Abstract
Dietary supplements, particularly those containing ingredients of natural origin, may contain microbiological contaminants, both bacterial and fungal.
The present study evaluated the microbiological purity of selected dietary supplements containing plant-based ingredients before their release to the market, as well as raw materials of plant origin which are used in the production of such supplements. A total of 122 samples of supplements and 30 materials of plant origin were studied, with 92.1% exhibiting different degrees of bacterial contamination. Eight samples (5.3%) were contaminated by aerobic bacteria in amounts exceeding 105 CFU/g. Five (3.3%) of the studied supplements were found to contain bacteria from the family Enterobacteriaceae at a level exceeding 103 CFU/g. Furthermore, a considerable proportion of the studied samples (86.8%) contained fungal contamination.
Microbiological contamination may contribute to a deterioration in quality and stability of dietary supplements. In addition, high levels of pathogenic bacteria and microorganisms may pose a risk to consumers.
Keywords: Dietary supplements, Microbiological contamination, Raw materials
1. Introduction
Dietary supplements (DS) are foodstuffs and must therefore conform to the definition of food contained in Regulation (EC) No. 178/2002 of the European Parliament and of the Council of 28 January 2002 which provides that: “food (or foodstuff) means any substance or product, whether processed, partially processed or unprocessed, intended to be, or reasonably expected to be, ingested by humans”. Recent decades have seen a marked increase in the popularity of dietary supplements, particularly in developed countries.
People purchase DS in order to improve and sustain their health and vitality, balance their diet, compensate for dietary deficiencies, improve their physical appearance or treat mental conditions. DS often come in forms which are similar to medicinal products (tablet, dragees, capsules). They are also commonly sold in pharmacies. However, they are subject to a variety of legal constraints. Significantly, patients frequently fail to differentiate between dietary supplements and medicinal products. The difference, however, is crucial. For approval purposes, manufacturers of medicinal products are obliged to submit a very detailed drug dossier including characteristics of the active ingredient, data obtained in qualitative, toxicological, preclinical and clinical studies, and verification of the safety of use, therapeutic efficacy and stability. In contrast, the sole requirement applicable to the marketing of dietary supplements in many countries is the submission of an application to appropriate state bodies which are in charge of food safety.
Annex I to the Commission Regulation (EC) No. 1441/2007 of 5 December 2007 specifies microbiological criteria for different groups of foodstuffs, however without identifying dietary supplements as a separate group. The Commission Regulation (EC) No. 1881/2006 of 19 December 2006 indicates maximum levels for certain contaminants in foodstuffs (e.g., nitrates, metals, dioxins, mycotoxins). Dietary supplements are not subject to any laws requiring DS control through conducting studies. There is no state/public institution evaluating the quality and safety of these products. Consequently, the full responsibility for the quality and safety of use of dietary supplements rests with the manufacturers.
A critical indicator of the safety of using dietary supplements is microbiological quality. DS often contain plant-based raw materials as a source of substances with health-promoting properties. Due to their origin, plant materials are frequently subject to contamination by microorganisms from the soil, air, and water. Consequently, they can also cause microbial contamination of dietary supplements.
A broad spectrum of microbes can be found either in or on the plant. Although spore-forming bacteria such as Bacillus spp. and moulds can be regarded as the two dominating groups of contaminants. Most species of the genus Bacillus is saprophytic bacteria but Bacillus cereus, often isolated from plants, is associated with foodborne illnesses and chronic skin infections (Asaeda et al., 2005, Pinna et al., 2001, Carraturo et al., 2018).
Some molds colonizing raw plant are known as potential producer of different mycotoxins, secondary metabolites which exhibit toxic, mutagenic, teratogenic and carcinogenic effects in humans and animals (Benedict et al., 2016, Keter et al., 2017). Pathogens microorganisms, e.g. Escherichia coli and coliform bacteria, Salmonella spp. and Clostridium spp. may also occur (Wilson et al., 2004). These bacteria are responsible for food poisoning and Clostridium spp. can cause gas gangrene and malignant edema too.
Solid dosage forms (capsules, tablets, dragees) are prone to microbial spoilage or degradation. The more serious problem arising from microbial contamination of solid dosage forms is the absence of obvious signs of spoilage.
Microbial infections are not only the result of the physical presence of microorganisms but also their metabolites/toxins that become harmful. Microorganisms can change the physical characteristics of DS, including the breaking of emulsions, the thinning of creams, fermentation of syrups, and can make turbidity or odors and color changes. These changes affect the therapeutic potency and dosage delivery.
