Abstract
Malignant melanoma is one of the untreatable cancers in which conventional therapeutic strategies, including chemotherapy, are hardly effective. Therefore, identification of novel therapeutic targets involved in melanoma progression is urgently needed for developing effective therapeutic methods. Overexpression of interleukin-13 receptor α2 (IL13Rα2) is observed in several cancer types including glioma and pancreatic cancer. Although IL13Rα2 is implicated in the progression of various types of cancer, its expression and roles in the malignant melanoma have not yet been elucidated. In the present study, we showed that IL13Rα2 was expressed in approximately 7.5% melanoma patients. While IL13Rα2 expression in human melanoma cells decreased their proliferation in vitro, it promoted in vivo tumour growth and angiogenesis in melanoma xenograft mouse model. We also found that the expression of amphiregulin, a member of the epidermal growth factor (EGF) family, was correlated with IL13Rα2 expression in cultured melanoma cells, xenograft tumour tissues and melanoma clinical samples. Furthermore, expression of amphiregulin promoted tumour growth, implicating causal relationship between the expression of IL13Rα2 and amphiregulin. These results suggest that IL13Rα2 enhances tumorigenicity by inducing angiogenesis in malignant melanoma, and serves as a potential therapeutic target of malignant melanoma.
Introduction
Malignant melanoma (melanoma) is the most aggressive type of skin cancer with high invasive and metastatic properties1. Much effort has been paid to develop molecular target drugs for melanoma aiming the inhibition of BRAF and MEK2–4, but those approaches still encounter problems of side effects5–7. Despite recent progress in immunotherapy8, there is an urgent need to develop more effective melanoma treatments being less harmful to normal cells. For this purpose, identification of new tumour markers specifically expressed in malignant melanoma will be of great importance.
We previously developed a screening method for selecting monoclonal antibodies that are recognised and internalised by target cells. Through the screening employing A375 malignant melanoma cells, we have identified antibodies that recognised interleukin-13 receptor α2 (IL13Rα2: encoded by IL13RA2)9. Several studies have demonstrated that IL13Rα2 is specifically expressed in multiple types of cancer10. IL-13 is one of the anti-inflammatory cytokines produced by activated CD4+T cells, mast cells, macrophages or dendritic cells11. There are two major IL-13 receptors, IL13Rα1 and IL13Rα2. IL-13 binds to its receptors in various combinations of receptor subtypes. The binding of IL-13 to its low-affinity receptor IL13Rα1 results in the activation of Janus kinase/signal transducers and activators of transcription (JAK/STAT) signalling pathway12. In contrast, IL13Rα2, a high-affinity receptor for IL-13, has been considered to function as a decoy receptor for IL-13-mediated signalling pathways, because it has a short intracellular domain, lacking signalling kinase part13,14. On the other hand, there have been also reports suggesting IL13Rα2 to play a role in IL-13 signalling15,16. Recently, Newman and colleagues reported that IL13Rα2 cooperated with epidermal growth factor receptor mutant (EGFRvIII) to activate the ERK and STAT3 pathways to promote the progression of glioblastoma multiforme (GBM)17, suggesting that IL13Rα2 actively participates in multiple signal transduction pathways.
A correlation between the expression level of IL13Rα2 and increased invasiveness as well as metastatic potential of human glioma18,19, breast cancer20 and pancreatic cancer21 have been reported. Among normal tissues, IL13Rα2 can be found only in spermatocytes22, suggesting that IL13Rα2 can be considered as a good candidate for developing new targeted therapeutics that potentially would exhibit fewer side effects. In fact, by taking advantage of the high affinity of IL13Rα2 for IL-13 as well as its cancer cell-specific expression, several therapeutic agents targeting IL13Rα2 have been designed. For example, glioma-targeted therapy based on the delivery of chimeric protein comprising IL-13 and Pseudomonas exotoxin A (PE), has been already under development19. While the expression of IL13Rα2 in melanoma has been also reported23, its expression profile and roles in melanoma progression remain to be elucidated. Thus in the present study, we studied the expression pattern of IL13Rα2 in malignant melanoma and elucidated the relationship between the expression of IL13Rα2 and tumour progression in melanoma.
Results
IL13Rα2 is highly expressed in a subgroup of patients with melanoma
We previously reported that A375 melanoma cells were recognised by anti-IL13Rα2 antibodies9. To examine the relative level of IL13Rα2 expression in melanoma cells, Cancer Cell Line Encyclopedia (CCLE) was used to analyse the frequency of IL13RA2 expression in various carcinoma cell lines. As shown in Fig. S1, IL13RA2 was highly expressed in some melanoma cell lines, suggesting that IL13Rα2 is highly expressed in certain regions of melanoma.
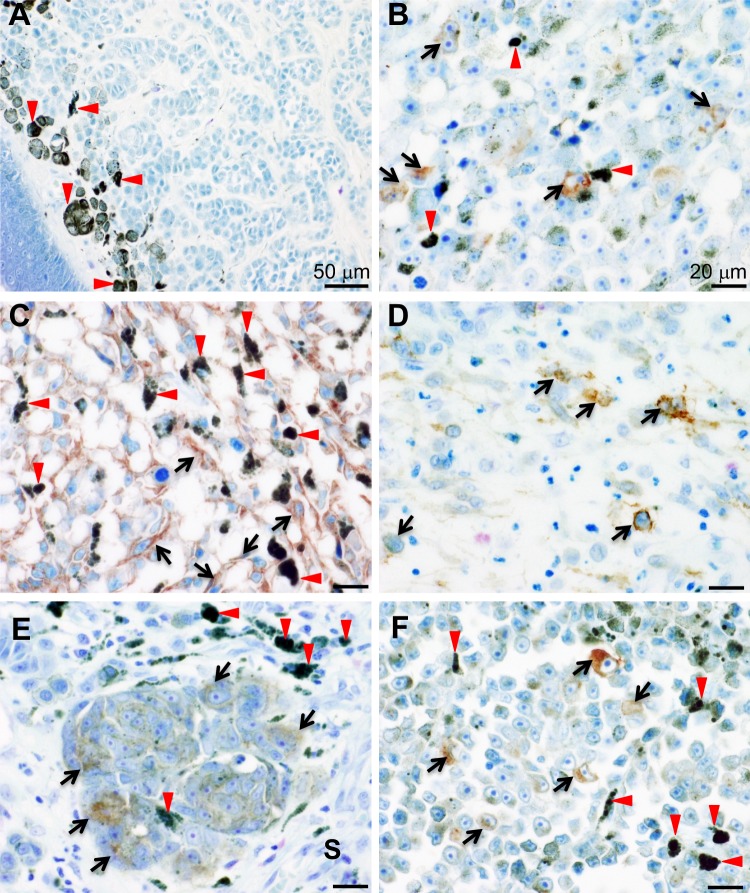
Next we examined the frequency of IL13Rα2 expression in human melanoma samples by using tissue microarrays. Our immunohistochemical analysis by using anti-IL13Rα2 antibody (KH7B9), detected IL13Rα2 in the xenograft tumour cells derived from A375, but not in IL13Rα2-negative cells (A375-IL13RA2 KO and A2058 cells) (Fig. S2A–C), thus confirming the specificity of the KH7B9. In addition, in agreement to the previous report, among normal human tissues, the signal corresponding to IL13Rα2 was only detected in spermatocytes22 (Fig. S2D–H). Moreover, IL13Rα2 expression was not detected in normal skin or benign naevus specimens (Fig. 1A). On the other hand, our data showed that substantial expression of IL13Rα2 was observed in various human melanoma tissues including metastatic malignant melanoma from the armpit (lymph node) (Fig. 1B), malignant melanoma from the thigh (Fig. 1C), cunnus (Fig. 1D), skin (Fig. 1E) and right sole (Fig. 1F). Positive staining for IL13Rα2 expression was detected in 14 samples (12 primary tumours; 2 metastatic tumours) out of 187 independent human melanoma samples (137 primary tumours; 50 metastatic tumours), which corresponded to 7.5% (14/187) of total cases examined, suggesting that IL13Rα2 was expressed in a group of human melanoma. IL13Rα2 staining pattern varied among tumour tissue samples examined (Supplementary Table 1) with IL13Rα2 staining observed in >90% tumour cells in a tumour tissue sample obtained from one patient (Fig. 1C). However, IL13Rα2 expression was observed only in a subset of tumour cells (≤10% tumour cells) in >50% tissue samples showing positive IL13Rα2 staining (Fig. 1B,D–F and Supplementary Table 1). No significant difference was observed in the rate of positive IL13Rα2 staining between the primary and metastatic tumour tissue samples examined (Supplementary Table 1). These expression profiles suggested that IL13Rα2 is a novel cancer-testis antigen.
