Abstract
Pseudomonas sp. strain PTH10 can utilize o-phthalate which is a key intermediate in the bacterial degradation of some polycyclic aromatic hydrocarbons. In this strain, o-phthalate is degraded to 2,3-dihydroxybenzoate and further metabolized via the 2,3-dihydroxybenzoate meta-cleavage pathway. Here, the opa genes which are involved in the o-phthalate catabolism were identified. Based on the enzymatic activity of the opa gene products, opaAaAbAcAd, opaB, opaC, and opaD were found to code for o-phthalate 2,3-dioxygenase, dihydrodiol dehydrogenase, 2,3-dihydroxybenzoate 3,4-dioxygenase, and 3-carboxy-2-hydroxymuconate-6-semialdehyde decarboxylase, respectively. Collectively, these enzymes are thought to catalyze the conversion of o-phthalate to 2-hydroxymuconate-6-semialdehyde. Deletion mutants of the above opa genes indicated that their products were required for the utilization of o-phthalate. Transcriptional analysis showed that the opa genes were organized in the same transcriptional unit. Quantitative analysis of opaAa, opaB, opaC, opaD, opaE, and opaN revealed that, except for opaB and opaC, all other genes were transcriptionally induced during growth on o-phthalate. The constitutive expression of opaB and opaC, and the transcriptional induction of opaD located downstream of opaB, suggest several possible internal promoters are existence in the opa cluster. Together, these results strongly suggest that the opa genes are involved in a novel o-phthalate catabolic pathway in strain PTH10.
Introduction
Phthalate isomers including o-phthalate (OPA), terephthalate, and isophthalate have been widely used as plasticizers to impart favorable properties such as flexibility and softness to various polymers. Furthermore, they are key intermediates in the bacterial degradation of phthalate esters, as well as some polycyclic aromatic hydrocarbons found in fossil fuels. They represent hazardous endocrine-disrupting substances capable of binding to the estrogen receptor1–3, which makes their release into the environment a serious concern worldwide4. Microbial degradation represents a promising technology for solving environmental contamination by phthalate isomers. OPA is known as a major intermediate metabolite and a key substance in the microbial degradation of phthalate esters5,6 and some polycyclic aromatic hydrocarbons, such as fluoranthene7, fluorene8, and phenanthrene9. The OPA catabolic pathway has been reported in many gram-negative and gram-positive bacteria. In gram-negative bacteria, including Burkholderia cepacia DBO110, Pseudomonas fluorescens PHK11, and Comamonas testosteroni12, OPA is reportedly oxidized by OPA 4,5-dioxygenase to form cis-4,5-dihydroxy-4,5-dihydrophthalate; the latter is then converted to 4,5-dihydroxyphthalate by a dehydrogenase. The resulting product is further transformed to protocatechuate by a decarboxylase, before entering the aromatic ring-cleavage pathway. In gram-positive bacteria such as Mycobacterium vanbaalenii PYR-113, Arthrobacter keyseri 12B14, Terrabacter sp. strain DBF6315, and Rhodococcus jostii RHA116, OPA is oxidized by OPA 3,4-dioxygenase to yield cis-3,4-dihydroxy-3,4-dihydrophthalate, which is then converted to 3,4-dihydroxyphthalate by a dehydrogenase. The resulting product is again transformed to protocatechuate by a decarboxylase and further catabolized through the aromatic ring-cleavage pathway.
Microbial degradation of terephthalate and isophthalate has been reported in several bacteria5,17–19. C. testosteroni YZW-D metabolizes terephthalate and isophthalate through catabolic pathways that involve the tph and iph genes, respectively20. These catabolic genes have been also characterized in Comamonas sp. strain E6, which is seemingly able to degrade terephthalate and isophthalate17,21. In these catabolic pathways, terephthalate and isophthalate are converted to protocatechuate and further degraded via the protocatechuate 4,5-cleavage pathway.
Of the catabolic pathways involved in the degradation of phthalate isomers, only the one proceeding via protocatechuate has been identified so far. In the present study, an OPA-assimilating bacterium, Pseudomonas sp. strain PTH10, was isolated. This strain is able to degrade OPA via 2,3-dihydroxybenzoate (23DHBA). However, not all the genes responsible for OPA degradation via 23DHBA have been characterized. This study is the report describing the identification of genes involved in the conversion of OPA to 23DHBA and implying the OPA degradation pathway via 23DHBA in strain PTH10. These results will improve our understanding of the OPA utilization system in the environmental microorganisms.
Results
Isolation and characterization of an OPA-degrading strain
An OPA-degrading mixed culture was enriched from soil that had been treated with 1 mM OPA. Strain PTH10 isolated from this enrichment culture was able to grow on OPA as the sole carbon and energy source; however, it was unable to utilize terephthalate and isophthalate. The 16S rRNA gene (1,537 bp) sequence which was found by the draft genome sequence analysis of strain PTH10 showed similarity with those of the Pseudomonas genus.
High-performance liquid chromatography (HPLC) analysis revealed the production of 23DHBA during growth of strain PTH10 on OPA. When the cells grown on OPA were incubated with 23DHBA, the reaction mixture turned yellow, suggesting the release of a meta-cleavage compound (data not shown). These results indicate that in this strain, OPA is degraded through the 23DHBA meta-cleavage pathway. However, no growth of this strain on 23DHBA was found. It seemed to be caused by lack of a positive uptake of 23DHBA in this strain.
Identification of 23DHBA meta-cleavage pathway genes
To identify the OPA degradation genes in strain PTH10, we first attempted to isolate the 23DHBA 3,4-dioxygenase gene. The 23DHBA meta-cleavage pathway in Pseudomonas reinekei MT1 has been reported by Marín and coworkers22. The extradiol ring cleavage dioxygenase, 23DHBA 3,4-dioxygenase (DhbA; accession number, AFN52421), is responsible for ring cleavage of 23DHBA in this species. To find the corresponding gene in strain PTH10, the draft genome sequence of this strain was determined and a tBLASTn homology search using the amino acid sequence of DhbA as the query. A gene, named opaC, that encoded a DhbA homolog was found. The amino acid sequence of OpaC showed 55% and 26% identity with those of DhbA and 2,3-dihydroxy-p-cumate 3,4-dioxygenase of Pseudomonas putida KL47 (CmtC; accession number, ABA10796), respectively (Table 1). Based on sequence similarity, the opaC gene product is thought to be involved in the extradiol ring cleavage of 23DHBA.
Table 1.
