Abstract
Saponins function as a natural self‐defense mechanism for plants to deter various insects due to their unpleasant taste and their toxicity. Here, we provide evidence that saponin from Quillaja saponaria functions as an antifeedant as well as an insecticide to ward off insects in both the larval and the adult stages. Using a behavioral screen of 26 mutant fly lines, we show that the Gr28b gene cluster plays a role in saponin avoidance in the labellum. The Gr28b mutant does not avoid saponin and exhibits increased lethality when fed saponin‐mixed food. Tissue‐specific rescue experiments with five different Gr28b isoforms revealed that only the Gr28b.c isoform is required for saponin sensation. We propose that in contrast to sensing many other bitter compounds, saponin sensing does not require the function of core taste receptors, such as GR32a, GR33a, and GR66a. Our results reveal a novel role for GR28b in taste. In addition, the ability of saponin to act as insecticides as well as antifeedants suggests its potential application in controlling insect pests.
Keywords: aversive behavior, electrophysiology, GR28b (C), insecticide
Subject Categories: Neuroscience
Introduction
Saponin is a class of chemical compounds found in many plants, mainly angiosperms including wild plants and cultivated crops. It is an amphipathic glycoside and used as a surfactant when shaken in water. The bark from Quillaja saponaria is one of the major sources of industrial triterpenoid saponins used for foaming agents. Saponins are also promoted commercially as human dietary supplements with a variety of functions such as anti‐inflammation and antivirus 1. For insects, however, saponin is toxic. When third‐instar Spodoptera littoralis larvae are fed on artificial diets containing saponin from Q. saponaria bark, their mortality rate is increased and the larval stages last longer than when fed a normal diet 2. In Aedes aegypti or Culex pipiens which is known to be a vector of dengue fever or Western Nile virus, Q. Saponaria bark induced larvicidal effect 3. This suggests that saponin can be used as an insecticide. In Drosophila melanogaster, saponin from Q. saponaria can activate specific sensilla in the labellum, although it does not activate any sensilla in the legs 4, 5. This indicates that insects, such as fruit flies, should be able to detect and avoid this toxic chemical to survive. However, the molecular sensor of saponin in insects remains to be elucidated. To discover the receptor participating in the sensation of saponin, we chose D. melanogaster as an ideal model organism.
Food preference largely depends on taste and smell, as well as vision. The gustatory organs in flies include the labellum, legs, and internal organs. The labellum, Drosophila's major taste organ, contains 31 taste sensilla, which are composed of large (L)‐, intermediate (I)‐, and small (S)‐type bristles depending on their length 6, 7. L‐ and S‐type sensilla contain four gustatory receptor neurons (GRNs), which respond to sugar, low concentrations of salt, and water or low osmolality 6, 7. Only S‐type, but not L‐type, sensilla can sense bitter compounds and high concentrations of salt. I‐type sensilla contain two GRNs, one that responds to sugar and low concentrations of salt, and another that responds to bitter substances and high concentrations of salt 6. These labellar taste sensilla provide gustatory information that allows the fly to decide whether to ingest or avoid a particular food. Additional taste sensilla in the pharynx are involved in reinforcing the swallowing reflex of food judged as nutritious or in regurgitating contaminated food 8, 9, 10. Evaluation of tastants by the sensilla in the legs is important in the fly's initial decision of whether a food is nutritious or toxic.
In Drosophila, the gustatory receptor (Gr) gene family is comprised of 60 members, which encode 68 receptors by alternative splicing 11, 12, 13. Molecular genetic analyses using the GAL4/UAS reporter system revealed that there are two major groups of Gr genes, with one group involved in sugar sensation and another larger group in bitter detection 5, 12, 14. Most Grs that are involved in sensing toxic compounds are expressed in bitter‐sensing GRNs 5.
Knockout studies for Gr genes have identified nine Gr genes required for sensing bitter compounds so far. Among these, three GRs, GR32a, GR33a, and GR66a, are expressed in all bitter‐sensing GRNs in the labellum and function broadly in the avoidance of various bitter compounds 5, 15, 16. In contrast, the other six known bitter‐sensing Grs are required for detecting relatively specific bitter compounds 17, 18, 19, 20, 21, 22, 23, 24.
Here, we report that flies avoid saponin‐laced food by dual mechanisms. One is direct activation of bitter GRNs. The other may be sugar inhibition of sweet GRNs. We screened 26 Gr mutants, including the broadly required Grs. We found that of these 26 mutants, only Gr28b was required for sensing saponin. To our knowledge, this is the first case where the three known broadly required Grs are dispensable for sensing a bitter compound. Gr28b has five alternative isoforms, with one of the five isoforms, Gr28b.d, known to have a role to sense temperature 25. This is the first report of a function of Gr28b in taste sensation. In addition, we identified the specific Gr28b isoform, Gr28b.c, which is essential for sensing saponin.
Results
Flies avoid saponin by dual mechanisms
To determine whether saponin can inhibit feeding, we carried out a binary food choice assay 16. Flies were allowed to choose between 1 mM sucrose and 5 mM sucrose that has been adulterated with different concentrations of saponin. Almost all flies preferred 5 mM sucrose to 1 mM sucrose (Fig 1A). However, when the 5 mM sucrose was contaminated with saponin, flies began to avoid the saponin‐laced food in a concentration‐dependent manner. This suggests that saponin acts as an antifeedant like caffeine and strychnine. Next, we determined the concentration of saponin that is necessary to induce action potentials from the dendritic tip of S6 sensilla (Fig 1B and C). We obtained enough spikes upon application of 0.1% saponin (Fig 1B and C); however, even 0.01% saponin induced ~ 10 spikes per second in these sensilla (Fig 1B and C). Application of 1% saponin produced a greater number of spikes than 0.1% saponin. Most bitter compounds have dual functions by inhibiting sugar responses, so we tested whether saponin also inhibits sugar responses 26. Application of 100 mM sucrose produces a robust response from L4 sensilla, eliciting ~ 103 spikes per second. We found that saponin suppressed the sucrose response in a dose‐dependent manner (Fig 1D and E). The number of spikes by sucrose was significantly reduced by concentrations of 0.1% saponin and higher (Fig 1D and E). This indicates that avoidance of saponin is carried out by dual mechanisms: directly by activation of bitter‐sensing sensilla and indirectly by inhibiting responses to sugar stimuli.
Figure 1. Behavioral avoidance and electrophysiology to saponin.