In addition, microbial contamination of final DS may be a result of improper handling during production and packing. Several microbes are important microbiological indicator in assessing safety, hygiene, and sanitary quality of DS and processing environments. Total viable bacteria, yeasts, and molds are indicators of hygiene, sanitation, and microbial quality of both raw material and final product. Presence of S. aureus (causative agent of food poisoning and skin infections) in DS and processing environment indicate extensive handling and poor personnel and general hygiene practices. E. coli and coliforms are useful indicators of raw material and water quality and efficacy of production hygiene program.
Despite known risks associated with the presence of different types of contaminants – such as heavy metals, agrochemical residues, and microbial metabolites – in plant-based dietary supplements limited research has been conducted on the microbial contamination of products of this type (Halt and Klapec, 2005, Kineman et al., 2002, Kosalec et al., 2009, Martins et al., 2001, Wilson et al., 2004, Ghisleni et al., 2016).
Dietary supplements and plant-based raw materials are available on the open market over the world, and the number of DS consumers is increasing every year. For these reasons, the microbial quality of dietary supplements is a global issue. The problem of microbiological quality of DS has been addressed in only a few publications. Consequently, the authors of the present study evaluated the level of microbiological contamination found in dietary supplements containing plant-based ingredients and in plant-derived raw materials used in the production of dietary supplements.
2. Materials and methods
The analysis encompassed results of microbiological purity tests of dietary supplements containing plant-derived ingredients (blue whortleberries, banana powder, hawthorn berries, Jerusalem artichoke root, red raspberries, linseeds, extract of globe artichoke leaves) performed as part of the procedure of batch release for marketing, and samples of plant-based materials used in the production of dietary supplements (extracts of nettle, tomato, elderberry, common mullein and gentian). The analyses samples were collected at random. The evaluated parameters were: total aerobic microbial count (TAMC), total yeast/moulds count (TYMC), presence and count of bile-tolerant Gram-negative bacteria in 1 g or 1 mL), and presence of Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus in 1 g or mL, and Salmonella spp. in 10 g or mL. Microbiological testing of the products was conducted in conformity with methods set out in relevant European Pharmacopoeia (Ph. Eur.) monographs as described previously (EP, 2017, Ratajczak et al., 2015).
Briefly, to quantitative enumerate mesophilic aerobic bacteria and fungi pour-plate method were used. After appropriate preparation (depending upon the physical characteristics of the product to be tested) and dilution of the sample, to the Petri dishes 1 mL of each level of dilution of the sample and 15–20 mL of Trypcase Soy bean digest Agar (TSA; MERC) or Sabouraud-Dextrose Agar (SDA; MERC) were added (in duplicate for each medium). After incubation at 34 ± 1 °C for 3–5 days (TSA – TAMC) and 22 ± 1 °C for 5–7 days (SDA – TYMC) the colonies were counted and the number of CFU per gram or milliliter of the product were calculated.
To estimate the number of bile-tolerant Gram-negative bacteria aliquots of sample containing respectively 0.1 g or mL, 0.01 g or mL and 0.001 g or mL of the product to be tested were added to the enterobacteria enrichment Broth-Mossel (BM, MERC). After incubation at 34 ± 1 °C for 24–48 h, samples were subcultured on a plate of Violet Red Bile Glucose agar (MERC) and incubated at 34 ± 1 °C for 18–24 h. Growth of colonies constituted a positive result, and the probable number of bacteria were determined.
To test the absence of bile-tolerant Gram-negative bacteria, appropriate prepared sample corresponding to 1 g or mL of the product was inoculated into BM and incubated and subcultured as described above. The product complied the test if there were no growth of colonies.
For assessing the absence of E. coli, P. aeruginosa and S. aureus prepared sample corresponding to 1 g or mL of the tested product was transferred into Trypcase Soy Broth (TSB; MERC), incubated at 34 ± 1 °C for 18–24 h and next subcultured on a plate of cetrimide agar (MERC), (for P. aeruginosa) or mannitol salt agar (MERC), (for S. aureus), and incubated at 34 ± 1 °C for 18–72 h. To isolate E. coli sample were transferred into MacConcey broth (MERC), incubated at 43 ± 1 °C for 24–48 h, subcultured on MacConcey agar (MERC), and incubated at 34 ± 1 °C for 18–72 h.
To estimate the presence of Salmonella 10 g or mL of the product was inoculated into TSB, incubated at 34 ± 1 °C for 18–24 h. Next, 0.1 mL of TSB were subcultured into 10 mL of Rappaport Vassiliadis Salmonella enrichment broth (MERC), incubated at 34 ± 1 °C for 18–24 h and then subcultured on xylose, lysine, deoxycholate agar (MERC) and incubated at 34 ± 1 °C for 18–48 h.
The possible presence of tested bacteria was confirmed by identification tests. The product complied these tests if colonies typical for specified microorganisms were not present or if the confirmatory identification tests were negative.
