Abstract
Affective states underlie daily decision-making and pathological behaviours relevant to obsessive–compulsive disorders (OCD), mood disorders and addictions. Deep brain stimulation targeting the motor and associative-limbic subthalamic nucleus (STN) has been shown to be effective for Parkinson’s disease (PD) and OCD, respectively. Cognitive and electrophysiological studies in PD showed responses of the motor STN to emotional stimuli, impairments in recognition of negative affective states and modulation of the intensity of subjective emotion. Here we studied whether the stimulation of the associative-limbic STN in OCD influences the subjective emotion to low-intensity positive and negative images and how this relates to clinical symptoms. We assessed 10 OCD patients with on and off STN DBS in a double-blind randomized manner by recording ratings of valence and arousal to low- and high-intensity positive and negative emotional images. STN stimulation increased positive ratings and decreased negative ratings to low-intensity positive and negative stimuli, respectively, relative to off stimulation. We also show that the change in severity of obsessive–compulsive symptoms pre- versus post-operatively interacts with both DBS and valence ratings. We show that stimulation of the associative-limbic STN might influence the negative cognitive bias in OCD and decreasing the negative appraisal of emotional stimuli with a possible relationship with clinical outcomes. That the effect is specific to low intensity might suggest a role of uncertainty or conflict related to competing interpretations of image intensity. These findings may have implications for the therapeutic efficacy of DBS.
Introduction
Affective states underlie daily decision-making and pathological behaviours relevant to obsessive–compulsive disorders (OCDs), mood disorders and addictions. Deep brain stimulation (DBS) is effective in Parkinson disease (PD)1 and OCD2 and critically offers the opportunity to investigate the neural underpinnings of cognitive and affective processes. DBS in OCD has shown efficacy in the anterior limb of the internal capsule (ALIC), the nucleus accumbens (NAcc) and ventral capsule/ventral striatum, and the subthalamic nucleus (STN)2–4. Effects on mood and anxiety are the most frequent stimulation-related reported side effects5–7. Thus, understanding the role of DBS on emotional processing may contribute to understanding the circuits underlying human emotional regulation and the mechanism of DBS in OCD.
Depressive reactions have been observed with acute stimulation in PD within different regions of the basal ganglia including the left8 or right substantia nigra9 and globus pallidus internus10. Acute induction of a positive emotional reaction (smile, laughter) intraoperatively during ALIC-NAcc DBS in OCD was suggested to predict DBS outcome11.
The STN is a small nucleus within the indirect pathway and receives significant hyper-direct prefrontal cortex connections highlighting its role as a nexus for integration12,13. The posterior dorsal motor STN is an effective target for PD and the anterior ventral limbic-associative STN1 is effective for OCD2. DBS targeting the motor STN in PD have reported acute positive emotion such as laughter/hilarity14 or euphoric manic behaviour13,15 and acute depressive reactions16,17, anger18, pathological crying19 and pseudo-bulbar crying20. These presumed acute stimulation effects of the STN have been suggested to be related to disinhibition of behaviour5 and may also be dependent upon baseline diagnoses21.
With chronic stimulation, STN DBS may be associated with frequent major depressive episodes (for review, see5) or apathy22, but reduction in dopaminergic pharmacotherapy is an important confounder. However, in contrast to discrete major depressive episodes, STN DBS in PD consistently improves overall depression23 and anxiety scores5. Post-operative hypomania/mania is the most consistently reported post-operative psychiatric stimulation-induced effect reported in PD (4%)6, which may be linked to antero-ventral STN stimulation13 and can be clinically addressed by decreasing voltage, dopaminergic dose or changing stimulation contacts dorsally24. Similarly, in OCD, mood effects of antero-ventral STN DBS have involved hypomania rather than depressive symptoms2. Stimulation duration may also be relevant: acute effects may involve euphoric feelings and improved motivation, which are less likely with chronic STN stimulation in PD16,22,25,26. Thus, the short-term2 and suggested long-term27 clinical benefit of antero-ventral STN DBS in OCD may not be dependent on mood impact although may influence quality of life28.
OCD is characterized by obsessions or repetitive intrusive thoughts and urges leading to compulsions or behaviours, which subjects feel driven to perform. Impairments in the processing of emotional stimuli in OCD with a more negative (or less positive) appraisal of emotional stimuli have been reported29,30, suggested to be related to a generalized negative appraisal bias. Studies on STN stimulation-locked emotional processing have focused on the motor STN in PD (for a review, see5); how DBS alters limbic function in OCD patients targeting the associative-limbic STN remains an open question. Here we sought to assess the role of the antero-ventral associative-limbic STN in limbic processing. We assessed the intensity of valence and arousal ratings in both pleasant and unpleasant imagery and divided the International Affective Picture System (IAPS)31 images into low and high valence intensity presuming that low valence images may have less ceiling effect and hence more sensitive to subjective interpretation and capacity for change. We hypothesized that STN DBS would increase subjective pleasant and decrease subjective unpleasant scores particularly with lower valence images.
Methods
Participants
Twelve OCD subjects were recruited from Grenoble University Hospital, tested On and Off DBS and compared with 24 healthy volunteers (HVs). OCD patients (eight females; mean age: 41.75 ± 7.94 years) had undergone bilateral STN DBS for mean 38.1 ± 18.8 months prior to testing (duration of the stimulation range prior to the study: 5–71 months). Patient characteristics are shown in Table 1. Disease duration before surgery was 18 ± 9.2 years, and Yale Brown Obsessive–Compulsive Scale (YBOCS) score (assessing OCD severity32) before surgery was 34.3 ± 3.2. At the time of the study, YBOCS baseline score was 20 ± 9.1 with a clinical improvement (compared with pre-surgery state) of 41 ± 28%. Patients had at least 5 years of treatment-resistant, severe, disabling OCD before DBS surgery. Several patients had some neuropsychiatric comorbidities (obsessive–compulsive spectra): one subject have comorbid Tourette’s syndrome; another subject have comorbid skin picking and another subject had a premorbid history of an eating disorder that was in remission 20 years before surgery. One patient had hypersomnia. All subjects were right handed and had normal or corrected to normal vision.
