Summary
Cell biological studies have shown that protofilament number, a fundamental feature of microtubules, can correlate with the expression of different tubulin isotypes. However, it is not known if tubulin isotypes directly control this basic microtubule property. Here, we report high-resolution cryo-EM reconstructions (3.5–3.65Å) of purified human α1B/β3 and α1B/β2B microtubules and find that the β-tubulin isotype can determine protofilament number. Comparisons of atomic models of 13- and 14-protofilament microtubules reveal how tubulin subunit plasticity, manifested in ‘accordion-like’ distributed structural changes, can accommodate distinct lattice organizations. Furthermore, compared to α1B/β3 microtubules, α1B/β2B filaments are more stable to passive disassembly and against depolymerization by MCAK or chTOG, microtubule-associated proteins with distinct mechanisms of action. Mixing tubulin isotypes in different proportions results in microtubules with protofilament numbers and stabilities intermediate to those of isotypically pure filaments. Together, our findings indicate that microtubule protofilament number and stability can be controlled through β-tubulin isotype composition.
Introduction
Microtubules are polymers of α/β-tubulin heterodimers with diverse and essential roles in eukaryotic cells. Within each microtubule, tubulin heterodimers are aligned head-to-tail to form protofilaments that associate laterally to form a hollow, cylindrical polymer (Nogales, 2000). The number of protofilaments in a microtubule is a fundamental feature that controls the width of the filament and the configuration of the tracks for intracellular cargoes. When 13 protofilaments are present they align parallel to the microtubule’s long axis and provide linear tracks for cargoes (Amos and Schlieper, 2005). By contrast, in non-13 protofilament microtubules there is a super-helical twist of the protofilaments along the polymer’s long axis that results in spiral trajectories for cargoes (Chretien and Wade, 1991), which have been suggested to pose challenges to directional transport in dense cellular environments (Amos and Schlieper, 2005). Microtubules with 13 protofilaments are common in many cells that have been examined and are considered to be the typical arrangement (Tilney et al., 1973). Importantly, microtubules with a non-13 protofilaments (e.g. 14 or 15 protofilaments) have also been found in diverse cell types across different species (Chaaban and Brouhard, 2017). However, we do not understand how protofilament number is regulated.
Cell biological studies have revealed a correlation between tubulin isotype expression and microtubule protofilament number. The α- and β-tubulin gene families have expanded in eukaryotes and different tubulin isotypes have cell-type specific expression profiles (Ludueña and Banerjee, 2008). In C. elegans, multiple cell types (e.g. nerve cord and pharyngeal cells) have 11-protofilament microtubules, while touch receptor neurons have 15-protofilament microtubules (Chalfie and Thomson, 1982). It has been shown that the expression of specific α- and β-tubulin isotypes (i.e. MEC-12 and MEC-7) can alter protofilament number in these cells (Fukushige et al., 1999; Savage et al., 1989). When a moth β2-tubulin is ectopically expressed in Drosophila testes, the accessory microtubules in the axoneme change from having 13- to 16-protofilament architectures (Hoyle and Raff, 1990; Raff et al., 1997). However, it is unclear if different tubulin isotypes can directly specify microtubule protofilament number.
The ‘lattice accommodation’ model has been proposed to explain how identical tubulin heterodimers can assemble into microtubules with different protofilament numbers (Chretien and Wade, 1991). This framework, which considers the 13-protofilament microtubules as the basic structure, suggests that protofilament number changes result in a geometrical mismatch in the microtubule lattice (Chretien and Wade, 1991). To maintain the lattice integrity in non-13-protofilament microtubules, protofilaments must slide along each other (i.e. lattice shear) or rotate around the microtubule’s long axis (i.e. lattice rotation) (Chretien and Wade, 1991). Recent breakthroughs in cryo-electron microscopy (cryo-EM) have led to near-atomic-resolution structures of microtubules and have identified the key residues that participate in the contacts between heterodimers (Zhang et al., 2015). The configuration of these key residues is likely maintained in microtubules with different geometries as suggested by low-resolution (~10 Å) cryo-EM reconstructions (Sui and Downing, 2010). However, it is unclear how sequence differences between isotypes are linked to structural changes in the microtubule lattice.
Microtubule stabilizing agents (MSAs), an important class of chemotherapeutic agents that suppress microtubule dynamics, exert contradictory effects on lattice organization, suggesting multiple mechanisms for lattice stabilization. In particular, taxanes, which broaden the distribution of protofilament number and enhance the structural plasticity of the microtubule lattice, promote deviations from cylindrical tubes relative to unstabilized microtubules or those stabilized with peluroside, which binds a different site on tubulin (Kellogg et al., 2017). Currently, it is unclear whether this enhanced lattice flexibility contributes to the stabilizing effects of taxanes. Differences in microtubule structural regularity have also been noted in the presence or absence of microtubule-associated proteins (MAPs) that influence microtubule dynamics, notably increased order in the presence of the microtubule plus-end-tracking protein EB3 (Zhang et al., 2015), which enhances both polymerization rates and catastrophe frequencies (Bieling et al., 2007; Vitre et al., 2008). While a more regular microtubule lattice has been assumed to be more stable (Zhang et al., 2015), experimental support for this assertion is currently lacking.
Tubulin purified from animal brains, which has been extensively used for biochemical and structural analyses, contains a mixture of different tubulin isotypes (Banerjee et al., 1988) and polymerizes into microtubules containing 9 to 16 protofilaments in vitro (Chaaban and Brouhard, 2017). Recent studies have described the purification and use of recombinant human tubulin and have shown differences in polymerization dynamics of microtubules assembled with different β-tubulin isotypes (Pamula et al., 2016; Vemu et al., 2016). Furthermore, studies of engineered yeast tubulin constructs with different C-terminal ‘tails’ have differences in binding to some motor proteins (Sirajuddin et al., 2014). However, the influence of tubulin isotypes on the binding and activity of MAPs that can depolymerize filaments has not been analyzed.
Here, we describe a strategy to generate isotypically pure recombinant human tubulin with no affinity-tags, and perform a comparative analysis of heterodimers differing only in the β-tubulin subunit. In the presence of GMPCPP, α1B/β2B forms microtubules with a broad distribution of protofilament numbers, while α1B/β3 forms almost exclusively canonical 13-protofilament microtubules. Taxol shifts the protofilament number distribution but maintains the relative difference. High-resolution cryo-EM structures of GMPCPP-bound 13-protofilament α1B/β3 and 14-protofilament α1B/β2B microtubules reveal enhanced structural plasticity of the β2B-subunit despite preservation of “lock-and-key” contacts between protofilaments, consistent with the lattice accommodation model. We also find that α1B/β2B microtubules are more resistant to passive disassembly or depolymerization by two different MAPs with distinct mechanisms of action. Microtubules composed of a mixture of the two isotypes have intermediate stability and protofilament numbers. Together, our studies indicate that β-tubulin isotype composition can modulate both microtubule protofilament number and stability, likely by modulating the intrinsic structural plasticity of the corresponding tubulin subunits.
Results
Purification and characterization of affinity tag-free recombinant human tubulin
To purify recombinant forms of different human tubulin isotypes we modified the purification strategy we previously developed to obtain recombinant human β-tubulin, which purified as heterodimers with a mixture of human and insect α-tubulin (Ti et al., 2016). We found that inclusion of an affinity tag on human α-tubulin, similar to the one we used for β-tubulin, substantially reduced protein yield. To address this, we evaluated several tagging strategies and finally selected a protease-cleavable N-terminal decahistidine tag for α-tubulin and a C-terminal Strep-tag II for β-tubulin (Figure 1A). The residues between these affinity tags and the protease sites for each tubulin also required optimization (Figure 1A). For these constructs we devised a multi-step purification that employed affinity chromatography, affinity tag cleavage, and size-exclusion chromatography (Figure 1B). This procedure yielded >95% pure human tubulin heterodimers at a yield (~3 mg/liter of cultured insect cells) sufficient for biochemical and biophysical analyses. We also chemically labeled this recombinant tubulin with fluorescent dyes and note that additional optimization of this protocol is needed to improve labeled tubulin yield (Figure 1B, see Methods).
Figure 1. Purification and analyses of affinity-tag free recombinant human α1B/β2B- and α1B/β3-tubulin.

(A) Schematic for constructs used for α-tubulin isotype 1B/β-tubulin isotype 2B (α1B/β2B-tubulin) and α-tubulin isotype 1B/β-tubulin isotype 3 (α1B/β3-tubulin) expression. (B) Schematic for the purification strategy and the preparation of labeled recombinant human tubulin. (C) SDS-PAGE analysis of purified human tubulin. (D) Elution profiles of α1B/β2B-tubulin (peak volume, 81.7 ml), α1B/β3-tubulin (peak volume, 80.2 ml) and bovine tubulin (peak volume, 80 ml) from size-exclusion chromatography. V0, void volume. (E and F) TIRF microscopy images of taxol-stabilized α1B/β2B (E) and α1B/β3 (F) microtubules. (G and H) TIRF microscopy images of GMPCPP-α1B/β2B (G) and -α1B/β3 (H) microtubules. Fluorescently labeled human tubulin (~3.5%) was added to visualize filaments that were immobilized on the kinesin-5-coated coverslips. Scale bar, 5 μm.
β2B and β3 are the two major β-tubulin isotypes identified in preparations of bovine brain tubulin, which is commonly used for biochemical and biophysical studies (Banerjee et al., 1988). In addition, human α1B, β2B and β3 are the major tubulin isotypes expressed in neuronal cells (Ludueña and Banerjee, 2008). Using our improved method we purified two different human tubulin heterodimers, α1B/β2B and α1B/β3 (Figure 1C) and initially characterized them using three approaches. First, mass spectrometry indicated that each of the purified tubulins consisted of >99% recombinant human α- and β-tubulin. No peptides corresponding to insect tubulin were detected (from ~5 μg of protein sample). Second, purified α1B/β2B- and α1B/β3-tubulin eluted from a size-exclusion column at similar volumes as bovine brain tubulin purified using standard methods (Figure 1D). Third, α1B/β2B- and α1B/β3-tubulin assembled into microtubules in the presence of paclitaxel (taxol), a MSA, or guanylyl 5′-α,β-methylenediphosphonate (GMPCPP), a slowly hydrolyzing GTP analogue (Figure 1E-H). Together, these data demonstrate that we can generate polymerization-competent affinity tag-free recombinant human tubulin isotypes.