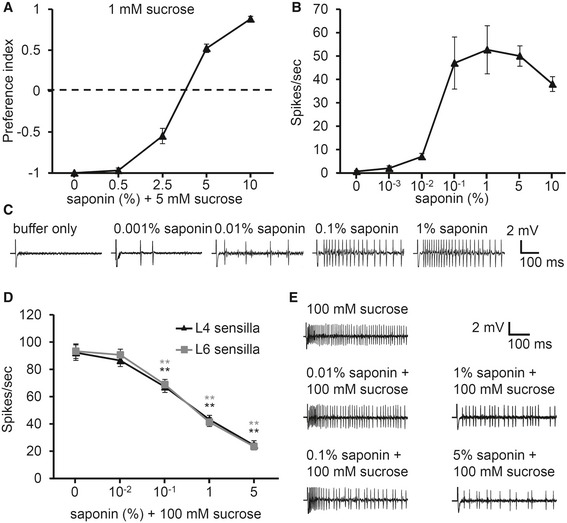
- Dose–response binary food choice assay of saponin (n ≥ 4).
- Dose–response curve of tip recordings using the indicated concentrations of saponin on S6 sensilla (n ≥ 8).
- Sample traces of saponin‐induced action potentials in (B).
- Dose–response curve for sugar inhibition using the indicated concentrations of saponin with 100 mM sucrose on L4 sensilla (n ≥ 8).
- Sample traces from (D).
Gr28b mutant has deficit to avoid saponin
In Drosophila, Gr genes mediate sensitivity to bitter compounds 7. To identify the GR required for sensing saponin, we performed an unbiased screen of 26 Gr mutants in a binary food choice assay with 5% saponin (Fig 2A). Historically, all bitter sensation has been shown to be at least partially mediated by one of the three broadly required Gr mutants: ΔGr32a, Gr33a 1, and Gr66a ex83. Therefore, we were surprised to find that these mutants had normal behavior to saponin. Instead, out of the mutants screened, only Gr28b Mi showed a strong defect in saponin avoidance (Fig 2A). We also confirmed the saponin avoidance without sucrose with control and mutant (Appendix Fig S1A). This is interesting since Gr28b has not previously been shown to have a role in taste. Furthermore, we found that Gr10a and Gr28a mutants have normal avoidance to saponin (Fig 2A), although these two genes are known to induce saponin‐induced action potentials from the sensilla, which are not activated by saponin in wild‐type flies22. We also confirmed that Gr10a and Gr28a mutants have normal responses to saponin in electrophysiology (Appendix Fig S1B). When we tested various concentrations of saponin with control, Gr28b Mi, and transheterozygotes of Gr28b Mi and Gr28b Deficiency strain (Gr28b Mi/Gr28b Df), we found that the mutants had a significant defect avoiding 5 and 10% saponin (Fig 2B). These results led us to consider whether Gr28b Mi mutant flies may ingest more saponin than wild‐type flies. To test this, we fed adult control and Gr28b Mi flies standard cornmeal with or without 5% saponin. After allowing the flies to feed for 15, 30, 60, and 240 min with the two different foods mixed with blue dye, we performed ingestion assays by calculating the difference in absorbance between normal cornmeal food and saponin‐laced cornmeal food (Fig 2C and Materials and Methods in detail). An ingestion index (I.I.) value of 0 means that flies ate the same amount of two different foods, while −1.0 or 1.0 indicates that flies ate only cornmeal food without saponin or with saponin, respectively. As expected, control flies ate much more standard cornmeal food without saponin, so the I.I. values for this group were negative for the tested period. However, we found that Gr28b Mi flies did not appear to discriminate between food with or without 5% saponin and ate similar amounts of both types of food throughout almost the entire testing period (Appendix Fig S1C). The I.I. values between control and Gr28b Mi flies were significantly different for the testing period. The binary food choice assay, as well as the ingestion assay, supports the idea that Gr28b is required for sensing saponin. To further analyze which gustatory organs are necessary for saponin avoidance, we performed a proboscis extension response (PER) assay after presenting sucrose or sucrose + saponin as a stimulus to the legs (Fig 2D) or labella (Fig 2E). When the sucrose + saponin stimulus was presented to the legs, control and Gr28b Mi flies exhibited a PER comparable to sucrose only, with control flies showing only a small reduction in PER in the first presentation but normal PER in the second presentation (Fig 2D). This is consistent with the previous finding that saponin does not activate any sensilla in the legs 4. However, when saponin was presented to the labella, control flies did not extend their proboscis, while Gr28b Mi flies extended their proboscis at a similar level as sucrose only (Fig 2E). These behavioral assays further support the idea that Gr28b is indispensable for sensing saponin via the labella.
Figure 2. Gr28b was required for saponin‐mediated avoidance and ingestion.
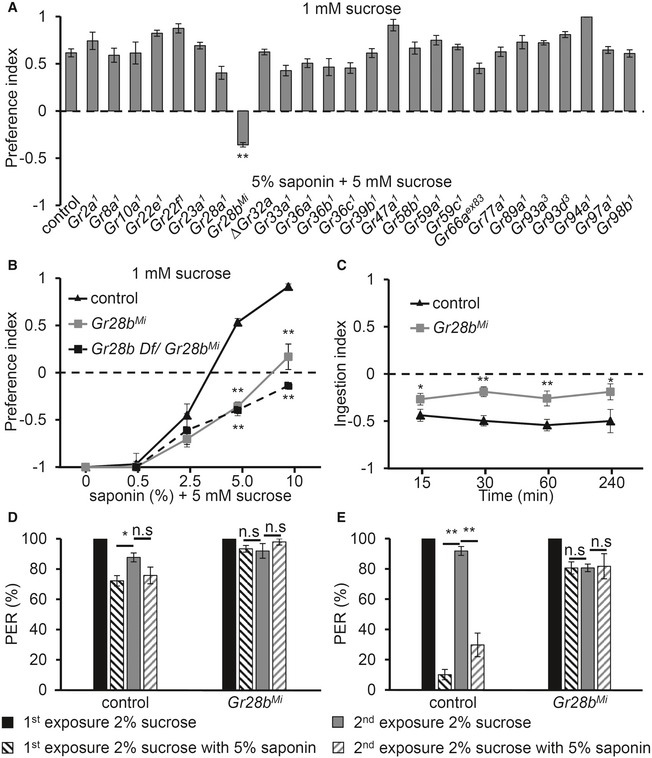
-
ABinary food choice assay with 26 Gr mutants for 5% saponin (n ≥ 4).
