Abstract
Background
At a glance commentary.
Scientific background on the subject
Since Adriamycin is used as an anticancer drug among various communities and is one of its side-effects, it is important to find a way to prevent this complication. Of these, medicinal plants such as Zataria multiflora can be important in preventing the complications.
What this study adds to the field
This study shows that carvacrol has a stronger effect on the liver antioxidant system as well as hepatic enzymes activity than the whole plant extract, and then carvacrol can be more helpful than whole plant in the treatment of hepatic damage caused by Adriamycin.
Due to antioxidant effects of Zataria multiflora (ZM) and Carvacrol (CAR) in many cases and the prominent role of reactive oxygen species (ROS) in hepatotoxicity induced by Adriamycin (ADR), the aim of this study is to investigate the effects of ZM and CAR on ADR-induced hepatotoxicity.
Methods
Twenty four male Wistar rats were randomly divided into four groups including: 1)Control, 2)Adriamycin (ADR), 3,4) ZM + ADR and CAR + ADR that received ZM and CAR for 28 consecutive days. Blood samples were collected on the days 0, 14 and 28 to determine the alkaline phosphatase (ALP), aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels. Also, the hepatic redox markers were evaluated.
Results
ADR significantly increased ALP, ALT and AST in comparison with the control (p < 0.05 - p < 0.001). In CAR + ADR group, the serum ALP, ALT and AST were significantly reduced compared to those of the ADR group (p < 0.01 to p < 0.001). Also, in ZM + ADR group, serum ALP and ALT compared to ADR was significantly reduced (p < 0.001). MDA level in the ADR group significantly increased compared to control (p < 0.01). The MDA level in ZM + ADR (p < 0.05) and CAR + ADR (p < 0.01) groups were significantly reduced compared to that of ADR. Thiol levels in ZM + ADR group significantly increased compared to the ADR group (p < 0.05). The activities of CAT in the ADR group was significantly reduced compared to control (p < 0.05) and increased in treatment groups in comparison with the ADR (p < 0.01).
Conclusion
Long-term administration of ZM extract and CAR could reduce the oxidative damage in the rat liver induced by ADR through the strengthening of the antioxidant system.
Keywords: Adriamycin, Zataria multiflora, Carvacrol, Hepatotoxicity
One of the chemotherapy agents that are used in the treatment of many cancers is Adriamycin (ADR). ADR has various side effects [1] as it is used in animal experiments to induce nephrotoxicity [2], [3], hepatotoxicity [4], [5], cardiotoxicity [6], [7] and even neurotoxicity [8]. Clearance of ADR from plasma is high but this drug and its metabolites are concentrated in many organs such as the liver and kidneys. Also, the drug excretion occurs via the liver [9]. Drug-induced liver damage is a major cause of acute and chronic liver disease, and the severity of this injury varies from non-specific changes in the liver structure to acute liver failure, cirrhosis and liver cancer. Some mechanisms are involved in hepatotoxicity induced by ADR such as intercalating into the nuclear DNA, impaired transcription and cell proliferation, inhibition of topoisomerase II activity, injuring the mitochondria and cell membrane structure. Also, production of reactive oxygen species (ROS) is the main mechanism [10].
Therefore, further studies in the herbal medicine field help us reduce the application of the chemical medications that bear numerous side effects and are expensive.
Zataria multiflora (ZM) is also known as Avishan Shirazi is a plant from the Lamiaceae family [11]. ZM has different effective compounds as the essential oils such as p-cymene (10%), thymol (16%) and Carvacrol (CAR) (52%) [12], [13]. CAR (2-methyl-5-(1-methylethyl)-phenol) is a potent monoterpenic phenol that is found in several essential oils of the Lamiaceae family [14]. ZM and CAR have an ameliorating effect on many conditions such as asthma [15], [16], cytotoxic effect on cancer [17], anti-inflammatory effect on ulcer [18] and COPD [19], [20], [21] and antioxidant effect [22], [23], [24].
Due to the antioxidant effects of ZM and CAR in many cases and the prominent role of the ROS in hepatotoxicity induced by ADR, the present study was designed to investigate the effects of hydroalcoholic extract of ZM and CAR on ADR-induced hepatotoxicity in rat.
Materials and methods
Extract preparation
ZM was purchased from an herbal shop in Mashhad, Khorasan Razavi, Iran and identified by Eng. Joharchi as a botanist in the Ferdowsi University of Mashhad herbarium (ID:35314).
ZM aerial parts, about 100 gr were dried, ground, weighed, and homogenized in 70% ethanol at a ratio of 1:10 of the plant to ethanol and macerated for 72 h at 37 °C with occasional shaking. The mixture was then filtered and the resulting liquid was concentrated under decreased pressure at 45 °C in a rotary evaporator named EYELA. The concentrated extract was then kept in the incubator at 45 °C for 72 h to evaporate the ethanol residue yielding the crude extract. Consequently, the extract was dissolved in saline before being gavaged to rats.
Extract standardization
The polyphenols content of the ZM extract evaluated based on the Folin–Ciocalteu method in which ZM extract has 37.1 mg gallic acid/g crude extract. Also, the flavonoids content of the ZM extract estimated based on the aluminum chloride colorimetric assay in which ZM extract has 13.7 mg quercetin/g crude extract. Finally, the anthocyanin content of the ZM extract evaluated based on the pH-differential method described by Rodriguez-Saona in which ZM extract has 1.9 mg/g crude extract. The line equations for standard curves of gallic acid and quercetin were Y = 0.0669X + 0.0116 and Y = 0.06632X − 0.01448, respectively.