The results were interpreted in line with requirements applicable to herbal drugs described in the European Pharmacopoeia.
To illustrate microbial contamination, microorganisms isolated in quantitative tests were identified to the genus or species using conventional microbiological methods.
3. Results
3.1. Quantitative microbial contamination
Tests were performed for a total of 122 samples of dietary supplements containing a variety of plant-derived ingredients, and 30 samples of plant-based raw materials. Table 1 lists results of microbial enumeration tests recorded for the studied DS and raw materials for the production of dietary supplements. The results of DS microbiological purity tests revealed the presence of a high number of aerobic bacteria and fungi in the tested samples.
Table 1.
Quantity of microbial contamination of dietary supplements and raw materials for the production of dietary supplements.
| Plant component | Number of samples tested (n) | TAMC |
TYMC |
||
|---|---|---|---|---|---|
| Number of CFU/g (range) | Samples showing contamination ()* | Number of CFU/g (range) | Samples showing contamination ()** | ||
| Dietary supplements | |||||
| European blueberry fruit (lac. Vaccinium myrtillus) | 50 | <1,0 × 101–2,0 × 105 | 48 (1) | 1,0 × 101–7 × 104 | 50 (2) |
| Powder from banana (lac. Musa L.) | 10 | <1,0 × 101–2,0 × 101 | 3 (0) | 2,0 × 101–6,0 × 101 | 10 (0) |
| Hawthorn berry (lac. Crataegus laevigata) | 10 | 4,0 × 101–4,0 × 103 | 10 (0) | 1,0 × 101–8,0 × 101 | 10 (0) |
| Jerusalem artichoke root (lac. Helianthus tuberosus L.) | 14 | 5,0 × 101–7,0 × 105 | 14 (2) | <1,0 × 101–7,0 × 102 | 11 (0) |
| Raspberry fruit (lac. Rubus idaeus L.) | 10 | <1,0 × 101–3,0 × 102 | 7 (0) | 1,0 × 101–4,0 × 104 | 10 (2) |
| Common flax semen (lac. Linum usitatissimum L.) | 20 | 1,0 × 101–6,0 × 106 | 20 (2) | <1,0 × 101–4,0 × 104 | 7 (1) |
| Globe artichoke leaves extract (lac. Cynara scolymus) | 8 | 1,0 × 101–3,0 × 105 | 8 (1) | 1,0 × 101–2,0 × 102 | 8 (0) |
| Raw materials for the production of dietary supplements | |||||
| Nettle herb (lac. Urtica dioica L.) | 5 | 8,0 × 101–2,5 × 105 | 5 (2) | 6,0 × 101–4,0 × 105 | 5 (2) |
| Tomatose extract (lac. Solanum lycopersicum) | 10 | 3,0 × 101–2,0 × 104 | 10 (0) | 2,0 × 101–6,0 × 104 | 10 (3) |
| Fructus extract (lac. Sambucus nigra L.) | 5 | 3,0 × 101–7,0 × 101 | 5 (0) | 1,0 × 101–9,0 × 104 | 5 (1) |
| Mullein flower extract (lac. Verbascum L.) | 5 | 1,0 × 101–8,0 × 101 | 5 (0) | <1,0 × 101–1,0 × 102 | 2 (0) |
| Gentian extract (lac. Gentiana L.) | 5 | 3,0 × 101–1,7 × 102 | 5 (0) | <1,0 × 101–1,0 × 102 | 4 (0) |
()* numer of samples showing contamination >105 CFU/g; ()** numer of samples showing contamination >104 CFU/g; CFU – colony forming units; TAMC – number of aerobic bacteria; TYMC – number of molds and yeasts.
Contamination of DS by aerobic bacteria varied between less than 1.0 × 101 CFU/g (the number of microbes was below the lower limit of detection) and 6.0 × 106 CFU/g. The results showed that six of the samples exceeded the tolerable limit according to Ph. Eur. The highest aerobic bacterial counts per 1 g of the product were noted in samples containing linseeds, globe artichoke and blue whortleberries.
The distribution of bacteria in raw materials for the production of dietary supplements ranged from 1.0 × 101 CFU/g to 2.5 × 101 CFU/g with two samples of nettle herb in which TAMC exceeded the acceptable limit.
Fungal contamination was noted in 106 (86.9%) samples of DS, ranging between less than 1.0 × 101 CFU/g and 7.0 × 104 CFU/g. The highest number of fungi was recovered from samples of blueberry, raspberry fruit and common flax semen among which 2, 2 and one samples respectively exceeded the limits. Other DS had much lower counts, with the lowest values found in samples of banana powder, hawthorn berry and globe artichoke leaves extract.