Human β-tubulin isotypes regulate protofilament number distributions
We next examined the architecture of these isotypically pure microtubules using cryo-EM. Microtubules were prepared from α1B/β2B- or α1B/β3-tubulin in the presence of GMPCPP (hereafter, GMPCPP-α1B/β2B or GMPCPP-α1B/β3 microtubules). A monomeric kinesin-1 mutant protein was used to distinguish between α- and β-tubulin in the microtubule lattice, as has been previously described (Alushin et al., 2014). The overall organization of frozen-hydrated GMPCPP-α1B/β2B and -α1B/β3 microtubules were similar and consistent with previous reports of GMPCPP-stabilized bovine microtubules (Figure 2A and B) (Alushin et al., 2014).
Figure 2. GMPCPP-α1B/β2B microtubules are wider and have more protofilaments than GMPCPP-α1B/β3 microtubules.
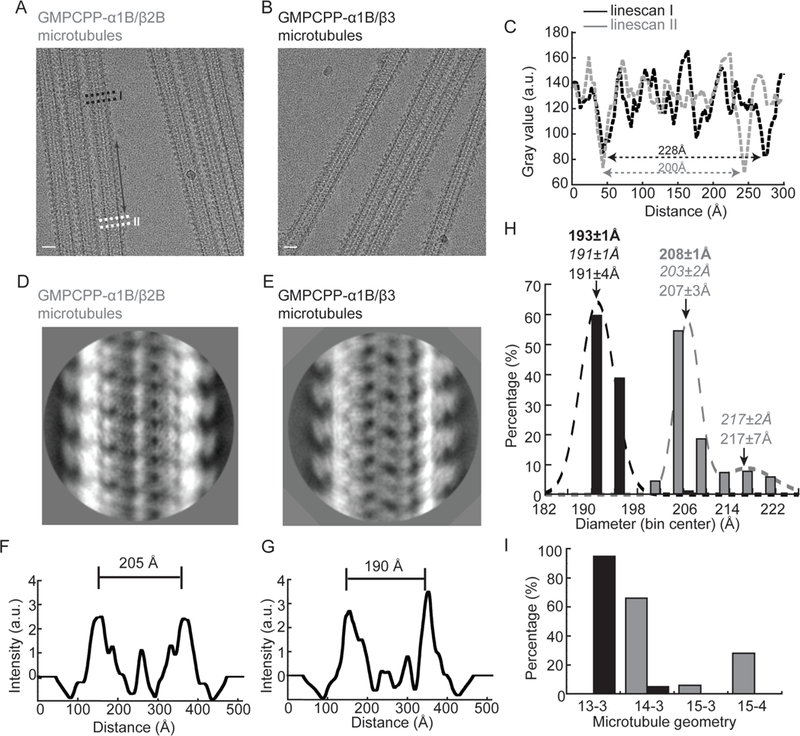
(A and B) Cryo-EM images of kinesin-1 motor domain-decorated GMPCPP-α1B/β2B microtubules (A) and GMPCPP-α1B/β3 microtubules (B). The double-headed arrows indicate transitions in microtubule diameter. Scale bar, 20 nm. (C) Linescans of highlighted regions I and II (dashed boxes in (A)). (D and E) Examples of 2D-class averages of GMPCPP-α1B/β2B and GMPCPP-α1B/β3 microtubules. (F and G) 1D projections of microtubules from the corresponding 2D-class averages shown in (D) and (E). The edge-to-edge distances are indicated. (H) Frequency distribution histograms for the diameters of GMPCPP-α1B/β2B (gray) and GMPCPP-α1B/β3 (black) microtubules measured from 2D-class averages. The peak values from the Gaussian fits (normal, mean±SD), the microtubule diameters measured from the 3D-reference models (italics, mean±SD), and the diameter of the final reconstructions (bold, mean±SD) are indicated. (I) Frequency distribution histograms for the protofilament numbers of GMPCPP-α1B/β2B microtubules (gray) and GMPCPP-α1B/β3 microtubules (black) measured from reference-based 3D classification. See also Figure S1.
Qualitative analyses of the cryo-EM micrographs revealed frequent and subtle transitions in diameter along the lengths of GMPCPP-α1B/β2B microtubules. In contrast, the diameter along GMPCPP-α1B/β3 microtubules was essentially constant (Figure 2A-C). To quantify the distribution of microtubule diameters in each sample, we used reference-free 2D classification (~80,000 segments with 80Å spacing in ~200 classes, Figure 2D, E). 1D projections perpendicular to the filament axis were generated from each class average, and the distance between the two highest peaks, which corresponds closely to the microtubule diameter (hereafter referred to as “2D-diameter”), was measured (Figure 2F and G). Frequency distribution histograms of 2D-diameter weighted by class membership revealed that GMPCPP-α1B/β3 microtubules have a unimodal distribution (peak: 189±4 Å; Figure 2H). In comparison, GMPCPP-α1B/β2B microtubules have a bimodal distribution (peaks: 205±3 Å and 215±7 Å; Figure 2H). This indicates that GMPCPP-α1B/β2B and -α1B/β3 microtubules likely have distinct protofilament number distributions (Chretien and Wade, 1991).
To further examine microtubule lattice distributions we adopted a previously reported reference-based 3D classification strategy (Sui and Downing, 2010). We generated microtubule models with feasible protofilament and start numbers, and classified our experimental segments against them utilizing a multi-reference alignment (MRA) procedure. This MRA revealed that GMPCPP-α1B/β3 microtubules are mainly 13 protofilament with a 3-start helical path if the tubulin monomers are considered identical, as is the historical convention (hereafter referred to as MT 13–3; 95% of segments). Only a small fraction (5%) of the microtubule segments are 14-protofilament with a 3-start helix (MT 14–3). These findings are consistent with the unimodal 2D-diameter distribution for this isotype (Figure 2H and I). In contrast, we find that 66% of GMPCPP-α1B/β2B microtubule segments were MT 14–3, 6% had 15 protofilaments with a 3-start helix (MT 15–3) and 28% had 15 protofilaments with a 4-start helix (MT 15–4), consistent with the bimodality of the corresponding 2D-diameter analysis (Figure 2H and I). Furthermore, the diameters of our 3D-reference models also agree with the peak values of the 2D-diameter measurements, validating our analysis (Figure 2H).
We next quantified the protofilament numbers of microtubules formed by α1B/β2B- and α1B/β3-tubulin polymerized in the presence of taxol (hereafter, taxol-α1B/β2B and taxol-α1B/β3 microtubules) (Figure S1A and B). In both cases, we observed transitions in diameter along the lengths of single microtubules, consistent with prior reports of microtubule “breathing” in the presence of taxol (Diaz et al., 1998) (Figure S1A-D). Microtubules were first processed by reference-free 2D-classification (~70,000 segments with 80Å spacing in ~150 classes, Figure S1E and F), and 2D-diameter analysis demonstrates that both microtubules have broader diameter distribution in comparison to their GMPCPP counterparts (Figure S1G-J), with taxol-α1B/β3 microtubules displaying a bimodal distribution and taxol-α1B/β2B microtubules a multimodal distribution (Figure S1I and J). Further, reference-based 3D classification indicated that 26% of taxol-α1B/β2B microtubules have 12 protofilaments with a 3-start helix (MT 12–3), 28% are MT 13–3, 18% are MT 14–3, and 28% are MT 15–4 (Figure S1K). In comparison, taxol-α1B/β3 microtubules contain 4% 11 protofilaments with a 3-start helix (MT 11–3), 78% of MT 12–3 and 18% of MT 13–3 filaments (Figure S1K). The relative quantity of microtubule geometries, diameters of 3D reference models, and 2D-diameter distributions are internally consistent (Figure S1I-K). Together, our cryo-EM structural analyses indicate that, on average, α1B/β2B microtubules have wider diameters and a broader protofilament distribution than α1B/β3 microtubules in the presence of either taxol or GMPCPP.
Atomic models of 14–3 GMPCPP-α1B/β2B and 13–3 GMPCPP-α1B/β3 microtubules
We next focused on determining high-resolution structures of 14–3 GMPCPP-α1B/β2B (hereafter 14-α1B/β2B) microtubules and 13–3 GMPCPP-α1B/β3 (hereafter 13-α1B/β3) microtubules, the most abundant lattice geometries we observed for each of these tubulin isotypes. Adapting a previously described pseudo-helical processing strategy (see Methods) (Alushin et al., 2014), we obtained reconstructions of both samples at 3.65 Å or better resolution (Fourier shell correlation [FSC] 0.143 criterion; Figure 3A-D, Table 1, Figure S2A-E). The axial rise and twist between laterally adjacent subunits of 14-α1B/β2B and 13-α1B/β3 microtubules were similar to those of prior lower-resolution bovine brain microtubule structures (Table 1) (Sui and Downing, 2010). The diameters of microtubules in our 3D-reconstructions were also consistent with 2D-diameter measurements (Figure 2H).
Figure 3. High-resolution cryo-EM reconstructions of 14-α1B/β2B and 13-α1B/β3 microtubules reveal distributed structural differences.

(A-D) Overviews of the pseudo-helical symmetrized cryo-EM reconstructions of kinesin-1 motor domain decorated GMPCPP-14-α1B/β2B (A and B) and GMPCPP-13-α1B/β3 (C and D) microtubules. (α1B-tubulin: green; β2B-tubulin: cyan; β3-tubulin: blue; kinesin-1 motor domain: orange). (E and F) Atomic models of tubulin subunits in 14-α1B/β2B (E) and 13-α1B/β3 (F) microtubules. Segmented cryo-EM densities corresponding to nucleotides are indicated (gray mesh). (G) Schematic for the tubulin dimers from adjacent protofilaments. The central α-tubulin (orange) used for the alignment of Cα positions, the adjacent two α-tubulin (green), and β-tubulin (blue) are highlighted. (H) Comparison of the Cα traces of three tubulin dimers from adjacent protofilaments in the 14-α1B/β2B (yellow) and 13-α1B/β3 microtubules (α1B-tubulin: green; β3-tubulin: blue). We considered only the globular core of tubulin subunits in these analyses (β2B- and β3-tubulin: amino acids 1–426; α1B-tubulin: amino acids 1–437 excluding the acetylation loop, S38-D46). The Cα traces of the central α-tubulins used for the alignment (orange) are highlighted. See also Figure S2 and S3.