Figure 1.
Tissue microarray analyses for IL13Rα2 expression. Multiple series of tissue microarrays were subjected to immunohistochemical analysis by using anti-IL13Rα2 antibody (KH7B9). Expression of IL13Rα2 was detected in the cytoplasm or membrane of melanoma cells (arrows). Red arrowheads indicate melanin pigment. (A) Benign naevus of the right face. (B) Metastatic malignant melanoma from the armpit (lymph node). (C) Malignant melanoma of the thigh. (D) Malignant melanoma of the cunnus. (E) Malignant melanoma of the skin. IL13Rα2 was expressed by melanoma cells (arrows) but not by stromal cells (S). (F) Malignant melanoma of the right sole. Scale bar: 50 μm (A), 20 μm (B–F).
IL13Rα2 suppresses in vitro proliferation of SK-MEL-28 melanoma cells
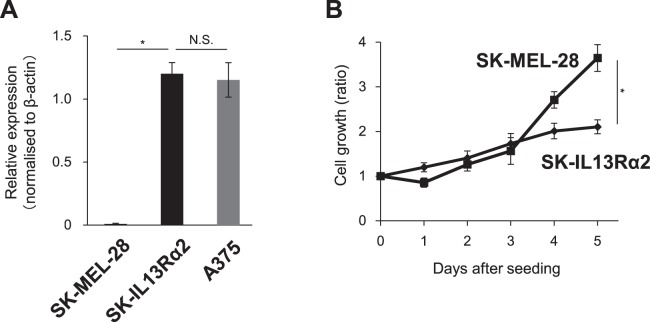
It is well known that different tumour cells show distinct morphological and phenotypic profiles, including cell morphology, gene expression and cell proliferation rate, which is termed tumour heterogeneity. Our finding revealing that IL13Rα2 expression was restricted to only a subset of melanoma cells prompted us to examine whether IL13Rα2 could regulate the oncogenic capacity of malignant melanoma. In order to address this issue, we used malignant melanoma SK-MEL-28 cells which show no detectable expression of IL13Rα2 (Fig. 2A). The SK-MEL-28 cells were transfected with an IL13Rα2 expression vector followed by quantitative RT-PCR (qRT-PCR). IL13Rα2 stable transfectants, SK-IL13Rα2 cells, showed similar expression level of IL13Rα2 compared to that of A375 melanoma cells. In order to study the effect of IL13Rα2 expression on the proliferation of malignant melanoma cells, the SK-MEL-28 cells and SK-IL13Rα2 cells were allowed to grow for 5 days and the number of cells was determined in vitro. We found that the expression of IL13Rα2 was inversely correlated with the proliferative activity of melanoma cells, because the SK-IL13Rα2 cells, expressing IL13Rα2 exhibited decreased cell growth rate compared with the parental SK-MEL-28 cells (Fig. 2B), suggesting that IL13Rα2 may play an inhibitory role in in vitro proliferation of melanoma cells. This inhibitory effect of IL13Rα2 on growth of SK-MEL-28 cells seem not to be mediated by the IL-13-related signals because the addition of IL-13 did not affect the proliferation of the SK-MEL-28, SK-IL13Rα2 or A375 cells (Fig. S3).
Figure 2.
Effects of IL13Rα2 expression on the in vitro proliferation of SK-MEL-28 melanoma cells. The SK-MEL-28 cells were transfected with the expression plasmid encoding IL13Rα2 to establish SK-IL13Rα2 to study the effect of IL13Rα2 on cell proliferation. (A) The expression of IL13Rα2 in the SK-MEL-28, SK-IL13Rα2 and A375 (IL13Rα2-positive) cells was determined by qRT-PCR. (B) 2 × 104 of SK-MEL-28 and SK-IL13Rα2 cells were seeded into 6-well plates and were allowed to grow for 5 days. The cells were harvested and were counted on the indicated day. All values are shown as the ratios to the number of the seeded cells on day 0 and are mean ± SD. *p < 0.05; Student’s t-test.
Size of tumours formed by SK-MEL-28 melanoma cells increased upon IL13Rα2 expression
Because IL13Rα2 decreased the in vitro proliferation of melanoma cells, we next attempted to study the effect of IL13Rα2 expression on in vivo tumour growth of melanoma cells. The SK-IL13Rα2 or SK-MEL-28 cells were subcutaneously xenografted into immunodeficient mice, followed by a measurement of formed tumours. In contrast to the in vitro data, tumours derived from the SK-IL13Rα2 cells were larger than those formed by parental SK-MEL-28 cells (Fig. 3A), suggesting that high level of IL13Rα2 expression promoted tumorigenicity in vivo.
Figure 3.
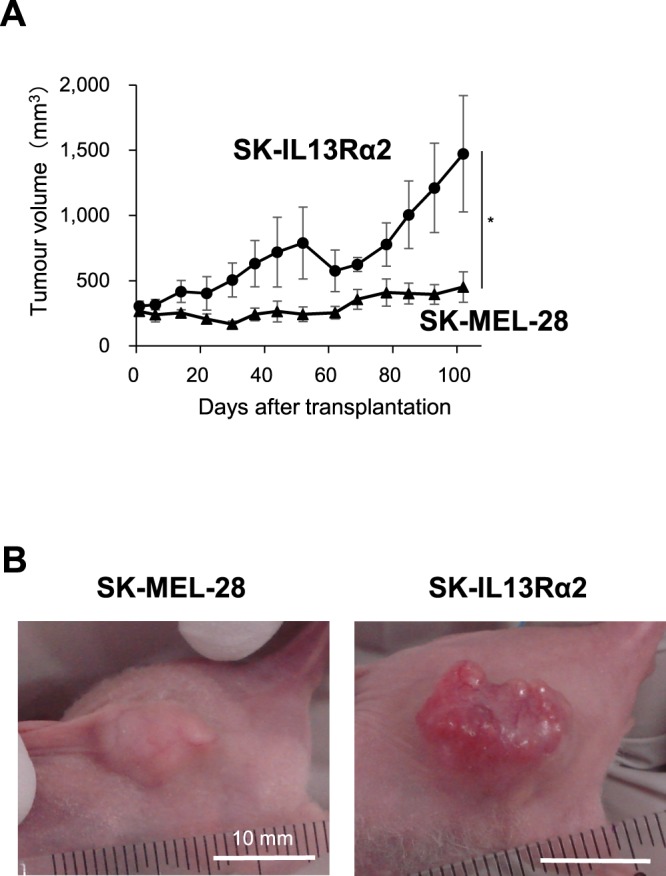
Effects of IL13Rα2 expression on the in vivo tumour formation of SK-MEL-28 melanoma cells. The SK-MEL-28 (n = 6) and SK-IL13Rα2 (n = 6) cells were subcutaneously transplanted into the immunodeficient mice. (A) Tumour growth was assessed for 102 days after transplantation by callipers and was calculated from minor axis and major radius. All values are mean ± SE. *p < 0.05; Student’s t-test. (B) Images of representative tumours at 102 days post-transplantation. Scale bar: 10 mm.
