Abstract
Collectivism is an important factor for coping with stress in one’s social life. To date, no imaging studies have revealed a direct association between collectivism and white matter structure. Collectivism is positively related to independence, harm avoidance, rejection sensitivity, cooperativeness, external locus of control, and self-monitoring and negatively related to need for uniqueness. Accordingly, we hypothesised that the neural structures underpinning collectivism are those that are also involved with its relationship using magnetic resonance imaging (MRI). This study aimed to identify the brain structures associated with collectivism in healthy young adults (n = 797), using regional grey and white matter volume, fractional anisotropy, and mean diffusivity (MD) analyses of MRI data. Scores on the collectivism scale were positively associated with MD values in the bilateral dorsolateral prefrontal cortex, left orbitofrontal cortex, inferior frontal gyrus, right superior temporal gyrus, ventral posterior cingulate cortex, globus pallidus, and calcarine cortex using the threshold-free cluster enhancement method with family-wise errors corrected to P < 0.05 at the whole-brain level. No significant associations between were found collectivism and other measures. Thus, the present findings supported our hypothesis that the neural correlates of collectivism are situated in regions involved in its related factors.
Introduction
Collective coping behaviours among culturally diverse populations are important for stress coping processes via collectivistic values1. Enhancing prosocial behaviour in collectivism is an effective means of social scrutiny2. Collectivism is defined as a social pattern that links individuals to parts of collectives (family, tribe, nation, etc.)3. Personal collectivism is defined as the tendency of an individual to prioritise the collective self over the private self, when those are in conflict4. Collectivism scale scores have been positively related to interdependence (highly correlated concept of collectivism), rejection from the group (related to harm avoidance), self-monitoring, and being an external locus of control, whereas they are negatively correlated with need for uniqueness4. Furthermore, collectivism increases cooperativeness5, particularly in Japanese participants6.
Collectivism has also been studied using functional magnetic resonance imaging (fMRI). Temporarily heightened awareness of individualist or collectivist orientations is sufficient to elicit greater activation within the medial prefrontal cortex (mPFC) and posterior cingulate cortex (PCC), respectively, during general or contextual self-descriptions7. Moreover, the degree of agreement with individualistic or collectivistic values, respectively, predicted mPFC responses to general or contextual self-descriptions8. Collectivists showed stronger activation in the left temporoparietal regions than individualists, who mainly recruited the medial prefrontal regions9. Importantly, fMRI is based in hemodynamics and is only an indirect measure of brain activation10. Excitatory brain activity remains unable to be distinguished from inhibitory actions by fMRI11. In brief, we could just say that ‘this area is associated with processing stimuli’11.
Numerous structural studies of the brain have assessed the neural correlates of factors related to collectivism. First, with respect to factors positively related to collectivism, interdependence using questionnaires was associated with decreased grey matter volume (GMV) limited in PFC (ventromedial PFC [vmPFC], dorsolateral PFC [DLPFC], rostrolateral PFC [RLPFC]12, and orbitofrontal cortex [OFC])13. Harm avoidance identified by the use of questionnaires was positively correlated with mean diffusivity (MD) in the globus pallidus14, putamen15, and superior temporal gyrus (STG)14. Cooperativeness, also assessed by questionnaire, was negatively correlated with fibre connectivity for the cognitive control system from the dorsal caudate to the rostral cingulate cortex and ventrolateral PFC16. Self-monitoring is inversely associated with white matter (WM) integrity in the anterior cingulum, which connects the dorsal anterior cingulate cortex and midline structures17. Furthermore, white matter volume (WMV) in the striatum showed significant negative correlations with a sense of being the external locus of control18. Second, with respect to factors negatively related to collectivism, individual need for uniqueness was also associated with larger WMV19 of the body of the corpus callosum.
Although MD and fractional anisotropy (FA) measure are related parameters of diffusion tensor imaging (DTI), they were different microstructural brain properties. Affection of MD includes capillaries, spines, and macromolecular proteins, properties of myelin, membrane, axon, the shape of neurons, protoplasmic glia, fibrous glia, and enhanced tissue organisation20. On the other hand, FA is more strongly associated with microstructural properties related to brain connectivity and sensitive to increases in axonal membrane thickness, diameter, and/or the amount of parallel organisation of the axons20. The general trend during youth is increasing FA and decreasing mean MD with age21. Accordingly, MD and FA may be used together to supplement the strengths of each method.
Previous studies have proved that neural correlates of collectivism were only the PFC, especially mPFC by fMRI studies7,8 and grey matter structure studies12,13, although collectivism could be related to several aforementioned psychological factors. Accordingly, the present study hypothesised that the degree of collectivism is related to the neural correlates of factors related to collectivism (the PFC, temporoparietal junction [TPJ], PCC, and striatum), and that this relationship can be demonstrated using regional WMV (rWMV), FA, and MD analyses. We also checked correlation between collectivism scores and regional GMV (rGMV) for reproducibility of the outcomes of the previous findings (i.e., the PFC)12,13. Thus, the present study aimed to identify the neuroanatomical correlates of collectivism in young people.