We next built and refined atomic models of tubulin subunits into these density maps. We could assign densities corresponding to the majority of the backbone in globular regions of the subunits, as well as side chains (Figure S2F and G), except for a few acidic residues (Glu and Asp), which are vulnerable to radiation damage (Allegretti et al., 2014). The densities corresponding to the nucleotides bound to α- and β-tubulin could also be assigned (Figure 3E and F). The positions and orientations of these nucleotides in the 14-α1B/β2B and 13-α1B/β3 microtubules are similar to those observed in other microtubule structures with comparable resolution (Zhang et al., 2015) (Figure 3E and F). In both 14-α1B/β2B and 13-α1B/β3 reconstructions, we noted that the densities corresponding to the α-tubulin acetylation loop (S38-D46), as well as the C-terminal ‘tails’ of both α- and β-tubulin, were not observed, likely due to the well-established flexibility of these regions (Nogales et al., 1998).
To understand how differences in protofilament number are linked to changes in α/β-tubulin subunit conformation, we compared atomic models of three tubulin heterodimers from adjacent protofilaments in the 14-α1B/β2B and 13-α1B/β3 microtubules (Figure 3G and H). Since the α-tubulin isotype is common in the two structures, we superimposed the Cα positions of the central α-subunit (Figure 3H, PF 0, orange) and considered 13-α1B/β3 as the reference state, as it forms canonical 13-protofilament microtubules. The 14-α1B/β2B tubulin subunits are laterally displaced relative to the central protofilament such that their overall width is narrower than those in the 13-α1B/β3 microtubules, as required by the lattice parameters. To quantify this displacement, we determined the root mean square deviation for the C-alpha atoms (Cα-RMSD) (Figure S3A, average Cα-RMSD=1.4 Å) and found that the displacements increased relative to the central protofilament, with Cα-RMSD reaching ~2 Å at the left and right edges of the adjacent protofilaments (Figure S3A). When the Cα atoms of β-tubulin subunits were used for alignment we obtained similar results that higher protofilament number is coupled to lateral contraction of the α/β-tubulin subunits in 14-α1B/β2B microtubules (Figure S3B-D).
We also to compared the models of three consecutive tubulin subunits within a single protofilament in the 14-α1B/β2B and 13-α1B/β3 microtubules (Figure S3E-H). This analysis revealed local displacements in some loops (e.g. H1-S2 loop), but no gross global structural displacements along the longitudinal lattice (Figure S3F and H). We next examined the specific residues that mediate lateral interactions, which have previously been reported to remain essentially constant across protofilament number, MAP, and drug-bound states (Figure S4A) (Kellogg et al., 2017; Sui and Downing, 2010; Vemu et al., 2016; Zhang et al., 2015). The S7-H9 loop (also called the M-loop) contributes an aromatic residue that acts as a ‘key’, which fits into a corresponding ‘lock’ formed by the H1-S2 and H2-S3 loops in the tubulin of the neighboring protofilament (Zhang et al., 2015). For both β-tubulin isotypes studied, a Tyr281 ‘key’ in the M-loop is nestled by ‘lock’ residues (e.g. Lys58 and Pro87) in the loops of the neighboring β-tubulin (Figure S4B and C). Similarly, at the α-tubulin lateral interface, a His283 ‘key’ in the M-loop inserts into ‘lock’ residues (e.g. Lys60, His88 and Pro89) in the loops of the neighboring α-tubulins (Figure S4D and E). Both the 13-α1B/β3 and 14-α1B/β2B atomic models feature this ‘lock-and-key’ configuration at lateral interfaces despite the lateral contraction of the 14-α1B/β2B subunits, with only very minor adjustments in positioning to accommodate lattice distortion.
Isotypes feature distributed structural differences
Since the major longitudinal and lateral contacts between tubulin subunits in both of our models are essentially unchanged at the residue level, we next examined if subtle remodeling at the backbone level could explain the relative narrowing of the 14-α1B/β2B tubulin subunits. A global superimposition of the Cα coordinates of β-tubulin subunits revealed that structural differences between β2B- and β3-tubulin are broadly distributed rather than localized at one specific contact or structural feature (Figure 4A). To quantify these structural differences, we calculated the per-residue Cα-RMSD (average=0.87 Å, Figure S4F and G). We were unable to analyze differences in the P-loop (S4-H4 loop) due to the limited local resolution for this glycine-rich region in the 14-α1B/β2B reconstruction. The Cα-RMSD analysis revealed three regions with substantial structural differences in β-tubulin, for which we empirically selected a cutoff of Cα-RMSD >1 Å. The first region is proximal to the M-loop (Figure 4B). There are five divergent residues in this region, and four of them are an alanine in β3-tubulin replaced by larger polar residues in β2B-tubulin (Figure 4B). In the second region, the H1-S2 loop undergoes conformational changes (Figure 4C). This loop is comprised of only a small fraction of the β-tubulin structured core (~9%, 39 out of 426), but contains >25% of the residues that diverge between β2B- and β3-tubulin (7 out of 27) (Figure S4G). The third region shows a displacement in helix H11’, which has been shown to be involved in inter-tubulin longitudinal contacts (Zhang et al., 2015); however, the residues in this motif are conserved between the two β-tubulin isotypes (Figure 4D).
Figure 4. Structural displacements are distributed throughout both α- and β-tubulin subunits, with enhanced flexibility apparent in α1B/β2B microtubules.
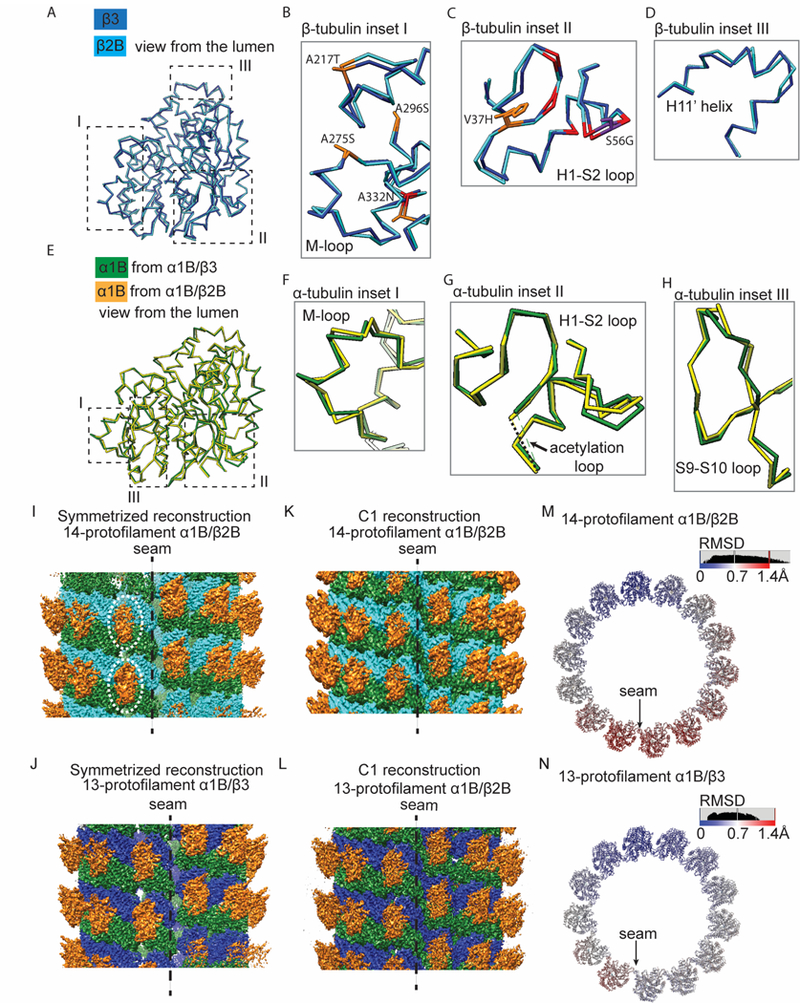
(A) Comparison of the Cα traces of β2B- (cyan) and β3-tubulin (blue). Regions I, II and III are highlighted (dashed boxes). (B-D) Enlarged views of Cα traces of β2B- (cyan) and β3-tubulin (blue) from regions highlighted in (A): around the M-loop (B), the H1-S2 loop (C) and the H11’ helix (D). Colors highlight β3-tubulin hydrophobic residues replaced by polar residues in β2B-tubulin (orange), β3-tubulin polar residues replaced by hydrophobic residues in β2B-tubulin (purple), and other divergent residues (red). (E) Comparison of the Cα traces of α1B-tubulin in 14-α1B/β2B (yellow) and 13-α1B/β3 (green) microtubules. Regions I, II and III are highlighted. (F-H) Enlarged views of Cα traces from regions highlighted in (E): around the M-loop (F), the H1-S2 loop (G) and the S9-S10 loop (H). (I-L) Overviews of the pseudo-helical symmetrized (I and J) and C1 (K and L) cryo-EM reconstructions of kinesin-1 motor domain decorated GMPCPP-14-α1B/β2B (I and K) and GMPCPP-13-α1B/β3 (J and L) microtubules at the seam. The less defined densities of kinesin motors at the seam of the symmetrized GMPCPP-14-α1B/β2B reconstruction are highlighted (dashed circles). α1B-tubulin: green; β2B-tubulin: cyan; β3-tubulin: blue; kinesin-1 motor domain: orange. (M and N) Cα-atom RMSD between superimposed models from the C1 and the pseudo-helical symmetrized reconstructions of 14-α1B/β2B (M) and 13-α1B/β3 (N) microtubules. One helical turn of αβ-tubulin heterodimers is shown. See also Figure S4.
Previous analyses of nucleotide-hydrolysis-dependent structural transitions in the microtubule lattice revealed greater changes in α-tubulin than in β-tubulin, suggesting extensive and obligatory allosteric communication between the subunits in the lattice (Alushin et al., 2014; Zhang et al., 2015). Consistent with this framework, per-residue Cα-RMSD analysis after superposition on the α1B-tubulin subunit also revealed distributed structural differences in α-tubulin (Figure 4E; n.b. the limited local resolution for the P-loop also restricted our analysis of this α-tubulin region). Indeed, the overall magnitude of structural displacements in α-tubulin was comparable to what we observed for β-tubulin (average Cα-RMSD= 0.75 Å, Figure S4H). α-tubulin regions featuring substantial structural displacements (Cα-RMSD >1 Å) include the M-loop and the H1-S2 loop (Figure 4F and G). Additionally, the region proximal to α-tubulin’s characteristic S9-S10 loop, which is 8 residues longer than the corresponding loop in β-tubulin, was also displaced (Figure 4H). Overall, our analyses reveal that distributed structural displacements between α1B/β2B- and α1B/β3-tubulin subunits accompany changes in protofilament number, with most changes corresponding to subtle tweaking of the conformation of loops that mediate lateral contacts to accommodate lattice reorganization, as has previously been predicted from low-resolution analysis (Sui and Downing, 2010).
