doi: 10.1111/j.1365-313X.2005.02595.x. [DOI] [PubMed] [Google Scholar]
- 42.Bharti N, Pandey SS, Barnawal D, Patel VK, Kalra A. Plant growth promoting rhizobacteria Dietzia natronolimnaea modulates the expression of stress responsive genes providing protection of wheat from salinity stress. Sci. Rep. 2016;6:34768. doi: 10.1038/srep34768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nadeem SM, Zahir ZA, Naveed M, Arshad M. Rhizobacteria containing ACC-deaminase confer salt tolerance in maize grown on salt-affected fields. Can. J. Microbiol. 2009;55:1302–1309. doi: 10.1139/W09-092. [DOI] [PubMed] [Google Scholar]
- 44.Wang Q, Dodd IC, Belimov AA, Jiang F. Rhizosphere bacteria containing 1-aminocyclopropane-1-carboxylate deaminase increase growth and photosynthesis of pea plants under salt stress by limiting Na + accumulation. Funct. Plant Biol. 2016;43:161–172. doi: 10.1071/FP15200. [DOI] [PubMed] [Google Scholar]
- 45.Hobbs G, Frazer C, Gardner DJ. J. Cullum & Oliver., S. Dispersed growth of Streptomyces in liquid culture. Appl. Microbiol. Biotechnol. 1989;31:272–277. doi: 10.1007/BF00258408. [DOI] [Google Scholar]
- 46.Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- 47.Bric JM, Bostock RM, Silverstone SE. Rapid In situ assay for indoleacetic acid production by bacteria immobilized on a nitrocellulose membrane. Appl. Environ. Microbiol. 1991;57:535–538. doi: 10.1128/aem.57.2.535-538.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gordon SA, Weber RP. Colorimetric estimation of indoleacetic acid. Plant Physiol. 1991;57:192–195. doi: 10.1104/pp.26.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Penrose DM, Glick BR. Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol. Plant. 2003;118:10–15. doi: 10.1034/j.1399-3054.2003.00086.x. [DOI] [PubMed] [Google Scholar]
- 50.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 51.Healy FG, et al. Characterization of γ-butyrolactone autoregulatory signaling gene homologs in the angucyclinone polyketide WS5995B producer Streptomyces acidiscabies. J. Bacteriol. 2009;191:4786–4797. doi: 10.1128/JB.00437-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.MacNeil DJ, et al. Analysis of Streptomyces avermitilis genes required for avermectin biosynthesis utilizaing a novel intergration vector. Gene. 1992;111:61–68. doi: 10.1016/0378-1119(92)90603-M. [DOI] [PubMed] [Google Scholar]
- 53.Xie P, Sheng Y, Ito T, Mahmud T. Transcriptional regulation and increased production of asukamycin in engineered Streptomyces nodosus subsp. asukaensis strains. Appl. Microbiol. Biotechnol. 2012;96:451–460. doi: 10.1007/s00253-012-4084-2. [DOI] [PubMed] [Google Scholar]
- 54.Yoshida, S., Formo, D. A., Cook, J. H. & Gomez, K. A. Laboratory Manual for Physiological Studies of Rice. (IRRI, 1976).
- 55.Cristescu SM, et al. Current methods for detecting ethylene in plants. Ann. Bot. 2013;111:347–360. doi: 10.1093/aob/mcs259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Porra RJ, Thompson WA, Kriedemann PE. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta. 1989;975:384–394. doi: 10.1016/S0005-2728(89)80347-0. [DOI] [Google Scholar]
- 57.Arnon DI. Copper enzymes in isolated chloroplasts, poluphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mostofa MG, Fujita M. Salicylic acid alleviates copper toxicity in rice (Oryza sativa L.) seedlings by up-regulating antioxidative and glyoxalase systems. Ecotoxicology. 2013;22:959–973. doi: 10.1007/s10646-013-1073-x. [DOI] [PubMed] [Google Scholar]
- 59.Smart RE, Bingham GE. Rapid estimates of relative water content. Plant Physiol. 1974;53:258–260. doi: 10.1104/pp.53.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johnson, C. M. & Ulrich, A. Analytical Methods for Use in Plant Analysis. (University of California, 1959).
- 61.Hodges DM, DeLong JM, Forney CF, Prange RK. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta. 1999;207:604–611. doi: 10.1007/s004250050524. [DOI] [PubMed] [Google Scholar]
- 62.Kumar D, Yusuf MA, Singh P, Sardar M, Sarin NB. Histochemical detection of superoxide and H2O2 accumulation in Brassica juncea seedlings. Bio Protoc. 2014;4:e1108. doi: 10.21769/BioProtoc.1108. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.