Chemicals and drugs
All chemicals including ethanol and CAR were obtained from Sigma. The ADR was obtained from Pharma Iran Company.
Animals and treatments
Twenty four male Wistar rats (250–300 g) were kept on a 12 h light–dark cycle, under constant temperature (22 ± 2 °C), and were allowed free access to standard laboratory diet and water. The ethical code is IR.MUMS.fm.REC.1396.470.
Animals were randomly divided into four groups (n = 6 in each group) including:
-
1.
The control group that received saline via the tail vein on the 1st day of the study.
-
2.
Adriamycin group (ADR) that received ADR (5 mg/kg) [5] via the tail vein on the 1st day of the study and saline for 28 consecutive days [25].
-
3.
ZM extract plus ADR group (ZM + ADR) which received ZM (200 mg/kg) [26] by gavage for 28 consecutive days.
-
4.
CAR extract plus ADR group (CAR + ADR) which received CAR (20 mg/kg) [27] by gavage for 28 consecutive days.
Preparation of samples
Orbital blood samples (about 2 ml) were collected on the days 0 (before ADR injection), 14 and 28 (After ADR injection) and after centrifugation (3000 g), the obtained serum (about 1 ml) was used for determination of the serum bilirubin, albumin, Alkalin phosphatase (ALP), Aspartate aminotransferase (AST) and Alkaline aminotransferase (ALT) levels (50 μl of serum was used to each of the above determinations) by using the related kits (Pars Azmoon Company, Tehran, Iran).
For bilirubin determination, direct bilirubin in the presence of diazo 2 and 4 di-chlorine aniline forms a red-colored acetic acid compound in acidic media. A combination of detergents makes it possible to measure the total bilirubin measurements. Direct bilirubin measures the amount of conjugated bilirubin and water soluble. The nonconjugated bilirubin value can be determined from the difference between total bilirubin and direct bilirubin.
Base of the ALP determination is below formula:
| P-Nitrophenylphosphate + H2O→(In presence of ALP)→ Phosphate + P-Nitrophenol |
The activity of the AST and ALT enzymes measured by the Ritman and Frankel methods. The activity of the enzyme directly depends on the amount of oxaloadtate and pyruvate produced in the reaction based on this method.
Also, weighing the rats was performed at the beginning and the end of the experimental period (difference (Δ) weight = second weight − first weight). On the 28th day of the experiment the animals were deeply anesthetized by urethane and the liver tissues were rapidly excised and after weighing, stored at −80 °C for redox marker evaluation.
Total thiol contents were evaluated by using DTNB (2, 2′-dinitro- 5, 5′-dithiodibenzoic acid), a reagent that reacts with the SH groups and produces a yellow colored complex that has a peak absorbance at 412 nm [28]. Generally, 1 ml Tris–EDTA buffer (pH = 8.6) was added to 50 μl of liver homogenate in 1 ml cuvettes and the absorbance was read at 412 nm versus Tris–EDTA buffer (A1). Then 20 μl of DTNB reagents (10 mM in methanol) was added to the mixture and after 15 min incubation in room temperature, the absorbance was read again (A2). Also, the absorbance of DTNB reagent was read as a blank (B). A total thiol content (mM) was calculated based on an equation described by Hosseini et al. [29], [30].
Malondialdehyde (MDA) concentrations as an index of lipid peroxidation were evaluated in the liver tissue. MDA reacts with thiobarbituric acid (TBA) as a thiobarbituric acid reactive substance (TBARS) and produces a red colored complex which has a peak absorbance at 535 nm. Briefly, 2 ml TBA/trichloroacetic acid (TCA)/hydrochloric acid (HCL) reagent was added to 1 ml of homogenate and the solution was incubated in a boiling water bath for 40 min. After cooling, the whole solutions were centrifuged at 1000 g for 10 min. The absorbance of the supernatant was read at 535 nm. The MDA concentration (C) was calculated as follows [29], [30].
| C (m) = Absorbance/ (1.65 × 105) |
Catalase (CAT) activity was estimated by using the Aebi method. The principle of the assay is based on a determination of the rate constant, k, (dimension: s-1, k) of hydrogen peroxide decomposition. By measuring the reduction in absorbance at 240 nm per minute, the rate constant of the enzyme was evaluated. Activities were expressed as k (rate constant) per liter [31].
Superoxide dismutase (SOD) activity was evaluated by the procedure explained by Madesh and Balasubramanian [32]. A colorimetric assay involving the production of superoxide by pyrogallol auto-oxidation and the inhibition of superoxide-dependent reduction of the tetrazolium dye, MTT (3-(4, 5-dimethylthiazol-2-yl) 2, 5-diphenyltetrazolium bromide) to its formazan by SOD was determined at 570 nm. One unit of SOD activity was defined as the amount of enzyme causing 50% inhibition in the MTT reduction rate.
Liver index percentage was calculated according to the following formula:
| % liver index = liver weight/body weight |
Statistical analysis
All data were expressed as means ± SEM. Normality test (Kolmogorov–Smirnov) was performed. Serum parameters in the different days in each group were compared by two way repeated measures of ANOVA and redox parameters in different groups were compared by one way ANOVA followed by LSD Post Hoc comparison test using the SPSS software 11.5. Differences were considered statistically significant when p < 0.05.
Results
Weight measurement
The weight changes in the ADR group significantly decreased compared to the control group (p < 0.01) while in the CAR + ADR group significantly increased compared to ADR group (p < 0.01). Also, the liver index percentage in the ADR group significantly increased compared to the control group (p < 0.01) while in treatment groups no significant increase was observed compared to the ADR group [Table 1].