Results
Behavioral Data
Table 1 shows the means and standard deviations (SD) for age, and scores for the Raven’s Advanced Progressive Matrix (RAPM) and collectivism scale. Figure 1 depicts the distributions of the collectivism scores among men and women.
Table 1.
Sex differences in age and scores (mean ± SD) on the RAPM, and collectivism; and one-way ANOVA results.
| Measure | Total (SD) | Males (N = 439) |
Females (N = 358) |
P | F |
|---|---|---|---|---|---|
| Age | 20.6 (1.8) | 20.7 (1.9) | 20.5 (1.6) | 0.037* | 4.4 |
| RAPM | 28.5 (3.8) | 28.9 (3.7) | 28.0 (3.9) | 0.002** | 10.1 |
| Collectivism | 43.0 (6.7) | 42.7 (6.7) | 43.3 (6.8) | 0.171 | 1.9 |
*P < 0.05, **P < 0.01.
ANOVA, analysis of variance; RAPM, Raven’s Advanced Progressive Matrix; SD, standard deviation.
Figure 1.
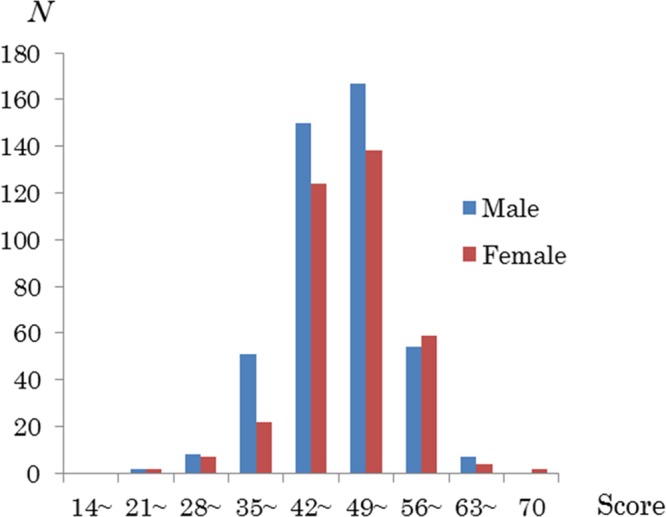
Distribution of collectivism scores in males and females. Histograms showing the distribution of collectivism scores in men and women.
After adjustment for the effects of age, sex, and RAPM, significant correlation among the collectivism scores was observed among the 797 subjects (P < 0.001).
There was significant difference between men and women in the RAPM scores (P < 0.05, one-way analysis of variance [ANOVA]) but not the collectivism scores (P = 0.171).
Magnetic resonance imaging Data
Analysis of voxel-based morphometry (VBM) data
After adjustment for sex, age, total intracranial brain volume (TIV; total GM volume + total WM volume + total cerebrospinal fluid [CSF] volume), and RAPM scores, there were no significant positive or negative correlations between collectivism scores and rGMV (or rWMV) at each voxel at a family-wise errors (FWE)-corrected threshold of P < 0.05 based on the threshold-free cluster enhancement (TFCE) method at the whole-brain level. There were negative correlations between collectivism scores and rGMV (or rWMV) at each voxel at a threshold of P < 0.001 uncorrected (t = 3.5) at the whole-brain level. For detailed results, please see Supplemental Table 1.
Regions of interest (ROI) analysis of the association between collectivism and rGMV
After adjustment for the aforementioned confounding factors, there were no significant positive or negative correlations between collectivism scores and rGMV in ROIs at the aforementioned threshold.