Characteristics of opa genes.
| Gene | Deduced molecular mass (Da)a | Representative homolog | Identity (%)b | Accession no. |
|---|---|---|---|---|
| opaAa | 48,985 | Anthranilate 1,2-dioxygenase large subunit (AntA) from Acinetobacter baylyi ADP1 | 30 | AAC34813 |
| (433) | Benzoate 1,2-dioxygenase large subunit (BenA) from A. baylyi ADP1 | 30 | AAC46436 | |
| opaAb | 19,403 | Anthranilate 1,2-dioxygenase small subunit (AntB) from A. baylyi ADP1 | 27 | AAC34814 |
| (171) | Benzoate 1,2-dioxygenase small subunit (BenB) from A. baylyi ADP1 | 26 | AAC46437 | |
| opaAc | 11,911 | Carbazole 1,9a-dioxygenase ferredoxin component (CarAc) from Pseudomonas resinovorans CA10 | 47 | BAA21733 |
| (110) | Anthranilate 1,2-dioxygenase ferredoxin component (AndAb) from Burkholderia cepacia DBO1 | 36 | AAO83641 | |
| opaAd | 36,575 | Acenaphthene dioxygenase ferredoxin reductase component (ArhA4) from Sphingomonas sp. strain A4 | 46 | BAE93942 |
| (337) | Carbazole 1,9a-dioxygenase ferredoxin reductase component (CarAd) from P. resinovorans CA10 | 42 | BAA21735 | |
| opaB | 29,067 | Benzoate diol dehydrogenase (BenD) from A. baylyi ADP1 | 50 | AAC46439 |
| (269) | 1,2-Dihydroxycyclohexa-3,4-diene carboxylate dehydrogenase (XylL) from Pseudomonas putida mt-2 (pWW0) | 49 | AAA26050 | |
| opaC | 32,431 | DBA 3,4-dioxygenase (DhbA) from Pseudomonas reinekei MT1 | 55 | AFN52421 |
| (285) | 2,3-Dihydroxy-p-cumate 3,4-dioxygenase (PsbC2) from Rhodopseudomonas palustris no. 7 | 51 | BAA82122 | |
| opaD | 36,819 | 2-Amino-3-carboxymuconate 6-semialdehyde decarboxylase (NbaD) from Pseudomonas fluorescens KU-7 | 30 | BAC65312 |
| (327) | 5-Carboxy-2-hydroxymuconate-6-semialdehyde decarboxylase (PraH) from Paenibacillus sp. strain JJ-1b | 28 | BAH79106 | |
| opaE | 52,750 | HMS dehydrogenase (NahI) from P. putida G7 (NAH7) | 75 | BAE92168 |
| (486) | HMS dehydrogenase (XylG) from P. putida mt-2 (pWW0) | 71 | AAA26053 | |
| opaF | 7,356 | OCA tautomerase (DhbF) from P. reinekei MT1 | 44 | AFN52426 |
| (66) | OCA tautomerase (DmpI) from Pseudomonas sp. strain CF600 | 38 | CAA43229 | |
| opaG | 28,313 | OCA decarboxylase (DmpH) from Pseudomonas sp. strain CF600 | 89 | CAA43228 |
| (264) | OCA decarboxylase (XylI) from P. putida mt-2 (pWW0) | 87 | AAA25693 | |
| opaH | 30,679 | HMS hydrolase (DmpD) from Pseudomonas sp. strain CF600 | 66 | CAA36993 |
| (276) | HMS hydrolase (DhbI) from P. reinekei MT1 | 59 | AFN52439 | |
| opaI | 27,888 | HPD hydratase (XylJ) from P. putida mt-2 (pWW0) | 71 | AAA26055 |
| (261) | HPD hydratase (DhbD) from P. reinekei MT1 | 63 | AFN52424 | |
| opaJ | 37,358 | HOV aldolase (DhbH) from P. reinekei MT1 | 89 | AFN52428 |
| (345) | HOV aldolase (XylK) from P. putida mt-2 (pWW0) | 87 | AAA25692 | |
| opaK | 32,998 | Acetaldehyde dehydrogenase (DhbG) from P. reinekei MT1 | 76 | AFN52427 |
| (312) | Acetaldehyde dehydrogenase from (NbaJ) from P. fluorescens KU-7 | 68 | BAC65307 | |
| opaL | 37,594 | TRAP transporter solute receptor from Virgibacillus halodenitrificans | 29 | CDQ31851 |
| (349) | Putative periplasmic binding protein from Aromatoleum aromaticum | 27 | CAI10584 | |
| opaM | 44,696 | Phosphate-selective porin from Alcanivorax pacificus W11-5 | 61 | EKE36862 |
| (412) | Phosphate-selective porin from Methylophaga thiooxydans DMS010 | 33 | EEF79304 | |
| opaN | 70,229 | TRAP transporter from Roseobacter litoralis Och 149 | 38 | AEI95128 |
| (660) | TRAP transporter from Bacillus wakoensis JCM 9140 | 33 | GAE27593 | |
| opaO | 32,346 | Hypothetical protein from Halobacterium sp. strain DL1 | 29 | EHB57276 |
| (300) | Hypothetical protein from Natrialba magadii ATCC 43099 | 28 | ADD06240 |
aThe values in parentheses are the numbers of amino acid residues.
bPercentages of identity by aligning the deduced amino acid sequences by use of EMBOSS alignment tool.
The opaD gene, thought to encode a decarboxylase, was found in the vicinity of opaC (Fig. 1a). As the amino acid sequence of OpaD shares 30% and 28% identity with those of 2-amino-3-carboxymuconate-6-semialdehyde (ACMS) decarboxylase from P. fluorescens KU-7 (NbaD; BAC65312) and 5-carboxy-2-hydroxymuconate-6-semialdehyde decarboxylase from Paenibacillus sp. strain JJ-1b (PraH; AAU25434), respectively, OpaD might be involved in the decarboxylation of 3-carboxy-2-hydroxymuconate-6-semialdehyde (CHMS) to 2-hydroxymuconate-6-semialdehyde (HMS).
Figure 1.
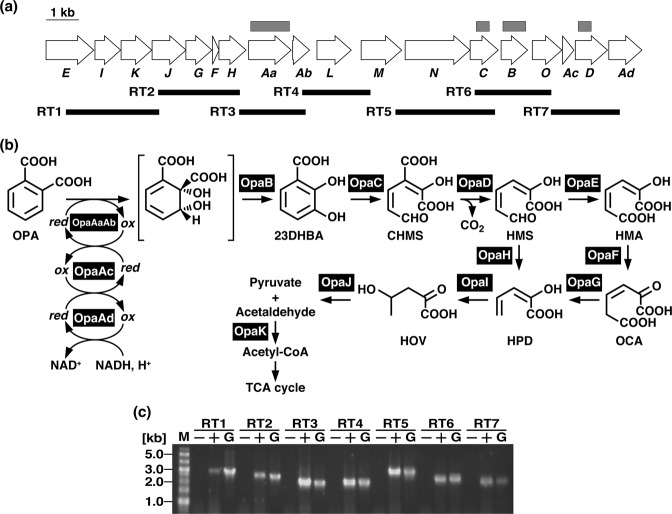
The OPA catabolic pathway genes in Pseudomonas sp. strain PTH10. (a) Open arrows indicate the sizes, locations, and transcriptional directions of ORFs. The deletion regions of the mutants are indicated by gray boxes above the gene cluster diagram. Boldface bars below the gene cluster diagram indicate the locations of the amplified RT-PCR products shown in panel (c). (b) OpaAaAb, large and small subunits, respectively, of oxygenase component of OPA 2,3-dioxygenase; OpaAc, ferredoxin component of OPA 2,3-dioxygenase; OpaAd, ferredoxin reductase component of OPA 2,3-dioxygenase; OpaB, dihydrodiol dehydrogenase; OpaC, 23DHBA 3,4-dioxygenase; OpaD, CHMS decarboxylase; OpaE, HMS dehydrogenase; OpaF, OCA tautomerase; OpaG, OCA decarboxylase; OpaH, HMS hydrolase; OpaI, HPD hydratase; OpaJ, HOV aldolase; and OpaK, acetaldehyde dehydrogenase (acylating). (c) The results of agarose gel electrophoresis of RT-PCR products obtained with primers targeting RT1 (expected size 2,865 bp), RT2 (expected size 2,501 bp), RT3 (expected size 2,039 bp), RT4 (expected size 2,100 bp), RT5 (expected size 3,054 bp), RT6 (expected size 2,367 bp), and RT7 (expected size 2,124 bp) are shown. The amplified regions and the primer sequences are indicated in panel (a) and Table S1, respectively. Lanes M, molecular size markers; lanes G, control PCR with the genomic DNA of strain PTH10; lanes + and −, RT-PCR with and without RT, respectively. Because, the image of the gel was cropped, full-length gel image is presented in Supplementary Fig. S4.