The test results for raw materials (RM) showed that the highest fungal contaminations were found in samples of nettle herb, tomatoes and fructus extract with the TYMC ranging from 1.0 × 101 CFU/g to 4.0 × 105 CFU/g.
3.2. Qualitative microbial contamination
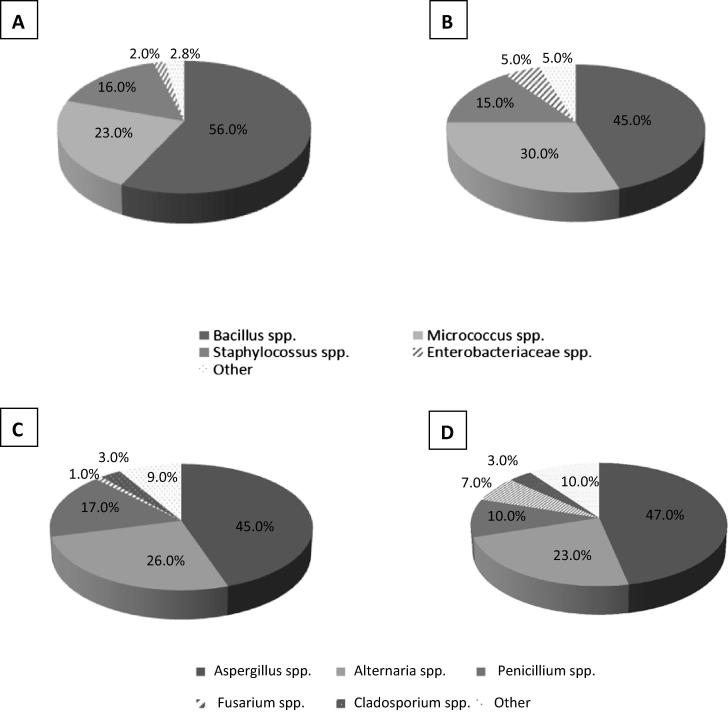
The dominant types of microbial contamination of DS and RM are shown in Fig. 1, while Table 2 shows the percentage of microbial contamination species.
Fig. 1.
The dominant microbial contaminants of dietary supplements and raw materials for the production of dietary supplements: A – bacterial contamination of dietary supplements; B – bacterial contamination of raw materials; C – fungal contamination of dietary supplements; D – fungal contamination of raw materials.
Table 2.
Percentage of each microbial group in the examined dietary supplements and raw materials.
| Identified bacteria | Dietary supplements |
Raw materials |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| European blueberry fruit | Powder from banana | Hawthorn berry | Jerusalem artichoke root | Raspberry fruit | Common flax semen | Globe artichoke leaves extract | Nettle herb | Tomatose extract | Fructus extract | Mullein flower extract | Gentian extract | |
| Bacillus spp. | 48 (96,0)a | 2 (20,0) | 9 (90,0) | 14 (100,0) | 6 (60,0) | 20 (100,0) | 8 (100,0) | 5 (100,0) | 10 (100,0) | 5 (100,0) | 5 (100,0) | 5 (100,0) |
| Staphylococcus spp. | 15 (30,0) | 3 30,0) | – | 5 (35,7) | 4 (40,0) | 1 (5,0) | – | 1 (20,0) | 5 (50,0) | 3 (60,0) | – | – |
| Micrococcus spp. | 18 (36,0) | – | 3 (30) | 7 (50) | – | 11 (55) | 3 (37,5) | 4 (80,0) | 9 (90,0) | 2 (40,0) | 2 (40,0) | 1 (20,0) |
| Enterobacteriaceae | 4 (8,0) | – | – | 6 (42,8) | 2 (20,0) | – | – | 2 (40,0) | 5 (50,0) | – | 1 (20,0) | – |
| Other | 7 (14,0) | – | – | 2 (14,0) | 1 (10,0) | 3 (15,0) | – | 1 (20,0) | 3 (30,0) | – | – | – |
| Identified fungi | ||||||||||||
| Aspergillus spp. | 33 (66,0) | 3 (30,0) | 4 (40,0) | 2 (14,0) | 8 (80,0) | 6 (30,0) | 3 (37,0) | 4 (80,0) | 7 (70,0) | 3 (60,0) | 1 (20,0) | 1 (20,0) |
| Alternaria spp. | 19 (38,0) | 4 (40,0) | 3 (30,0) | 3 (21,0) | 2 (20,0) | 3 (15,0) | 4 (50,0) | 1 (20,0) | 5 (50,0) | 2 (40,0) | – | – |
| Penicillium spp. | 12 (24,0) | 5 (50,0) | 2 (20,0) | – | 1 (10,0) | 2 (10,0) | – | 1 (20,0) | 2 (20,0) | – | 1 (20,0) | – |
| Fusarium spp. | 2 (4,0) | – | – | 1 (7,0) | 1 (10,0) | – | – | 1 (20,0) | 1 (10,0) | – | – | – |
| Cladosporium spp. | 1 (2,0) | – | 2 (20,0) | – | – | 1 (5,0) | 1 (12,0) | – | 1 (10,0) | 1 (20,0) | – | – |
| Other | 6 (12,0) | 2 (20,0) | – | – | 2 (20,0) | – | 2 (25,0) | 1 (20,0) | 2 (20,0) | – | – | 1 (20,0) |
Percent of the test product.