Our pseudo-helical symmetrization procedure does not faithfully average a discontinuity present in 3-start microtubule lattices, referred to as the ‘seam’, where α- and β-tubulin form heterotypic (α-β or β-α) rather than homotypic (α-α or β-β) lateral contacts (Figure 4I and J). We noted that the densities corresponding to kinesin motor heads at the seam are less defined in the 14-α1B/β2B reconstruction compared to 13-α1B/β3 (Figure 4I and J). We reasoned that the pseudo-helical symmetrization procedure could be generating artifacts at the seam in this reconstruction due to greater inherent deviations from a true cylindrical lattice in α1B/β2B vs. α1B/β3 microtubules. To test this hypothesis, we also calculated reconstructions without applying symmetry (C1) at 4.8 Å or better resolution for both specimens (Figure 4K and L). We rigid-body docked tubulin heterodimers to form one-helical turn, then compared these C1 models to the corresponding symmetric model by superimposing the tubulin dimers on the opposite side of the microtubule from the seam. By quantifying the Cα-RMSD, we find substantially larger deviations from ideality in 14-α1B/β2B than 13-α1B/β3 (Figure 4M and N). This suggests that the positioning of tubulin subunits is indeed more variable in 14-α1B/β2B than 13-α1B/β3, indicative of enhanced flexibility in α1B/β2B microtubules.
α1B/β2B microtubules are intrinsically more stable and against MCAK-dependent depolymerization than α1B/β3 microtubules
In the course of our experiments with recombinant tubulins we noted that α1B/β2B microtubules appeared to be more stable than α1B/β3 microtubules. To examine this effect in detail we used a total internal reflection fluorescence microscopy (TIRF) assay in which GMPCPP-stabilized microtubules were immobilized on coverslips and then the assay chamber was perfused with tubulin-free buffer (Figure S5A). Time-lapse images were collected to monitor microtubule length changes due to disassembly (Figure S5B-G). For this assay, we copolymerized recombinant tubulins with rhodamine-labeled bovine tubulin (~3.5%) for imaging and with biotin-labeled bovine tubulin (~3.5%) for coverslip attachment (Figure S5A). In parallel, to exclude the effects of the added bovine tubulin, we used rhodamine-labeled recombinant human tubulin for visualization and kinesin-5 to attach microtubules to the coverslip (Figure S5H-J). In both cases, we found that GMPCPP-α1B/β3 microtubules depolymerized 4~6-fold faster than GMPCPP-α1B/β2B microtubules (Figure S5K-L).
We hypothesized that depolymerases could have distinct activities against these microtubules with different intrinsic stabilities. To test this hypothesis, we used MCAK, a kinesin-13 family protein that functions as a microtubule depolymerase (Hunter et al., 2003). We generated a GFP-tagged monomeric MCAK construct (hereafter, GFP-MCAK) that contains the neck and motor domains but lacks the C- and N-terminal dimerization domains (Figure 5A). This GFP-MCAK construct is the minimal functional module that can depolymerize microtubules (Cooper et al., 2010).
Figure 5. MCAK stimulates faster depolymerization of GMPCPP-stabilized α1B/β3 than α1B/β2B microtubules.

(A) Schematic for MCAK constructs with motor domain (black box) highlighted. (B and C) Images of GMPCPP-stabilized α1B/β2B (B) and α1B/β3 (C) microtubules in the presence of 5 nM GFP-MCAK at t=0. Recombinant human tubulin was copolymerized with rhodamine-labeled bovine tubulin (~3.5%) and biotin-labeled bovine tubulin (~3.5%) to visualize and immobilize filaments on the biotin-PEG functionalized coverslips. (D and E). Images of GMPCPP-stabilized α1B/β2B (D) and α1B/β3 (E) microtubules in the same fields as in (B) and (C) at t=10 min. (F and G) Kymographs of α1B/β2B (F) and α1B/β3 (G) microtubules in the presence of 5 nM GFP-MCAK. (H and I) Microtubule depolymerization rates in the presence of 5 nM GFP-MCAK (H: α1B/β2B, 0.28±0.14 μm/min (n= 57); α1B/β3 microtubules, 1.88±0.6 μm/min (n= 54)) or 50 nM GFP-MCAK (I: α1B/β2B, 1.3±0.53 μm/min (n= 43); α1B/β3, 7.2±1.44 μm/min (n= 41)). Events (n>40) from 3 independent experiments were pooled for each condition and analyzed to determine the mean and standard deviation. (J and K) Kymographs show the depolymerization of α1B/β2B (J) and α1B/β3 (K) microtubules in the presence of 50 nM GFP-MCAK. Microtubules were polymerized from unlabeled recombinant human tubulin and were attached to the kinesin-5-coated coverslips. GFP signal along microtubules was used to track filament length. (L) Microtubule depolymerization rates in the presence of 50 nM GFP-MCAK (α1B/β2B, 1.28±0.51 μm/min (n= 40); α1B/β3, 6.54±0.94 μm/min (n= 44)). Events (n>40) from 2 independent experiments were pooled for each condition and analyzed to determine the mean and standard deviation. All experiments include 1 mM MgATP. Horizontal scale bars, 3 μm; vertical scale bars, 1 min. See also Figure S5 and S6.
We set up a two-color TIRF-based assay to track filament length and GFP-MCAK binding (Figure 5B-G, Figure S6A-E and S6H-K). We first used rhodamine-labeled and biotin-labeled bovine tubulin in this assay, as at this stage our protocols do not yield labeled recombinant human tubulins in amounts sufficient for multiple assays. At close to physiologic MCAK concentrations (5 and 50 nM) (Hunter et al., 2003), the depolymerization rates of GMPCPP- or taxol-stabilized α1B/β2B microtubules were similar to those previously reported with microtubules polymerized from brain tubulins (Figure 5H and I, Figure S6L and M) (Cooper et al., 2010; Helenius et al., 2006). Strikingly, stabilized α1B/β3 microtubules shortened 3- to 7-fold faster than α1B/β2B filaments in the examined conditions (Figure 5H and I, Figure S6L and M). The GFP fluorescence intensity per unit length of α1B/β2B microtubules was similar to that of α1B/β3 microtubules (Figure S6F, G, N and O) and, therefore, the significantly faster MCAK-stimulated depolymerization rates are unlikely due to higher amounts of MCAK bound to α1B/β3 filaments.
To exclude the effects of adding the labeled bovine tubulin, we tested the MCAK-stimulated depolymerization of GMPCPP- or taxol-stabilized unlabeled recombinant human microtubules that were attached to kinesin-5-coated coverslips (Figure S6P). Because the MCAK construct is GFP tagged, it is possible to track the microtubule depolymerization through the GFP signal along the filaments (Figure 5J and K, Figure S6Q and R). The average depolymerization rates of α1B/β3 microtubules were ~5-fold faster than the rates of α1B/β2B microtubules (Figure 5L, Figure S6S). Together, these data indicate that α1B/β2B microtubules are more stable against disassembly by a kinesin-13 family microtubule depolymerase compared to α1B/β3 microtubules.
α1B/β3 microtubules depolymerize faster than α1B/β2B microtubules in the presence of chTOG
We next tested the activity of a MAP with a mechanism of microtubule depolymerization that is distinct from that of MCAK. Human chTOG, an XMAP215/Dis1 family protein, functions as a catalyst that can increase both the association rate constant and the dissociation rate constant of GTP-bound tubulin at microtubule ends (Brouhard et al., 2008; Roostalu et al., 2015). Therefore, in the absence of free tubulin in the buffer, XMAP215/chTOG facilitates the depolymerization of stabilized microtubules (Brouhard et al., 2008; Roostalu et al., 2015).
We investigated the depolymerization of GMPCPP- or taxol-stabilized microtubules at a physiologically relevant concentration (50 nM) of GFP-tagged full-length human chTOG (Kinoshita et al., 2001) (Figure 6A). We first used a two-color TIRF assay to image the chTOG-GFP signal that accumulated on filaments and rhodamine-labeled bovine tubulin copolymerized (~3.5%) with α1B/β2B- or α1B/β3-tubulin to track filament length. In separate experiments, we examined the chTOG-stimulated depolymerization of unlabeled human microtubules that were attached to kinesin-5-coated coverslips (Figure S6P). In this case, we used the chTOG-GFP signal along microtubules to track filament length (Figure 6I and J, Figure S7L and M).
Figure 6. chTOG stimulates faster depolymerization of GMPCPP-stabilized α1B/β3 than α1B/β2B microtubules.
