Abstract
Background
In a multicenter study from Taiwan, we aimed to investigate the outcome of patients who received different antimicrobial therapy in carbapenem-resistant Enterobacteriaceae bloodstream infections and proposed a new definition for tigecycline use.
Methods
Patients from 16 hospitals in Taiwan who received appropriate therapy for bloodstream infections due to carbapenem-resistant Klebsiella pneumoniae and Escherichia coli were enrolled in the study between January 2012 and June 2015. We used a cox proportional regression model for multivariate analysis to identify independent risk factors of 14-day mortality. Tigecycline was defined as appropriate when the isolates had a minimum inhibitory concentration (MIC) ≤0.5 mg/L, and we investigated whether tigecycline was associated with mortality among patients with monotherapy.
Results
Sixty-four cases with carbapenem-resistant K pneumoniae (n = 50) and E coli (n = 14) bloodstream infections were analyzed. Of the 64 isolates, 17 (26.6%) had genes that encoded carbapenemases. The 14-day mortality of these cases was 31.3%. In the multivariate analysis, Charlson Comorbidity Index (hazard ratio [HR], 1.21; 95% confidence interval [CI], 1.03–1.42; P = .022) and colistin monotherapy (HR, 5.57; 95% CI, 2.13–14.61; P < .001) were independently associated with 14-day mortality. Among the 55 patients with monotherapy, the 14-day mortality was 30.9% (n = 17). Tigecycline use was not associated with mortality in the multivariate analysis.
Conclusions
Tigecycline monotherapy was a choice if the strains exhibited MIC ≤0.5 mg/L, and colistin monotherapy was not suitable. Our findings can initiate additional clinical studies regarding the efficacy of tigecycline in carbapenem-resistant Enterobacteriaceae infections.
Keywords: antimicrobial therapy, bloodstream infection, carbapenem, Enterobacteriaceae, tigecycline
The rapid spread of carbapenem-resistant (nonsusceptible) Enterobacteriaceae has become a great challenge for physicians [1–3]. Clinical studies have demonstrated a high mortality rate among patients with carbapenem-resistant Enterobacteriaceae infection [1–3]. An optimal antimicrobial regimen is important in the treatment of carbapenem-resistant Enterobacteriaceae infection. Tigecycline and colistin are considered as a last-resort treatment for these infections [1–3].
In previous studies of carbapenem-resistant Enterobacteriaceae bloodstream infections [4–14], an appropriate antimicrobial regimen was defined as at least 1 in vitro active agent according to breakpoints established by the Clinical and Laboratory Standards Institute (CLSI) [15] or the European Committee on Antimicrobial Susceptibility Testing (EUCAST) [16]. However, the CLSI did not issue interpretative criteria for tigecycline susceptibility. The EUCAST recommends tigecycline susceptibility breakpoints in Enterobacteriaceae of susceptible minimum inhibitory concentration (MIC) ≤1 mg/L and resistant MIC >2 mg/L [16]. In addition, most of the studies used the interpretative criteria from the US Food and Drug Administration (FDA) for tigecycline (susceptible, MIC ≤2 mg/L; intermediate, MIC = 4 mg/L; resistant, MIC >4 mg/L) [17]. Therefore, defining an appropriate therapy with tigecycline against Enterobacteriaceae is challenging. Moreover, the steady-state maximal serum concentrations of tigecycline (0.6 mg/L) [18] were lower than the current breakpoints proposed by the EUCAST or FDA. However, the issue of low serum concentration had not been addressed in the above-mentioned studies [4–14], and the efficacy of tigecycline in the treatment of bacteremia is also debated in the literature [19].
In this multicenter study, we investigated the independent risk factors for mortality among patients with bloodstream infections caused by carbapenem-resistant Klebsiella pneumoniae and Escherichia coli. We proposed a new definition of appropriate antimicrobial therapy with tigecycline in these infections. We aimed to investigate the impact of different regimens of antimicrobial therapy, especially tigecycline, on mortality in patients who received appropriate therapy in these infections.
MATERIALS AND METHODS
Study Setting and Patients
Patients with at least 1 positive blood culture were defined as having bloodstream infections. Bloodstream infections caused by carbapenem-resistant K pneumoniae and E coli were identified from 16 hospitals (12 medical centers and 4 regional hospitals [Supplementary Data]) in Taiwan between January 1, 2012 and June 30, 2015. Carbapenem resistance was defined as nonsusceptibility to imipenem or meropenem (MIC ≥2 mg/L) based on the interpretative criteria from CLSI published in 2012 [15]. Only the first episode of bloodstream infections was included for each patient. The clinical data were retrospectively collected, and patients aged <20 years, polymicrobial infections, and those with incomplete medical records were excluded. Patients who did not receive at least 48 hours of at least 1 appropriate antibiotic were also excluded. The detailed information of appropriate therapy is described under the following section. The study protocol was approved by the institutional review board of each participating hospital.