Table 1.
Clinical and demographical characteristics of the OCD patients
| Patient number/age (years)/gender (F/M) | Age at surgery (years) | Duration of disease before surgery (years) | Age at onset of OCD (years) | Duration of DBS (months) | YBOCS before surgery | YBOCS Baseline at time of study | Medications at the time of the study |
|---|---|---|---|---|---|---|---|
| 1/46/M | 39 | 18 | 21 | 71 | 37 | 25 | Fluvoxamine 200 mg/day; Lorazepam 4 mg/day |
| 2/49/F | 42 | 25 | 17 | 64 | 30 | 28 | Aripiprazole 30 mg/day; Olanzapine 5 mg/day; Escitalopram 20 mg/day; Clomipramine 75 mg/day |
| 3/39/M | 36 | 17 | 19 | 32 | 32 | 28 | Paroxetine 60 mg/day |
| 4/53/F | 49 | 39 | 10 | 51 | 35 | 29 | Fluoxetine 20 mg/day; Clomipramine 25 mg/day |
| 5/37/M | 34 | 13 | 21 | 22 | 32 | 27 | Clomipramine 150 mg/day; Oxazepam 175 mg/day; Alimemazine 50 mg/day |
| 6/41/F | 38 | 11 | 27 | 35 | 36 | 6 | None |
| 7/43/F | 40 | 15 | 25 | 32 | 36 | 23 | Fluvoxamine 200 mg/day; Hydroxyzine 50 mg/day; Clomipramine 25 mg/day |
| 8/41/F | 37 | 5 | 32 | 44 | 32 | 2 | Venlafaxine 37.5 mg/day; Clotiazepam1.5 mg/day |
| 9/30/M | 27 | 10 | 17 | 25 | 38 | 24 | Sertraline 50 mg/day; Aripiprazole 20 mg/day; Methylphenidate 60 mg/day; Pitolisant 20 mg/day |
| 10/56/F | 52 | 25 | 27 | 51 | 40 | 18 | Zopiclone 7.5 mg/day; Aripiprazole 2.5 mg/day; Hydroxyzine 100 mg/day |
| 11/33/F | 33 | 21 | 12 | 5 | 30 | 11 | Venlafaxine 150 mg/day |
| 12/33/F | 33 | 26 | 7 | 25 | 34 | 19 | Fluoxetine 20 mg/day; Levothyroxine 125 µg/day |
M male, F female, YBOCS Yale Brown Obsessive–Compulsive Scale, DBS deep brain stimulation, OCD obsessive–compulsive disorder
The OCD subjects were implanted bilaterally with two electrodes 3389 connected to a Kinetra stimulator (Medtronic, Minneapolis, Minnesota, USA), accordingly to the STN DBS protocol already published elsewhere2,4. The surgical procedure targeted the antero-ventral non-motor part of the STN. Indirect targeting was defined as 1 mm anterior to the mid-commissural point, 10 mm lateral from the midline and 4 mm below the AC-PC line. The final target was adapted laterally according to the visualization of the medial border of the STN. The antero–posterior coordinates were defined 2 mm anterior to the anterior border of the red nucleus. Stimulation frequency and pulse width were set at 130 Hz and 60 µs, respectively; stimulation voltage and activated contacts were adjusted individually to obtain the best clinical response.
The 24 age-matched (± 5y) and gender-matched HVs (16 females; 42.67 ± 8.34 years old) were recruited from the University and community in Grenoble. Subjects were screened by a psychiatrist with the Structured Clinical Interview (SCID) for DSM IV in order to check the exclusion criteria. Exclusion criteria for HVs were past or present serious psychiatric or medical disorders, as well as any psychotropic medications. The research protocol was approved by the Ethics Committee of Grenoble University Hospital (ancillary study to protocol N° ID RCB: 2012-A00490-43). All participants volunteered to participate in the study and gave written informed consent.
Procedure
DBS has the advantage of reversibility, which enables studying its effects within DBS On/Off paradigms. Thus, the patients performed the tasks with STN-DBS On and with STN-DBS Off, in a randomized double-blind within-subject design over 2 successive days to allow a sufficiently long washout of DBS effects (Table 2). All patients were under STN DBS On when included in the study. Patients were randomized (stratification by gender) to one of two arms: (1) DBS was switched off in the morning of Day 1 and patient tested 4 h later; DBS was kept off overnight and in the morning of Day 2 the DBS was switched on and the patient was tested again 4 h later (after 4 h DBS on). (2) Alternatively, in the other arm, DBS was kept On on Day 1 and patient tested similarly at the same moment of the day, 4 h after the blind control of his DBS device; the DBS was kept on overnight and, in the morning of Day 2, the DBS was switched off and patient tested 4 h later. Both the tester and patient were blinded to the condition of stimulation (the control of the DBS device and the DBS switches were performed by another investigator). The blind was maintained during the study as patients were unable to identify properly the stimulation condition (On/Off). The patients continued their usual medication during the study. HVs were tested once.
Table 2.