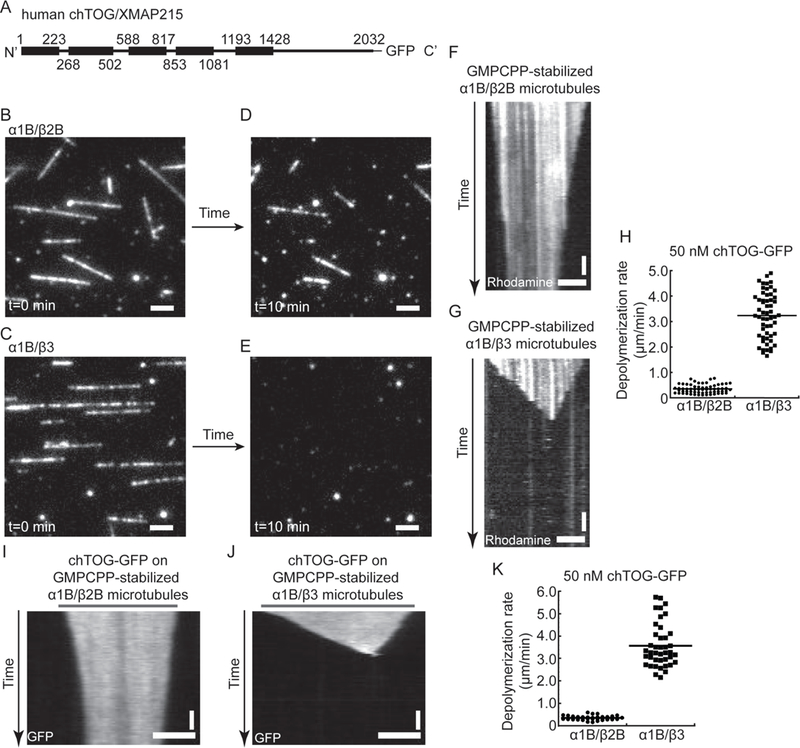
(A) Schematic for chTOG constructs with TOG domains (black box) highlighted. (B and C) Images of GMPCPP-stabilized α1B/β2B (B) and α1B/β3 (C) microtubules in the presence of 50 nM chTOG-GFP at t=0. Recombinant human tubulin was copolymerized with rhodamine-labeled bovine tubulin (~3.5%) and biotin-labeled bovine tubulin (~3.5%) to visualize and immobilize filaments on the biotin-PEG functionalized coverslips. (D and E) Images of GMPCPP-stabilized α1B/β2B (D) and α1B/β3 (E) microtubules in the same fields as in (B) and (C) at t=10 min. (F and G) Kymographs of GMPCPP-stabilized α1B/β2B (F) and α1B/β3 (G) microtubules in the presence of 50 nM chTOG-GFP. (H) Microtubule depolymerization rates in the presence of 50 nM chTOG-GFP (α1B/β2B, 0.35±0.17 μm/min (n= 63); α1B/β3, 3.23±0.91 μm/min (n= 56)). Events (n>55) from 3 independent experiments were pooled for each condition and analyzed to determine the mean and standard deviation. (I and J) Kymographs show the depolymerization of α1B/β2B (I) and α1B/β3 (J) microtubules in the presence of 50 nM chTOG-GFP. Microtubules were polymerized from unlabeled recombinant human tubulin and were attached to the kinesin-5-coated coverslips. GFP signal along microtubules was used to track filament length. (K) Microtubule depolymerization rates in the presence of 50 nM chTOG-GFP (α1B/β2B, 0.35±0.09 μm/min (n= 39); α1B/β3, 3.57±0.97 μm/min (n= 41)). Events (n>35) from 2 independent experiments were pooled for each condition and analyzed to determine the mean and standard deviation. Horizontal scale bars, 3 μm; vertical scale bars, 1 min. See also Figure S7.
We found that α1B/β3 microtubules were also more sensitive to chTOG-stimulated depolymerization than α1B/β2B microtubules (Figure 6B-E). Kymographs of individual microtubules indicated that chTOG stimulated depolymerization from both ends of human microtubules (Figure 6F, G, I and J, Figure S7A-D, F-I and L-M). In all the tested conditions, the average depolymerization rates of α1B/β3 microtubules were 7- to 9-fold faster than the depolymerization rates of α1B/B2B microtubules (Figure 6H and K, Figure S7J and N). The GFP fluorescence intensity per unit length of microtubules again suggested that a similar amount of chTOG bound to α1B/β2B and α1B/β3 microtubules (Figure S7E and K). Together, our findings indicate that α1B/β2B microtubules are more stable than α1B/β3 microtubules.
β2B- and β3-tubulin additively regulate microtubule protofilament number and lattice stability
We next polymerized microtubules with mixtures of β2B- and β3-tubulins to examine their relative effects on the regulation of protofilament number and MAP-dependent depolymerization. First, we used cryo-EM to analyze taxol-stabilized microtubules polymerized from a mixture containing equal amount of α1B/β2B- and α1B/β3-tubulin (hereafter taxol-α1B/β2B/β3 microtubules) (Figure 7A). 2D-diameter analysis (~110,000 segments with 80Å spacing in ~200 distinct classes) showed that taxol-α1B/β2B/β3 microtubules have a bimodal distribution (Figure 7B). Further, reference-based 3D classification indicated that 3% of taxol-α1B/β2B/β3 microtubules are MT 11–3, 43% are MT 12–3, 39% are MT 13–3, 7% are MT 14–3, and 7% are MT 15–4 (Figure 7C). The relative distributions of microtubule geometries are consistent with the 2D-diameter analyses (Figure 7B and C). Our results indicate that taxol-α1B/β2B/β3 microtubules have intermediate protofilament numbers compared to taxol-α1B/β3 microtubules and taxol-α1B/β2B microtubules (Figure S1K).
Figure 7. β-tubulin isotypes modulate microtubule protofilament number and stability.
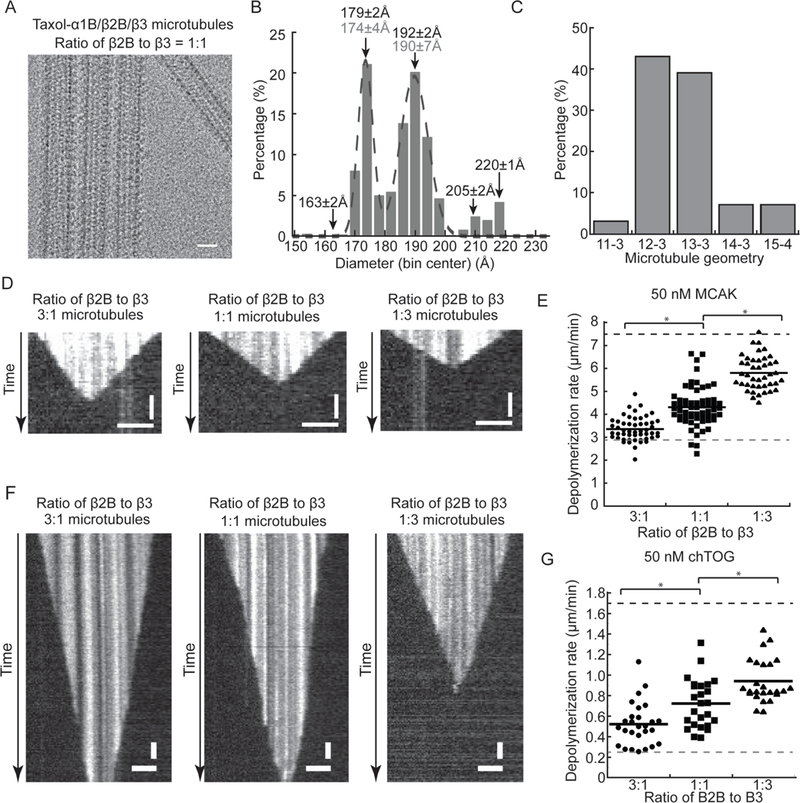
(A) Cryo-EM images of kinesin-1 motor domain decorated taxol-α1B/β2B/β3 microtubules that are polymerized using a mixture of equal amounts of α1B/β2B- and α1B/β3-tubulin. Scale bar, 20 nm. (B) Frequency distribution histograms for the diameters of taxol-α1B/β2B/β3 microtubules measured from 2D-class averages. The peak values from Gaussian fits (gray, mean±SD), and the microtubule diameters measured from 3D-reference models (black, mean±SD) are indicated. (C) Frequency distribution histograms for the protofilament numbers of taxol-α1B/β2B/β3 microtubules measured from reference-based 3D classification. (D) Kymographs showing the depolymerization of taxol-stabilized microtubules containing different ratios of β2B- to β3-tubulin in the presence of 50 nM GFP-MCAK. (E) Microtubule depolymerization rates in the presence of 50 nM GFP-MCAK (β2B:β3=3:1, 3.36±0.52 μm/min (n= 51); β2B:β3=1:1, 4.31±0.86 μm/min (n= 61); β2B:β3=1:3, 5.81±0.73 μm/min (n= 42)). The depoloymerization rates of α1B/β2B (gray dashed line) and α1B/β3 (black dashed line) microtubules are indicated. Events (n>40) from 3 independent experiments were pooled for each condition and analyzed to determine the mean and standard deviation. All experiments include 1 mM MgATP. (F) Kymographs showing the depolymerization of taxol-stabilized microtubules containing different ratios of β2B- to β3-tubulin in the presence of 50 nM chTOG-GFP. (G) Microtubule depolymerization rates in the presence of 50 nM chTOG-GFP (β2B:β3=3:1, 0.52±0.21 μm/min (n= 27); β2B:β3=1:1, 0.72±0.24 μm/min (n= 25); β2B:β3=1:3, 0.94±0.21 μm/min (n= 25)). The depoloymerization rates of α1B/β2B (gray dashed line) and α1B/β3 (black dashed line) microtubules are indicated. Events (n>25) from 2 independent experiments were pooled for each condition and analyzed to determine the mean and standard deviation. For (D-G), recombinant human tubulin was copolymerized with rhodamine-labeled bovine tubulin (~3.5%) and biotin-labeled bovine tubulin (~3.5%) to visualize and immobilize filaments on the biotin-PEG functionalized coverslips. Horizontal scale bars, 3 μm; vertical scale bars, 1 min.
Second, we used a two-color TIRF-assay to examine the MAP-dependent depolymerization of taxol-stabilized microtubules that were polymerized from mixtures of α1B/β2B- and α1B/β3-tubulins. At physiologically relevant concentrations of MCAK or chTOG (50 nM), we observed depolymerization from both ends of the taxol-stabilized microtubules (Figure 7D and F). In particular, analyses of kymographs suggest that microtubules depolymerization rates are faster with increasing amounts of β3-tubulin in the polymers (Figure 7D and F). The average depolymerization rates of taxol-α1B/β2B/β3 microtubules are 2~3-fold faster than the rates of taxol-α1B/β2B microtubules and ~2-fold slower than the rates of taxol-α1B/β3 microtubules (Figure 7E and G). Together, our results show that human β-tubulin isotypes can control microtubule protofilament number and stability in an additive fashion, producing a microtubule population with properties that are essentially a weighted average of the component isotypes.
Discussion
We have used isotypically pure, affinity-tag free recombinant human tubulin to show that β-tubulin isotypes can determine microtubule protofilament number and stability. Atomic models of microtubules with different protofilament numbers indicate that variations in the primary sequence of human β-tubulin can encode distinct microtubule architectures. Collectively, our results support a link between tubulin subunit plasticity, microtubule protofilament number, and microtubule stability.
The 13-α1B/β3 and 14-α1B/β2B structures we present here do not differ substantially from previous high-resolution reconstructions of GMPCPP-bound microtubules polymerized from brain tubulin. This finding is consistent with the observation that heterogeneous mixtures of bovine (or porcine) tubulin produce near-atomic resolution reconstructions, suggesting all component isotypes have a similar structure, which can be averaged to 3–4 Å resolution. Indeed, a consensus is emerging that certain structural properties of all microtubule lattices appear to be invariant; notably, identical ‘lock and key’ residue-level contacts which mediate lateral interactions are present in all high-resolution microtubule structures to date, regardless of isotype composition, nucleotide state, drug binding, or MAP engagement (Alushin et al., 2014; Benoit et al., 2018; Kellogg et al., 2017; Vemu et al., 2016; von Loeffelholz et al., 2017; Zhang et al., 2015).
