Abstract
Islet autoantibodies are typically associated with type 1 diabetes, but have been found in patients diagnosed with type 2 diabetes in whom they are associated with lower adiposity. The significance of autoantibody positivity in overweight and obese patients is not well understood. The aim of this study was to determine the prevalence and clinical significance of islet autoantibodies in overweight/obese adults diagnosed with type 2 diabetes. This study includes 204 participants at one site of the multicenter Look AHEAD (Action for Health in Diabetes) trial (ClinicalTrials.gov identifier: NCT00017953) which randomized overweight/obese adults diagnosed with type 2 diabetes to an intensive lifestyle intervention or diabetes support and education. We measured antibodies to glutamic acid decarboxylase, insulinoma antigen-2, and zinc transporter 8. Participants with and without autoantibodies were compared with respect to baseline clinical features, and longitudinal changes in weight, hemoglobin A1c, and antihyperglycemic medications. We found that 13 participants (6.4%) were autoantibody positive, including six of 47 participants (12.8%) with BMI ≥40 kg/m2. At baseline, autoantibody positive participants had higher HDL cholesterol (1.27 vs. 1.09 mmol/L, P=0.034) and lower fasting C-peptide (0.32 vs. 0.57 nmol/L, P=0.049). Over four years, autoantibody positive participants lost 5.1 kg more weight than autoantibody negative participants (P=0.056). Longitudinal changes in hemoglobin A1c did not differ by autoantibody status, though autoantibody positive participants were more likely to increase the number of antihyperglycemic medications or initiate insulin (P=0.011). In conclusion, islet autoantibodies were present in 6.4% of overweight/obese adults with type 2 diabetes including those with severe obesity, and were associated with distinct clinical features. The effect of autoantibody positivity on weight loss interventions requires further study.
Keywords: Type 2 diabetes, autoantibodies, islet cell antibody, autoimmunity, obesity, weight loss
Introduction
An ongoing challenge in the care of patients with type 2 diabetes is to identify individual pathophysiologic differences contributing to heterogeneity in clinical features and prognosis. Autoimmune damage to pancreatic beta cells is the central feature of type 1 diabetes, but may also contribute to the disease process of some patients diagnosed with type 2 diabetes [1]. Among adults clinically diagnosed with type 2 diabetes, approximately 2 to 12% have autoantibodies to islet cell antigens detected in peripheral blood, and their presence has been associated with characteristics closer to those of patients with type 1 diabetes [1–8]. Antigen-specific islet autoantibodies include the glutamic acid decarboxylase 65 antibody (GADA), insulinoma antigen-2 antibody (IA2A), and zinc transporter 8 antibody (ZnT8A) [1, 9]. In cross-sectional and longitudinal studies of adults diagnosed with type 2 diabetes, the presence of one or more of these autoantibodies has been associated with differences in clinical features including lower C-peptide levels, lower adiposity, less favorable glycemic control, and a greater need for insulin therapy [2, 3, 8, 10–13]. The mean body mass index (BMI) of patients diagnosed with type 2 diabetes who were autoantibody positive has ranged from 23.9 to 31.4 kg/m2 [2–7, 10–13], with most studies finding autoantibody positivity associated with a significantly lower BMI [2–7, 10–12].
Among patients with undifferentiated diabetes or Ketosis Prone Diabetes, the presence of islet autoantibodies may help to improve classification of diabetes subtype and predict future beta cell dysfunction [14–16]. However, the clinical utility of islet autoantibody testing is less clear in patients with an established diagnosis of type 2 diabetes [9]. In the UKPDS 25 report, patients with islet autoantibodies were less likely to achieve glycemic control in response to an initial three-month trial of dietary change, suggesting this may be important in considering nonpharmacologic diabetes therapy [2, 17]. As lifestyle change, including weight loss for overweight and obese patients, is a component of the initial therapy for all patients with type 2 diabetes [18], determining whether islet autoantibody status affects the results of diabetes lifestyle interventions may have important consequences for diabetes care. In addition, the effects of islet autoantibody status on longitudinal changes in weight and adiposity among patients with type 2 diabetes are not known.
In this study, we tested for the presence of islet autoantibodies in a subgroup of patients in the Look AHEAD (Action for Health in Diabetes) study. Look AHEAD is a randomized trial testing the effects of an intensive lifestyle intervention in overweight and obese patients with clinically diagnosed type 2 diabetes on cardiovascular morbidity and mortality and other diabetes-related outcomes. We performed cross-sectional and prospective analyses to determine the association between islet autoantibody positivity and participants’ clinical features, including longitudinal changes in weight, glycemic control, and antihyperglycemic medication use.
Materials and Methods
Study population
Look AHEAD enrolled 5145 overweight and obese adults with type 2 diabetes from 16 U.S. study centers. The current study included 204 participants at the Johns Hopkins University study center who had consented to the use of stored blood. Descriptions of the Look AHEAD study design and primary outcomes have been published [19, 20]. Key eligibility criteria were type 2 diabetes, age 45 to 76 years, hemoglobin A1c (HbA1c) < 11%, BMI ≥ 25 kg/m2 or ≥ 27 kg/m2 if using insulin, and ability to complete a maximal exercise test [19]. Type 2 diabetes was determined by self-report with verification by either review of medical records, report of the patient’s healthcare provider, use of antihyperglycemic medications, or glycemic testing. Exclusion criteria included a clinical history suggestive of type 1 diabetes, exercise-limiting cardiovascular disease, or significant renal disease [19].
Look AHEAD randomized participants 1:1 to an Intensive Lifestyle Intervention (ILI) or Diabetes Support and Education (DSE) which was the control arm. Participants in the ILI arm received behavioral, lifestyle, and pharmacologic interventions (orlistat) intended to produce weight loss of 5% to 10% of initial body weight [21]. The DSE arm received several group support sessions annually [22]. Diabetes care during the trial was provided by participants’ personal physicians, except for protocolized medication monitoring and adjustment in the ILI arm to prevent hypoglycemia [19, 21]. Enrollment began in 2001; the study intervention was terminated in September, 2012 after a median duration of 9.6 years of follow-up; participants continue to be followed [20]. Look AHEAD is registered with ClinicalTrials.gov (NCT00017953). The current study focused on weight and glycemic control over four years and antihyperglycemic medications over six years based on availability of yearly data.