Study design for OCD subjects
| Day 1 | Day 2 |
|---|---|
| 9 a.m.: YBOCS (baseline) | 9 a.m.: YBOCS |
| 10 a.m.: DBS device control | 10 a.m.: DBS device control |
| A. On | A. Switch Off |
| B. Switch Off | B. Switch On |
| 2 p.m.: IAPS testing | 2 p.m.: IAPS testing |
YBOCS Yale Brown Obsessive–Compulsive Scale, DBS deep brain stimulation, OCD obsessive–compulsive disorder, IAPS International Affective Picture System
Tasks
Affect task
Subjects were shown 20 visual stimuli out of a dataset from the International Affective Picture System (IAPS31). Subjects were shown neutral (four images) and high pleasant and unpleasant images (four images of each condition) and low pleasant and unpleasant images (four images of each condition). All the images were balanced between emotional categories (human, animals, objects, scenery) and were presented on a computer screen in a pseudorandomized order with no time limitation for responding. The image ceased when the subject responded with an interstimulus interval of 2 s during which a fixation-cross was presented in the screen’s centre. Each image was presented once. Subjects were instructed to look at the picture and rate the degree of pleasantness and unpleasantness (emotional valence) by moving the mouse cursor along a line anchored at 0 (unpleasant emotion) to 5 (neutral) and 10 (pleasant emotion) (visual analogue scale). A similar procedure was conducted for degree of arousal. The arousal referred to the intensity of the emotional activation: from calm (score 0) to very excited (score 10). We focused on the outcome measure of ratings of pleasantness and arousal.
Statistical analysis
The behavioural scores were first assessed for outliers and normality of distribution using Shapiro–Wilkes test. We first compared the effects of On and Off DBS on the neutral stimuli to assess the influence of stimulation on neutral images using a paired t-test prior to focusing on the affective stimuli. We then compared the scores for affective ratings (emotional valence) and arousal ratings separately using a repeated-measures analysis of variance (ANOVA with a within-subject On-Off factor and within-subject Affect factor (Pleasant and Unpleasant). This was separately conducted for the high valence and low valence stimuli. The p < 0.05 was considered significant for the main hypothesis of a DBS effect focusing on low valence stimuli (Bonferroni correction for multiple corrections p < 0.0125). We then used a repeated-measures ANOVA to compare OCD patients On and Off DBS and HV with a within-subject Affect factor (Pleasant and Unpleasant) separately conducted for high and low valence stimuli. We further asked if the effect on valence was related to clinical parameters by including YBOCS change (pre–post) as a covariate in the repeated-measures ANOVA.
Results
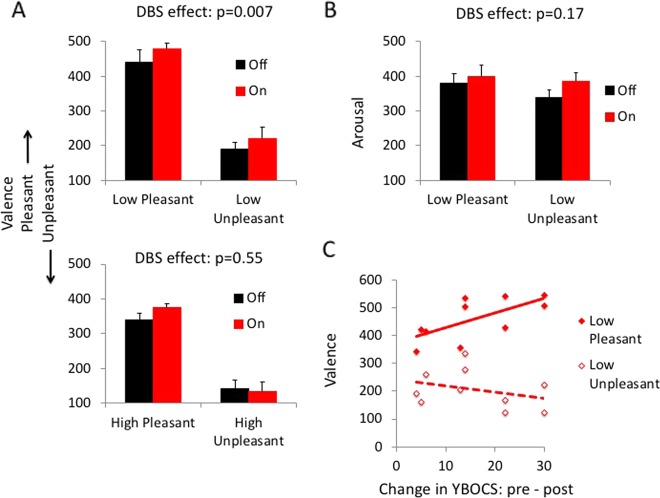
As two subjects did not complete the low valence component of the task due to an examiner error during testing, we restricted our analyses to the 10 OCD subjects who had completed the full task. The data were normally distributed. There were no effects of DBS on neutral stimuli (valence: t = –0.84, df = 9, p = 0.43; arousal: t = 0.14, df = 9, p = 0.89). In the critical within-subjects comparison of low valence stimuli, there was a main effect of valence (F(1, 9) = 63.11, p < 0.0001) and of DBS (F(1, 9) = 11.84, p = 0.007) but no interaction effect (F(1, 9) = 0.04, p = 0.85) (Fig. 1). OCD subjects On DBS had more positive ratings across both positive and negative valences relative to Off DBS. Although we had a hypothesis specific to the low valence stimuli, the findings were also significant after Bonferroni correction for multiple comparisons. This effect of STN DBS was specific to the low valence condition as in the comparison of high valence stimuli, there was a main effect of valence (F(1, 9) = 49.16, p < 0.0001) but no effect of DBS (F(1, 9) = 0.39, p = 0.55) or DBS × valence interaction (F(1, 9) = 2.79, p = 0.13) (Fig. 1).
Fig. 1. Subthalamic deep brain stimulation effects on affective valence and relationship to clinical outcomes.
a Valence ratings for emotional images in obsessive-compulsive disorder (OCD) subjects On and Off deep brain stimulation (DBS) targeting the subthalamic nucleus. Top: low intensity. Bottom: high intensity. b Arousal ratings for low pleasant and low unpleasant emotional images. c Valence ratings for low pleasant and unpleasant images Off DBS as a function of change in pre-operative and post-operative Yale Brown Obsessive–Compulsive Scale (YBOCS). Error bars represent standard error of the mean
In the assessment of arousal in the low valence condition, there was no effect of arousal (F(1, 9) = 0.69, p = 0.43), DBS (F(1, 9) = 2.26, p = 0.17) or DBS × arousal interaction (F(1, 9) = 0.71, p = 0.42) (Fig. 1). In the high valence condition, there was an effect of arousal (F(1, 9) = 5.96, p = 0.04), and no effect of DBS (F(1, 9) = 0.04, p = 0.84) or DBS × arousal interaction (F(1, 9) = 0.25, p = 0.63). We conducted a further assessment demonstrating using paired t-tests that there were no differences in subjective arousal between positive and negative stimuli Off stimulation (positive: 400.88 (80.95); negative: 431.47 (50.38), t = –1.30, df = 9, p = 0.23) as expected but the difference in arousal arose On stimulation (positive: 388.77 (60.20); negative: 433.42 (70.41), t = –2.49, df = 9, p = 0.03). We confirm that the subjective assessment of arousal in this patient group Off stimulation was no different as a function of valence consistent with IAPS arousal ratings. The effects On stimulation suggest a potential effect of stimulation on decreasing arousal of positive images; however, the DBS effects and interaction effects were both not significant.
