Abstract
Carbon nanotubes (CNTs) have been applied in a wide range of fields, such as materials, electronics, energy storages, and biomedicine. With the rapid increase in CNTs industrialization, more and more CNT-containing wastewater is being produced. Since concerns about the toxic effects of CNTs on human health persist, CNT-containing wastewater should not be released into the environment without purification, but no effective methods have been reported. In the present study, we report a simple method to eliminate CNTs from industrial or laboratorial wastewater using sodium hypochlorite. Direct treatment of aqueous dispersions with sodium hypochlorite solution completely degraded CNTs into carbon oxides or carbonates ions. Since hypochlorite is environmentally friendly and frequently used as a disinfectant or bleaching agent in domestic cleaning, this method is practical for purification of CNT-contaminated industrial wastewater.
Introduction
Due to their unique porous structures and outstanding properties, including tremendous flexibility, light weight, high strength and extremely high electrical and thermal conductivity, carbon nanomaterials (CNM) such as carbon nanotubes (CNTs)1,2, nanohorns3 and graphene4 have been explored for various applications in the fields of electronics, materials, and even nanomedicines5–8. The number of CNM-containing products appearing in the market is therefore increasing rapidly. By 2015, there were over 100 CNT-related companies worldwide, and the number is increasing year by year. Annual global production of CNTs is estimated to be in the order of hundreds of tons9. However, accompanying the expansion of the CNT industry is an increasing amount of CNT-containing industrial wastewater that represents a significant threat to the natural environment if discarded. The release of CNT-containing waste water has the potential to damage aquatic life in water columns and sediment compartments10 as well as human health11–13. CNT exposure affects cell viability and algal growth14 and CNTs can act as pesticide carriers affecting fish survival, metabolism, and behavior15. Moreover, toxicology studies have shown that some CNTs might have harmful effects on human health11–13. Compared with micrometer-sized carbon-based particles such as carbon black, multi-walled carbon nanotubes (MWNTs) of similar size induce pulmonary inflammation at a lower dose in animal studies, and inhalation exposure of MWNTs induces malignant mesothelioma in mice, suggesting that MWNTs may pose a similar hazard to asbestos12. Damage to other organs due to pulmonary exposure to MWNTs has also been reported in animal studies12,13. Although most other CNTs are reported to have low acute toxicity, long-term safety is yet to be clarified16–20. To protect the environment and mitigate health problems caused by CNTs in water, they must be removed from industrial wastewater before it is released into the environment. It is easy to remove most CNTs from aqueous solution by filtration, but a few types may pass through the filter pores and enter the environment.
To date, no effective method has been ported for the removal of CNTs from waste systems or other liquids. In general, CNTs are chemically extremely stable substances that are difficult to degrade other than by strong acid or oxidation treatment at high temperature, and such methods are not suitable for their removal from aqueous systems. Recent studies show that CNTs can be degraded by enzymatic oxidation using horseradish peroxidase (HRP) and myeloperoxidase (MPO) from human neutrophils21–24. The biodegradation mechanism of CNTs is thought to involve oxidation by hypochlorous acid that is generated during enzymatic reaction19–22, and sodium hypochlorite or hypochlorous acid have since been shown to oxidize and degrade CNTs24–28. Interestingly, 2D graphene oxide sheets are degraded faster than 1D oxidized CNTs29, and the wall thickness of CNTs might be not a crucial factor for their degradation by hypochlorite30. However, it is still not obvious whether CNTs are completely degraded. In the present study, we demonstrate that hypochlorite can completely remove CNTs from aqueous solutions, suggesting a potential method for the removal of CNTs from CNT-containing industrial wastewater. Sodium hypochlorite and hypochlorous acid are inexpensive and environmentally friendly oxidizing agents that are commonly used as household bleaching or sterilizing agents, suggesting they may be suitable for application in CNT industries and research laboratories.