Table 1.
Weight changes in different groups of rat. Values are means ± SEM (n = 6).
| Weight (g) | Body weight Day 0 |
Body weight Day 14 |
Body weight Day 28 |
Liver weight Day 28 |
Weight changes | % Liver index |
|---|---|---|---|---|---|---|
| Groups | ||||||
| Control | 266 ± 11.1 | 254 ± 10.3 | 284 ± 12.0 | 11.0 ± 0.3 | 18.0 ± 4.6 | 3.9 ± 0.1 |
| ADR | 243 ± 7.8 | 200 ± 6.2 | 193.6 ± 5.8** | 12.6 ± 0.5 | −49.4 ± 17.6** | 5.5 ± 0.6** |
| ZM + ADR | 221 ± 6.7 | 200 ± 7.9 | 185 ± 8.7 | 10.2 ± 1.1 | −36 ± 9.3 | 5.1 ± 0.1 |
| CAR + ADR | 236 ± 6.0 | 230 ± 9.4 | 235.6 ± 12.6## | 8.4 ± 0.4 | 1.0 ± 10.8## | 4.4 ± 0.3 |
**p < 0.01 compared to control group, ##p < 0.01 compared to ADR group. Statistical analyses were made using the one-way ANOVA followed by the LSD post hoc.
Liver enzymes assay
Results showed that the total and direct bilirubin in all groups in each time did not show any significant difference [Table 2, Table 3].
Table 2.
Comparison the total bilirubin concentration between four groups (n = 6 in each group).
| Total Bilirubin (mg/dl) | Day 0 | Day 14 | Day 28 |
|---|---|---|---|
| Control | 0.68 ± 0.02 | 0.70 ± 0.03 | 0.64 ± 0.02 |
| ADR | 0.74 ± 0.02 | 0.76 ± 0.04 | 0.68 ± 0.03 |
| ZM + ADR | 0.70 ± 0.00 | 0.69 ± 0.02# | 0.64 ± 0.02 |
| CAR + ADR | 0.70 ± 0.00 | 0.70 ± 0.00 | 0.66 ± 0.02 |
ADR: Adriamycin, Z.M: Zataria multiflora, CAR: Carvacrol.
Values are the Mean ± SEM Statistical analyses were made using the one-way ANOVA followed by the LSD post hoc.#:p < 0.05, compared to ADR group.
Table 3.
Comparison the direct bilirubin concentration between four groups (n = 6 in each group).
| Direct Bilirubin (mg/dl) | Day 0 | Day 14 | Day 28 |
|---|---|---|---|
| Control | 0.12 ± 0.02 | 0.12 ± 0.02 | 0.1 ± 0.00 |
| ADR | 0.12 ± 0.02 | 0.12 ± 0.02 | 0.12 ± 0.02 |
| ZM + ADR | 0.1 ± 0.00 | 0.1 ± 0.00 | 0.1 ± 0.00 |
| CAR + ADR | 0.1 ± 0.00 | 0.1 ± 0.00 | 0.1 ± 0.00 |
ADR: Adriamycin, Z.M: Zataria multiflora, CAR: Carvacrol.
Values are the Mean ± SEM. Statistical analyses were made using the one-way ANOVA followed by the LSD post hoc.
Effect of ADR, ZM and CAR on serum ALP, ALT and AST have been shown in Table 4, Table 5, Table 6, respectively. ADR significantly increased the ALP, ALT and AST in comparison with the control group in days 14 and 28 (p < 0.05- p < 0.001). In CAR + ADR group, the serum ALP, ALT and AST were significantly reduced compared to ADR group in days 14 and 28 (p < 0.01 to p < 0.001). Also, in ZM + ADR group, the serum ALP and ALT compared to ADR group in days 14 and 28 (p < 0.01 to p < 0.001) were significantly reduced.
Table 4.
Comparison the Alkalin Phosphatase (ALP) concentration between four groups (n = 6 in each group).
| ALP (IU/L) | Day 0 | Day 14 | Day 28 |
|---|---|---|---|
| Control | 242 ± 20.59 | 227.8 ± 30.44 | 248.4 ± 16.32 |
| ADR | 259.8 ± 18.77 | 805.4 ± 12.41*** | 771.4 ± 52.75*** |
| ZM + ADR | 266.4 ± 29.63 | 264.8 ± 28.08### | 175 ± 17.95### |
| CAR + ADR | 247.8 ± 29.48 | 232.8 ± 11.86### | 196 ± 23.15### |
***:p < 0.001, compared to Co group.
###:p < 0.001, compared to ADR group.
ADR: Adriamycin, Z.M: Zataria multiflora, CAR: Carvacrol.
Values are the Mean ± SEM. Statistical analyses were made using the one-way ANOVA followed by the LSD post hoc.
Table 5.
Comparison the Alanine aminotransferase (ALT) concentration between four groups (n = 6 in each group).
| ALT (IU/L) | Day 0 | Day 14 | Day 28 |
|---|---|---|---|
| Control | 54.6 ± 8.82 | 51.6 ± 10.43 | 55 ± 2.19 |
| ADR | 57.2 ± 6.61 | 71 ± 2.36* | 162.2 ± 9.78*** |
| ZM + ADR | 48.6 ± 4.16 | 36.2 ± 2.97### | 39.6 ± 3.88### |
| CAR + ADR | 52.8 ± 10.07 | 48.2 ± 3.10## | 45 ± 1.51### |
*:p < 0.05, ***:p < 0.001 compared to Co group.