To assess the effects of order of stimulation in the low valence condition, we conducted a separate analysis comparing low valence with Order (On-Off N = 5; Off-On N = 5) as a between-subjects factor. There were no effects of Order (F(1, 8) = 4.41, p = 0.07) and the DBS effects remained significant (F(1, 8) = 10.94, p = 0.01).
We then asked if there was a relationship between the low valence condition and change in YBOCS scores pre- and post-STN DBS by including the change in YBOCS scores (YBOCS change = pre–post) as a covariate. There was a main effect of DBS (F(1, 8) = 23.31, p = 0.001) and of valence (F(1, 8) = 8.07, p = 0.02) and no DBS × valence interaction (F(1, 8) = 0.54, p = 0.48). There was also an interaction between DBS × YBOCS change (F(1, 8) = 8.40, p = 0.02) and a valence × YBOCS change interaction (F(1, 8) = 9.02, p = 0.02). Given these interactions with YBOCS change, we conducted exploratory Pearson correlation analyses with YBOCS change. The change in YBOCS with stimulation (YBOCS at the time of experiment – YBOCS pre-op), the more pleasant the subjective ratings of the low pleasant stimuli Off DBS (Pearson correlation coefficient R2 = 0.67, p = 0.03) (Fig. 1) with no significant correlation observed On DBS or with low unpleasant (p > 0.05).
The repeated-measures ANOVAs comparing valence or arousal for all groups including healthy controls were not significant (p > 0.05).
We further assessed any relationship between the difference between On and Off in the current findings with risk taking choices to reward and loss, K-values of delay discounting and reflection impulsivity measures previously reported in this same population. As the risk choices were not normally distributed, Spearman rank correlation was used. No significant findings were noted.
Discussion
We showed that STN DBS enhances positive (or decreases negative) subjective ratings of low-intensity stimuli irrespective of valence. This effect was not observed with high valence or arousal ratings. These findings may be related to greater variability and capacity for change in low valence ratings (rated subjectively more negative in the Off state with greater positive shift in the On state) with the high valence conditions demonstrating ceiling effects. We also show that the change in YBOCS or severity of OCD pre- versus post-op interacts with both DBS and valence ratings. On exploratory analysis, the greater the YBOCS improvement, the higher the valence ratings Off DBS in the low pleasant condition with no correlation observed On DBS. This is the first study to suggest a role for emotional processing of the antero-ventral STN with DBS in OCD patients with previous studies focusing on the STN in PD patients. These findings are consistent with our previous confirmation that the clinically optimal coordinates in this same OCD population converge within the antero-ventral associative-limbic STN with resting state functional connectivity with limbic and associative prefrontal regions33,34.
OCD is associated with impaired processing of emotional stimuli with a more negative (or less positive) appraisal of emotional stimuli. In response to both unpleasant and pleasant odours, OCD subjects showed both enhanced disgust sensitivity and intensity and greater insular activity correlating with severity and anxiety35. A perception bias towards negative facial emotion (disgust) was also observed in OCD36. Similarly, OCD subjects overestimate the valence of negative/unpleasant stimuli30, whereas pleasant stimuli were rated as less pleasant/more unpleasant. Along these lines, enhanced functional connectivity of a salience network was observed during early conditioning to a fearful threatening stimulus37 with impairments in ventromedial prefrontal cortex activity to conditioning to the safety signal. Together, these observations in OCD suggest that this negative cognitive bias underlying emotional appraisal and processing may be a generalized impairment that might underlie impairments in associative learning processes to negative, threatening or fearful stimuli35. Thus, targeting this negative cognitive bias and decreasing the negative (or less positive) appraisal of emotional stimuli irrespective of the valence may have therapeutic efficacy. Here we showed that stimulation of the anterior limbic-associative STN in OCD enhances the positive appraisal of low-intensity stimuli irrespective of valence, consistent with an improvement in negative cognitive bias.
That the effects were shown in the low valence stimuli, which may have greater range for subjective interpretation of intensity and capacity for change rather than the high valence stimuli with likely greater ceiling effects, may also implicate a role for uncertainty. Here uncertainty is defined as greater variance or alternative outcomes in subjective interpretation of intensity. Uncertainty has also been suggested to be relevant in OCD: greater accumulation of evidence has been reported to greater perceptual and probabilistic uncertainty in OCD38–40, although these findings are not always consistent41 or only significant after controlling for neuroticism42. Uncertainty (e.g., the possibility of alternative outcomes) has been suggested to increase the gathering excessive evidence to support their decision with some43,44 but not all studies38,45. Using a delayed matching-to-sample task with choice verification, poor insight triggered checking behaviours in OCD patients, which indexed uncertainty46,47. OCD subjects have also shown greater explicit subjective ratings of uncertainty for low but not higher uncertainty evidence in a probabilistic reasoning task44.
Emotion processing and STN
Many but not all studies in PD patients have shown an effect of DBS targeting the motor STN on emotion recognition, particularly with negative emotions. In PD patients, tested 3 months before and after STN DBS, recognition of facial emotions was impaired48,49, particularly with fearful50 and angry faces51 and also with emotional prosody48. Disgust recognition was impaired on STN DBS and off Levodopa, whereas fear recognition was impaired in the off state in both therapies52. Combined DBS and Levodopa also improved general emotion recognition53. However, some studies reported no differences on emotion recognition as a function of DBS in both late54 and early PD55. The decoding of prosody was also impaired in patients irrespective of stimulator status; however, STN DBS was associated with more rapid responses to highly conflicting contradictory emotional stimuli suggested to reflect impaired inhibition of irrelevant stimulus dimensions with competing response alternatives56. This latter observation is consistent with the common observation of hastened responding and a decrease in the decision-making threshold with conflict with STN DBS33,57,58.