Results
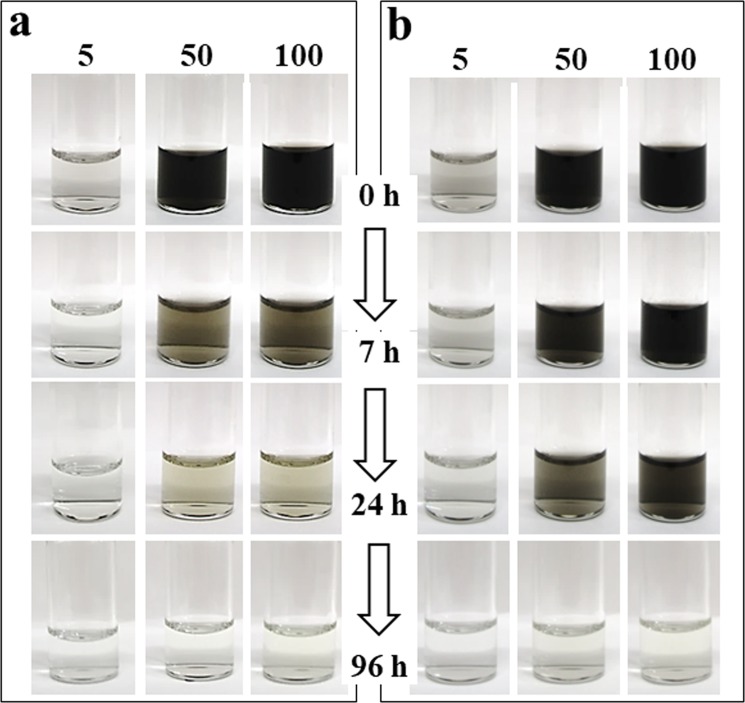
As a demonstration, we show that two typical commercially available CNTs, single-walled carbon nanotubes (SWNTs; SG CNT) and MWNTs (NC 7000), could be completely degraded by treatment with NaClO. To obtain aqueous dispersions, SWNTs or MWNT were pretreated with acids (see Experimental Methods below) and washed with water. The concentrations of CNTs dispersed in water were 5, 50 and 100 mg/L. To monitor the elimination of CNTs from solution, CNT dispersions were mixed with NaClO (1.1%, w/w) aqueous solution and incubated at 37 °C for 1–120 h. The black color of dispersions of SWNTs (Fig. 1a) or MWNTs (Fig. 1b) faded gradually with increasing treatment time, and 5 mg/L dispersions of SWNTs became completely transparent after 24 h, while 50 mg/L and 100 mg/L dispersions became completely transparent after 96 h.
Figure 1.
Images of dispersions of single-walled nanotubes (SWNTs; a) and multi-walled nanotubes (MWNTs; b) after treatment with sodium hypochlorite solution for 0–96 h.
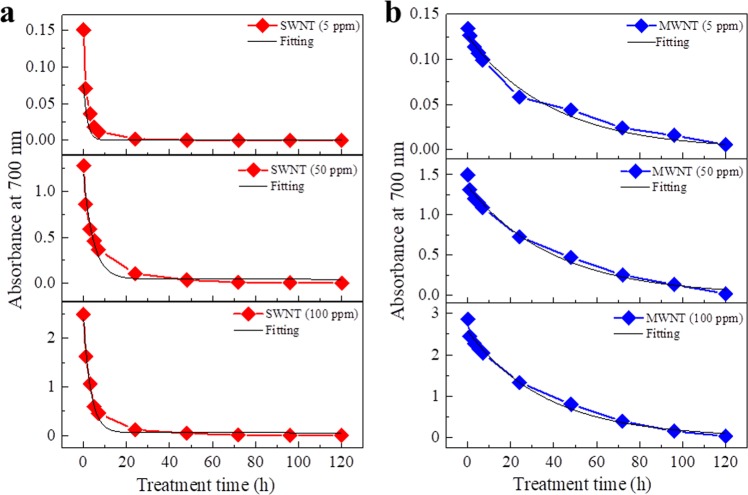
The quantity of CNTs in dispersions was also estimated by measurement of the absorbance at 700 nm. The results showed that SWNTs were dramatically decreased in the first 1 h, then decreased to almost zero over 24 h (Fig. 2a). Degradation of MWNTs at all three concentrations was slower than that of SWNTs (Fig. 2b). In addition, the degradation rates for both SWNTs and MWNTs under the conditions used in this study fitted well to the first-order reaction rate curve Y = y0 + A1e−kt (black curves in Fig. 2a,b), where Y is the concentration of CNTs, y0 is the final concentration of CNTs (approximately zero), A1 is the initial concentration of CNTs, k is reaction constant, and t is the reaction time. The half-value periods for complete degradation of SWNTs and MWNTs were estimated to be 1.25–2.5 h and 21–24 h, respectively.