##:p < 0.01, ###:p < 0.001 compared to ADR group.
ADR: Adriamycin, Z.M: Zataria multiflora, CAR: Carvacrol.
Values are the Mean ± SEM. Statistical analyses were made using the one-way ANOVA followed by the LSD post hoc.
Table 6.
Comparison the Aspartate aminotransferase (AST) concentration between four groups (n = 6 in each group).
| AST (IU/L) | Day 0 | Day 14 | Day 28 |
|---|---|---|---|
| Control | 119.6 ± 22.77 | 122 ± 5.41 | 118 ± 1.58 |
| ADR | 120.6 ± 23.02 | 187.4 ± 7.66*** | 145 ± 4.04 |
| ZM + ADR | 114 ± 10.04 | 199.8 ± 10.14 | 129.8 ± 21.53 |
| CAR + ADR | 113.6 ± 5.23 | 179.6 ± 5.45 | 122.6 ± 11.88 |
***:p < 0.001 compared to Co group.
ADR: Adriamycin, Z.M: Zataria multiflora, CAR: Carvacrol.
Values are the Mean ± SEM. Statistical analyses were made using the one-way ANOVA followed by the LSD post hoc.
Oxidative stress parameters assay
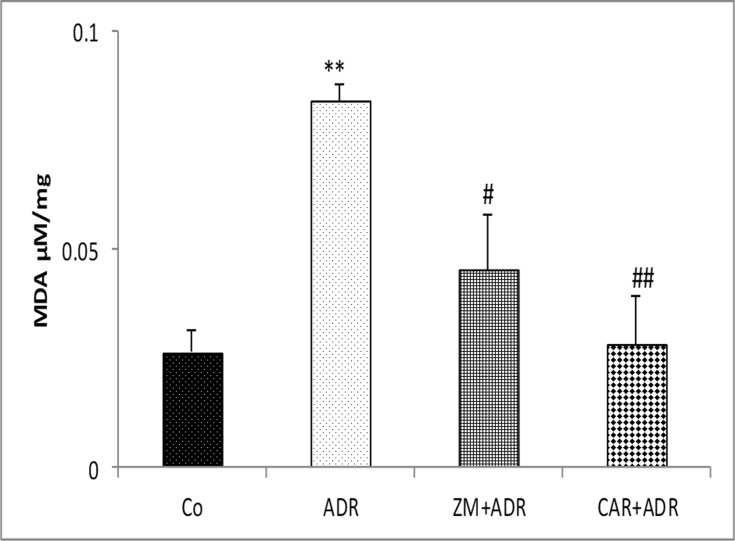
MDA level in the liver tissue in ADR group significantly increased compared to control group (p < 0.01). The MDA level in ZM + ADR (p < 0.05) and CAR + ADR (p < 0.01) groups was significantly reduced in comparison with the ADR group [Fig. 1].
Fig. 1.
Comparison of the MDA concentration in liver tissue of four groups. Data are presented as Mean ± SEM (n = 6 in each group). **p < 0.01 compared with Co group. #p < 0.05 and ##p < 0.01 compared with ADR group. Abbreviations used: Co: Control; ADR: Adriamycin; Z.M: Zataria multiflora; CAR: Carvacrol.
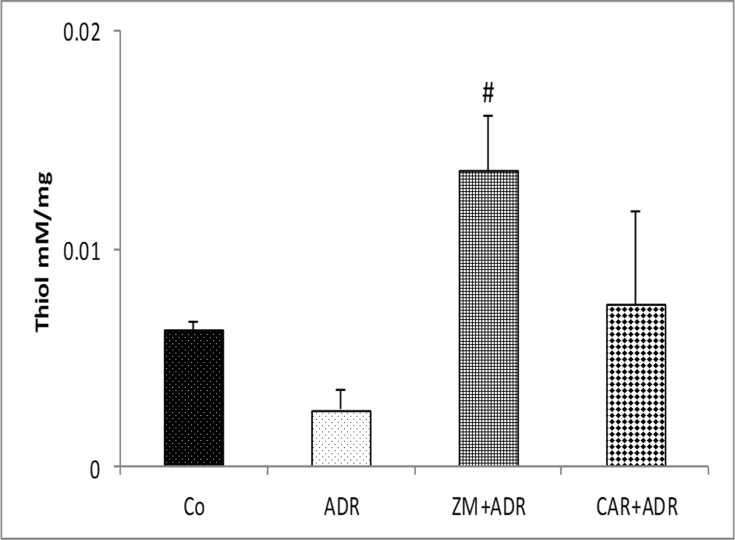
Thiol levels in ADR group showed no significant decrease compared to Co group. Thiol levels in ZM + ADR group significantly increased compared to ADR group (p < 0.05) [Fig. 2].
Fig. 2.
Comparison of the Thiol concentration in liver tissue of four groups. Data are presented as Mean ± SEM (n = 6 in each group). #p < 0.05 compared with ADR group. Abbreviations used: Co: Control; ADR: Adriamycin; Z.M: Zataria multiflora; CAR: Carvacrol.