STN DBS also influences subjective intensity of valence mostly by decreasing the intensity of negative and enhancing positive stimuli. STN DBS in PD have been reported to enhance emotional intensity of prosody48, lower intensity to aversive stimuli59,60 and with ventral STN stimulation, enhance ratings of positive stimuli61. Our findings suggest a generalized effect irrespective of valence focusing particularly on low-intensity stimuli. We suggest that these may be more ecologically valid as much emotional stimuli in the environment are of low intensity and perhaps more amenable to shifts in subjective perception.
PD patients showed decreased alpha event-related desynchronization (ERD) to pleasant and unpleasant stimuli 1–2 s after stimuli onset62,63 with ERD correlating with individual subjective ratings. Alpha band (8–12 Hz) activity has been suggested to represent a marker of limbic activity in non-STN regions including the bed nucleus of stria terminalis or subgenual cingulate area, or pathologies such as major depressive disorder and OCD64. Beta oscillations have also been used to map dorsal oscillatory and ventral non-oscillatory STN activity12. Emotive auditory stimuli evoked ventral right STN activity suggesting both regional specialization and hemispheric asymmetry65. Similarly, enhanced activity to both angry and happy auditory stimuli was observed in the right STN with differential timing of activity66. Using perioperative micro-recordings, 17% of single STN neurons responded in the alpha band activity (500–2000 ms) to emotional stimuli67, a measure analogous to alpha band local field potential activity62,63. Affective neurons were recorded in the sensorimotor regions consistent with integration of functional STN territories with no evidence of laterality, but with a posterior valence and anterior arousal segregation67.
Neuroimaging studies in STN DBS PD patients have identified a neural network implicated in emotional processing. Decreased fear recognition correlated with decreased glucose metabolism of the right orbitofrontal cortex68. STN DBS increases anterior cingulate activity and decreases putaminal activity to emotional imagery69. DBS diminished frontal polar oxy-haemoglobin to positive stimuli and diminished frontopolar and right lateral prefrontal cortex and increased bilateral inferior ventrolateral prefrontal cortex oxy-haemoglobin to negative stimuli70. Finally, a fluorodeoxyglucose-positron emission tomography (FDG-PET) study showed that the decrease in intensity to disgust ratings after STN DBS in PD correlated with prefrontal–insular–cerebellar activity71.
Limitations
This study is not without limitations. The sample size is low; however, we note that this population is very difficult to recruit and further, the randomized controlled trial study of STN DBS in OCD consisted of only 16 subjects. Notably, the within-subject design of this current study reduces inter-individual variability and tends to minimize the heterogeneity potentially related to the different types of OCD. Medication status can also interfere with emotional state, but this has not been modified during the study. Healthy controls were only tested once, which limits comparisons due to an order effect in the experimental condition.
Conclusions
We suggest two plausible main mechanisms that might underlie these and our previous observations. We have previously shown that STN DBS enhances reflection impulsivity and delay discounting and decreases risk taking to rewards in the same population, an effect we suggested may be related to enhanced evidence accumulation in the context of conflict33,34. We did not show any relationship in our current findings with our previous findings on decisional impulsivity or risk taking33,34. However, since we observed a change only in low valence stimuli, our findings may similarly be related to a specific effect of STN DBS on greater competing interpretation alternatives and hence greater conflict56. That the direction of effect is an enhancement in positive intensity is in keeping with previous reports of STN DBS impairing negative emotion recognition and enhancing positive or decreasing negative intensity ratings. We have also previously reported dissociable findings on risk taking to rewards and losses with STN DBS influencing decreased risk taking to rewards and impairing discrimination of loss magnitude. In the latter, we suggested that impaired discrimination of loss magnitude may be specifically related to STN DBS influencing the indirect NoGo pathway mediated via low affinity D2 receptors34. Thus, STN DBS may impair subjective discrimination of negative value and recognition of negative affect and here we show a specific shift towards more subjective positive valence attribution away from negative. Whether the impairment may be specific to negative valence and value, or have an additional separate effect on positive valence is not clear. In either case, this will shift the cost–benefit analysis.
In conclusion, our findings support the ‘tuner' role of STN in emotional processing by decreasing the negative appraisal of low valence stimuli in OCD. These findings highlight potential mechanisms involved in the therapeutic benefit of STN DBS in OCD and suggesting its putative application in resistant major depression disorder.
Acknowledgements
We would like to thank the patients who took part in this study. This work was supported by Agence Nationale de la Recherche (grant number ANR-14-CE13-0030-01 Physiobs); and University Hospital of Grenoble (Direction de la Recherche Clinique et de l’Innovation).