Figure 2.
Estimation of the quantity of carbon nanotubes (CNTs) in aqueous dispersions following treatment with sodium hypochlorite solution. Red lines (a) and blue lines (b) indicate the absorbance at 700 nm for dispersions of SWNTs and MWNTs, respectively, after treatment for 0–96 h at 37 °C. Black lines represent curves fitted using the formula Y = Y0 + Ae−x/t (Origin software).
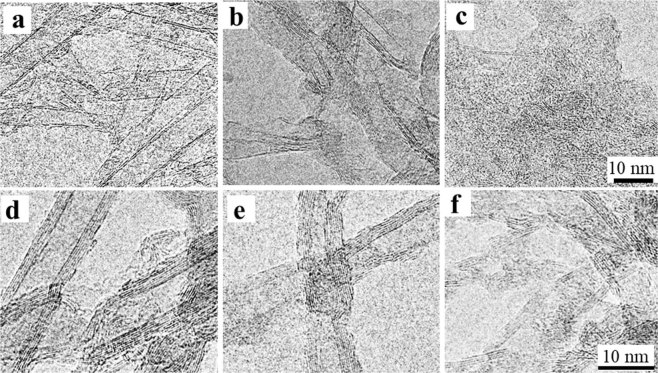
To investigate the structural changes of CNTs, CNT dispersion solutions (100 mg/L) were ultra-filtrated through a 10 kDa molecular weight cut-off membrane and rinsed with water before and after NaClO treatment for 1, 3, and 24 h. The obtained CNTs were analyzed by transmission electron microscopy (TEM) and Raman spectroscopy. TEM images showed that SWNT samples contained single walls, double walls (DWNTs) and a few MWNTs (Fig. 3a), in which DWNTs and MWNTs were impurities. After treatment with NaClO solution for 3 h, single-walled CNTs were no longer visible, and only DWNTs and thin graphite-like structures (or MWNTs) were observed (Fig. 3b). When the treatment time was extended to 24 h, the residues consisted of amorphous-like fragments, and only very few DWNTs or thin graphite-like fragments remained (Fig. 3c). For MWNT samples, structural changes after a 3 h treatment were not obvious (Fig. 3d,e), but a 24 h treatment resulted in residues with more amorphous-like structures, more greater damage to the walls of MWNTs (Fig. 3f).
Figure 3.
Transmission electron microscopy (TEM) images of SWNTs (a–c) and MWNTs (d–f) after treatment with sodium hypochlorite solution for 0, 3, and 24 h.
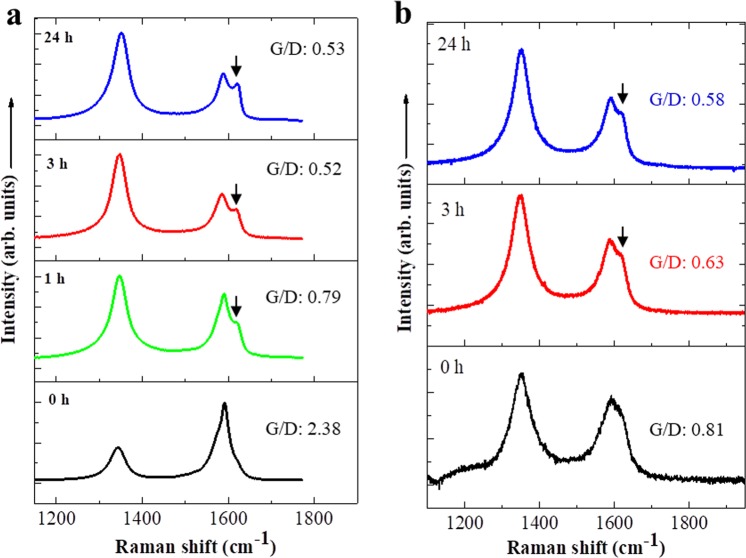
These TEM observations indicate that SWNTs were degraded faster than MWNTs, consistent with the quantity change results described above (Figs 1 and 2). Raman spectra of SWNTs and MWNTs at 100 mg/L after NaClO treatment for 0–24 h (Fig. 4a,b) revealed G-band peaks (graphite) at 1591 cm−1, D-band (disordered) peaks at ~1350 cm−1, and D’ (second-order Raman scattering from D-band variation) peaks at ~1620 cm−1. The ratio of D-band to G-band intensities increased and D’ peaks at ~1620 cm−1 became more obvious in both samples after prolonged NaClO treatment, indicating increased wall damage and amorphous-like structure31 after NaClO treatment. In addition, when the treatment was longer than 120 h for both CNTs samples, no CNTs were detected by TEM or Raman measurements (data not shown).