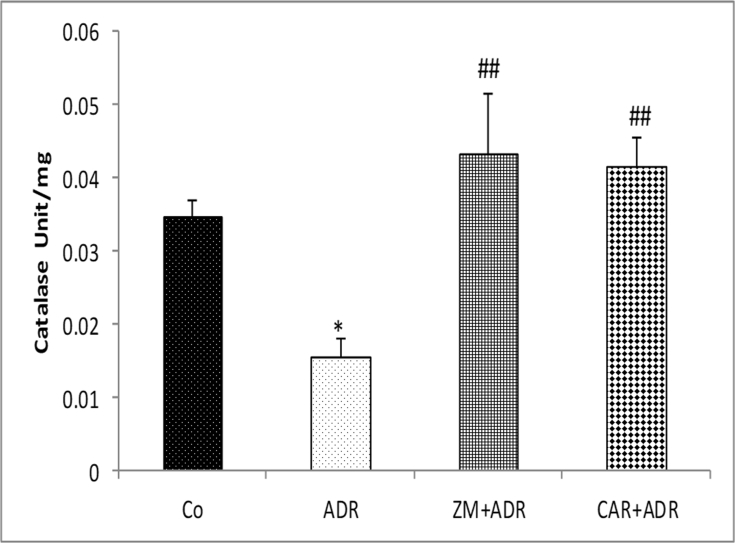
The activities of CAT in the liver tissues of the ADR group were significantly reduced compared to control group (p < 0.05) and increased in treatment groups in comparison with the ADR group (p < 0.01) [Fig. 3].
Fig. 3.
Comparison of the CAT activity in liver tissue of four groups. Data are presented as Mean ± SEM (n = 6 in each group). *p < 0.05 compared with Co group. ##p < 0.01 compared with ADR group. Abbreviations used: Co: Control; ADR: Adriamycin; Z.M: Zataria multiflora; CAR: Carvacrol.
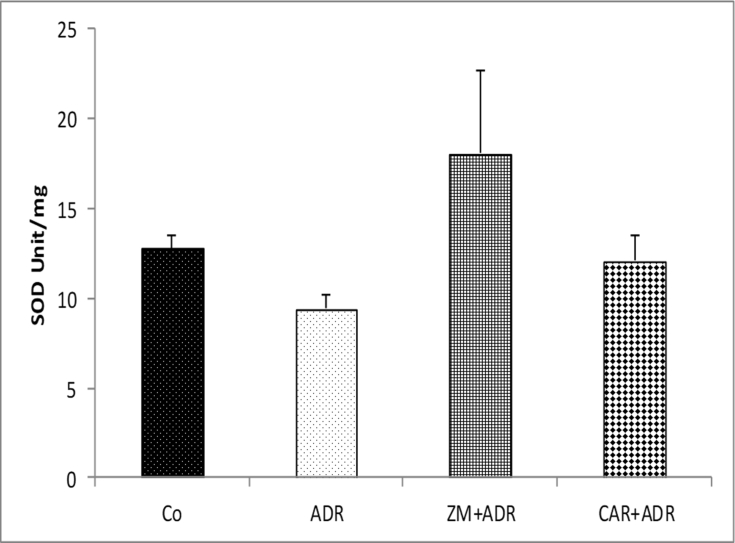
The activity of SOD enzyme in all four groups did not show any significant difference [Fig. 4].
Fig. 4.
Comparison of the SOD activity in liver tissue of four groups. Data are presented as Mean ± SEM (n = 6 in each group). There is no difference between groups. Abbreviations used: Co: Control; ADR: Adriamycin; Z.M: Zataria multiflora; CAR: Carvacrol.
Discussion
Forthcoming articles confirm the ADR-induced hepatotoxicity by the increase in the MDA, ALT, AST and ALP levels and the reduction of SOD, CAT and total thiol groups. In one of these studies, injection of ADR intraperitoneally (2 mg/kg) for six weeks in rats induced hepatotoxicity by increasing the MDA (lipid peroxidation indicator), ALT and AST (important liver enzymes) and reduced the SOD activity (important cellular antioxidant enzyme) [33]. Also, another study showed that ADR injection as a single dose (10 mg/kg) in rats induced hepatotoxicity by increasing the MDA, ALT and AST levels and decreasing the SOD and CAT activity in the liver tissue (antioxidant enzymes) [34]. Previous studies confirm this method for hepatotoxicity induction in rats [5]. The rise in the AST and ALT levels has been attributed to the injured structural integrity of the liver because the enzymes above are cytoplasmic and released into circulation after the cellular injury [35]. Probably, CAR preserves the structural integrity of the liver cell membrane as evidenced by the remarkable decrease in the enzymes activities. Also, ALP is a glycoprotein enzyme and located in the endothelium of the portal veins. It has an important role in the metabolites transport through cell membranes, glycogen metabolism, peptide synthesis, and secretory activities. Therefore, the increase in the ALP activity in ADR-induced hepatotoxic rats may be the result of secretory activity disturbance or in metabolites transport or alternation of enzymes synthesis that CAR and approximately ZM improve them [14].
The possible mechanism of ADR hepatotoxicity is the activation of reactive oxygen species (ROS) in which free radicals such as hydrogen peroxide, superoxide radical and hydroxyl radical have an important role in the oxidative stress induction [36]. ADR promotes MDA production by enhancing the NADPH-dependent lipid peroxidation in the liver microsomal and formation of free radicals [37].
The biological ingredients with antioxidant properties may have an important role in protecting the organs against ROS effects induced by ADR.
Our results showed that administration of ZM and CAR in ADR-induced hepatotoxic rats improved the aforementioned parameters compared to the ADR treated rats.