Islet autoantibody assays and other markers
We tested for three islet autoantibodies: GADA, IA2A, and ZnT8A using stored plasma samples that had not previously been thawed, collected at study baseline in the fasting state. Nonspecific antibodies to islet cell cytoplasm were not measured due to a low specificity for endogenous insulin deficiency in prior studies [23, 24]. In 2016, GADA and IA2A were measured at the Northwest Lipid Research Laboratories (NWRL) in Seattle, Washington using radiobinding assays according to the protocol approved by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Autoantibody Harmonization Committee [25]. Radiolabeled proteins were produced in vitro using pThGAD65 and IA2ic clones for GADA and IA2A, respectively, and the analyses were performed using a set of NIDDK provided calibrators, and negative control serum. Three quality control samples with low, medium, and high levels were used in each assay to monitor the analytical performance. Cutoffs for autoantibody positivity were 33 DK units for GADA and 5 DK units for IA2A which were determined by the Diabetes Autoantibody Harmonization Program sponsored by the NIDDK, and are the 97th percentile of control subjects [25]. As the GADA assay used in this study did not have a predefined high-titer cutoff, we defined the cutoff for high-titer GADA as four times the upper limit of the standard cutoff, or 132 DK units. Znt8A was measured by radioimmunoassay at the Barbara Davis Center for Diabetes in Aurora, Colorado using their protocol [26, 27]. 35-S methionine labeled Znt8 protein was used as a tracer, with results expressed as an index value based on results of 50 patients with new onset type 1 diabetes (positive controls) and 100 healthy subjects (negative controls). The cutoff for Znt8A positivity was determined as an index value of 0.020 which is the 99th percentile on the receiver operating characteristic curve. International proficiency evaluations found coefficients of variation of 6.2%, 8.4%, and 12.5% for GADA, IA2A, and Znt8A assays, respectively [25, 28].
We also measured C-peptide from the same stored plasma samples. The C-peptide assays were performed at the Northwest Lipid Research Laboratories using a two site immunoenzymometric assay with the Tosoh 2000 auto-analyzer calibrated to World Health Organization 84/510 reference reagent, with coefficients of variation for low, medium, and high C-peptide levels of 3.2%, 1.6% and 1.8%, respectively.
Study measures
The remainder of the data were collected as part of the main Look AHEAD study [20]. Sociodemographics and race/ethnicity were self-reported. HbA1c was measured yearly by dedicated ion exchange high-performance liquid chromatography. Albuminuria was defined by a urine albumin creatinine ratio ≥ 30 mg/g. Glomerular filtration rate (GFR) was estimated by the CKD-Epi equation [29]. Diabetes complications were ascertained by questionnaire in the clinic setting. Metabolic syndrome was defined according to the National Cholesterol Education Program - Adult Treatment Panel III guidelines [30]. Homeostatic model assessment (HOMA) was calculated from fasting glucose and fasting C-peptide using the HOMA calculator V2.2.4 [31, 32]. Medications were recorded by trial staff with participants instructed to bring home medications to study visits for review. For longitudinal analysis of medications data, single-visit gaps were imputed such that if the number of antihyperglycemic medications and insulin use status remained the same before and after the missing visit, the missing data were replaced with the surrounding values; otherwise it was left as missing. Prior to imputation 123 of 1428 (8.6%) medication values were missing; after imputation 92 of 1428 (6.4%) values were missing.
Statistical analysis
Baseline characteristics were described stratified by islet autoantibody positivity (any positive autoantibody as described above) using non-parametric summary statistics. Differences in baseline characteristics by autoantibody status were determined by the Wilcoxon rank-sum test (continuous variables) or Fisher’s exact test (categorical variable). The association between autoantibody positivity and C-peptide levels/HOMA measures were additionally analyzed stratified by insulin treatment as insulin treatment may suppress endogenous insulin secretion [33]. Differences in insulin use and the number of antihyperglycemic medications over follow-up were compared by autoantibody status using Fisher’s exact test. Outlier values were determined as those exceeding 1.5 times the interquartile range (IQR) above the third quartile, or exceeding 1.5 times the IQR below the first quartile. Effects of autoantibody positivity on longitudinal changes in weight and HbA1c were determined using marginal effects generalized least squares regression with an unstructured covariance matrix, with indicators for autoantibody positivity and treatment arm specified at each follow-up time; this method adjusted for baseline differences in weight and HbA1c by autoantibody status. The interaction between autoantibody positivity and treatment arm for weight change was determined by including indicators for the multiplicative interaction term between autoantibody positivity and treatment arm at each follow-up time in the previously described regression model, with statistical significance determined by the likelihood-ratio test. Two-sided P ≤ 0.05 was considered statistically significant. Analyses were performed using STATA version 14 (StataCorp LP, College Station, Texas, USA).
This study was reviewed and approved by the local institutional review board. All participants gave written informed consent.
Results
Among 204 participants at baseline, the mean age was 60.2 years, 50.5% were women, and 66.2%, 27.5%, and 6.4% were white, black American (non-Hispanic), and other race/ethnicities, respectively. Participants were diagnosed with diabetes at a mean age of 53.2 years (range 32 to 72 years) and had a mean diabetes duration of 7.0 years (range 0 to 35 years). The mean baseline HbA1c was 7.0% (53 mmol/mol), the mean BMI was 35.5 kg/m2, and 94% of participants had metabolic syndrome.
