Abstract
Apigenin (APG) is a poorly soluble bioactive compound/nutraceutical which shows poor bioavailability upon oral administration. Hence, the objective of this research work was to develop APG solid dispersions (SDs) using different techniques with the expectation to obtain improvement in its in vitro dissolution rate and in vivo bioavailability upon oral administration. Different SDs of APG were prepared by microwave, melted and kneaded technology using pluronic-F127 (PL) as a carrier. Prepared SDs were characterized using “thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), Fourier transform infra-red (FTIR) spectrometer, powder X-ray diffraction (PXRD) and scanning electron microscopy (SEM)”. After characterization, prepared SDs of APG were studied for in vitro drug release/dissolution profile and in vivo pharmacokinetic studies. The results of TGA, DSC, FTIR, PXRD and SEM indicated successful formation of APG SDs. In vitro dissolution experiments suggested significant release of APG from all SDs (67.39–84.13%) in comparison with control (32.74%). Optimized SD of APG from each technology was subjected to in vivo pharmacokinetic study in rats. The results indicated significant improvement in oral absorption of APG from SD prepared using microwave and melted technology in comparison with pure drug and commercial capsule. The enhancement in oral bioavailability of APG from microwave SD (319.19%) was 3.19 fold as compared with marketed capsule (100.00%). Significant enhancement in the dissolution rate and oral absorption of APG from SD suggested that developed SD systems can be successfully used for oral drug delivery system of APG.
Keywords: Apigenin, Microwave technology, Pluronic-F127, Solid dispersion, Bioavailability
1. Introduction
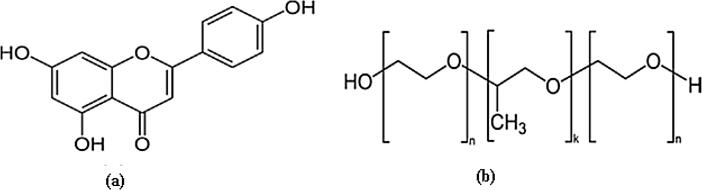
Apigenin (APG, Fig. 1a) is a naturally occurring bioactive flavonoid which is obtained from several fruits and vegetables in the form of either O-glycosides or C-glycosides (Peterson and Dwyer, 1998, Shukla and Gupta, 2010, Soto et al., 2011). The most common sources for APG are parsley, celery, celeriac and chamomile tea (Gupta et al., 2001, Shukla and Gupta, 2010, Shibata et al., 2014). Various therapeutic activities including “anti-inflammatory, anti-cancer, antibiotic, anti-viral, antidiabetic and anti-oxidant” activities for APG have been reported in literature (Gupta et al., 2001, Laorko et al., 2010, Choi et al., 2014, Shibata et al., 2014, Morimoto et al., 2015, Hong et al., 2018, Ozbey et al., 2018, Wang et al., 2018, Zhu et al., 2018). APG has also been reported as a potent inhibitor of CYP4F2 in humans (Steuck et al., 2016). Its formulation development is difficult because it comes under biopharmaceutical classification system (BCS) class II (presents low solubility and high permeability) (Zhang et al., 2012, Altamimi et al., 2018). It has been proposed as practically insoluble/poorly soluble in water (solubility: 15.60 µg/ml at ambient temperature) and aqueous buffers (Zhang et al., 2012, Shakeel et al., 2017, Shakeel et al., 2018). Its oral bioavailability in rats and humans has been reported as very low (Elhennawy and Lin, 2018, Perez-Moral et al., 2018). Upon single dose administration of APG in rats, its oral bioavailability was obtained as very low i.e. 7.06% (Elhennawy and Lin, 2018).
Fig. 1.
Molecular structures of (a) APG and (b) PL.
Pluoronic F-127 (PL) (Fig. 1b) is a synthetic amphiphilic copolymer of ethylene oxide and propylene oxide which has been investigated extensively for improvement of wettability, solubility/dissolution, stability and bioavailability of drugs in different formulations (Wong et al., 2006, Dumortier et al., 2006, Park et al., 2009, Cespi et al., 2012, Liu et al., 2015). The chemical name of PL is 2-[2-(2-hydroxyethoxy)propoxy]ethanol (Wong et al., 2006). It is approved as an excipient for human use by FDA (Diniz et al., 2015, Gioffredi et al., 2016). It has various applications in drug delivery, controlled release and mesenchymal stem cells encapsulation (Ruel-Gariepy and Leroux, 2004, Diniz et al., 2015, Gioffredi et al., 2016).
Various techniques have been used in the investigation of solid dispersions (SDs) of various weakly soluble drugs and other organic compounds (Moneghini et al., 2008, Aso et al., 2009, Moneghini et al., 2009, Menéndez et al., 2010, Maurya et al., 2010, Van Eardenbrugh and Taylor, 2010, Issa et al., 2013, Paudel et al., 2013, Li et al., 2014, Xiqiang et al., 2014, Fulop et al., 2015, Kang et al., 2015, Wang et al., 2015, Altamimi and Neau, 2016). Microwave technology has been investigated rarely in literature in order to enhance solubility, in vitro dissolution, therapeutic efficacy and in vivo bioavailability of such drugs. Recently, the drug release profile and anti-inflammatory efficacy of mefenamic acid and flufenamic acid have been successfully enhanced using SD systems prepared by microwave technology (Alshehri et al., 2017a). Various drugs delivery systems such as microemulsion (Zhao et al., 2016), liposomes (Arsic et al., 2011), ethosomes (Shen et al., 2014), phytosomes (Telange et al., 2017), transferosmes (Jangdey et al., 2017), nanocrystal (Al Shaal et al., 2011, Zhang et al., 2013), nanocapsule (Ding et al., 2013), polymeric micelles (Zhai et al., 2013), nanoparticles (Das et al., 2013), cyclodextrin complexation (Huanget al., 2016), spray-dried microparticles (Altamimi et al., 2018), dendrimers (Zhu et al., 2018) and self-microemulsifying drug delivery system (Zhao et al., 2013) of APG have been studied so far. Microwave SDs of APG have not been investigated for its dissolution, solubility and oral bioavailability enhancement. Therefore, the objective of this work was to prepare various SDs of APG using PL as a carrier with the expectation to obtain improvement in its in vitro dissolution rate and bioavailability upon oral administration. Various SDs of APG were developed by three different techniques including melting technology, kneading technology and microwave technology using PL as a carrier. Optimized melted, kneaded and microwave SD systems of APG were chosen for in vivo bioavailability/pharmacokinetic evaluation in male “Wistar rats”.
2. Materials and methods
2.1. Materials
APG (purity: 98.20% by HPLC) was acquired from “Beijing Mesochem Technology Co., Ltd. (Beijing, China)”. Poloxamer-F-127 was obtained from “BASF (Ludwigshafen, Germany)”. HPLC grades methanol and ethanol were acquired from “Sigma Aldrich (St. Louis, MO, USA)”. Other chemicals/materials were of analytical/pharmaceutical grades with high purity.
2.2. Preparation of SDs
2.2.1. Kneaded method
APG-PL physical mixtures at different weight ratios such as 1:1, 1:2 and 1:4 were kneaded with appropriate amounts of ethanol (0.5 ml of ethanol in water/g of physical mixture) using a mortar and pestle for about 10 min. The mass was dried (room temperature overnight), crushed, sieved and dried again in an Oven (Heraeus, Germany) at 40 °C for 24 h.
2.2.2. Melted method
APG-PL physical mixtures at different weight ratios (1:1, 1:2 and 1:4) were prepared by mixing in a mortar and pestle for about 10 min. The resultant mixture was heated at 65 0C with stirring in a thermostatic water bath in order to obtain a homogenous dispersion. The resulting dispersion was cooled at room temperature, crushed, sieved and dried (Issa et al., 2013).