Seven open reading frames (ORFs) including the opaEIKJGFH genes, which share identity with those of the aromatic ring meta-cleavage pathway, were found in the opa gene cluster (Fig. 1a). BLAST analysis showed that these genes encoded a putative HMS dehydrogenase (opaE), 2-hydroxypenta-2,4-dienoate (HPD) hydratase (opaI), acetaldehyde dehydrogenase (opaK), 4-hydroxy-2-oxovalerate (HOV) aldolase (opaJ), 4-oxalocrotonate (OCA) decarboxylase (opaG), OCA tautomerase (opaF), and HMS hydrolase (opaH) (Fig. 1a). Based on sequence similarity, these gene products are likely to share the same function (Fig. 1b).
Characterization of the opaC and opaD gene products
To determine the enzymatic activity of the opaC and opaD gene products, each gene was expressed in E. coli BL21(DE3). When 100 μM 23DHBA, which has maximum absorption at 240 nm, was incubated with the cell extract containing OpaC at 30 °C, it was converted to a product with maximum absorption at 343 nm (see Fig. S1a in the supplemental material), which is identical to that of CHMS22. No conversion was observed when the cell extract of E. coli harboring the empty vector was used (data not shown), indicating that 23DHBA was converted to CHMS by the opaC-encoding 23DHBA 3,4-dioxygenase.
When the cell extract containing OpaD was added to the resulting mixture from the OpaC reaction, a spectrum with a maximum at 375 nm was detected (see Fig. S1b in the supplemental material). According to previous studies23,24, this spectrum is characteristic of HMS, which is produced from CHMS by decarboxylation. As before, conversion of CHMS did not occur in the presence of crude extract from E. coli harboring the empty vector (data not shown). These results strongly suggest that opaD encodes a CHMS decarboxylase, which catalyzes the decarboxylation of CHMS to HMS.
Identification of the OPA degradation gene cluster
In the opa gene cluster, the genes designated opaAa and opaAb, appeared to code for the large and small subunits, respectively, of the terminal oxygenase of an aromatic-ring-hydroxylating dioxygenase (Fig. 1 and Table 1). The amino acid sequence of OpaAa showed ca. 30% identity to those of the large subunits of anthranilate 1,2-dioxygenase (AntA) and benzoate 1,2-dioxygenase (BenA) from Acinetobacter baylyi ADP125. The amino acid sequence of OpaAb shared 27% and 26% identity with those of the small subunits of anthranilate 1,2-dioxygenase (AntB) and benzoate 1,2-dioxygenase (BenB) from strain ADP1, respectively25. Further analysis revealed the existence of two genes named opaAc and opaAd whose predicted amino acid sequences showed similarity to those of [2Fe-2S]-type ferredoxin and FNRN-type reductase, respectively. The amino acid sequence of opaB, which was found immediately downstream of opaC, showed ca. 50% identity to those of other known dihydrodiol dehydrogenases (Fig. 1a and Table 1). Based on amino acid sequence similarity, OPA seems to be converted to 23DHBA by the sequential reactions of OpaA and OpaB in strain PTH10.
Expression of genes responsible for the conversion of OPA to 23DHBA
To determine the function of the opaAaAb, opaAc, opaAd, and opaB gene products, these genes were individually expressed in E. coli BL21(DE3) cells harboring plasmids pCPHAab, pEPHAc, pCPHAd, and pEPHB, respectively. The degradation of 100 μM OPA was assayed with 100 μg each of the crude cell extracts containing OpaAaAb, OpaAc, OpaAd, and OpaB in the presence of 1 mM NADH. When the reaction mixture was analyzed by HPLC, depletion of OPA was accompanied by formation of a compound with a retention time of 2.9 min (see Fig. S2 in the supplemental material). Based on a comparison of the retention time and corresponding absorption spectrum with those of authentic 23DHBA (data not shown), the reaction compound was identified as 23DHBA. These results strongly suggested that OpaA and OpaB were involved in the transformation of OPA to 23DHBA. The amount of OPA decreased in the absence of OpaB, suggesting that the putative dihydrodiol compound was formed from OPA by the OpaA reaction. However, formation of said product could not be detected in this condition. Furthermore, no degradation of OPA was observed in the absence of single OpaA components. These data demonstrate that the terminal oxygenase, OpaAaAb, requires a proper electron transfer system, which could be conveniently provided by the specific ferredoxin and ferredoxin reductase encoded by opaAc and opaAd, respectively. To investigate substrate specificity, OpaA was incubated with 100 μM terephthalate, isophthalate, and benzoate. No depletion of these compounds was observed, suggesting that OpaA was unable to degrade these phthalate isomers.
Disruption of the opa genes in strain PTH10
To investigate the involvement of the opa genes in the catabolism of OPA in vivo, opaAa, opaB, opaC, and opaD were inactivated by internal deletion using gene replacement. The opaAa, opaB, opaC, and opaD mutants, DOA, DOB, DOC, and DOD, respectively, were unable to grow on OPA as the sole source of carbon and energy (Fig. 2a). To verify that growth deficiency was solely due to the deletion of the opa genes, the respective plasmids harboring opaAa, opaB, opaC, or opaD in the shuttle vector pJB866 were introduced into the corresponding mutants for complementation. The opa genes were expressed using the Pm promoter regulated by XylS in the presence of m-toluate, which is not a growth substrate of strain PTH10. Introduction of the plasmids fully restored growth to wild type levels (Fig. 2b). These results indicate that opaAa, opaB, opaC, and opaD are essential for the catabolism of OPA in strain PTH10.
Figure 2.
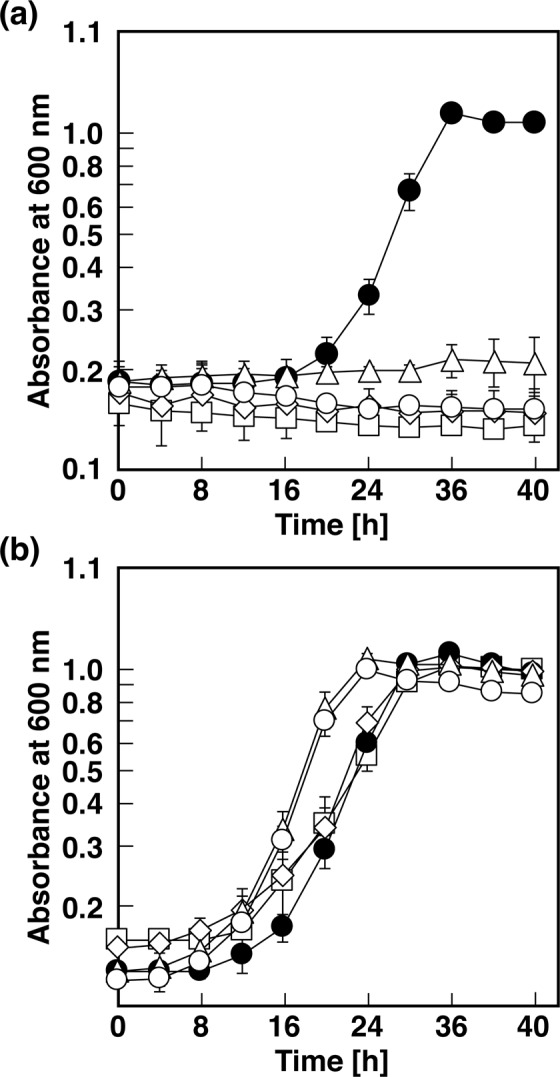
Growth of strain PTH10 and its mutant derivatives on OPA. (a) PTH10 (closed circles), DOA (open squares), DOB (open diamonds), DOC (open triangles), and DOD (open circles). (b) PTH10 (closed circles), opaAa-complementated DOA (open squares), opaB-complementated DOB (open diamonds), opaC-complementated DOC (open triangles), and opaD-complementated DOD (open circles). These strains were grown in W medium containing 10 mM OPA. The data are averages ± standard deviations of three independent experiments performed in parallel.