-
BConcentration‐dependent avoidance of saponin in control, Gr28b Mi, and Gr28b Df/Gr28b Mi (n ≥ 4).
-
CAmount of ingestion calculated at the indicated time points (15, 30, 60, and 240 min) using spectrophotogram analysis for control and 5% saponin‐containing food (n = 8).
-
D, EPER assay for control and Gr28b Mi flies in the (D) legs or (E) proboscis. Flies were initially given 2% sucrose, then 2% sucrose in combination with 5% saponin (n = 4).
The taste response to saponin in the bitter‐sensing GRNs is essential for avoidance
To further dissect which sensilla in the labellum respond to 5% saponin, we surveyed all the sensilla in control and Gr28b Mi flies with tip recordings. We found that six sensilla, S1, S3, S5, S6, S7, and S10 (Tanimura's nomenclature), showed strong responses to saponin (Fig 3A) 27. Other S‐type sensilla, except S4, S8, and S9, showed relatively mild, but robust responses (Fig 3A). In addition, most I‐type sensilla, except I7, I8, I9, and I10, did not produce robust action potentials to saponin (Fig 3A), and no L‐type bristles produced any action potentials upon application of saponin. However, consistent with our behavioral results, Gr28b Mi flies showed significantly reduced responses from any of the sensilla to 5% saponin and from S6 sensilla at different ranges of saponin concentration (Fig 3A and B, and Appendix Fig S1D). This indicates that Gr28b is essential in the labella to sense saponin. However, we found that saponin suppressed the sucrose response of wild‐type and Gr28b Mi flies at the similar level (Appendix Fig S1E). This indicates that Gr28b has a role in only bitter‐sensing GRNs. To test any possible hemolytic effect of saponin in GRNs, we also tested caffeine response after wild‐type and Gr28b Mi flies were exposed to saponin (Appendix Fig S1F). We detected normal level of caffeine response in both wild‐type and Gr28b Mi flies. We could exclude any damage to GRNs by saponin‐like surfactant. Next, we decided to test the specificity of Gr28b. When we tested the ability of Gr28b Mi to respond to other bitter compounds, we found that this mutant exhibited normal electrophysiological responses to 12 well‐known bitter compounds, including sodium dodecyl sulfate (SDS) (Fig 3C). We additionally found that SDS sensation is mediated by broadly tuned Grs, including Gr32a, Gr33a, and Gr66a in avoidance as well as tip recording (Appendix Fig S2A and B). To confirm if saponin sensation is mediated via bitter‐sensing GRNs, we expressed cell death gene, hid in bitter‐sensing GRNs under the control of Gr33a‐GAL4 as well as Gr66a‐GAL4, both of which induce GAL4 expression in 22 bitter‐sensing GRNs (Fig 3D) 15. We found that cell ablation of bitter‐sensing GRNs no longer avoids saponin, compared with control and parent strains (Fig 3D). Furthermore, we checked any residual response from S‐type sensilla from the same bitter cell ablated flies (Fig 3E). We found that saponin‐induced action potentials are almost completely absent in UAS‐hid/Gr66a‐GAL4 flies (Fig 3E). This indicates that saponin sensation is mediated via bitter‐sensing GRNs.
Figure 3. S‐type and I‐type sensilla in labellum‐mediated saponin avoidance.
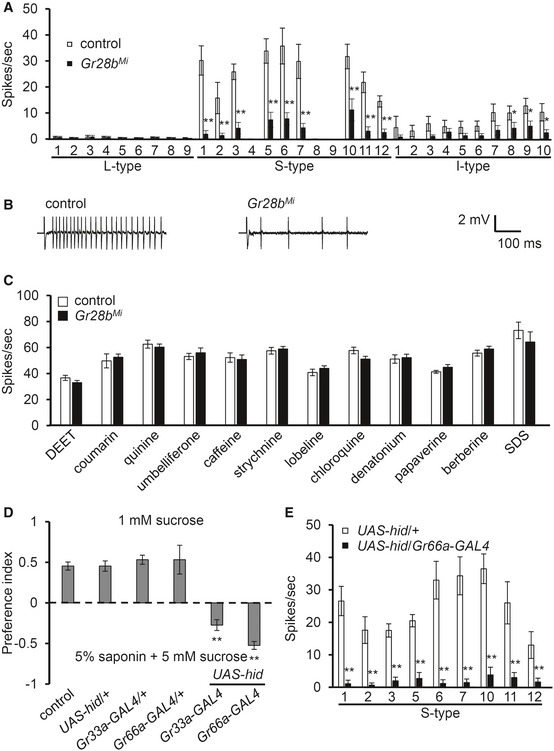
- Mapping analysis of all the sensilla following stimulation with 5% saponin in control and Gr28b Mi flies (we followed Tanimura's nomenclature) (n ≥ 8).
- Sample traces of saponin‐induced action potentials on S6 sensilla.
- Tip recording analysis of S6 sensilla from control and Gr28b Mi flies. Stimuli used are 0.2% DEET, 1.0 mM coumarin, 0.5 mM quinine, 10 mM umbelliferone, 10 mM caffeine, 0.3 mM strychnine, 0.3 mM lobeline, 0.1 mM chloroquine, 0.2 mM denatonium, 0.5 mM papaverine, 0.1 mM berberine, and 0.1% SDS (n ≥ 7).
- Binary food choice assays were conducted after GRNs were ablated by expressing the cell death gene (hid), under Gr33a‐GAL4 or Gr66a‐GAL4. All heterozygote controls (UAS‐hid/+, Gr33a‐GAL4/+, and Gr66a‐GAL4/+) are shown (n ≥ 4).
- Tip recording analysis of the indicated S‐type sensilla and genotypes (n ≥ 7).