Expression of IL13Rα2 stimulated vessel formation in SK-MEL-28 cell-derived tumours
Although IL13Rα2 suppressed in vitro proliferation of the SK-MEL-28 cells (Fig. 2B), the tumours formed by the SK-IL13Rα2 cells grew faster than those derived from the SK-MEL-28 cells (Fig. 3A). This result suggested that IL13Rα2 may specifically promote tumour growth only while being expressed by the cells residing within tumour microenvironment (TME). The TME is composed of not only cancer cells, but also other components such as blood vessels and fibroblasts, which also actively participate in tumour formation. Especially blood vessels play an essential role in promoting growth of tumour tissue by supplying oxygen and nutrients necessary for the growth of tumour. Interestingly, the tumour mass formed by the SK-IL13Rα2 cells had more reddish appearance than the tumour mass derived from the control SK-MEL-28 cells (Fig. 3B), implying that the formation of large tumour mass by SK-IL13Rα2 may depend on enhanced angiogenesis. In order to study whether IL13Rα2-dependent tumour growth was mediated by enhanced angiogenesis, we investigated whether altered expression of IL13Rα2 would affect new vessel formation in melanoma tumour tissues. As shown in Figs 4 and S4, the PECAM-1-positive vascular area in the tumour tissue derived from the SK-IL13Rα2 cells was significantly larger when compared with the tumour derived from the SK-MEL-28 cells, implying that the expression of IL13Rα2 in malignant melanoma enhanced angiogenesis.
Figure 4.
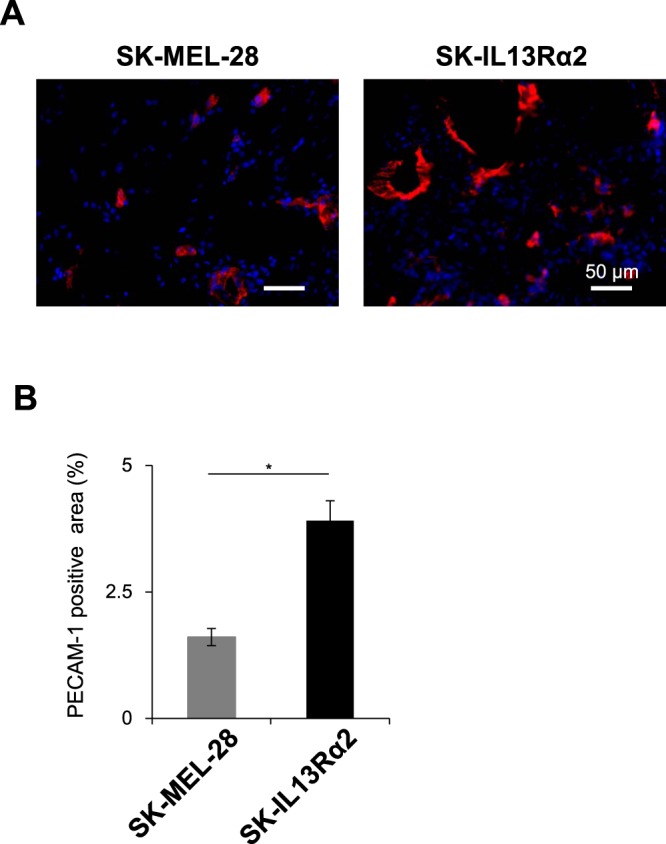
Effects of IL13Rα2 expression on tumour angiogenesis. (A) Sections of the tumours derived from the SK-MEL-28 (n = 5) and SK-IL13Rα2 (n = 6) cells were subjected to immunofluorescence staining with the anti-PECAM-1 antibodies. Scale bar: 50 μm. (B) PECAM-1 positive area represented as a fraction of the total image area. All values are mean ± SE. *p < 0.05; Student’s t-test.
Endogenous IL13Rα2 expression in A375 melanoma cells is indispensable for in vivo tumour formation and angiogenesis
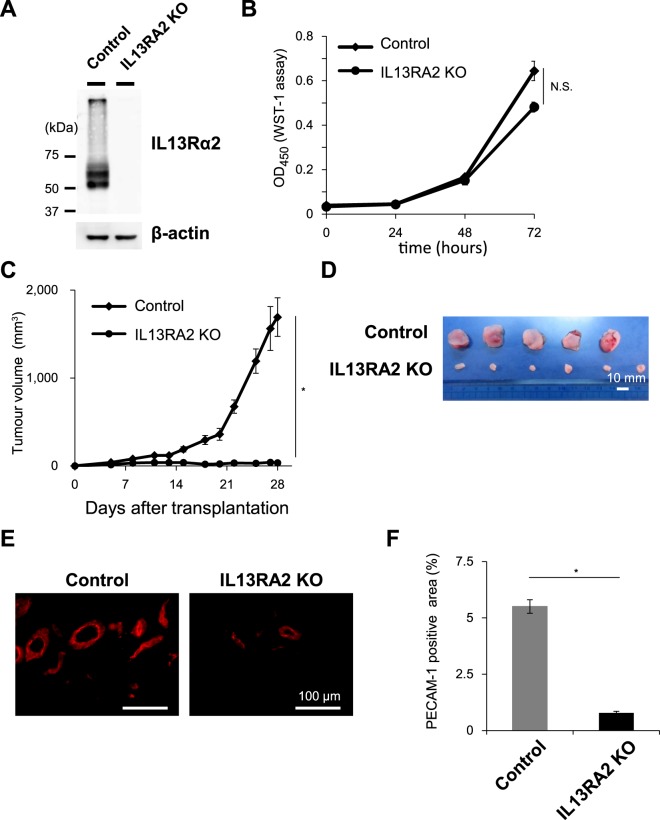
Since we found that increased level of IL13Rα2 expression in the SK-MEL-28 cells promoted in vivo tumorigenicity and angiogenesis (Fig. 3), we next questioned whether loss of endogenous IL13Rα2 expression in A375 cells would affect in vivo tumour growth. For this purpose we generated A375-IL13RA2 knockout (KO) cells using CRISPR-Cas9 regulated by transcription and nuclear-shuttling (CRONUS) system, a recently developed, inducible CRISPR-Cas9 system24 (Figs 5A and S7). Although the loss of IL13Rα2 expression in A375 cells did not affect in vitro proliferation (Fig. 5B), tumours formed after the subcutaneous inoculation of the A375-IL13RA2 KO cells were significantly smaller than those derived from the control cells (Fig. 5C,D), suggesting that endogenous IL13Rα2 expression in A375 melanoma cells is necessary for in vivo tumour formation. In addition, a detailed analysis of the tumour tissues derived from the A375-IL13RA2 KO cells showed a dramatic decrease in the number of PECAM-1-positive vessels (Fig. 5E,F) indicating that new vessel formation was strongly affected by the absence of IL13Rα2 expression and revealing it to be indispensable for A375 tumour formation and angiogenic events.