Microbiologic Methods
Carbapenem-resistant K pneumoniae and E coli isolates were collected from blood culture in the microbiological laboratories of each participating hospitals. The isolates were sent to the National Health Research Institutes (Miaoli, Taiwan) and were stored at −70°C in 10% glycerol Luria-Bertani medium before analysis. Bacterial identification was performed by a VITEK 2 automated system (bioMérieux, Marcy l’Etoile, France). Minimum inhibitory concentrations were determined by broth microdilution (Sensititre; Trek Diagnostic Systems, Cleveland, OH) for all antibiotics except tigecycline. The MICs for tigecycline were determined using the Etest (bioMérieux) on Mueller-Hinton medium. The results were interpreted according to the breakpoints published by CLSI, except those for colistin and tigecycline. Colistin was interpreted according to breakpoints established by EUCAST, and tigecycline was interpreted according to breakpoints established by the FDA. Carbapenem-resistant K pneumoniae and E coli isolates were screened for carbapenemase genes, plasmid-borne AmpC-like genes, and extended-spectrum β-lactamases (ESBL) genes using polymerase chain reaction detection as described previously [20–23]. Bacterial outer membrane proteins (OMPs) were isolated, and the OMP profiles (OmpK35 and OmpK36 for K pneumoniae, and OmpC and OmpF for E coli) were identified by sodium dodecyl sulfate polyacrylamide gel electrophoresis followed by coomassie blue staining (Bio-Rad). Klebsiella pneumoniae American Type Culture Collection (ATCC) 13883 and E coli ATCC25922 were used as the control strains [24, 25].
Definitions
The probable source of bloodstream infections, including pneumonia, urinary tract infection, surgical site infection, intra-abdominal infection, catheter-related infection, or primary bacteremia, was determined on the basis of microbiological results and physicians’ findings. The definition of healthcare- associated infection was described previously [26]. Severity of illness at the time of onset of infection was assessed by the Acute Physiology and Chronic Health Evaluation II (APACHE II) score. Appropriate antimicrobial therapy, including carbapenems, in patients enrolled in this study was defined as treatment with at least 1 agent to which the isolate was susceptible in vitro according to EUCAST breakpoints [16]. For tigecycline, target values of area under curve/MIC ≥6.96 have been reported to be predictive of clinical response [27]. The steady-state maximal serum concentration of tigecycline was 0.6 mg/L [28]. To achieve the target values with a standard dose (100-mg loading dose followed by 50 mg twice daily), >90% probability of target attainment could be expected at tigecycline MIC ≤0.5 mg/L [28]. Therefore, we defined appropriate antimicrobial therapy with tigecycline when the strains exhibited MIC ≤0.5 mg/L. Appropriate antimicrobial therapy with colistin was defined as an isolate being susceptible in vitro according to the EUCAST breakpoint (MIC ≤2 mg/L) [16]. Antimicrobial therapy in these patients usually varied, making it hard to classify them to a specific regimen; therefore, patients were assigned to a regimen only if it was initiated during the first 5 days after the sampling of blood culture and maintained for at least 48 hours [29]. Appropriate combination therapy was defined as 2 or more appropriate antibiotics administrated simultaneously for >48 hours.
Predictors of Mortality and Treatment Regimens
The primary outcome was death within 14 days from the onset of bloodstream infection. Risk factors for mortality in patients with bloodstream infections due to carbapenem-resistant K pneumoniae and E coli were investigated by comparing clinical variables of survivor and nonsurvivor subgroups.
The therapeutic regimens for patients with carbapenem-resistant K pneumoniae and E coli bloodstream infections were selected at the discretion of the attending physicians. There was no standard hospital guideline for antimicrobial therapy in carbapenem-resistant K pneumoniae and E coli infections. The recommended total daily dose of colistin was usually 9 million IU given in 2 or 3 divided dosages, and for tigecycline the total daily dose was 100 administered in 2 divided dosages. Usual doses of carbapenems were used: 500 mg for imipenem every 6 hours, 500 mg for doripenem, and 1 gram for meropenem every 8 hours. Dosages were adjusted to creatinine clearance when indicated.
Statistical Analysis
Categorical variables were evaluated with the χ2 or 2-tailed Fisher’s exact test. Continuous variables were compared with the Student t test (for normally distributed variables) or the Mann-Whitney U test (for nonnormally distributed variables). We used cox proportional regression model to identify independent predictors of mortality. All biologically plausible variables with P < .20 in univariate testing were incorporated into the model using a backward approach. Hazard ratio (HR) and 95% confidence interval (CI) were calculated. Two-tailed tests were used to determine statistical significance and P < .05 was considered significant. Sensitivity analysis was performed as well among patients who received monotherapy only. All statistical analyses were performed using SPSS, version 17 (SPSS Inc., Chicago, IL).
RESULTS
Characteristics of Patients With Carbapenem-Resistant Klebsiella pneumoniae and Escherichia coli Bloodstream Infections
A total of 125 cases with bloodstream infections caused by carbapenem-resistant K pneumoniae and E coli were identified during the study period. Sixty-one cases were excluded because of polymicrobial infection (n = 39), mortality within 48 hours (n = 14), or inappropriate therapy (n = 8). Finally, 64 cases were analyzed in this study. Klebsiella pneumoniae accounted for the majority of infections (n = 50, 78.1%). The demographic and clinical characteristics of the patients are shown in Table 1. The ages of the patients ranged from 20 to 94 years, with a median age of 71 years, and 39 patients were male. The 14-day mortality rate was 31.3% and the overall in-hospital mortality rate was 53.1%.
Table 1.