GR28b (C) is a receptor for saponin
The Gr28b locus encodes five isoforms such as GR28b (A), GR28b (B), GR28b (C), GR28b (D), and GR28b (E) with its own 5′ regulatory region and unique first exons, which are spliced to three common exons (Fig 4A) 13. Gr28b is involved in light avoidance in class IV dendritic arborization neurons in the body wall 28, and Gr28b.d controls rapid warmth avoidance 25. So, the functions of the other four Gr28b genes remains elusive, and it is still currently unknown which isoform of Gr28b is required for light avoidance. We confirmed that Gr28b Mi is a null mutant by performing reverse transcription–polymerase chain reaction (RT–PCR) for the common exons of the Gr28b isoform, with tubulin used as a control for the reaction (Fig 4B). To determine which specific Gr28b isoform is involved in sensing saponin, we expressed each UAS‐Gr28b isoform under the control of Gr28b.a or Gr28b.c‐GAL4 (Fig 4C and D). The Gr28b.a‐GAL4 drives expression of each downstream gene of UAS in all S‐type sensilla and three I‐type sensilla (I8, I9, and I10) in the labellum, which is narrowly expressed GAL4 compared with Gr66a‐GAL4 in the labellum 5. We found that only Gr28b.c recovered the defect of Gr28b Mi flies for behavioral avoidance (Fig 4C) and electrophysiological responses (Fig 4D). This suggests that only the GR28b (C) isoform out of the five isoforms forms part of the receptor for saponin. We also rescued the defect of Gr28b Mi flies using Gr28b.c‐GAL4 (Fig 4C and D). This indicates that Gr28b.c‐GAL4 is expressed in bitter‐sensing GRNs. We indeed investigated no avoidance to saponin and no saponin‐induced action potential with the induction of apoptosis using Gr28b.c‐GAL4 (Fig 5A and B). To further investigate the expression of Gr28b.c, we analyzed Gr28b.c‐GAL4 reporter after crossing with UAS‐DsRed (Fig 5C). The Gr28b.c‐GAL4 reporter overlapped partially with Gr66a‐I‐GFP reporter, which was ubiquitously expressed in all the bitter‐sensing GRNs, and labeled all the S‐type sensilla, half of I‐type sensilla, and other unidentified sensilla (Fig 5C). Thus, we conclude that Gr28b.c is indeed required in saponin‐responsive GRNs. However, when we performed tip recordings from I‐type sensilla or L‐type sensilla after expressing Gr28b.c under the control of Gr33a‐GAL4 or Gr5a‐GAL4 respectively, we did not see responses to 0.1% saponin, indicating that GR28b (C) cannot recapitulate the saponin receptor in other bitter‐sensing or sugar‐sensing neurons (Appendix Fig S3A and B) 22. This indicates that, like other bitter‐sensing receptors, other gustatory receptors may be required in addition to GR28b (C) to form a functional saponin receptor 23, 24. Furthermore, we could not find any significant increase in action potentials elicited by saponin from I‐a (I2, I3, and I4)‐ and I‐b (I9, and I10)‐type sensilla by misexpression of Gr10a or Gr28a driven by Gr33a‐GAL4 (Appendix Fig S3B) 22.
Figure 4. The structure of Gr28b genome and specific requirement of GR28b(C) for saponin‐mediated avoidance and electrophysiology.
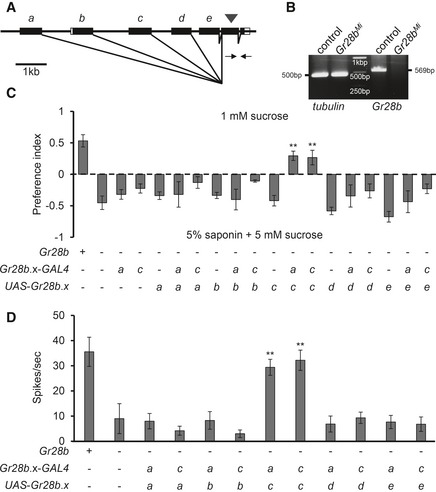
-
AThe genomic structure of Gr28b. It is composed of five different forms by alternative splicing. The arrowhead indicates the P‐element insertion. White boxes indicate predicted untranslated regions in flybase. Arrows indicate primers for RT–PCR.
-
BRT–PCR analysis with the indicated primers in (A) for control and Gr28b Mi flies with tubulin as a control. From left to right, the band of tubulin of wild‐type, tubulin of Gr28b Mi, DNA ladder, Gr28b of wild‐type, and Gr28b of Gr28b Mi flies.
-
C, DEach isoform from Gr28b.a to Gr28b.e was driven by Gr28b.a‐GAL4 or Gr28b.c‐GAL4 in a Gr28b Mi background. Error bars represent the standard error of the mean (SEM). The asterisks indicate significant differences from that of the Gr28b mutant detected by a single‐factor ANOVA with Scheffe's analysis as a post hoc test to compare two sets of data (**P < 0.01). (C) Binary food choice assay for recovery experiments with each of the five alternative Gr28b isoforms using the GAL4/UAS systems (n ≥ 4). (D) Saponin‐induced action potential frequencies from the indicated transgenic flies on S6 sensilla (n = 7).
Figure 5. The expression of Gr28b.c‐GAL4 reporter in bitter‐sensing GRNs.
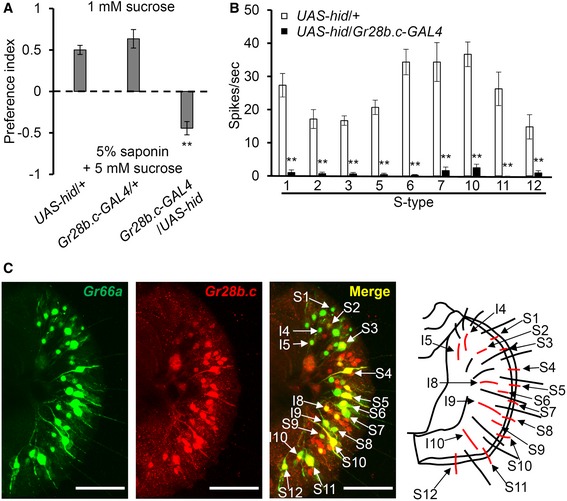
- Binary food choice assays were conducted after GRNs were ablated by expressing the cell death gene (hid), under Gr28b.c‐GAL4 (n = 4).
- Tip recording analysis of the indicated S‐type sensilla and genotypes (n ≥ 7).
- Expression of the Gr28b.c‐GAL4 reporter in subsets of Gr66a‐positive GRNs. First panel shows that all bitter‐responsive GRNs are labeled by Gr66a‐I‐GFP (anti‐GFP, green). Second panel shows the expression of the Gr28b.c‐GAL4, crossed with UAS‐DsRed (anti‐DsRed, red). Third panel shows merged image of first and second panels. Forth panel shows a schematic diagram of sensilla arrangement. Red‐marked sensilla indicate the expression of Gr28b.c‐GAL4 reporter in second panel. Scale bars represent 40 μm.