2.2.3. Microwave method
Different SDs of APG were developed with the help of “Domestic Microwave Irradiation (Samsung Model ME0113M1)” as reported in literature (Maurya et al., 2010; Menendez et al., 2010). APG was blended with PL at various drug:carrier ratios such as 1:1, 1:2 and 1:4% w/w and sieved with the help of mesh size number 45 in order to get the particles of uniform size. The precise amounts of APG and PL were gently mixed for about 5 min. The power of microwave was adjusted at 900 W and rest of the procedure was same as reported for mefenamic and flufenamic acids SDs using microwave technology in our previous publications (Alshehri et al., 2017a). The temperature of microwave was not adjusted but samples were heated till melting of mixtures.
2.3. Thermal gravimetric analysis (TGA)
TGA experiments were carried out with the help of “Pyris 1 TGA (Perkin Elmer, Waltham, MA, USA)” equipment which was equipped with auto sampler. These experiments were carried out for the analysis of the thermal stabilities of materials (pure APG and pure PL). TGA experiments were performed with the heating range of 25–400 °C at the rate of 10 °C/min. The amount of each pure material was around 3.0 mg which was quantified using TGA. The flow rate of nitrogen was set at 20 ml/min for this analysis. The results of TGA analysis were interpreted based on the loss in the weights of the materials with respect to from the original weights of the powder (Alshehri et al., 2017a).
2.4. Differential scanning calorimetry (DSC)
DSC experiments on pure APG and optimized SDs were performed with the help of “DSC apparatus (DSC-8000, Perkin Elmer, Waltham, MA, USA)” with the temperature range of 30.0–400.0 °C at the heating rate of 10.0 °C/min. The flow rate of nitrogen was set at 20 ml/min. DSC apparatus was attached with an auto sampler and chiller. Accurately weighed amounts of the samples of pure APG and optimized SDs (approximately 3.0 mg) were taken and placed in an aluminium pan which was hermetically sealed. The software used for DSC data analysis was “Pyris Manager Software (Perkin Elmer, Waltham, MA, USA)”. DSC analysis was performed for the characterization of the solid state of materials (Alshehri et al., 2017a).
2.5. Fourier transform infrared spectroscopy (FTIR)
The FTIR spectra of APG powder and various SDs prepared by different techniques were recorded with the help a “Perkin Elmer FTIR Spectrum BX (Perkin Elmer, Marlborough, MA, USA)”. The disc for each sample matrices was prepared using potassium bromide pellets. The FTIR spectra were recorded in the wavenumber range of 4400–350 cm−1 (Ding et al., 2013, Zhai et al., 2013). The FTIR spectra were interpreted with the help of “IR Solution Software (version 1.10, Marlborough, MA, USA)”.
2.6. Scanning electron microscopy
The surface morphology/shape of pure APG, pure PL and various SDs prepared by different techniques were studied using “Scanning Electron Microscopy (SEM)”. The samples from each material were fixed on the stubs with the help of “Adhesive Carbon Tape (SPI Supplies, West Chester, PA, USA)” and coated with gold under vacuum in a “Q150R Sputter Coater Unit (Quorum Technologies Ltd., East Sussex, UK)” in an argon atmosphere at 20 mA for about 60 sec. The photomicrographs of each sample were recorded with the help of “SEM Microscope (Zeiss EVO LS10; Cambridge, UK)” (Alshehri et al., 2017a).
2.7. Powder X-Ray diffraction
Powder X-Ray diffraction (PXRD) profile of APG powder, pure PL and various SDs prepared by different techniques were recorded using “Ultima IV Diffractometer (Rigaku Inc. Tokyo, Japan at College of Pharmacy, King Saud University, KSA)” in the range of 3–60° (2 θ) at a scan speed of 1°/min. The tube anode used was “Cu with Ka = 0.1540562 nm mono chromatized with a graphite crystal (Rigaku Inc., Tokyo, Japan)”. The patterns of each sample were recorded at tube voltage and tube current of 40 kV and 40 mA, respectively in step scan mode (step size 0.02°, counting time 1 sec/step). This analysis was performed in order to evaluate the crystalline nature of APG powder and APG in various SD systems prepared by different techniques (Alshehri et al., 2017a).
2.8. UPLC-UV analysis of APG
The analysis of APG in pure drug, various SDs and dissolution samples was conducted with the help of an “Ultra-Performance Liquid Chromatography-Ultra-Violet (UPLC-UV)” technique. The contents of APG were quantified by injecting the appropriate amounts of analytes into a “UPLC (Acquity® UPLC, Waters Inc., Bedford, MA, USA)”. The detection was performed at the wavelength of 336 nm. The separation was carried out at reverse-phase isocratic elution mode. The mixture of 0.05 M ammonium formate buffer and acetonitrile (28:72% v/v) was used as a mobile phase. The binary mixture of mobile phase was delivered at flow rate of 0.3 ml/min and the volume of injection was set at 1.0 µl. The PDA detector was set to acquire 3D data in the range of 210–400 nm while the 2D channel was processed at 336 nm. The column “(Acquity® UPLC BEH C18 column, 2.1 × 50 mm, 1.7 µm, Waters, Bedford, MA, USA)” was utilized for this purpose and the temperature of column was maintained at 40 °C.
2.9. In vitro dissolution/release studies
In vitro dissolution/release studies were conducted in order to compare the release profile of APG from different SDs with that of pure drug and marketed capsule. These studies were performed with the help of “USP-24 Dissolution Test Apparatus Type II” rotating at 100 rpm using an “Automated Dissolution Tester (Logan Instrument Corp., Somerset, NJ, USA)”. Dissolution medium used was 900 ml of purified water with 1% SLS. The capsules containing the amount equivalent to 10 mg of APG were taken in a dissolution medium which was maintained at 37 °C. Dissolution studies were performed for the period of 2.0 h. The appropriate amount of samples were withdrawn at regular intervals of time and replaced each time with drug free dissolution medium. The samples were subjected for the quantification of APG contents using proposed UPLC-UV technique at 336 nm. The amount of APG released was calculated from the calibration curve of APG and plotted against the time.
2.10. Pharmacokinetic investigations in rats
Male Wister Albino rats (weighing 200–220 g) were taken from the “College of Pharmacy, Experimental Animal Care Center (King Saud University, Riyadh, Saudi Arabia)”. The animals were fasted for 24 h prior the administration of the drug. The drug was administered using the oral route using oral gavage in order to assure the dose uniformity. The protocol for this work was approved by the “Ethical Committee of the College of Pharmacy (King Saud University, Riyadh, Saudi Arabia)”. Before gavage administration of the rats, pure APG and different SDs of APG were dispersed homogenously in 0.5% sodium carboxymethyl cellulose aqueous solution in order to obtain suspensions. Animals were provided for free access to food and water 3 h after oral administration of each sample.
Five groups containing 6 rats in each group were tested for the study i.e. G1, G2, G3, G4 and G5 with dose of 10 mg/kg. The animals were fasted overnight before starting experiment. About 1.0 ml of blood samples were taken from each rat following time intervals of 0, 1, 2, 4, 6, 12, 24 h from the retro orbital plexus vein into a heparinized test tubes from each group. Total seven times blood was drawn from each rat and blood draw procedures were also approved by “Ethical Committee of the College of Pharmacy (King Saud University, Riyadh, Saudi Arabia)”. The volume was maintained by providing sufficient amount of water to each rat. Finally, plasma was separated through the centrifugation of the samples at 50,000 rpm and for 20 min and then kept at -80 °C pending further analysis.
2.11. Analysis of APG in rat plasma by UPLC-MS/MS method
The plasma was separated from blood and APG from plasma was extracted using protein precipitation method. In this method, 0.1 ml of rat plasma was combined with 50 µl of prednisolone (200 µg/ml) which was used as an internal standard (IS) and 0.75 ml of methanol. The mixture obtained was vortexed for about 1.0 min. The samples were subjected to centrifugation at 15,000 rpm for about 10 min. After centrifugation, around 800 µl of the supernatant liquid was taken and transferred to a sample vial. About 5 µl sample was injected into the UPLC-MS/MS system for the quantification of APG in plasma samples.