Conflict of interest
M.P. has received reimbursement for travelling costs for participation in scientific meetings by Medtronic and honoraria for lecturing or consultation from the Movement Disorder Society, Lundbeck. P.K. has received research support from Orkyn, Novartis, UCB, Medtronic, LVL, Boston Scientific, and St Jude, and honoraria for lecturing or consultation from the Movement Disorder Society, Lundbeck, Boehringer Ingelheim, Novartis, UCB, Medtronic, Orkyn, Abbott, Orion, TEVA, and Boston Scientific. S.C. has received honoraria for consultation from Medtronic, Boston Scientific. V.V. is a Medical Research Council Senior Clinical Fellow (MR/P008747/1). She has acted as an expert witness for the court related to dopamine agonists and deep brain stimulation. The remaining authors declare that they have no conflict of interest.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Deuschl G, et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. N. Engl. J. Med. 2006;355:896–908. doi: 10.1056/NEJMoa060281. [DOI] [PubMed] [Google Scholar]
- 2.Mallet L, et al. Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N. Engl. J. Med. 2008;359:2121–2134. doi: 10.1056/NEJMoa0708514. [DOI] [PubMed] [Google Scholar]
- 3.Kohl S, et al. Deep brain stimulation for treatment-refractory obsessive compulsive disorder: a systematic review. BMC Psychiatry. 2014;14:214. doi: 10.1186/s12888-014-0214-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chabardès S, et al. Deep brain stimulation for obsessive-compulsive disorder: subthalamic nucleus target. World Neurosurg. 2013;80:S31.e1–S31.e8. doi: 10.1016/j.wneu.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Castrioto A, Lhommée E, Moro E, Krack P. Mood and behavioural effects of subthalamic stimulation in Parkinson’s disease. Lancet Neurol. 2014;13:287–305. doi: 10.1016/S1474-4422(13)70294-1. [DOI] [PubMed] [Google Scholar]
- 6.Voon V, Kubu C, Krack P, Houeto JL, Tröster AI. Deep brain stimulation: neuropsychological and neuropsychiatric issues. Mov. Disord. 2006;21:S305–S327. doi: 10.1002/mds.20963. [DOI] [PubMed] [Google Scholar]
- 7.Widge AS, et al. Predictors of hypomania during ventral capsule/ventral striatum deep brain stimulation. J. Neuropsychiatry Clin. Neurosci. 2016;28:38–44. doi: 10.1176/appi.neuropsych.15040089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bejjani BP, et al. Transient acute depression induced by high-frequency deep-brain stimulation. N. Engl. J. Med. 1999;340:1476–1480. doi: 10.1056/NEJM199905133401905. [DOI] [PubMed] [Google Scholar]
- 9.Blomstedt P, et al. Acute severe depression induced by intraoperative stimulation of the substantia nigra: a case report. Park. Relat. Disord. 2008;14:253–256. doi: 10.1016/j.parkreldis.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Philipsson J, Sjöberg RL, Yelnik J, Blomstedt P. Acute severe depression induced by stimulation of the right globus pallidus internus. Neurocase. 2017;00:1–4. doi: 10.1080/13554794.2017.1284243. [DOI] [PubMed] [Google Scholar]
- 11.Haq IU, et al. Smile and laughter induction and intraoperative predictors of response to deep brain stimulation for obsessive-compulsive disorder. Neuroimage. 2011;54:S247–S255. doi: 10.1016/j.neuroimage.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haynes WIA, Haber SN. The organization of prefrontal-subthalamic inputs in primates provides an anatomical substrate for both functional specificity and integration: implications for basal ganglia models and deep brain stimulation. J. Neurosci. 2013;33:4804–4814. doi: 10.1523/JNEUROSCI.4674-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mallet L, et al. Stimulation of subterritories of the subthalamic nucleus reveals its role in the integration of the emotional and motor aspects of behavior. Proc. Natl. Acad. Sci. USA. 2007;104:10661–10666. doi: 10.1073/pnas.0610849104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krack P, et al. Mirthful laughter induced by subthalamic nucleus stimulation. Mov. Disord. 2001;16:867–875. doi: 10.1002/mds.1174. [DOI] [PubMed] [Google Scholar]
- 15.Ulla M, et al. Manic behaviour induced by deep-brain stimulation in Parkinson’s disease: evidence of substantia nigra implication? J. Neurol., Neurosurg. Psychiatry. 2006;77:1363–1366. doi: 10.1136/jnnp.2006.096628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berney A, et al. Effect on mood of subthalamic DBS for Parkinson’s disease A consecutive series of 24 patients. Neurology. 2002;59:1427–1429. doi: 10.1212/01.WNL.0000032756.14298.18. [DOI] [PubMed] [Google Scholar]
- 17.Tommasi G, et al. Transient acute depressive state induced by subthalamic region stimulation. J. Neurol. Sci. 2008;273:135–138. doi: 10.1016/j.jns.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 18.Burdick AP, et al. Do patient’s get angrier following STN, GPi, and thalamic deep brain stimulation. Neuroimage. 2011;54:S227–S232. doi: 10.1016/j.neuroimage.2010.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wojtecki L, et al. Pathological crying induced by deep brain stimulation. Mov. Disord. 2007;22:1314–1316. doi: 10.1002/mds.21266. [DOI] [PubMed] [Google Scholar]
- 20.Okun MS. Pseudobulbar crying induced by stimulation in the region of the subthalamic nucleus. J. Neurol. Neurosurg. Psychiatry. 2004;75:921–923. doi: 10.1136/jnnp.2003.016485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eisenstein SA, et al. Acute changes in mood induced by subthalamic deep brain stimulation in Parkinson disease are modulated by psychiatric diagnosis. Brain Stimul. 2014;7:701–708. doi: 10.1016/j.brs.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Funkiewiez A. Long term effects of bilateral subthalamic nucleus stimulation on cognitive function, mood, and behaviour in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry. 2004;75:834–839. doi: 10.1136/jnnp.2002.009803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Combs HL, et al. Cognition and depression following deep brain stimulation of the subthalamic nucleus and globus pallidus pars internus in Parkinson’s disease: a meta-analysis. Neuropsychol. Rev. 2015;25:439–454. doi: 10.1007/s11065-015-9302-0. [DOI] [PubMed] [Google Scholar]