Figure 5.
Roles of IL13Rα2 in the in vivo tumour formation and angiogenesis in A375 xenograft model. IL13RA2 gene was knocked out in A375 melanoma cells. (A) The expression of IL13Rα2 in A375-Control and A375-IL13RA2 KO cells was determined by immunoblotting analysis with the anti-IL13Rα2 (KH7B9) and anti-β-actin antibodies. Cropped images from the same blots are shown. Full-length blots are presented in Supplementary Fig. S7. (B) In all, 3 × 103 of A375-Control (n = 6) and A375-IL13RA2 KO (n = 6) cells were seeded into 12-well plates and allowed to grow for 3 days. Cells were harvested and were counted at the indicated period. (C) The A375-Control and A375-IL13RA2 KO cells were subcutaneously transplanted into immunodeficient mice. Tumour growth was measured using callipers and was calculated from minor axis and major radius. (D) Images of representative tumours. Scale bar: 10 mm. (E) Sections of tumours derived from A375 Control (n = 5) and A375-IL13RA2 KO (n = 6) cells were subjected to immunofluorescence staining with the anti-PECAM-1 antibodies. Scale bar: 100 μm. (F) PECAM-1 positive area represented as the fraction of total image area. All values are mean ± SE. *p < 0.05; Student’s t-test.
IL13Rα2 increases amphiregulin expression in various types of melanoma cells
Our findings revealed that IL13Rα2 accelerated tumour growth in vivo not by promoting cell proliferation but rather by affecting in vivo tumour angiogenesis. Therefore, we hypothesised that IL13Rα2 in malignant melanoma would stimulate the secretion of angiogenesis-inducing factors. In order to determine the molecules involved in IL13Rα2-mediated angiogenesis in melanoma, we performed Angiogenesis Antibody Array using the lysates of tumour tissues derived from xenografted SK-MEL-28 and SK-IL13Rα2 cells (unpublished data) and identified multiple angiogenesis-related molecules including amphiregulin.
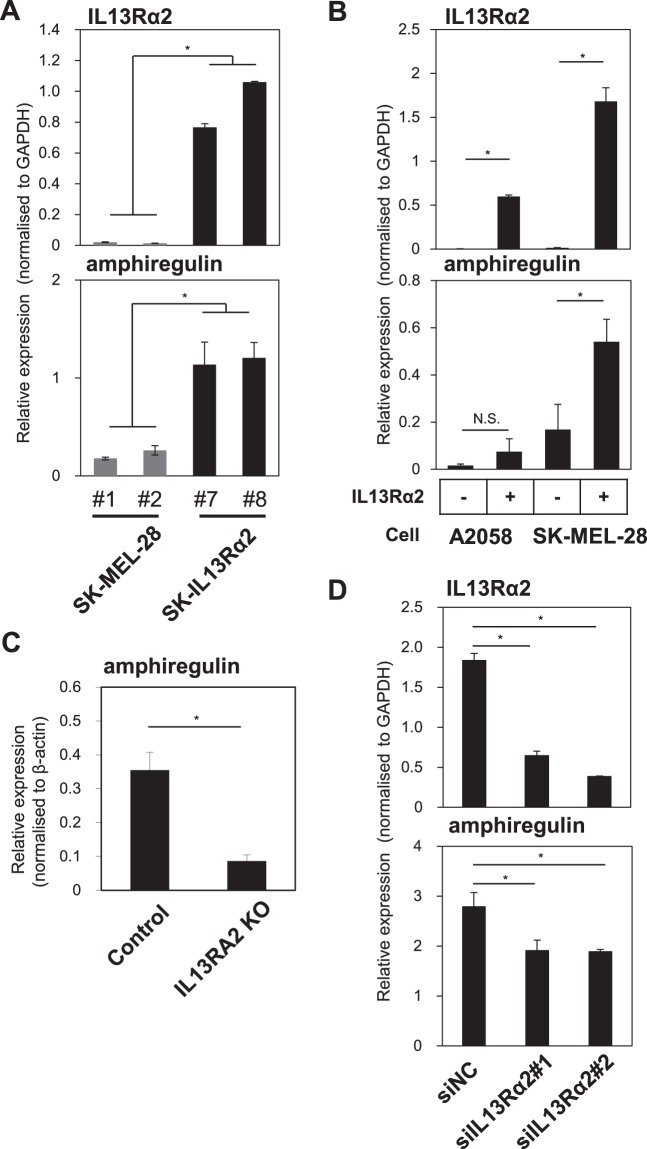
Amphiregulin (encoded by AREG) is a member of the epidermal growth factor (EGF) family, and is known to promote the growth of multiple types of cells25. In order to determine whether expression of amphiregulin transcript is also upregulated by IL13Rα2, we performed qRT-PCR analyses for the expression of IL13Rα2 and amphiregulin using the cDNAs prepared from tumours originated from SK-MEL-28 and SK-IL13Rα2 cells. As shown in Fig. 6A, the level of amphiregulin expression was increased in the tumours originated from the SK-IL13Rα2 cells as compared with those from the SK-MEL-28 cells, suggesting a positive correlation between IL13Rα2 and amphiregulin expressions.
Figure 6.
Effects of IL13Rα2 on amphiregulin expression in various types of melanoma cells. (A) Total RNAs were prepared from tumour tissues derived from the SK-MEL-28 (#1, #2) and SK-IL13Rα2 (#7, #8) cells and were subjected to quantitative RT-PCR analyses for the expression of IL13Rα2 (top) and amphiregulin (bottom). (B) The A2058 and SK-MEL-28 melanoma cells were transfected with IL13Rα2 expression vector, followed by qRT-PCR analysis for the expression of IL13Rα2 (top) and amphiregulin (bottom). (C) IL13RA2 was knocked out in the A375 melanoma cells, followed by qRT-PCR analysis for the expression of IL13Rα2. (D) The A375 melanoma cells were transfected with negative control (NC) siRNAs or siRNAs for IL13Rα2 (#1 and 2), followed by qRT-PCR analysis for the expression of IL13Rα2 (top) and amphiregulin (bottom). All values are mean ± SD. *p < 0.05; Student’s t-test.
In addition, in order to investigate whether the increased expression of amphiregulin in melanoma cells is cell-autonomously regulated by IL13Rα2, we studied the amphiregulin expression in various melanoma cells in which IL13Rα2 expression was altered. Increased expression of IL13Rα2 in A2058 and SK-MEL-28 melanoma cells, which do not express endogenous IL13Rα2, the expression of amphiregulin was upregulated (Fig. 6B). In contrast, when IL13RA2 was deleted in A375 cells, amphiregulin expression was significantly decreased (Fig. 6C). To confirm the effects of loss-of-function of IL13Rα2 on amphiregulin expression, the A375 melanoma cells were subjected to RNA silencing using siRNA specific for IL13Rα2. Downregulation of IL13Rα2 expression in the A375 cells resulted in decreased level of amphiregulin expression (Fig. 6D) thus confirming a positive correlation between IL13Rα2 and amphiregulin expressions. These results indicate that the level of IL13Rα2 expression regulates mRNA transcription of amphiregulin.