In this study, a validated “UPLC-MS/MS (UPLC, Waters Acquity, Milford, MA, USA)” was utilized to determine the amount of APG in plasma samples. The purpose of this study was to enhance oral bioavailability of APG from SDs prepared using different techniques and hence only APG was analyzed in rat plasma. Phase II conjugates of APG such as APG glucuronide and APG sulfate were not analyzed in rat plasma. The chromatographic conditions involved the use of a “BEH C18 column (50 mm × 2.1 mm, 1.7 µm)” with a mobile phase of acetonitrile and 0.1% formic acid (35:65% v/v) at a flow rate of 0.25 ml/min using prednisolone as the IS. The eluted compounds were detected by tandem mass spectrometry using “TQ detector (Waters Corp., Milford, MA, USA)” equipped with an electrospray ionisation (ESI) source operating in positive ionization mode. The quantification was performed with multiple reactions monitoring (MRM) mode. Selection of ionization pairs (m/z) was shown as follows: APG: 270.99 → 152.9 (cone voltage 57 V, collision energy 34 V) and prednisolone: 403.172 → 385.224 (cone voltage 42 V, collision energy 13 V).
2.12. Pharmacokinetic (PK) data analysis
The APG concentrations in rat plasma at various time intervals were employed to investigate its PK profiles by plotting drug concentration–time profile curves. The values of PK parameters are expressed as the mean ± standard deviation (SD). The software utilized for the calculation of PK parameters of APG was “WinNonlin software (Pharsight Co., Mountain View, CA, USA)”. The noncompartmental PK model was applied to calculate various PK parameters including maximum plasma concentration (Cmax) and time to reach maximum concentration (Tmax), area under the curve from 0 to t (AUC0–24), elimination rate constant (λz), half-life (T½) and mean residence time (MRT). Relative bioavailability was calculated using marketed capsule as a standard.
2.13. Statistical analysis
In vitro drug release data are presented as mean ± SD of three independent experiments. PK data are presented as mean ± SD of six independent experiments. The data of in vitro and PK studies were analyzed by one way ANOVA using Dunnett’s test. The software used for this analysis was “Graphpad Instat Software (San Diego, CA, USA)” and P < 0.05 was taken as statistically significant.
3. Results and discussion
3.1. TGA analysis
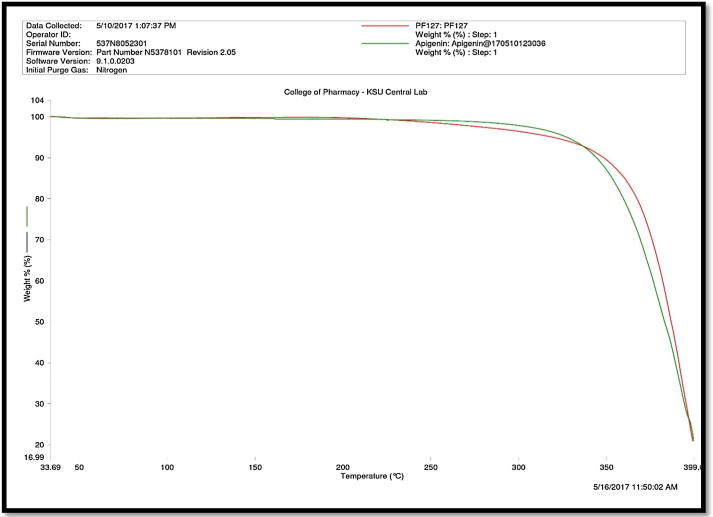
The TGA spectra of APG powder and pure PL are shown in Fig. 2. The TGA spectra of pure APG showed the loss in weight of APG at around 352.94 °C (Fig. 2). This loss in weight was due to the decomposition of APG at 352.94 °C. These results suggested that APG does not transform into polymorphic forms and it was pure crystalline compound. The loss in weight of APG due to this decomposition was recorded as 0.54% (w/w). The TGA spectra of pure PL presented decomposition at little higher temperature than APG (362.3 °C), suggesting that PL exists in semi-crystalline form.
Fig. 2.
TGA spectra of pure APG and pure PL.
3.2. DSC spectral analysis
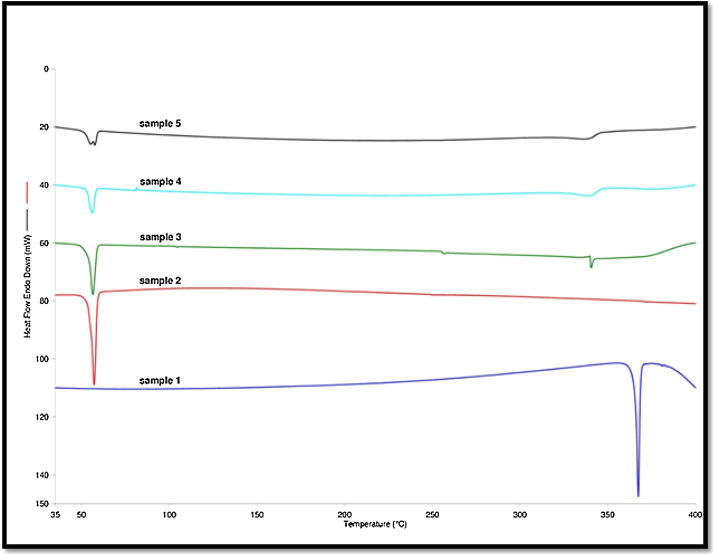
The DSC spectral analysis was conducted for the investigation of possible interactions between APG and carrier PL in various SDs prepared using different techniques (Alshehri et al., 2015, Alshehri et al., 2017b). The DSC spectra of APG powder and various SDs prepared by different techniques are shown in Fig. 3. The DSC spectra of pure APG presented a sharp endothermic peak at melting temperature of 367.46 °C. However, the DSC spectra of pure PL showed a small peak at melting temperature of around 56 °C. While the DSC spectra of various SDs prepared by different techniques presented a very poor peak. The endothermic peaks of APG and PL were shifted to lower temperatures in various SDs prepared using different techniques. These results suggested the amorphization of APG into various SDs prepared using different techniques. It was also observed that all SDs presented a small DSC peak at around 50.0 °C which was possible due to the presence of PL in all SDs. This observation might be possible due to molecular interactions of APG with PL which was used as the carrier for SDs. In this study, PL works by forming eutectic mixture with APG in all SDs as indicated by DSC thermograms of different SDs. DSC thermograms of SDs prepared by kneaded, melted and microwave technology showed two peaks (one at around 50 0C) and second at around 345 0C, suggesting formation of eutectic mixture between PL and APG in all SDs (Karolewicz et al., 2017). The melting temperature of APG was recorded as 366.36 °C by Huang et al. (2016). Moreover, it was recorded as 366.52 °C by Shakeel et al. (2017). The melting temperature of APG was recorded as 367.46 °C in the present study. The melting temperature of APG obtained in this study was much closed with those reported by Huang et al. (2016), Shakeel et al. (2017). This literature comparison suggested good agreement of DSC spectra obtained in this study with literature values of APG. The single and crystalline endothermic peak of pure APG suggested that APG exists in pure crystalline form and did not transform into polymorphic form.
Fig. 3.
DSC spectra of pure APG and various SDs prepared by different techniques; sample 1: pure APG, sample 2: pure PL, sample 3: microwave SDs, sample 4: melted SDs and sample 5: kneaded SDs.