Among 204 participants, 13 (6.4%) were positive for any autoantibody; eleven (5.4%) were GADA positive, one (0.5%) was IA2A positive, and two (1.0%) were Znt8A positive (Table 1). Four participants (2.0%) were high-titer GADA positive; these participants had GADA values ranging from 197 to 825 DK units. The participant who was IA2A positive also had high-titer GADA. Autoantibody clustering among participants is shown in Figure 1. The levels of islet autoantibodies among autoantibody positive participants, and their cutoff values, are shown in Figure 2.
Table 1.
Distribution of islet autoantibody positivity
| Autoantibody status | Number of participants (%) |
|---|---|
| GADA positivea | 11 (5.4%) |
| IA2A positive | 1 (0.5%) |
| Znt8A positive | 2 (1.0%) |
| GADA + IA2A positiveb | 1 (0.5%) |
| GADA + Znt8A positive | 0 |
| IA2A + Znt8A positive | 0 |
| Any autoantibody positive | 13 (6.4%) |
| Any two autoantibodies positive | 1 (0.5%) |
| All three autoantibodies positive | 0 |
Abbreviations: GADA, glutamic acid decarboxylase 65 antibody; IA2A, insulinoma antigen-2 antibody; ZnT8A, zinc transporter 8 antibody.
4 participants (2.0%) had high-titer GADA (>132 DK units).
The participant who was IA2A positive was also high-titer GADA positive.
Figure 1.
Venn diagram of islet autoantibody clustering
GADA, glutamic acid decarboxylase 65 antibody; IA2A, insulinoma antigen-2 antibody; ZnT8A, zinc transporter 8 antibody.
Figure 2.
Dot plot of islet autoantibody levels among islet autoantibody positive participants
Horizontal dashed lines represent cutoff values for autoantibody positivity. Horizontal dash-dot line represents cutoff value for high-titer GADA. GADA, glutamic acid decarboxylase 65 antibody; IA2A, insulinoma antigen-2 antibody; ZnT8A, zinc transporter 8 antibody.
Table 2 shows participant baseline characteristics stratified by islet autoantibody status. Five of 101 (5.0%) men and eight of 103 (7.8%) women participants were autoantibody positive (P=0.568). Autoantibody positivity was found in six of 135 (4.4%) white participants, five of 56 (8.9%) black American participants, and two of 13 (15.4%) other races, with no significant difference by race (P=0.129). Participants who were autoantibody positive had significantly higher median HDL cholesterol (1.27 vs. 1.09 mmol/L, P=0.034); other clinical features did not differ significantly by autoantibody status. Notably, the median age at diabetes diagnosis was 54 years in both autoantibody positive and negative participants; the youngest age of diabetes diagnosis for an autoantibody positive participant was 39 years. A non-significant greater proportion of autoantibody positive participants were using insulin at baseline (30.8 vs. 14.7%, P=0.127) and using insulin as the only antihyperglycemic medication (15.4 vs. 4.7%, P=0.149). Autoantibody positive participants had a higher median BMI (39.8 vs. 34.8 kg/m2), however this difference did not reach statistical significance (P=0.149). BMI ranged from 25.4 to 52.8 kg/m2 among autoantibody negative participants and 26.6 to 45.9 kg/m2 among autoantibody positive participants. Among the 47 participants with BMI ≥40 kg/m2, six (12.8%) were autoantibody positive, including one who was high-titer GADA positive.
Table 2.
Baseline characteristics of study participants by islet autoantibody status
| Autoantibody Negative | Any Autoantibody Positive | P-valuea | |
|---|---|---|---|
| n | 191 | 13 | |
| Age, years | 59 [56–64] | 62 [59–63] | 0.271 |
| Women | 95 (49.7) | 8 (61.5) | 0.568 |
| Race/ethnicity | 0.129 | ||
| White, non-Hispanic | 129 (67.5) | 6 (46.2) | |
| Black, non-Hispanic | 51 (26.7) | 5 (38.5) | |
| Other | 11 (5.8) | 2 (15.4) | |
| Weight, kg | 101 [90–115] | 113 [102–120] | 0.146 |
| BMI, kg/m2 | 34.8 [31.5–39.3] | 39.8 [33.7–42.8] | 0.149 |
| HbA1c, % | 6.8 [6.3–7.8] | 7.2 [6.9–7.7] | 0.178 |
| HbA1c, mmol/mol | 51 [45–62] | 55 [52–61] | 0.178 |
| Fasting glucose, mg/dL | 137 [117–166] | 141 [132–178] | 0.383 |
| Diabetes duration, years | 5 [2–10] | 6 [2–11] | 0.825 |
| Age at diabetes diagnosis, years | 54 [48–58] | 54 [51–58] | 0.512 |
| Family history of diabetes | 124 (64.9) | 8 (61.5) | 0.773 |
| Metabolic syndrome | 180 (94.2) | 12 (92.3) | 0.557 |
| Waist circumference, cm | 116 [106–126] | 117 [101–125] | 0.553 |
| Systolic blood pressure, mmHg | 133 [122–140] | 131 [115–133] | 0.103 |
| Diastolic blood pressure, mmHg | 74 [68–81] | 70 [62–76] | 0.099 |
| Antihypertensive use | 147 (77.0) | 10 (76.9) | 1.000 |
| Total cholesterol, mmol/L | 4.69 [4.20–5.44] | 4.92 [4.64–5.57] | 0.192 |
| HDL cholesterol, mmol/L | 1.09 [0.91–1.32] | 1.27 [1.09–1.63] | 0.034 |
| LDL cholesterol, mmol/L | 2.75 [2.33–3.44] | 3.08 [2.49–3.52] | 0.430 |
| Triglycerides, mmol/L | 3.24 [2.43–4.87] | 2.54 [1.79–4.82] | 0.194 |
| Lipid lowering drug use | 103 (54.5) | 7 (53.9) | 1.000 |
| Estimated GFR | 89 [75–100] | 94 [69–96] | 0.542 |
| Albuminuria | 27 (14.8) | 1 (7.7) | 0.697 |
| Diabetic neuropathy | 27 (14.1) | 2 (15.4) | 1.000 |
| Diabetic retinopathy | 9 (4.7) | 2 (15.4) | 0.149 |
| Cardiovascular disease | 29 (15.2) | 3 (23.1) | 0.434 |
| Antihyperglycemic treatment intensity | 0.248 | ||
| 0 antihyperglycemics | 29 (15.2) | 1 (7.7) | |
| 1 non-insulin antihyperglycemic | 71 (37.2) | 7 (53.9) | |
| 2 non-insulin antihyperglycemics | 43 (22.5) | 1 (7.7) | |
| ≥3 non-insulin antihyperglycemics | 20 (10.5) | 0 | |
| Insulin | 28 (14.7) | 4 (30.8) | |
| Insulin is only antihyperglycemic | 9 (4.7) | 2 (15.4) | 0.149 |
| Metformin use | 112 (59.6) | 7 (53.9) | 0.773 |
| Sulfonylurea use | 79 (41.8) | 2 (15.4) | 0.080 |
| Thiazolidinedione use | 61 (32.3) | 4 (30.7) | 1.000 |
| Randomized to ILI arm | 98 (51.3) | 7 (53.9) | 1.000 |
Data expressed as n (%) or median [IQR].