Saponin is an insecticide
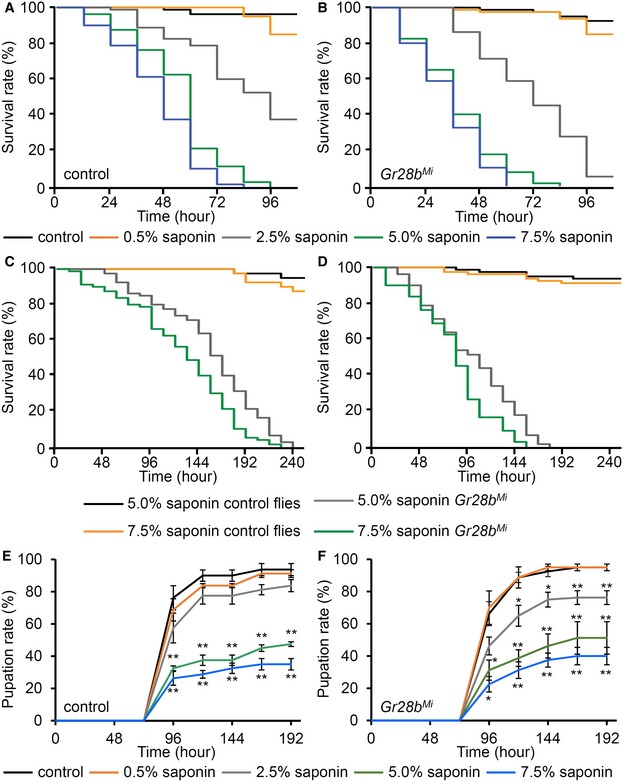
Previous results suggest that saponin can be used as an antifeedant. Some antifeedants play dual roles and can also act as insecticides 16. To determine whether saponin is an insecticide, we examined the survival rate of adult flies when fed on five different concentrations of saponin: 0, 0.5, 2.5, 5.0, and 7.5% saponin. We found that 5.0 and 7.5% saponin clearly killed control and Gr28b Mi flies, while 0 and 0.5% saponin did not affect the survival rate of control or mutant flies (Fig 6A and B, and Appendix Table S1). The LT50s of control and Gr28b Mi flies after feeding on 7.5% saponin were 48 (± 2.74) h and 36 (± 2.40) h, respectively. The Gr28b Mi flies died much quicker than controls when fed 2.5 and 5.0% saponin, which is likely due to their inability to detect and avoid ingesting saponin‐laced food (Fig 2C). To further verify that saponin can act as an insecticide, we allowed the flies to choose between 100 mM fructose alone and 100 mM or 200 mM fructose mixed with 5% or 7.5% saponin (Fig 6C and D). Almost all of the control survived for 10 days, but Gr28b Mi flies showed high levels of lethality in both cases (Fig 6C and D, and Appendix Table S2). The findings support the idea that feeding on saponin induces lethality. Next, we examined whether it also causes lethality during the larval stage. To test this, we collected first‐instar larva and reared them on the same five different concentrations of saponin. In our experimental conditions, wild‐type larvae started pupation 4 days after transfer, and 93.8% of the larvae successfully pupated when raised on control food (Fig 6C). We found that only 83.8% of the larvae pupated after feeding on 2.5% saponin, indicating that the other 16.2% of the larvae died (Fig 6E). Less than 50% of the larvae fed 5.0–7.5% saponin survived to pupation (Fig 6E). This effect was comparable in Gr28b Mi larvae (Fig 6F).
Figure 6. Saponin toxicity during adult stage and larval growth.
-
A, BSurvival rate of (A) control and (B) Gr28b Mi adult raised on control and the indicated concentrations of saponin‐containing cornmeal diet (n = 4). Statistics and LD50 are presented in Appendix Table S1.
-
C, DSurvival rate based on binary food choice. Control and Gr28b Mi were allowed to feed 100 mM fructose versus 100 mM (C) or 200 mM fructose (D) mixed with 5 or 7.5% saponin (n = 4). Statistics and LD50 are presented in Appendix Table S2.
-
E, FCumulative pupation rate of (E) control and (F) Gr28b Mi larvae raised on the indicated saponin‐containing food as well as control food (n = 4). The error bars represent SEMs. The asterisks indicate significant differences from normal diet fed, which were detected by a single‐factor ANOVA with Scheffe's analysis as a post hoc test to compare two sets of data (*P < 0.05, **P < 0.01).
Discussion
We extensively studied the role of saponin in taste, ingestion, and survival. Saponin is a well‐known supplement that has been shown to have benefits to humans. However, insects, such as fruit flies, avoid saponin because it acts as an insecticide as well as an antifeedant. One of the surprising findings in this study is that only the Gr28b mutant from 26 tested mutants is required for sensing saponin, but other well‐known broadly required Grs, including Gr32a, Gr33a, and Gr66a, are not required. For example, Gr33a mutant flies have deficits in sensing most bitter compounds, such as caffeine, DEET, strychnine, lobeline, denatonium, chloroquine, and others, with the exception of L‐canavanine 16, 23, 24. However, we failed to recapitulate the saponin receptor using expression of Gr28b.c in sugar neurons, suggesting that more Gr genes may be required to form a functional saponin receptor and for saponin avoidance. This finding also suggests that there may be other broadly required Grs in the fly genome. In this study, we used relatively lower concentration of saponin in electrophysiology than behavioral assay. A cellular taste coding mechanism, called the labeled line model, would apply in cases in which distinct taste qualities, are each sensed by different receptor cells, which communicates, uninterrupted to the brain. This differs from a distributive model, in which taste receptor cells respond to multiple types of taste qualities to varying amounts, and the brain then interprets the taste quality based on the intensity of the repertoire of the cellular activities 29, 30, 31. Detection of saponin in food, through Gr28b.c leads to a negative behavioral valence—gustatory repulsion. So depending on the concentration of a positive valence, the effect of saponin is indeed repulsive or masked in behavior. In our behavioral assay, we used fivefold higher concentration of sugar mixed with saponin, compared with the other side, so the negative valence of saponin should be enough to overcome fivefold positive valence. Indeed, we found that much lower concentration of saponin is enough to induce avoidance without sucrose.
Another common feature of bitter compounds is that they inhibit feeding in flies through dual mechanisms: activation of bitter GRNs and inhibition of sugar GRNs. However, saponin‐induced inhibition of sugar GRNs is not dependent on Gr28b, since this effect is not attenuated in Gr28b Mi mutant flies. The mechanism through which saponin inhibits sugar GRNs remains to be determined. In the case of other bitter compounds, they inhibit sugar GRNs through two mechanisms: Stimulation of bitter GRNs activate GABAergic interneurons, which then inhibit sweet GRNs 32, and bitter compounds bind with an odorant‐binding protein in the endolymph, which in turn inhibits sugar‐activated gustatory receptors 33.