The main bacterial contaminants of dietary supplements and raw materials belong to the Bacillus (56,0%; 45,0%), Micrococcus (23,2%; 30,0%) and Staphylococcus (16,0%; 15,0%) genera. Five samples were highly contaminated with Gram-negative bacteria from the family Enterobacteriaceae in amounts more than103 CFU/g (date not shown). These included two samples of a dietary supplement containing jerusalem artichoke root from which Enterobacter cloacae were isolated, and three samples of a RM – tomato extract (with Klebsiella pneumoniae isolated twice and Klebsiella oxytoca isolated once).
The indicator bacteria E. coli were detected in two samples. They were isolated from a supplement containing Jerusalem artichoke root and raspberries. Mixed microflora was frequently noted. None of tested samples were contaminated with S. aureus, P. aeruginosa and Salmonella spp.
The majority of all tested samples (n = 106) had a mixed fungal contamination pattern with more than one genus present. The dominant genera isolated from DS and RM were respectively: Aspergillus spp. (45.0%; 47.0%), Alternaria spp. (26.3%; 23.0%), Penicillium spp. (17.0%; 10,0%), Fusarium spp. (1.0%; 7.0%), Cladosporium spp. (3.0%; 3.0%) and others (yeasts, Rhizopus spp., Mucor spp.) (9.0%; 10.0%).
4. Discussion
The use of dietary supplements is continually increasing and more information is needed regarding, among others, the microbiological safety of these products. Therefore, the microbial quality of DS is of utmost importance.
The microbial count is one of the DS quality indicators. Our results show that controlled DS and raw materials were contaminated with various levels of microbes. In some samples of even the same tested products, the level of contamination was low but in some samples we found that the microbial contamination exceeded the tolerable limits of 105 CFU/g (6 samples of DS and 2 samples of raw material) for the total aerobic microbial count (TAMC) and 104 CFU/g (5 samples of DS and 6 samples of raw material) for the total combined yeast/moulds count (TYMC).
Similar results were obtained by the authors in a previous study encompassing results of microbiological tests conducted for 1165 dietary supplements containing both synthetic and natural ingredients. The study found that 6.5% of all studied supplements failed to meet microbiological criteria pertaining to medicines. The non-compliances were mainly a result of exceeding the maximum acceptable aerobic bacteria count (50% of non-compliances) and total fungal count (25% of non-compliances). In a lower percentage of cases, they were caused by exceeding the maximum acceptable count of Enterobacteriaceae (17.0%) and the presence of the indicator bacteria E. coli (8.0%) (Ratajczak et al., 2015).
The results obtained by the authors are consistent with the literature data. In her study, Tournas VH examined a total of 138 different dietary supplements, demonstrating that all of them contained microbiological contaminants. The highest aerobic bacterial count was noted for products formulated with addition of alfalfa (5.2 × 106–3.8 × 107 CFU/g), and the lowest – in vitamin and mineral dietary supplements (<100–5.5 × 102 CFU/ (Tournas, 2009).
Brown and Jiang studied a group of 28 herbal supplements, evaluating not only bacterial contamination but also drug sensitivity. The total bacterial count ranged from <5 to 2.9 × 105 CFU/g (Brown and Jiang, 2008).
Microbial contamination of DS may differ depending on the composition, row material used, manufacture process, moisture content, storage conditions (Brown and Jiang, 2008). Therefore microbial counts may vary significantly between products of the same type.
Dietary supplements may be contaminated both by bacteria and fungi (Czech et al., 2001, Raman et al., 2004, Ratajczak et al., 2015, Tournas et al., 2013, Tournas, 2009). These products dietary supplements often include a variety of raw materials of botanic origin. These are mainly raw medicinal plant traditionally used in Europe, including pharmacopoeial, but also plants from Asia and South America. Plant raw materials in dietary supplements are not object to quality control, as it is in the case of herbal medicines. Microbiological contaminants of plant-based materials typically include microbes which occur naturally in the soil, air and water (Kneifel et al., 2002). They can be a source of microbiological contamination due to improper methods of cultivation, collection, drying and storage conditions. Moreover, volatility and sensitivity of the active components of the (medicinal) plants to heat, UV, β and γ irradiation do not permit the use of many decontamination methods (Kneifel et al., 2002). Microbial contamination of dietary supplements may also result from improper handling during production and packing (contaminated processing facilities, human origin).