Characteristics of Patients With Carbapenem-Resistant Klebsiella pneumoniae and Escherichia coli Bloodstream Infectionsa
| Variables | Total (n = 64) |
14-Day Survivors (n = 44) |
14-Day Nonsurvivors (n = 20) |
P |
| Demographics | ||||
| Age, years, median (IQR) | 71 (61–77) | 72 (62–77) | 67 (60–80) | .875 |
| Male sex | 39 (60.9) | 26 (59.1) | 13 (65.0) | .653 |
| Nosocomial-acquired infection | 62 (96.9) | 42 (95.5) | 20 (100) | 1.000 |
| Healthcare-associated infection | 2 (3.1) | 2 (4.5) | 0 (0.0) | 1.000 |
| ICU-acquired isolate | 30 (46.9) | 19 (43.2) | 11 (55.0) | .380 |
| Previous hospitalizationb | 32 (50) | 20 (45.5) | 12 (60.0) | .281 |
| Microbiology | ||||
| K pneumoniae | 50 (78.1) | 34 (77.3) | 16 (80.0) | .807 |
| E coli | 14 (21.9) | 10 (22.7) | 4 (20.0) | .807 |
| Carbapenemase | 17 (26.6) | 12 (27.3) | 5 (25.0) | .849 |
| Clinical Syndrome | ||||
| Pneumonia | 19 (29.7) | 11 (25.0) | 8 (40.0) | .223 |
| Urinary tract infection | 3 (4.7) | 3 (6.8) | 0 (0.0) | .546 |
| Intra-abdominal infection | 19 (29.7) | 11 (25.0) | 8 (40.0) | .223 |
| Catheter-associated infection | 5 (7.8) | 3 (6.8) | 2 (10.0) | .644 |
| Skin and soft tissue infection | 3 (4.7) | 1 (2.3) | 2 (10.0) | .214 |
| Primary bacteremia | 15 (23.4) | 15 (34.1) | 0 (0.0) | .003 |
| Comorbidities | ||||
| Diabetes mellitus | 21 (32.8) | 14 (31.8) | 7 (35.0) | .802 |
| Chronic obstructive pulmonary disease | 6 (9.4) | 4 (9.1) | 2 (10.0) | 1.000 |
| Chronic respiratory failure with mechanical ventilator | 17 (26.6) | 9 (20.5) | 8 (40.0) | .101 |
| Congestive heart failure | 12 (18.8) | 10 (22.7) | 2 (10.0) | .227 |
| Cerebrovascular disease | 14 (21.9) | 10 (22.7) | 4 (20.0) | .807 |
| Chronic kidney diseasec | 20 (31.3) | 13 (30.2) | 7 (36.8) | .608 |
| Liver cirrhosis | 7 (10.9) | 4 (9.1) | 3 (15.0) | .668 |
| Malignancy | 26 (40.6) | 14 (31.8) | 12 (60.0) | .033 |
| Immunocompromised stated | 12 (18.8) | 8 (18.2) | 4 (20.0) | .863 |
| Previous surgerye | 24 (37.5) | 17 (38.6) | 7 (35.0) | .781 |
| Charlson Comorbidity Index, median (IQR) | 4 (2–7) | 4 (2–7) | 6 (2–8) | .173 |
| Invasive Procedures 7 Days Preceding Onset of infection | ||||
| Indwelled central venous catheter | 35 (54.7) | 26 (59.1) | 9 (45.0) | .294 |
| Indwelled urinary catheter | 33 (51.6) | 23 (52.3) | 10 (50.0) | .866 |
| Surgical drainage | 13 (20.3) | 7 (15.9) | 6 (30.0) | .194 |
| Mechanically ventilated | 29 (45.3) | 18 (40.9) | 11 (55.0) | .294 |
| Renal replacement therapy | 16 (25.0) | 10 (22.7) | 6 (30.0) | .533 |
| Severity of Illness | ||||
| Septic shock | 13 (20.3) | 7 (15.9) | 6 (30.0) | .194 |
| APACHE II score, median (IQR) | 25 (17–30) | 23 (17–28) | 28 (18–34) | .048 |
| Therapy | ||||
| Monotherapy, No. (%) | 55 (85.9) | 38 (86.4) | 17 (85.0) | .884 |
| Colistin monotherapy | 21 (32.8) | 9 (20.5) | 12 (60.0) | .002 |
| Tigecycline monotherapy | 11 (17.2) | 9 (20.5) | 2 (10.0) | .304 |
| Other monotherapy | 23 (35.9) | 20 (45.5) | 3 (15.0) | .019 |
| Combination therapy, No. (%) | 9 (14.1) | 6 (13.6) | 3 (15.0) | .884 |
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; HIV, human immunodeficiency virus; ICU, intensive care unit; IQR, interquartile range.
aData are expressed as number (%) unless specified otherwise.
bWithin 3 months preceding onset of infection.
cChronic kidney disease stage 4 and stage 5.
dUse of steroid dose equivalent to ≥20 mg of prednisolone or immunosuppressants 30 days preceding onset of infection, patients as transplantation recipient, or patients with HIV infection.
eWithin 30 days preceding onset of infection.