The Gr28b cluster has multiple functions to avoid harmful stimuli in nature, such as bright light and too warm environments 25, 28. Here, we show a new function of the gene in taste. Gr28b.c may contribute to the detection of changes in the viscosity or surface tension of the solutions containing a surfactant. Since the mixture of saponin tested might have hemolytic features, it is possible that saponin would directly harm the taste neurons and that this noxious effect could be detected by GR28b (C). However, this is not a case, because Gr28b mutant shows normal response to SDS. GR28b (C) is specifically essential for saponin sensation. We also tested normal electrophysiological response to caffeine after saponin application. So saponin does not have such an abrupt hemolytic feature in taste.
The insecticidal effect of saponin is intriguing because it caused lethality in the larval as well as the adult stage. It would be interesting to examine whether saponin could be used as an effective insecticide for harmful pests, such as mosquitoes, especially since it is not harmful for humans.
Materials and Methods
Fly stocks
We previously obtained and studied the following mutants from the Bloomington Stock Center: Gr2a 1, Gr8a 1, Gr10a 1, Gr22e 1, Gr22f 1, Gr23a 1, Gr28b Mi, Gr33a 1, Gr36b 1, Gr36c 1, Gr47a 1, Gr58b 1, Gr59a 1, Gr66a ex83, Gr77a 1, Gr93a 3, Gr93d 1, Gr94a 1 , Gr97a 1, and Gr98b 1 15, 17, 34. We also obtained the following mutants from the GIST Laboratory Animal Resource Center: Gr36a 1, Gr39b 1, Gr59c 1, Gr89a 1 35. H. Amrein, S.J. Moon, and K. Kang respectively provided the ΔGr32a 36, UAS‐Gr28a and the following UAS‐Gr28b 25: Gr28b Mi; UAS‐Gr28b.a, Gr28b Mi; UAS‐Gr28b.b, Gr28b Mi; UAS‐Gr28b.c, Gr28b Mi; UAS‐Gr28b.d, Gr28b Mi; UAS‐Gr28b.e, Gr28b Mi. We generated UAS‐Gr10a for an other study (Rimal S and Lee Y submitted). Gr28b.a‐GAL4 was provided by J.Y. Kwon 37. Gr28b.c‐GAL4 was provided by H. Amrein 38. We used w 1118 as the “wild‐type” control.
Chemical sources
Sucrose (CAS No 57‐50‐1, Cat No S9378), sulforhodamine B (CAS No 3520‐42‐1, Cat No 230162), saponin (CAS No 8047‐15‐2, Cat No S4531), caffeine (CAS No 58‐08‐2, Cat No C0750), strychnine (CAS No 1421‐86‐9, Cat No S8753), quinine (CAS No 6119‐47‐7, Cat No Q1125), lobeline (CAS No 134‐63‐4, Cat No 141879), papaverine (CAS No 61‐25‐6, Cat No P3510), denatonium (CAS No 6234‐33‐6, Cat No D5765), umbelliferone (CAS No 93‐35‐6, Cat No H24003), and coumarin (CAS No 91‐64‐5, Cat No C4261) were purchased from Sigma‐Aldrich Company (St. Louis, MO, USA). Brilliant Blue FCF (CAS No 3844‐45‐9, Cat No 027‐12842) and berberine (CAS No 141443‐60‐5, Cat No 020‐05502) were obtained from Wako Pure Chemical Industry, Ltd (Osaka, Japan).
RT–PCR analysis
Whole‐fly mRNA from wild‐type and Gr28b Mi flies was extracted (TRIzol), and AMV reverse transcription was used to generate cDNAs (Promega). We used the following primers: 5′‐GTGTTGAAGAACCTAGCC‐3′ and 5′‐CCGCTGATCGTGAAATAC‐3′ for Gr28b and 5′‐TCCTTGTCGCGTGTGAAACA‐3′ and 5′‐CCGAACGAGTGGAAGATGAG‐3′ for tubulin.
Binary food choice assay
We performed binary food choice assays as described 34, 39. At first, 40–50 flies (3‐ to 6‐day‐old) were starved for 18 h on 1% agarose. We prepared two mixtures with each dye and distributed the mixtures in a zigzag pattern. One mixture contained 1% agarose and the indicated concentration of saponin and 5 mM sucrose with red dye (sulforhodamine B, 0.2 mg/ml), and the other mixture contained 1% agarose and 1 mM sucrose with blue dye (Brilliant Blue FCF, 0.125 mg/ml). Flies were transferred to a prepared 72‐well microtiter dish, which was placed in a dark, humid chamber. After feeding, flies were sacrificed by freezing at −20°C, and then, we segregated them by analyzing their abdomen colors under the microscope for the presence of red, blue, or purple dye. The preference index (P.I) was calculated using the following equation: (NB − NR)/(NR + NB + NP) or (NR − NB)/(NR + NB + NP), depending on the dye/tastant combinations. P.I.s of −1.0 or 1.0 indicated a complete preference to either 5 mM sucrose with saponin or 1 mM sucrose alone, respectively. A P.I. of 0.0 indicated no bias between the two food alternatives.
Tip recording assay
Tip recordings were carried out based on Tanimura's nomenclature. The average action potential frequencies (spikes/s) evoked in response are shown, and only spikes evoked at 50–550 ms were counted. We conducted tip recordings as previously described, using the indicated concentration of saponin in distilled water with 1 mM KCl or 30 mM tricholine citrate (TCC) for the assay 34. The 1 mM KCl or 30 mM TCC functions as the electrolyte for recording. For the recordings, we first immobilized 3‐ to 7‐day‐old flies by placing them on ice. We then inserted a reference glass electrode filled with Ringer's solution through the back thorax and passed it through the proboscis. We stimulated the sensilla on the labial palp with a compound dissolved in the buffer solution of the recording pipette (10–20 μm tip diameter). The recording electrode was connected to a pre‐amplifier (Taste PROBE, Syntech, Germany), which amplified the signals 10× using a signal connection interface box (Syntech) in conjunction with a 100–3,000 Hz band‐pass filter. Recorded action potentials were acquired using a 12 kHz sampling rate and analyzed using Autospike 3.1 software (Syntech). We then performed recordings on the indicated sensilla on the labial palp.