The natural environment may be a source of potentially disease-causing microorganisms.
The result from this study revealed that occurrence of Bacillus spp. was observed in almost all of the both DS (107/122) and raw materials (30/30) samples. The abundant presence of spore-forming bacteria from the genus Bacillus stems from the fact that this bacteria is widely distributed in nature and its spores are resistant to various physical factors (humidity, temperature) which help to survive manufacturing processes such as drying, heating, etc. The second most dominant genus was Micrococcus (42/122 and 18/30). This is not surprising because microbes belonging to this genus can survive in dry condition, are widely distributed in the environment and can colonize the plants during growth, harvesting and storage. Staphylococcus spp. were found quite often (in 23% of DS and 30% of the raw material), but no samples were containing S. aureus. S. aureus does not represent a natural contaminant of plant-derived materials. It is identified extremely rarely and may be associated with the presence of human carriers of the bacteria. Staphylococcal contamination of final product usually indicates the absence of good manufacturing practices (GMPs) and hygienic conditions during processing and packaging. A few products assessed in the present study were contaminated with enterobacteria. Enterobacteriaceae in quantities exceeding 103 CFU/g were found in five (3.3%) of the supplements under study. The most commonly isolated species included Enterobacter cloacae, Klebsiella pneumoniae and Klebsiella oxytoca. E. coli was detected in two samples. It needs to be stressed, though, that none of the samples was found to contain bacteria with a higher disease-causing potential such as, for example, Salmonella spp.
Furthermore, the presence of bacteria from the family Enterobacteriaceae, chiefly E. coli, points to contamination of fecal origin and may indicate that the hygienic conditions in the manufacturing plant are inadequate. It may also be indicative of the use of natural fertilizers in farming systems.
In their study, Czech et al. (2001) examined a total of 138 herbal medications to determine the presence of various microbiological contaminants. The evaluated parameters included TAMC and the occurrence of different types of pathogenic bacteria. None of the studied samples was shown to contain Salmonella spp., Pseudomonas aeruginosa, Listeria spp., Staphylococcus aureus and Candida albicans. Four samples were contaminated by E. coli, two by Campylobacter jejuni and nine by potentially toxin-producing moulds (Czech et al., 2001).
The presence of bacteria in drugs or dietary supplements may lead to undesirable processes such as decomposition of active ingredients, accumulation of secondary metabolites or iatrogenic infections. Products contaminated by microorganisms, especially drug-resistant types, may contribute to the spreading of resistance genes. In the study reported above Brown and Jiang assessed the resistance of isolated bacteria to nine antibiotics used for therapeutic purposes. Resistant bacteria belonged to several genera including Bacillus, Erwinia, Ewingella, Staphylococcus, Enterobacter and Stenotrophomonas (Brown and Jiang, 2008).
A number of problems arise due to fungal contamination (Mandeel, 2005, Ratajczak et al., 2015, Rizzo et al., 2004, Tournas et al., 2006). Moulds are very widespread and, therefore, they represent a very common contaminant in materials of plant origin. Spores produced by mould fungi spread very easily in the environment by air, and cannot always be eliminated in dietary supplement production processes. Other factors contributing to the contamination of the finished product include the storage and transport conditions of plant-derived raw materials (Keter et al., 2017, Carraturo et al., 2018).
The present study clearly demonstrated that a considerable proportion of the studied samples (86.8%) contained fungal contamination. The most commonly isolated moulds belonged to three genera: Aspergillus spp., Alternaria spp. and Penicillium spp. Every single sample of dietary supplements containing blue whortleberries, banana powder, hawthorn berries, red raspberries and globe artichoke leaf extract was contaminated by fungi. However, only in five samples (two samples of supplements containing raspberries, two with blue whortleberries and one with linseeds), the level of contamination exceeded 104 CFU/g. In their 2015 study, the authors also showed dietary supplements to be contaminated by fungi, and in 19 samples the content of fungi was higher than 103 CFU/g (Ratajczak et al., 2015).
The problem of microbial contamination of dietary supplements by moulds was addressed by Tournas et al. (2006) who reported that seventy-eight percent of ginseng herb supplements, 100% of Siberian, 56% of Chinese and 48% of American ginseng root samples exhibited fungal contamination. Fungi found in ginseng herb were Alternaria alternate, Aspergillus niger, Aspergillus spp., Cladosporium spp., Penicillium spp., Rhizopus spp. and yeasts.