Microbiological Characteristics of Carbapenem-Resistant Klebsiella pneumoniae and Escherichia coli Isolates
Of the 64 isolates, 17 (26.6%) had genes that encoded carbapenemases, including K pneumoniae carbapenemase (KPC)-2 (n = 11), imipenemase (IMP)-8 (n = 1), Verona integron-encoded metallo-β-lactamase (VIM)-1 (n = 2), oxacillinase (OXA)-48 (n = 1), New Delhi metallo-β-lactamase (NDM)-1 (n = 1), and 1 with both KPC-2 and IMP-8. Almost all the carbapenemase-producing strains were K pneumoniae, and only 1 E coli with carbapenemase (NDM-1) was identified. Other noncarbapenemase-producing strains had genes that encoded plasmid-borne AmpC/ESBL and the loss of outer membrane porins (Supplementary Data). All of the strains were resistant to ceftriaxone or ceftazidime. The MIC of tigecycline was ≥0.5 mg/L for 46 (71.9%), 1–2 mg/L for 16 (25%), and >2 mg/L for 2 (3.1%). The MIC of imipenem or meropenem was ≥8 mg/L for 48 (75%) isolates and 4 mg/L for 9 isolates (6.3%).
Risk Factors for 14-Day Mortality
Table 2 showed the risk factors for 14-day mortality among patients with carbapenem-resistant K pneumoniae and E coli bloodstream infections. Chronic respiratory failure with mechanical ventilator, malignancy, Charlson Comorbidity Index, surgical drain, septic shock, APACHE II score, colistin monotherapy, and monotherapy other than colistin or tigecycline were incorporated into multivariate cox regression. Combination therapy was not associated with 14-day survival in the univariate analysis. In multivariate analysis, Charlson Comorbidity Index (HR, 1.21; 95% CI, 1.03–1.42; P = .022) and colistin monotherapy (HR, 5.57; 95% CI, 2.13–14.61; P < .001) were independently associated with 14-day mortality.
Table 2.
Cox Proportional Hazards Regression Analysis of Predictors Associated With 14-Day Mortality of Patients With Carbapenem-Resistant Klebsiella pneumoniae and Escherichia coli Bloodstream Infectionsa
| Variables | Univariate Analysis | Multivariable Analysis | ||
| HR (95% CI) |
P | HR (95% CI) |
P | |
| Chronic respiratory failure with mechanical ventilator | 2.14 (0.87–5.24) | .096 | ||
| Malignancy | 2.29 (0.94–5.62) | .069 | ||
| Charlson Comorbidity Index | 1.11 (0.96–1.29) | .147 | 1.21 (1.03–1.42) | .022 |
| Surgical drain | 1.93 (0.74–5.02) | .180 | ||
| Septic shock | 2.08 (0.80–5.42) | .134 | ||
| APACHE II score | 1.07 (1.01–1.13) | .024 | ||
| Colistin monotherapy | 4.05 (1.65–9.96) | .002 | 5.57 (2.13–14.61) | <.001 |
| Other monotherapy | 0.25 (0.07–0.86) | .028 |
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; CI, confidence interval; HR, hazard ratio; ICU, intensive care unit.
aAll biologically relevant variables with P < .20 in the univariate cox proportional regression analysis were included in the multivariate analysis.