3.3. FTIR spectral analysis
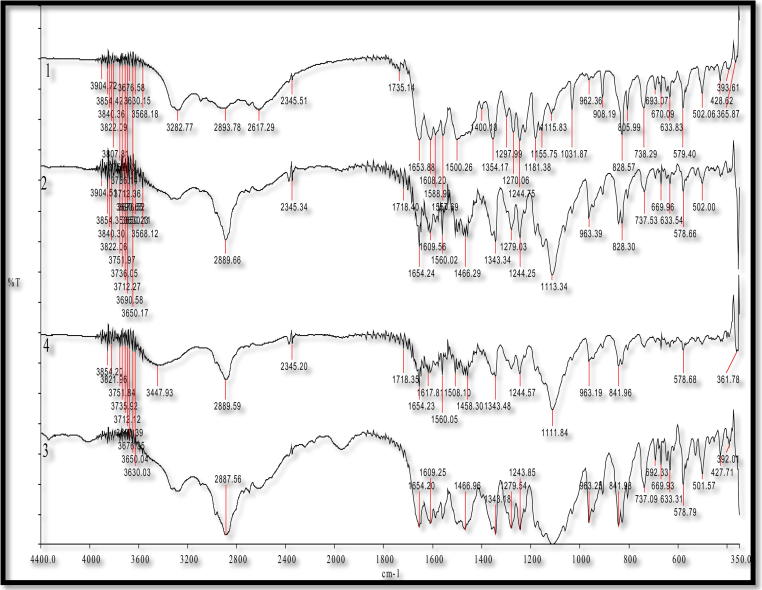
The FTIR spectra of APG powder and various SDs prepared by different techniques are presented in Fig. 4. The FTIR spectra of pure APG presented vibrational bands with characteristic peak at the wave number of 3278 cm−1 for O—H group. However, C—H group presented multiple small peaks at 2800 cm−1. The characteristic peaks at 1653.6 and 1607.8 cm−1 were obtained for the C O functional group. All these characteristics peaks of APG were due to the intermolecular interaction between the carbonyl and hydroxyl groups. These interactions were originated from the high electronegativity of oxygen forcing a hydrogen bonding with the hydroxyl group (Huang et al., 2013; Shagautdinova et al., 2015, Telange et al., 2017). The stretching vibrational peaks of APG were also presented in the SDs prepared by different techniques, suggesting no change in the chemical structure of APG during the formation of SDs (Zhang et al., 2013). However, the intensities of APG peaks intensities were reduced, indicating the amorphization of APG in SDs prepared by different techniques.
Fig. 4.
FTIR spectra for pure APG and various SDs prepared by different techniques; 1: pure APG, 2: microwave SDs, 3: melted SDs and 4: kneaded SDs.
3.4. SEM analysis
The SEM photomicrographs of APG powder, pure PL and various SDs prepared by different techniques are shown in Fig. 5. The SEM images of pure APG presented clear crystals of APG which were larger in particle size (Fig. 5). While, the SEM photomicrographs of pure PL presented its amorphous character. When, APG was formulated into various SDs, the morphology of APG converted to amorphous forms and their particle size were much smaller than pure APG (Fig. 5). Overall, the results of SEM analysis suggested that SDs of APG prepared by different techniques were in amorphous states.
Fig. 5.
SEM images of pure APG, pure PL and various SDs prepared by different techniques.
3.5. PXRD spectral analysis
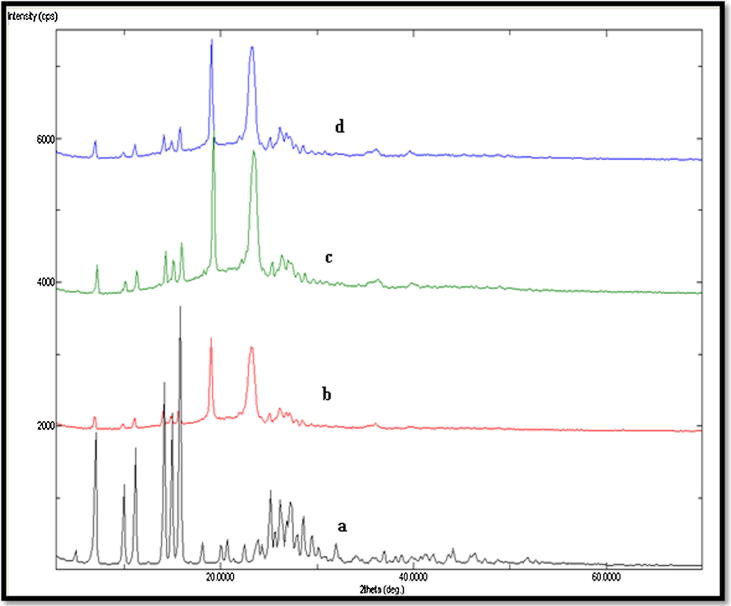
The PXRD spectral analysis was performed for the evaluation of crystalline and amorphous characteristics of various SDs prepared using different techniques. The PXRD spectra of APG powder and various SDs of APG prepared by different techniques are shown in Fig. 6. The PXRD spectra of APG powder presented sharp crystalline peaks at various 2 θ values which were possible due to crystalline character of APG. While, the SDs of APG prepared using different techniques showed either disappearance or shifting of main peaks of APG. These results again suggested the amorphization of APG into SDs prepared by different techniques. This amorphization of APG could be due to molecular interactions of APG with PL used as a carrier for SDs.
Fig. 6.
PXRD spectra of pure APG and various SDs prepared by different techniques; (a): pure APG, (b): microwave SDs, (c): melted SDs and (d): kneaded SDs.
3.6. In vitro dissolution studies
The in vitro drug release studies were conducted to compare the release profile of APG from various SDs with those of pure APG and marketed capsule. The in vitro dissolution/drug release profiles of APG from different SD systems prepared by kneading, melting and microwave techniques are illustrated in Fig. 7, Fig. 8, Fig. 9, respectively and the dissolution parameters are furnished in Table 1. Untreated APG/control showed a slow dissolution with an initial release of 32.74% and dissolution efficiency of 33.43% after 60 min of study. Only 46% of the drug was dissolved within 30 min, after which a slow release profile was observed.
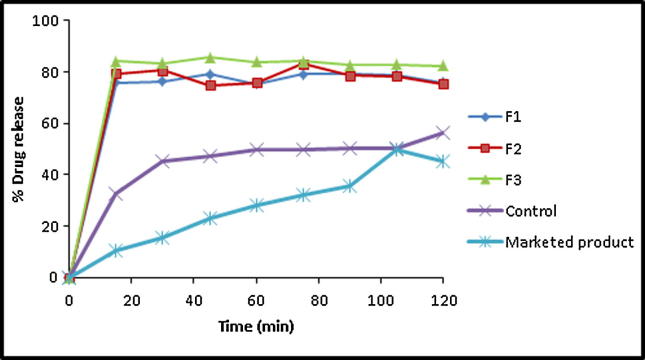
Fig. 7.
In vitro drug release profile of APG from pure drug, marketed product (Swanson®) and its kneaded SDs in the ratio of 1:1 (F1), 1:2 (F2) and 1:4 (F3).
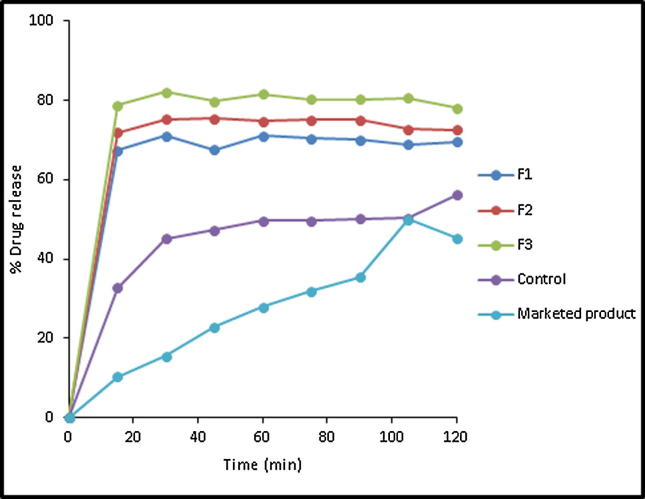
Fig. 8.
In vitro drug release profile of APG from pure drug, marketed product (Swanson®) and its melted SDs in the ratio of 1:1 (F1), 1:2 (F2) and 1:4 (F3).
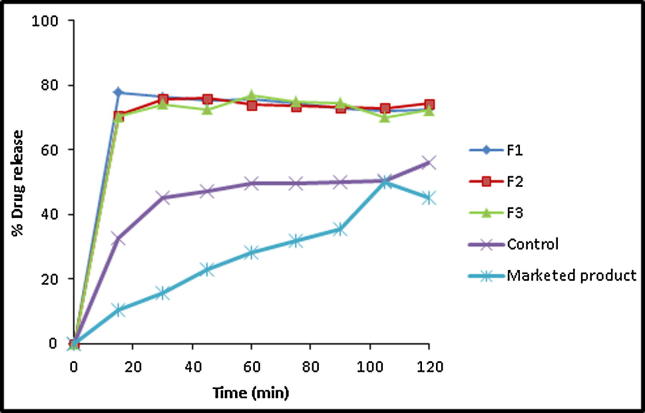
Fig. 9.
