Abstract
Liposomes mimic natural cell membranes and have long been investigated as drug carriers due to excellent entrapment capacity, biocompatibility and safety. Despite the success of parenteral liposomes, oral delivery of liposomes is impeded by various barriers such as instability in the gastrointestinal tract, difficulties in crossing biomembranes, and mass production problems. By modulating the compositions of the lipid bilayers and adding polymers or ligands, both the stability and permeability of liposomes can be greatly improved for oral drug delivery. This review provides an overview of the challenges and current approaches toward the oral delivery of liposomes.
Abbreviations: APC, antigen-presenting cell; AUC, area under curve; BSA, bovine serum albumin; DC, dendritic cells; DMPC, dimyristoyl phosphatidyl choline; DPPC, dipalmitoyl phosphotidylcholine; FAE, follicle-associated epithelia; FITC, fluorescein isothiocyannate; GIT, gastrointestinal tract; LUV, large unilamellar vesicles; MLV, multilamellar vesicles; MRT, mean residence time; MVL, multivesicular liposomes; PC, phosphatidylcholine; PEG, polyethylene glycol; rhEGF, recombinant human epithelial growth factor; RES, reticulo-endothelial; SC, sodium cholate; SDC, sodium deoxycholate; SGC, sodium glycocholate; STC, sodium taurocholate; SPC, soy phosphatidylcholine; SUV, small unilamellar vesicles; Tgel, gelling temperature; Tp, phase transition temperature; TPGS, tocopherol polyethylene glycol succinate; UEA 1, ulex europaeus agglutinin 1; WGA, wheat germ agglutinin
KEY WORDS: Liposomes, Oral, Drug delivery, Stability, Absorption, Bioavailability
Graphical abstract
Despite the success of parenteral liposomes, oral delivery of liposomes is impeded by various barriers such as instability, poor permeability and mass production difficulties. By modulating bilayer compositions and decorating with polymers or ligands, both the stability and permeability of liposomes can be greatly improved, bettering liposomes for oral delivery.
1. Introduction
Since the discovery of liposomes by Bangham and Horne in 19641, the potential of liposomes as drug delivery carriers has been extensively explored via versatile administrative routes such as parenteral, oral, pulmonary, nasal, ocular and transdermal routes2., 3., 4.. In 1974, AmBisome®, a formulation of amphotericin B, became the first injectable liposome product to be licensed3., 4.. Nevertheless, primitive parenteral liposomes have one severe drawback: they are always cleared from blood very quickly and end up in organs and tissues in the reticulo-endothelial system (RES, e.g., liver, spleen, and lung). The clearing occurs by plasma opsonization and subsequent sequestration from circulation5., 6., 7., 8.. By pegylation, a process of coating with long-chain polyethylene glycols (PEG), liposomes are camouflaged with layers of hydrophilic coatings to evade RES clearance and achieve long circulation in the body9., 10., 11., 12., 13., 14., 15., 16.. The successful marketing of Doxil®, a pegylated liposomal doxorubicin product, represents a milestone in the development of parenteral liposomes17.
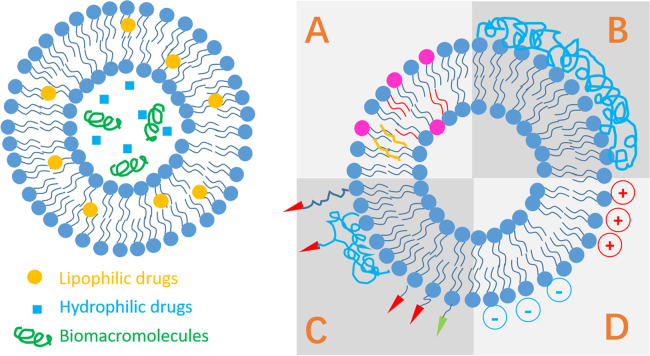
Liposomes consist of enclosed vesicles of concentric self-assembling lipid bilayers composed of phospholipids and cholesterols in common1., 4., 5.. According to the structure of lipid bilayers and the size of the vesicles, liposomes are commonly classified into large unilamellar vesicles (LUV), small unilamellar vesicles (SUV), multilamellar vesicles (MLV) and multivesicular vesicles (MVV)4., 5.. While LUV, SUV and MLV are candidate carriers for versatile routes including the oral route, MVV are used for parenteral delivery only. The inner aqueous phase of liposomes is well protected by the lipid bilayers and is able to load hydrophilic entities, whereas the hydrophobic region in the lipid bilayers is able to load hydrophobic entities (Fig. 1). The most remarkable advantages of liposomes are their biocompatibility and safety due to resemblance to biomembranes. Moreover, it is easy to modify the liposomal surfaces by conjugation to polymers and/or ligands so as to endow the vesicles with special properties (Fig. 1). See recent reviews2., 18., 19., 20., 21., 22., 23., 24. for a better understanding of the history and various application aspects of liposomes.
Figure 1.
Drug loading patterns and strategies for surface modification of liposomes. A, modification of liposomal compositions; B, polymer coating; C, surface charging; D, modification with ligands.
Oral delivery of liposomes has a long history as well and can be traced to as early as the late 1970s25., 26., 27.. It is interesting to see that the initial application of oral liposomes was with the delivery of insulin28, emphasizing the continual challenge in the field of oral drug delivery. Despite the initial ardor, the efficacy of oral liposomes was not reproducible or predictable. For instance, only 54% of the normal rats and 67% of the diabetic rabbits responded to the treatment of oral liposomal insulin29. More negative results added to the disappointment of using liposomes as oral delivery carriers30., 31., and there seemed to be a period of quiescence in the 1980s. However, attempts to use liposomes as drug carrier systems for oral delivery resurged in recent years32., 33., 34., 35., 36., 37., 38., 39., thanks to modern modification technologies to enhance liposomal stability and permeation.
By addition of polymer coatings40., 41., 42., 43. and modulating liposomal compositions44., 45., 46., 47., both the stability of liposomes in the gastrointestinal tract (GIT) and trans-epithelial absorption of active components have been significantly improved. It is worth noting that once again oral delivery of biomacromolecules, especially proteins and peptides, becomes the hot topic of research and discussion48., 49.. In addition to improved oral bioavailability, the pharmacokinetic and pharmacodynamic profiles are improved as well50., 51.. In this review, the status quo will be summarized with emphasis on challenges and strategies taken to adapt liposomes for oral delivery.
2. Challenges confronting liposomes as oral drug delivery systems
2.1. Instability
Conventional (i.e., non-modified) liposomes, are susceptible to combined detrimental effects of gastric acid, bile salts and pancreatic lipases in the GIT, all of which lead to reduced concentrations of intact liposomes and payload leakage52. Following incubation with artificial intestinal fluid for 120 min, a majority of liposomes show irregular shapes and obviously damaged membranes, whereas only a small proportion of liposomes maintain intact structures53. Bile salts are able to disrupt the lipid bilayers of liposomes composed of lipids with lower phase transition temperatures such as phosphatidylcholine (PC) and dimyristoyl phosphatidyl choline (DMPC)54., 55.. Pancreatic fluid, which contains lipolytic enzymes such as lipases, phospholipase A2 and cholesterol esterases, hydrolyses liposomal phospholipids thereby disrupting liposomal structure55., 56..
Generally, there are widespread concerns with the physical stability of liposomes in the GIT. For labile biomacromolecules, liposomes are apparently not ideal carrier systems because of the instability of liposomes and instant degradation of leaked payloads upon disruption of the liposomal structure40. However, the situation differs for poorly water-soluble drugs; in this case, the remnants of liposomes can form new mixed micelles, in which the encapsulated drugs are transferred to the new vehicles and transported to intestinal epithelia for absorption40., 54..
2.2. Poor permeability
Conventional liposomes have poor permeability across intestinal epithelia because of the relatively large size of particles and the presence of various epithelial barriers. There are mainly two proposed pathways for enhancement of oral drug delivery by liposomes. The first is via drug release in the gastrointestinal lumen or via transformation of vesicles into mixed micelles, and subsequent permeation of drug molecules across the intestinal epithelia40. As mentioned above, this approach is apparently not workable for labile biomacromolecules (e.g., insulin47., 52.). The improved absorption of biomacromolecules is apparently via the second pathway; that is via uptake of intact liposomes by M cells residing in the follicle-associate epithelia (FAE) of Peyer׳s patches57. However, M cell-mediated uptake sets an upper limit on oral absorption of liposomes40., 47. because M cells represent only 5% of human FAE and 1% of total intestinal epithelial cell population58., 59.. On the other hand, the rapid secretion and shedding of gastrointestinal mucus significantly restrict the oral absorption of liposomes as well, which are likely trapped in the mucus layers via hydrophobic interaction60. There is so far no direct evidence confirming the transport of intact liposomes across intestinal enterocytes.
2.3. Formulation challenges
Although several liposomal formulations (e.g., Doxil®) have been successfully marketed, the production of liposomes is not without challenges. In fact, the mass production of liposomes is largely unsatisfactory due to batch-to-batch variations. Although it may meet the demands for parenteral products, the biggest batch sizes so far are not big enough for oral use, which usually require higher doses and extended courses of treatment. Owing to the instability of liposomes in aqueous dispersion, there is always a need to formulate liposomes into solid dosage forms61., 62., 63., 64.. Traditionally, freeze-drying is employed to produce solid liposomal formulations with good reconstituting capacities64., 65., 66.. However, the freeze-drying technology is less efficient and consumes much time and money. More efficient technologies are desired for mass manufacturing of solid liposomal products.
3. Recent advances in modulating liposomes for oral drug delivery
3.1. Stabilization
In view of the poor stability of liposomes during production, storage and transit across GIT, a series of approaches such as modulation of lipid compositions, surface coating and interior thickening have been explored to stabilize liposomes.
3.1.1. Modulation of lipid compositions
Conventional liposomes are commonly comprised of phospholipids and cholesterols, mimicking the physiological compositions of biomembranes. Although liposomes demonstrate certain degree of stability both in vitro and in vivo, they are susceptible to the harsh gastrointestinal environment. Liposomes containing phospholipids with phase transition temperatures (Tp) below 37 °C are completely disrupted by bile salts, but this effect is less pronounced for those with Tp higher than 37 °C67. In early developmental stages, it is an easy option to improve the physical stability of liposomes by optimizing lipid compositions. By incorporating stearylamine, liposomes are charged positively and are capable of suppressing the digestion of insulin by trypsin68 and enhancing the hypoglycemic effect26., 69.. Replacing phospholipids or cholesterols with specific lipids or sterols improves the performance of oral liposomes due to enhanced stability in the GIT70., 71., 72., 73.. Insulin-loaded liposomes prepared with dipalmitoyl phosphatidylcholine (DPPC) and a soybean-derived sterol mixture exhibit a better hypoglycemic effect than conventional liposomes, which was ascribed by the authors to increased rigidity of the lipid bilayers72.
As a type of surfactant secreted by hepatocytes, bile salts have been considered to be the main factor for the disruption of liposomes in GIT74., 75.. Paradoxically, studies revealed that prior incorporation of bile salts into liposomal bilayers stabilized the membranes against the destructive effect of physiological bile salts44., 45., 52., 76.. It is well accepted that physiological phospholipids and bile salts readily form colloidal mixed micelles, which is the main mechanism for oral absorption of aliphatic acids and glycerides44., 45.. Bile salts always have a tendency to associate with phospholipids actively, even from lipid bilayers of plain liposomes, thereby compromising the integrity of liposomes30., 31., 40.. However, the prior incorporation of bile salts in liposomal bilayers offsets the destructive effects of outside bile salts47., 52.. To date, liposomes containing bile salts, also named as bilosomes, have been widely investigated for both oral immunization45., 77. and oral delivery of poorly water-soluble drugs and biomacromolecules47., 78., 79., 80., 81.. Various types of bile salts including sodium glycocholate (SGC), sodium taurocholate (STC) and sodium deoxycholate (SDC) have been incorporated into liposomes to protect enclosed insulin from enzymatic degradation by pepsin, trypsin and α-chymotrypsin52., 81.. A better protection of insulin is observed for liposomes containing SGC than liposomes containing STC or SDC and conventional liposomes47., 81.. It is believed that improved stability of liposomes by bile salts contributes at least partly to enhanced oral bioavailability of insulin81.