Our findings suggest a simple model for how variations in β-tubulin isotypes can lead to microtubules with different protofilament number. As detailed in the lattice accommodation model, small changes in lattice shear and rotation lead to microtubules with different protofilament numbers (Chretien and Wade, 1991). Our atomic models reveal that sequence differences between β-tubulin result in ‘accordion-like’ relatively small structural displacements distributed across the subunit and also in the associated α-tubulin. The specific conformation of the heterodimer is compatible with a narrow range of lattice parameters, such as shear or rotation, to control the configuration and number of protofilaments in a microtubule. The conformation of the α1B/β3-tubulin heterodimer is more restricted and can only assemble microtubules with rather limited choices of protofilament numbers. By contrast, the α1B/β2B-tubulin heterodimers likely sample more conformations and can generate microtubules with a broader distribution of protofilament numbers. This differential preference persists in the presence of taxol or GMPCPP, which have distinct mechanisms of action, indicating that the plasticity of tubulin conformation is an intrinsic property encoded in the β-tubulin primary sequence.
To better understand the effects of amino acid side chains on tubulin subunit plasticity, exhibited in ‘accordion-like’ distributed changes in tubulin conformation, we examined the difference in the residue-by-residue ‘buriability’, i.e. the contribution of an amino acid residue to protein stability as the buried surface area increases. Hydrophobic side chains have a generally higher ‘buriability’ than hydrophilic side chains (Zhou and Zhou, 2004). We find that the primary sequence of β2B-tubulin has an overall lower ‘buriability’ than β3-tubulin. Previous studies have also found that buried residues are more rigid while solvent-exposed residues are more flexible (Schlessinger and Rost, 2005). Therefore, isotype-specific residue substitutions could control the plasticity of the tubulin dimer and thereby, the protofilament configuration and number in the lattice. Our studies also suggest that enhanced tubulin flexibility increases microtubule stability, consistent with a recent report that deformations of the microtubule lattice facilitate softening, and thus buckling rather than breakage, in response to mechanical forces (Memet et al., 2018). By extension, the lattice plasticity enhancing properties of taxanes (Kellogg et al., 2017) may thus actually contribute to their microtubule stabilizing activity, which should be considered in future efforts to design and optimize MSAs.
A study by Brouhard and co-workers (Chabaan et al., Dev Cell, In Press), contemporaneous with our work, has analyzed the structure and polymerization properties of C. elegans tubulin, which at the level of primary sequence is nearly identical to human tubulin (e.g. ~90% identify for β-tubulins). Their study shows that C. elegans tubulin, purified as a mixture of isotypes, can form microtubules with fewer protofilaments than the canonical 13, indicating that the intrinsic properties of tubulin contribute to the smaller protofilament numbers observed for microtubules in embryos as well as in other cell types in the adult animal (Chalfie and Thomson, 1982). Interestingly, the purified tubulin has the unusually fast microtubule growth rates observed in C. elegans embryos (Honda et al., 2017; Srayko et al., 2005). The authors provide evidence that these rapid polymerization rates are likely due to the H1-S2 and M loops in C. elegans tubulin adopting secondary structure more readily than other slower polymerizing tubulins, effectively pre-paying the entropic costs that limit polymerization rates. Together, these data and our findings for human tubulins show how subtle changes in the primary sequence of nanometer-sized tubulin subunits can alter the structure and polymerization dynamics of micrometer-sized filaments.
We find that the majority (~95%) of our GMPCPP-α1B/β3 microtubules have 13-protofilaments. A previous report demonstrated that GMPCPP-α1A/β3 microtubules have mainly (~92%) 14-protofilaments (Vemu et al., 2016). The recombinant α1A/β3-tubulin used in the earlier study had an internal histidine-tag in the α-tubulin acetylation loop, which is part of the H1-S2 loop (S38-D46 in human α1B-tubulin), and is not resolved in high-resolution structures (von Loeffelholz et al., 2017; Zhang et al., 2015). Nonetheless, comparison of our atomic models reveals substantial displacements in the H1-S2 loop of microtubules with different protofilament numbers. Further, α-tubulin acetylation has been shown to weaken microtubule inter-protofilament lateral interactions (Portran et al., 2017). Accordingly, it is likely that introducing a positively charged histidine-tag in the α1A-tubulin acetylation loop could change protofilament numbers. This possibility and the roles of tubulin acetylation need to be further examined, particularly by combining non-natural amino acid incorporation by amber-suppression and recombinant tubulin expression (Kleiner et al., 2013).
An open question is how protofilament number is specified and maintained in cells expressing different tubulin isotypes. The γ-tubulin ring complex (γ-TuRC) is a microtubule-nucleating factor thought to specify 13-protofilament microtubules at the time of nucleation (Moritz et al., 2000; Zheng et al., 1995). It is possible that the efficiency of γ-TuRC-mediated microtubule nucleation depends on the compatibility of tubulin conformation to the γ-TuRC template, i.e., some tubulin isotypes could be better substrates for γ-TuRCs. Another possibility is that γ-TuRCs are able to nucleate certain isotypes of tubulin to form non-13-protofilament microtubules that have been described in certain cell types. These strategies, however, may not prevent the transitions in protofilament numbers from occurring once microtubules have nucleated. Cells may also use other protofilament number-specifying MAPs (e.g. doublecortin) (Bechstedt and Brouhard, 2012), to further regulate the lattice geometries. Additional mechanisms of regulating microtubule protofilament number are also likely, as evident from studies of human blood platelets, where activation (e.g. by ADP addition) leads to 14-protofilament, rather than the standard 13-protofilament, microtubules (Xu and Afzelius, 1988). In vitro reconstitutions will be essential to dissect the functions of, and the relationship between, tubulin isotypes and the different mechanisms regulating protofilament number.
Dynamic microtubules play critical roles in growth cone migration (Tanaka et al., 1995), and microtubule stabilization can specify the position of axons during neuronal polarization (Witte et al., 2008). At least two mechanisms have been proposed to regulate microtubule stability in neurons. These include post-translational modifications (e.g. acetylation and polyamination) (Portran et al., 2017; Song et al., 2013; Xu et al., 2017), and neuronal MAPs (e.g. tau and MAP2) (Cleveland et al., 1977; Conde and Caceres, 2009; Kim et al., 1979). In addition, kinesin-13 family depolymerases such as Kif2a have also been shown to contribute to axon outgrowth and regeneration (Ghosh-Roy et al., 2012; Homma et al., 2003; Walczak et al., 2013). Interestingly, recent studies have revealed distinct subcellular localizations of β2- and β3-tubulin in differentiated human neuroblastoma cells (Guo et al., 2010). As our data show that α1B/β2B microtubules are more stable against kinesin-13-stimulated depolymerization than α1B/β3 microtubules, we propose that the subcellular microtubule dynamics can be regulated by adjusting tubulin isotype composition at specific cellular locations.
Diverse tubulin isotypes can specify interactions with MAPs and regulate parameters of dynamic instability (Pamula et al., 2016; Sirajuddin et al., 2014; Vemu et al., 2016). Our finding that tubulin isotypes can control protofilament number should help better interpret these results in terms of microtubule structure. The availability of recombinant tubulins will allow studies of how nucleation templates and post-translational modifications, along with the specific species or isotypes of tubulin, control basic microtubule properties, such as protofilament number, in different cellular contexts.
STAR Methods
Contact for Reagent and Resource Sharing
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Tarun M. Kapoor (kapoor@rockefeller.edu)
Method Details
Purification of recombinant human tubulin
The codon optimized cDNA (Epoch Life Science Inc.) encoding Homo sapiens α-tubulin 1B (NP_006073.2), β-tubulin 2B (NM_178012.4) and β-tubulin 3 (NP_006077.2) were cloned into pFastBac Dual vector (ThermoFisher 10712024). For affinity purification, a sequence encoding a Tobacco Etch Virus (TEV) protease site, a Gly-Gly-Ser-Gly-Gly linker and Strep-tag II were fused to the 3’ end of the β-tubulin cDNA sequence. In addition, sequence encoding a decahistidine tag, a Tobacco Etch Virus (TEV) protease site, and a Ala-Pro linker were fused to the 5’ end of the α-tubulin cDNA sequence. An L21 enhancer (AACTCCTAAAAAACCGCCACC, (Sano et al., 2002) was added at the 5’ of the start codon (ATG). We used the Bac-to-Bac system (Life Technologies) to generate recombinant baculovirus. High Five cells (Life Technologies 10486–025), grown to 3.0–3.5 X 106 cells/ml in Express Five SFM (Life Technologies 10902–096) supplemented with 1X antibiotic-antimyocotic (Life Technologies 15240–062) and 18 mM L-glutamine (Life Technologies 25030–081), were infected with P3 viral stocks. Cells were cultured in suspension at 27 ºC and harvested at 60 hours after infection. The following steps were done on ice or at 4 ºC. Cells were lysed in an equal volume of lysis buffer (50 mM HEPES, 20 mM imidazole, 100 mM KCl, 1 mM MgCl2, 0.5 mM β-mercaptoethanol, 0.1 mM GTP, 3 U/ml benzonase, 1X Roche Complete EDTA-free protease inhibitor, pH 7.2) by dounce homogenization (20 strokes) and the homogenate was centrifuged at 55,000 rpm in a Type 70 Ti rotor (Beckman Coulter) for 1 hr. The supernatant was then filtered through a 0.22 μm Millex-GP PES membrane (Millipore SLGP033RS) and loaded onto two 1 ml HisTrap HP columns connected in tandem (GE life science 17–5247-01) pre-equilibrated with lysis buffer. The column was washed with 35 ml lysis buffer until the UV absorption reached baseline, then eluted with nickel elution buffer (1X BRB80 (80 mM PIPES, 1mM MgCl2, 1mM EGTA), 500 mM imidazole, 0.2 mM GTP, 2 mM β-mercaptoethanol, pH 7.2). The fractions containing proteins were pooled, diluted 3-fold with lysis buffer and loaded onto two 1 ml StrepTrap HP columns connected in tandem (GE life science 29–0486-53). The columns were washed with 25 ml 66% lysis buffer + 33% nickel elution buffer, 25 ml of wash buffer 1 (1X BRB80, 1 mM β-mercaptoethanol, 0.1 mM GTP, 0.1 % Tween-20, 10% glycerol, pH 7.2), and 25 ml of wash buffer 2 (1X BRB80 1 mM β-mercaptoethanol, 0.1 mM GTP, 10 mM MgCl2, 5 mM ATP, pH 7.2). The bound protein was then eluted with ~5 ml StrepTrap elution buffer (1XBRB80, 20 mM Imidazole, 2 mM β-mercaptoethanol, 0.2 mM GTP, 3 mM desthiobiotin, pH 7.2). The StrepTrap eluate was mixed with 4 mg of previously purified TEV protease (~8 mg/ml stored in 40 mM HEPES, 150 mM KCl, 30%(w/v) glycerol, 1 mM MgCl2, 3 mM β-mercaptoethanol, pH 7.5, and diluted into 5 ml StrepTrap elution buffer) and incubated for 2 hr on ice. The TEV-digested protein solution was loaded onto two 1 ml HiTrap SP Sepharose FF columns (GE life science 17–5054-01) followed, in tandem, by two 1 ml HisTrap HP columns, and washed with strep-elution buffer. The flow-through containing tubulin was pooled, concentrated with an Amicon Ultra 50K MWCO centrifugal filter unit (Millipore UFC901024), and loaded on to a Superdex 200 16/60 column (GE life science 17–1069-01) equilibrated in size-exclusion buffer (1XBRB80, 5%(w/v) glycerol, 0.2 mM GTP, 2 mM β-mercaptoethanol, pH 6.8). Tubulin eluted at ~80 ml and was concentrated to ~5 mg/ml with an Amicon Ultra 50K MWCO centrifugal filter unit. The purified tubulin was snap frozen in liquid nitrogen and stored at −80 ºC.