Furthermore, in order to determine whether IL13RA2 expression level in melanoma clinical samples was correlated with AREG expression, we analysed IL13RA2 and AREG expression by using the TCGA (The Cancer Genome Atlas) database. In accordance with the present in vitro and in vivo analyses in various melanoma cells, TCGA analysis showed that IL13RA2 expression was correlated with AREG expression in malignant melanoma patients (Fig. S5), suggesting that IL13Rα2 regulated amphiregulin expression in human melanoma patients.
Size of tumours derived from SK-MEL-28 cells is positively regulated by amphiregulin expression
We showed that expression of IL13Rα2 in the malignant melanoma SK-MEL-28 cells increased expression of angiogenic factor, amphiregulin as well as enhanced tumour angiogenesis and tumorigenicity in vivo. However, it remained to be clarified whether expression of amphiregulin enhanced the tumorigenicity of malignant melanoma. Therefore, we investigated whether amphiregulin augmented the progression of malignant melanoma in vivo by using SK-MEL-28 cells overexpressing amphiregulin, SK-amphiregulin cells. The tumours originated from the SK-amphiregulin cells were significantly larger than those formed by control cells, SK-GFP (Fig. 7A). To study whether amphiregulin-dependent tumour growth was mediated by enhanced angiogenesis, we investigated whether altered amphiregulin expression would affect new vessel formation in melanoma tumour tissues. We observed that PECAM-1-positive vascular area in the tumours derived from the SK-amphiregulin cells was significantly larger than that in the tumours derived from SK-GFP cells, implying that amphiregulin expression in malignant melanoma enhanced angiogenesis. These results suggest that expression of amphiregulin in malignant melanoma promotes tumour growth by inducing tumour angiogenesis.
Figure 7.
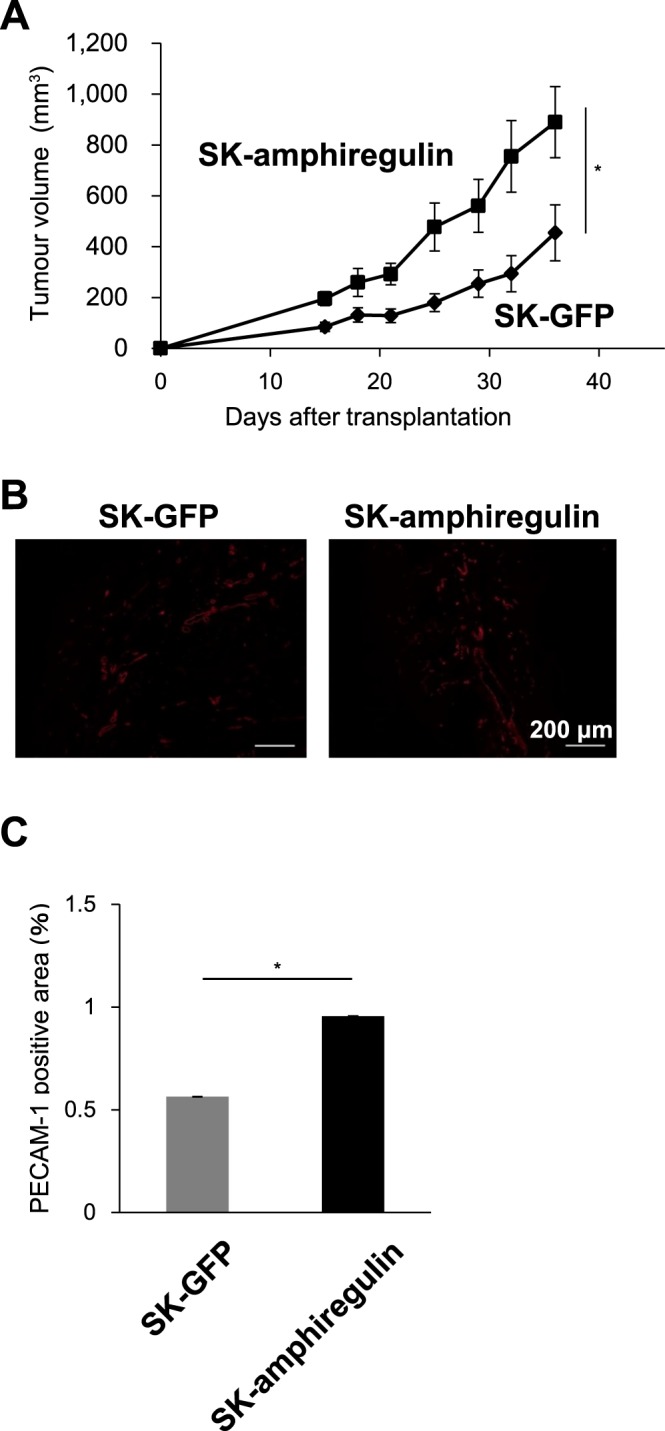
Effects of amphiregulin expression on the in vivo tumour formation and angiogenesis in the SK-MEL-28 xenograft model. The SK-MEL-28 cells were transfected with the expression plasmid encoding amphiregulin to establish SK-amphiregulin cells. The SK-amphiregulin and SK-GFP cells, were then subcutaneously transplanted into the immunodeficient mice, followed by the measurement of tumour size (A). (B) Sections of the tumours derived from the SK-GFP (n = 6) and SK-amphiregulin (n = 6) cells were examined by performing immunofluorescence staining with the anti-PECAM-1 antibodies. Scale bars, 200 μm. (C) PECAM-1 positive area represented as the fraction of the total image area. All values are mean ± SE. *p < 0.05; Student’s t-test.
Discussion
In this study we investigated the expression of IL13Rα2 in malignant melanoma and its role in the progression of melanoma. Our data revealed that the expression of IL13Rα2, which was detected in a subgroup of human melanoma specimens, was correlated with the tumorigenicity of multiple types of melanoma cells. While high level of IL13Rα2 expression inhibited cell proliferation in vitro, it also promoted the expression of amphiregulin, a proangiogenic factor, which in turn resulted in enhanced angiogenesis and increased tumour formation in vivo.
We showed that IL13Rα2 in malignant melanoma promoted tumorigenicity by stimulating angiogenesis using gain-of-function (Figs 3 and 4) and loss-of-function (Fig. 5) studies. Previous reports showed that the expression of IL13Rα2 in other types of cancers had been associated with the progression of cancer by promoting metastatic and invasive abilities of cancer cells17,18,20. Although the role of IL13Rα2 in melanoma progression is likely attributed to its proangiogenic function, its roles in other aspects of melanoma progression such as metastasis need to be further characterised.
The present study indicated that IL13Rα2 expression in melanoma cells enhanced tumour angiogenesis (Figs 4 and 5). Although IL13Rα2 expression in the SK-MEL-28 cells slightly upregulated the expression of vascular endothelial growth factor (VEGF), a well-known tumour angiogenic factor, the upregulation can be considered minimal (1.5-fold), compared to that of amphiregulin (3.2-fold) (Figs 6 and S6). The loss of IL13Rα2 expression in the A375 melanoma cells did not alter the VEGF expression (Fig. S6). Instead, our comprehensive analysis using Angiogenesis Antibody Array revealed that the expression of IL13Rα2 was positively correlated with the enhanced expression of amphiregulin (Fig. 6), but not that of VEGF (unpublished data). Amphiregulin is a member of the EGF family. Its binding to the EGFR activates intracellular signals leading to various physiological responses including enhanced cell proliferation and cardiac hypertrophy25,26. Elevated expression of amphiregulin has been detected in various types of cancers25 where in addition to promoting the proliferation of tumour cells, it also induces tumour vascularisation27. Consistent with these reports, we showed that elevated expression of amphiregulin promoted tumorigenesis of melanoma (Fig. 7). Our results, taken together with the previous reports, suggest that the expression of IL13Rα2 enhances melanoma tumorigenesis in vivo via promoting angiogenesis through the expression of angiogenesis-triggering factors such as amphiregulin.