In vitro drug release profile of APG from pure drug, marketed product (Swanson®) and its microwave SDs in the ratio of 1:1 (F1), 1:2 (F2) and 1:4 (F3).
Table 1.
Dissolution parameters of APG from its-PL binary systems prepared using different solid dispersion techniques.
| System | IRD (%) | DE%60 | RDR (%)60 |
|---|---|---|---|
| API untreated | 32.74 | 33.43 | – |
| Kneaded (API-Pl 1: 1) | 75.91 | 57.81 | 1.52 |
| Kneaded (API-Pl 1: 2) | 79.30 | 58.30 | 1.53 |
| Kneaded (API-Pl 1: 4) | 84.13 | 63.30 | 1.69 |
| Microwave (API-Pl 1: 1) | 77.83 | 57.09 | 1.52 |
| Microwave (API-Pl 1: 2) | 70.71 | 56.04 | 1.49 |
| Microwave (API-Pl 1: 4) | 70.21 | 55.09 | 1.55 |
| Melting (API-Pl 1: 1) | 67.39 | 51.91 | 1.43 |
| Melting (API-Pl 1: 2) | 78.67 | 60.48 | 1.65 |
| Melting (API-Pl 1: 4) | 71.48 | 55.79 | 1.51 |
IRD (%): Initial dissolution rate (after 15 min)).
DE%60: Dissolution efficiency percentages after 60 min.
RDR (%)60: Relative dissolution rate after 60 min.
APG exhibited enhanced dissolution rates in its SDs in PL matrices at different weight ratios prepared by kneading method (Fig. 7). Increasing polymer weight ratio in the kneaded mixtures resulted in enhanced drug dissolution rate. For example, APG exhibited an initial dissolution values of 75.93, 75.31 and 84.13 in case of its kneaded mixtures at weight ratios of 1:1, 1:2 and 1:4, respectively with DE%60 values of 57.81, 58.3 and 63.30% and relative dissolution rates (RDR) of 1.52, 1.353 and 1.69, respectively. The dissolution profiles of APG SDs in different weight ratios of PL matrix prepared by melting method are illustrated in Fig. 8. A pronounced enhancement in the initial drug dissolution rate was exhibited in all drug-PL weight ratios. Unfortunately, a slow dissolution rates were observed thereafter. APG exhibited DE %60 values of 51.91, 60.48 and 55.79 in case of its-PL melted SDs prepared at weight ratios of 1:1, 1:2 and 1:4, respectively.
In case of APG-PL binary systems prepared by microwave technique, the drug dissolution rate was noticeably enhanced when dispersed in the polymeric matrix by microwave in comparison to the untreated/control form (Fig. 9). In addition, an enhanced IDR was obtained in case of drug:PL ratio of 1:1 (77.83). However, the PL weight ratio did not have a pronounced effect on the drug dissolution rate from these SDs. The recorded DE %60 values of the drug from its-PL microwave-based SDs were 57.09, 56.04 and 55.09 for weight ratios of 1:1, 1:2 and 1:4, respectively. Alshehri et al. showed that in vitro dissolution rates of mefenamic and flufenamic acids were remarkably enhanced by SDs of the drugs in the polyethylene glycol 400 matrix by microwave technique (Alshehri et al., 2017a).
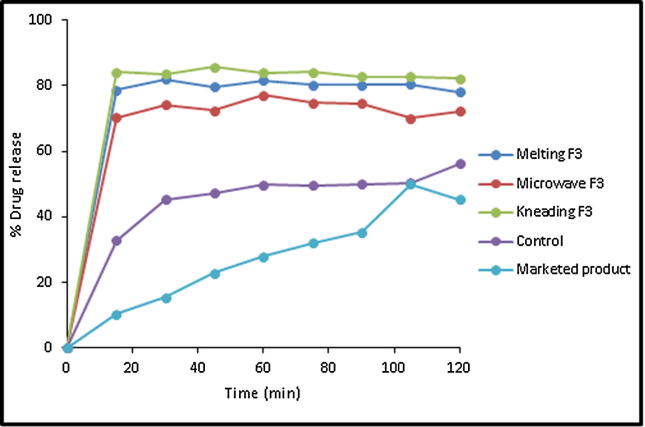
The enhanced drug dissolution rate in its-PL binary mixtures prepared by three different techniques could be attributed to the simultaneous drug crystallization out in very minute crystals embodied in water-soluble carrier as revealed by DSC data. This can result in an increased surface area of the drug particles leading to enhanced dissolution rate (Newa et al., 2008, Monti et al., 2010, El-Badry et al., 2013). Moreover, rapid dissolution of the water-soluble matrices (PL) is associated by fast dissolution of incorporated drug minute crystals. In addition, the hydrophilic polymer encircling the drug particles which could helpful in minimizing the aggregation of APG. This would allow the drug to dissolve readily due to rapid contact with the dissolution medium and wetting of the drug particles causing enhanced dissolution rate of APG (Shazly et al., 2012). Comparative dissolution profile of APG from optimized SDs prepared from different techniques in comparison with control/pure APG and marketed capsule is presented in Fig. 10. The maximum release of APG was obtained from kneaded SD followed by melted SD, microwave SD, pure APG and marketed capsule. The release profile of APG from each optimized SD system was significantly higher in comparison with pure APG and commercial capsule (P < 0.05).
Fig. 10.
Comparative in vitro drug release profile of APG from pure drug and optimized SDs prepared by different techniques.
3.7. PK studies in rats
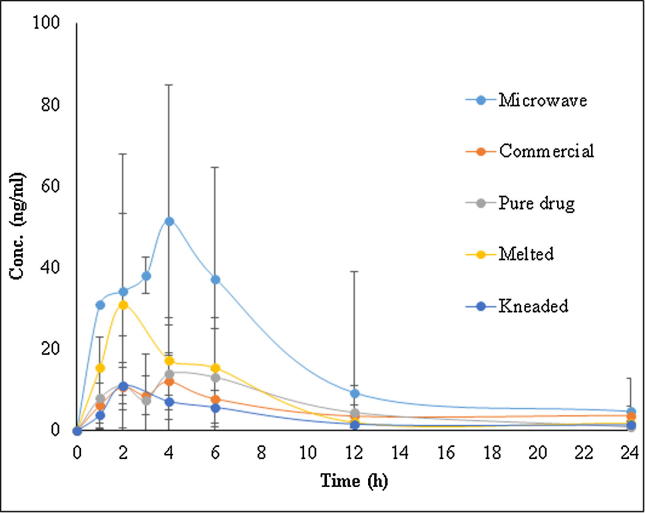
On the basis of physicochemical characterization and in vitro drug release profile, one SD from each technique including kneaded (F3; 1:4), melted (F3; 1:4) and microwave (F3; 1:4) were chosen in order to perform in vivo PK studies in male Albino Wistar rats. The PK studies were performed to analyze APG concentrations in rat plasma after oral administration of optimized SDs, pure APG and marketed APG capsule. The plasma concentration of APG was determined with the help of a validated UPLC-MS/MS method using prednisolone as IS. The plasma concentration-time profile curve of APG for optimized SDs suggested that microwave SD enhanced the oral absorption of APG in comparison with pure APG and marketed capsule (Fig. 11). The PK data of optimized SDs, pure APG and marketed capsule are furnished in Table 2. The value of Tmax was obtained as 2.8 h for microwave and melted SDs and 3.2 h for kneaded SD in comparison with 3.6 and 4.0 h for pure APG and marketed capsule, respectively. The Tmax value for microwave and melted SDs was significantly different from pure APG and marketed capsule (P < 0.05). However, the value of Cmax was obtained as 60.54 µg/ml, 31.78 µg/ml and 11.76 µg/ml for microwave, melted and kneaded SDs, respectively in comparison with 21.38 µg/ml and 17.48 µg/ml for pure APG and marketed capsule, respectively. The Cmax of microwave and melted SDs was significantly (P < 0.05) different from pure APG and marketed capsule. AUC0-24 of microwave, melted and kneaded SDs were obtained as 453.20, 187.36 and 83.85 µg.h/ml, respectively in comparison with 146.54 and 141.98 µg.h/ml for pure APG and marketed capsule, respectively. AUC0-24 values of microwave and melted SDs were significantly higher (P < 0.05) in comparison with AUC0-24 of pure APG and marketed capsule. The MRT values for microwave (9.08 h) and melted (14.03 h) SDs were also significantly different (P < 0.05) from marketed capsule (28.67 h). The relative bioavailability of microwave and melted SDs with respect to marketed capsule was obtained as 319.19 and 131.96%, respectively. These values of relative bioavailability were also significantly higher (P < 0.05) in comparison with marketed capsule. The oral absorption of APG from optimized microwave SD resulted in 3.19 fold enhancement in oral bioavailability in comparison with marketed capsule. The enhancement in oral absorption and bioavailability of APG from SDs was possible due to the presence of hydrophilic carrier PL in SDs in comparison with pure APG and marketed capsule. PL is known to enhance the dissolution rate, wettability, solubility and surface area of lipophilic drugs which could finally results in enhanced oral bioavailability (Newa et al., 2008, El-Badry et al., 2013). Moreover, PL is also capable to convert crystalline drugs into amorphous form (Ruel-Gariepy and Leroux, 2004). Therefore, the significant enhancement in oral absorption and bioavailability of APG from optimized microwave SD was probably due to the presence of PL which was responsible for thr amorphization of APG in SD form (Ruel-Gariepy and Leroux, 2004, Newa et al., 2008, Monti et al., 2010, El-Badry et al., 2013).