Labeling recombinant human tubulin with X-rhodamine succinimidyl ester
To label recombinant human tubulin with X-rhodamine succinimidyl ester, we polymerized microtubules using tubulin present in the flow-through of HiTrap SP/HisTrap columns as described above. The tubulin solution was concentrated to ~10 mg/ml with an Amicon Ultra 50K MWCO centrifugal filter unit, mixed with GMPCPP (final 1 mM), and incubated on ice for 5 min, and then polymerized by incubation at 37 °C for 45 mins. The polymerized microtubules were centrifuged through a high pH cushion (0.1 M Na-HEPES, 1 mM MgCl2, 1 mM EGTA, 60% glycerol, pH 8.6) at 90,000 rpm for 20 mins at 37 °C (TLA120.1 Beckman Coulter). At 37 °C, the microtubule pellet was rinsed with labeling buffer (0.1 M Na-HEPES, 1 mM MgCl2, 1 mM EGTA, 40% glycerol, pH 8.6) and suspended in 400 μl labeling buffer supplemented with 0.1 mM GMPCPP. A 20-fold molar excess of X-rhodamine succinimidyl ester (Fisher Scientific C6125, dissolved in DMSO) was added and incubated with microtubules at 37 °C for 45 mins, and then quenched with 400 μl quench buffer (2X BRB80, 100 mM K-glutamate, 40% glycerol). The labeled microtubules was centrifuged through a low pH cushion (1X BRB80, 60% glycerol) at 90,000 rpm for 20 mins at 37 °C (TLA120.2 Beckman Coulter). The microtubule pellet was rinsed with 1X BRB80 at 37 °C, suspended in 200 μl cold (4 °C) 1X IB buffer (50 mM K-glutamate, 0.5 mM MgCl2, pH 7.0), and dounce homogenized on ice every 5 mins for 60 mins, and then centrifuged at 90,000 rpm for 10 mins at 4 °C (TLA120.1 Beckman Coulter). The depolymerized tubulin was supplemented with 5X BRB80 (final 1X), MgCl2 (final 4 mM) and GMPCPP (final 1 mM), incubated on ice for 5 mins, then at 37 °C for 1 hr, and then centrifuged at 90,000 rpm for 10 mins at 37 °C (TLA120.1 Beckman Coulter). The microtubule pellet was rinsed with warm (37 °C) 1X BRB80, suspended in 100 μl 1X IB, and then depolymerized on ice for 45 mins. The labeled tubulin was further clarified by centrifugation at 90,000 rpm for 10 mins at 4 °C (TLA120.1 Beckman Coulter). The concentrations of tubulin and X-rhodamine were measured by the absorption at 280 nm and 585 nm using the following equations. X-rhodamine labeling of α1B/β2B-tubulin: ~90%; and α1B/β3-tubulin: ~50%.
[Tub]={[A280nm-(A585nm*dye contribution at 280 nm)] x dilution factor}/ ε280nm, tubX-Rh dye contribution at 280 nm is 0.2
ε280nm, tub=115000
[X-Rh]=(A585nm*dilution factor)/ ε585nm, Rh
ε585nm, Rh=80000
Purification of MAPs
We purified rigor kinesin-1 motor domain (K347 CLM), GFP-tagged MCAK and GFP-tagged chTOG according to published protocols (Cooper et al., 2010; Miller et al., 2016; Rice et al., 1999).
Microtubule preparation for cryo-EM
We observed that the polymerization of GTP-bound α1B/β2B- or α1B/β3-tubulin was not efficient when we employed published protocols for bovine tubulin (i.e., glycerol or DMSO). Therefore, to polymerize GMPCPP-stabilized microtubules, we revised the published protocol (Alushin et al., 2014).
We first prepared α1B/β2B or α1B/β3 microtubule seeds. Tubulin were thawed, mixed with GMPCPP (final 1.5 mM), diluted to ~ 1.5 mg/ml with 1XBRB80+5% glycerol, centrifuged at 90,000 rpm for 10 min at 4 °C (TLA120.1 Beckman Coulter), and then polymerized by incubation at 37 °C for 30 mins. The microtubules were pelleted at 90,000 rpm for 10 min at 37 °C (TLA120.1 Beckman Coulter) and re-suspended in warm (37 °C) 1XBRB80 supplemented with 1 mM TCEP. Next, we used these microtubule seeds to polymerize GTP-bound α1B/β2B- or α1B/β3-tubulin. Another aliquot of recombinant tubulin was thawed, diluted to a final concentration ~3 mg/ml (1XBRB80, 33% glycerol, 1 mM GTP), and spun at 90,000 rpm for 10 min at 4 °C (TLA120.1 Beckman Coulter). After incubation at 37 °C for 2 mins, the supernatant was mixed with GMPCPP-seeds from the prior step and then incubated at 37 °C for 30 mins followed by centrifugation at 90,000 rpm for 10 min at 37 °C (TLA120.1 Beckman Coulter). The microtubule pellets were rinsed twice with 100 μl warm (37 °C) EM buffer (1X BRB80, 1 mM DTT, 0.1 mM ATP, 0.05% Nonidet P-40) before suspending in 30 μl cold EM buffer and then incubated on ice for 1 hr. After a centrifugation at 90,000 rpm for 10 min at 4 °C, the supernatant containing depolymerized GDP-tubulin (~2 mg/ml, measured by Bradford assay) was mixed with GMPCPP (final 2 mM) and then incubated on ice for 10 mins. After an incubation at 37 °C for 2 mins, the protein solution was mixed with 30 μl warm (37 °C) EM buffer followed by 37 °C incubation for another 1 hr. The polymerized GMPCPP-microtubules were pelleted by 90,000 rpm for 10 min at 37 °C (TLA120.1 Beckman Coulter) and suspended in warm (37 °C) EM buffer.
For taxol-stabilized microtubules, α1B/β2B and α1B/β3 tubulin were thawed, mixed with GTP (final concentration 2 mM), diluted to ~ 1 mg/ml with 1XBRB80+5% glycerol, and centrifuged at 90,000 rpm for 10 min at 4 °C (TLA120.1 Beckman Coulter) to remove any aggregates from the freeze-thaw cycle. After incubation at 37 °C for 2 mins, the supernatant was mixed with 1 μM taxol (final 0.1 μM, 37 °C 10 mins), followed by mixing with 10 μM taxol (final 1 μM, 37 °C 10 mins), and then mixed with 100 μM taxol (final 10 μM, 37 °C 15 mins). The microtubules were pelleted at 90,000 rpm for 10 min at 37 °C (TLA120.1 Beckman Coulter) and re-suspended in warm (37 °C) EM buffer containing 15 μM taxol.
Cryo-EM microscopy grid preparation and imaging
Taxol- and GMPCPP-microtubules were diluted to ~0.25 mg/ml in EM buffer. Microtubules were applied to a glow discharged C-flat grid (Electron Microscopy Sciences, 71150) in the chamber of a Vitrobot (ThermoFisher, Mark IV) set to 25 °C and 100% relative humidity. Microtubules were allowed to adhere to the grid for 30 sec, and rigor kinesin-1 motor domain in EM buffer was then added to the grid. After another 30 sec incubation, the grid was then blotted for 4 s and plunged into ethane slush. Micrographs were collected on a Titan Krios electron microscope operated at 300kV (GMPCPP-microtubules) or a Titan Arctica electron microscope operated at 200kV (taxol-microtubules) both equipped with Gatan K2 direct electron detectors. Micrographs were collected in superresolution mode using SerialEM with a nominal magnification of 29,000× (Krios) or 28,000× (Arcitca), giving a final non-superresolution pixel size of 1 Å per pixel (Krios) or 1.4375 Å per pixel (Arctica). Fifty frames of 200 ms each were collected with a defocus range from 1 to 2.4 μm at a dose rate of 8 e- per pixel per sec.
Cryo-EM image analysis and data processing
Movie frames were gain-normalized using the “clip” function from the IMOD processing suite (Mastronarde and Held, 2017), then aligned with Unblur (Grant and Grigorieff, 2015) for CTF estimation and particle picking. Unless otherwise noted, 2D processing steps were carried out using Relion (Scheres, 2012). Contrast transfer function (CTF) estimation was performed using CTFFIND4 (Rohou and Grigorieff, 2015) on summed images which had not been dose-weighted. Overlapping microtubule segments were then picked with a 512 pixel window every 80 Å along the filament axis, resulting in approximately 1 layer of unique tubulin dimers per segment, and extracted with alignparts_lmbfgs from frames 2 to 50 using per-particle alignments and dose-weighting (Rubinstein and Brubaker, 2015). Segments were then subjected to reference-free 2D classification, and those contributing to well-defined class averages were selected for further processing.