3.1.2. Surface coating
To protect liposomes from the harsh gastrointestinal environment, another workable approach is to coat liposomal surfaces with layers of polymers such as enteric polymers, proteins and chitosans50., 82., 83.. Enteric coatings are well known to prevent liposomes from disintegration in the stomach thereby improving absorption as more liposomes survive and are exposed in small intestine. Liposomes coated with Eudragit L100 enhance the oral bioavailability of alendronate sodium by 12-fold in rats as compared with the commercial tablets50. However, in some cases a layer of coating with enteric polymers such as Eudragit S100 does not protect damage by bile salts82. To this end, a design of liposomes-in-microspheres delivery systems comprising chitosan-coated liposomes within Eudragit S100 microspheres was found to be highly effective to resist the attack by bile salts83.
Polysaccharides are another kind of functional coating materials used to stabilize liposomes in the GIT84., 85., 86., 87.. Arabinoside-loaded liposomes coated with O-palmitoylpullutan (OPP), a polysaccharide derivative, are able to withstand the damage caused by sodium cholate (SC) at a concentration up to 16 mmol/L at pH 5.6 or pH 7.484. Moreover, OPP-coated liposomes showed a reduced release rate at pH 2.0 and 5.6 at 37 °C as compared to uncoated liposomes84. Polysaccharide-coated liposomes loading bovine serum albumin (BSA) are capable of producing higher levels of serum IgA and IgG in comparison with naked liposomes, indirectly verifying improved stability of the model drug85. In addition to OPP, O-palmitoylcurdlan sulfate86 and O-palmitoylscleroglucan87 have been utilized to protect liposomes from SC and pancreatin. Well-known as a gelling agent88, pectin has also been studied as a stabilizer for liposomal drug delivery systems89. Low- and high-methoxylated pectins show improved liposomal stability upon storage without disturbing membrane permeability89. Among various polysaccharides, chitosan may be the choice of coating materials because it is positively charged and readily interacts with the negatively charged liposomal surfaces to ensure firm coating. On the contrary, positive charges should be introduced onto liposomal surfaces to achieve firm coating with negatively charged polymers such as pectins via electrostatic interaction41. In vitro studies show that chitosan-coated liposomes achieve better protection of liposomes as well as the protein payloads in artificial gastrointestinal media90., 91.. Further observation of enhanced oral bioavailability confirms the effectiveness of coating with chitosan91. Moreover, the stability of chitosan-coated liposomes can be strengthened by subsequent cross-linking using β-glycerolphosphate92.
Pegylation, a technique originally developed for extending drug half-life in blood93, has also found applications in the oral delivery of liposomes43., 69., 94., 95., 96.. Pegylation of DPPC and PC liposomes significantly enhances the oral bioavailability of recombinant human epithelial growth factor (rhEGF), which was ascribed by the authors to suppression of enzymatic degradation by coating with PEG95. Liposomes coated with PEG 2000 or mucin are able to withstand bile salts and improve the stability of encapsulated insulin in GIT69.
In addition to the coating materials mentioned above, there are many other compounds available for chemical modifications of liposomes. For instance, polyelectrolytes perform well to stabilize liposomes loading doxorubin97 or paclitaxel98 by layer-by-layer (LBL) coating in artificial gastrointestinal fluids with enhanced oral bioavailability by 4–6 folds vs. conventional liposomes. Inorganic materials such as silica99., 100. and silica nanoparticles101 are among other stabilizers for oral delivery of liposomes. The formation of layers of protective coatings, as a result of surface adsorption of silica particles, is believed to contribute to enhanced liposomal stability99., 100., 101..
3.1.3. Interior thickening
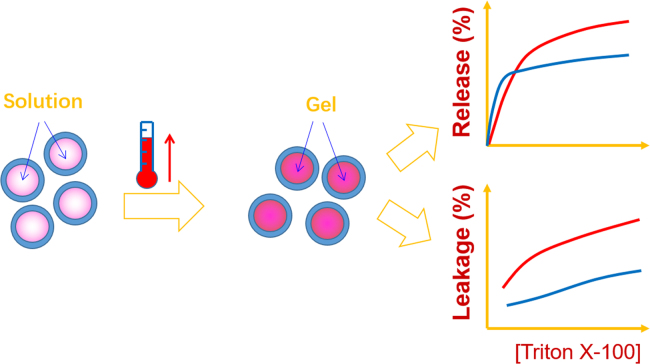
The physical stability of liposomes can also be improved by thickening the interior aqueous phases. Normally, interior thickening is initiated by increasing the viscosity of the interior aqueous phases, or alternatively by reconstitution of lipid bilayers to enclose hydrogel beads upon mixing the beads with liposomes102. The so-called Supermolecular Biovector (SMBVTM), which consists of charged, cross-linked polysaccharide cores surrounded by lipid membranes, was found to be an amiable carrier for proteins103., 104.. Another group reported a kind of lipobeads prepared by self-assembling of lipid bilayers around hydrogel beads initiated by acrylamine-functionalized lipids tethered to the bead surfaces105. In vitro evaluation indicated enhanced stability of lipid bilayers even at temperatures below Tp106. Interior thickening can also be attained via in situ gelling after formation of liposomes in response to physical stimuli. UV-induced polymerization within liposomes has been utilized to prepare lipobeads with increased mechanical strength and enhanced stability107., 108.. By incorporating reverse-phase thermosensitive in situ gel into the aqueous phase of liposomes, interior thickening was achieved when liposomes were heated to a temperature above the gelling temperature (Tgel) (Fig. 2)109., 110.. Tgel can be tailored within the range of room temperature and physiological temperature (37 °C) through adjusting the ratio of the thermosensitive gel (poloxamer 407/poloxamer 188). Therefore, the liposomes were prepared under conditions similar to conventional liposomes at ambient temperature109., 110.. After administration, the liposomal interior gelates in response to increased temperature. Further study showed that interior gelling improves physical stability and protects the lipid bilayers against membrane destabilizers (Fig. 2)110. Significantly prolonged elimination time after intravenous injection suggests enhanced liposomal performance in vivo109. Interior thickening improves some of the physicochemical properties of liposomes such as increased rigidity of the lipid bilayers, modified shape, improved physical stability and sustained release of the payloads. However, the utility of these liposome formulations for oral delivery of liposomes awaits experimental validation.
Figure 2.
Rationale of interior thickening of liposomes with thermosensitive poloxamer 407/188 in situ gels. Adapted from Ref. [109] with permission.
3.1.4. Other strategies
In addition to the methods mentioned above, other strategies have also been utilized to improve the stability of liposomes. For example, novel double liposomes, prepared by filtering preformed inner liposomes through a glass filter painted with lipid bilayers, demonstrate even more improved stability111. The outer bilayers serve as protective coatings against the destruction by intestinal enzymes; as a result, significantly enhanced hypoglycemic effect (insulin)111 or hypocalcemia effect (salmon calcitonin)112 was achieved. In another study, liposomes were embedded into gelatin matrices to stabilize the lipid bilayers and attained controlled release of the vesicles113, although no in vivo data were provided.
3.2. Absorption enhancement
3.2.1. Enhanced absorption due to mucoadhesion
Mucoadhesion endows liposomes with prolonged GIT residence, allowing prolonged contact of liposomes and/or the payloads with intestinal epithelia and subsequently enhancing opportunities for oral absorption of either liposomal vesicles or the payloads. Enhancement of mucoadhesion is attainable through coating with polymers or modulating surface charges. Positively charged liposomes gain not only mucoadhesion but also resistance to enzyme destruction42, and thus improve oral bioavailability of the payloads26. Coating liposomes with mucoadhesive polymers such as polysaccharides seems to be one of the most promising approaches to achieve mucoadhesion41., 114., 115., 116.. Pectins are one class of preferable polysaccharides commonly used115., 117.. Pectin-coated liposomes show adhesion to mucin with high-methoxylated pectin-coated liposomes performing the best115. In another study, mucoadhesive pectin-liposome nanocomplexes (PLNs) gave better intestinal absorption of calcitonin than uncoated liposomes41. High density of fluorescently labeled PLNs, observed by confocal laser scanning microscopy, were found adhering to intestinal epithelia and remained for a prolonged duration, suggesting strong mucoadhesion41.
As a natural cationic polysaccharide derived from chitin via deacetylation, chitosan represents one of the most popular coating materials for oral liposomes due to low toxicity, biocompatibility, biodegradability and mucoadhesion. Various chitosan derivatives are reported to improve mucoadhesive properties of liposomes by either chemical coupling118 or physical coating119., 120., 121.. Insulin-loaded liposomes coated with mucoadhesive polymers such as chitosan, polyvinyl alcohol and poly (acrylic acid) show better and more prolonged hypoglycemic effect than uncoated ones122. The type of chitosans also influences the degree of mucoadhesion and thereby the in vivo behaviors; low-molecular-weight chitosans show stronger mucoadhesion114. A comparison of different materials on mucoadhesion confirms that chitosan is the best coating materials for liposomes following the order of chitosan-coated liposomes≥carbopol-coated liposomes>positively charged non-coated liposomes>negatively charged non-coated liposomes42. Combinatory use of chitosan with other mucoadhesive materials such as tocopherol polyethylene glycol succinate (TPGS) reinforces mucoadhesiveness123.
Apart from polysaccharides, many other mucoadhesive polymers are also used to coat liposomes. Coating with PEG and mucin not only improves the stability of liposomes69 but also extends the residence time in GIT, which altogether contribute to the hypoglycemic effect of insulin43. In contrast to the mechanism of prolonged residence of pegylated nanocarriers in circulation following intravenous administration, the extended residence of PEG-coated liposomes in the GIT is due to deep penetration of the PEG chains into the mucus layers lining the GIT wall and inter-weaving with mucin. The extended retention in the GIT thus strengthens the uptake of the vesicles by M cells and subsequent efficacy of oral immunization95.
3.2.2. Enhancer-facilitated absorption
Various absorption enhancers have been studied to facilitate the oral absorption of liposomal payloads. TPGS 400, cetylpyridinium chloride and cholylsarcosine, in combination with stearylamine, were confirmed to enhance the oral absorption of liposomal fluorescein isothiocyanate (FITC)-dextran, a hydrophilic macromolecule124. Tween-80, a surfactant commonly used as a solubilizer, enhances the oral bioactivity of insulin when it is incorporated into liposomes at a level of 1%46. In a comparative study, cetylpyridinium chloride performed better on enhancement of oral bioavailability of human growth hormone than a few other absorption enhancers including d-α-TPGS 400, phenylpiperazine, sodium caprate and octadacanehiol125.
Bile salts are physiological surfactants that play a very important role in lipid absorption. By incorporating bile salts into the lipid bilayers of liposomes, the oral bioavailability of a variety of hydrophilic and lipophilic drugs has been significantly enhanced79., 126.. Owing to their structural resemblance to cholesterol, bile salts can be easily incorporated into liposomal membranes to form bilosomes. Among the bile salt family, SC, STC, SDC and SGC are popular candidates used in bilosomes for enhancement of oral absorption127. The oral bioavailability of cyclosporine A was significantly enhanced by bilosomes in comparison with conventional liposomes. The enhancement is probably due to facilitated absorption by SDC rather than improved release because drug release from liposomes is very slow78.
Non-ionic surfactants are also used as absorption enhancers. Tween 80-reinforced liposomes composed of SPC and cholesterol significantly enhanced the absorption of (+)-catechin following oral administration with increased area under the curve (AUC) and prolonged mean residence time (MRT) as compared to the solution control129. Enzyme inhibitors are always used in combination with enhancers to improve the absorption of liposomal biomacromolecules. This was demonstrated by the significantly enhanced hypocalcemic effect of calcitonin when chitosan conjugated with an inhibitor aprotinin was used to coat liposomes119.
3.2.3. Polymer-facilitated absorption
Besides enhancement of liposomal stability and mucoadhesion, polymers also enhance intestinal permeability. By opening tight junctions130, N-trimethyl chitosan has become a preferable polymer to coat liposomes for oral delivery of various ingredients36., 39., 131., 132., 133.. Another chitosan derivative, methylated N-(4-N,N-dimethylaminobenzyl) chitosan, was applied to coat FITC-conjugated liposomes to enhance the permeability of a model protein BSA across Caco-2 cell monolayers91. The combined use of cell-penetrating peptide such as oligoarginine further enhanced the efficacy of chitosans133. It should be noted that opening epithelials junctions with these agents can have both positive and negative effects. The latter may include risks of concurrent entry of toxins as well as payload. The loss and gain of using chitosans are still awaiting systemic evaluation.