Abbreviations: ILI, Intensive Lifestyle Intervention.
Rank-sum test for continuous variables; Fisher’s exact test for categorical variables.
Table 3 shows baseline tests of glucose homeostasis by islet autoantibody status. Autoantibody positive participants had significantly lower fasting C-peptide (0.32 vs. 0.57 nmol/L, P=0.049), HOMA beta cell function (33 vs. 51%, P=0.018), and HOMA insulin resistance (0.81 vs. 1.37, P=0.050). This pattern was consistent in both insulin users and non-users, though differences by autoantibody status were not significant after stratification by insulin use.
Table 3.
Tests of glucose homeostasis by islet autoantibody status, overall and by insulin use
| Autoantibody Negative | Any Autoantibody Positive | P-valuea | |
|---|---|---|---|
| All participants | n = 191 | n = 13 | |
| Fasting C-peptide, nmol/L | 0.57 [0.31–0.89] | 0.32 [0.24–0.77] | 0.049 |
| HOMA beta cell function, % | 51 [31–73] | 33 [17–42] | 0.018 |
| HOMA insulin resistance | 1.37 [0.82–2.38] | 0.81 [0.61–1.82] | 0.050 |
| Insulin non-users | n = 163 | n = 9 | |
| Fasting C-peptide, nmol/L | 0.61 [0.37–0.94] | 0.41 [0.30–0.79] | 0.282 |
| HOMA beta cell function, % | 54 [34–77] | 38 [28–50] | 0.114 |
| HOMA insulin resistance | 1.49 [0.94–2.44] | 0.99 [0.78–1.98] | 0.286 |
| Insulin users | n = 28 | n = 4 | |
| Fasting C-peptide, nmol/L | 0.31 [0.17–0.45] | 0.13 [0.01–0.33] | 0.154 |
| HOMA beta cell function, % | 30 [15–50] | 11 [3–20] | 0.117 |
| HOMA insulin resistance | 0.83 [0.45–1.17] | 0.35 [0.01–0.84] | 0.139 |
Data expressed as median [IQR].
Abbreviations: HOMA, homeostatic model assessment.
Rank-sum test.
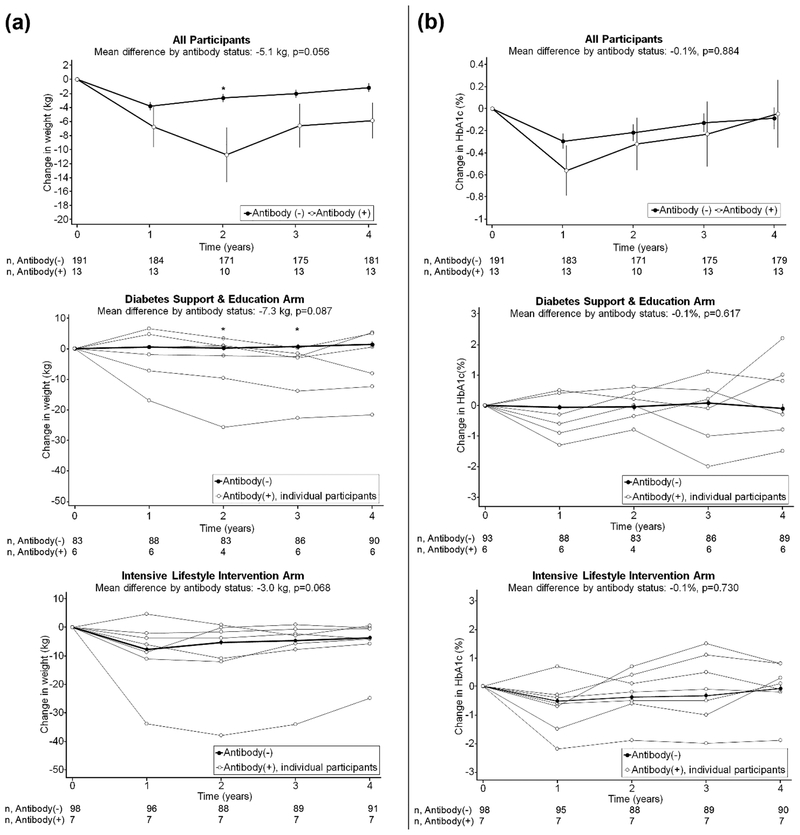
Autoantibody positive participants lost significantly more weight at study year two than autoantibody negative participants (P=0.008): autoantibody positive participants lost 10.8 kg or 9.4% of initial body weight (IBW) while antibody negative participants lost 2.7 kg or 2.5% of IBW. Differences in weight at other individual time points were not significant (Figure 3). Averaged over four years, autoantibody positive participants lost 5.1 kg more weight (4.2% of IBW) than autoantibody negative participants, independent of treatment arm, which nearly reached statistical significance (P=0.056). In the DSE (control) arm, autoantibody negative participants gained, on average, 0.7 kg (0.7% of IBW) while autoantibody positive participants lost 6.6 kg (6.0% of IBW). In the ILI arm, autoantibody negative participants lost, on average, 5.4 kg (5.0% of IBW) while autoantibody positive participants lost 8.3 kg (7.0% of IBW).