- 24.Chopra A, et al. Underlying neurobiology and clinical correlates of mania status after subthalamic nucleus deep brain stimulation in Parkinson’s disease: a review of the literature. J. Neuropsychiatry Clin. Neurosci. 2012;24:102–110. doi: 10.1176/appi.neuropsych.10070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim JS, Kim HJ, Lee JY, Kim JM, Yun JY. Hypomania induced by subthalamic nucleus stimulation in a Parkinson’s disease patient: does it suggest a dysfunction of the limbic circuit? Mov. Disord. 2012;5:14–17. doi: 10.14802/jmd.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Funkiewiez A, et al. Acute psychotropic effects of bilateral subthalamic nucleus stimulation and levodopa in Parkinson’s disease. Mov. Disord. 2003;18:524–530. doi: 10.1002/mds.10441. [DOI] [PubMed] [Google Scholar]
- 27.Polosan M, et al. Long-term improvement in obsessions and compulsions with subthalamic stimulation. Neurology. 2016;87:1843–1844. doi: 10.1212/WNL.0000000000003248. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez-Salgado B, et al. Perceived quality of life in obsessive-compulsive disorder: related factors. BMC Psychiatry. 2006;6:1094–1097. doi: 10.1186/1471-244X-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berlin HA, et al. Neural correlates of emotional response inhibition in obsessive-compulsive disorder_ a preliminary study. Psychiatry Res. Neuroimaging. 2015;234:259–264. doi: 10.1016/j.pscychresns.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 30.Casado Y, Cobos P, Godoy A, Machado-Pinheiro W, Vila J. Emotional processing in obsessive-compulsive disorder. J. Anxiety Disord. 2011;25:1068–1071. doi: 10.1016/j.janxdis.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Lang P., Bradley M. M. The International Affective Picture System (IAPS) in the study of emotion and attention. (eds Coan, J. A. & Allen J. J. B.) Handbook of emotion elicitation and assessment. (Oxford University Press, Oxford, New York, 2007) p 29-46.
- 32.Goodman WK, et al. Obsessive compulsive scale. I. Development, use, and reliability. Arch. Gen. Psychiatry. 1989;46:1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- 33.Voon V, et al. Decisional impulsivity and the associative-limbic subthalamic nucleus in obsessive-compulsive disorder: stimulation and connectivity. Brain. 2017;140:442–456. doi: 10.1093/brain/aww309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Voon V, et al. Dissociable effects of subthalamic stimulation in obsessive compulsive disorder on risky reward and loss prospects. Neuroscience. 2018;382:105–114. doi: 10.1016/j.neuroscience.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 35.Berlin HA, et al. Altered olfactory processing and increased insula activity in patients with obsessive-compulsive disorder_ an fMRI study. Psychiatry Res. Neuroimaging. 2017;262:15–24. doi: 10.1016/j.pscychresns.2017.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang JI, Namkoong K, Yoo SW, Jhung K, Kim SJ. Abnormalities of emotional awareness and perception in patients with obsessive–compulsive disorder. J. Affect Disord. 2012;141:286–293. doi: 10.1016/j.jad.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 37.Apergis-Schoute AM, et al. Neural basis of impaired safety signaling in obsessive compulsive disorder. Proc. Natl Acad. Sci. USA. 2017;114:3216–3221. doi: 10.1073/pnas.1609194114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Banca P. et al. Evidence accumulation in obsessive-compulsive disorder: the role of uncertainty and monetary reward on perceptual decision-making thresholds. Neuropsychopharmacology40, 1192–1202 (2015). [DOI] [PMC free article] [PubMed]
- 39.Fear CF, Healy D. Probabilistic reasoning in obsessive-compulsive and delusional disorders. Psychol. Med. 1997;27:199–208. doi: 10.1017/S0033291796004175. [DOI] [PubMed] [Google Scholar]
- 40.Pélissier MC, O’Connor KP. Deductive and inductive reasoning in obsessive-compulsive disorder. Br. J. Clin. Psychol. 2002;41:15–27. doi: 10.1348/014466502163769. [DOI] [PubMed] [Google Scholar]
- 41.Grassi G, et al. Think twice: impulsivity and decision making in obsessive–compulsive disorder. J. Behav. Addict. 2015;4:263–272. doi: 10.1556/2006.4.2015.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Volans PJ. Styles of decision-making and probability appraisal in selected obsessional and phobic patients. Br. J. Soc. Clin. Psychol. 1976;15:305–317. doi: 10.1111/j.2044-8260.1976.tb00038.x. [DOI] [PubMed] [Google Scholar]
- 43.Dar R. Elucidating the mechanism of uncertainty and doubt in obsessive-compulsive checkers. J. Behav. Ther. Exp. Psychiatry. 2004;35:153–163. doi: 10.1016/j.jbtep.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 44.Stern ER, et al. Subjective uncertainty and limbic hyperactivation in obsessive-compulsive disorder. Hum. Brain. Mapp. 2012;34:1956–1970. doi: 10.1002/hbm.22038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sarig S, Dar R, Liberman N. Obsessive-compulsive tendencies are related to indecisiveness and reliance on feedback in a neutral color judgment task. J. Behav. Ther. Exp. Psychiatry. 2012;43:692–697. doi: 10.1016/j.jbtep.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 46.Rotge JY, et al. Provocation of obsessive-compulsive symptoms: a quantitative voxel-based meta-analysis of functional neuroimaging studies. J. Psychiatry Neurosci. 2008;33:405–412. [PMC free article] [PubMed] [Google Scholar]
- 47.Jaafari N, et al. The relationship between insight and uncertainty in obsessive-compulsive disorder. Psychopathology. 2011;44:272–276. doi: 10.1159/000323607. [DOI] [PubMed] [Google Scholar]