The trapping capacity and fast turnover of mucus are known factors which impede the permeability of liposomes across mucus layers60. Recently, mucus-penetrating polymers were used to coat liposomes to facilitate permeation. For instance, liposomes coated with chitosan-thioglycolic acid 6-mercaptonicotinamide-conjugate (an S-protected thiomer chitosan with mucus-penetrating capabilities) achieved 8.2-fold enhancement of physiological bioavailability (areas above curves of the blood calcium levels) of calcitonin following oral administration in rats134. Pluronic F127-coated liposomes were reported to enhance oral absorption of lipophilic ingredients due to the intestinal mucus-penetrating properties135., 136., 137.. A 1.84-fold enhancement of AUC0-t of cyclosporine A-loaded pluronic F127-coated liposomes was seen following oral administration vs. chitosan-coated liposomes136. Additionally, polymers with polyethylene oxide tags such as pluronic P85 and PEGylated G5 PAMAM dendrimer inhibit the P-glycoprotein efflux system and enhance overall oral bioavailability when used as coating materials for liposomes138.
3.2.4. Ligand-mediated targeting to epithelial cells
To overcome the poor permeability of conventional liposomes, ligands have been investigated to enhance intestinal uptake by epithelial cells via receptor-mediated endocytosis. Since most cell proteins and lipids in cell membranes of the GIT are glycosylated, lectins have been widely utilized to modify liposomes for oral immunization139., 140., 141. or oral drug delivery142. This is possible due to the specific recognition and binding by lectins to glycans. Wheat germ agglutinin (WGA)-modified liposomes containing insulin achieved superior control of blood glucose as compared with ulex europaeus agglutinin 1 (UEA 1)-modified ones142. However, the results are not consistent with the findings obtained by another group, who reported that UEA 1 performed better than WGA139. By taking advantage of the interaction between lectins and glucans, mannose derivatives were applied to modify liposomes to target mannosyl receptors expressed in antigen-presenting cells (APCs)143., 144.. Antibodies were attached to liposomes to enhance gastrointestinal permeability as well. In this case, IgA-coated liposomes containing ferritin showed enhanced immune responses145. The authors ascribed the enhancement to increased uptake via M cells, but did not mention the relevant receptors145. In a recent work, Fc fragments were used as ligands to modify liposomes for active targeting to neonatal Fc receptors. Results with these liposomes showed significantly improved hypoglycemic effects of insulin146. In view of the instability of peptide ligands in the GIT, non-peptide ligands such as folic acid (FA)147., 148. and biotin are preferred for liposomal surface modification149. FA-modified polymer-stabilized multilayer liposomes gave an approximately 20% relative bioavailability of insulin following oral administration vs. results from subcutaneous administration150. Similarly, functionalization of bilosomes with glycomannan improved liposomal targeting and stability151. In addition to the ligands mentioned above, ligands employed in the oral delivery of other types of nanoparticles152., 153. can also be utilized to modify liposomes.
3.3. Mass production
The practice of developing liposomes as oral drug delivery systems has motivated investigations on the mass production of liposomes on an industrial scale. On a laboratory scale, liposomes can be prepared using a variety of methods such as thin-film dispersion, reversed-phase evaporation, detergent dialysis, solvent injection and a few other methods154. So far, these methods are only successful for small scale production of liposomes. Problems encountered with scale-up include poor size distribution, poor batch-to-batch reproducibility, physicochemical instability, residues of organic solvent and high production costs155.
Considerable effort has been made in recent decades to overcome these problems. A continuous high-pressure extrusion apparatus was developed to prepare liposomes with uniform size on a one-liter scale156. The leakage of drugs upon extrusion is seen as a drawback of this method156. A high-speed dispersion method has been developed to prepare liposomes with high physical stability and encapsulation efficency157. One concern with this technique may be the production of smaller-sized liposomes, ranging from 280–350 nm157. High-pressure homogenization/extrusion has been applied to downsize large liposomes containing plasmid DNA with commercially available instrumentation. Although this methodology has the capability for large-scale continuous (1–1000 L/h) production158, but drug leakage and high production costs of this complex process restrict its industrial application.
The ethanol injection technique is probably the most suitable present method for implementation at industrial scale due to its simplicity and safety. Regarding this method, size distribution is controllable by modulating the aqueous phase temperature in large-scale production159. A novel ethanol injection method using a microengineered nickel membrane was recently developed160. Depending on the size of the membrane, this technique can be easily scaled up to a very large scale. Moreover, the size and size distribution of liposomes can be controlled via the oscillating membrane system during scale-up160. Another scalable production technology based on ethanol injection produces liposomes regardless of production scale under fully sterile conditions161. Economical evaluation of liposome production by ethanol injection suggests economic feasibility for a plant with a daily production capacity of 288 L of liposomal suspension162.
Owing to the physical instability of liposomes in aqueous media, the storage problems must also be considered. Therefore, there has been consistent effort to prepare liposomes as solid dosage formulations. Spray-drying and freeze-drying are commonly used to address this problem. However, factors such as high cost of freeze-drying and heat liability of the payloads in spray-drying limit industrial applications. In contrast, proliposomes are an alternative for mass production and storage of liposomes due to the solid state formulations and simplicity in production. Preliminary evaluation of proliposomes containing amphotericin B163 or cyclosporine A164 demonstrated promising features for large-scale industrial applications. More importantly, the final dosage forms of proliposomes resemble conventional oral solid dosage forms and can be easily adapted to conventional manufacturing facilities and processes. This was demonstrated by BSA proliposome tablets coated with Eudragit L100 that could be completely reconstituted into liposomes165.
In spite of the progress in mass production of liposomes, only a few parenteral, and no oral liposomal products are successfully marketed. There are significant remaining impediments to successfully developing liposomes as oral drug delivery carriers.
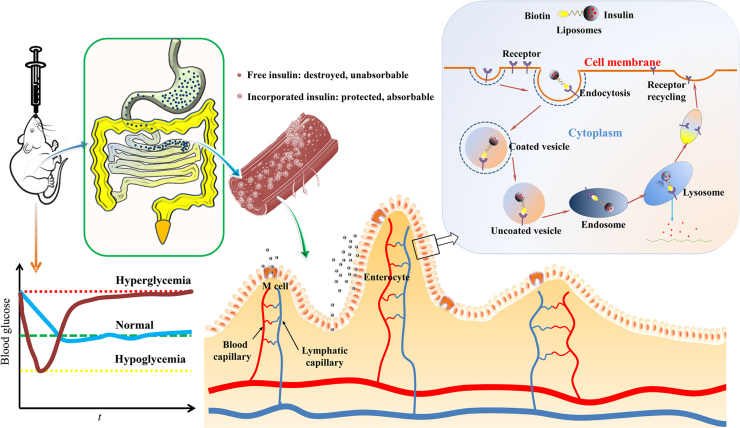
4. Mechanisms of oral absorption of liposomes
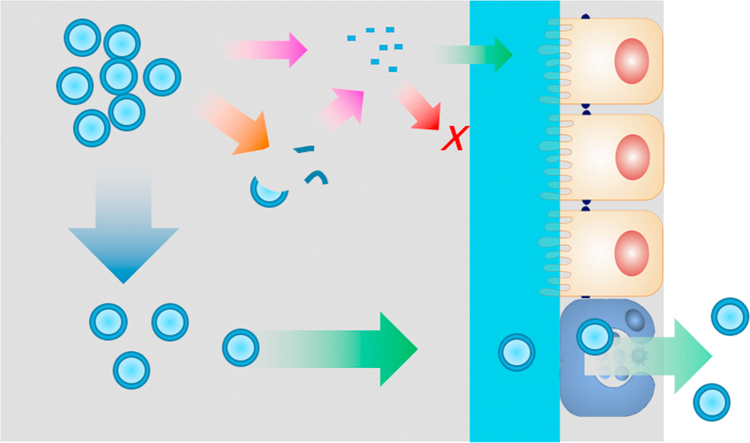
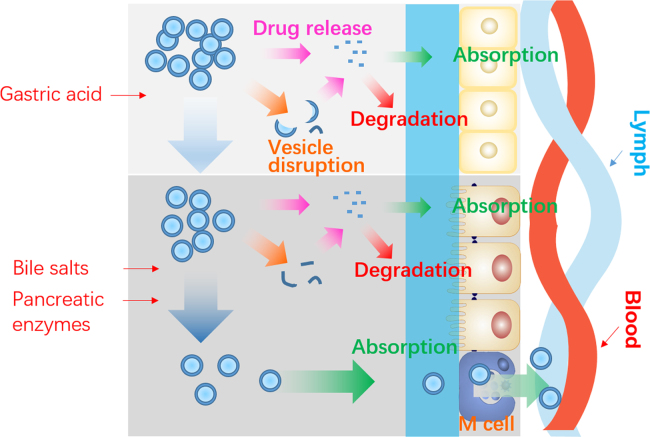
Despite the advances outlined above, the mechanisms of oral delivery of liposomes have yet to be elucidated. To begin this topic, it is important to outline the general fate of liposomes as well as the embedded drug payloads following oral administration (Fig. 3). Orally administered liposomes are partially destroyed following exposure to gastric acid. Although some of the payload drug is released, other liposomes and their cargo survive40., 47., 52.. While free drugs follow their own fate, surviving liposomes are emptied from stomach and transit into small intestine, where another fraction is destroyed by intestinal surfactants and enzymes53., 54., 55., 56.. Liposomes surviving this step penetrate the mucus layers and make close contact with intestinal epithelia47., 61.. There is still the possibility of destruction of liposomes at this stage as well as release of embedded drugs. However, the fractions of liposomes that survive the whole digestion process are able to be absorbed as integral vesicles via the M cell-to-lymph pathway47. Liposomes may be taken up by enterocytes as well, but their fate following this step is unknown. Several mechanisms are proposed as follows.
Figure 3.
Schematic presentation of the fate of liposomes following oral administration.
4.1. Enhanced gastrointestinal stability
As mentioned above, liposomes are prone to degradation in response to the combined effects of gastric acids, bile salts and pancreatic lipases. Degradation of liposomes leads to the leakage of the payloads, which further leads to inactivation or degradation of labile drugs (e.g., peptides and proteins). Leakage also causes precipitation of lipophilic ingredients, thus decreasing the total fraction of oral absorption. Many studies show that enhancing the stability of liposomes or their payloads significantly improves oral bioavailability. In a sense, improving the stability means to enhance the surviving rate of liposomes and thereby enhance the opportunities to be taken up by intestinal epithelia.
Several strategies have been applied to enhance the stability of liposomes, and the underlying mechanisms have been partly elucidated. For example, phospholipids with a higher Tp endow liposomes with rigid membranes at physiological temperature, and thus help to resist the gastrointestinal destabilizing factors166., 167., 168.. Incorporation with bile salts improves the flexibility of the lipid biomembranes and helps to withstand the detrimental effects of bile acids in the GIT45., 52.. Imaging evidence show that bilosomes surviving the gastrointestinal environment can be absorbed as intact vesicles169. By exterior coating, the liposomal membranes are separated from the harsh environment in the GIT due to steric hindrance induced by polymers or polymer-formed water layers69, protecting the membranes from the influence of gastric acid50., 69., 82., bile salts69., 84. and pancreatic lipases87., 101.. Moreover, enzyme inhibitors can stabilize the proteins released from liposomes by inhibiting various enzymes in the GIT114., 119..
There is currently a disagreement about whether the payloads are released first before absorption or the liposomes are absorbed as integral vesicles. In the first case, the payloads such as proteins are released in the gastrointestinal lumen and inhibitors must be used together to suppress enzymatic degradation99., 119.. Secondly, uptake of intact liposomes via clathrin-dependent endocytosis, caveolae-dependent endocytosis, macrocytosis or fusion may be alternative routes for oral absorption of liposomes132. Abundant evidence indicates that free insulin without concomitant use of enzymatic inhibitors elicits no hypoglycemic efficacy47. Our previous work that validates the transcellular transit of bilosomes also provides a reference for trans-enterocytic internalization of oral liposomes169.
4.2. Mucoadhesion
It is logical to assume that mucoadhesion of liposomes to intestinal epithelia prolongs the exposure of the vesicles in small intestine (the ideal site for oral absorption) and enhances opportunities for oral absorption. Polymers such as polysaccharides41., 116., 132., PEGs43 and carbopols42 are good coating materials to improve mucoadhesion of liposomes. Mucoadhesion of various polymers is mainly due to the ionic interaction between positively charged polymers and negatively charged constituents (i.e., sulfonic and sialic acid residues) of the mucus layers39., 116., 132.. Furthermore, disulfide bridges form between thiolated polymers with cysteine-rich subdomains of mucus glycoproteins118., 134., as well as the interpenetration of polymers within mucus43., 92.. Mucins, a family of glycoproteins, have been generally used to evaluate the mucoadhesion of polymer-coated liposomes in vitro116., 132., 170., as mucins are largely responsible for mucus viscoelastic and adhesive properties. There are ex vivo42., 92., 118. and in vivo132 models for this purpose. Following oral administration of mucoadhesive polymer-coated liposomes, prolonged elimination half-life39 and extended pharmacological action41., 43., 132. of the payloads have been observed, which is ascribable to prolonged drug-residence time due to mucoadhesion. It is speculated that mucoadhesion increases partition of liposomal payloads from the gastrointestinal lumen to the epithelial wall in comparison with free drugs, and ultimately results in enhanced passive permeation across intestinal epithelia. A mechanism was proposed for insulin-122 or calcitonin-loaded chitosan-coated liposomes42 suggesting that the drugs are released in the mucus layers upon interaction with mucin and degradation of the liposomes, and subsequently absorbed without enzymatic degradation. Other studies ascribe enhanced oral absorption to adherence of the polymers to the mucus layers and prolonged retention therein, facilitating penetration of liposomes and payloads across intestinal epithelial cells41., 116..