Figure 3.
Longitudinal changes in weight and HbA1c by islet autoantibody status over four years
Panel A shows changes in weight from baseline, and Panel B shows changes in hemoglobin A1c (HbA1c) from baseline, with standard error bars. The upper graphs show results for all participants; the middle and lower graphs show participants randomized to the diabetes support and education (control) arm and intensive lifestyle intervention arm, respectively. In the graphs stratified by study arm, changes in weight and HbA1c are shown for each individual autoantibody positive participant. The number of participants with available data at each study visit is shown on the lines below each graph. Asterisks denote P≤0.05 comparing autoantibody positive to autoantibody negative participants at the designated study visit, using linear regression adjusted for the baseline value of the outcome and study arm (unless stratified by study arm).
Among autoantibody positive participants, there were two outliers in weight loss who lost 25.0 kg (20.4% of IBW) and 21.7 kg (20.5% of IBW) over four years. These participants were both insulin users, were randomized to the ILI and DSE arms respectively, had baseline BMI of 39.8 kg/m2 and 33.7 kg/m2, and baseline HbA1c of 7.7% (61 mmol/mol) and 6.9% (52 mmol/mol), respectively. These participants had C-peptide, HOMA beta cell function, and HOMA insulin resistance values of <0.01 and 0.25 nmol/L, 5.5% and 17.2%, and 0.01 and 0.69, respectively. Their mean HbA1c over four years follow-up was 8.3% (67 mmol/mol) and 5.5% (37 mmol/mol), respectively.
We also examined the 4-year effect of the Look AHEAD intervention (difference in weight change between intervention and control arms) by autoantibody status. The 4-year effect of the intervention was a weight difference of −6.1 kg (−5.7% of IBW) in autoantibody negative participants and −1.7 kg (−1.0% of IBW) in autoantibody positive participants; the interaction between autoantibody positivity and treatment arm was not significant (P=0.163). Longitudinal changes in HbA1c were similar by autoantibody status.
Longitudinal changes in antihyperglycemic medication use over six years of follow-up are shown in Figure 4, and are reported stratified by treatment arm in Table 4. Among participants not using insulin at baseline, two of nine (22.2%) autoantibody positive participants started insulin during follow-up, compared to 27 of 163 (16.6%) autoantibody negative participants (P=0.649). All nine (100%) autoantibody positive participants not on insulin at baseline either increased the number of antihyperglycemic medications or started insulin during follow-up, compared to 91 of 163 (55.8%) autoantibody negative participants (P=0.011). Longitudinal differences in antihyperglycemic intensity by autoantibody status were more pronounced in the ILI arm as fewer autoantibody negative participants increased antihyperglycemic treatment intensity in this group.
Figure 4.
Longitudinal changes in antihyperglycemic medication use by islet autoantibody status over six years
The proportion of participants using 1, 2, or 3+ non-insulin antihyperglycemic medications, or insulin, is shown at each study visit among autoantibody negative participants (top graph) and autoantibody positive participants (bottom graph). Some missing medication data was imputed based on surrounding values. The number of participants included in the analysis at each time point after imputation is shown below each graph.
Table 4.
Longitudinal changes in antihyperglycemic treatment intensity and insulin use by islet autoantibody status over six years, among participants not using insulin at baseline
| Autoantibody Negative | Any Autoantibody Positive | P-valuea | |
|---|---|---|---|
| All participants | n=163 | n=9 | |
| Increased treatment intensity | 91 (55.8) | 9 (100) | 0.011 |
| Initiated insulin | 27 (16.6) | 2 (22.2) | 0.649 |
| DSE arm | n=78 | n=4 | |
| Increased treatment intensity | 55 (70.5) | 4 (100) | 0.573 |
| Initiated insulin | 16 (20.5) | 2 (50) | 0.208 |
| ILI arm | n=85 | n=5 | |
| Increased treatment intensity | 36 (42.4) | 5 (100) | 0.017 |
| Initiated insulin | 11 (12.9) | 0 (0) | 1.000 |
Data expressed as n (%). Increased treatment intensity defined as increasing the number of glucose lowering medications or starting insulin.
Abbreviations: DSE, Diabetes Support & Education; ILI, Intensive Lifestyle Intervention.
Fisher’s exact test.
Discussion
In this study of overweight and obese adults clinically diagnosed with type 2 diabetes, positivity to GADA, IA2A, or Znt8A was found in 6.4% of patients, and in 12.8% of patients with severe obesity (BMI ≥ 40 kg/m2). In addition, autoantibody positivity was associated with higher baseline HDL cholesterol, lower baseline fasting C-peptide, a greater likelihood of increasing antihyperglycemic treatment intensity during follow-up, and a trend towards greater weight loss during follow-up. Therefore, islet autoantibodies may be present in a minority of overweight and obese patients clinically diagnosed with type 2 diabetes in whom they are associated with distinct clinical features.
In this study, we found that islet autoantibody positivity was associated with a trend towards greater weight loss longitudinally, which nearly reached statistical significance. To our knowledge, this is the first study to examine this. Autoantibody positive participants lost weight in both the lifestyle intervention and control arms, and lost more weight than autoantibody negative participants in both treatment arms. The two autoantibody positive participants who were outliers in terms of a large amount of weight loss were both insulin users with low values of C-peptide, HOMA beta cell function, and HOMA insulin resistance. These findings suggest that a metabolic phenotype closer to type 1 diabetes may be driving weight loss in these individuals. We considered whether the greater weight loss in autoantibody positive participants was due to a higher starting BMI in these participants; we feel that this is unlikely as the outliers who lost the most weight were not those with the highest starting BMI, and differences in weight loss by autoantibody status were consistent whether examining absolute weight change or percent of initial body weight. The ability of this study to detect an interaction between autoantibody positivity and the Look AHEAD intensive lifestyle intervention was limited by a small number of autoantibody positive patients in each treatment arm. Overall, we found no significant association between autoantibody status and longitudinal changes in weight, although the borderline significant difference merits further research that is powered to determine the impact of islet autoimmunity on changes in weight and the efficacy of diabetes lifestyle interventions.