We further evaluated the risk factors for 28-day mortality among the 64 patients. The result was similar to that in the analysis for 14-day mortality. Charlson Comorbidity Index (HR, 1.21; 95% CI, 1.04–1.40; P = .011) and colistin monotherapy (HR, 5.31; 95% CI, 2.24–12.6; P < .001) were still independently associated with 28-day mortality.
Monotherapy With Tigecycline in Carbapenem-Resistant Klebsiella pneumoniae and Escherichia Bloodstream Infections
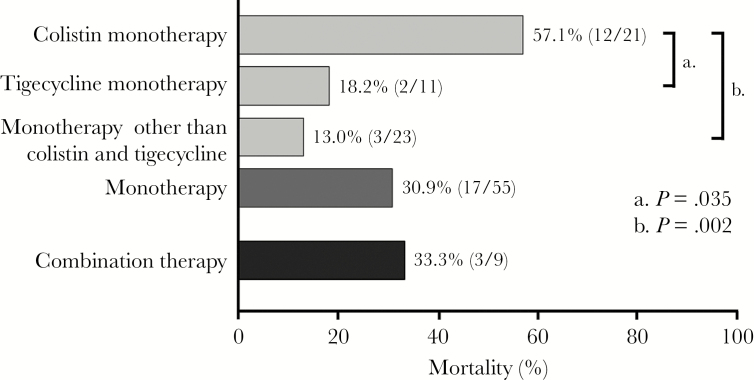
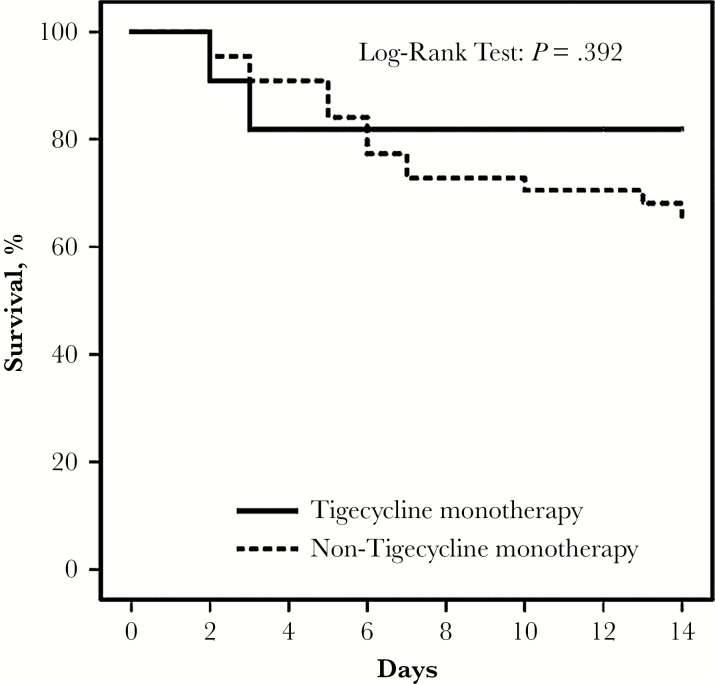
Table 3 showed detailed antimicrobial regimens among the 64 patients. We compared the 14-day mortality among the 64 patients who received different antimicrobial regimen (Figure 1). Most of the cases (n = 55) in the current study received monotherapy. Colistin monotherapy was associated with a higher mortality than that in tigecycline monotherapy (57.1% versus 18.2%, P = .035) and monotherapy other than colistin or tigecycline (57.1% versus 13.0%, P = .002). No mortality difference was noted among other regimen comparison. We defined appropriate therapy with tigecycline when the strains exhibited MIC ≤0.5 mg/L in the current study. We further compared the 11 patients with tigecycline monotherapy and those with other monotherapy (n = 44). The clinical characteristics and 14-day mortality were not different significantly between these 2 groups (Table 4). Figure 2 showed no significant difference in mortality between the 2 groups in the survival curve (log-rank test, P = .392). Among the 55 patients with monotherapy, the 14-day mortality was 30.9% (n = 17). Tigecycline was not associated with survival benefit independently in the multivariate cox regression model (data not shown).
Table 3.
Detailed Antimicrobial Therapy of Patients With Carbapenem-Resistant Klebsiella pneumoniae and Escherichia coli Bloodstream Infectionsa
| Antimicrobial Regimens | n (%) | 14-Day Mortality, n (%) |
| Monotherapy | 55 | 17/55 (30.9) |
| Colistin | 21 (38.2) | 12/21 (57.1) |
| Tigecycline | 11 (20.0) | 2/11 (18.2) |
| Aminoglycosideb | 7 (12.7) | 0/7 (0) |
| Cefepime | 6 (10.9) | 1/6 (16.7) |
| Carbapenemc | 5 (9.1) | 1/5 (20.0) |
| Fluoroquinoloned | 4 (7.3) | 1/4 (25.0) |
| Piperacillin + tazobactam | 1 (1.8) | 0/1 (0) |
| Combination therapy | 9 | 3/9 (33.3) |
| Aminoglycoside+ carbapenem | 1 (11.1) | 0/1 (0) |
| Aminoglycoside + tigecycline | 1 (11.1) | 0/1 (0) |
| Colistin + carbapenem | 2 (22.2) | 1/2 (50) |
| Colistin + tigecycline | 2 (22.2) | 1/2 (50) |
| Colistin + tigecycline + carbapenem | 1 (11.1) | 0/1 (0) |
| Colistin + tigecycline + aminoglycoside | 1 (11.1) | 0/1 (0) |
| Tigecycline + carbapenem | 1 (11.1) | 1/1 (100) |
aData are presented as number (%) unless specified otherwise.
bAmikacin or gentamicin.
cGroup 2 carbapenem (imipenem, meropenem, or doripenem).
dCiprofloxacin or levofloxacin.
Figure 1.
The 14-day mortality among the 64 patients who received different antimicrobial regimen. The mortality of colistin monotherapy was significantly higher than that in the other regimen.
Table 4.