Figure 4.
Raman spectra of SWNTs (a) and MWNTs (b) after treatment with sodium hypochlorite solution for 0, 1, 3, and 24 h.
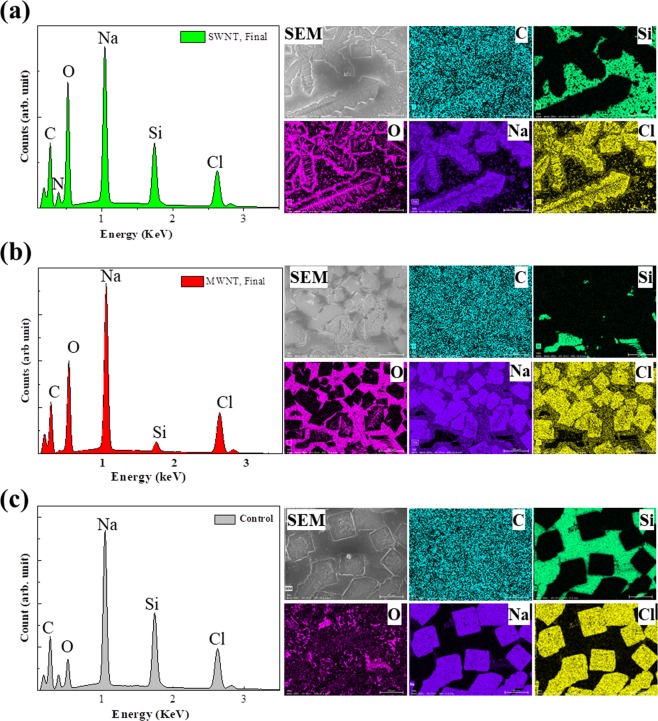
To confirm whether CNTs were completely degraded, we investigated the final colorless solution obtained from NaClO treatment after 120 h (hereafter referred to as the final solution). We heat-treated the obtained final solutions at 75 °C for 1 h to decompose most of NaClO, then analyzed them. First, we added CaCl2 solution to the resultant solution and found that the colorless transparent solution immediately changed into a white turbid solution, indicating the existence of carbonate ions in the final solution (Fig. SI-1). Second, we extracted aromatic hydrocarbons from the resultant solution using CH2Cl2, and materials dissolved in CH2CL2 were analyzed by mass spectrometry. No obvious peaks corresponding to aromatic hydrocarbon molecules were detected (Fig. SI-2), indicating the complete degradation of CNTs. One intermediate product with a mass-peak at 131 m/z (probably C9H8O) was observed in SWNTs treated for 24 h and in MWNTs treated for 96 h (Fig. SI-2a,c), but this peak almost disappeared after further treatment (Fig. S-2b). These results were slightly different from those obtained from enzyme-catalyzed degradation of SWNTs, in which several types of aromatic hydrocarbons were detected25. In addition, the final solutions after removal of ClO− by adding HCl were placed on SiN wafers and dried for analysis by scanning electron microscopy (SEM) in combination with energy dispersive X-ray spectroscopy (SEM-EDS). The spectra indicated that there were elements of C, O, Cl, and Na in all samples, as well as Si and N of the SEM-plate of SiN wafer (Fig. 5). C-mapping images of the final solutions for SWNTs and MWNTs (Fig. 5a,b) were almost the same as that of the control sample (Fig. 5c), indicating that there was little or no C in the samples. The control specimen was prepared by mixing water with NaClO solution and treating with HCl. The results of semi-quantitative analysis confirmed that the percentage of C in all samples was almost the same. Although it is difficult to confirm the exact quantity of C in the samples due to background C from the SiN wafer and the EDS detector, we can confirm that the resultant products in final solutions were mainly NaCl from the 1:1 atom ratio of Na and Cl.