The prevalence of islet autoantibody positivity in this study was comparable to other cohorts recruiting adults with type 2 diabetes that were not restricted by BMI (2–12% prevalence in large cohorts) [2–7]. Among cohorts of adults clinically diagnosed with type 2 diabetes, islet autoantibody positivity has been associated with a phenotype of higher HDL, lower C-peptide, and a need for more intensive antihyperglycemic therapy [2, 3, 10–13]; our study supports that these effects of islet autoimmunity extend to overweight and obese patients. Notably, we found that autoantibody positive participants had a non-significant higher BMI, while most prior studies have found autoantibody positivity in type 2 diabetes to be associated with a lower BMI [2, 5–7, 10–12]. It is possible that these findings were due to the exclusion of individuals with BMI ≤ 25 kg/m2 who have been found to have the highest autoantibody prevalence in prior studies [34]. Also, the presence of islet autoantibodies was associated with worse glycemic control in prior studies [12, 13], whereas in this study HbA1c was similar by autoantibody status. It is possible that the absence of an effect of autoantibody positivity on glycemic control was due to a significantly greater intensity of antihyperglycemic therapy in autoantibody positive participants. It is also possible that significant differences in HbA1c and other clinical outcomes were not detected due to lack of statistical power.
In addition, we found that islet autoantibody positivity was associated with lower HOMA estimated beta cell function and insulin resistance which is consistent with prior studies of patients with type 2 diabetes not restricted by BMI [2–4, 10]. Given that insulin treatment may decrease endogenous insulin secretion [33], we considered whether autoantibody positive patients had lower HOMA measures due to a non-significant greater proportion of baseline insulin use. We felt that this is unlikely as differences in C-peptide and HOMA measures by autoantibody status were consistent after stratifying by insulin use. Therefore, even in overweight and obese patients diagnosed with type 2 diabetes, islet autoantibodies are associated with physiologic differences such that hyperglycemia is driven to a greater degree by endogenous insulin deficiency rather than insulin resistance.
This study examined islet autoantibody positivity in a multiethnic population. Though we found no significant differences in the prevalence of autoantibody positivity by race/ethnicity, this may have been limited by small numbers. Autoantibody positivity appeared to be over twice as common in black Americans and other race/ethnicities, compared to white Americans. There have been only limited prior studies examining racial/ethnic differences in islet autoimmunity among adults with type 2 diabetes [3, 4, 35]. Further research in multiethnic populations is needed.
While participants in this study were excluded if they had type 1 diabetes by self-report or clinical history, we may consider whether some autoantibody positive participants were misdiagnosed as having type 2 diabetes and more careful evaluation would have classified them as having type 1 diabetes. The frequency of diabetes misclassification in clinical practice and research studies is unclear due to the lack of an objective gold standard test by which to distinguish diabetes subtypes [36–38]. In clinical practice, patients are classified as having type 1 diabetes by clinical features (younger age at diagnosis, lower adiposity, signs of absolute insulin deficiency) and longitudinal observation [36]. Autoantibody positive participants in this study were diagnosed with diabetes at 39 years of age or older, and less than one third were using insulin at enrollment, so the majority of autoantibody positive participants lacked key features of type 1 diabetes at enrollment. Therefore, it is unlikely that autoantibody positive participants in this study were misdiagnosed type 1 diabetes, and more likely that they represent the natural heterogeneity of patients appropriately diagnosed with type 2 diabetes. Indeed, recent findings have identified distinct subgroups of patients with newly diagnosed diabetes that cluster in terms of clinical and metabolic characteristics, with some patients diagnosed with type 2 diabetes having relative leanness and endogenous insulin deficiency more typical of type 1 diabetes [39]. Our findings highlight the variable phenotype of overweight and obese patients diagnosed with type 2 diabetes, and that islet autoimmunity plays a role in that variability.
Furthermore, there may be specific mechanisms by which obesity contributes to autoimmunity in patients with type 2 diabetes. Obesity induces inflammatory changes which may promote the development of autoantibody positivity and increase the risk for autoimmune disorders [40, 41]. Recent studies have identified distinct epitopes of IA2A and GADA that may be differentially associated with endogenous insulin deficiency and obesity [34, 41, 42]. To determine how these distinct autoimmune phenotypes impact diabetes-related outcomes, further longitudinal studies of islet autoimmunity in overweight and obese patients with type 2 diabetes are needed.
This study has several limitations. There were relatively few autoantibody positive participants, resulting in limited power to detect the association between autoantibody positivity and differences in clinical features and interactions with the Look AHEAD intervention. In addition, participants included in this study were derived from a single site of the Look AHEAD trial, and are thus limited to a single geographic region in the U.S. and may not be representative of all study participants or of the U.S. population.
The major strength of this study is that it examined three antigen-specific islet autoantibodies among a group of participants who were closely followed with standardized longitudinal measures of weight, glycemic control, and diabetes medication use. Overall, this study suggests that islet autoantibodies are present in a small but notable minority of overweight and obese adults diagnosed with type 2 diabetes and are associated with phenotypic differences. More research is warranted to evaluate whether islet autoimmunity differs by race/ethnicity and impacts weight change and the efficacy of diabetes lifestyle interventions.
