A common observation in hematological cancer cells, including follicular lymphoma, diffuse large B-cell lymphoma (DLBCL) and chronic lymphocytic leukemia (CLL), is the upregulation of the anti-apoptotic B-cell lymphoma 2 (Bcl-2) protein, the founding member of the Bcl-2-protein family1. Bcl-2 overexpression enables cancer cell survival despite pro-apoptotic challenges related to oncogenic stress such as genomic aberrations1. Bcl-2 provides this protection by acting at the mitochondrial outer membrane, scaffolding pro-apoptotic Bcl-2-family members such as Bax and Bak (multi-domain executioners of mitochondrial outer membrane permeabilization), and Bim (a BH3-only protein activating Bax and Bak) via its hydrophobic cleft, that is formed by the B-cell homology (BH)1, -2, and -3 domains1. In cancer cells, pro-apoptotic factors (such as Bim) are often upregulated, establishing a dependency on anti-apoptotic Bcl-2 to prevent apoptosis. This dependency is exploited by BH3-mimetic anticancer agents, such as ABT-737 and ABT-199 (venetoclax), which antagonize Bcl-2 at the level of the hydrophobic cleft1. Recently, venetoclax has been approved by the Food and Drug Administration (FDA) for the treatment of patients with relapsed CLL2.
However, it has become clear that Bcl-2 overexpression can also protect cells against apoptosis through means other than its canonical anti-apoptotic function3. Indeed, work from several labs indicated that Bcl-2 is present at the endoplasmic reticulum (ER) Ca2+ stores, where it diminishes Ca2+ efflux from the ER4. Although different mechanisms have been proposed, it is clear that Bcl-2, via its BH4 domain, can directly bind IP3 receptors (IP3Rs)—intracellular Ca2+-release channels—and limit their Ca2+-flux properties, thereby preventing cell death driven by Ca2+ overload5.
Bcl-2-IP3R disrupter-2 (BIRD-2), a cell-permeable peptide tool that targets Bcl-2’s BH4 domain has been developed by fusing the TAT sequence to a stretch of 20 amino acids representing the Bcl-2-binding site present in the central, modulatory region of the IP3R6,7. This peptide is able to disrupt the interaction between the IP3R and Bcl-28. BIRD-2 provoked spontaneous IP3R-mediated Ca2+ signaling and cell death in several Bcl-2-dependent cancer cell models, including CLL, multiple myeloma and follicular lymphoma9, small cell lung cancer, and DLBCL7. Interestingly, in DLBCL at least, we discovered a negative correlation between the sensitivity towards venetoclax and BIRD-210. Therefore, we may speculate that a cancer cell needs to choose to deploy Bcl-2 for its canonical role at the mitochondria, preventing Bax/Bak activity, or an alternative function at the ER, inhibiting IP3R activity. The former depends on Bcl-2’s hydrophobic cleft, whereas its BH4 domain is involved in the latter.
Recent work from our lab has shed more light on the mechanism of action of BIRD-2. A paper by Bittremieux et al. highlights the importance of intra- and extracellular Ca2+ for BIRD-2 to work11. We initially hypothesized that store-operated Ca2+ entry (SOCE) is an important process in BIRD-2-induced cell death. After all, BIRD-2 promotes Ca2+ release from the ER, which would be refilled upon depletion by SOCE. During Ca2+ depletion, the luminal ER Ca2+ sensor STIM1, interacts with ORAI, a plasma membrane resident Ca2+-influx channel. This interaction results in the activation of ORAI and Ca2+ influx, refilling the ER. However, Bittremieux et al. showed that SOCE is not necessary for BIRD-2-induced cell death. They did this by using several well-characterized pharmacological tools, including DPB162-AE, YM-58483, and GSK-7975A. All compounds were shown to inhibit SOCE, but, interestingly, only DPB162-AE could reduce BIRD-2-induced cell death. This discrepancy was explained by DPB162-AE’s effect on ER Ca2+ store filling, since treatment with thapsigargin and cyclopiazonic acid, two other molecules reducing the ER Ca2+ store but without effect on SOCE, too, could protect against BIRD-2-induced cell death. These experiments confirm and highlight the importance of ER Ca2+ in BIRD-2’s working mechanism. The case against the involvement of SOCE in BIRD-2-mediated cell death was strengthened by a knock-down of STIM1. Cell death experiments comparing the knock-down and the wild-type showed no significant difference between the two conditions11. Caution with the interpretation of these results is warranted, since both the pharmacological and genetic approaches may not have completely annihilated SOCE and thus remnant SOCE could have been sufficient for BIRD-2-induced cell death.