Figure 5.
Scanning electron microscopy (SEM) images, energy dispersive X-ray spectroscopy (EDS) spectra and elemental mapping of C, Si, O, Na and Cl. The results are for SWNTs (a), MWNTs (b) and water controls (c) after sodium hypochlorite treatment for >120 h.
Taking into account the above results, we concluded that CNTs were completely removed by treatment with NaClO, and the resultant products of CNT degradation are [Na+], [Cl−], and [CO32−] or [HCO3−]. NaClO is a powerful oxidizing agent in which [ClO−] donates an oxygen atom and its electrons to the species being oxidized, releasing a chloride ion (Cl−). Thus, the complete degradation of CNTs could be summarized by formula CCNT + 2NaClO → CO2 + 2NaCl, where CO2 is partially or fully changed to [CO32−] or [HCO3−] in aqueous solutions containing NaClO (pH 10).
To determine whether the method proposed in this study is suitable to all types of CNTs or other carbon nanomaterials (CNM), we treated six types of CNMs containing carbon nanohorns (CNHs), SWNTs, and MWNTs, which had not been acid treated but dispersed using bovine serum albumin assisted with sonication31–33. The results (Fig. S-3) showed that the six types of CNMs were completely degraded and that the degradation rates differed depending on CNM type. The degradation rates seemed to be dependent on the diameters of the CNTs in dispersion, in agreement with the results showing that the degradation rates of SWNTs were faster than those of MWNTs (Figs 1 and 2) and previous results showing that CNHs are degraded faster than MWNTs29. We suggest that the larger surface areas of CNTs with smaller diameters provide more reaction sites for reaction with NaClO, resulting in higher reaction rates for CNTs with smaller diameters than for those with larger diameters. We also found that CNTs dispersed by two different surfactants (data not shown) had almost the same degradation rates and were degraded completely. Together, the data indicate all CNT types in dispersion undergo complete degradation by hypochlorite, independent of whether they had been oxidized or not and the type of dispersant employed.
We also found that graphene oxide might be an intermediate product during SWNT degradation (Fig. 3), suggesting that CNT degradation might involve an unzipping process involving oxidization similar to the oxidation of CNTs by strong acid treatment34–36. However, the reaction rates with hypochlorite were much faster than those with strong acid treatment, and no obvious nanoribbon structures were found. In addition, because components in raw waste water are more complex than those in the CNT-dispersions employed in this study, the practicability of the proposed method needs to be confirmed using raw or simulated wastewater.
In summary, our results indicate that CNTs in aqueous solution such as wastewater can be removed completely using simple NaClO treatment. The degradation rates of CNTs with NaClO are dependent on their type: SWNTs were degraded faster than DWNTs, and MWNTs were degraded more slowly than SWNTs or DWNTs. CNTs were completely degraded and left no trace of hydrocarbon components, unlike degradation of CNTs by enzymatic oxidation. Although some issues remain to be clarified, such as the detailed mechanism of degradation of CNTs by NaClO, the relationship between the degradation rate and type of CNTs, and the influence of impurities as well as other substrates in the practical waste water on the degradation of CNTs, we believe that the results reported herein reveal a novel route for establishing a suitable method to eliminate CNTs from industrial wastewater, which is of significance in advancing the environmental sustainability of the CNT industry.
Experimental Methods
Preparation of CNT dispersions
For preparation of CNT aqueous dispersions, 50 mg of SWNTs produced by the super-growth method37 (SG-CNTs, Zeon Nanotechnology Co., Tokyo, Japan) and MWNTs (NC 7000, Nanocyl SA, Belgium) were dispersed and stirred in a 50 mL acidic solution of H2SO4/HNO3 (3:1) at 70 °C for 40 min. After treatment, CNT dispersions were immediately filtered through a 0.2 µm membrane, particles retained on the filter were resuspended in Milli-Q water, and this was repeated five times until the pH was neutral. The obtained CNTs were dispersed in 50 mL water and sonicated using a bath-type sonicator for 20 min to obtain CNT dispersions. The concentration of CNTs was estimated to be ~1 mg/mL.