Acknowledgements
This research was funded in part by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH). S.J.P. was supported by NIH training grant 5T32HL007180-40. A.B. was supported by NIH/NIDDK grant U01DK057177-18. D.M.N. was supported by NIH/NIDDK grant U01DK057154-18. X.P. was supported by NIH/NIDDK grant U01DK057178-18. J.M.C. and N.M.M. were supported by NIH/NIDDK grant U01DK057149-17. S.J.P performed the analysis and prepared the manuscript. A.B., W.C.K., M.L., D.M.N. and X.P. each contributed to the study design and interpretation and reviewed/edited the manuscript. J.M.C. contributed to the data collection, study design, and interpretation, and reviewed/edited the manuscript. N.M.M. contributed to the study design, analysis, and interpretation and reviewed/edited the manuscript. The authors would like to thank James Potter and the Translational Research Enhancement Core of the Hopkins Conte Digestive Diseases Basic and Translational Research Development Center for the processing, storage, and shipment of specimens. We would like to thank Dr. Santica Marcovina of the University of Washington and the Northwest Lipid Research Laboratories in Seattle, Washington, for assistance with islet autoantibody assays.
Footnotes
Disclosure of Interest
The authors report no conflicts of interest in this work.
REFERENCES
- 1.Naik RG, Brooks-Worrell BM, Palmer JP. Latent autoimmune diabetes in adults. J Clin Endocrinol Metab. 2009. December;94(12):4635–44. doi: 10.1210/jc.2009-1120. [DOI] [PubMed] [Google Scholar]
- 2.Turner R, Stratton I, Horton V, et al. UKPDS 25: autoantibodies to islet-cell cytoplasm and glutamic acid decarboxylase for prediction of insulin requirement in type 2 diabetes. UK Prospective Diabetes Study Group. Lancet. 1997. November 1;350(9087):1288–93. doi: 10.1016/s0140-6736(97)03062-6. [DOI] [PubMed] [Google Scholar]
- 3.Davis TM, Wright AD, Mehta ZM, et al. Islet autoantibodies in clinically diagnosed type 2 diabetes: prevalence and relationship with metabolic control (UKPDS 70). Diabetologia. 2005. April;48(4):695–702. doi: 10.1007/s00125-005-1690-x. [DOI] [PubMed] [Google Scholar]
- 4.Zinman B, Kahn SE, Haffner SM, et al. Phenotypic characteristics of GAD antibody-positive recently diagnosed patients with type 2 diabetes in North America and Europe. Diabetes. 2004. December;53(12):3193–200. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Z, Xiang Y, Ji L, et al. Frequency, immunogenetics, and clinical characteristics of latent autoimmune diabetes in China (LADA China study): a nationwide, multicenter, clinic-based cross-sectional study. Diabetes. 2013. February;62(2):543–50. doi: 10.2337/db12-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hawa MI, Buchan AP, Ola T, et al. LADA and CARDS: a prospective study of clinical outcome in established adult-onset autoimmune diabetes. Diabetes Care. 2014. June;37(6):1643–9. doi: 10.2337/dc13-2383. [DOI] [PubMed] [Google Scholar]
- 7.Maddaloni E, Lessan N, Al Tikriti A, et al. Latent Autoimmune Diabetes in Adults in the United Arab Emirates: Clinical Features and Factors Related to Insulin-Requirement. PLoS One. 2015;10(8):e0131837. doi: 10.1371/journal.pone.0131837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trabucchi A, Faccinetti NI, Guerra LL, et al. Detection and characterization of ZnT8 autoantibodies could help to screen latent autoimmune diabetes in adult-onset patients with type 2 phenotype. Autoimmunity. 2012. March;45(2):137–42. doi: 10.3109/08916934.2011.604658. [DOI] [PubMed] [Google Scholar]
- 9.van Deutekom AW, Heine RJ, Simsek S. The islet autoantibody titres: their clinical relevance in latent autoimmune diabetes in adults (LADA) and the classification of diabetes mellitus. Diabet Med. 2008. February;25(2):117–25. doi: 10.1111/j.1464-5491.2007.02316.x. [DOI] [PubMed] [Google Scholar]
- 10.Desai M, Cull CA, Horton VA, et al. GAD autoantibodies and epitope reactivities persist after diagnosis in latent autoimmune diabetes in adults but do not predict disease progression: UKPDS 77. Diabetologia. 2007. October;50(10):2052–60. doi: 10.1007/s00125-007-0745-6. [DOI] [PubMed] [Google Scholar]
- 11.Davies H, Brophy S, Fielding A, et al. Latent autoimmune diabetes in adults (LADA) in South Wales: incidence and characterization. Diabet Med. 2008. November;25(11):1354–7. doi: 10.1111/j.1464-5491.2008.02580.x. [DOI] [PubMed] [Google Scholar]
- 12.Andersen CD, Bennet L, Nystrom L, et al. Worse glycaemic control in LADA patients than in those with type 2 diabetes, despite a longer time on insulin therapy. Diabetologia. 2013. February;56(2):252–8. doi: 10.1007/s00125-012-2759-y. [DOI] [PubMed] [Google Scholar]
- 13.Olsson L, Grill V, Midthjell K, et al. Mortality in Adult-Onset Autoimmune Diabetes Is Associated With Poor Glycemic Control Results from the HUNT Study. Diabetes Care. 2013. December;36(12):3971–3978. doi: 10.2337/dc13-0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maldonado M, Hampe CS, Gaur LK, et al. Ketosis-prone diabetes: dissection of a heterogeneous syndrome using an immunogenetic and beta-cell functional classification, prospective analysis, and clinical outcomes. J Clin Endocrinol Metab. 2003. November;88(11):5090–8. doi: 10.1210/jc.2003-030180. [DOI] [PubMed] [Google Scholar]
- 15.Balasubramanyam A, Garza G, Rodriguez L, et al. Accuracy and predictive value of classification schemes for ketosis-prone diabetes. Diabetes Care. 2006. December;29(12):2575–9. doi: 10.2337/dc06-0749. [DOI] [PubMed] [Google Scholar]
- 16.Littorin B, Sundkvist G, Hagopian W, et al. Islet cell and glutamic acid decarboxylase antibodies present at diagnosis of diabetes predict the need for insulin treatment - A cohort study in young adults whose disease was initially labeled as type 2 or unclassifiable diabetes. Diabetes Care. 1999. March;22(3):409–412. doi: DOI 10.2337/diacare.22.3.409. [DOI] [PubMed] [Google Scholar]