ZM and its active ingredient, CAR, have been considered as hepatoprotective herbs. Treatment with ZM has been reported to ameliorate Cyclophosphamide-induced liver toxicity. Also, Atayi et al. have been shown that ZM attenuates the hepatotoxicity induced by long-term administration of the albendazole [38]. In one study it was shown that ZM with a high concentration of phenolic ingredients and antioxidant ingredients have an important role in reducing the DNA disruption induced by radiation in lymphocytes [39]. In addition to phenolic compounds, flavonoids as the main ingredients of ZM extract are a group of phenolic compounds with significant chelating and antioxidant properties. Their positive effects result in their ability to lipid peroxidation inhibition, chelating redox-active metals and ameliorating other procedure involving ROS [40].
Hydro alcoholic extract of ZM and CAR indicate powerful antioxidant activity, comparable to that of vitamins [41]. The rate constant for proxy radicals scavenging for CAR is 3.92 × l05 M−1 s−1 [42]. CAR is an antioxidant that does not have any pro-oxidant effect or accelerative effect in DNA damaging unlike some antioxidants [42]. In one study, CAR (25 and 50 mg/kg) attenuates thioacetamide-induced hepatotoxicity by destroying the oxidative stress in a dose-dependent manner. CAR in the study above has an important beneficial role in the thioacetamide-induced hepatotoxicity prevention, probably by scavenging reactive free radicals by promoting the endogenous antioxidant system [43]. In another study performed by Canbek et al., CAR at the dose of 73 mg/kg has a hepatoprotective effect against ischemia/reperfusion during 60 min. In this study CAR with the mentioned dose does not have hepatotoxic effects [44]. Current results showed that CAR even at 20 mg/kg has a protective role against hepatotoxic agents. Also, CAR Treatment in ADR-treated rats, maintained the level of enzymatic antioxidants at normal. Palabiyik et al. reported that CAR and thymol for 24 h ameliorated this inflammation and oxidative stress in the liver and suggested that the antioxidant effect of CAR and thymol have caused these results [45].
According to this opinion, the important mechanism in which ZM and to a great extent CAR improve the hepatotoxicity induced by ADR is the reinforcement of the antioxidant system in liver organ.
Conclusion
The results showed that long-term administration of ZM hydroalcoholic extracts and CAR could reduce the oxidative stress damage through strengthening the antioxidant system, although administration of CAR showed better improvement in the Adriamycin-induced hepatotoxicity in rat.
Conflicts of interest
The authors declare that there is no conflict of interests in this study.
Acknowledgment
The authors would like to thank the Research Affairs of Mashhad University of Medical Sciences for their financial support and Pharmacological Research Center of Medicinal Plants for preparation of Zataria multiflora extract.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Minami M., Matsumoto S., Horiuchi H. Cardiovascular side-effects of modern cancer therapy. Circ J. 2010;74:1779–1786. doi: 10.1253/circj.cj-10-0632. [DOI] [PubMed] [Google Scholar]
- 2.Mohebbati R., Abbsnezhad A., Khajavi Rad A., Mousavi S.M., Haghshenas M. Effect of hydroalcholic extract of Nigella sativa on doxorubicin-induced functional damage of kidney in rats. Horizon Med Sci. 2016;22:13–20. [Google Scholar]
- 3.Rad A.K., Mohebbati R., Hosseinian S. Drug-induced nephrotoxicity and medicinal plants. Iran J Kidney Dis. 2017;11 [PubMed] [Google Scholar]
- 4.Anandakumar P.P., Malarkodi S.P., Sivaprasad T.R., Saravanan G.D. 2007. Antioxidant DL-alpha lipoic acid as an attenuator of adriamycin induced hepatotoxicity in rat model. [PubMed] [Google Scholar]
- 5.Khazdair M.R., Mohebbati R., Karimi S., Abbasnezhad A., Haghshenas M. The protective effects of Curcuma longa extract on oxidative stress markers in the liver induced by Adriamycin in rat. Physiol Pharmacol. 2016;20:31–37. [Google Scholar]
- 6.Olson R.D., Boerth R.C., Gerber J.G., Nies A.S. Mechanism of adriamycin cardiotoxicity: evidence for oxidative stress. Life Sci. 1981;29:1393–1401. doi: 10.1016/0024-3205(81)90001-1. [DOI] [PubMed] [Google Scholar]
- 7.Khajavi Rad A., Mohebbati R. Zataria multiflora extract and carvacrol affect cardiotoxicity induced by Adriamycin in rat. J Basic Clin Physiol Pharmacol. 2018;30:73–79. doi: 10.1515/jbcpp-2018-0008. [DOI] [PubMed] [Google Scholar]
- 8.Mohebbati R., Jalili-Nik M., Paseban M., Shafei M.N., Rad A.K. 2018. Effects of Zataria multiflora extract and Carvacrol on doxorubicin-induced oxidative stress in rat brain. [Google Scholar]
- 9.Blum R.H., Carter S.K. Adriamycin: a new anticancer drug with significant clinical activity. Ann Intern Med. 1974;80:249–259. doi: 10.7326/0003-4819-80-2-249. [DOI] [PubMed] [Google Scholar]