When DOA cells were incubated with 100 μM OPA, the strain completely lost its capacity to catabolize OPA under the assay conditions used (Fig. 3). These results strongly suggest that opaAa encodes an oxygenase component of OPA 2,3-dioxygenase (OpaA) and is essential for the hydroxylation of OPA. DOB cells degraded only 20% of OPA after 6 h of incubation, whereas strain PTH10 degraded all the OPA. When the respective plasmids carrying opaAa or opaB were introduced into the corresponding mutants, their degradation activity was restored to wild type levels (Fig. 3). This finding indicates that the lack of utilization is due to the inactivated gene and is not caused by a polar effect.
Figure 3.
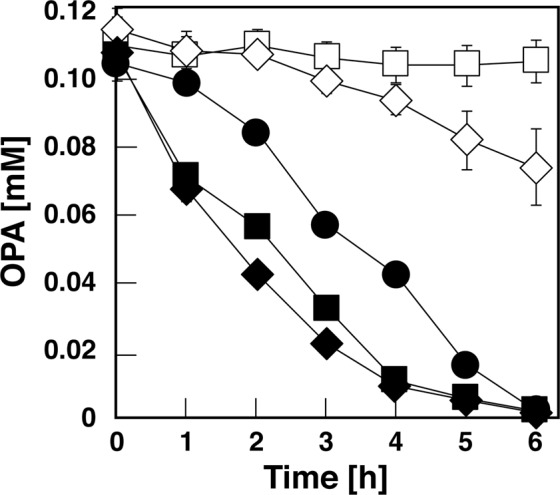
Degradation of OPA by strain PTH10 and its mutant derivatives. The cells of PTH10 (closed circles), DOA (open squares), DOB (open diamonds), opaAa-complementated DOA (closed squares), and opaB-complementated DOB (closed diamonds) were incubated with 100 μM OPA. The remaining amount of each substrate was determined by HPLC analysis. The values represent the averages ± standard deviations of three independent experiments.
DOC cells degraded OPA at the same rate as the wild type, leading to accumulation of an almost identical amount of 23DHBA (see Fig. S3 in the supplemental material). Furthermore, this strain lost completely its ability to degrade 23DHBA, indicating that the opaC-dependent 23DHBA meta-cleavage pathway was essential for OPA degradation. When DOD cells were incubated with OPA or 23DHBA, these compounds were depleted to the same extent as when the wild type strain was used (see Fig. S3 in the supplemental material). However, accumulation of CHMS (λmax = 343 nm) was observed during the degradation of 23DHBA (Fig. 4), indicating that opaD was required for the decarboxylation of CHMS in strain PTH10.
Figure 4.
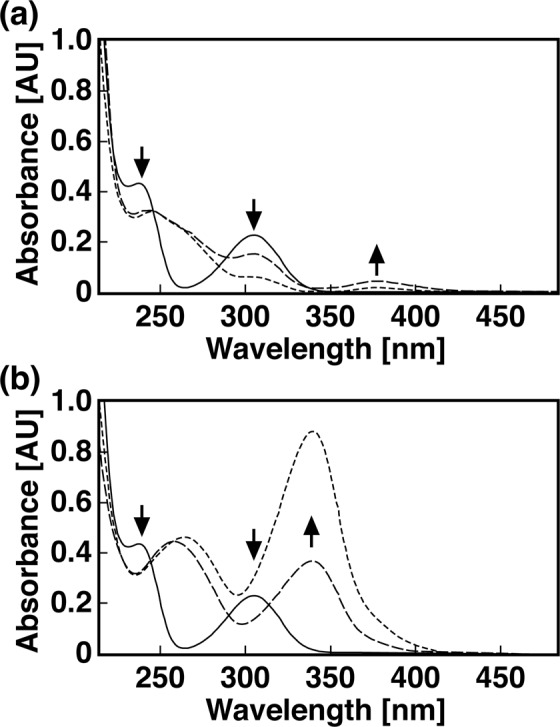
Conversion of 23DHBA by strain PTH10 and its mutant derivative. The reaction mixtures containing 100 μM 23DHBA and the cells of PTH10 (a) and DOD (b) were incubated at 30 °C. UV-visible spectra were recorded at the start, after 3, and 6 hours of incubation, which are represented by the solid, dashed, and dotted lines, respectively.
Transcriptional analysis of the opaAa gene
To identify the operon structure of the opa genes, reverse transcription PCR (RT-PCR) analysis was performed with total RNA extracted from strain PTH10 grown on OPA as the sole carbon and energy source. RT-PCR amplification products of the expected size were detected for the intergenic regions of opaE-opaJ, opaJ-opaH, opaH-opaAb, opaAb-opaM, opaM-opaC, opaC-opaO, and opaO-opaAd (Fig. 1b,c). These results suggest that the opa genes are organized in the same transcriptional unit.
To determine whether transcription of the opa operon was induced in response to OPA, mRNA levels of opaAa, opaB, opaC, opaD, opaE, and opaN were measured by quantitative reverse transcription-PCR (qRT-PCR) analysis using the total RNA harvested from the cells grown on OPA or succinate. When the PTH10 cells grew on succinate, mRNAs of opaAa, opaD, and opaE were not detected in our analytical condition (Fig. 5). The transcription of opaN was extremely low level. On the other hand, these genes transcription was significantly increased in the cells grown on OPA, suggesting that the transcription of these opa genes is induced during OPA utilization. At the same time, the opaB and opaC genes were constitutively expressed at a high level under non-induced conditions (Fig. 5). This suggests the possible existence of an additional internal promoter for the constitutive expressions of opaB and opaC. Furthermore, transcription of opaD located downstream of opaB was induced in the presence of OPA, suggesting that an additional inducible promoter is available to control also the opaD transcription.
Figure 5.