4.3. Facilitated translocation across the mucus layers
The intestinal permeability of liposomes is known to be restricted by the trapping and fast turnover of the mucus layers. The turnover time of the mucus layers are supposed to be a limiting factor that determines the transit time of mucoadhesive liposomes171. Considering the intestinal mucin turnover time is between 50 and 270 min, mucoadhesive liposomes are not expected to adhere to the mucus for more than 4–5 h172, a factor that greatly limits the efficacy of mucoadhesive polymer-coated liposomes. Therefore, facilitating mucus penetration potentially enhances residence time of liposomes in mucus, thereby increasing the oral absorption of liposomes and their payloads. A series of polymers possessing mucoadhesive properties have been utilized to coat liposomes to render them mucus-penetrating instead of mucus entrapment41., 134.. Pluronic F127 has very good mucus-penetrating ability and has been used to modify liposomes for oral drug delivery136. It is reported that facilitated penetration in the mucus layers promotes direct contact of liposomes with epithelia, and thus improves liposomal uptake by caveolae- or clathrin-mediated endocytosis135., 137.. The mucus-penetrating ability is thought to be attributable to the PEG chains of Pluronic F127 on the surface of liposomes that ease hydrophobic and electrostatic interaction of liposomes with mucins136. Besides liposomes, PEG modification has also been used for mucus-penetrating polymeric nanoparticles173., 174..
4.4. Enhanced permeation across the enteric epithelia
The oral bioavailability of liposomes is limited by poor intestinal permeability of both the vesicles and the payloads, especially biomacromolecules. Incorporation of absorption enhancers along with polymer coatings has been shown to efficiently enhance permeation across enteric epithelia. As for small molecular weight drugs, the effects and mechanisms of absorption enhancers are clear175. However, enhancers for absorption of integral liposomes may have different mechanisms. Carrier-mediated transmembrane absorption128 and penetration through intercellular regions132 are proposed for enhancing oral absorption of deformable liposomes containing surfactants. Another in vitro study124 using Caco-2 cell models shows that some bioenhancers incorporated in liposomes may act via interfering with cellular lipid bilayer structure, which leads to facilitated uptake of payloads or higher fusion affinity of liposomes with cell membranes. The opening tight junctions that facilitate paracellular absorption of drugs is another potential mechanism. Furthermore, some absorption enhancers also enhance the oral bioavailability of liposomal payloads by forming lipophilic ion-pair complexes with various organic cations, which increase permeability of the cations across biological membranes79. It is worth noting that many absorption enhancers such as bile salts act via multiple rather than a single mechanisms79., 127., 128..
Polymer coating enhances permeability of liposomal payloads through epithelial cells as well. Chitosans and derivatives are unique types of polymers widely investigated to coat liposomes for oral delivery39., 91., 133.. The interaction of chitosan with cellular membranes is reported to initiate a structural re-organization of tight junction-associated proteins, thus facilitating paracellular transport of hydrophilic macromolecules176., 177.. However, a majority of mechanistic studies with chitosan-coated liposomes are carried out in Caco-2 models91., 133.. Moreover, P-gp, a multidrug transporter, is responsible for the efflux of various drug substrates, and P-gp inhibition represents another potential mechanism for enhancement of oral absorption of liposomal payloads36., 124., 138., 178., 179..
4.5. Ligand-mediated endocytosis
Inspired by the fact that some nutrients are absorbed via active absorption, liposomes can be modified with nutritional ligands to achieve active targeting to specific receptors in the enteric epithelia. Ligands are able to further enhance the cellular uptake and trans-epithelial transport of liposomes and thus improve oral absorption. The ligand-receptor interaction probably brings about two aspects of functions: i.e., receptor-mediated transport and accumulation of liposomes at the sites of absorption. The former is comprised of the mechanisms of pinocytosis and phagocytosis, mainly restricted to M cells180. The latter refers to the ligand-receptor interaction that achieves adherence and accumulation of liposomes at the site of absorption, thus facilitating absorption of the payloads if they are meant to be released there. In general, receptor-mediated pinocytosis occur by clathrin-mediated endocytosis (CME) or caveolae-mediated endocytosis (CvME)180. Compared to CME, CvME is not concerned with lysosomal biodegradation. Therefore, the use of liposomes exploiting CvME may be advantageous for oral delivery of enzyme-sensitive drugs. Size seems to be an important factor that determines the patterns of cellular internalization via either CME or CvME181.
Significantly enhanced oral absorption has been reported for liposomes modified with FA147., 148., biotin149,182, lectins140., 168. and mannose143., 144.. FA and biotin interact with their own receptors, (both of which are expressed widely by intestinal epithelia) to improve liposomal uptake via receptor-mediated endocytosis147., 148., 149.. Moreover, CME rather than CvME may be an important route for endocytosis, as confirmed by utilizing endocytosis inhibitors (Fig. 4)149. Lectins interact with the specific glycosylation patterns expressed in M cells or absorptive cells or both to enhance liposomal uptake139., 140.. APCs in the GIT, including the macrophages and dendritic cells (DC) (the major APCs present in the vicinity of Peyer׳s patches), abundantly express the mannose receptors (also called C-type lectin) and thus can be utilized as targeting cells for oral liposomes143., 183.. It is worth mentioning that phagocytosis plays an important role in receptor-mediated endocytosis in M cells and APC targeting. In spite of its high efficacy, receptor-mediated endocytosis may not be the sole mechanism for enhanced oral absorption of ligand-modified liposomes150. Accumulation of liposomes at the sites of absorption and sustained release of payloads prior to absorption contribute to enhanced oral absorption as well.
Figure 4.
Schematic presentation of enhanced oral absorption of biotin-decorated liposomes via ligand-mediated endocytosis following active targeting to intestinal epithelia. Adapted from Ref. [148] with permission.
4.6. Uptake by M cells
M cells are specialized epithelial cells locating in the FAE of Peyer׳s patches. They are able to transport a broad range of particles, such as bacteria, viruses and antigens, from the intestinal lumen to the underlying lymphoid tissues184. Despite the small population of M cells, liposomal absorption through the M cell pathway has many advantages, including less glycocalyx, reduced levels of membrane hydrolases, few lysosomes and high endocytosis capabilities150. Furthermore, M cells are the least protected cells by mucus in enteric epithelia and the most exposed to chyme because M cells do not secrete mucus. Therefore, M cells are easily accessible for liposomes via mechanisms of adsorptive endocytosis, fluid phase endocytosis and phagocytosis185. It was shown that the M cell pathway contributes to total oral absorption of liposomes57., 186., and the liposomal surface charges influenced the efficency187. In addition, prolonged residence of liposomes in GIT increases the opportunity of uptake by M cells188., 189., which partly explains the contribution of stabilization of liposomes to enhanced oral absorption69., 95.. Polymer-coated liposomes can be transcytosed by M cells as well due to prolonged contact with intestinal epithelia190. To further increase oral absorption, ligands such as lectins141., 168. have been utilized to modify liposomes to target M cells as mentioned above. In conclusion, the M cell pathway has been shown to be an important route for the oral absorption of liposomes.
5. Conclusions and perspectives
Despite the growing number of investigations on the oral delivery of liposomes, essential breakthroughs are still needed to develop and market these products for clinical use. The bottleneck to development of oral liposomes lies in the poor understanding of the absorption mechanisms. Following the transit of liposomes from the stomach to small intestine, liposomes are gradually broken down. The drug payloads can be released immediately into the gastrointestinal lumen or be transferred into secondary carriers like mixed micelles and transported to the intestinal epithelia for absorption. This represents the first mode of drug absorption. As for labile biomacromolecules, released fractions are degraded quickly and will not be absorbed; only liposomes that survive the gastrointestinal environment and manage to penetrate the mucus layers can reach the intestinal epithelia and be absorbed together with the payloads. To enhance the oral absorption of liposomes as well as the payloads, the initial challenge is to maintain the integrity of liposomes and prolong gastrointestinal residence, thereby enhancing penetration of the mucus layers. Recent advances are focused on modulating the compositions of the lipid bilayers or modifying the liposomal surfaces with polymers or ligands to modulate the in vivo fate of liposomes after oral administration.
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (81573363 and 81690263) and National Key Basic Research Program (2015CB931800).
Footnotes
Invited for Special Column.Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Contributor Information
Zhongjian Chen, Email: aajian818@163.com.
Wei Wu, Email: wuwei@shmu.edu.cn.