Fig. 11.
Drug concentration–time profile curve of APG after oral administration of pure APG, commercial capsule and various SDs prepared by different techniques.
Table 2.
Pharmacokinetic parameters of APG after oral administration of optimized SDs, pure drug and commercial capsule (10 mg/kg) in rats.
| Parameters | Pure APG (Mean ± SD) | Capsule (Mean ± SD) | Microwave (Mean ± SD) | Melted (Mean ± SD) | Kneaded (Mean ± SD) |
|---|---|---|---|---|---|
| Cmax (µg/ml) | 21.38 (6.42) | 17.48 (1.33) | 60.54* (24.70) | 31.78* (21.21) | 11.76 (4.41) |
| Tmax (h) | 3.60 (0.75) | 4.00 (0.73) | 2.80* (1.30) | 2.80* (1.78) | 3.20 (1.78) |
| AUC0-24 (µg h/ml) | 146.54 (62.44) | 141.98 (30.10) | 453.20* (328.78) | 187.36* (92.40) | 83.35 (19.40) |
| λz (h−1) | 0.12 (0.03) | 0.11 (0.06) | 0.11 (0.01) | 0.10 (0.06) | 0.07 (0.05) |
| T ½ (h) | 7.78 (2.25) | 19.94 (8.80) | 6.38* (1.01) | 11.60* (11.73) | 31.07* (46.70) |
| MRT (h) | 11.41 (1.92) | 28.67 (11.46) | 9.08* (0.46) | 14.03* (10.78) | 42.39* (63.36) |
| Relative bioavailability (%) | 103.21 | 100.00 | 319.19* | 131.96* | 58.70 |
P < 0.05 significant compared to commercial capsule.
4. Conclusions
In the present study, various SDs of APG were prepared using PL as a carrier by three different techniques including microwave, melting and kneading technologies. Developed SDs of APG were characterized using TGA, DSC, PXRD, SEM and FTIR techniques and investigated for in vitro dissolution/drug release studies. Physicochemical evaluation of developed SDs suggested that APG SDs were developed successfully by three different techniques and changed crystalline APG to amorphous APG. In vitro dissolution studies suggested that SDs of APG developed by different techniques showed significant drug release profile as compared with pure APG and marketed capsule. Optimized SD from each technique was chosen for in vivo PK studies in male Wistar rats. In vivo PK studies in rats suggested that APG SD prepared by microwave technology showed maximum in vivo absorption in comparison with pure APG, marketed capsule and SD prepared by melting and kneading technology. The enhancement in oral bioavailability of APG from microwave SD was 3.19 fold in comparison with marketed capsule. Overall, these results suggested that SDs of APG developed in this study could be successfully applied in the dissolution and bioavailability enhancement of APG.
Acknowledgments
Acknowledgement
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding the research through research group project number RGP-1438-013.
Conflict of interest
The authors have declared that no competing interests exist.
Footnotes
Peer review under responsibility of King Saud University.
References
- Al Shaal L., Shegokar R., Muller R.H. Production and characterization of antioxidant apigenin nanocrystals as a novel UV skin protective formulation. Int. J. Pharm. 2011;420:133–140. doi: 10.1016/j.ijpharm.2011.08.018. [DOI] [PubMed] [Google Scholar]
- Alshehri S.M., Park J.B., Alsulays B.B., Tiwari R.V., Almutairy B., Alshetaili A.S., Morott J., Shah S., Kulkarni V., Majumdar S., Martin S.T. Mefenamic acid taste-masked oral disintegrating tablets with enhanced solubility via molecular interaction produced by hot melt extrusion technology. J. Drug Deliv. Sci. Technol. 2015;27:18–27. doi: 10.1016/j.jddst.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshehri S., Shakeel F., Ibrahim M., Elzayat E., Tamimi M., Shazly G., Mohsin K., Alkholief M., Alsulays B., Alshetaili A., Alshahrani A., Almalki B., Alanazi F. Influence of microwave technology on solid dispersions of mefenamic acid and flufenamic acid. Plos One. 2017;12:E0182011. doi: 10.1371/journal.pone.0182011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshehri S.M., Tiwari R.V., Alsulays B.B., Ashour E.A., Alshetaili A.S., Almutairy B., Park J.B., Morott J., Sandhu B., Majumdar S., Repka M.A. Investigation of the combined effect of MgO and PEG on the release profile of mefenamic acid prepared via hot-melt extrusion techniques. Pharm. Develop. Technol. 2017;22:740–753. doi: 10.3109/10837450.2016.1138129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altamimi M.A., Neau S.H. Use of the Flory-Huggins theory to predict the solubility of nifedipine and sulfamethoxazole in the triblock, graft copolymer Soluplus. Drug Develop. Ind. Pharm. 2016;42:446–455. doi: 10.3109/03639045.2015.1075033. [DOI] [PubMed] [Google Scholar]
- Altamimi M.A., Elzayat E.M., Alshehri S.M., Mohsin K., Ibrahim M.A., Al Meanazel O.T., Shakeel F., Alanazi F.K., Alsarra I.A. Utilizing spray drying technique to imrove oral bioavailability of apigenin. Adv. Powder Technol. 2018;29:1676–1684. [Google Scholar]
- Arsic I., Tadic V., Vlaovic D., Homšek I., Vesic S., Isailovic G., Vuleta G. Preparation of novel apigenin-enriched, liposomal and non-Liposomal, antiinflammatory topical formulations as substitutes for corticosteroid therapy. Phytother. Res. 2011;25:228–233. doi: 10.1002/ptr.3245. [DOI] [PubMed] [Google Scholar]
- Aso Y., Yoshioka S., Miyazaki T., Kawanishi T. Feasibility of 19F-NMR for assessing the molecular mobility of flufenamic acid in solid dispersions. Chem. Pharm. Bull. 2009;57:61–64. doi: 10.1248/cpb.57.61. [DOI] [PubMed] [Google Scholar]
- Cespi M., Bonacucina G., Casettari L., Mencarelli G., Palmieri G.F. Poloxamer thermogel systems as medium for crystallization. Pharm. Res. 2012;29:818–826. doi: 10.1007/s11095-011-0606-3. [DOI] [PubMed] [Google Scholar]
- Choi J.S., Islam M.N., Ali M.Y., Kim E.J., Kim Y.M., Jung H.A. Effects of C-glycosylation on anti-diabetic, anti-Alzheimer’s disease and anti-inflammatory potential of apigenin. Food Chem. Toxicol. 2014;64:27–33. doi: 10.1016/j.fct.2013.11.020. [DOI] [PubMed] [Google Scholar]
- Das S., Das J., Samadder A., Paul A., Khuda-Bukhsh A.R. Strategic formulation of apigenin-loaded PLGA nanoparticles for intracellular trafficking, DNA targeting and improved therapeutic effects in skin melanoma in vitro. Toxicol. Lett. 2013;223:124–138. doi: 10.1016/j.toxlet.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Ding B., Chen H., Wang C., Zhai Y., Zhai G. Preparation and in vitro evaluation of apigenin loaded lipid nanocapsules. J. Nanosci. Nanotechnol. 2013;13:6546–6552. doi: 10.1166/jnn.2013.7763. [DOI] [PubMed] [Google Scholar]