Analysis of MD and FA data
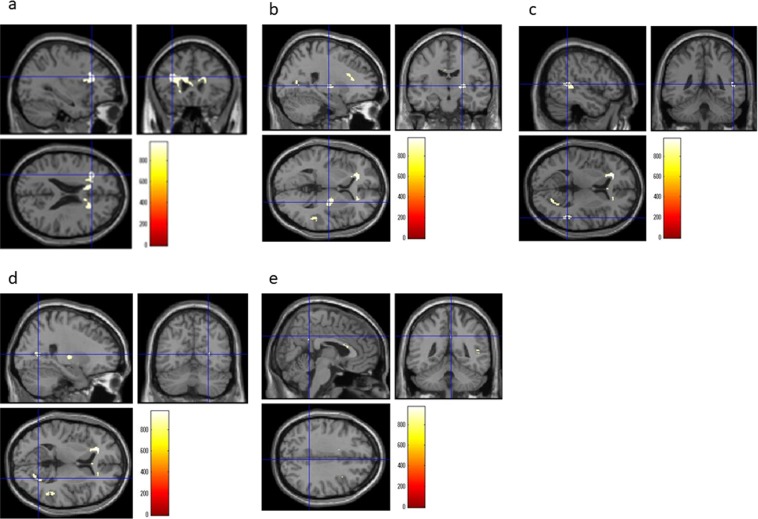
A whole-brain multiple regression analysis after adjustment for sex, age, TIV, and RAPM scores, revealed a significant positive correlation between collectivism scores and MD in the bilateral DLPFC, left OFC, inferior frontal gyrus (IFG), right globus pallidus, STG along with the posterior superior temporal sulcus, calcarine cortex, and ventral PCC (vPCC), using the TFCE method with FWE corrected to P < 0.05 at the whole-brain level (Table 2; Figs 2 and 3). There were no significant negative correlations between collectivism scores and MD at the same threshold at the whole-brain level. Most peak voxels of the significant regions except for one peak voxel of vPCC and that of the globus pallidus fall in white matter.
Table 2.
Brain regions exhibiting a significant correlation between MD and collectivism scores.
| Brain region | G/W | R/L | x | y | z |
TFCE
Value |
Corrected P-value (FWE) |
Cluster size (kE) |
β |
|---|---|---|---|---|---|---|---|---|---|
| DLPFC | W | L | −32 | 26 | 23 | 971.7 | 0.034* | 2309 | 0.129 |
| OFC | W | L | −17 | 35 | −6 | 971.1 | 0.034* | ||
| IFG | W | L | −21 | 33 | 8 | 963.3 | 0.034* | ||
| DLPFC | W | R | 36 | 11 | 38 | 868.7 | 0.046* | 24 | 0.145 |
| Globus pallidus | G | R | 24 | −14 | 3 | 909.2 | 0.040* | 175 | 0.142 |
| STG | W | R | 51 | −44 | 8 | 906.7 | 0.040* | 160 | 0.136 |
| MTG | W | R | 53 | −36 | 3 | 895.4 | 0.041 | ||
| MTG | W | R | 56 | −35 | 3 | 847.9 | 0.050* | 1 | 0.117 |
| Calcarine cortex | W | R | 26 | −63 | 8 | 886.7 | 0.043* | 84 | 0.144 |
| vPCC | W | R | 5 | −45 | 35 | 849.2 | 0.050* | 3 | 0.136 |
| vPCC | G | R | 5 | −47 | 29 | 846.6 | 0.050* | 2 | 0.130 |
*P < 0.05, FWE corrected.
DLPFC, dorsolateral prefrontal cortex; FWE, family-wise errors; G, grey matter; IFG, inferior frontal gyrus; L, left; R, right; MD, Mean diffusivity; OFC, orbitofrontal cortex; STG, Superior temporal gyrus; TFCE, threshold-free cluster enhancement; vPCC, ventral posterior cingulate cortex; W, white matter.
Figure 2.
Regions correlated with MD and collectivism scores. The present results were determined based on an FWE-corrected threshold of P < 0.05 with a TFCE based on 5000 permutations; the results were corrected at the whole-brain level. Regions showing correlations were overlaid on a single T1 image in the SPM8 toolbox. The red-to-yellow colour scale indicates the strength of the TFCE value for the positive correlation between the MD and collectivism scores; areas with significant correlations were identified in the left DLPFC (a), the right globus pallidus (b), STG (c), calcarine (d), and vPCC (e). DLPFC: dorsolateral prefrontal cortex, FWE: family-wise errors, vPCC: ventral posterior cingulate cortex, MD: Mean diffusivity, STG: Superior temporal gyrus, TFCE: threshold-free cluster enhancement.
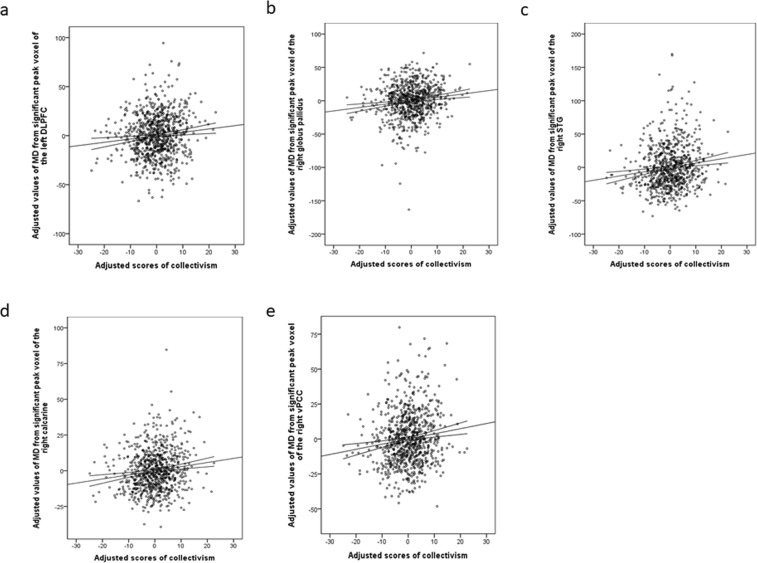
Figure 3.