Comparison Between Patients Treated With Tigecycline Monotherapy and Non-Tigecycline Monotherapya
| Variables | Tigecycline Monotherapy (n = 11) |
Non-Tigecycline Monotherapy (n = 44) |
P |
| Demographics | |||
| Age, years, median (IQR) | 67 (56–77) | 72 (63–77.75) | .335 |
| Male sex | 8 (72.7) | 26 (59.1) | .405 |
| Nosocomial-acquired infection | 11 (100) | 43 (97.7) | 1.000 |
| Healthcare-associated infection | 0 (0.0) | 1 (2.3) | 1.000 |
| ICU-acquired isolate | 4 (36.4) | 23 (52.3) | .345 |
| Previous hospitalizationb | 4 (36.4) | 24 (54.5) | .281 |
| Microbiology | |||
| Klebsiella pneumoniae | 9 (81.8) | 35 (79.5) | .866 |
| Escherichia coli | 2 (18.2) | 9 (20.5) | .866 |
| Carbapenemase-producing strains | 3 (27.3) | 13 (29.5) | .882 |
| Clinical Syndrome | |||
| Pneumonia | 5 (45.5) | 11 (25.0) | .182 |
| Urinary tract infection | 0 (0.0) | 2 (4.5) | 1.000 |
| Intra-abdominal infection | 3 (27.3) | 13 (29.5) | .882 |
| Catheter-associated infection | 0 (0.0) | 5 (11.4) | .571 |
| Skin and soft tissue infection | 0 (0.0) | 2 (4.5) | 1.000 |
| Primary bacteremia | 3 (27.3) | 11 (25.0) | .877 |
| Comorbidities | |||
| Diabetes mellitus | 3 (27.3) | 13 (29.5) | .882 |
| Chronic obstructive pulmonary disease | 2 (18.2) | 3 (6.8) | .259 |
| Chronic respiratory failure with mechanical ventilator | 2 (18.2) | 11 (25.0) | .634 |
| Congestive heart failure | 2 (18.2) | 7 (15.9) | .855 |
| Cerebrovascular disease | 5 (45.5) | 5 (11.4) | .009 |
| Chronic kidney diseasec | 4 (36.4) | 13 (29.5) | .662 |
| Liver cirrhosis | 1 (9.1) | 6 (13.6) | .686 |
| Malignancy | 3 (27.3) | 19 (43.2) | .335 |
| Immunocompromised stated | 3 (27.3) | 8 (18.2) | .500 |
| Previous surgerye | 4 (36.4) | 19 (43.2) | .682 |
| Charlson Comorbidity Index, median (IQR) | 6 (3–12) | 7 (3–9) | .135 |
| Invasive Procedures 7 Days Preceding Onset of Infection | |||
| Indwelled central venous catheter | 6 (54.5) | 24 (54.5) | 1.000 |
| Indwelled urinary catheter | 6 (54.5) | 23 (52.3) | .893 |
| Surgical drainage | 3 (27.3) | 9 (20.5) | .624 |
| Mechanically ventilated | 3 (27.3) | 22 (50.0) | .176 |
| Renal replacement therapy | 4 (36.4) | 9 (20.5) | .267 |
| Severity of Illness | |||
| Septic shock | 2 (18.2) | 8 (18.2) | 1.000 |
| APACHE II score, median (IQR) | 22 (17–30) | 25.5 (16.25–28.75) | .758 |
| 14-day mortality | 2 (18.2) | 15 (34.1) | .307 |
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; HIV, human immunodeficiency virus; ICU, intensive care unit; IQR, interquartile range.
aData are expressed as number (%) unless specified otherwise.
bWithin 3 months preceding onset of infection.
cChronic kidney disease stage 4 and stage 5.
dUse of steroid dose equivalent to ≥20 mg of prednisolone or immunosuppressants 30 days preceding onset of infection, patients as transplantation recipient, or patients with HIV infection.
eWithin 30 days preceding onset of infection.
Figure 2.
Kaplan-Meier survival curve of patients treated with monotherapy. The survival of tigecycline monotherapy (solid line) was not significantly different from that in non-tigecycline monotherapy (dotted line) (log-rank test; P = .392).
DISCUSSION
In this study, we investigated patients with bloodstream infections caused by carbapenem-resistant K pnuemoniae and E coli who received appropriate antimicrobial therapy. We proposed a new definition of appropriate antimicrobial therapy with tigecycline. We found that colistin monotherapy and Charlson Comorbidity Index were the independent predictors for 14-day mortality. Tigecycline monotherapy was not associated with a higher mortality among patients with monotherapy.
In Taiwan, the major mechanism for carbapenem-resistant Enterobacteriaceae was not mediated by carbapenemase until 2015 [20–23]. We included both carbapenemase-producing and noncarbapenemase-producing strains in this study, aiming to generalize our findings in the real-life practice. Many microbiology laboratories do not perform the molecular detection of carbapenemase routinely, and the physicians usually treat these infections according to the MICs interpreted by the CLSI or EUCAST. Therefore, the current study is able to help physicians manage bloodstream infections caused by carbapenem-resistant K pnuemoniae and E coli according to the MICs, regardless of the mechanisms of carbapenem resistance.
Most studies regarding treatment for carbapenem-resistant Enterobacteriaceae bloodstream infections [4–10, 12–14] defined appropriate use of tigecycline according to the FDA criteria (susceptibile, MIC ≤2 mg/L). Tumbarello et al [11] used EUCAST criteria to define the appropriate use of tigecycline (susceptibile, MIC ≤1 mg/L) in KPC-producing K pneumoniae bacteremia. We defined tigecycline use in bloodstream infection as appropriate only when the isolates exhibited MIC ≤0.5 mg/L, based on previous pharmacodynamics study [27, 28]. With tigecycline MIC ≤0.5 mg/L, a >90% probability of target attainment could be expected, and the cumulative fraction of response was 82.0%, based on previous pharmacokinetics and EUCAST wild-type MIC distributions of K pneumoniae [28]. Tigecycline is considered as a potent therapeutic option for carbapenem-resistant Enterobacteriaceae infections [30], but the efficacy of tigecycline cannot be clearly defined because a suitable definition of appropriate therapy is lacking. One recent meta-analysis showed that the efficacy of tigecycline in treating carbapenem-resistant Enterobacteriaceae infections is similar to that of other antibiotics [31], but the issue of suboptimal concentrations of tigecycline was still not discussed [31]. In the literature, cases treated with tigecycline monotherapy in bloodstream infections due to carbapenem-resistant K pneumoniae are limited. The current study first adopted a new definition of appropriate tigecycline in bloodstream infections caused by carbapenem-resistant K pneumoniae and E coli taking into account the low serum level of tigecycline. Our study found that tigecycline monotherapy was not associated with 14-day mortality if the strains exhibited MIC ≤0.5 mg/L. Our results provided some insight into tigecycline treatment in carbapenem-resistant Enterobacteriaceae bacteraemia and might initiate additional prospective studies to solidify the findings.