Degradation of CNTs by hypochlorite
The NaClO solution was prepared by dissolving 2.5 g NaClO∙5H2O powder (Wako, Japan) in water (10 mL), resulting in a NaClO concentration of ~11%. A 0.5 mL sample of NaClO solution was mixed with 5 mL of each of the CNT dispersions, resulting in a NaClO concentration of ~1.0%. The concentration of CNTs in dispersions was 5, 50 or 100 mg/L. After incubating CNT/NaClO mixtures at 37 °C for 0, 1, 3, 7, 24, 48, 72, 96, and 120 h, CNT dispersions were immediately analyzed using a UV-Vis-NIR spectrophotometer (Lambda 19, PerkinElmer Japan Co., Ltd.). The absorbance of each solution at 700 nm was recorded.
Evaluation of structural changes in CNTs during degradation
SWNT and MWNTs were treated with NaClO for 0, 3, or 24 h, filtered using an ultra-centrifuge filter with a 10 kDa molecular weight cut-off, and washed with water five times. The obtained residues were then dispersed in water (0.2 mL) and a single drop of the resultant dispersion was placed on a glass slide and dried at room temperature overnight for Raman spectroscopy (LabRAM HR Evolution, Horiba, Japan). Measurements were taken at an excitation wavelength of 532 nm. The morphology of CNTs was observed using TEM (120 keV, 36 µA; 002B HRTEM, Topcon Corporation). Samples were prepared by placing a drop of each CNT dispersion on a copper grid and drying at room temperature.
Analysis of the final products of CNTs after complete degradation
To assess the final products of CNTs after degradation with NaClO, the resultant final colorless transparent solutions (and the control solution prepared with water without CNTs) were heat-treated at 75 °C for 1 h to remove most of the NaClO, then analyzed. First, 0.5 mL CaCl2 solution (0.05 M) was added to 0.5 mL of the resultant solution to test for color changes. Second, the resultant solution (1 mL) was mixed with CH2Cl2 (1 mL) to extract any organic compounds, and extracts were subjected to liquid chromatography-mass spectrometry (JEOL, MStation, JMS 700, Tokyo, Japan).
For SEM combined with EDS using a Hitachi SU8220 and a BRUKER 5060FlatQUAD, respectively, HCl solution (100 µL, 6.5 M) was added to the final solution (1 mL) and heated at 70 °C for 2–3 min. The obtained solution was checked using a chlorine test-strip (WAP-ClO, Kyoritsu Chemical-Check Lab., Corp. Japan) to confirm that NaClO was completely removed. The pH of the solution was adjusted to neutral by adding NaOH solution, and the final solution was placed on a SiN wafer and dried at room temperature. SEM-EDS measurements were performed at an acceleration voltage of 5.0 kV and an emission current of 10 µA.
Supplementary information
Acknowledgements
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI (Grant-in-Aid for Scientific Research B, 17H02742).
Author Contributions
M.Z. designed the study. M.Z., Y.D. M.Y. and H.N. performed the experiments. M.Z., M.Y., I.S. and T.O. analysed the data. M.Z. wrote the manuscript. All authors discussed the results and contributed to the final manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-38307-7.
References
- 1.Iijima S. S. Helical microtubules of graphitic carbon. Nature. 1991;354:56–58. doi: 10.1038/354056a0. [DOI] [Google Scholar]
- 2.Iijima S, Ichihashi T. Single-shell carbon nanotubes of 1-nm diameter. Nature. 1993;363:603–605. doi: 10.1038/363603a0. [DOI] [Google Scholar]
- 3.Iijima S, et al. Nano-aggregates of single-walled graphitic carbon nano-horns. Chem. Phys. Lett. 1999;309:165–170. doi: 10.1016/S0009-2614(99)00642-9. [DOI] [Google Scholar]
- 4.Novoselov KS, et al. Electric field effect in atomically thin carbon films. Science. 2004;306:666–669. doi: 10.1126/science.1102896. [DOI] [PubMed] [Google Scholar]
- 5.Baughman RH, Zakhidov AA, Heer WA. Carbon nanotubes—the route toward applications. Science. 2002;297:797–792. doi: 10.1126/science.1060928. [DOI] [PubMed] [Google Scholar]
- 6.Volder MD, Tawfick SH, Baughman RH, John Hart A. Carbon nanotubes: Present and future commercial applications. Science. 2013;339:535–539. doi: 10.1126/science.1222453. [DOI] [PubMed] [Google Scholar]
- 7.Ajayan P. M., Zhou O. Z. Applications of Carbon Nanotubes. (eds Dresselhaus M.S., Dresselhaus G. & Avouris P.) Carbon Nanotubes. (Springer, Berlin, Heidelberg, 2001).