Another study (Tournas, 2009) involved an analysis of 138 different dietary supplements containing alfalfa, Circu-Care, coriander, cumin, echinacea, Garlic, ginger, ginkgo, juniper berries and valerian. The highest levels of fungal contamination were determined for supplements containing alfalfa (5.6 × 106 CFU/g) and the lowest in ginger supplements (1.0 × 102 CFU/g).
In the studies by Tournas et al. (2013), the most commonly isolated fungi represented the genera Aspergillus, Eurotium and Penicillium. Less common were Alternaria alternata, Fusarium, Cladosporium, Rhizopus spp. and Phoma spp. Also, in another study, the same author indicated that 60% of samples of milk thistle dietary supplements were contaminated with fungi (Tournas et al., 2013).
Moulds are capable of surviving even in environments with very low humidity levels. Furthermore, they multiply despite very limited availability of nutrients. The growth of moulds has a potential to reduce the quality and usefulness of DS. The presence of fungi can also lead to the secretion of toxic secondary metabolites. Examples of such toxins are fungal mycotoxins which can be carcinogenic and mutagenic. They can also cause acute and chronic poisoning, allergies, diseases of the respiratory and digestive systems, and liver damage. Plant-based products have repeatedly been shown to contain moulds which may produce mycotoxins posing a threat to human health (Bugno et al., 2006, Benedict et al., 2016, Rajeshwari and Raveesha, 2016, Fanelli et al., 2017). Bugno et al. (2006) tested a total of 91 samples of medicinal plants to determine contamination by potentially toxigenic moulds. Mould contamination levels exceeding 2 × 103 CFU/g were found in 54.9% of the samples. The isolated fungi were dominated by two genera: Aspergillus and Penicillium. Nearly 22% of the Aspergillus and Penicillium isolates had a capacity to produce mycotoxins (aflatoxins, ochratoxin A).
The results of this study implicate an impending danger for consumers. The presence of even low levels of pathogenic microorganisms, higher levels of opportunistic pathogens or toxic microbial metabolites, which persist even after the elimination of primary contaminants, may result in products being ineffective. Microbial infections, not only regarding the physical presence of microorganisms but also their metabolites/toxins, could produce harmful effects even at low levels.
The results of our research and literature data presented in this work indicate the urgent need for introducing legal regulations concerning the microbiological quality of DS, defining both acceptance criteria and test methods. It seems that due to the same form (tablets, capsules, dragees) and composition of DS as drugs, and usually high recommended doses, acceptance criteria for DS should be not less than for plant medicines according to the criteria contained in chapter 5.1.8. Microbiological Quality of Herbal Medicinal Products for Oral Use and Extracts Used in their Preparation of European Pharmacopoeia.
The concept of good agricultural, collection and manufacturing practices, as well as quality assurance, should be similar to those employed in the manufacture of medicines too.
Footnotes
Peer review under responsibility of King Saud University.
References
- Asaeda G., Caicedow G., Swanson C. Fried rice syndrome. JEMS. 2005;30:30–32. doi: 10.1016/S0197-2510(05)70258-8. [DOI] [PubMed] [Google Scholar]
- Benedict K., Chiller T.M., Mody R.K. Invasive fungal infections acquired from contaminated food or nutritional supplements: a review of the literature. Foodborne Pathog Dis. 2016;13:343–349. doi: 10.1089/fpd.2015.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.C., Jiang X. Prevalence of antibiotic – resistant bacterial on herbal products. J. Food Prot. 2008;71:1486–1490. doi: 10.4315/0362-028x-71.7.1486. [DOI] [PubMed] [Google Scholar]
- Bugno A., Almodovar A.A.B., Pereira T.C., Andreoli Pinto T.J., Sabino M. Occurrence of toxigenic fungi in herbal drugs. B.J.M. 2006;37:47–51. [Google Scholar]
- Carraturo F., De Castro O., Troisi J., De Luca A., Masucci A., Cennamo P., Trifuoggi M., Aliberti F., Guida M. Comparative assessment of the quality of commercial black and green tea using microbiology analyses. BMC Microbiol. 2018;18:4. doi: 10.1186/s12866-017-1142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commission Regulation (EC) No. 1441/2007 of 5 December 2007 amending Regulation (EC) No. 2073/2005 on microbiological criteria for foodstuffs.
- Commission Regulation (EC) No. 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law.
- Czech E., Kneifel W., Kopp B. Microbiological status of commercially available medicinal herbal drugs – a screening study. Planta Med. 2001;67:263–269. doi: 10.1055/s-2001-12007. [DOI] [PubMed] [Google Scholar]
- European Pharmacopoeia 9th Edition, 2017. European Directorate for the Quality of Medicines – EDQM, pp. 163–170.