In this study, we found that colistin monotherapy was independently associated with poor outcome. We cannot demonstrate whether colistin-based combination therapy is better, because of the limited number of cases with combination therapy in our analysis. One recent study conducted by de Oliveira et al [32] demonstrated that polymyxins was associated with a higher risk of mortality in KPC-producing Enterobacteriaceae infections, and dosage was the major concern. The dosing guidance of cilistin is necessary because of the extensive interpatient variability in pharmacokinetics [33]. One recent study proposed clinician-friendly dosing algorithms and suggested that monotherapy with intravenous colistin may be suboptimal [33]. In our study, the difficulty in selecting an optimal dose in bloodstream infections may be the reason for the low efficacy of colistin. We also identified that the Charlson Comorbidity Index influenced the outcome, which emphasized the role of host factors in combating carbapenem-resistant bacteria.
One major limitation of this study was that clinical data were obtained retrospectively from medical records. Several missing variables, such as source control, might have had potential effects on outcome. An additional limitation was the limited number of cases, especially patients with combination therapy, which precluded further analysis. Finally, the limited cases treated with tigecycline monotherapy was another limitation of this analysis. Nevertheless, our study provided the first new definition of appropriate tigecycline monotherapy in these serious infections.
CONCLUSIONS
In conclusion, we used a new definition of appropriate antimicrobial therapy with tigecycline in the treatment of bloodstream infections caused by carbapenem-resistant K pneumoniae and E coli. Our findings suggested that tigecycline monotherapy therapy was an appropriate choice if the strains exhibited MIC ≤0.5 mg/L, and colistin monotherapy is not suitable. In the era of limited new drugs, our findings can initiate additional clinical studies regarding the efficacy of tigecycline in carbapenem-resistant Enterobacteriaceae infections.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank the Medical Science and Technology Building of Taipei Veterans General Hospital for providing experimental space and facilities.
Disclaimer. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was funded by grants from the Ministry of Science and Technology in Taiwan, Taipei Veterans General Hospital (V103B-016, V104B-001, V105B-001, and V106B-001), the Ministry of Science and Technology in Taiwan (MOST 105-2628-B-010-015-MY3), and Centers for Disease Control, R.O.C. (Taiwan) (DOH101-DC-1204, DOH102-DC-1508, MOHW103-CDC-C-114-134504, and MOHW104-CDC-C-114-144406).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Metan G, Akova M. Reducing the impact of carbapenem-resistant Enterobacteriaceae on vulnerable patient groups: what can be done? Curr Opin Infect Dis 2016; 29:555–60. [DOI] [PubMed] [Google Scholar]
- 2. Lee CR, Lee JH, Park KS, et al. . Global dissemination of carbapenemase-producing Klebsiella pneumoniae: epidemiology, genetic context, treatment options, and detection methods. Front Microbiol 2016; 7:895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Duin D, Kaye KS, Neuner EA, Bonomo RA. Carbapenem-resistant Enterobacteriaceae: a review of treatment and outcomes. Diagn Microbiol Infect Dis 2013; 75:115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mouloudi E, Protonotariou E, Zagorianou A, et al. . Bloodstream infections caused by metallo-β-lactamase/Klebsiella pneumoniae carbapenemase-producing K. pneumoniae among intensive care unit patients in Greece: risk factors for infection and impact of type of resistance on outcomes. Infect Control Hosp Epidemiol 2010; 31:1250–6. [DOI] [PubMed] [Google Scholar]
- 5. Tumbarello M, Viale P, Viscoli C, et al. . Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis 2012; 55:943–50. [DOI] [PubMed] [Google Scholar]
- 6. Zarkotou O, Pournaras S, Tselioti P, et al. . Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin Microbiol Infect 2011; 17:1798–803. [DOI] [PubMed] [Google Scholar]
- 7. Qureshi ZA, Paterson DL, Potoski BA, et al. . Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother 2012; 56:2108–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clancy CJ, Chen L, Shields RK, et al. . Epidemiology and molecular characterization of bacteremia due to carbapenem-resistant Klebsiella pneumoniae in transplant recipients. Am J Transplant 2013; 13:2619–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Navarro-San Francisco C, Mora-Rillo M, Romero-Gómez MP, et al. . Bacteraemia due to OXA-48-carbapenemase-producing Enterobacteriaceae: a major clinical challenge. Clin Microbiol Infect 2013; 19:E72–9. [DOI] [PubMed] [Google Scholar]
- 10. Daikos GL, Tsaousi S, Tzouvelekis LS, et al. . Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob Agents Chemother 2014; 58:2322–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tumbarello M, Trecarichi EM, De Rosa FG, et al. . Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study. J Antimicrob Chemother 2015; 70:2133–43. [DOI] [PubMed] [Google Scholar]
- 12. Gomez-Simmonds A, Nelson B, Eiras DP, et al. . Combination regimens for treatment of carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Antimicrob Agents Chemother 2016; 60:3601–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gutiérrez-Gutiérrez B, Salamanca E, de Cueto M, et al. . Effect of appropriate combination therapy on mortality of patients with bloodstream infections due to carbapenemase-producing Enterobacteriaceae (INCREMENT): a retrospective cohort study. Lancet Infect Dis 2017; 17:726–34. [DOI] [PubMed] [Google Scholar]
- 14. Machuca I, Gutiérrez-Gutiérrez B, Gracia-Ahufinger I, et al. . Mortality associated with bacteremia due to colistin-resistant Klebsiella pneumoniae with high-level meropenem resistance: importance of combination therapy without colistin and carbapenems. Antimicrob Agents Chemother 2017; 61:406–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-First Informational Supplement. CLSI document M100-S24. Wayne, PA; 2014. [Google Scholar]
- 16. European Committee on Antimicrobial Susceptibility Testing. Available at: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_5.0_Breakpoint_Table_01.pdf Accessed 22 March 2015.