We showed that IL13Rα2 expression not only enhanced the expression of amphiregulin (Fig. 6) but also suppressed in vitro proliferation of SK-MEL-28 cells (Fig. 2), suggesting that IL13Rα2 transduces intracellular signals that regulated these events in melanoma cells. IL-13 did not affect the proliferation of the SK-MEL-28 or A375 melanoma cells in the present study (Fig. S3); however, previous study in a colorectal cancer epithelial cell line HT-29 revealed that IL-13 signal interfered with cell proliferation by inducing cell death28. While binding of IL-13 to IL13Rα1 results in recruitment of interleukin 4 receptor (IL4R), followed by formation of IL13Rα1-IL4R complex and activation of intracellular signals12, these effects of IL-13 can be mediated independently of IL13Rα1-IL4R signalling axis because the IL-13-induced injury-related responses in the mouse intestinal mucosa take place even under IL4R deficiency29. The interaction of IL13Rα2 with EGFRvIII induces progression of GBM by activating intracellular signalling17. However, detailed mechanisms how IL13Rα2 regulates the expression of amphiregulin and proliferation of melanoma cells need to be elucidated in the future.
Melanin affects various cellular processes both in normal melanocytes and melanoma cells as well as in their surrounding microenvironment30. Moreover, melanin level is positively correlated with melanoma aggressiveness and radiotherapy resistance31,32. Melanogenesis induction in melanoma cells upregulates HIF-1α-related pathways, including stress response-related pathways, glucose metabolism and angiogenesis33,34. In the present study we used multiple melanoma cell lines, which are characterised by different level of melanin content. A375 cells are amelanotic melanoma cells, SK-MEL-28 cells are lightly pigmented, while A2058 cells represents pigmented melanoma cell line. No obvious correlation was observed between expression of IL13Rα2 and pigmentation in the melanoma cells used in the present study. However, the role of IL13Rα2 with respect to the pigmentation in the pathogenesis and progression of melanoma should be elucidated in the future.
Our previous study suggested that IL13Rα2 could be a novel biomarker for malignant melanoma9. IL-13-related pathway has been already targeted while developing drug for gliomas. The approach employs a chimeric protein composed of IL-13 and PE that exploits the high affinity to IL13Rα221. We previously reported functional antibodies that targeted IL13Rα2 and became internalised by targeted cells9. These anti-IL13Rα2 antibodies would facilitate development of antibody-drug conjugates, drugs developed by conjugation of an anticancer agent to a monoclonal antibody. The present finding that IL13Rα2 is expressed in a subgroup of melanoma cells and promotes tumorigenesis suggest IL13Rα2 to be a promising target for the development of novel therapeutic strategy against melanoma.
Materials and Methods
Cells and cell culture
Human melanoma A375, SK-MEL-28 and A2058 cells were obtained from American Type Culture Collection (ATCC), RIKEN Cell Bank and the Japanese Collection of Research Bioresource, respectively. The A375 and A2058 cells were maintained in DMEM; high glucose (4.5 g/L, Nacalai Tesque, Kyoto, JAPAN) supplemented with 10% fetal calf serum (FCS, Sigma-Aldrich, St. Louis, MO, USA), 100 units/mL penicillin and 100 μg/mL streptomycin. The SK-MEL-28 cells were maintained in Eagle-modified MEM (E-MEM, Wako, Saitama, JAPAN) supplemented with 10% FCS (Sigma-Aldrich), 1 mM sodium pyruvate (Nacalai Tesque), 100 units/mL penicillin and 100 μg/mL streptomycin. All the cells were grown in humidified incubator at 37 °C and 5% CO2.
Generation of anti-IL13Rα2 monoclonal antibody (KH7B9)
The anti-IL13Rα2 antibodies were generated by immunising mice with denatured recombinant human IL13Rα2, which yielded monoclonal antibody KH7B9. The antibody was purified from hybridoma culture supernatant using Prosep-A (Merck Millipore, Burlington, MA, USA) according to the instructions of the manufacturer.
Immunohistochemistry
Human melanoma tissue microarray slides, ME208, ME1002a, and ME1004e, were purchased from US Biomax (Rockville, MD, USA). ME208 consisted of 208 cores representing 69 cases in triplicate: 30 were primary melanoma tissues, 30 were metastatic melanoma tissues and 9 were normal skin tissues. ME1002a consisted of 100 cores representing 50 cases in duplicate: 45 were malignant melanoma, 1 was cancer adjacent a normal skin tissue, 4 were normal skin tissues. ME1004e consisted of 100 cores representing 100 cases (single core per case): 62 were malignant melanoma, 20 were metastatic malignant melanoma and 18 were naevus tissues. All core diameters were 1 mm. Immunostaining for IL13Rα2 (by using KH7B9 antibody, 1:200) was performed according to standard protocols using an autostainer (BenchMark XT; Ventana Medical Systems, Inc., Tucson, AZ, USA). Sections were counterstained with 2% Giemsa solution (Sigma-Aldrich). The Giemsa solution changes the melanin pigment to green, which facilitates discrimination from brown positive signals35. IL13Rα2 immunostaining was considered positive when at least 1% of tumour cells showed cytoplasmic or membranous staining. Immunofluorescent staining of human melanoma xenografts was done as described previously36. Briefly, the formation of blood vessels in xenografted tumours were evaluated after the tumours were harvested on day 102 (Fig. 4), day 28 (Fig. 5) and day 53 (Fig. 7) by immunostaining using anti-platelet and endothelial cell adhesion molecule (PECAM-1) antibodies (BD Biosciences, San Jose, CA, USA, cat. no. 55370, 1:500).
RNA isolation and quantitative RT-PCR
Total RNA was prepared with RNeasy reagent (QIAGEN, Hilden, Germany) and was reverse transcribed by random priming and PrimeScript II 1st strand cDNA Synthesis Kit (Takara Bio, Otsu, JAPAN). Quantitative RT-PCR analysis was performed using the GeneAmp 5700 Sequence Detection System and StepOne Plus Real-Time PCR System (Applied Biosystems/Thermo Fisher Scientific, Waltham, MA, USA). All expression data were normalised using the expression of GAPDH or β-actin gene. The primer sequences used for analysis are listed in Supplementary Table 2.
Cell proliferation assay
The cells were seed into 6-well plate and allowed to grow for an indicated period of time, followed by direct cell counting with hemocytometer or WST-1 assay by measuring absorbance of reduced formazan at OD450. The experiments were performed in quintuplicate (direct cell counting) or duplicate (WST-1 assay).
Construction of lentiviral vectors and infection
The cDNAs encoding IL13RA2 and AREG were cloned from A375 cells and were subcloned into pCSII-EF-RfA lentivirus vectors as described previously36. 293FT cells were co-transfected with the expression plasmids and packaging plasmids (pCMV-VSV-G-RSV-Rev and pCAG-HIVgp). The viral supernatants were collected 72 hours after transfection. SK-MEL-28 cells (5.0 × 104 cells/well in 6-well tissue culture plates) were infected with lentiviral particles resulting in the SK-IL13Rα2, SK-amphiregulin or SK-GFP cells (negative control). All the experiments were approved by “the Safety Control Committee for Experiments Using Genetically Modified Organisms, Etc.” and done according to the guideline of “Safety Control Regulations for Experiments Using Genetically Modified Organisms, Etc., Tokyo Medical and Dental University” (registration number: 2016-032C8).