Ingestion assay
The ingestion assay was carried out as previously described 37 with a slight modification. Without starvation, 3‐ to 6‐day‐old flies were allowed to feed on 0.1% (w/v) Brilliant Blue FCF food with 5% saponin for the indicated times. After feeding control diet and saponin‐containing diet at the same time, they were sacrificed by freezing at −20°C. We transferred six flies (three males and three females) into individual 1.5‐ml epi‐tubes filled with 200 μl PBST (1× PBS with 0.2% Triton X‐100). The flies were completely ground, then 800 μl of PBST was added and blended. The tube was centrifuged for 5 min at 15,871 g force at 4°C. Subsequently, supernatant was loaded in cuvettes for spectrophotogram. The supernatant was measured at 630 nm, and PBST alone was used as the blank control. The ingestion index (I.I.) was calculated based on the difference in optical density (O.D.) between control‐diet and saponin‐diet groups. The formula of I.I is (O.D.saponin‐diet‐O.D.control‐diet)/(O.D.control‐diet). Each experiment was carried out eight times.
Survival assay
For the survival assay, twenty 2‐ to 4‐day‐old flies (10 male and 10 female flies) were placed in standard cornmeal food vials containing the indicated concentrations of saponin and kept at 25°C in a 60% humidity chamber. Dead flies were counted every 12 h and transferred into new vials. For the survival assay based on binary food choice in Fig 5C and D, flies introduced into egg‐laying petri dishes were allowed to choose between 100 mM fructose versus 100 mM or 200 mM fructose and 5 or 7.5% saponin. Each experiment was carried out four times.
Proboscis extension response assay
Proboscis extension response assay was performed as previously described 19 with slight modification. Twenty flies (3‐ to 6‐day‐old) were starved for 18 h in vials containing 1% agarose. For the PER assay, we used two different fixing methods depending on where the stimulus was presented. For leg stimulation, the wings of the flies were fixed on a slide glass and the flies were recovered in a humidified incubator for at least 1 h. For labellum stimulation, each fly was immobilized in a yellow pipette tip and the head was exposed by cutting the edge of the tip. Two percent sucrose was given for the initial stimuli. If the flies did not respond to sucrose, they were excluded from the experiment. The test stimulus used in the PER assay was 5% saponin with 2% sucrose applied to Kim‐wipe paper wicks as medium for presenting the tastant stimuli. Wet wicks stimulated proboscis extension through contact of only forelegs or labellum. Second exposures were repeated using the same conditions as the initial exposures. More than 10 flies were used for one test. Each experiment was carried out ≥ 4 times.
Immunohistochemistry
Immunohistochemistry was performed as previously described 21. The labella of Gr66a‐I‐GFP, UAS‐DsRed/+; Gr28b.c‐GAL4/+ flies were dissected. 4% paraformaldehyde was used for fixing in PBST (1× PBS with 0.2% Triton X‐100) for 15 min at room temperature. The fixed labella were washed three times with PBST for 20 min each. Labella were cut in half and blocked with 0.5% goat serum in PBST for 30 min at room temperature. They were transferred to primary antibody buffer [blocking buffer containing the primary antibodies, which are mouse anti‐GFP (Molecular Probe, 1:1,000) and rabbit anti‐DsRed (Clontech 1:1,000)] for overnight at 4°C. They were washed three times with PBST. Labella were transferred to secondary antibody buffer (blocking buffer containing the secondary antibodies, which are goat anti‐mouse Alexa Fluor 488 and goat anti‐rabbit Alexa Fluor 568) for 1 h at room temperature. Tissues were washed with PBST and mounted in mount buffer (37.5% glycerol, 187.5 mM NaCl, 62.5 mM Tris pH 8.8). The samples were viewed using Nikon's C1 Digital Eclipse Modular Confocal Microscope.
Pupation assay
For collecting larvae, 3‐ to 6‐day‐old female flies were allowed to lay eggs for 12 h. After 1 day, we transferred 20 larvae into vials containing standard cornmeal food with or without various concentrations of saponin. Pupation rate was normalized by this formula: (pupation number of saponin‐diet)/(pupation number of control‐diet). Control‐diet pupation value was divided by 20 control‐diet larvae.
Statistical analysis
All error bars represent the standard error of the means (SEMs). With the exception of the survival assay, single‐factor analysis of variance (ANOVA) with Scheffe's analysis as a post hoc test was used to compare multiple sets of data. Asterisks indicate statistically significant differences (*P < 0.05, **P < 0.01). For the survival assay, statistics followed a Kaplan–Meier analysis. Log‐rank values were calculated by comparing with each experiment.
Author contributions
YL and JS conceived and designed the experiments. JS and RS performed and analyzed all the data. YL and JS wrote the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Appendix
Review Process File
Acknowledgements
We thank Dr. Melissa Fowler for comments. We thank J. Kwon, S.J. Moon, and K. Kang for Gr28b.a‐GAL4, UAS‐Gr28a, and UAS‐Gr28b lines, respectively. This work is supported by grants to Y. L. from the Basic Science Research Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF‐2016R1D1A1B03931273 and NRF‐2018R1A2B6004202). S.R. was supported by the Global Scholarship Program for Foreign Graduate Students at Kookmin University in Korea.