- Diniz I.M.A., Chen C., Xu X., Ansari S., Zadeh H.H., Marques M.M., Shi S., Moshavarinia A. Pluronic F-127 hydrogel as a promising scaffold for encapsulation of dental-derived mesenchymal stem cells. J. Mater. Sci: Mater. Med. 2015;26:E153. doi: 10.1007/s10856-015-5493-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumortier G., Grossiord J.L., Agnely F., Chaumeil J.C. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm. Res. 2006;23:2709–2728. doi: 10.1007/s11095-006-9104-4. [DOI] [PubMed] [Google Scholar]
- El-Badry M., Hassan M.A., Ibrahim M.A., Elsaghir H. Performance of Poloxamer 407 as hydrophilic carrier on the binary mixtures with nimesulide. Farmacia. 2013;61:1137–1150. [Google Scholar]
- Elhennawy M.G., Lin H.S. Dose- and time-dependent pharmacokinetics of apigenin trimethyl ether. Eur. J. Pharm. Sci. 2018;118:96–102. doi: 10.1016/j.ejps.2018.03.022. [DOI] [PubMed] [Google Scholar]
- Fulop I., Gyeresi A., Kiss L., Deli M.A., Croitoru M.D., Szabo-Revesz P., Aigner Z. Preparation and investigation of mefenamic acid-polyethylene glycol-sucrose ester solid dispersions. Acta Pharm. 2015;65:453–462. doi: 10.1515/acph-2015-0035. [DOI] [PubMed] [Google Scholar]
- Gioffredi E., Boffito M., Calzone S., Giannitelli S.M., Rainer A., Trombetta M., Mozetic P., Chiono V. Pluronic F-127 hydrogel characterization and biofabrication in cellularized constructs for tissue engineering applications. Proced. CIRP. 2016;49:125–132. [Google Scholar]
- Gupta S., Afaq F., Mukhtar H. Selective growth-inhibitory, cell-cycle deregulatory and apoptotic response of apigenin in normal versus human prostate carcinoma cells. Biochem. Biophys. Res. Comm. 2001;287:914–920. doi: 10.1006/bbrc.2001.5672. [DOI] [PubMed] [Google Scholar]
- Hong J., Fristiohady A., Nguyen C.H., Milovanovic D., Huttary N., Krieger S., Hong J., Geleff S., Birner P., Jäger W., Özmen A., Krenn L., Krupitza G. Apigenin and luteolin attenuate the breaching of MDA-MB231 breast cancer spheroids through the lymph endothelial barrier in vitro. Front. Pharmacol. 2018;9:E220. doi: 10.3389/fphar.2018.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Zu Y., Zhao X., Wu M., Feng Z., Deng Y., Zu C., Wang L. Preparation of inclusion complex of apigenin-hydroxypropyl-β-cyclodextrin by using supercritical antisolvent process for dissolution and bioavailability enhancement. Int. J. Pharm. 2016;511:921–930. doi: 10.1016/j.ijpharm.2016.08.007. [DOI] [PubMed] [Google Scholar]
- Issa A.A., Marchidan D., Cojocaru V., Anuta V. Preparation and evaluation of meloxicam solid dispersions by melting method. Farmacia. 2013;61:1216–1232. [Google Scholar]
- Jangdey M.S., Gupta A., Saraf S., Saraf S. Development and optimization of apigeninloaded transfersomal system for skin cancer delivery: in vitro evaluation. Art. Cells Nanomed. Biotechnol. 2017;45:1452–1462. doi: 10.1080/21691401.2016.1247850. [DOI] [PubMed] [Google Scholar]
- Kang N., Lee J., Choi J.N., Mao C., Lee E.H. Cryomilling-induced solid dispersions of poor glass forming/poorly water-soluble mefenamic acid with polyvinylpyrollidone K12. Drug Develop. Ind. Pharm. 2015;41:978–988. doi: 10.3109/03639045.2014.920024. [DOI] [PubMed] [Google Scholar]
- Karolewicz B., Gajda M., Gorniak A., Owczarek A., Mucha I. Pluronic F127 as a suitable carrier for preparing the imatinib base solid dispersions and its potential in development of a modified release dosage forms. J. Therm. Anal. Calorim. 2017;130:383–390. [Google Scholar]
- Laorko A., Li Z., Tongchitpakdee S., Chantachum S., Youravong W. Effect of membrane property and operating conditions on phytochemical properties and permeate flusx durng clarification of pineapple juice. J. Food Eng. 2010;100:514–521. [Google Scholar]
- Li Y., Pang H., Guo Z., Dong Y., Li G., Lu M., Wu C. Interactions between drugs and polymers inluencing hot melt extrusion. J. Pharm. Pharmacol. 2014;66:148–166. doi: 10.1111/jphp.12183. [DOI] [PubMed] [Google Scholar]
- Liu S., Bao H., Li L. Role of PPO–PEO–PPO triblock copolymers in phase transitions of a PEO–PPO–PEO triblock copolymer in aqueous solution. Eur. Polym. J. 2015;71:423–439. [Google Scholar]
- Maurya D., Belgamwar V., Tekade A. Microwave induced solubility enhancement of poorly water soluble atorvastatin calcium. J. Pharm. Pharmacol. 2010;62:1599–1606. doi: 10.1111/j.2042-7158.2010.01187.x. [DOI] [PubMed] [Google Scholar]
- Menéndez J.A., Arenillas A., Fidalgo B., Fernández Y., Zubizarreta L., Calvo E.G., Bermúdez J.M. Microwave heating processes involving carbon materials. Fuel Proc. Technol. 2010;91:1–8. [Google Scholar]
- Moneghini M., Bellich B., Baxa P., Princivalle F. Microwave generated solid dispersions containing ibuprofen. Int. J. Pharm. 2008;361:125–130. doi: 10.1016/j.ijpharm.2008.05.026. [DOI] [PubMed] [Google Scholar]
- Moneghini M., Zingone G., Zordi N.D. Influence of microwave technology on the physical-chemical properties of solid dispersion with nimesulide. Powder Technol. 2009;195:259–263. [Google Scholar]
- Monti S., Burgalassi S., Rossato M.S., Albertini B., Passerini N., Rodriguez I., Chetoni P. Poloxamer 407 microspheres for orotransmucosal drug delivery. Part II: In vitro/in vivo evaluation. Int. J. Pharm. 2010;400:32–36. doi: 10.1016/j.ijpharm.2010.08.018. [DOI] [PubMed] [Google Scholar]
- Morimoto Y., Baba T., Sasaki T., Hiramatsu K. Apigenin as an anti-quinolone-resistance antibiotic. Int. J. Antimic. Agents. 2015;46:666–673. doi: 10.1016/j.ijantimicag.2015.09.006. [DOI] [PubMed] [Google Scholar]
- Newa M., Bhandari K.H., Oh D.H., Kim Y.R., Sung J.H., Kim J.O., Woo J.S., Choi H.G., Yong C.S. Enhancement dissolution of ibuprofen using solid dispersion with poloxamer 407. Arch. Pharm. Res. 2008;31:1497–1507. doi: 10.1007/s12272-001-2136-8. [DOI] [PubMed] [Google Scholar]
- Ozbey U., Attar R., Romero M.A., Alhewairini S.S., Afshar B., Sabitaliyevich U.Y., Hanna-Wakim L., Ozcelik B., Farooqi A.A. Apigenin as an effective anticancer natural product: Spotlight on TRAIL, WNT/β-catenin, JAK-STAT pathways, and microRNAs. J. Cell Biochem. 2018 doi: 10.1002/jcb.27575. [DOI] [PubMed] [Google Scholar]
- Park Y.J., Kwon R., Quan Q.Z., Oh D.H., Kim J.O., Hwang M.R., Koo Y.B., Woo J.S., Yong C.S., Choi H.G. Development of novel ibuprofen-loaded solid dispersion with improved bioavailability using aqueous solution. Arch. Pharm. Res. 2009;32:767–772. doi: 10.1007/s12272-009-1516-3. [DOI] [PubMed] [Google Scholar]