Although SOCE was excluded as a major factor in the cell death mechanism underlying BIRD-2, there was an indication that extracellular Ca2+ is important for proper cell death induction by BIRD-211. Experiments performed with ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA) in the extracellular medium showed that the intracellular Ca2+ signal, elicited by BIRD-2, is not present when Ca2+ is chelated in the extracellular environment. This implies that extracellular Ca2+ is involved in killing the cells with BIRD-2. However, the molecular identity of the pathway mediating Ca2+ influx from the extracellular medium remains elusive and requires further investigation11.
Independently from this, our lab has also identified other factors that contribute to the sensitivity of DLBCL cancer cells towards BIRD-2 exposure (Fig. 1). A first factor is the expression of particular IP3R isoforms12. We found that cells displaying high IP3R2 subtype expression are most sensitive towards BIRD-2. It is hypothesized that these cells are more sensitive to disinhibition of the IP3R due to Bcl-2 removal from the channel, because the IP3R2 has the highest affinity for its ligand IP312. A second factor that contributes to BIRD-2 sensitivity is constitutive IP3 signaling13. B-cell cancers are often characterized by chronic or tonic B-cell receptor (BCR) activity. Importantly, phospholipase γ2, an enzyme producing IP3 and diacyl glycerol from phosphatidylinositol 4,5-bisphosphate (PIP2) present in the cell membrane, acts downstream of this hyperactive BCR, thus providing a constant source of IP3 that helps to promote cell survival and growth14. Treatment of DLBCL and primary CLL cells with a chemical inhibitor of phospholipase C suppressed the ability of BIRD-2 to provoke cell death. At least in DLBCL cell lines, these pharmacological experiments were independently validated by the overexpression of an IP3 sponge that buffers free IP3, thereby dampening BIRD-2-induced cell death. So, although these tumor cells use constitutive IP3 signaling as a pro-survival mechanism, this signaling system can be converted into a pro-death signal by BIRD-213. Now, further research is needed to examine whether BIRD-2 can also kill other primary cancer cells besides the ones derived from CLL patients and whether BIRD-2 sensitivity is dependent on IP3R2 expression and IP3 signaling in these primary cells.
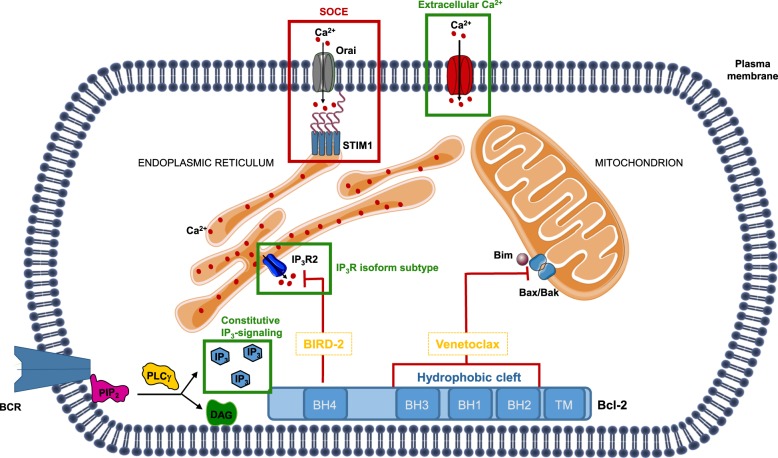
Fig. 1. Antagonizing B-cell lymphoma 2 (Bcl-2) to induce cell death in B-cell cancer cells.