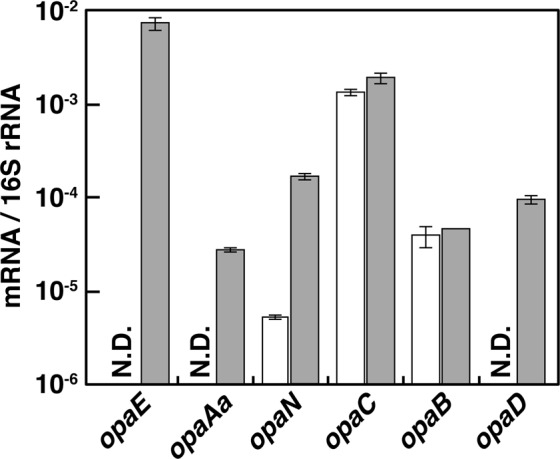
Quantification of the expression levels of the opa genes. Total RNA was isolated from the cells of PTH10 grown in W medium containing 10 mM succinate (open bars) or 10 mM OPA (grey bars). The mRNA expression levels were calculated as a ratio of 16S rRNA gene expression. The data are mean values ± standard deviations for three independent experiments. N.D.: Not detected.
Discussion
The present study reports the identification of the opa gene cluster, which is involved in the OPA catabolic pathway in Pseudomonas sp. strain PTH10. Degradation via 23DHBA is notably different from other known OPA catabolic pathways12,14. Although, the 23DHBA meta-cleavage pathway has been found in P. reinekei MT122, growth ability on OPA and the genes responsible for its conversion to 23DHBA have never been reported. The amino acid sequence of OpaC presents similarity to that of DhbA from MT1, which belongs to the type I extradiol dioxygenase family26. In the presence of either OpaC or DhbA, CHMS was generated as a reaction product from 23DHBA, suggesting that these enzymes share a similar reaction mechanism for the aromatic ring cleavage of 23DHBA. The dhbB gene-encoding class II aldolase is involved in the decarboxylation of CHMS in MT122. In contrast, the CHMS decarboxylation of strain PTH10 requires OpaD, which belongs to a metallo-dependent hydrolase superfamily27. Crystallographic analysis of NbaD has revealed that the active site Zn ion is directly bound to His9, His11, His177, Asp294, and a water molecule that is coordinated by hydrogen bonds with His228 and Asp29428. Given that the corresponding amino acid residues of OpaD are well conserved, they might be involved in the coordination of this enzyme’s active site.
Deduced amino acid sequence similarities of opaAaAbAcAd revealed that OpaA belonged to the type III Rieske non-heme iron oxygenase family29. Amino acid sequence alignment between OpaAa and the oxygenase large subunit of naphthalene 1,2-dioxygenase from Pseudomonas sp. NCIB 9816-4, which belongs to the same family of protein30, revealed high conservation of the amino acid residues responsible for the binding of mononuclear non-heme iron (His208, His213, and Asp362) and Rieske-type [2Fe-2S] centers (Cys81, Cys101, His83, and His104) are well conserved30,31. We speculate that the corresponding residues in OpaAa act as ligands for mononuclear non-heme iron and as an [2Fe-2S] cluster.
As shown in Fig. 3, OPA degradation was affected by deletion of opaB, opening the possibility that OPA hydroxylation was inhibited by accumulation of the dihydrodiol compound in this strain. Transcription of opaB might be driven by a constitutive promoter located in the internal region of the opa operon. A similar case has been reported for the 2-chloronitrobenzene degradation gene cluster of P. stutzeri ZWLR2-132. There, the internal promoter enhances gene expression to optimize the degradation of 2-chloronitrobenzene. In the case of PTH10, the constitutive expression of opaB and opaC is most likely required for OPA catabolism, as it allows cells to prevent the accumulation of the dihydrodiol and diol compounds and utilize the substrate efficiently.
According to their sequence similarity and the transcriptional induction during the growth of PTH10 on OPA (Fig. 5), the opaEIKJGFH gene products might be involved in the lower branch of the OPA catabolism pathway (Fig. 1a). Organization of the opaEIKJGFH genes is almost identical to that of the catechol meta-cleavage pathway genes of P. putida mt-2 (xyl; accession number AJ344068)33,34 and Pseudomonas sp. CF600 (dmp; accession numbers M33263, X52805, and X60835)35–37. In the catechol meta-cleavage pathway gene clusters, the HMS hydrolase genes (xylF and dmpD) are located between the HMS dehydrogenase genes (xylG and dmpC) and HPD hydratase genes (xylJ and dmpE). However, in strain PTH10, the HMS hydrolase gene (opaH) was located in the terminal region of this cluster. Furthermore, the gene corresponding to xylT encoding a chloroplast-type ferredoxin was not observed in the opa gene cluster.
The amino acid sequence of opaL and opaN products showed similarity to a solute-binding protein (SBP) and an integral membrane protein, respectively, of tripartite ATP-independent periplasmic (TRAP) transporter38. TRAP transporter is implicated in the transport of aromatic compounds and has been characterized in Comamonas sp. DJ-1239, Aromatoleum aromaticum EbN140, and Rhodopseudomonas palustris41. These strains have been reported to have a SBP belonging to the DctP family42, and small and large integral membrane proteins related to DctQ and DctM, respectively, which are involved in the uptake of aromatic compounds. In contrast, the opaL gene product is related to another family of SBP, the TRAP-associated extracytoplasmic immunogenic (TAXI) protein family43. The TAXI-TRAP system, which contains a TAXI-SBP and a DctQ-DctM fused integral membrane protein, has been invariably found in Archaea43. Because the primary structure of OpaN consists of contiguous segments corresponding to DctQ and DctM, which are joined to form a single protein, opaL and opaN seemed to code for the TAXI-TRAP system for the uptake of OPA. However, to our knowledge, no functional characterization of the TAXI-TRAP system has been reported. Therefore, further characterization of these genes will be required to gain a better understanding of the transport of OPA by the TAXI-TRAP system.
Transcriptional analysis revealed that the opa genes consisted of a single transcriptional unit induced during OPA utilization. The transcription of the opa genes was thought to be regulated by an unknown transcriptional regulator. However, no genes encoding such a transcriptional regulator were found in this gene cluster. Instead, the constitutive expression of opaB and opaC and the transcriptional induction of opaD located downstream of opaB, suggest the presence of several internal promoters in the opa operon, which are involved in the transcription of these genes. Unfortunately, the consensus promoter sequence could not be found in the upstream region of these opa genes. Further studies must be conducted to elucidate the transcriptional regulation of the opa operon.
Methods
Bacterial strains, plasmids, and culture conditions
Pseudomonas sp. strain PTH10 and its mutant derivatives were routinely grown at 30 °C in Luria-Bertani (LB), 1/5 LB, or W minimal salt medium44 containing 10 mM OPA or 10 mM succinate. Escherichia coli strains were grown in LB medium at 30 °C or 37 °C. If necessary, ampicillin, chloramphenicol, tetracycline, and kanamycin were added into the medium at the concentrations of 100, 20, 10, and 50 mg/l, respectively. For enrichment culture, 100 ml of W minimal salt medium containing 10 g of soil and 1 mM OPA was incubated at 30 °C for a week.
DNA manipulations, sequencing, and analysis
DNA manipulations including chromosomal and plasmid DNA isolation, electroporation, and nucleotide sequencing were performed according to methods described previously45–47. Analyses of nucleotide and amino acid sequences were carried out as previously described48.
The draft genome sequence of strain PTH10 was generated using the 454 GS-FLX Titanium (Roche, Basel, Switzerland) and the Illumina HiSeq 2000 system (Illumina, San Diego, CA, USA). The reads from these two systems were assembled by using Newbler version 2.6 (Roche). The assembly contains 40 contigs with a total genome size of 4,184,609 bp (N50 of 199,694 bp; G + C content of 63.2%). The genome sequence was annotated using RAST server49.