- 48.Péron J, et al. Recognition of emotional prosody is altered after subthalamic nucleus deep brain stimulation in Parkinson’s disease. Neuropsychologia. 2010;48:1053–1062. doi: 10.1016/j.neuropsychologia.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 49.Dujardin K, et al. Subthalamic nucleus stimulation induces deficits in decoding emotional facial expressions in Parkinson’s disease. J. Neurol., Neurosurg. Psychiatry. 2004;75:202–208. [PMC free article] [PubMed] [Google Scholar]
- 50.Biseul I, et al. Fear recognition is impaired by subthalamic nucleus stimulation in Parkinson’s disease. Neuropsychologia. 2005;43:1054–1059. doi: 10.1016/j.neuropsychologia.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 51.Schroeder U. Facial expression recognition and subthalamic nucleus stimulation. J. Neurol. Neurosurg. Psychiatry. 2004;75:648–650. doi: 10.1136/jnnp.2003.019794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shapira NA, et al. Brain activation by disgust-inducing pictures in obsessive-compulsive disorder. Biol. Psychiatry. 2003;54:751–756. doi: 10.1016/S0006-3223(03)00003-9. [DOI] [PubMed] [Google Scholar]
- 53.Mondillon L, et al. The combined effect of subthalamic nuclei deep brain stimulation and l-dopa increases emotion recognition in Parkinson’s disease. Neuropsychologia. 2012;50:2869–2879. doi: 10.1016/j.neuropsychologia.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 54.Albuquerque L, et al. STN-DBS does not change emotion recognition in advanced Parkinson’s disease. Park. Relat. Disord. 2014;20:166–169. doi: 10.1016/j.parkreldis.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 55.McIntosh LG, et al. Emotion recognition in early Parkinson’s disease patients undergoing deep brain stimulation or dopaminergic therapy: a comparison to healthy participants. Front. Aging Neurosci. 2015;6:349. doi: 10.3389/fnagi.2014.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brück C, Wildgruber D, Kreifelts B, Krüger R, Wächter T. Effects of subthalamic nucleus stimulation on emotional prosody comprehension in Parkinson’s disease. PLoS. ONE. 2011;6:e19140–12. doi: 10.1371/journal.pone.0019140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Frank MJ, Samanta J, Moustafa AA, Sherman SJ. Hold your horses: impulsivity, deep brain stimulation, and medication in Parkinsonism. Science. 2007;318:1309–1312. doi: 10.1126/science.1146157. [DOI] [PubMed] [Google Scholar]
- 58.Zavala BA, et al. Midline frontal cortex low-frequency activity drives subthalamic nucleus oscillations during conflict. J. Neurosci. 2014;34:7322–7333. doi: 10.1523/JNEUROSCI.1169-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Serranová T, et al. Subthalamic nucleus stimulation affects incentive salience attribution in Parkinson’s disease. Mov. Disord. 2011;26:2260–2266. doi: 10.1002/mds.23880. [DOI] [PubMed] [Google Scholar]
- 60.Vicente S, et al. Subthalamic nucleus stimulation affects subjective emotional experience in Parkinson’s disease patients. Neuropsychologia. 2009;47:1928–1937. doi: 10.1016/j.neuropsychologia.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 61.Greenhouse I, et al. Stimulation at dorsal and ventral electrode contacts targeted at the subthalamic nucleus has different effects on motor and emotion functions in Parkinson’s disease. Neuropsychologia. 2011;49:528–534. doi: 10.1016/j.neuropsychologia.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 62.Kühn AA, et al. Activation of the subthalamic region during emotional processing in Parkinson disease. Neurology. 2005;65:707–713. doi: 10.1212/01.wnl.0000174438.78399.bc. [DOI] [PubMed] [Google Scholar]
- 63.Brücke C, et al. The subthalamic region is activated during valence-related emotional processing in patients with Parkinson’s disease. Eur. J. Neurosci. 2007;26:767–774. doi: 10.1111/j.1460-9568.2007.05683.x. [DOI] [PubMed] [Google Scholar]
- 64.Neumann WJ, et al. Different patterns of local field potentials from limbic DBS targets in patients with major depressive and obsessive compulsive disorder. Mol. Psychiatry. 2014;19:1186–1192. doi: 10.1038/mp.2014.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eitan R, et al. Asymmetric right/left encoding of emotions in the human subthalamic nucleus. Front. Syst. Neurosci. 2013;7:69. doi: 10.3389/fnsys.2013.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Péron J, et al. Vocal emotion decoding in the subthalamic nucleus: an intracranial ERP study in Parkinson’s disease. Brain Lang. 2017;168:1–11. doi: 10.1016/j.bandl.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 67.Sieger T, et al. Distinct populations of neurons respond to emotional valence and arousal in the human subthalamic nucleus. Proc. Natl. Acad. Sci. USA. 2015;112:3116–3121. doi: 10.1073/pnas.1410709112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Le Jeune F, et al. Subthalamic nucleus stimulation affects orbitofrontal cortex in facial emotion recognition: a PET study. Brain. 2008;131:1599–1608. doi: 10.1093/brain/awn084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Geday J, Ostergaard K, Gjedde A. Stimulation of subthalamic nucleus inhibits emotional activation of fusiform gyrus. Neuroimage. 2006;33:706–714. doi: 10.1016/j.neuroimage.2006.06.056. [DOI] [PubMed] [Google Scholar]
- 70.Bick SKB, et al. Subthalamic nucleus deep brain stimulation alters prefrontal correlates of emotion induction. Neuromodulation. 2017;20:233–237. doi: 10.1111/ner.12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ory S, et al. Pre-frontal-insular-cerebellar modifications correlate with disgust feeling blunting after subthalamic stimulation: a positron emission tomography study in Parkinson’s disease. J. Neuropsychol. 2017;11:378–395. doi: 10.1111/jnp.12094. [DOI] [PubMed] [Google Scholar]