- 8.Zhuang L, Tabakman S, Welsher K, Dai H. Carbon nanotubes in biology and medicine: In vitro and in vivo detection, imaging and drug delivery. Nano Res. 2009;2:85–120. doi: 10.1007/s12274-009-9009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zion Market Research “Carbon Nanotubes Market, By Type (Single-Walled Nanotubes (SWNT), Multi-Walled Nanotubes (MWNT)) for Polymers, Electrical and Electronics, Energy, Composites and Other Applications: Global Industry Perspective, Comprehensive Analysis, and Forecast, 2016–2021”. https://www.zionmarketresearch.com/report/carbon-nanotubes-market (2016).
- 10.Blaise C, Gagné F, Férard JF, Eullaffroy P. Ecotoxicity of selected nano-materials to aquatic organisms. Environmental Toxicology. 2008;23:591–598. doi: 10.1002/tox.20402. [DOI] [PubMed] [Google Scholar]
- 11.Poland C, et al. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos like pathogenicity in a pilot study. Nature Nanotechnol. 2008;3:423–428. doi: 10.1038/nnano.2008.111. [DOI] [PubMed] [Google Scholar]
- 12.Takagi A, et al. Induction of mesothelioma in p53+/− mouse by intraperitoneal application of multi-wall carbon nanotube. J. Toxicol. Sci. 2008;33:105–116. doi: 10.2131/jts.33.105. [DOI] [PubMed] [Google Scholar]
- 13.Takagi A, Hirose A, Futakuchi M, Tsuda H, Kanno J. Dose-dependent mesothelioma induction by intraperitoneal administration of multi-wall carbon nanotubes in p53 heterozygous mice. Cancer Science. 2012;103:1440–1444. doi: 10.1111/j.1349-7006.2012.02318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pereira, M. et al. Ecotoxicological effects of carbon nanotubes and cellulose nanofibers in Chlorella vulgaris. Journal of Nanobiotechnology2014, 12:15, 10.1186/1477-3155-12-15 (2014). [DOI] [PMC free article] [PubMed]
- 15.Campos-Garcia J, Martinez DST, Alves OL, Leonardo AFG, Barbieri E. Ecotoxicological effects of carbofuran and oxidised multiwalled carbon nanotubes on the freshwater fish Nile tilapia: Nanotubes enhance pesticide ecotoxicity. Ecotoxicology and Environmental Safety. 2015;111:131–137. doi: 10.1016/j.ecoenv.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Donaldson K, et al. Carbon Nanotubes: A review of their properties in relation to pulmonary toxicology and workplace safety. Toxicol. Sci. 2006;92:5–22. doi: 10.1093/toxsci/kfj130. [DOI] [PubMed] [Google Scholar]
- 17.Kostarelos K. The long and short of carbon nanotube toxicity: toxicological and pharmacological studies suggest guidelines for the safe use of carbon nanotubes in medicine. Nature Biotechnology. 2008;26:774–776. doi: 10.1038/nbt0708-774. [DOI] [PubMed] [Google Scholar]
- 18.Miyawaki J, Yudasaka M, Azami T, Kubo Y, Iijima S. Toxicity of single-walled carbon nanohorns. ACS Nano. 2008;2:213–226. doi: 10.1021/nn700185t. [DOI] [PubMed] [Google Scholar]
- 19.Khalid P, Hussain MA, Suman VB, Arun AB. Toxicology of carbon nanotubes - A review. International Journal of Applied Engineering Research. 2016;11:159–168. [Google Scholar]
- 20.Ong LC, Chung FFL, Tan YF, Leong C. Toxicity of single-walled carbon nanotubes. Arch Toxicol. 2016;90:103–118. doi: 10.1007/s00204-014-1376-6. [DOI] [PubMed] [Google Scholar]
- 21.Allen BL, et al. Biodegradation of single-walled carbon nanotubes through enzymatic catalysis. Nano Lett. 2008;8:3899–3903. doi: 10.1021/nl802315h. [DOI] [PubMed] [Google Scholar]