References
- 1.Bangham A.D., Horne R.W. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J Mol Biol. 1964;8:660–668. doi: 10.1016/s0022-2836(64)80115-7. [DOI] [PubMed] [Google Scholar]
- 2.Torchilin V.P. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 3.Gregoriadis G., Swain C., Wills E., Tavill A. Drug-carrier potential of liposomes in cancer chemotherapy. Lancet. 1974;303:1313–1316. doi: 10.1016/s0140-6736(74)90682-5. [DOI] [PubMed] [Google Scholar]
- 4.Gregoriadis G., Florence A.T. Liposomes in drug delivery. Clin, Diagn Ophthalmic Potential Drugs. 1993;45:15–28. doi: 10.2165/00003495-199345010-00003. [DOI] [PubMed] [Google Scholar]
- 5.Gregoriadis G. Fate of injected liposomes: observations on entrapped solute retention, vesicle clearance and tissue distribution in vitro. In: Gregoriadis G., editor. Liposomes as drug carriers: recent trends and progress. Plenum Press; New York: 1988. pp. 3–18. [Google Scholar]
- 6.Moghimi S.M., Szebeni J. Stealth liposomes and long circulating nanoparticles: critical issues in pharmacokinetics, opsonization and protein-binding properties. Prog Lipid Res. 2003;42:463–478. doi: 10.1016/s0163-7827(03)00033-x. [DOI] [PubMed] [Google Scholar]
- 7.Yan X., Scherphof G.L., Kamps J.A. Liposome opsonization. J Liposome Res. 2005;15:109–139. doi: 10.1081/lpr-64971. [DOI] [PubMed] [Google Scholar]
- 8.Palchetti S., Colapicchioni V., Digiacomo L., Caracciolo G., Pozzi D., Capriotti A.L. The protein corona of circulating PEGylated liposomes. Biochim Biophys Acta. 2016;1858:189–196. doi: 10.1016/j.bbamem.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Tang J., Kuai R., Yuan W., Drake L., Moon J.J., Schwendeman A. Effect of size and pegylation of liposomes and peptide-based synthetic lipoproteins on tumor targeting. Nanomedicine. 2017;13:1869–1878. doi: 10.1016/j.nano.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pereira S., Egbu R., Jannati G., Al-Jamal W.T. Docetaxel-loaded liposomes: the effect of lipid composition and purification on drug encapsulation and in vitro toxicity. Int J Pharm. 2016;514:150–159. doi: 10.1016/j.ijpharm.2016.06.057. [DOI] [PubMed] [Google Scholar]
- 11.Huang X., Li M., Bruni R., Messa P., Cellesi F. The effect of thermosensitive liposomal formulations on loading and release of high molecular weight biomolecules. Int J Pharm. 2017;524:279–289. doi: 10.1016/j.ijpharm.2017.03.090. [DOI] [PubMed] [Google Scholar]
- 12.Signorell R.D., Papachristodoulou A., Xiao J., Arpagaus B., Casalini T., Grandjean J. Preparation of PEGylated liposomes incorporating lipophilic lomeguatrib derivatives for the sensitization of chemo-resistant gliomas. Int J Pharm. 2017;536:388–396. doi: 10.1016/j.ijpharm.2017.11.070. [DOI] [PubMed] [Google Scholar]
- 13.Bunker A., Magarkar A., Viitala T. Rational design of liposomal drug delivery systems, a review: combined experimental and computational studies of lipid membranes, liposomes and their PEGylation. Biochim Biophys Acta. 2016;1858:2334–2352. doi: 10.1016/j.bbamem.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 14.Suk J.S., Xu Q., Kim N., Hanes J., Ensign L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99:28–51. doi: 10.1016/j.addr.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J.X., Xin B., Li C., Xie N.H., Gong W.L., Huang Z.L. PEGylated perylenemonoimide-dithienylethene for super-resolution imaging of liposomes. ACS Appl Mater Interfaces. 2017;9:10338–10343. doi: 10.1021/acsami.6b15076. [DOI] [PubMed] [Google Scholar]
- 16.Griffin J.I., Wang G., Smith W.J., Vu V.P., Scheinman R., Stitch D. Revealing dynamics of accumulation of systemically injected liposomes in the skin by intravital microscopy. ACS Nano. 2017;11:11584–11593. doi: 10.1021/acsnano.7b06524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barenholz Y. Doxil®—the first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160:117–134. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 18.Weissig V. Liposomes came first: the early history of liposomology. Methods Mol Biol. 2017;1522:1–15. doi: 10.1007/978-1-4939-6591-5_1. [DOI] [PubMed] [Google Scholar]
- 19.Xing H., Hwang K., Lu Y. Recent developments of liposomes as nanocarriers for theranostic applications. Theranostics. 2016;6:1336–1352. doi: 10.7150/thno.15464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daraee H., Etemadi A., Kouhi M., Alimirzalu S., Akbarzadeh A. Application of liposomes in medicine and drug delivery. Artif Cells Nanomed Biotechnol. 2016;44:381–391. doi: 10.3109/21691401.2014.953633. [DOI] [PubMed] [Google Scholar]
- 21.Noble G.T., Stefanick J.F., Ashley J.D., Kiziltepe T., Bilgicer B. Ligand-targeted liposome design: challenges and fundamental considerations. Trends Biotechnol. 2014;32:32–45. doi: 10.1016/j.tibtech.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Caritá A.C., Eloy J.O., Chorilli M., Lee R.J., Leonardi G.R. Recent advances and perspectives in liposomes for cutaneous drug delivery. Curr Med Chem. 2018;25:606–635. doi: 10.2174/0929867324666171009120154. [DOI] [PubMed] [Google Scholar]
- 23.Agrawal M., Ajazuddin, Tripathi D.K., Saraf S., Saraf S., Antimisiaris S.G. Recent advancements in liposomes targeting strategies to cross blood–brain barrier (BBB) for the treatment of Alzheimer׳s disease. J Control Release. 2017;260:61–77. doi: 10.1016/j.jconrel.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 24.Kapoor M., Lee S.L., Tyner K.M. Liposomal drug product development and quality: current US experience and perspective. AAPS J. 2017;19:632–641. doi: 10.1208/s12248-017-0049-9. [DOI] [PubMed] [Google Scholar]
- 25.Patel H., Ryman B.E. Oral administration of insulin by encapsulation within liposomes. FEBS Lett. 1976;62:60–63. doi: 10.1016/0014-5793(76)80016-6. [DOI] [PubMed] [Google Scholar]
- 26.Hashimoto A., Kawada J. Effects of oral administration of positively charged insulin liposomes on alloxan diabetic rats: preliminary study. Endocrinol Jpn. 1979;26:337–344. doi: 10.1507/endocrj1954.26.337. [DOI] [PubMed] [Google Scholar]
- 27.Dapergolas G., Gregoriadis G. Hypoglycaemic effect of liposome-entrapped insulin administered intragastrically into rats. Lancet. 1976;308:824–827. doi: 10.1016/s0140-6736(76)91209-5. [DOI] [PubMed] [Google Scholar]
- 28.He H., Lu Y., Qi J., Zhao W., Dong X., Wu W. Biomimetic thiamine- and niacin-decorated liposomes for enhanced oral delivery of insulin. Acta Pharm Sin B. 2018;8:97–105. doi: 10.1016/j.apsb.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arrieta-Molero J., Aleck K., Sinha M., Brownscheidle C., Shapiro L., Sperling M. Orally administered liposome-entrapped insulin in diabetic animals. Horm Res. 1982;16:249–256. doi: 10.1159/000179509. [DOI] [PubMed] [Google Scholar]
- 30.Chiang C.M., Weiner N. Gastrointestinal uptake of liposomes. I. In vitro and in situ studies. Int J Pharm. 1987;37:75–85. [Google Scholar]
- 31.Chiang C.M., Weiner N. Gastrointestinal uptake of liposomes. II. In vivo studies. Int J Pharm. 1987;40:143–150. [Google Scholar]
- 32.Tang W.L., Tang W.H., Chen W.C., Diako C., Ross C.F., Li S.D. Development of a rapidly dissolvable oral pediatric formulation for mefloquine using liposomes. Mol Pharm. 2017;14:1969–1979. doi: 10.1021/acs.molpharmaceut.7b00077. [DOI] [PubMed] [Google Scholar]
- 33.Song Z., Lin Y., Zhang X., Feng C., Lu Y., Gao Y. Cyclic RGD peptide-modified liposomal drug delivery system for targeted oral apatinib administration: enhanced cellular uptake and improved therapeutic effects. Int J Nanomed. 2017;12:1941–1958. doi: 10.2147/IJN.S125573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Y., Luo X., Xu X., Gao N., Liu X. Preparation, characterization and in vivo pharmacokinetic study of PVP-modified oleanolic acid liposomes. Int J Pharm. 2017;517:1–7. doi: 10.1016/j.ijpharm.2016.11.056. [DOI] [PubMed] [Google Scholar]
- 35.Verma A.K., Sharma S., Gupta P., Singodia D., Kansal S., Sharma V. Vitamin B12 grafted layer-by-layer liposomes bearing HBsAg facilitate oral immunization: effect of modulated biomechanical properties. Mol Pharm. 2016;13:2531–2542. doi: 10.1021/acs.molpharmaceut.6b00274. [DOI] [PubMed] [Google Scholar]
- 36.Chen W.L., Yuan Z.Q., Liu Y., Yang S.D., Zhang C.G., Li J.Z. Liposomes coated with N-trimethyl chitosan to improve the absorption of harmine in vivo and in vitro. Int J Nanomed. 2016;11:325–336. doi: 10.2147/IJN.S95540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uhl P., Helm F., Hofhaus G., Brings S., Kaufman C., Leotta K. A liposomal formulation for the oral application of the investigational hepatitis B drug Myrcludex B. Eur J Pharm Biopharm. 2016;103:159–166. doi: 10.1016/j.ejpb.2016.03.031. [DOI] [PubMed] [Google Scholar]
- 38.Jensen S.M., Christensen C.J., Petersen J.M., Treusch A.H., Brandl M. Liposomes containing lipids from Sulfolobus islandicus withstand intestinal bile salts: an approach for oral drug delivery? Int J Pharm. 2015;493:63–69. doi: 10.1016/j.ijpharm.2015.07.026. [DOI] [PubMed] [Google Scholar]
- 39.Chen H., Wu J., Sun M., Guo C., Yu A., Cao F. N-trimethyl chitosan chloride-coated liposomes for the oral delivery of curcumin. J Liposome Res. 2012;22:100–109. doi: 10.3109/08982104.2011.621127. [DOI] [PubMed] [Google Scholar]
- 40.Wu W., Lu Y., Qi J. Oraldelivery ofliposomes. Ther Deliv. 2015;6:1239–1241. doi: 10.4155/tde.15.69. [DOI] [PubMed] [Google Scholar]
- 41.Thirawong N., Thongborisute J., Takeuchi H., Sriamornsak P. Improved intestinal absorption of calcitonin by mucoadhesive delivery of novel pectin–liposome nanocomplexes. J Control Release. 2008;125:236–245. doi: 10.1016/j.jconrel.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 42.Takeuchi H., Matsui Y., Yamamoto H., Kawashima Y. Mucoadhesive properties of carbopol or chitosan-coated liposomes and their effectiveness in the oral administration of calcitonin to rats. J Control Release. 2003;86:235–242. doi: 10.1016/s0168-3659(02)00411-x. [DOI] [PubMed] [Google Scholar]
- 43.Iwanaga K., Ono S., Narioka K., Kakemi M., Morimoto K., Yamashita S. Application of surface-coated liposomes for oral delivery of peptide: effects of coating the liposome׳s surface on the GI transit of insulin. J Pharm Sci. 1999;88:248–252. doi: 10.1021/js980235x. [DOI] [PubMed] [Google Scholar]
- 44.Conacher M., Alexander J., Brewer J.M. Oral immunisation with peptide and protein antigens by formulation in lipid vesicles incorporating bile salts (bilosomes) Vaccine. 2001;19:2965–2974. doi: 10.1016/s0264-410x(00)00537-5. [DOI] [PubMed] [Google Scholar]
- 45.Shukla A., Khatri K., Gupta P.N., Goyal A.K., Mehta A., Vyas S.P. Oral immunization against hepatitis B using bile salt stabilized vesicles (bilosomes) J Pharm Pharm Sci. 2008;11:59–66. doi: 10.18433/j3k01m. [DOI] [PubMed] [Google Scholar]
- 46.Choudhari K., Labhasetwar V., Dorle A. Liposomes as a carrier for oral administration of insulin: effect of formulation factors. J Microencapsul. 1994;11:319–325. doi: 10.3109/02652049409040461. [DOI] [PubMed] [Google Scholar]
- 47.Niu M., Lu Y., Hovgaard L., Guan P., Tan Y., Lian R. Hypoglycemic activity and oral bioavailability of insulin-loaded liposomes containing bile salts in rats: the effect of cholate type, particle size and administered dose. Eur J Pharm Biopharm. 2012;81:265–272. doi: 10.1016/j.ejpb.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 48.Bozzuto G., Molinari A. Liposomes as nanomedical devices. Int J Nanomed. 2015;10:975–999. doi: 10.2147/IJN.S68861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamano E., Miyauchi M., Furusyo H., Kawazoe A., Ishikado A., Makino T. Inhibitory effects of orally administrated liposomal bovine lactoferrin on the LPS-induced osteoclastogenesis. Lab Invest. 2010;90:1236–1246. doi: 10.1038/labinvest.2010.80. [DOI] [PubMed] [Google Scholar]