- Fanelli F., Cozzi G., Raiola A., Dini I., Mulè G., Logrieco A.F., Ritieni A. Raisins and currants as conventional nutraceuticals in Italian market: natural occurrence of ochratoxin A. J. Food Sci. 2017;82:2306–2312. doi: 10.1111/1750-3841.13854. [DOI] [PubMed] [Google Scholar]
- Ghisleni D.D., Braga Mde S., Kikuchi I.S., Braşoveanu M., Nemţanu M.R., Dua K., Pinto Tde J. The microbial quality aspects and decontamination approaches for the herbal medicinal plants and products: an in-depth review. Curr. Pharm. Des. 2016;22:4264–4287. doi: 10.2174/1381612822666160623070829. [DOI] [PubMed] [Google Scholar]
- Halt M., Klapec T. Microbial populations in medicinal and aromatic plants and herbal teas from Croatia. Ital. J. Food Sci. 2005;17:349–354. [Google Scholar]
- Keter L., Too R., Mwikwabe N., Mutai C., Orwa J., Mwamburi L., Ndwigah S., Bii C., Korir R. Risk of fungi associated with aflatoxin and fumonisin in medicinal herbal products in the Kenyan market. Sci. World J. 2017 doi: 10.1155/2017/1892972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kineman B., Nahikian-Nelms M.L., Frazier C. A pilot investigation of the microbial contamination of herbal supplements: a potential risk for immunocompromised populations. HIV Nutr. Update. 2002;7:1–9. [Google Scholar]
- Kneifel W., Czech E., Kopp B. Microbial contamination of medicinal plants. Planta Med. 2002;68:5–15. doi: 10.1055/s-2002-20060. [DOI] [PubMed] [Google Scholar]
- Kosalec I., Cvek J., Tomic S. Contaminants of medicinal herbs and herbal products. Arh. Hig. Rada Toksikol. 2009;60:485–501. doi: 10.2478/10004-1254-60-2009-2005. [DOI] [PubMed] [Google Scholar]
- Mandeel Q.A. Fungal contamination of some imported spices. Mycopathologia. 2005;159:291–298. doi: 10.1007/s11046-004-5496-z. [DOI] [PubMed] [Google Scholar]
- Martins H.M., Martins M.L., Dias M.I., Bernardo F. Evaluation of microbiological quality of medicinal plants used in natural infusions. Int. J. Food Microbiol. 2001;68:149–153. doi: 10.1016/s0168-1605(01)00480-9. [DOI] [PubMed] [Google Scholar]
- Pinna A., Sechi L.A., Zanetti S., Usai D., Delogu G., Cappuccinelli P., Carta F. Bacillus cereus keratitis associated with contact lens wear. Ophthalmology. 2001;108:1830–1834. doi: 10.1016/s0161-6420(01)00723-0. [DOI] [PubMed] [Google Scholar]
- Rajeshwari P., Raveesha K. Mycological analysis and aflatoxin B1 contaminant estimation of herbal drug raw materials. Afr J Tradit Complement Altern Med. 2016;12:13123–13131. doi: 10.21010/ajtcam.v13i5.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman P., Patino L.C., Nair M.G. Evaluation of metal and microbial contamination in botanical supplements. J. Agric. Food Chem. 2004;52:7822–7827. doi: 10.1021/jf049150+. [DOI] [PubMed] [Google Scholar]
- Ratajczak M., Kubicka M.M., Kamińska D., Sawicka P., Długaszewska J. Microbial contamination of select dietary supplements. SPJ. 2015;23:303–307. doi: 10.1016/j.jsps.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo I., Vedoya G., Maurutto S., Haidukowski M., Varsavsky E. Assessment of toxigenic fungi on Argentinean medicinal herbs. Microbiol. Res. 2004;159:113–120. doi: 10.1016/j.micres.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Tournas V.H. Microbial contamination of select dietary supplements. J. Food Saf. 2009;29:430–442. [Google Scholar]
- Tournas V.H., Katsoudas E., Miracco E.J. Moulds, yeasts and aerobic plate counts in ginseng supplements. Int. J. Food Microbiol. 2006;108:178–181. doi: 10.1016/j.ijfoodmicro.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Tournas V.H., Rivera Calo J., Sapp C. Fungal profiles in various milk thistle botanicals from US retail. Int. J. Food Microbiol. 2013;64:87–91. doi: 10.1016/j.ijfoodmicro.2013.03.026. [DOI] [PubMed] [Google Scholar]
- Wilson C., Dettenkofer M., Jonas D., Daschner F.D. Pathogen growth in herbal teas used in clinical settings: a possible source of nosocomial infection? Am. J. Infect. Control. 2004;32:117–119. doi: 10.1016/j.ajic.2003.09.004. [DOI] [PubMed] [Google Scholar]