- 22.Kagan VE, et al. Carbon nanotubes degraded by neutrophil myeloperoxidase induce less pulmonary inflammation. Nat. Nanotech. 2010;5:354–359. doi: 10.1038/nnano.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang M, et al. Biodegradation of carbon nanohorns in macrophage cells. Nanoscale. 2015;7:2834–2840. doi: 10.1039/C4NR06175F. [DOI] [PubMed] [Google Scholar]
- 24.Chiu CF, et al. Nanoemitters and innate immunity: the role of surfactants and bio-coronas in myeloperoxidase-catalyzed oxidation of pristine single-walled carbon nanotubes. Nanoscale. 2017;9:5948–5956. doi: 10.1039/C6NR07706D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allen BL, et al. Mechanistic investigations of horseradish peroxidase-catalyzed degradation of single-walled carbon nanotubes. J. Am. Chem. Soc. 2009;131:17194–17205. doi: 10.1021/ja9083623. [DOI] [PubMed] [Google Scholar]
- 26.Vlasova II, Sokolov AV, Chekanov AV, Kostevich VA, Vasil’ev VB. Myeloperoxidase-induced biodegradation of single-walled carbon nanotubes is mediated by hypochlorite. Russ J Bioorg Chem. 2011;37:510–521. doi: 10.1134/S1068162011040157. [DOI] [PubMed] [Google Scholar]
- 27.Vlasova II, et al. PEGylated single-walled carbon nanotubes activate neutrophils to increase production of hypochlorous acid, the oxidant capable of degrading nanotubes. Toxicol. Appl. Pharmacol. 2012;264:131–142. doi: 10.1016/j.taap.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 28.Lu N, Li J, Tian R, Peng YY. Binding of human serum albumin to single-walled carbon nanotubes activated neutrophils to increase production of hypochlorous acid, the oxidant capable of degrading nanotubes. Chem. Res. Toxicol. 2014;27:1070–1077. doi: 10.1021/tx5001317. [DOI] [PubMed] [Google Scholar]
- 29.Newman L, et al. Hypochlorite degrades 2D graphene oxide sheets faster than 1D oxidised carbon nanotubes and nanohorns. 2D Materials and Applications. 2017;39:774–776. [Google Scholar]
- 30.Masyutin AG, et al. Wall thickness of industrial multi-walled carbon nanotubes is not a crucial factor for their degradation by sodium hypochlorite. Nanomaterials (Basel) 2018;8:715. doi: 10.3390/nano8090715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kinoshita, K. Electrochemical and Physicochemical Properties. Carbon, John Wiley & Sons, New York, Chichester, Brisbane, Toronto, Singapore (1988).
- 32.Fujita K, et al. Physical properties of single-wall carbon nanotubes in cell culture and their dispersal due to alveolar epithelial cell response. Toxicol. Mech. Methods. 2013;23:598e609. doi: 10.3109/15376516.2013.811568. [DOI] [PubMed] [Google Scholar]
- 33.Zhang M, et al. Size-dependent cell uptake of carbon nanotubes by macrophages: A comparative and quantitative study. Carbon. 2018;127:93–101. doi: 10.1016/j.carbon.2017.10.085. [DOI] [Google Scholar]
- 34.Kosynkin DV, et al. Longitudinal unzipping of carbon nanotubes to form graphene nanoribbons. Nature. 2009;458:872–876. doi: 10.1038/nature07872. [DOI] [PubMed] [Google Scholar]
- 35.Dimiev AM, et al. Revisiting the mechanism of oxidative unzipping of multiwall carbon nanotubes to graphene nanoribbons. ACS Nano. 2018;12:3985–3993. doi: 10.1021/acsnano.8b01617. [DOI] [PubMed] [Google Scholar]
- 36.Hu X, et al. One-step oxidation preparation of unfolded and good soluble graphene nanoribbons by longitudinal unzipping of carbon nanotubes. Nanotechnology. 2018;29:145705. doi: 10.1088/1361-6528/aaac1d. [DOI] [PubMed] [Google Scholar]
- 37.Hata K, et al. Water-assisted highly efficient synthesis of impurity-free single-walled carbon nanotubes. Science. 2004;306:1362–1364. doi: 10.1126/science.1104962. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.