- 50.Hosny K.M., Ahmed O.A., Al-Abdali R.T. Enteric-coated alendronate sodium nanoliposomes: a novel formula to overcome barriers for the treatment of osteoporosis. Exp Opin Drug Deliv. 2013;10:741–746. doi: 10.1517/17425247.2013.799136. [DOI] [PubMed] [Google Scholar]
- 51.Yang Z., Lu A., Wong B.C., Chen X., Bian Z., Zhao Z. Effect of liposomes on the absorption of water-soluble active pharmaceutical ingredients via oral administration. Curr Pharm Des. 2013;19:6647–6654. doi: 10.2174/1381612811319370008. [DOI] [PubMed] [Google Scholar]
- 52.Hu S., Niu M., Hu F., Lu Y., Qi J., Yin Z. Integrity and stability of oral liposomes containing bile salts studied in simulated and ex vivo gastrointestinal media. Int J Pharm. 2013;441:693–700. doi: 10.1016/j.ijpharm.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 53.Liu W., Ye A., Liu W., Liu C., Han J., Singh H. Behaviour of liposomes loaded with bovine serum albumin during in vitro digestion. Food Chem. 2015;175:16–24. doi: 10.1016/j.foodchem.2014.11.108. [DOI] [PubMed] [Google Scholar]
- 54.Tian J.N., Ge B.Q., Shen Y.F., He Y.X., Chen Z.X. Thermodynamics and structural evolution during a reversible vesicle-micelle transition of a vitamin-derived bolaamphiphile induced by sodium cholate. J Agric Food Chem. 2016;64:1977–1988. doi: 10.1021/acs.jafc.5b05547. [DOI] [PubMed] [Google Scholar]
- 55.Kokkona M., Kallinteri P., Fatouros D., Antimisiaris S.G. Stability of SUV liposomes in the presence of cholate salts and pancreatic lipases: effect of lipid composition. Eur J Pharm Sci. 2000;9:245–252. doi: 10.1016/s0928-0987(99)00064-0. [DOI] [PubMed] [Google Scholar]
- 56.Cohn J.S., Kamili A., Wat E., Chung R.W., Tandy S. Dietary phospholipids and intestinal cholesterol absorption. Nutrients. 2010;2:116–127. doi: 10.3390/nu2020116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shukla A., Mishra V., Kesharwani P. Bilosomes in the context of oral immunization: development, challenges and opportunities. Drug Discov Today. 2016;21:888–899. doi: 10.1016/j.drudis.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 58.Lopes M.A., Abrahim B.A., Cabral L.M., Rodrigues C.R., Seiça R.M., de Baptista Veiga F.J. Intestinal absorption of insulin nanoparticles: contribution of M cells. Nanomedicine. 2014;10:1139–1151. doi: 10.1016/j.nano.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 59.Giannasca P.J., Giannasca K.T., Leichtner A.M., Neutra M.R. Human intestinal M cells display the sialyl Lewis A antigen. Infect Immun. 1999;67:946–953. doi: 10.1128/iai.67.2.946-953.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ensign L.M., Cone R., Hanes J. Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers. Adv Drug Deliv Rev. 2012;64:557–570. doi: 10.1016/j.addr.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guan P., Lu Y., Qi J., Niu M., Lian R., Wu W. Solidification of liposomes by freeze-drying: the importance of incorporating gelatin as interior support on enhanced physical stability. Int J Pharm. 2015;478:655–664. doi: 10.1016/j.ijpharm.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 62.van den Hoven J.M., Metselaar J.M., Storm G., Beijnen J.H., Nuijen B. Cyclodextrin as membrane protectant inspray-dryingand freeze-drying of PEGylatedliposomes. Int J Pharm. 2012;438:209–216. doi: 10.1016/j.ijpharm.2012.08.046. [DOI] [PubMed] [Google Scholar]
- 63.Wang L., Hu X., Shen B., Xie Y., Shen C., Lu Y. Enhanced stability of liposomes against solidification stress during freeze-drying and spray-drying by coating with calcium alginate. J Drug Deliv Sci Tech. 2015;30:163–170. [Google Scholar]
- 64.Chen C., Han D., Cai C., Tang X. An overviewofliposome lyophilization and its future potential. J Control Release. 2010;142:299–311. doi: 10.1016/j.jconrel.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 65.Kannan V., Balabathula P., Thoma L.A., Wood G.C. Effect of sucrose as a lyoprotectant on the integrity of paclitaxel-loadedliposomesduring lyophilization. J Liposome Res. 2015;25:270–278. doi: 10.3109/08982104.2014.992023. [DOI] [PubMed] [Google Scholar]
- 66.Ali M.E., Lamprecht A. Spray freeze drying as an alternative technique for lyophilization of polymeric and lipid-based nanoparticles. Int J Pharm. 2016;516:170–177. doi: 10.1016/j.ijpharm.2016.11.023. [DOI] [PubMed] [Google Scholar]
- 67.Richards M.H., Gardner C.R. Effects of bile salts on the structural integrity of liposomes. Biochim Biophys Acta. 1978;543:508–522. doi: 10.1016/0304-4165(78)90305-7. [DOI] [PubMed] [Google Scholar]
- 68.Kato Y., Hosokawa T., Hayakawa E., Ito K. Influence of liposomes on tryptic digestion of insulin. Biol Pharm Bull. 1993;16:457–461. doi: 10.1248/bpb.16.457. [DOI] [PubMed] [Google Scholar]
- 69.Iwanaga K., Ono S., Narioka K., Morimoto K., Kakemi M., Yamashita S. Oral delivery of insulin by using surface coating liposomes: improvement of stability of insulin in GI tract. Int J Pharm. 1997;157:73–80. [Google Scholar]
- 70.Parmentier J., Thewes B., Gropp F., Fricker G. Oral peptide delivery by tetraether lipid liposomes. Int J Pharm. 2011;415:150–157. doi: 10.1016/j.ijpharm.2011.05.066. [DOI] [PubMed] [Google Scholar]
- 71.Cui M., Wu W., Hovgaard L., Lu Y., Chen D., Qi J. Liposomes containing cholesterol analogues of botanical origin as drug delivery systems to enhance the oral absorption of insulin. Int J Pharm. 2015;489:277–284. doi: 10.1016/j.ijpharm.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 72.Muramatsu K., Maitani Y., Nagai T. Dipalmitoylphosphatidylcholine liposomes with soybean-derived sterols and cholesterol as a carrier for the oral administration of insulin in rats. Biol Pharm Bull. 1996;19:1055–1058. doi: 10.1248/bpb.19.1055. [DOI] [PubMed] [Google Scholar]
- 73.Parmentier J., Becker M.M., Heintz U., Fricker G. Stability of liposomes containing bio-enhancers and tetraether lipids in simulated gastro-intestinal fluids. Int J Pharm. 2011;405:210–217. doi: 10.1016/j.ijpharm.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 74.Birru W.A., Warren D.B., Ibrahim A., Williams H.D., Benameur H., Porter C.J. Digestion of phospholipids after secretion of bile into the duodenum changes the phase behavior of bile components. Mol Pharm. 2014;11:2825–2834. doi: 10.1021/mp500193g. [DOI] [PubMed] [Google Scholar]
- 75.Andrieux K., Forte L., Lesieur S., Paternostre M., Ollivon M., Grabielle-Madelmont C. Solubilisation of dipalmitoylphosphatidylcholine bilayers by sodium taurocholate: a model to study the stability of liposomes in the gastrointestinal tract and their mechanism of interaction with a model bile salt. Eur J Pharm Biopharm. 2009;71:346–355. doi: 10.1016/j.ejpb.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 76.Arafat M., Kirchhoefer C., Mikov M., Sarfraz M., Löbenberg R. Nanosized liposomes containing bile salt: a vesicular nanocarrier for enhancing oral bioavailability of BCS class III drug. J Pharm Pharm Sci. 2017;20:305–318. doi: 10.18433/J3CK88. [DOI] [PubMed] [Google Scholar]
- 77.Aburahma M.H. Bile salts-containing vesicles: promising pharmaceutical carriers for oral delivery of poorly water-soluble drugs and peptide/protein-based therapeutics or vaccines. Drug Deliv. 2016;23:1847–1867. doi: 10.3109/10717544.2014.976892. [DOI] [PubMed] [Google Scholar]
- 78.Guan P., Lu Y., Qi J., Niu M., Lian R., Hu F. Enhanced oral bioavailability of cyclosporine A by liposomes containing a bile salt. Int J Nanomed. 2011;6:965–974. doi: 10.2147/IJN.S19259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Song K.H., Chung S.J., Shim C.K. Enhanced intestinal absorption of salmon calcitonin (sCT) from proliposomes containing bile salts. J Control Release. 2005;106:298–308. doi: 10.1016/j.jconrel.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 80.Ayogu I., Ogbonna O., Ayolugbe C., Attama A. Evaluation of the pharmacodynamic activity of insulin from bilosomal formulation. Curr Drug Deliv. 2009;6:415–418. doi: 10.2174/156720109789000573. [DOI] [PubMed] [Google Scholar]
- 81.Niu M., Lu Y., Hovgaard L., Wu W. Liposomes containing glycocholate as potential oral insulin delivery systems: preparation, in vitro characterization, and improved protection against enzymatic degradation. Int J Nanomed. 2011;6:1155–1166. doi: 10.2147/IJN.S19917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Barea M., Jenkins M., Gaber M., Bridson R. Evaluation of liposomes coated with a pH responsive polymer. Int J Pharm. 2010;402:89–94. doi: 10.1016/j.ijpharm.2010.09.028. [DOI] [PubMed] [Google Scholar]
- 83.Barea M., Jenkins M., Lee Y., Johnson P., Bridson R. Encapsulation of liposomes within pH responsive microspheres for oral colonic drug delivery. Int J Biomater. 2012;2012:458712. doi: 10.1155/2012/458712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sehgal S., Rogers J.A. Polymer-coated liposomes: improved liposome stability and release of cytosine arabinoside (Ara-C) J Microencapsul. 1995;12:37–47. doi: 10.3109/02652049509051125. [DOI] [PubMed] [Google Scholar]
- 85.Venkatesan N., Vyas S. Polysaccharide coated liposomes for oral immunization—development and characterization. Int J Pharm. 2000;203:169–177. doi: 10.1016/s0378-5173(00)00442-7. [DOI] [PubMed] [Google Scholar]
- 86.Lee C.M., Lee H.C., Lee K.Y. O-palmitoylcurdlan sulfate (OPCurS)-coated liposomes for oral drug delivery. J Biosci Bioeng. 2005;100:255–259. doi: 10.1263/jbb.100.255. [DOI] [PubMed] [Google Scholar]
- 87.Carafa M., Marianecci C., Annibaldi V., Di Stefano A., Sozio P., Santucci E. Novel O-palmitoylscleroglucan-coated liposomes as drug carriers: development, characterization and interaction with leuprolide. Int J Pharm. 2006;325:155–162. doi: 10.1016/j.ijpharm.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 88.Willats W.G., Knox J.P., Mikkelsen J.D. Pectin: new insights into an old polymer are starting to gel. Trends Food Sci Technol. 2006;17:97–104. [Google Scholar]
- 89.Smistad G., Bøyum S., Alund S.J., Samuelsen A.B.C., Hiorth M. The potential of pectin as a stabilizer for liposomal drug delivery systems. Carbohydr Polym. 2012;90:1337–1344. doi: 10.1016/j.carbpol.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 90.Nguyen T.X., Huang L., Liu L., Abdalla A.M.E., Gauthier M., Yang G. Chitosan-coated nano-liposomes for the oral delivery of berberine hydrochloride. J Mater Chem B. 2014;2:7149–7159. doi: 10.1039/c4tb00876f. [DOI] [PubMed] [Google Scholar]
- 91.Kowapradit J., Apirakaramwong A., Ngawhirunpat T., Rojanarata T., Sajomsang W., Opanasopit P. Methylated N-(4-N,N-dimethylaminobenzyl) chitosan coated liposomes for oral protein drug delivery. Eur J Pharm Sci. 2012;47:359–366. doi: 10.1016/j.ejps.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 92.Manconi M., Nacher A., Merino V., Merino-Sanjuan M., Manca M.L., Mura C. Improving oral bioavailability and pharmacokinetics of liposomal metformin by glycerolphosphate-chitosan microcomplexation. AAPS PharmSciTech. 2013;14:485–496. doi: 10.1208/s12249-013-9926-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hoffman A.S. The early days of PEG and PEGylation (1970s–1990s) Acta Biomater. 2016;40:1–5. doi: 10.1016/j.actbio.2016.05.029. [DOI] [PubMed] [Google Scholar]
- 94.Patel K., Doddapaneni R., Sekar V., Chowdhury N., Singh M. Combination approach of YSA peptide anchored docetaxel stealth liposomes with oral antifibrotic agent for the treatment of lung cancer. Mol Pharm. 2016;13:2049–2058. doi: 10.1021/acs.molpharmaceut.6b00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Minato S., Iwanaga K., Kakemi M., Yamashita S., Oku N. Application of polyethyleneglycol (PEG)-modified liposomes for oral vaccine: effect of lipid dose on systemic and mucosal immunity. J Control Release. 2003;89:189–197. doi: 10.1016/s0168-3659(03)00093-2. [DOI] [PubMed] [Google Scholar]