EMBO Reports (2019) 20: e47328
References
- 1. Dinda B, Debnath S, Mohanta BC, Harigaya Y (2010) Naturally occurring triterpenoid saponins. Chem Biodivers 7: 2327–2580 [DOI] [PubMed] [Google Scholar]
- 2. De Geyter E, Lambert E, Geelen D, Smagghe G (2007) Novel advances with plant saponins as natural insecticides to control pest insects. Pest Technol 1: 96–105 [Google Scholar]
- 3. Pelah D, Abramovich Z, Markus A, Wiesman Z (2002) The use of commercial saponin from Quillaja saponaria bark as a natural larvicidal agent against Aedes aegypti and Culex pipiens . J Ethnopharmacol 81: 407–409 [DOI] [PubMed] [Google Scholar]
- 4. Ling F, Dahanukar A, Weiss LA, Kwon JY, Carlson JR (2014) The molecular and cellular basis of taste coding in the legs of Drosophila . J Neurosci 34: 7148–7164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weiss LA, Dahanukar A, Kwon JY, Banerjee D, Carlson JR (2011) The molecular and cellular basis of bitter taste in Drosophila . Neuron 69: 258–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liman ER, Zhang YV, Montell C (2014) Peripheral coding of taste. Neuron 81: 984–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee Y, Poudel S (2014) Taste sensation in Drosophila melanogaster . Hanyang Med Rev 34: 130–136 [Google Scholar]
- 8. Joseph RM, Sun JS, Tam E, Carlson JR (2017) A receptor and neuron that activate a circuit limiting sucrose consumption. Elife 6: e24992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. LeDue EE, Chen Y‐C, Jung AY, Dahanukar A, Gordon MD (2015) Pharyngeal sense organs drive robust sugar consumption in Drosophila . Nat Commun 6: 6667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen Y‐CD, Dahanukar A (2017) Molecular and cellular organization of taste neurons in adult Drosophila pharynx. Cell Rep 21: 2978–2991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Clyne PJ, Warr CG, Carlson JR (2000) Candidate taste receptors in Drosophila . Science 287: 1830–1834 [DOI] [PubMed] [Google Scholar]
- 12. Thorne N, Chromey C, Bray S, Amrein H (2004) Taste perception and coding in Drosophila . Curr Biol 14: 1065–1079 [DOI] [PubMed] [Google Scholar]
- 13. Dunipace L, Meister S, McNealy C, Amrein H (2001) Spatially restricted expression of candidate taste receptors in the Drosophila gustatory system. Curr Biol 11: 822–835 [DOI] [PubMed] [Google Scholar]
- 14. Wang Z, Singhvi A, Kong P, Scott K (2004) Taste representations in the Drosophila brain. Cell 117: 981–991 [DOI] [PubMed] [Google Scholar]
- 15. Moon SJ, Lee Y, Jiao Y, Montell C (2009) A Drosophila gustatory receptor essential for aversive taste and inhibiting male‐to‐male courtship. Curr Biol 19: 1623–1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee Y, Kim SH, Montell C (2010) Avoiding DEET through insect gustatory receptors. Neuron 67: 555–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee Y, Moon SJ, Montell C (2009) Multiple gustatory receptors required for the caffeine response in Drosophila . Proc Natl Acad Sci USA 106: 4495–4500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Poudel S, Kim Y, Kim YT, Lee Y (2015) Gustatory receptors required for sensing umbelliferone in Drosophila melanogaster . Insect Biochem Mol Biol 66: 110–118 [DOI] [PubMed] [Google Scholar]
- 19. Poudel S, Lee Y (2016) Gustatory receptors required for avoiding the toxic compound coumarin in Drosophila melanogaster . Mol Cells 39: 310–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee Y, Moon SJ, Wang Y, Montell C (2015) A Drosophila gustatory receptor required for strychnine sensation. Chem Senses 40: 525–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee Y, Kang MJ, Shim J, Cheong CU, Moon SJ, Montell C (2012) Gustatory receptors required for avoiding the insecticide L‐canavanine. J Neurosci 32: 1429–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Delventhal R, Carlson JR (2016) Bitter taste receptors confer diverse functions to neurons. Elife 5: e11181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shim J, Lee Y, Jeong YT, Kim Y, Lee MG, Montell C, Moon SJ (2015) The full repertoire of Drosophila gustatory receptors for detecting an aversive compound. Nat Commun 6: 8867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Poudel S, Kim Y, Kwak J, Jeong S, Lee Y (2017) Gustatory receptor 22e is essential for sensing chloroquine and strychnine in Drosophila melanogaster . Insect Biochem Mol Biol 88: 30–36 [DOI] [PubMed] [Google Scholar]
- 25. Ni L, Bronk P, Chang EC, Lowell AM, Flam JO, Panzano VC, Theobald DL, Griffith LC, Garrity PA (2013) A gustatory receptor paralogue controls rapid warmth avoidance in Drosophila . Nature 500: 580–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. French AS, Sellier M‐J, Agha MA, Guigue A, Chabaud M‐A, Reeb PD, Mitra A, Grau Y, Soustelle L, Marion‐Poll F (2015) Dual mechanism for bitter avoidance in Drosophila . J Neurosci 35: 3990–4004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hiroi M, Marion‐Poll F, Tanimura T (2002) Differentiated response to sugars among labellar chemosensilla in Drosophila . Zoolog Sci 19: 1009–1018 [DOI] [PubMed] [Google Scholar]
- 28. Xiang Y, Yuan Q, Vogt N, Looger LL, Jan LY, Jan YN (2010) Light‐avoidance‐mediating photoreceptors tile the Drosophila larval body wall. Nature 468: 921–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Erickson RP (2000) The evolution of neural coding ideas in the chemical senses. Physiol Behav 69: 3–13 [DOI] [PubMed] [Google Scholar]
- 30. Erickson RP (2008) A study of the science of taste: on the origins and influence of the core ideas. Behav Brain Sci 31: 59–75 [DOI] [PubMed] [Google Scholar]
- 31. Reiter S, Rodriguez CC, Sun K, Stopfer M (2015) Spatiotemporal coding of individual chemicals by the gustatory system. J Neurosci 35: 12309–12321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Charlu S, Wisotsky Z, Medina A, Dahanukar A (2013) Acid sensing by sweet and bitter taste neurons in Drosophila melanogaster . Nat Commun 4: 2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jeong YT, Shim J, Oh SR, Yoon HI, Kim CH, Moon SJ, Montell C (2013) An odorant‐binding protein required for suppression of sweet taste by bitter chemicals. Neuron 79: 725–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moon SJ, Köttgen M, Jiao Y, Xu H, Montell C (2006) A taste receptor required for the caffeine response in vivo . Curr Biol 16: 1812–1817 [DOI] [PubMed] [Google Scholar]
- 35. Sung HY, Jeong YT, Lim JY, Kim H, Oh SM, Hwang SW, Kwon JY, Moon SJ (2017) Heterogeneity in the Drosophila gustatory receptor complexes that detect aversive compounds. Nat Commun 8: 1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Miyamoto T, Amrein H (2008) Suppression of male courtship by a Drosophila pheromone receptor. Nat Neurosci 11: 874–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim H, Choi MS, Kang K, Kwon JY (2015) Behavioral analysis of bitter taste perception in Drosophila larvae. Chem Senses 41: 85–94 [DOI] [PubMed] [Google Scholar]
- 38. Thorne N, Amrein H (2008) Atypical expression of Drosophila gustatory receptor genes in sensory and central neurons. J Comp Neurol 506: 548–568 [DOI] [PubMed] [Google Scholar]
- 39. Meunier N, Marion‐Poll F, Rospars JP, Tanimura T (2003) Peripheral coding of bitter taste in Drosophila . J Neurobiol 56: 139–152 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Review Process File