- 10.Minotti G., Menna P., Salvatorelli E., Cairo G., Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004;56:185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- 11.Taheri S., Motallebi A., Fazlara A., Aghababyan A. Effect of Zataria multiflora Boiss (Avishan shirazi) essential oil on oxidative progress in frozen cobia fish fillets during storage. J Aquat Food Prod Technol. 2013;22:310–321. [Google Scholar]
- 12.Shafiee A., Javidnia K. Composition of essential oil of Zataria multiflora. Planta Med. 1997;63:371–372. doi: 10.1055/s-2006-957707. [DOI] [PubMed] [Google Scholar]
- 13.Kavoosi G., Teixeira da Silva J.A., Saharkhiz M.J. Inhibitory effects of Zataria multiflora essential oil and its main components on nitric oxide and hydrogen peroxide production in lipopolysaccharide-stimulated macrophages. J Pharm Pharmacol. 2012;64:1491–1500. doi: 10.1111/j.2042-7158.2012.01510.x. [DOI] [PubMed] [Google Scholar]
- 14.Aristatile B., Al-Numair K.S., Veeramani C., Pugalendi K.V. Effect of carvacrol on hepatic marker enzymes and antioxidant status in d-galactosamine-induced hepatotoxicity in rats. Fund Clin Pharmacol. 2009;23:757–765. doi: 10.1111/j.1472-8206.2009.00721.x. [DOI] [PubMed] [Google Scholar]
- 15.Boskabady M.H., Tabanfar H., Gholamnezhad Z., Sadeghnia H.R. Inhibitory effect of Zataria multiflora Boiss and carvacrol on histamine (H1) receptors of Guinea-pig tracheal chains. Fund Clin Pharmacol. 2012;26:609–620. doi: 10.1111/j.1472-8206.2011.00971.x. [DOI] [PubMed] [Google Scholar]
- 16.Boskabady M.H., Kaveh M., Eftekhar N., Nemati A. Zataria multiflora Boiss and carvacrol affect β2-adrenoceptors of Guinea pig trachea. Evid base Compl Alternative Med. 2011;2011:857124. doi: 10.1155/2011/857124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shokrzadeh M., Azadbakht M., Ahangar N., Naderi H., Saravi S.S.S. Comparison of the cytotoxic effects of Juniperus sabina and Zataria multiflora extracts with Taxus baccata extract and Cisplatin on normal and cancer cell lines. Phcog Mag. 2010;6:102–105. doi: 10.4103/0973-1296.62894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silva F.V., Guimarães A.G., Silva E.R.S., Sousa-Neto B.P., Machado F.D.F., Quintans-Júnior L.J. Anti-inflammatory and anti-ulcer activities of carvacrol, a monoterpene present in the essential oil of oregano. J Med Food. 2012;15:984–991. doi: 10.1089/jmf.2012.0102. [DOI] [PubMed] [Google Scholar]
- 19.Mahtaj L.G., Feizpour A., Kianmehr M., Soukhtanloo M., Boskabady M.H. The effect of carvacrol on systemic inflammation in Guinea pigs model of COPD induced by cigarette smoke exposure. Pharmacol Rep. 2015;67:140–145. doi: 10.1016/j.pharep.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 20.Boskabady M.H., Mahtaj L.G. Lung inflammation changes and oxidative stress induced by cigarette smoke exposure in Guinea pigs affected by Zataria multiflora and its constituent, carvacrol. BMC Complement Altern Med. 2015;15:39. doi: 10.1186/s12906-015-0574-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boskabady M.H., Gholami Mhtaj L. Effect of the Zataria multiflora on systemic inflammation of experimental animals model of COPD. BioMed Res Int. 2014;2014:802189. doi: 10.1155/2014/802189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saei-Dehkordi S.S., Tajik H., Moradi M., Khalighi-Sigaroodi F. Chemical composition of essential oils in Zataria multiflora Boiss. from different parts of Iran and their radical scavenging and antimicrobial activity. Food Chem Toxicol. 2010;48:1562–1567. doi: 10.1016/j.fct.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 23.Khazdair M.R., Ghorani V., Alavinezhad A., Boskabady M.H. Pharmacological effects of Zataria multiflora Boiss L. and its constituents, focus on their anti-inflammatory, antioxidant and immunomodulatory effects. Fundam Clin Pharmacol. 2017;32:26–50. doi: 10.1111/fcp.12331. [DOI] [PubMed] [Google Scholar]
- 24.Khazdair M.R., Ghorani V., Alavinezhad A., Boskabady M.H. Pharmacological effects of Zataria multiflora Boiss L. and its constituents focus on their anti-inflammatory, antioxidant, and immunomodulatory effects. Fund Clin Pharmacol. 2018;32:26–50. doi: 10.1111/fcp.12331. [DOI] [PubMed] [Google Scholar]
- 25.Mohebbati R., Khazdair M.R., Karimi S., Abbasnezhad A. Hepatoprotective effects of combination hydroalcoholic extracts of Nigella Sativa and Curcuma Longa on adriamycin-induced oxidative stress in rat. J Rep Pharmaceut Sci. 2017;6:105–114. [Google Scholar]
- 26.Hosseinimehr S.J., Ahmadashrafi S., Naghshvar F., Ahmadi A., Ehasnalavi S., Tanha M. Chemoprotective effects of Zataria multiflora against genotoxicity induced by cyclophosphamide in mice bone marrow cells. Integr Canc Ther. 2010;9:219–223. doi: 10.1177/1534735409360361. [DOI] [PubMed] [Google Scholar]