Peak regional MD values of the significant regions and scores of collectivism. Residual plots with trend lines depicting the correlations between residuals in the multiple regression analysis with collectivism scores as the dependent variable and other confounding factors as independent variables; 95% confidence intervals for the trend lines are shown. The peak regional MD values of the significant regions in the left DLPFC (a), the right globus pallidus (b), STG (c), calcarine cortex (d), and vPCC (e). DLPFC: dorsolateral prefrontal cortex, vPCC: ventral posterior cingulate cortex, MD: Mean diffusivity, STG: Superior temporal gyrus.
We found no significant correlations between collectivism scores and FA using the same analyses mentioned above. Further, there was no correlations between collectivism scores and FA at each voxel at a threshold of P < 0.001 uncorrected (t = 3.5) at the whole-brain level.
Interaction effects of sex and collectivism on brain structures
Using data from both sexes with respect to the covariates of age, RAPM, TIV, and collectivism scores, an analysis of covariance (ANCOVA) revealed no significant interaction effect between collectivism scores and sex on rGMV, rWMV, FA, or MD using the TFCE method with FWE corrected to P < 0.05 at the whole-brain level.
Discussion
To our knowledge, the present study is the first to investigate the direct associations between collectivism and white matter structures in healthy individuals at the whole-brain level. Consistent with the stated hypothesis, total collectivism scores were most positively associated with MD values in the left PFC (including DLPFC, OFC, and IFG), the right DLPFC, STG (including TPJ), vPCC, and globus pallidus. These brain structural outcomes confirmed that the regions implicated in functional studies of collectivism are also associated with the neural correlates of interdependence, rejection sensitivity, cooperativeness, self-monitoring, and external locus of control.
There are several mechanisms potentially underlying the significant relationship between collectivism and the regions implicated here: the PFC (the bilateral DLPFC, left orbitofrontal cortex, and IFG) in accordance with the previous grey matter structural studies associated with independence12,13. Furthermore, cooperativeness is positively related to MD in the PFC14, whereas self-monitoring is inversely associated with WM integrity in the anterior cingulum17. Thus, low function of the PFC including anterior cingulum could be main neural correlates of collectivism as aspects of interdependence, cooperativeness, and self-monitoring.
We also demonstrated that collectivism scores were related to higher MD values in the globus pallidus and the vPCC. Harm avoidance (positively related to collectivism) was positively correlated with MD in the basal ganglia14. Moreover, the external locus of control (positively related to collectivism) was negatively correlated with WMV in the striatum18. With respect to the vPCC, GMV in the region of the PCC has been negatively associated with rejection sensitivity22. Uniquely, one of the peak voxels of the significant regions at vPCC falls in the grey matter. Increasing MD, especially in grey matter includes reduction of spines and macromolecular proteins, protoplasmic glia20. Accordingly, the low function of the globus pallidus and vPCC seemed to be neural correlates of collectivism as aspects of harm avoidance, rejection sensitivity, and external locus of control.
The calcarine cortex was unexpectedly related to collectivism. Collectivism scale scores were positively related to social anxiety4. Interestingly, compared with healthy controls, decreased functional connectivity between the frontal and occipital lobes might be related to abnormal information processing in patients with social anxiety disorder in resting-state fMRI23. Furthermore, lower nodal centralities in patients with social anxiety disorder were seen in the calcarine, DLPFC, and insula than those of healthy controls in resting-state fMRI24. Accordingly, based on the social anxiety, the dysfunction of the calcarine cortex is therefore presumed to be a legitimate neural correlate of collectivism. Notably, all MD values in regions significantly related to collectivism were not negative but positive relationships. MD decreases are extraordinarily sensitive to white matter development, reflecting underlying changes in tissue water content and cytoarchitecture25. Nonetheless, the lower tissue density in the regions was related to the tendency for collectivism. This interpretation is in accordance with the idea that collectivists would perceive disruption to their interdependence, social harmony, and security as more threatening and stressful than individualists, as recently suggested in a review of the subject1.