Analysis of disruption mutants
To construct the opa gene disruption mutants, the opaAa, opaB, opaC, and opaD genes were disrupted by in-flame deletion of their central region. The fragments containing the N- and the C-terminal sequences were ligated and inserted in pK18mobsacB50. Each of the resulting plasmid was introduced into the cells of PTH10 by electroporation, and candidates for the deletion mutants were isolated as described previously51. Disruption of the genes was confirmed by diagnostic PCR using specific primer sets and subsequently by DNA sequencing of the PCR amplified regions flanking the deletions.
To construct the complementary plasmids, the specific primer pairs (Table S1) were used to amplify DNA fragments spanning each opa gene. The amplified fragments were cloned into the shuttle vector pJB86652 using In-Fusion HD cloning kit (Clontech, CA, USA). The resulting plasmids carrying opaAa, opaB, opaC, or opaD were independently introduced into the corresponding mutant cells by electroporation for complementation studies. The plasmid maintained in each cell was confirmed by the plasmid extraction form the resulting transformants and PCR amplification of the corresponding gene regions.
To determine the degradation ability of PTH10 and its mutant derivatives toward OPA or 23DHBA, the cells were grown on LB medium to mid-logarithmic phase. Then, the cells were corrected by the centrifugation and inoculated into 1-ml W minimal salt medium containing 1 mM OPA or 23DHBA. Then the cells were incubated at 30 °C, and the concentration of these substrates were measured by HPLC analysis.
Expression of the opa genes in E. coli
The opaAaAb, opaAd, and opaD genes were independently PCR amplified using the specific primer pairs (Table S1) and ligated into the expression vector pColdIV using In-Fusion HD cloning kit to construct pCPHAab, pCPHAd, and pCPHD, respectively. The opaB and opaC genes were independently PCR amplified and ligated into pET21a using In-Fusion HD cloning kit to construct pEPHB and pEPHC, respectively. The opaAc gene was PCR amplified and cloned into pET16b by In-Fusion cloning to construct pEHTAc. The transformants of E. coli BL21(DE3) harboring the pColdIV, pET21a, and pET16b derivatives were grown at 30 °C. Expressions of the opaAc, opaB, and opaC were induced for 4 h by adding 1 mM isopropyl-β-D-thiogalactopyranoside (IPTG) when the absorbance of the culture at 600 nm reached 0.5. The opaAaAb, opaAd, and opaD expressions were induced by cultivation at 15 °C for 24 h after the addition of 1 mM IPTG. After the induction of the gene expression, the cells were harvested by centrifugation at 5,000 × g for 10 min and resuspended in 50 mM Tris-HCl buffer (pH 7.5). Then they were disrupted by an ultrasonic disintegrator (UD-201; Tomy Seiko Co., Tokyo, Japan) and centrifuged at 15,000 × g for 15 min. The resulting supernatants were used as crude enzymes.
Enzymatic assays
Conversion of OPA to 23DHBA was monitored by HPLC analysis. The 1-ml assay mixture consisted of 50 mM Tris-HCl buffer (pH 7.5), 100 μM OPA, 1 mM NADH, and the cell extracts containing OpaAaAb, OpaAc, OpaAd, and OpaB (100 μg of protein each) was incubated at 30 °C. A portion of the reaction mixture was removed at various sampling times and analyzed using HPLC.
23DHBA 3,4-dioxygenase activity was monitored using a DU-800 spectrophotometer (Beckman coulter, Fullerton, Calif.). The reaction mixture (final volume, 1 ml) containing 50 mM Tris-HCl buffer (pH 7.5), 100 μM 23DHBA, and the cell extract of BL21(DE3) harboring pEPHC (10 μg of protein) was preincubated without the substrate for 1 min at 30 °C, and then the reaction was started by adding 23DHBA. 23DHBA 3,4-dioxygenase activity was also assayed by measuring the substrate-dependent oxygen consumption rate. A 2-ml assay mixture contained 50 mM GTA buffer (pH 7.5) consisting of 50 mM 3,3-dimethylglutarate, 50 mM Tris, and 50 mM 2-amino-2-methyl-1,3-propanediol, the cell extract (10 µg of protein), and 1 mM 23DHBA. The reaction mixture was incubated at 30 °C. The oxygen consumption rate was determined with an oxygen electrode (B-505; Iijima Electronics Manufacturing Co., Ltd., Aichi, Japan). One unit of enzyme activity was defined as the amount of enzyme that resulted in consumption of 1 µmol of O2 per 1 min at 30 °C. Specific activity was expressed in units per milligram of protein.
The CHMS decarboxylase activity was assayed spectrophotometrically by monitoring the production of HMS from CHMS using a preassay mixture that consisted of 100 μM 23DHBA and the cell extract including OpaC (10 μg of protein) in 50 mM Tris-HCl buffer (pH 7.5) in a total volume of 900 μl. The mixture was incubated for 5 min at 30 °C. After the reaction was completed, the cell extract of BL21(DE3) harboring pCPHD and pG-Tf2 (the crude OpaD; 10 μg of protein) was added in a final volume of 1 ml, and then the decrease in the absorbance at 343 nm and the increase in the absorbance at 375 nm were monitored at 30 °C.
Analytical methods
The protein concentration was determined by the method of Bradford53. The sizes of the proteins expressed in E. coli were determined by sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis (SDS-PAGE). The proteins in the gels were stained with Coomassie brilliant blue R-250. HPLC analysis was performed with 1290 Infinity LC (Agilent technologies, Santa Clara, CA) equipped with a HC-C18(2) column (150 mm by 4.6 mm; Agilent technologies). The mobile phase was a mixture of water (74.5%), acetonitrile (24.5%), and phosphoric acid (1%) at a flow rate of 1.5 ml/min. The retention times of OPA and 23DHBA were 2.3 and 2.9 min, respectively.
RT-PCR and qRT-PCR analyses
The cells of PTH10 were grown in W minimal salt medium supplemented with 10 mM OPA or succinate at 30 °C. When the absorbance of the culture at 600 nm reached approximately 0.8, the cells were harvested by centrifugation at 5,000 × g at 4 °C for 10 min. Total RNA was isolated with NucleoSpin RNA Plus (Macherey-Nagel, Düren, Germany) and treated with TURBO DNase (Thermo Fisher Scientific, Waltham, MA, USA). Single-stranded cDNA was synthesized from 1.0 μg of total RNA with 200 U of PrimeScript II reverse transcriptase (Takara Bio, Inc., Shiga, Japan) and random 6-mer primers in a 20-μl reaction mixture. The cDNA mixture of 1.0 μl was used to perform PCR amplification in a 25-μl mixture using specific primers (Table S1) and 0.65 U of PrimeStar GXL DNA polymerase (TaKaRa Bio Inc., Otsu, Japan) under the following conditions: 98 °C for 5 min plus 25 cycles of 98 °C for 10 s, 57 °C for 15 s, and 68 °C for 30 s. A control without reverse transcriptase was used for each reaction to verify the absence of genomic DNA contamination. PCR-amplified samples were electrophoresed on a 0.8% agarose gel and visualized with ethidium bromide. qRT-PCR analysis was carried out in a 25-μl mixture using the specific primers (Table S1) according to the previous report54.
Nucleotide sequence accession number
The nucleotide sequence of 16S rRNA gene and the opa genes reported in this paper have been deposited in the DDBJ, EMBL, and GenBank nucleotide sequence databases under accession number LC427665 and LC314585, respectively.