Two functional domains, the hydrophobic cleft and the BH4 domain, are important for Bcl-2’s anti-apoptotic function. The hydrophobic cleft of Bcl-2 prevents apoptosis by scaffolding and neutralizing several pro-apoptotic Bcl-2 family members, including Bax/Bak and BH3-only proteins such as Bim, at the mitochondrial outer membranes. The hydrophobic cleft of Bcl-2 can be targeted by so-called BH3 mimetics, including the recently FDA-approved small molecule venetoclax/ABT-199, provoking cell death in Bcl-2-dependent cancer cells. The BH4 domain suppresses apoptosis by binding and inhibiting the IP3R, intracellular Ca2+-release channels present in the endoplasmic reticulum (ER). A decoy peptide, the Bcl-2 IP3R disruptor-2 (BIRD-2), can target Bcl-2’s BH4 domain, thereby disrupting Bcl-2/IP3R complexes and provoking Ca2+-driven apoptosis in Bcl-2-dependent cancer cells. The IP3R isoform subtype (IP3R2), constitutive IP3 signaling and extracellular Ca2+ are critical factors that contribute to the sensitivity of Bcl-2-dependent cancer cells towards BIRD-2 (indicated in green), while store-operated Ca2+ entry likely may not be involved (indicated in red)
Finally, BIRD-2 can be used to eradicate cancer cells, even when it is not directly killing the cells itself. In ovarian cancer cells, Bcl-2 has been implicated in cisplatin resistance. Recent work by Xie et al. shows that BIRD-2 can overcome cisplatin resistance, thereby re-sensitizing ovarian cancer cells towards cisplatin15. At the mechanistic level, BIRD-2 augmented cisplatin-induced Ca2+ release and cell death without causing cell death by itself in these cells. These findings would advocate for opportunities to apply BIRD-2 as an adjuvant for other anticancer treatments that impinge on Ca2+ signaling15.
Acknowledgements
Research in the authors’ laboratory related to this topic has been supported by the Research Foundation—Flanders (FWO) (G.0C91.14 N, G.0A34.16 N), the Research Council—KU Leuven (OT14/101). M.K. and M.B. are holders of a Ph.D. fellowship from the FWO. We also thank all co-authors of the original research papers for their important contributions to the work. We also wish to apologize to all authors whose papers could not be cited due to space limitations.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Montero J, et al. Cell Death Differ. 2018;25:56–64. doi: 10.1038/cdd.2017.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green DR. Cell. 2016;165:1560. doi: 10.1016/j.cell.2016.05.080. [DOI] [PubMed] [Google Scholar]
- 3.Akl H, et al. Biochim. Biophys. Acta Mol. Cell Res. 2014;1843:2240–2252. doi: 10.1016/j.bbamcr.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 4.Vervliet T, et al. Oncogene. 2016;35:5079–5092. doi: 10.1038/onc.2016.31. [DOI] [PubMed] [Google Scholar]
- 5.Rong YP, et al. Proc. Natl. Acad. Sci. USA. 2009;106:14397–14402. doi: 10.1073/pnas.0907555106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Distelhorst, C. W. Creating a New Cancer Therapeutic Agent by Targeting Bcl-2-IP3R Interaction. Cold Spring Harb. Perspect. Biol. (2019) (in press). [DOI] [PMC free article] [PubMed]
- 7.Distelhorst CW. Biochim. Biophys. Acta Mol. Cell Res. 2018;11:1795–1804. doi: 10.1016/j.bbamcr.2018.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhong F, et al. Blood. 2011;117:2924–2934. doi: 10.1182/blood-2010-09-307405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lavik AR, et al. Oncotarget. 2015;6:27388–27402. doi: 10.18632/oncotarget.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vervloessem T, et al. Oncotarget. 2017;8:111656–111671. doi: 10.18632/oncotarget.22898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bittremieux M, et al. Cell Death Discov. 2018;4:101. doi: 10.1038/s41420-018-0118-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akl H, et al. Cell Death Dis. 2013;4:e632. doi: 10.1038/cddis.2013.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bittremieux, M. et al. Constitutive IP3 signaling underlies the sensitivity of B-cell cancers to the Bcl-2/IP3 receptor disruptor BIRD-2. Cell Death Differ. 10.1038/s41418-018-0142-3 (2018) [DOI] [PMC free article] [PubMed]
- 14.Fowler N, et al. Hematol. Am. Soc. Hematol. Educ. Program. 2013;2013:553–560. doi: 10.1182/asheducation-2013.1.553. [DOI] [PubMed] [Google Scholar]
- 15.Xie Q, et al. Int J. Mol. Med. 2018;41:809–817. doi: 10.3892/ijmm.2017.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]