Moreover, there were no significant associations between collectivism and other measures (rGMV, rWMV, and FA) at the standard threshold. A plausible reason for this is that proliferation of glial cells, such as oligodendrocytes, astrocytes, and microglia might be particularly affected by decreasing MD in white matter. These cells substantially impact neuronal function and activities26, for example, by supporting axons by regulating the extracellular ion concentration27. In addition, DTI results particularly indicate white matter maturation during adolescence28. Young adults in this study might show higher collectivism scores due to developmentally slower maturation. Most MD regions (i.e., frontal regions and STG [including TPJ]) significantly associated with collectivism seem to develop even after adolescence. Notably, the frontal-temporal connections have the most prolonged development29. Furthermore, FA values were reduced 3–11% after the peak (20–42 years old), whereas MD values increased 4–20% after the minimum (18–43 years old)29. This difference in the magnitude of changing values might affect the sensitivity to detect regions associated with collectivism. It has been postulated that people typically become more collectivist with age, with one of the main reasons being that older people might have more established social relationships than younger people3. With respect to differences among similar studies, the mean age of participants of the study by Kitayama et al.13 was 26.4 (range 20–39) years, which was older than that of the participants in our study. The finding by Wang et al.12 does not seem to survive at our stringent statistical threshold; their statistical threshold was uncorrected P < 0.005 at whole brain level. Accordingly, only MD could be significantly associated with the values of collectivism in our study.
Finally, as we have previously noted30,31, there are a few limitations of this study. Because the present study used a cross-sectional design, the results cannot be used to determine the causality between collectivism and brain regions with which it is significantly associated. Thus, to overcome this limitation, a prospective study that confirms such causality is necessary. Furthermore, we used young healthy subjects who possessed high levels of education, which may affect the ability of our results to be generalised to other groups. Education tends to individualism due to exposure to cultural diversity3. Accordingly, future research is necessary to study neural correlates of collectivism with subjects differing in culture, education, and generation.
Methods
Subjects
The present study included 797 healthy right-handed individuals (439 men and 358 women; mean age: 20.6 ± 1.8 years). Written informed consent was obtained from each participant prior to the study in accordance with the Declaration of Helsinki (1991). All study procedures were approved by the Ethics Committee of Tohoku University, and all experiments were performed in accordance with the approved guidelines. For more details regarding the study procedures, please see our previous studies32–34.
Psychological Outcome Measures
Assessment of collectivism
We used Yamaguchi’s (revised) Collectivism Scale to measure the allocentric tendency to prioritise a group goal over personal goal when the two goals are in conflict. The scale was developed by adding four individualist items to an original scale4 for application to both collectivists and individualists35: for example, ‘I stick with my group even through difficulties’, ‘I maintain harmony in my group’, and ‘I respect decisions made by my group’. The validity of the revised scale for the variables has been demonstrated to be consistent across cultural groups because the total collectivism scores are associated with higher affiliative tendency, higher rejection sensitivity, and lower need for uniqueness in three countries (the United States, Korea and Japan)35. Thus, this psychometric scale appears to represent a cognitive component present across groups and yields meaningful relationships with other psychological constructs. The total score of the revised scale has satisfactory internal consistency for all countries35. This scale includes a 14-item questionnaire, each item rated on a 5-point Likert scale from describes me not at all (1) to describes me very well (5), which results in a total score ranging from 14 to 70, where higher scores indicate a greater sense of collectivism (9 reverse scores are included). One can define a group referred to in the scale in various ways. Examples of the questions include ‘I sacrifice self-interest for my group’ and ‘I act as fellow group members would prefer’.
Psychometric measures of general intelligence
RAPM, which is a widely used measure of general intelligence36, was utilised in the present study. This measure was adjusted to examine the effects of general intelligence on brain structures. For more details, please refer to our previous study20.
Behavioural data analyses
All behavioural data were analysed using IBM SPSS Statistics 22.0 software package (IBM Corp.; Armonk, NY, USA). Differences between males and females in terms of age and scores on the cognitive measures (RAPM and the collectivism score) were analysed using one-way ANOVA; a two-tailed P value < 0.05 was considered to indicate statistical significance.
Image Acquisition
Structural MRI
All MRI data were acquired using a 3-T Philips Achieva scanner (Philips Medical Systems, Best, Netherlands). Three-dimensional high-resolution T1-weighted images were collected using a magnetisation-prepared rapid gradient-echo sequence with the following parameters: 240 × 240 matrix, TR = 6.5 ms, TE = 3 ms, TI = 711 ms, FOV = 24 cm, 162 slices, in-plane resolution = 1.0 × 1.0 mm, slice thickness = 1.0 mm, and scan duration = 483 s.