- Paudel A., Worku Z.A., Meeus J., Guns S., Van den Mooter G. Manufacturing of solid dispersions of poorly water soluble drugs by spray drying: formulation and process considerations. Int. J. Pharm. 2013;453:253–284. doi: 10.1016/j.ijpharm.2012.07.015. [DOI] [PubMed] [Google Scholar]
- Perez-Moral N., Saha S., Philo M., Hart D.J., Winterbone M.S., Hollands W.J., Spurr M., Bows J., Velpen V.V.D., Kroon P.A., Curtis P.J. Comparative bio-accessibility, bioavailability and bioequivalence of quercetin, apigenin, glucoraphanin and carotenoids from freeze-dried vegetables incorporated into a baked snack versus minimally processed vegetables: Evidence from in vitro models and a human bioavailability study. J. Func. Foods. 2018;48:410–419. doi: 10.1016/j.jff.2018.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J., Dwyer J. Flavonoids: dietary occurrence and biochemical activity. Nutr. Res. 1998;18:1995–2018. [Google Scholar]
- Ruel-Gariepy E., Leroux J.C. In situ-forming hydrogels—review of temperature-sensitive systems. Eur. J. Pharm. Biopharm. 2004;58:409–426. doi: 10.1016/j.ejpb.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Shagautdinova I., Elkin M., Likhter A. Identification of the vibrational spectra of apigenin and luteolin. J. Surf. Invest. 2015;9:753–760. [Google Scholar]
- Shakeel F., Alshehri S., Ibrahim M.A., Elzayat E.M., Tamimi M.A., Mohsin K., Alanazi F.K., Alsarra I.A. Solubility and thermodynamic parameters of apigenin in different neat solvents at different temperatures. J. Mol. Liq. 2017;234:73–80. [Google Scholar]
- Shakeel F., Alshehri S., Haq N., Elzayat E., Ibrahim M., Altamimi M.A., Mohsin K., Alanazi F.K., Alsarra I.A. Solubility determination and thermodynamic data of apigenin in binary Transcutol® + water mixtures. Ind. Crops Prod. 2018;116:56–63. [Google Scholar]
- Shazly G.A., Ibrahim M.A., Badran M.M., Khairy M.A., Zoheir M.A. Utilizing Pluronic F 127 and Gelucire 50/13 dolid dispersions for enhanced skin delivery of flufenamic acid. Drug Develop. Res. 2012;73:299–307. [Google Scholar]
- Shen L.N., Zhang Y.T., Wang Q., Xu L., Fenga N.P. Enhanced in vitro and in vivo skin deposition of apigenin delivered using ethosomes. Int. J. Pharm. 2014;460:280–288. doi: 10.1016/j.ijpharm.2013.11.017. [DOI] [PubMed] [Google Scholar]
- Shibata C., Ohno M., Otsuka M., Goto K., Muroyama R., Kato N., Yoshikawa T., Takata A., Koike K. The flavonoid apigenin inhibits hepatitis C virus replication by decreasing mature microRNA122 levels. Virol. 2014;462:42–48. doi: 10.1016/j.virol.2014.05.024. [DOI] [PubMed] [Google Scholar]
- Shukla S., Gupta S. Apigenin: a promising molecule for cancer prevention. Pharm. Res. 2010;27:962–978. doi: 10.1007/s11095-010-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto M.L., Moure A., Dominguez H., Parajo J.C. Recovery, concentration and purification of phenolic compounds by adsorption: a review. J. Food Eng. 2011;105:1–27. [Google Scholar]
- Steuck M., Hellhake S., Schebb N.H. Food polyphenol apigenin inhibits the cytochrome 450 monooxygenase branch of the arachidonic acid cascade. J. Agric. Food Chem. 2016;64:8973–8976. doi: 10.1021/acs.jafc.6b04501. [DOI] [PubMed] [Google Scholar]
- Telange D.R., Patil A.T., Pethe A.M., Fegade H., Anand S., Dave V.S. Formulation and characterization of an apigenin-phospholipid phytosome (APLC) for improved solubility, in vivo bioavailability, and antioxidant potential. Eur. J. Pharm. Sci. 2017;108:36–49. doi: 10.1016/j.ejps.2016.12.009. [DOI] [PubMed] [Google Scholar]
- Van Eardenbrugh B., Taylor L.S. Small scale screening to determine the ability of different polymers to inhibit drug crystallization upon rapid solvent evaporation. Mol. Pharm. 2010;7:1328–1337. doi: 10.1021/mp1001153. [DOI] [PubMed] [Google Scholar]
- Wang X., Wang W., Wang J.Z., Yang C., Liang C.Z. Effect of apigenin on apoptosis induced by renal ishchemia/reperfusion injury in vivo and in vitro. Ren. Fail. 2018;40:498–505. doi: 10.1080/0886022X.2018.1497517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Zhao C., Sun J., Wang X., Zhao X., Mao Y., Li X., Song Z. Quantitative measurement of energy utilization efficiency and study of influence factors in typical microwave heating process. Energy. 2015;87:678–685. [Google Scholar]
- Wong S., Kellaway I., Murdan S. Enhancement of the dissolution rate and oral absorption of a poorly water soluble drug by formation of surfactant-containing microparticles. Int. J. Pharm. 2006;317:61–68. doi: 10.1016/j.ijpharm.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Xiqiang Z., Wenlong W., Hongzhen L., Yanpeng M., Chunyuan M., Zhanlong S. Temperature rise and weight loss characteristics of wheat straw under microwave heating. J. Anal. Appl. Pyrol. 2014;107:59–66. [Google Scholar]
- Zhai Y., Guo S., Liu C., Yang C., Dou J., Li L., Zhai G. Preparation and in vitro evaluation of apigenin-loaded polymeric micelles. Coll. Surf. A. 2013;429:24–30. [Google Scholar]
- Zhang J., Liu D., Huang Y., Gao Y., Qian S. Biopharmaceutics classification and intestinal absorption study of apigenin. Int. J. Pharm. 2012;436:311–317. doi: 10.1016/j.ijpharm.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Zhang J., Huang Y., Liu D., Gao Y., Qian S. Preparation of apigenin nanocrystals using super critical antisolvent process for dissolution and bioavailability enhancement. Eur. J. Pharm. Sci. 2013;48:740–747. doi: 10.1016/j.ejps.2012.12.026. [DOI] [PubMed] [Google Scholar]
- Zhao X., Wang Z., Li X. Preparation, in vitro release and antioxidant potential of formulation of apigenin with hydroxypropyl-β-cyclodextrin modified microemulsion. J. Incl. Phen. Macrocyc. Chem. 2016;86:93–102. [Google Scholar]
- Zhao L., Zhang L., Meng L., Wang J., Zhai G. Design and evaluation of a selfmicroemulsifying drug delivery system for apigenin. Drug Develop. Ind. Pharm. 2013;39:662–669. doi: 10.3109/03639045.2012.687378. [DOI] [PubMed] [Google Scholar]
- Zhu B., Li X., Xu X., Li J., Ding C., Zhao C., Li J. One-step phosphorylated poly(amide-amine) dendrimer loaded with apigenin for simultaneous remineralization and antibacterial of dentine. Coll. Surf. B. 2018;172:760–768. doi: 10.1016/j.colsurfb.2018.09.036. [DOI] [PubMed] [Google Scholar]