- 17.UKPDS Study Group. UK Prospective Diabetes Study (UKPDS). VIII. Study design, progress and performance. Diabetologia. 1991. December;34(12):877–90. [PubMed] [Google Scholar]
- 18.American Diabetes Association. 4. Lifestyle Management: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018. January;41(Suppl 1):S38–S50. doi: 10.2337/dc18-S004. [DOI] [PubMed] [Google Scholar]
- 19.Ryan DH, Espeland MA, Foster GD, et al. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Controlled clinical trials. 2003. October;24(5):610–28. [DOI] [PubMed] [Google Scholar]
- 20.The Look AHEAD Research Group, Wing RR, Bolin P, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013. July 11;369(2):145–54. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The Look AHEAD Research Group, Wadden TA, West DS, et al. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring). 2006. May;14(5):737–52. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wesche-Thobaben JA. The development and description of the comparison group in the Look AHEAD trial. Clin Trials. 2011. June;8(3):320–9. doi: 10.1177/1740774511405858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bottazzo GF, Bosi E, Cull CA, et al. IA-2 antibody prevalence and risk assessment of early insulin requirement in subjects presenting with type 2 diabetes (UKPDS 71). Diabetologia. 2005. April;48(4):703–8. doi: 10.1007/s00125-005-1691-9. [DOI] [PubMed] [Google Scholar]
- 24.Torn C, Landin-Olsson M, Lernmark A, et al. Combinations of beta cell specific autoantibodies at diagnosis of diabetes in young adults reflects different courses of beta cell damage. Autoimmunity. 2001;33(2):115–20. [DOI] [PubMed] [Google Scholar]
- 25.Bonifacio E, Yu L, Williams AK, et al. Harmonization of glutamic acid decarboxylase and islet antigen-2 autoantibody assays for national institute of diabetes and digestive and kidney diseases consortia. J Clin Endocrinol Metab. 2010. July;95(7):3360–7. doi: 10.1210/jc.2010-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu L, Boulware DC, Beam CA, et al. Zinc transporter-8 autoantibodies improve prediction of type 1 diabetes in relatives positive for the standard biochemical autoantibodies. Diabetes Care. 2012. June;35(6):1213–8. doi: 10.2337/dc11-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wenzlau JM, Juhl K, Yu L, et al. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci U S A. 2007. October 23;104(43):17040–5. doi: 10.1073/pnas.0705894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schlosser M, Mueller PW, Achenbach P, et al. Diabetes Antibody Standardization Program: First evaluation of assays for autoantibodies to IA-2beta. Diabetes Care. 2011. November;34(11):2410–2. doi: 10.2337/dc11-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009. May 5;150(9):604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001. May 16;285(19):2486–97. [DOI] [PubMed] [Google Scholar]
- 31.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998. December;21(12):2191–2. [DOI] [PubMed] [Google Scholar]
- 32.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004. June;27(6):1487–95. [DOI] [PubMed] [Google Scholar]
- 33.Garvey WT, Revers RR, Kolterman OG, et al. Modulation of insulin secretion by insulin and glucose in type II diabetes mellitus. J Clin Endocrinol Metab. 1985. March;60(3):559–68. doi: 10.1210/jcem-60-3-559. [DOI] [PubMed] [Google Scholar]
- 34.Buzzetti R, Spoletini M, Zampetti S, et al. Tyrosine phosphatase-related islet antigen 2(256–760) autoantibodies, the only marker of islet autoimmunity that increases by increasing the degree of BMI in obese subjects with type 2 diabetes. Diabetes Care. 2015. March;38(3):513–20. doi: 10.2337/dc14-1638. [DOI] [PubMed] [Google Scholar]
- 35.Juneja R, Hirsch IB, Naik RG, et al. Islet cell antibodies and glutamic acid decarboxylase antibodies, but not the clinical phenotype, help to identify type 1(1/2) diabetes in patients presenting with type 2 diabetes. Metabolism. 2001. September;50(9):1008–13. [DOI] [PubMed] [Google Scholar]
- 36.American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018. January;41(Suppl 1):S13–S27. doi: 10.2337/dc18-S002. [DOI] [PubMed] [Google Scholar]
- 37.Shields BM, Peters JL, Cooper C, et al. Can clinical features be used to differentiate type 1 from type 2 diabetes? A systematic review of the literature. BMJ Open. 2015. November 2;5(11):e009088. doi: 10.1136/bmjopen-2015-009088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pilla SJ, Maruthur NM, Schweitzer MA, et al. The Role of Laboratory Testing in Differentiating Type 1 Diabetes from Type 2 Diabetes in Patients Undergoing Bariatric Surgery. Obes Surg. 2018. January;28(1):25–30. doi: 10.1007/s11695-017-2804-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahlqvist E, Storm P, Karajamaki A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018. May;6(5):361–369. doi: 10.1016/S2213-8587(18)30051-2. [DOI] [PubMed] [Google Scholar]
- 40.Versini M, Jeandel PY, Rosenthal E, et al. Obesity in autoimmune diseases: not a passive bystander. Autoimmun Rev. 2014. Sep;13(9):981–1000. doi: 10.1016/j.autrev.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 41.Tiberti C, Zampetti S, Capoccia D, et al. Evidence of diabetes-specific autoimmunity in obese subjects with normal glucose tolerance. Diabetes Metab Res Rev. 2018. August 20:e3055. doi: 10.1002/dmrr.3055. [DOI] [PubMed] [Google Scholar]
- 42.Bansal N, Hampe CS, Rodriguez L, et al. DPD epitope-specific glutamic acid decarboxylase (GAD)65 autoantibodies in children with Type 1 diabetes. Diabet Med. 2017. May;34(5):641–646. doi: 10.1111/dme.13077. [DOI] [PMC free article] [PubMed] [Google Scholar]