Diffusion-weighted data were acquired using a spin-echo echo-planar imaging (EPI) sequence with the following parameters: TR = 10293 ms, TE = 55 ms, FOV = 22.4 cm, 2 × 2 × 2 mm3 voxels, 60 slices, SENSE reduction factor = 2, and number of acquisitions = 1. The diffusion weighting was isotropically distributed along 32 directions (b value = 1,000 s/mm2), and three images with no diffusion weighting (b value = 0 s/mm2; b = 0 images) were acquired using the spin-echo EPI sequence (TR = 10293 ms, TE = 55 ms, FOV = 22.4 cm, 2 × 2 × 2 mm3 voxels, 60 slices). Acquisitions for phase correction and signal stabilisation were performed, but these data were not used as part of the reconstructed images. For more details regarding these procedures, please see our previous studies20,30,37.
Pre-processing and Analyses of Structural Data
VBM data
All pre-processing of the T1 weighted image data was performed using Statistical Parametric Mapping software (SPM8; Wellcome Department of Cognitive Neurology, London, UK) according to the protocol described for voxel-based morphometry analyses in our previous study18. Using the new segmentation algorithm implemented in SPM8, T1-weighted structural images of each individual were segmented and normalised to the Montreal Neurological Institute space to yield images with 1.5 × 1.5 × 1.5 mm3 voxels using diffeomorphic anatomical registration through exponentiated lie algebra (DARTEL) registration process implemented in SPM8. In addition, we performed a volume change correction (modulation)38. All images were smoothed by convolving them using an isotropic Gaussian kernel of 6 mm full-width at half maximum (FWHM). These methods have been adapted from a previous study18. For additional details, please refer to our previous study39.
MD and FA data
All pre-processing and analyses of the imaging data were performed using SPM8 implemented in Matlab (Mathworks Inc.; Natick, MA, USA). The FA and MD maps were calculated from the collected images using a commercially available diffusion tensor analysis package (Philips Medical Systems, Best, Netherlands) on the MR console. These procedures involved corrections for motion and distortion caused by eddy currents, and all calculations were performed using a previously described method40. Briefly, the MD and FA images of the participants were normalised using a previously validated DARTEL-based registration process to develop images with 1.5 × 1.5 × 1.5 mm3 voxels. Furthermore, tissues that were least likely to be grey or white matter were carefully removed, and the images were smoothed by convolving them using an isotropic Gaussian kernel of 6-mm FWHM and normalised FA images were masked by a custom mask image indicating features highly likely to be white matter and then smoothed by a Gaussian Kernel of 6-mm FWHM. These methods have been adapted from a previous study14. For additional details, please see our previous studies30,31.
Statistical Group-level Analyses of Imaging Data
Correction for multiple comparisons was performed using TFCE41 with randomised (5,000 permutations) nonparametric testing in the TFCE toolbox. A FWE-corrected threshold of P < 0.05 was applied. These methods have been adapted from a previous study14.
VBM data
A whole-brain multiple regression analysis performed in SPM8 was used to assess the association between rGMV (or rWMV) and collectivism scores. The covariates included sex, age, RAPM score, and TIV. For each covariate, the overall mean was used for mean centering. Analyses for rGMV and rWMV were performed in voxels for all subjects that showed a signal intensity of >0.05.
Subsequently, based on a priori hypothesis, we used ROI approaches to determine whether the rGMV correlates of the PFC areas included the Brodmann area 8, 9, 10, 11, 44, 45, 46, and 47. The ROI was constructed using the Brodmann area option of the WFU_PickAtlas tool (http://www.fmri.wfubmc.edu/cms/software#PickAtlas)42,43.
Sex differences in the correlates of collectivism were not the main purpose of the study; however, we also investigated whether the relationship between rGMV or rWMV and collectivism scores differed between males and females in a whole-brain analysis. We used a voxel-wise ANCOVA in which sex difference was a group factor using t-contrasts (using the full factorial option of SPM8). In this analysis, age, RAPM, TIV, and collectivism scores were modelled to enable unique relationships with rGMV (using the interaction option in SPM8 for each sex). TIV was modelled to have a common relationship with rGMV and rWMV across sexes.
MD and FA data
A voxel-by-voxel regression analysis was performed in SPM8 using the MD or FA value at each voxel as the dependent variable and age, sex, RAPM score, and collectivism score as the independent variables. The analysis using MD was limited to areas within the grey and white matter masks that were created using the procedures, whereas the analysis using FA was limited to areas within the white matter. We also investigated whether the relationship between MD or FA and collectivism scores differed between men and women by using the same method, rGMV or rWMV.
Supplementary information
Acknowledgements
Dr. H.T. was supported by JST/RISTEX, JST/CREST, a Grant-in-Aid for Young Scientists (B) (KAKENHI 23700306), and a Grant-in-Aid for Young Scientists (A) (KAKENHI 25700012) from the Ministry of Education, Culture, Sports, Science, and Technology, and Health Science Center Foundation. Dr. S.N. was financially supported by the Division of Psychiatry, Tohoku Medical and Pharmaceutical University. We respectfully thank Yuki Yamada for operating the MRI scanner and Haruka Nouchi for administering the psychological tests. Furthermore, we would like to thank the participants in the study, the other individuals who administered psychological tests, and our colleagues in the Institute of Development, Ageing and Cancer at Tohoku University for their support. We would like to thank Editage (www.editage.jp) for English language editing.