- 96.Daeihamed M., Haeri A., Ostad S.N., Akhlaghi M.F., Dadashzadeh S. Doxorubicin-loaded liposomes: enhancing the oral bioavailability by modulation of physicochemical characteristics. Nanomedicine (Lond) 2017;12:1187–1202. doi: 10.2217/nnm-2017-0007. [DOI] [PubMed] [Google Scholar]
- 97.Jain S., Patil S.R., Swarnakar N.K., Agrawal A.K. Oral delivery of doxorubicin using novel polyelectrolyte-stabilized liposomes (layersomes) Mol Pharm. 2012;9:2626–2635. doi: 10.1021/mp300202c. [DOI] [PubMed] [Google Scholar]
- 98.Jain S., Kumar D., Swarnakar N.K., Thanki K. Polyelectrolyte stabilized multilayered liposomes for oral delivery of paclitaxel. Biomaterials. 2012;33:6758–6768. doi: 10.1016/j.biomaterials.2012.05.026. [DOI] [PubMed] [Google Scholar]
- 99.Dwivedi N., Arunagirinathan M., Sharma S., Bellare J. Silica-coated liposomes for insulin delivery. Int J Pharm. 2010;392:285–293. [Google Scholar]
- 100.Li C., Zhang Y., Su T., Feng L., Long Y., Chen Z. Silica-coated flexible liposomes as a nanohybrid delivery system for enhanced oral bioavailability of curcumin. Int J Nanomed. 2012;7:5995–6002. doi: 10.2147/IJN.S38043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mohanraj V.J., Barnes T.J., Prestidge C.A. Silica nanoparticle coated liposomes: a new type of hybrid nanocapsule for proteins. Int J Pharm. 2010;392:285–293. doi: 10.1016/j.ijpharm.2010.03.061. [DOI] [PubMed] [Google Scholar]
- 102.Kazakov S., Levon K. Liposome-nanogel structures for future pharmaceutical applications. Curr Pharm Des. 2006;12:4713–4728. doi: 10.2174/138161206779026281. [DOI] [PubMed] [Google Scholar]
- 103.De Miguel I., Ioualalena K., Bonnefous M., Peyrot M., Nguyen F., Cervilla M. Synthesis and characterization of supramolecular biovector (SMBV) specifically designed for the entrapment of ionic molecules. Biochim Biophys Acta. 1995;1237:49–58. doi: 10.1016/0005-2736(95)00079-i. [DOI] [PubMed] [Google Scholar]
- 104.von Hoegen P. Synthetic biomimetic supra molecular Biovector™ (SMBV™) particles for nasal vaccine delivery. Adv Drug Deliv Rev. 2001;51:113–125. doi: 10.1016/s0169-409x(01)00175-2. [DOI] [PubMed] [Google Scholar]
- 105.Ng C.C., Cheng Y.-L., Pennefather P.S. One-step synthesis of a fluorescent phospholipid-hydrogel conjugate for driving self-assembly of supported lipid membranes. Macromolecules. 2001;34:5759–5765. [Google Scholar]
- 106.Buck S., Pennefather P.S., Xue H.Y., Grant J., Cheng Y.L., Allen C.J. Engineering lipobeads: properties of the hydrogel core and the lipid bilayer shell. Biomacromolecules. 2004;5:2230–2237. doi: 10.1021/bm049751+. [DOI] [PubMed] [Google Scholar]
- 107.Kazakov S., Kaholek M., Teraoka I., Levon K. UV-induced gelation on nanometer scale using liposome reactor. Macromolecules. 2002;35:1911–1920. [Google Scholar]
- 108.Petralito S., Spera R., Pacelli S., Relucenti M., Familiari G., Vitalone A. Design and development of PEG-DMA gel-in-liposomes as a new tool for drug delivery. React Funct Polym. 2014;77:30–38. [Google Scholar]
- 109.Zhang B., Lu Y., Chen J., Wu W. Effects of interior gelation on pharmacokinetics and biodistribution of liposomes encapsulating an anti-cancer drug cytarabine. J Biomed Nanotechnol. 2010;6:704–709. doi: 10.1166/jbn.2010.1162. [DOI] [PubMed] [Google Scholar]
- 110.Zhang B., Chen J., Lu Y., Qi J., Wu W. Liposomes interiorly thickened with thermosensitive nanogels as novel drug delivery systems. Int J Pharm. 2013;455:276–284. doi: 10.1016/j.ijpharm.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 111.Katayama K., Kato Y., Onishi H., Nagai T., Machida Y. Double liposomes: hypoglycemic effects of liposomal insulin on normal rats. Drug Dev Ind Pharm. 2003;29:725–731. doi: 10.1081/ddc-120021771. [DOI] [PubMed] [Google Scholar]
- 112.Ebato Y., Kato Y., Onishi H., Nagai T., Machida Y. In vivo efficacy of a novel double liposome as an oral dosage form of salmon calcitonin. Drug Dev Res. 2003;58:253–257. [Google Scholar]
- 113.Pantze S.F., Parmentier J., Hofhaus G., Fricker G. Matrix liposomes: a solid liposomal formulation for oral administration. Eur J Lipid Sci Technol. 2014;116:1145–1154. [Google Scholar]
- 114.Thongborisute J., Tsuruta A., Kawabata Y., Takeuchi H. The effect of particle structure of chitosan-coated liposomes and type of chitosan on oral delivery of calcitonin. J Drug Target. 2006;14:147–154. doi: 10.1080/10611860600648346. [DOI] [PubMed] [Google Scholar]
- 115.Klemetsrud T., Jonassen H., Hiorth M., Kjøniksen A.L., Smistad G. Studies on pectin-coated liposomes and their interaction with mucin. Colloids Surf B Biointerfaces. 2013;103:158–165. doi: 10.1016/j.colsurfb.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 116.Han H.K., Shin H.J., Ha D.H. Improved oral bioavailability of alendronate via the mucoadhesive liposomal delivery system. Eur J Pharm Sci. 2012;46:500–507. doi: 10.1016/j.ejps.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 117.Nguyen S., Alund S.J., Hiorth M., Kjøniksen A.L., Smistad G. Studies on pectin coating of liposomes for drug delivery. Colloids Surf B Biointerfaces. 2011;88:664–673. doi: 10.1016/j.colsurfb.2011.07.058. [DOI] [PubMed] [Google Scholar]
- 118.Gradauer K., Vonach C., Leitinger G., Kolb D., Frohlich E., Roblegg E. Chemical coupling of thiolated chitosan to preformed liposomes improves mucoadhesive properties. Int J Nanomed. 2012;7:2523–2534. doi: 10.2147/IJN.S29980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Werle M., Takeuchi H. Chitosan-aprotinin coated liposomes for oral peptide delivery: development, characterisation and in vivo evaluation. Int J Pharm. 2009;370:26–32. doi: 10.1016/j.ijpharm.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 120.Manconi M., Mura S., Manca M.L., Fadda A.M., Dolz M., Hernandez M.J. Chitosomes as drug delivery systems for C-phycocyanin: preparation and characterization. Int J Pharm. 2010;392:92–100. doi: 10.1016/j.ijpharm.2010.03.038. [DOI] [PubMed] [Google Scholar]
- 121.Sugihara H., Yamamoto H., Kawashima Y., Takeuchi H. Effectiveness of submicronized chitosan-coated liposomes in oral absorption of indomethacin. J Liposome Res. 2012;22:72–79. doi: 10.3109/08982104.2011.621128. [DOI] [PubMed] [Google Scholar]
- 122.Takeuchi H., Yamamoto H., Niwa T., Hino T., Kawashima Y. Enteral absorption of insulin in rats from mucoadhesive chitosan-coated liposomes. Pharm Res. 1996;13:896–901. doi: 10.1023/a:1016009313548. [DOI] [PubMed] [Google Scholar]
- 123.Shao Y., Yang L., Han H.K. TPGS-chitosome as an effective oral delivery system for improving the bioavailability of coenzyme Q10. Eur J Pharm Biopharm. 2015;89:339–346. doi: 10.1016/j.ejpb.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 124.Parmentier J., Hartmann F.J., Fricker G. In vitro evaluation of liposomes containing bio-enhancers for the oral delivery of macromolecules. Eur J Pharm Biopharm. 2010;76:394–403. doi: 10.1016/j.ejpb.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 125.Parmentier J., Hofhaus G., Thomas S., Cuesta L.C., Gropp F., Schröder R. Improved oral bioavailability of human growth hormone by a combination of liposomes containing bio-enhancers and tetraether lipids and omeprazole. J Pharm Sci. 2014;103:3985–3993. doi: 10.1002/jps.24215. [DOI] [PubMed] [Google Scholar]
- 126.Jalali A., Moghimipour E., Akhgari A. Enhancing effect of bile salts on gastrointestinal absorption of insulin. Trop J Pharm Res. 2014;13:1797–1802. [Google Scholar]
- 127.Degim Z., Ünal N., Eşsiz D., Abbasoglu U. The effect of various liposome formulations on insulin penetration across Caco-2 cell monolayer. Life Sci. 2004;75:2819–2827. doi: 10.1016/j.lfs.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 128.Chen Y., Lu Y., Chen J., Lai J., Sun J., Hu F. Enhanced bioavailability of the poorly water-soluble drug fenofibrate by using liposomes containing a bile salt. Int J Pharm. 2009;376:153–160. doi: 10.1016/j.ijpharm.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 129.Huang Y.B., Tsai M.J., Wu P.C., Tsai Y.H., Wu Y.H., Fang J.Y. Elastic liposomes as carriers for oral delivery and the brain distribution of (+)-catechin. J Drug Target. 2011;19:709–718. doi: 10.3109/1061186X.2010.551402. [DOI] [PubMed] [Google Scholar]
- 130.Benediktsdóttir B.E., Gudjónsson T., Baldursson Ó., Másson M. N-alkylation of highly quaternized chitosan derivatives affects the paracellular permeation enhancement in bronchial epithelia in vitro. Eur J Pharm Biopharm. 2014;86:55–63. doi: 10.1016/j.ejpb.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 131.Cao J., Sun J., Wang X., Li X., Deng Y. N-trimethyl chitosan-coated multivesicular liposomes for oxymatrine oral delivery. Drug Dev Ind Pharm. 2009;35:1339–1347. doi: 10.3109/03639040902902427. [DOI] [PubMed] [Google Scholar]
- 132.Huang A., Makhlof A., Ping Q., Tozuka Y., Takeuchi H. N-trimethyl chitosan-modified liposomes as carriers for oral delivery of salmon calcitonin. Drug Deliv. 2011;18:562–569. doi: 10.3109/10717544.2011.596585. [DOI] [PubMed] [Google Scholar]
- 133.Huang A., Su Z., Li S., Sun M., Xiao Y., Ping Q. Oral absorption enhancement of salmon calcitonin by using both N-trimethyl chitosan chloride and oligoarginines-modified liposomes as the carriers. Drug Deliv. 2014;21:388–396. doi: 10.3109/10717544.2013.848247. [DOI] [PubMed] [Google Scholar]
- 134.Gradauer K., Barthelmes J., Vonach C., Almer G., Mangge H., Teubl B. Liposomes coated with thiolated chitosan enhance oral peptide delivery to rats. J Control Release. 2013;172:872–878. doi: 10.1016/j.jconrel.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhu Q., Guo T., Xia D., Li X., Zhu C., Li H. Pluronic F127-modified liposome-containing tacrolimus–cyclodextrin inclusion complexes: improved solubility, cellular uptake and intestinal penetration. J Pharm Pharmacol. 2013;65:1107–1117. doi: 10.1111/jphp.12074. [DOI] [PubMed] [Google Scholar]
- 136.Chen D., Xia D., Li X., Zhu Q., Yu H., Zhu C. Comparative study of Pluronic® F127-modified liposomes and chitosan-modified liposomes for mucus penetration and oral absorption of cyclosporine A in rats. Int J Pharm. 2013;449:1–9. doi: 10.1016/j.ijpharm.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 137.Li X., Chen D., Le C., Zhu C., Gan Y., Hovgaard L. Novel mucus-penetrating liposomes as a potential oral drug delivery system: preparation, in vitro characterization, and enhanced cellular uptake. Int J Nanomed. 2011;6:3151–3162. doi: 10.2147/IJN.S25741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhou Y., Ning Q., Yu D.N., Li W.G., Deng J. Improved oral bioavailability of breviscapine via a Pluronic P85-modified liposomal delivery system. J Pharm Pharmacol. 2014;66:903–911. doi: 10.1111/jphp.12215. [DOI] [PubMed] [Google Scholar]
- 139.Chen H., Torchilin V., Langer R. Lectin-bearing polymerized liposomes as potential oral vaccine carriers. Pharm Res. 1996;13:1378–1383. doi: 10.1023/a:1016030202104. [DOI] [PubMed] [Google Scholar]
- 140.Li K., Chen D., Zhao X., Hu H., Yang C., Pang D. Preparation and investigation of Ulex europaeus agglutinin I-conjugated liposomes as potential oralvaccine carriers. Arch Pharm Res. 2011;34:1899–1907. doi: 10.1007/s12272-011-1110-3. [DOI] [PubMed] [Google Scholar]
- 141.Gupta P.N., Vyas S.P. Investigation of lectinized liposomes as M-cell targeted carrier-adjuvant for mucosal immunization. Colloids Surf B Biointerfaces. 2011;82:118–125. doi: 10.1016/j.colsurfb.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 142.Zhang N., Ping Q.N., Huang G.H., Xu W.F. Investigation of lectin-modified insulin liposomes as carriers for oral administration. Int J Pharm. 2005;294:247–259. doi: 10.1016/j.ijpharm.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 143.Wang N., Wang T., Zhang M., Chen R., Niu R., Deng Y. Mannose derivative and lipid A dually decorated cationic liposomes as an effective cold chain free oral mucosal vaccine adjuvant-delivery system. Eur J Pharm Biopharm. 2014;88:194–206. doi: 10.1016/j.ejpb.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 144.Wang T., Zhen Y., Ma X., Wei B., Li S., Wang N. Mannosylated and lipid A-incorporating cationic liposomes constituting microneedle arrays as an effective oral mucosal HBV vaccine applicable in the controlled temperature chain. Colloids Surf B Biointerfaces. 2015;126:520–530. doi: 10.1016/j.colsurfb.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 145.Zhou F., Kraehenbuhl J.-P., Neutra M.R. Mucosal IgA response to rectally administered antigen formulated in IgA-coated liposomes. Vaccine. 1995;13:637–644. doi: 10.1016/0264-410x(94)00029-m. [DOI] [PubMed] [Google Scholar]