Accession Code
The nucleotide sequence reported in this paper has been deposited in the DDBJ, EMBL, and GenBank nucleotide sequence databases under accession number LC314585.
Supplementary information
Author Contributions
D.K., T.N. and M.F. designed the study. D.K., T.I., K.N. and N.A. performed experiments. D.K. and T.I. analyzed data and wrote the manuscript. All authors reviewed and approved the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-38077-2.
References
- 1.Nakai M, et al. Binding characteristics of dialkyl phthalates for the estrogen receptor. Biochem Biophys Res Commun. 1999;254:311–314. doi: 10.1006/bbrc.1998.9928. [DOI] [PubMed] [Google Scholar]
- 2.Harris CA, Henttu P, Parker MG, Sumpter JP. The estrogenic activity of phthalate esters in vitro. Environ Health Perspect. 1997;105:802–811. doi: 10.1289/ehp.97105802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ganning AE, Brunk U, Dallner G. Phthalate esters and their effect on the liver. Hepatology. 1984;4:541–547. doi: 10.1002/hep.1840040331. [DOI] [PubMed] [Google Scholar]
- 4.Giam CS, Chan HS, Neff GS, Atlas EL. Phthalate ester plasticizers: a new class of marine pollutant. Science. 1978;199:419–421. doi: 10.1126/science.199.4327.419-a. [DOI] [PubMed] [Google Scholar]
- 5.Vamsee-Krishna C, Phale PS. Bacterial degradation of phthalate isomers and their esters. Indian J Microbiol. 2008;48:19–34. doi: 10.1007/s12088-008-0003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hara H, Stewart GR, Mohn WW. Involvement of a novel ABC transporter and monoalkyl phthalate ester hydrolase in phthalate ester catabolism by Rhodococcus jostii RHA1. Appl Environ Microbiol. 2010;76:1516–1523. doi: 10.1128/AEM.02621-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sepic E, Bricelj M, Leskovsek H. Degradation of fluoranthene by Pasteurella sp. IFA and Mycobacterium sp. PYR-1:isolation and identification of metabolites. J Appl Microbiol. 1998;85:746–754. doi: 10.1111/j.1365-2672.1998.00587.x. [DOI] [PubMed] [Google Scholar]
- 8.Grifoll M, Selifonov SA, Chapman PJ. Evidence for a novel pathway in the degradation of fluorene by Pseudomonas sp. strain F274. Appl Environ Microbiol. 1994;60:2438–2449. doi: 10.1128/aem.60.7.2438-2449.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnsley EA. Bacterial oxidation of naphthalene and phenanthrene. J Bacteriol. 1983;153:1069–1071. doi: 10.1128/jb.153.2.1069-1071.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang HK, Zylstra GJ. Novel organization of the genes for phthalate degradation from Burkholderia cepacia DBO1. J Bacteriol. 1998;180:6529–6537. doi: 10.1128/jb.180.24.6529-6537.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pujar BG, Ribbons DW. Phthalate metabolism in Pseudomonas fluorescens PHK: purification and properties of 4,5-dihydroxyphthalate decarboxylase. Appl Environ Microbiol. 1985;49:374–376. doi: 10.1128/aem.49.2.374-376.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakazawa T, Hayashi E. Phthalate metabolism in Pseudomonas testosteroni: accumulation of 4,5-dihydroxyphthalate by a mutant strain. J Bacteriol. 1977;131:42–48. doi: 10.1128/jb.131.1.42-48.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stingley RL, Brezna B, Khan AA, Cerniglia CE. Novel organization of genes in a phthalate degradation operon of Mycobacterium vanbaalenii PYR-1. Microbiology. 2004;150:3749–3761. doi: 10.1099/mic.0.27263-0. [DOI] [PubMed] [Google Scholar]
- 14.Eaton RW. Plasmid-encoded phthalate catabolic pathway in Arthrobacter keyseri 12B. J Bacteriol. 2001;183:3689–3703. doi: 10.1128/JB.183.12.3689-3703.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Habe H, et al. Phthalate catabolic gene cluster is linked to the angular dioxygenase gene in Terrabacter sp. strain DBF63. Appl Environ Microbiol. 2003;61:44–54. doi: 10.1007/s00253-002-1166-6. [DOI] [PubMed] [Google Scholar]
- 16.Patrauchan MA, et al. Catabolism of benzoate and phthalate in Rhodococcus sp. strain RHA1: redundancies and convergence. J Bacteriol. 2005;187:4050–4063. doi: 10.1128/JB.187.12.4050-4063.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukuhara Y, et al. Characterization of the isophthalate degradation genes of Comamonas sp. strain E6. Appl. Environ. Microbiol. 2010;76:519–527. doi: 10.1128/AEM.01270-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schläfli HR, Weiss MA, Leisinger T, Cook AM. Terephthalate 1,2-dioxygenase system from Comamonas testosteroni T-2: purification and some properties of the oxygenase component. J. Bacteriol. 1994;176:6644–6652. doi: 10.1128/jb.176.21.6644-6652.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi KY, et al. Molecular and biochemical analysis of phthalate and terephthalate degradation by Rhodococcus sp. strain DK17. FEMS Microbiol. Lett. 2005;252:207–213. doi: 10.1016/j.femsle.2005.08.045. [DOI] [PubMed] [Google Scholar]
- 20.Wang YZ, Zhou Y, Zylstra GJ. Molecular analysis of isophthalate and terephthalate degradation by Comamonas testosteroni YZW-D. Environ. Health Perspect. 1995;103(Suppl 5):9–12. doi: 10.1289/ehp.95103s49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sasoh M, et al. Characterization of the terephthalate degradation genes of Comamonas sp. strain E6. Appl. Environ. Microbiol. 2006;72:1825–1832. doi: 10.1128/AEM.72.3.1825-1832.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marín M, Plumeier I, Pieper DH. Degradation of 2,3-dihydroxybenzoate by a novel meta-cleavage pathway. J Bacteriol. 2012;194:3851–3860. doi: 10.1128/JB.00430-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crawford RL, Bromley JW, Perkins-Olson PE. Catabolism of protocatechuate by Bacillus macerans. Appl Environ Microbiol. 1979;37:614–618. doi: 10.1128/aem.37.3.614-618.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sala-Trepat JM, Evans WC. The meta cleavage of catechol by Azotobacter species. 4-Oxalocrotonate pathway. Eur J Biochem. 1971;20:400–413. doi: 10.1111/j.1432-1033.1971.tb01406.x. [DOI] [PubMed] [Google Scholar]
- 25.Bundy BM, Campbell AL, Neidle EL. Similarities between the antABC-encoded anthranilate dioxygenase and the benABC-encoded benzoate dioxygenase of Acinetobacter sp. strain ADP1. J Bacteriol. 1998;180:4466–4474. doi: 10.1128/jb.180.17.4466-4474.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaillancourt FH, Bolin JT, Eltis LD. The ins and outs of ring-cleaving dioxygenases. Crit Rev Biochem Mol Biol. 2006;41:241–267. doi: 10.1080/10409230600817422. [DOI] [PubMed] [Google Scholar]