Author Contributions
S.N., H.T., Y.T. and R.K. designed the study. S.N., H.T., R.N., Y.K., T.S., T.M., A.S., K.I., R.Y., Y.Y., S.H., T.A., C.M.M., D.M., K.S., H.J., and Y.S. collected the data. S.N. and H.T. analysed the data and prepared the manuscript. S.N. prepared Figures 1–3. All authors reviewed the manuscript.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-37995-5.
References
- 1.Kuo BC. Collectivism and coping: current theories, evidence, and measurements of collective coping. Int J Psychol. 2013;48:374–388. doi: 10.1080/00207594.2011.640681. [DOI] [PubMed] [Google Scholar]
- 2.Rossano MJ. Supernaturalizing Social Life: Religion and the Evolution of Human Cooperation. Hum Nat. 2007;18:272–294. doi: 10.1007/s12110-007-9002-4. [DOI] [PubMed] [Google Scholar]
- 3.Triandis, H. C. Individualism and collectivism. (Routledge, 2018).
- 4.Yamaguchi, S. Collectivism among the Japanese: A perspective from the self. In Kim, U. et al. (Ed.), Cross-cultural research and methodology series, Vol. 18. Individualism and collectivism: Theory, method, and applications 175–188 (Sage Publications, 1994).
- 5.Aritzeta A, Balluerka N. Cooperation, competition and goal interdependence in work teams: a multilevel approach. Psicothema. 2006;18:757–765. [PubMed] [Google Scholar]
- 6.Guzley RM, Araki F, Chalmers LE. Cross‐cultural perspectives of commitment: Individualism and collectivism as a framework for conceptualization. Southern Journal of Communication. 1998;64:1–19. doi: 10.1080/10417949809373114. [DOI] [Google Scholar]
- 7.Chiao JY, et al. Dynamic cultural influences on neural representations of the self. Journal of cognitive neuroscience. 2010;22:1–11. doi: 10.1162/jocn.2009.21192. [DOI] [PubMed] [Google Scholar]
- 8.Chiao JY, et al. Neural basis of individualistic and collectivistic views of self. Hum Brain Mapp. 2009;30:2813–2820. doi: 10.1002/hbm.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sul S, Choi I, Kang P. Cultural modulation of self-referential brain activity for personality traits and social identities. Soc Neurosci. 2012;7:280–291. doi: 10.1080/17470919.2011.614001. [DOI] [PubMed] [Google Scholar]
- 10.Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453:869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- 11.Bandettini PA. What’s new in neuroimaging methods? Ann N Y Acad Sci. 2009;1156:260–293. doi: 10.1111/j.1749-6632.2009.04420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang F, Peng K, Chechlacz M, Humphreys GW, Sui J. The Neural Basis of Independence Versus Interdependence Orientations: A Voxel-Based Morphometric Analysis of Brain Volume. Psychol Sci. 2017;28:519–529. doi: 10.1177/0956797616689079. [DOI] [PubMed] [Google Scholar]
- 13.Kitayama S, et al. Reduced orbitofrontal cortical volume is associated with interdependent self-construal. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:7969–7974. doi: 10.1073/pnas.1704831114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takeuchi H, et al. Mean diffusivity of globus pallidus associated with verbal creativity measured by divergent thinking and creativity-related temperaments in young healthy adults. Hum Brain Mapp. 2015;36:1808–1827. doi: 10.1002/hbm.22739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laricchiuta D, et al. Linking novelty seeking and harm avoidance personality traits to basal ganglia: volumetry and mean diffusivity. Brain Structure and Function. 2014;219:793–803. doi: 10.1007/s00429-013-0535-5. [DOI] [PubMed] [Google Scholar]
- 16.Lei X, et al. Striatum-Centered Fiber Connectivity Is Associated with the Personality Trait of Cooperativeness. PLoS One. 2016;11:e0162160. doi: 10.1371/journal.pone.0162160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang J, et al. Macro and micro structures in the dorsal anterior cingulate cortex contribute to individual differences in self-monitoring. Brain imaging and behavior. 2016;10:477–485. doi: 10.1007/s11682-015-9398-0. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto T, et al. Neuroanatomical correlates of the sense of control: Gray and white matter volumes associated with an internal locus of control. Neuroimage. 2015;119:146–151. doi: 10.1016/j.neuroimage.2015.06.061. [DOI] [PubMed] [Google Scholar]
- 19.Takeuchi H, et al. A voxel-based morphometry study of gray and white matter correlates of a need for uniqueness. Neuroimage. 2012;63:1119–1126. doi: 10.1016/j.neuroimage.2012.08.037. [DOI] [PubMed] [Google Scholar]