- 17. Food and Drug Administration. Highlights of prescribing information Tygacil. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021821s021lbl.pdf Accessed 22 March 2015.
- 18. Meagher AK, Ambrose PG, Grasela TH, Ellis-Grosse EJ. The pharmacokinetic and pharmacodynamic profile of tigecycline. Clin Infect Dis 2005; 41(Suppl 5):S333–40. [DOI] [PubMed] [Google Scholar]
- 19. Kelesidis T, Karageorgopoulos DE, Kelesidis I, Falagas ME. Tigecycline for the treatment of multidrug-resistant Enterobacteriaceae: a systematic review of the evidence from microbiological and clinical studies. J Antimicrob Chemother 2008; 62:895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu PF, Chuang C, Su CF, et al. . High minimum inhibitory concentration of imipenem as a predictor of fatal outcome in patients with carbapenem non-susceptible Klebsiella pneumoniae. Sci Rep 2016; 6:32665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chang YY, Chuang YC, Siu LK, et al. . Clinical features of patients with carbapenem nonsusceptible Klebsiella pneumoniae and Escherichia coli in intensive care units: a nationwide multicenter study in Taiwan. J Microbiol Immunol Infect 2015; 48:219–25. [DOI] [PubMed] [Google Scholar]
- 22. Lin YT, Chuang C, Su CF, et al. . Efficacy of appropriate antimicrobial therapy on the survival of patients with carbapenem nonsusceptible Klebsiella Pneumoniae infection: a multicenter study in Taiwan. Medicine (Baltimore) 2015; 94:e1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Su CF, Chuang C, Lin YT, et al. . Treatment outcome of non-carbapenemase-producing carbapenem-resistant Klebsiella pneumoniae infections: a multicenter study in Taiwan. Eur J Clin Microbiol Infect Dis 2018; 37:651–9. [DOI] [PubMed] [Google Scholar]
- 24. Chiu SK, Wu TL, Chuang YC, et al. . National surveillance study on carbapenem non-susceptible Klebsiella pneumoniae in Taiwan: the emergence and rapid dissemination of KPC-2 carbapenemase. PLoS One 2013; 8:e69428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ma L, Siu LK, Lin JC, et al. . Updated molecular epidemiology of carbapenem-non-susceptible Escherichia coli in Taiwan: first identification of KPC-2 or NDM-1-producing E. coli in Taiwan. BMC Infect Dis 2013; 13:599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Friedman ND, Kaye KS, Stout JE, et al. . Health care–associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med 2002; 137:791–7. [DOI] [PubMed] [Google Scholar]
- 27. Passarell JA, Meagher AK, Liolios K, et al. . Exposure-response analyses of tigecycline efficacy in patients with complicated intra-abdominal infections. Antimicrob Agents Chemother 2008; 52:204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pai MP. Serum and urine pharmacokinetics of tigecycline in obese class III and normal weight adults. J Antimicrob Chemother 2014; 69:190–9. [DOI] [PubMed] [Google Scholar]
- 29. Gonzalez-Padilla M, Torre-Cisneros J, Rivera-Espinar F, et al. . Gentamicin therapy for sepsis due to carbapenem-resistant and colistin-resistant Klebsiella pneumoniae. J Antimicrob Chemother 2015; 70:905–13. [DOI] [PubMed] [Google Scholar]
- 30. Pournaras S, Koumaki V, Spanakis N, et al. . Current perspectives on tigecycline resistance in Enterobacteriaceae: susceptibility testing issues and mechanisms of resistance. Int J Antimicrob Agents 2016; 48:11–8. [DOI] [PubMed] [Google Scholar]
- 31. Ni W, Han Y, Liu J, et al. . Tigecycline treatment for carbapenem-resistant enterobacteriaceae infections: a systematic review and meta-analysis. Medicine 2016; 95:e3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. de Oliveira MS, de Assis DB, Freire MP, et al. . Treatment of KPC-producing Enterobacteriaceae: suboptimal efficacy of polymyxins. Clin Microbiol Infect 2015; 21:179.e1–7. [DOI] [PubMed] [Google Scholar]
- 33. Nation RL, Garonzik SM, Thamlikitkul V, et al. . Dosing guidance for intravenous colistin in critically-ill patients. Clin Infect Dis 2017; 64:565–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.