Establishment of A375 cells deficient for IL13RA2 gene using CRONUS system
IL13RA2 knockout cells were established using CRONUS (CRISPR-Cas9 regulated by transcription and nuclear-shuttling) system as described previously24. A gRNA target sequence for IL13RA2; 5′-GAGAGATAACCTAAGTATCCTGG-3′ (PAM sequence underlined) was designed with CRISPRdirect (https://crispr.dbcls.jp/). The primer sequences used for cloning are as follows: sgRNA-IL13RA2-127/149-fwd primer; 5′- GAGACCACTTGGATCCGAGAGATAACCTAAGTATCCGTTTTAGAGCTAGAAATAGCA (underlined sequence corresponds to gRNA spacers), sgRNA-Universal-rev primer; 5′-GCCCGGGTTTGAATTCAAAAAAAGCACCGACTCGGTGCCACTTTTTCAAGTTGATAACGGACTAGCCTTATTTTAACTTGCTATTTCTAGCTCTAA-3′. Stable transfectants of A375 melanoma cells were established by transfection with the plasmids described previously24, and selection by combined puromycin and hygromycin treatment. The induction of Cas9 expression and its nuclear translocation was achieved by incubating resistant cells with a mixture of 5 μM doxycycline (LKT Laboratories, St. Paul, MN, USA) and 10 μM dexamethasone (Wako) for 5 days. A375-IL13RA2 KO clones were then isolated by performing limiting dilution. Effective knocking-out of IL13RA2 was confirmed at the protein level by immunoblotting with anti-human IL13Rα2 antibody KH7B9.
Subcutaneous xenograft melanoma model
Animal experiments were approved by the Institutional Animal Care and Use Committee at Tokyo University of Pharmacy and Life Sciences (approval number: L16-13, LS27-007) and Tokyo Medical and Dental University (registration number: A2018-210C) and were done according to the guidelines of the Animal Care Standards of both the institutes. The SK-IL13Rα2, SK-MEL-28, A375-IL13RA2 KO, A375-Control, SK-amphiregulin or SK-GFP cells were inoculated subcutaneously (s.c.) into the 6-week-old female immunodeficient nude mice by injection of 1 × 107 cells of SK-MEL-28 and its derivatives, and 1 × 105 cells of A375 and its derivatives in 100 μL of Matrigel (Corning, New York, NY, USA), respectively. Tumour formation was examined at the indicated time points. Tumour volume (V) was calculated using the formula: V (mm3) = L × W2 × 0.5, in which L corresponds to the length of the tumour in mm and W to the width of the tumour in mm, respectively.
Immunoblotting analysis
Immunoblotting analysis was performed as described previously37 using antibodies to IL13Rα2 (KH7B9) and β-actin (A1978, SIGMA, St. Louis, MO, USA).
Angiogenesis array
Upregulation of angiogenesis-related factors was determined using a Proteome Profiler™ Human Angiogenesis Antibody Array (R&D Systems, Minneapolis, MN, USA, cat. no. ARY007), which allows the identification of angiogenic factors present in tumour lysates. The array consists 59 types of nitrocellulose membrane-bound antibodies specific for various angiogenic factors. The array was conducted according to the manufacturer’s protocol. Briefly, tumour tissue extracts were prepared from xenografted tumours derived from the SK-MEL-28 and SK-IL13Rα2 cells and incubated with a cocktail of biotinylated antibodies against various angiogenic factors. The mixture was then incubated with membrane-bound anti-angiogenic factors antibodies, followed by incubation of trapped complexes with HRP-conjugated streptavidin, and detection with chemiluminescent detection reagent.
RNA interference
Predesigned small interfering RNAs for IL13Rα2 and negative control were purchased from Invitrogen/Thermo Fisher Scientific. Obtained siRNAs (Stealth RNAi, Cat. no. 1299001 and 1330001) were introduced into cells by using RNA iMAX (Invitrogen) according to the protocol suggested by the manufacturer.
Statistical analyses
Results were compared by Student’s t-test. Differences were considered significant when P < 0.05. All statistical tests were two sided except for animal experiments (one sided).
Supplementary information
Acknowledgements
We thank Dr. Hiroyuki Miyoshi for providing the lentiviral vectors, Ms. Kei Sakuma for technical assistance with histology, and the members of Laboratory of Oncology of Tokyo University of Pharmacy and Life Sciences, and Department of Biochemistry of Tokyo Medical and Dental University for critical discussion. This work was supported in part by a grant for the Precursory Research for Embryonic Science and Technology (PRESTO: JPMJPR12M3) (to TW) from the Japan Science and Technology Agency; Grants-in-Aid for Scientific Research on Innovative Areas, Cellular, and Molecular Basis for Neuro-vascular Wiring (23122504) (to TW) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (MEXT); MEXT-Supported Program for the Strategic Research Foundation at Private Universities at Tokyo University of Pharmacy and Life Sciences (S1411014) (to TW and TF); MEXT KAKENHI Grant Number JP25830123 and JP16K07184 (to TF), Grant-in-Aid for Young Scientists (B) (15K21394 to YY) and Scientific Research (C) (17K07157 to YY) from the Japan Society for the Promotion of Science (JSPS); and grants from Uehara Memorial Foundation (to TW). This study was also conducted as part of a research program of the Project for Cancer Research and Therapeutic Evolution (P-CREATE), the Japan Agency for Medical Research and Development (AMED) (18xx0000000h0001 to TW).
Author Contributions
T.W. and Y.Y. conceived and designed the experiments, analysed the experimental data and wrote the manuscript; H.O., Y.Y., T.T., A.K., R.T., T.M., D.K., K.T., T.O., M.S. and M.K. performed the experiments; K.I., H.U., H.H., K.F., S.I., M.F., and T.F. helped in conceiving and/or analysing the experiments and provided reagents.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hayato Okamoto, Yasuhiro Yoshimatsu, Taishi Tomizawa, Akiko Kunita and Rina Takayama contributed equally.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-019-39018-3.