- 20.Takeuchi H, et al. Impact of videogame play on the brain’s microstructural properties: cross-sectional and longitudinal analyses. Molecular psychiatry. 2016;21:1781–1789. doi: 10.1038/mp.2015.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmithorst VJ, Yuan W. White matter development during adolescence as shown by diffusion MRI. Brain and cognition. 2010;72:16–25. doi: 10.1016/j.bandc.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Sun J, et al. Regional gray matter volume is associated with rejection sensitivity: a voxel-based morphometry study. Cognitive, affective & behavioral neuroscience. 2014;14:1077–1085. doi: 10.3758/s13415-014-0249-z. [DOI] [PubMed] [Google Scholar]
- 23.Ding J, et al. Disrupted functional connectivity in social anxiety disorder: a resting-state fMRI study. Magnetic resonance imaging. 2011;29:701–711. doi: 10.1016/j.mri.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Yang, X. et al. Network analysis reveals disrupted functional brain circuitry in drug-naive social anxiety disorder. Neuroimage, 10.1016/j.neuroimage.2017.12.011 (2017). [DOI] [PubMed]
- 25.Neil J, Miller J, Mukherjee P, Huppi PS. Diffusion tensor imaging of normal and injured developing human brain - a technical review. NMR in biomedicine. 2002;15:543–552. doi: 10.1002/nbm.784. [DOI] [PubMed] [Google Scholar]
- 26.Yamamuro K, Kimoto S, Rosen KM, Kishimoto T, Makinodan M. Potential primary roles of glial cells in the mechanisms of psychiatric disorders. Frontiers in cellular neuroscience. 2015;9:154. doi: 10.3389/fncel.2015.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nilsson M, van Westen D, Stahlberg F, Sundgren PC, Latt J. The role of tissue microstructure and water exchange in biophysical modelling of diffusion in white matter. Magma (New York, N.Y.) 2013;26:345–370. doi: 10.1007/s10334-013-0371-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Correlation of white matter diffusivity and anisotropy with age during childhood and adolescence: a cross-sectional diffusion-tensor MR imaging study. Radiology. 2002;222:212–218. doi: 10.1148/radiol.2221010626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lebel C, et al. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage. 2012;60:340–352. doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- 30.Takeuchi H, et al. White matter structures associated with empathizing and systemizing in young adults. Neuroimage. 2013;77:222–236. doi: 10.1016/j.neuroimage.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Nakagawa, S. et al. Lenticular nucleus correlates of general self-efficacy in young adults. Brain Struct Funct, 10.1007/s00429-017-1406-2 (2017). [DOI] [PMC free article] [PubMed]
- 32.Nakagawa S, et al. Basal ganglia correlates of fatigue in young adults. Scientific reports. 2016;6:21386. doi: 10.1038/srep21386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takeuchi H, et al. Degree centrality and fractional amplitude of low-frequency oscillations associated with Stroop interference. Neuroimage. 2015;119:197–209. doi: 10.1016/j.neuroimage.2015.06.058. [DOI] [PubMed] [Google Scholar]
- 34.Nakagawa S, et al. Sex-Related Differences in the Effects of Sleep Habits on Verbal and Visuospatial WorkingMemory. Frontiers in psychology. 2016;7:1128. doi: 10.3389/fpsyg.2016.01128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamaguchi S, Kuhlman DM, Sugimori S. Personality correlates of allocentric tendencies in individualist and collectivist cultures. Journal of Cross-Cultural Psychology. 1995;26:658–672. doi: 10.1177/002202219502600609. [DOI] [Google Scholar]
- 36.Raven, J. Manual for Raven’s progressive matrices and vocabulary scales. (Oxford Psychologists Press, 1998).
- 37.Takeuchi H, et al. Training of Working Memory Impacts Structural Connectivity. J. Neurosci. 2010;30:3297–3303. doi: 10.1523/JNEUROSCI.4611-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ashburner J, Friston KJ. Voxel-based morphometry-the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 39.Takeuchi H, et al. The structure of the amygdala associates with human sexual permissiveness: Evidence from voxel-based morphometry. Hum. Brain Mapp. 2015;36:440–448. doi: 10.1002/hbm.22638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le Bihan D, et al. Diffusion tensor imaging: concepts and applications. Journal of Magnetic Resonance Imaging. 2001;13:534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- 41.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 42.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/S1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 43.Maldjian JA, Laurienti PJ, Burdette JH. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage. 2004;21:450–455. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.