- 27.Liu A, Zhang H. Transition metal-catalyzed nonoxidative decarboxylation reactions. Biochemistry. 2006;45:10407–10411. doi: 10.1021/bi061031v. [DOI] [PubMed] [Google Scholar]
- 28.Martynowski D, et al. Crystal structure of α-amino-β-carboxymuconate-ε-semialdehyde decarboxylase: insight into the active site and catalytic mechanism of a novel decarboxylation reaction. Biochemistry. 2006;45:10412–10421. doi: 10.1021/bi060903q. [DOI] [PubMed] [Google Scholar]
- 29.Kweon O, et al. A new classification system for bacterial Rieske non-heme iron aromatic ring-hydroxylating oxygenases. BMC biochemistry. 2008;9:11. doi: 10.1186/1471-2091-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kauppi B, et al. Structure of an aromatic-ring-hydroxylating dioxygenase-naphthalene 1,2-dioxygenase. Structure. 1998;6:571–586. doi: 10.1016/S0969-2126(98)00059-8. [DOI] [PubMed] [Google Scholar]
- 31.Parales RE. The role of active-site residues in naphthalene dioxygenase. J Ind Microbiol Biotechnol. 2003;30:271–278. doi: 10.1007/s10295-003-0043-3. [DOI] [PubMed] [Google Scholar]
- 32.Gao YZ, Liu H, Chao HJ, Zhou NY. Constitutive expression of a Nag-like dioxygenase gene through an internal promoter in the 2-chloronitrobenzene catabolism gene cluster of Pseudomonas stutzeri ZWLR2-1. Appl Environ Microbiol. 2016;82:3461–3470. doi: 10.1128/AEM.00197-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franklin FC, Bagdasarian M, Bagdasarian MM, Timmis KN. Molecular and functional analysis of the TOL plasmid pWWO from Pseudomonas putida and cloning of genes for the entire regulated aromatic ring meta cleavage pathway. Proc Natl Acad Sci USA. 1981;78:7458–7462. doi: 10.1073/pnas.78.12.7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harayama S, Lehrbach PR, Timmis KN. Transposon mutagenesis analysis of meta-cleavage pathway operon genes of the TOL plasmid of Pseudomonas putida mt-2. J Bacteriol. 1984;160:251–255. doi: 10.1128/jb.160.1.251-255.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nordlund I, Shingler V. Nucleotide sequences of the meta-cleavage pathway enzymes 2-hydroxymuconic semialdehyde dehydrogenase and 2-hydroxymuconic semialdehyde hydrolase from Pseudomonas CF600. Biochim Biophys Acta. 1990;1049:227–230. doi: 10.1016/0167-4781(90)90046-5. [DOI] [PubMed] [Google Scholar]
- 36.Bartilson M, Shingler V. Nucleotide sequence and expression of the catechol 2,3-dioxygenase-encoding gene of phenol-catabolizing Pseudomonas CF600. Gene. 1989;85:233–238. doi: 10.1016/0378-1119(89)90487-3. [DOI] [PubMed] [Google Scholar]
- 37.Shingler V, Powlowski J, Marklund U. Nucleotide sequence and functional analysis of the complete phenol/3,4-dimethylphenol catabolic pathway of Pseudomonas sp. strain CF600. J Bacteriol. 1992;174:711–724. doi: 10.1128/jb.174.3.711-724.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mulligan C, Fischer M, Thomas GH. Tripartite ATP-independent periplasmic (TRAP) transporters in bacteria and archaea. FEMS Microbiol Rev. 2011;35:68–86. doi: 10.1111/j.1574-6976.2010.00236.x. [DOI] [PubMed] [Google Scholar]
- 39.Chae JC, Kim Y, Kim YC, Zylstra GJ, Kim CK. Genetic structure and functional implication of the fcb gene cluster for hydrolytic dechlorination of 4-chlorobenzoate from Pseudomonas sp. DJ-12. Gene. 2000;258:109–116. doi: 10.1016/S0378-1119(00)00419-4. [DOI] [PubMed] [Google Scholar]
- 40.Trautwein K, et al. Benzoate mediates repression of C4-dicarboxylate utilization in “Aromatoleum aromaticum” EbN1. J Bacteriol. 2012;194:518–528. doi: 10.1128/JB.05072-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salmon RC, Cliff MJ, Rafferty JB, Kelly DJ. The CouPSTU and TarPQM transporters in Rhodopseudomonas palustris: redundant, promiscuous uptake systems for lignin-derived aromatic substrates. PloS one. 2013;8:e59844. doi: 10.1371/journal.pone.0059844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shaw JG, Hamblin MJ, Kelly DJ. Purification, characterization and nucleotide sequence of the periplasmic C4-dicarboxylate-binding protein (DctP) from Rhodobacter capsulatus. Mol Microbiol. 1991;5:3055–3062. doi: 10.1111/j.1365-2958.1991.tb01865.x. [DOI] [PubMed] [Google Scholar]
- 43.Kelly DJ, Thomas GH. The tripartite ATP-independent periplasmic (TRAP) transporters of bacteria and archaea. FEMS Microbiol Rev. 2001;25:405–424. doi: 10.1111/j.1574-6976.2001.tb00584.x. [DOI] [PubMed] [Google Scholar]
- 44.Araki N, et al. Identification and characterization of uptake systems for glucose and fructose in Rhodococcus jostii RHA1. J Mol Microb Biotech. 2011;20:125–136. doi: 10.1159/000324330. [DOI] [PubMed] [Google Scholar]
- 45.Abe T, Masai E, Miyauchi K, Katayama Y, Fukuda M. A tetrahydrofolate-dependent O-demethylase, LigM, is crucial for catabolism of vanillate and syringate in Sphingomonas paucimobilis SYK-6. J Bacteriol. 2005;187:2030–2037. doi: 10.1128/JB.187.6.2030-2037.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ausubel, F. M. et al. Current protocols in molecular biology. (John Wiley & Sons, Inc., 1990).
- 47.Sambrook, J., Fritsch, E. F. & Maniatis, T. Molecular cloning: a laboratory manual, 2nd ed., (Cold Spring Harbor Laboratory Press, 1989).
- 48.Kasai D, et al. Uncovering the protocatechuate 2,3-cleavage pathway genes. J Bacteriol. 2009;191:6758–6768. doi: 10.1128/JB.00840-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aziz, R. K. et al. The RAST Server: rapid annotations using subsystems technology. BMC Genomics, 10.1186/1471-2164-9-75 (2008). [DOI] [PMC free article] [PubMed]
- 50.Schäfer A, et al. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene. 1994;145:69–73. doi: 10.1016/0378-1119(94)90324-7. [DOI] [PubMed] [Google Scholar]
- 51.Masai E, et al. Genetic and biochemical characterization of a 2-pyrone-4,6-dicarboxylic acid hydrolase involved in the protocatechuate 4,5-cleavage pathway of Sphingomonas paucimobilis SYK-6. J Bacteriol. 1999;181:55–62. doi: 10.1128/jb.181.1.55-62.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blatny JM, Brautaset T, Winther-Larsen HC, Karunakaran P, Valla S. Improved broad-host-range RK2 vectors useful for high and low regulated gene expression levels in gram-negative bacteria. Plasmid. 1997;38:35–51. doi: 10.1006/plas.1997.1294. [DOI] [PubMed] [Google Scholar]
- 53.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 54.Kasai D, Kitajima M, Fukuda M, Masai E. Transcriptional regulation of the terephthalate catabolism operon in Comamonas sp. strain E6. Appl Environ Microbiol. 2010;76:6047–6055. doi: 10.1128/AEM.00742-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.