References
- 1.Shain AH, Bastian BC. From melanocytes to melanomas. Nat Rev Cancer. 2016;16:345–358. doi: 10.1038/nrc.2016.37. [DOI] [PubMed] [Google Scholar]
- 2.Chapman PB, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hauschild A, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–365. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 4.Schadendorf D, et al. Functional and symptom impact of trametinib versus chemotherapy in BRAF V600E advanced or metastatic melanoma: quality-of-life analyses of the METRIC study. Ann Oncol. 2014;25:700–706. doi: 10.1093/annonc/mdt580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flaherty KT, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107–114. doi: 10.1056/NEJMoa1203421. [DOI] [PubMed] [Google Scholar]
- 6.Robert C, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30–39. doi: 10.1056/NEJMoa1412690. [DOI] [PubMed] [Google Scholar]
- 7.Long GV, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet. 2015;386:444–451. doi: 10.1016/S0140-6736(15)60898-4. [DOI] [PubMed] [Google Scholar]
- 8.Franklin C, Livingstone E, Roesch A, Schilling B, Schadendorf D. Immunotherapy in melanoma: Recent advances and future directions. Eur J Surg Oncol. 2017;43:604–611. doi: 10.1016/j.ejso.2016.07.145. [DOI] [PubMed] [Google Scholar]
- 9.Yamaguchi M, et al. Development of a sensitive screening method for selecting monoclonal antibodies to be internalized by cells. Biochem Biophys Res Commun. 2014;454:600–603. doi: 10.1016/j.bbrc.2014.10.133. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki A, Leland P, Joshi BH, Puri RK. Targeting of IL-4 and IL-13 receptors for cancer therapy. Cytokine. 2015;75:79–88. doi: 10.1016/j.cyto.2015.05.026. [DOI] [PubMed] [Google Scholar]
- 11.Wynn TA. IL-13 effector functions. Annu Rev Immunol. 2003;21:425–456. doi: 10.1146/annurev.immunol.21.120601.141142. [DOI] [PubMed] [Google Scholar]
- 12.Zurawski SM, Vega F, Jr, Huyghe B, Zurawski G. Receptors for interleukin-13 and interleukin-4 are complex and share a novel component that functions in signal transduction. EMBO J. 1993;12:2663–2670. doi: 10.1002/j.1460-2075.1993.tb05927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tabata Y, Khurana Hershey GK. IL-13 receptor isoforms: breaking through the complexity. Curr Allergy Asthma Rep. 2007;7:338–345. doi: 10.1007/s11882-007-0051-x. [DOI] [PubMed] [Google Scholar]
- 14.Hershey GK. IL-13 receptors and signaling pathways: an evolving web. J Allergy Clin Immunol. 2003;111:677–690. doi: 10.1067/mai.2003.1333. [DOI] [PubMed] [Google Scholar]
- 15.Hold GL, Untiveros P, Saunders KA, El-Omar EM. Role of host genetics in fibrosis. Fibrogenesis Tissue Repair. 2009;2:6. doi: 10.1186/1755-1536-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blease K, et al. Stat6-deficient mice develop airway hyperresponsiveness and peribronchial fibrosis during chronic fungal asthma. Am J Pathol. 2002;160:481–490. doi: 10.1016/S0002-9440(10)64867-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newman JP, et al. Interleukin-13 receptor α 2 cooperates with EGFRvIII signaling to promote glioblastoma multiforme. Nat Commun. 2017;8:1913. doi: 10.1038/s41467-017-01392-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawakami K, Kawakami M, Snoy PJ, Husain SR, Puri RK. In vivo overexpression of IL-13 receptor α2 chain inhibits tumorigenicity of human breast and pancreatic tumors in immunodeficient mice. J Exp Med. 2001;194:1743–1754. doi: 10.1084/jem.194.12.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balyasnikova IV, et al. Characterization and immunotherapeutic implications for a novel antibody targeting interleukin (IL)-13 receptor α2. J Biol Chem. 2012;287:30215–30227. doi: 10.1074/jbc.M112.370015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawakami K, Kawakami M, Puri RK. Specifically targeted killing of interleukin-13 (IL-13) receptor-expressing breast cancer by IL-13 fusion cytotoxin in animal model of human disease. Mol Cancer Ther. 2004;3:137–147. [PubMed] [Google Scholar]
- 21.Fujisawa T, Joshi B, Nakajima A, Puri RK. A novel role of interleukin-13 receptor α2 in pancreatic cancer invasion and metastasis. Cancer Res. 2009;69:8678–8685. doi: 10.1158/0008-5472.CAN-09-2100. [DOI] [PubMed] [Google Scholar]
- 22.Jarboe JS, Johnson KR, Choi Y, Lonser RR, Park JK. Expression of interleukin-13 receptor α2 in glioblastoma multiforme: implications for targeted therapies. Cancer Res. 2007;67:7983–7986. doi: 10.1158/0008-5472.CAN-07-1493. [DOI] [PubMed] [Google Scholar]
- 23.Beard RE, et al. Gene expression profiling using nanostring digital RNA counting to identify potential target antigens for melanoma immunotherapy. Clin Cancer Res. 2013;19:4941–4950. doi: 10.1158/1078-0432.CCR-13-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishida K, et al. Site-specific randomization of the endogenous genome by a regulatable CRISPR-Cas9 piggyBac system in human cells. Sci Rep. 2018;8:310. doi: 10.1038/s41598-018-30977-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Busser B, Sancey L, Brambilla E, Coll JL, Hurbin A. The multiple roles of amphiregulin in human cancer. Biochim Biophys Acta. 2011;1816:119–131. doi: 10.1016/j.bbcan.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Fujiu K, et al. A heart-brain-kidney network controls adaptation to cardiac stress through tissue macrophage activation. Nat Med. 2017;23:611–622. doi: 10.1038/nm.4326. [DOI] [PubMed] [Google Scholar]
- 27.Ma L, et al. Antisense expression for amphiregulin suppresses tumorigenicity of a transformed human breast epithelial cell line. Oncogene. 1999;18:6513–6520. doi: 10.1038/sj.onc.1203042. [DOI] [PubMed] [Google Scholar]
- 28.Wright K, Kolios G, Westwick J, Ward SG. Cytokine-induced apoptosis in epithelial HT-29 cells is independent of nitric oxide formation. Evidence for an interleukin-13-driven phosphatidylinositol 3-kinase-dependent survival mechanism. J Biol Chem. 1999;274:17193–17201. doi: 10.1074/jbc.274.24.17193. [DOI] [PubMed] [Google Scholar]
- 29.Kawashima R, et al. IL-13 receptor α2 promotes epithelial cell regeneration from radiation-induced small intestinal injury in mice. Gastroenterology. 2006;131:130–141. doi: 10.1053/j.gastro.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 30.Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155–1228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 31.Brożyna AA, VanMiddlesworth L, Slominski AT. Inhibition of melanogenesis as a radiation sensitizer for melanoma therapy. Int J Cancer. 2008;123:1448–1456. doi: 10.1002/ijc.23664. [DOI] [PubMed] [Google Scholar]
- 32.Brożyna AA, Jóźwicki W, Carlson JA, Slominski AT. Melanogenesis affects overall and disease-free survival in patients with stage III and IV melanoma. Hum Pathol. 2013;44:2071–2074. doi: 10.1016/j.humpath.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brożyna AA, Jóźwicki W, Roszkowski K, Filipiak J, Slominski AT. Melanin content in melanoma metastases affects the outcome of radiotherapy. Oncotarget. 2016;7:17844–17853. doi: 10.18632/oncotarget.7528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Slominski A, et al. The role of melanogenesis in regulation of melanoma behavior: melanogenesis leads to stimulation of HIF-1α expression and HIF-dependent attendant pathways. Arch Biochem Biophys. 2014;563:79–93. doi: 10.1016/j.abb.2014.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Omori K, et al. Lipocalin-type prostaglandin D synthase-derived PGD(2) attenuates malignant properties of tumor endothelial cells. J Pathol. 2018;244:84–96. doi: 10.1002/path.4993. [DOI] [PubMed] [Google Scholar]
- 36.Yoshimatsu Y, et al. Bone morphogenetic protein-9 inhibits lymphatic vessel formation via activin receptor-like kinase 1 during development and cancer progression. Proc Natl Acad Sci USA. 2013;110:18940–18945. doi: 10.1073/pnas.1310479110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goto K, Kamiya Y, Imamura T, Miyazono K, Miyazawa K. Selective inhibitory effects of Smad6 on bone morphogenetic protein type I receptors. J Biol Chem. 2007;282:20603–20611. doi: 10.1074/jbc.M702100200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.