- 27.Dagli Gul A.S., Fadillioglu E., Karabulut I., Yesilyurt A., Delibasi T. The effects of oral carvacrol treatment against H2O2 induced injury on isolated pancreas islet cells of rats. Islets. 2013;5:149–155. doi: 10.4161/isl.25519. [DOI] [PubMed] [Google Scholar]
- 28.Ellman G.L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 29.Hosseini M., Pourganji M., Khodabandehloo F., Soukhtanloo M., Zabihi H. Protective effect of L-Arginine against oxidative damage as a possible mechanism of its beneficial properties on spatial learning in ovariectomized rats. Basic Clin Neurosci. 2012;3:36–44. [Google Scholar]
- 30.Khodabandehloo F., Hosseini M., Rajaei Z., Soukhtanloo M., Farrokhi E., Rezaeipour M. Brain tissue oxidative damage as a possible mechanism for the deleterious effect of a chronic high dose of estradiol on learning and memory in ovariectomized rats. Arq Neuropsiquiatr. 2013;71:313–319. doi: 10.1590/0004-282x20130027. [DOI] [PubMed] [Google Scholar]
- 31.Mohebbati R., Shafei M.N., Soukhtanloo M., Roshan N.M., Rad A.K., Anaeigoudari A. Adriamycin-induced oxidative stress is prevented by mixed hydro-alcoholic extract of Nigella sativa and Curcuma longa in rat kidney. Avicenna J Phytomed. 2016;6:86–94. [PMC free article] [PubMed] [Google Scholar]
- 32.Madesh M., Balasubramanian K.A. Microtiter plate assay for superoxide dismutase using MTT reduction by superoxide. Indian J Biochem Biophys. 1998;35:184–188. [PubMed] [Google Scholar]
- 33.Sakr S.A., Mahran H.A., Lamfon H.A. Protective effect of ginger (Zingiber officinale) on adriamycin-induced hepatotoxicity in albino rats. J Med Plants Res. 2011;5:133–140. [Google Scholar]
- 34.Sakr S.A., Abo-El-Yazid S.M. Effect of fenugreek seed extract on adriamycin-induced hepatotoxicity and oxidative stress in albino rats. Toxicol Ind Health. 2012;28:876–885. doi: 10.1177/0748233711425076. [DOI] [PubMed] [Google Scholar]
- 35.Ahmed M.B., Khater M.R. Evaluation of the protective potential of Ambrosia maritima extract on acetaminophen-induced liver damage. J Ethnopharmacol. 2001;75:169–174. doi: 10.1016/s0378-8741(00)00400-1. [DOI] [PubMed] [Google Scholar]
- 36.Qin X.J., He W., Hai C.X., Liang X., Liu R. Protection of multiple antioxidants Chinese herbal medicine on the oxidative stress induced by adriamycin chemotherapy. J Appl Toxicol. 2008;28:271–282. doi: 10.1002/jat.1276. [DOI] [PubMed] [Google Scholar]
- 37.Sterrenberg L., Julicher R.H., Bast A., Noordhoek J. Adriamycin stimulates NADPH-dependent lipid peroxidation in liver microsomes not only by enhancing the production of O2 and H2O2, but also by potentiating the catalytic activity of ferrous ions. Toxicol Lett. 1984;22:153–159. doi: 10.1016/0378-4274(84)90059-6. [DOI] [PubMed] [Google Scholar]
- 38.Atayi Z., Borji H., Moazeni M., Darbandi M.S., Heidarpour M. Zataria multiflora would attenuate the hepatotoxicity of long-term albendazole treatment in mice with cystic echinococcosis. Parasitol Int. 2018;67:184–187. doi: 10.1016/j.parint.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 39.Hosseinimehr S.J., Mahmoudzadeh A., Ahmadi A., Ashrafi S.A., Shafaghati N., Hedayati N. The radioprotective effect of Zataria multiflora against genotoxicity induced by γ irradiation in human blood lymphocytes. Cancer Biother Radiopharm. 2011;26:325–329. doi: 10.1089/cbr.2010.0896. [DOI] [PubMed] [Google Scholar]
- 40.Setchell K.D.R., Cassidy A. Dietary isoflavones: biological effects and relevance to human health. J Nutr. 1999;129:758S–767S. doi: 10.1093/jn/129.3.758S. [DOI] [PubMed] [Google Scholar]
- 41.Jayakumar S., Madankumar A., Asokkumar S., Raghunandhakumar S., Kamaraj S., Divya M.G.J. Potential preventive effect of carvacrol against diethylnitrosamine-induced hepatocellular carcinoma in rats. Mol Cell Biochem. 2012;360:51–60. doi: 10.1007/s11010-011-1043-7. [DOI] [PubMed] [Google Scholar]
- 42.Aeschbach R., Löliger J., Scott B.C., Murcia A., Butler J., Halliwell B. Antioxidant actions of thymol, carvacrol, 6-gingerol, zingerone and hydroxytyrosol. Food Chem Toxicol. 1994;32:31–36. doi: 10.1016/0278-6915(84)90033-4. [DOI] [PubMed] [Google Scholar]
- 43.Nafees S., Ahmad S.T., Arjumand W., Rashid S., Ali N., Sultana S. Carvacrol ameliorates thioacetamide-induced hepatotoxicity by abrogation of oxidative stress, inflammation, and apoptosis in liver of Wistar rats. Hum Exp Toxicol. 2013;32:1292–1304. doi: 10.1177/0960327113499047. [DOI] [PubMed] [Google Scholar]
- 44.Canbek M., Uyanoglu M., Bayramoglu G., Senturk H., Erkasap N., Koken T. Effects of carvacrol on defects of ischemia-reperfusion in the rat liver. Phytomedicine. 2008;15:447–452. doi: 10.1016/j.phymed.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 45.Palabiyik S.S., Karakus E., Halici Z., Cadirci E., Bayir Y., Ayaz G. The protective effects of carvacrol and thymol against paracetamol–induced toxicity on human hepatocellular carcinoma cell lines (HepG2) Hum Exp Toxicol. 2016;35:1252–1263. doi: 10.1177/0960327115627688. [DOI] [PubMed] [Google Scholar]