- 146.Pridgen E.M., Alexis F., Kuo T.T., Levy-Nissenbaum E., Karnik R., Blumberg R.S. Transepithelial transport of Fc-targeted nanoparticles by the neonatal Fc receptor for oral delivery. Sci Transl Med. 2013;5:213ra167. doi: 10.1126/scitranslmed.3007049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Anderson K.E., Eliot L.A., Stevenson B.R., Rogers J.A. Formulation and evaluation of a folic acid receptor-targeted oral vancomycin liposomal dosage form. Pharm Res. 2001;18:316–322. doi: 10.1023/a:1011002913601. [DOI] [PubMed] [Google Scholar]
- 148.Anderson K.E., Stevenson B.R., Rogers J.A. Folic acid–PEO-labeled liposomes to improve gastrointestinal absorption of encapsulated agents. J Control Release. 1999;60:189–198. doi: 10.1016/s0168-3659(99)00072-3. [DOI] [PubMed] [Google Scholar]
- 149.Zhang X., Qi J., Lu Y., He W., Li X., Wu W. Biotinylated liposomes as potential carriers for the oral delivery of insulin. Nanomedicine. 2014;10:167–176. doi: 10.1016/j.nano.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 150.Agrawal A.K., Harde H., Thanki K., Jain S. Improved stability and antidiabetic potential of insulin containing folic acid functionalized polymer stabilized multilayered liposomes following oral administration. Biomacromolecules. 2014;15:350–360. doi: 10.1021/bm401580k. [DOI] [PubMed] [Google Scholar]
- 151.Jain S., Harde H., Indulkar A., Agrawal A.K. Improved stability and immunological potential of tetanus toxoid containing surface engineered bilosomes following oral administration. Nanomedicine. 2014;10:431–440. doi: 10.1016/j.nano.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 152.des Rieux A., Pourcelle V., Cani P.D., Marchand-Brynaert J., Préat V. Targeted nanoparticles with novel non-peptidic ligands for oral delivery. Adv Drug Deliv Rev. 2013;65:833–844. doi: 10.1016/j.addr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 153.Zhang X., Wu W. Ligand-mediated active targeting for enhanced oral absorption. Drug Discov Today. 2014;19:898–904. doi: 10.1016/j.drudis.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 154.Patil Y.P., Jadhav S. Novel methods for liposome preparation. Chem Phys Lipids. 2014;177:8–18. doi: 10.1016/j.chemphyslip.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 155.Shukla D., Chakraborty S., Singh S., Mishra B. Lipid-based oral multiparticulate formulations-advantages, technological advances and industrial applications. Exp Opin Drug Deliv. 2011;8:207–224. doi: 10.1517/17425247.2011.547469. [DOI] [PubMed] [Google Scholar]
- 156.Schneider T., Sachse A., Röbling G., Brandl M. Large-scale production of liposomes of defined size by a new continuous high pressure extrusion device. Drug Dev Ind Pharm. 1994;20:2787–2807. [Google Scholar]
- 157.Pons M., Lizondo M., Gallardo M., Freixas J., Estelrich J. Enrofloxacin loaded liposomes obtained by high speed dispersion method. Chem Pharm Bull. 1995;43:983–987. [Google Scholar]
- 158.Pupo E., Padrón A., Santana E., Sotolongo J., Quintana D., Dueñas S. Preparation of plasmid DNA-containing liposomes using a high-pressure homogenization–extrusion technique. J Control Release. 2005;104:379–396. doi: 10.1016/j.jconrel.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 159.Justo O.R., Moraes A.M. Analysis of process parameters on the characteristics of liposomes prepared by ethanol injection with a view to process scale-up: effect of temperature and batch volume. Chem Eng Res Des. 2011;89:785–792. [Google Scholar]
- 160.Laouini A., Charcosset C., Fessi H., Holdich R., Vladisavljević G. Preparation of liposomes: a novel application of microengineered membranes—from laboratory scale to large scale. Colloids Surf B Biointerfaces. 2013;112:272–278. doi: 10.1016/j.colsurfb.2013.07.066. [DOI] [PubMed] [Google Scholar]
- 161.Wagner A., Platzgummer M., Kreismayr G., Quendler H., Stiegler G., Ferko B. Gmp production of liposomes—a new industrial approach. J Liposome Res. 2006;16:311–319. doi: 10.1080/08982100600851086. [DOI] [PubMed] [Google Scholar]
- 162.Justo O.R., Moraes A.M. Economical feasibility evaluation of an ethanol injection liposome production plant. Chem Eng Technol. 2010;33:15–20. [Google Scholar]
- 163.Singodia D., Verma A., Khare P., Dube A., Mitra K., Mishra P.R. Investigations on feasibility of in situ development of amphotericin B liposomes for industrial applications. J Liposome Res. 2012;22:8–17. doi: 10.3109/08982104.2011.584317. [DOI] [PubMed] [Google Scholar]
- 164.Karn P.R., Jin S.-E., Lee B.J., Sun B.K., Kim M.-S., Sung J.H. Preparation and evaluation of cyclosporin A-containing proliposomes: a comparison of the supercritical antisolvent process with the conventional film method. Int J Nanomed. 2014;9:5079–5091. doi: 10.2147/IJN.S70340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tantisripreecha C., Jaturanpinyo M., Panyarachun B., Sarisuta N. Development of delayed-release proliposomes tablets for oral protein drug delivery. Drug Dev Ind Pharm. 2012;38:718–727. doi: 10.3109/03639045.2011.623168. [DOI] [PubMed] [Google Scholar]
- 166.Han M., Watarai S., Kobayashi K., Yasuda T. Application of liposomes for development of oral vaccines: study of in vitro stability of liposomes and antibody response to antigen associated with liposomes after oral immunization. J Vet Med Sci. 1997;59:1109–1114. doi: 10.1292/jvms.59.1109. [DOI] [PubMed] [Google Scholar]
- 167.Schubert R., Jaroni H., Schoelmerich J., Schmidt K. Studies on the mechanism of bile salt-induced liposomal membrane damage. Digestion. 1983;28:181–190. doi: 10.1159/000198984. [DOI] [PubMed] [Google Scholar]
- 168.Werle M., Makhlof A., Takeuchi H. Carbopol-lectin conjugate coated liposomes for oral peptide delivery. Chem Pharm Bull. 2010;58:432–434. doi: 10.1248/cpb.58.432. [DOI] [PubMed] [Google Scholar]
- 169.Niu M., Tan Y., Guan P., Hovgaard L., Lu Y., Qi J. Enhanced oral absorption of insulin-loaded liposomes containing bile salts: a mechanistic study. Int J Pharm. 2014;460:119–130. doi: 10.1016/j.ijpharm.2013.11.028. [DOI] [PubMed] [Google Scholar]
- 170.Rescia V.C., Takata C.S., de Araujo P.S., Bueno da Costa M.H. Dressing liposomal particles with chitosan and poly(vinylic alcohol) for oral vaccine delivery. J Liposome Res. 2011;21:38–45. doi: 10.3109/08982101003735988. [DOI] [PubMed] [Google Scholar]
- 171.Bernkop-Schnürch A. Mucoadhesive systems in oral drug delivery. Drug Discov Today Technol. 2005;2:83–87. doi: 10.1016/j.ddtec.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 172.Lehr C.M., Poelma F.G., Junginger H.E., Tukker J.J. An estimate of turnover time of intestinal mucus gel layer in the rat in situ loop. Int J Pharm. 1991;70:235–240. [Google Scholar]
- 173.Wang Y.Y., Lai S.K., Suk J.S., Pace A., Cone R., Hanes J. Addressing the PEG mucoadhesivity paradox to engineer nanoparticles that “slip” through the human mucus barrier. Angew Chem Int Ed Engl. 2008;47:9726–9729. doi: 10.1002/anie.200803526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yuan H., Chen C.Y., Chai G.H., Du Y.Z., Hu F.Q. Improved transport and absorption through gastrointestinal tract by PEGylated solid lipid nanoparticles. Mol Pharm. 2013;10:1865–1873. doi: 10.1021/mp300649z. [DOI] [PubMed] [Google Scholar]
- 175.Ganem-Quintanar A., Kalia Y.N., Falson-Rieg F., Buri P. Mechanisms of oral permeation enhancement. Int J Pharm. 1997;156:127–142. [Google Scholar]
- 176.Thanou M., Verhoef J., Junginger H. Chitosan and its derivatives as intestinal absorption enhancers. Adv Drug Deliv Rev. 2001;50:S91–S101. doi: 10.1016/s0169-409x(01)00180-6. [DOI] [PubMed] [Google Scholar]
- 177.Zambito Y., Zaino C., Di Colo G. Effects of N-trimethylchitosan on transcellular and paracellular transcorneal drug transport. Eur J Pharm Biopharm. 2006;64:16–25. doi: 10.1016/j.ejpb.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 178.Wang J., Li L., Du Y., Sun J., Han X., Luo C. Improved oral absorption of doxorubicin by amphiphilic copolymer of lysine-linked ditocopherol polyethylene glycol 2000 succinate: in vitro characterization and in vivo evaluation. Mol Pharm. 2015;12:463–473. doi: 10.1021/mp500833m. [DOI] [PubMed] [Google Scholar]
- 179.Ma Q., Han Y., Chen C., Cao Y., Wang S., Shen W. Oral absorption enhancement of probucol by PEGylated G5 PAMAM dendrimer modified nanoliposomes. Mol Pharm. 2015;12:665–674. doi: 10.1021/mp500388m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hillaireau H., Couvreur P. Nanocarriers׳ entry into the cell: relevance to drug delivery. Cell Mol Life Sci. 2009;66:2873–2896. doi: 10.1007/s00018-009-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Rejman J., Oberle V., Zuhorn I., Hoekstra D. Size-dependent internalization of particles via the pathways of clathrin-and caveolae-mediated endocytosis. Biochem J. 2004;377:159–169. doi: 10.1042/BJ20031253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhang X., Qi J., Lu Y., Hu X., He W., Wu W. Enhanced hypoglycemic effect of biotin-modified liposomes loading insulin: effect of formulation variables, intracellular trafficking, and cytotoxicity. Nanoscale Res Lett. 2014;9:1–10. doi: 10.1186/1556-276X-9-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Jain S., Indulkar A., Harde H., Agrawal A.K. Oral mucosal immunization using glucomannosylated bilosomes. J Biomed Nanotechnol. 2014;10:932–947. doi: 10.1166/jbn.2014.1800. [DOI] [PubMed] [Google Scholar]
- 184.des Rieux A., Fievez V., Garinot M., Schneider Y.-J., Préat V. Nanoparticles as potential oral delivery systems of proteins and vaccines: a mechanistic approach. J Control Release. 2006;116:1–27. doi: 10.1016/j.jconrel.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 185.Buda A., Sands C., Jepson M.A. Use of fluorescence imaging to investigate the structure and function of intestinal M cells. Adv Drug Deliv Rev. 2005;57:123–134. doi: 10.1016/j.addr.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 186.Ling S.S., Magosso E., Khan N.A., Yuen K.H., Barker S.A. Enhanced oral bioavailability and intestinal lymphatic transport of a hydrophilic drug using liposomes. Drug Dev Ind Pharm. 2006;32:335–345. doi: 10.1080/03639040500519102. [DOI] [PubMed] [Google Scholar]
- 187.Tomizawa H., Aramaki Y., Fujii Y., Kara T., Suzuki N., Yachi K. Uptake of phosphatidylserine liposomes by rat Peyer׳s patches following intraluminal administration. Pharm Res. 1993;10:549–552. doi: 10.1023/a:1018945902276. [DOI] [PubMed] [Google Scholar]
- 188.Aramaki Y., Tomizawa H., Hara T., Yachi K., Kikuchi H., Tsuchiya S. Stability of liposomes in vitro and their uptake by rat Peyer׳s patches following oral administration. Pharm Res. 1993;10:1228–1231. doi: 10.1023/a:1018936806278. [DOI] [PubMed] [Google Scholar]
- 189.Rogers J.A., Anderson K.E. The potential of liposomes in oral drug delivery. Crit Rev Ther Drug Carr Syst. 1998;15:421–480. [PubMed] [Google Scholar]
- 190.Channarong S., Chaicumpa W., Sinchaipanid N., Mitrevej A. Development and evaluation of chitosan-coated liposomes for oral DNA vaccine: the improvement of Peyer׳s patch targeting using a polyplex-loaded liposomes. AAPS PharmSciTech. 2011;12:192–200. doi: 10.1208/s12249-010-9559-9. [DOI] [PMC free article] [PubMed] [Google Scholar]