Adapting a workflow which has previously been described for initial 3D classification (Alushin et al., 2014; Zhang et al., 2015), alignment and reconstruction was performed utilizing functions from the EMAN2 / SPARX libraries (Hohn et al., 2007; Tang et al., 2007), followed by final refinement and reconstruction of individual classes in FREALIGN (Lyumkis et al., 2013). To generate references, a single protofilament was extracted from EMD-6352, and initial models of 11–3, 12–3, 13–3, 14–3, 14–4, 15–3, 15–4, 16–3 and 16–4 protofilament microtubules were generated using previously determined helical parameters (Sui and Downing, 2010) and low-pass filtered to 35 Å resolution. A multi-reference Iterative Helical Real Space Reconstruction (IHRSR) procedure adapted to appropriately symmetrize microtubules featuring a seam was then performed as described (Alushin et al., 2014), estimating helical parameters with the program hsearch_lorentz (Egelman, 2007).
Segments corresponding to 13–3 (for GMPCPP α1B/β3) and 14–3 microtubules (for GMPCPP α1B/β2B) were then selected for further processing. Reconstructions corresponding to these classes from the multi-reference refinement were low-pass filtered to 35 Å, and then used as initial models for independent half-dataset refinement beginning with a global parameter search to minimize noise bias in refinement (Scheres and Chen, 2012), utilizing a recently described EMAN2/SPARX procedure (Kim et al., 2016) here adapted to implement microtubule symmetrization. Final refinement and reconstruction was performed in FREALIGN v9.11 (Lyumkis et al., 2013) without refinement of independent-half datasets but restricting information used for alignment to 6 Å resolution. High-resolution reconstructions generated with FREALIGN were symmetrized with a previously described procedure (Alushin et al., 2014; Zhang et al., 2015).
Width analysis of 2D Class Averages
1-D density projections were generated from 2D class averages made vertical after determining their in-plane orientations from the radon transforms of their power spectra (Li et al., 2002) with a procedure implemented in the SPIDER processing suite (Shaikh et al., 2008). The two largest peaks in these density profiles were identified with a python script using the SciPy function “scipy.signal.find_peaks_cwt”, which correspond to the microtubule boundaries. Histograms of microtubule segment widths were then plotted based on the distribution of the distances between these peaks weighted by class membership. All custom software used in cryo-EM structure determination and analysis is available at [https://github.com/alushinlab/microtubules].
Atomic model building, analysis, and visualization
Initial atomic models were generated in Coot (Emsley et al., 2010) by mutating residues of existing GMPCPP-bound tubulin models (PDB ID 3JAT) to match the human β2B and β3 sequence and fixing obvious regions of poor fit. For model refinement, nine copies of this model were then docked into segmented density from the final 13 protofilament maps and further refined using Phenix (Adams et al., 2010). The models were validated using EMRinger and MolProbity (Barad et al., 2015; Chen et al., 2010). UCSF Chimera was used to perform RMSD calculations and prepare molecular graphics illustrations (Pettersen et al., 2004).
Microtubule preparation for fluorescence microscopy assay
GMPCPP- and taxol-microtubules for TIRF assays were polymerized as for cryo-EM with minor changes. First, instead of EM buffer, BRB80 supplemented with 1 mM TCEP was used. Second, to visualize and immobilize microtubules in TIRF assays, α1B/β2B or α1B/β3 tubulin was polymerized with ~3.5 mol% of X-rhodamine-labeled and ~3.5 mol% of biotin-labeled bovine brain tubulin. The bovine brain tubulin was purified and labeled with standard published protocols (Gell et al., 2011; Hyman et al., 1991). X-rhodamine labeling is ~92%, and biotin labeling is ~95%.
Fluorescence Microscopy assays
Microscope setup, TIRF sample chambers, and assay conditions were similar to published protocols (Ti et al., 2016). Assays were done in buffer containing 1XBRB80, 5% sucrose, 1 mM MgCl2, 0.25 mg/ml κ-casein and oxygen scanvenging mix (0.2 mg/ml glucose oxidase, 0.035 mg/ml catalase, 4.5 mg/ml glucose, 143 mM β-mercaptoethanol). 10 μM paclitaxel was added in the buffer for experiments using taxol-stabilized microtubules. 1 mM Mg-ATP was added in the buffer for MCAK experiments. Time-lapse images were acquired at a rate of 1 frame/1 min for passive depolymerization assays and at 1 frame/5 sec for MCAK- and chTOG-dependent depolymerization assays. All fluorescence microscopy experiments were carried out at room temperature.
Quantification and Statistical Analysis
Data presented in the text are expressed as the mean ± standard deviation. Statistical significance was determined using two-sample unequal variance t-test with two-tailed distribution. Replicates, number of quantified microtubules and statistical results are indicated in figure legends for the respective expreiments.
Supplementary Material
Acknowledgments
This research was supported by the NIH (GM65933, PI: TMK, 7DP5OD017885, PI: GMA). We also thank Mark Ebrahim and Johanna Sotiris (Cryo-EM Resource Center, Rockefeller University) for support with cryo-EM data collection, and Elizabeth Kellogg and Eva Nogales (UC Berkeley) for assistance with microtubule cryo-EM data processing software.
Footnotes
Declaration of Interests
The authors declare no competing financial interests.
Data and Software Availability
Microtubule cryo-EM reconstructions and corresponding atomic models have been deposited with the following accession numbers: 13-α1B/β3; EMDB (8997) RCSB-PDB (6E7B); 14-α1B/β2B; EMDB (8998) RCSB-PDB (6E7C). All custom software used in data analysis is available at https://github.com/alushinlab/microtubules.
References
- Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, et al. (2010). PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr 66, 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allegretti M, Mills DJ, McMullan G, Kuhlbrandt W, and Vonck J (2014). Atomic model of the F420-reducing [NiFe] hydrogenase by electron cryo-microscopy using a direct electron detector. Elife 3, e01963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alushin GM, Lander GC, Kellogg EH, Zhang R, Baker D, and Nogales E (2014). High-resolution microtubule structures reveal the structural transitions in alphabeta-tubulin upon GTP hydrolysis. Cell 157, 1117–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amos LA, and Schlieper D (2005). Microtubules and maps. Adv Protein Chem 71, 257–298. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Roach MC, Wall KA, Lopata MA, Cleveland DW, and Luduena RF (1988). A monoclonal antibody against the type II isotype of beta-tubulin. Preparation of isotypically altered tubulin. J Biol Chem 263, 3029–3034. [PubMed] [Google Scholar]
- Barad BA, Echols N, Wang RY, Cheng Y, DiMaio F, Adams PD, and Fraser JS (2015). EMRinger: side chain-directed model and map validation for 3D cryo-electron microscopy. Nature methods 12, 943–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechstedt S, and Brouhard GJ (2012). Doublecortin recognizes the 13-protofilament microtubule cooperatively and tracks microtubule ends. Dev Cell 23, 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit M, Asenjo AB, and Sosa H (2018). Cryo-EM reveals the structural basis of microtubule depolymerization by kinesin-13s. Nature communications 9, 1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieling P, Laan L, Schek H, Munteanu EL, Sandblad L, Dogterom M, Brunner D, and Surrey T (2007). Reconstitution of a microtubule plus-end tracking system in vitro. Nature 450, 1100–1105. [DOI] [PubMed] [Google Scholar]
- Brouhard GJ, Stear JH, Noetzel TL, Al-Bassam J, Kinoshita K, Harrison SC, Howard J, and Hyman AA (2008). XMAP215 is a processive microtubule polymerase. Cell 132, 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaaban S, and Brouhard GJ (2017). A microtubule bestiary: structural diversity in tubulin polymers. Mol Biol Cell 28, 2924–2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M, and Thomson JN (1982). Structural and functional diversity in the neuronal microtubules of Caenorhabditis elegans. J Cell Biol 93, 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen VB, Arendall WB 3rd, Headd JJ, Keedy DA, Immormino RM, Kapral GJ, Murray LW, Richardson JS, and Richardson DC (2010). MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr D Biol Crystallogr 66, 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chretien D, and Wade RH (1991). New data on the microtubule surface lattice. Biol Cell 71, 161–174. [DOI] [PubMed] [Google Scholar]
- Cleveland DW, Hwo SY, and Kirschner MW (1977). Purification of tau, a microtubule-associated protein that induces assembly of microtubules from purified tubulin. J Mol Biol 116, 207–225. [DOI] [PubMed] [Google Scholar]
- Conde C, and Caceres A (2009). Microtubule assembly, organization and dynamics in axons and dendrites. Nat Rev Neurosci 10, 319–332. [DOI] [PubMed] [Google Scholar]
- Cooper JR, Wagenbach M, Asbury CL, and Wordeman L (2010). Catalysis of the microtubule on-rate is the major parameter regulating the depolymerase activity of MCAK. Nature structural & molecular biology 17, 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz JF, Valpuesta JM, Chacon P, Diakun G, and Andreu JM (1998). Changes in microtubule protofilament number induced by Taxol binding to an easily accessible site. Internal microtubule dynamics. J Biol Chem 273, 33803–33810. [DOI] [PubMed] [Google Scholar]
- Egelman EH (2007). The iterative helical real space reconstruction method: surmounting the problems posed by real polymers. Journal of structural biology 157, 83–94. [DOI] [PubMed] [Google Scholar]
- Emsley P, Lohkamp B, Scott WG, and Cowtan K (2010). Features and development of Coot. Acta Crystallogr D Biol Crystallogr 66, 486–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushige T, Siddiqui ZK, Chou M, Culotti JG, Gogonea CB, Siddiqui SS, and Hamelin M (1999). MEC-12, an alpha-tubulin required for touch sensitivity in C. elegans. J Cell Sci 112 ( Pt 3), 395–403. [DOI] [PubMed] [Google Scholar]
- Gell C, Friel CT, Borgonovo B, Drechsel DN, Hyman AA, and Howard J (2011). Purification of tubulin from porcine brain. Methods Mol Biol 777, 15–28. [DOI] [PubMed] [Google Scholar]
- Ghosh-Roy A, Goncharov A, Jin Y, and Chisholm AD (2012). Kinesin-13 and tubulin posttranslational modifications regulate microtubule growth in axon regeneration. Dev Cell 23, 716–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant T, and Grigorieff N (2015). Measuring the optimal exposure for single particle cryo-EM using a 2.6 A reconstruction of rotavirus VP6. Elife 4, e06980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Walss-Bass C, and Luduena RF (2010). The beta isotypes of tubulin in neuronal differentiation. Cytoskeleton (Hoboken) 67, 431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius J, Brouhard G, Kalaidzidis Y, Diez S, and Howard J (2006). The depolymerizing kinesin MCAK uses lattice diffusion to rapidly target microtubule ends. Nature 441, 115–119. [DOI] [PubMed] [Google Scholar]
- Hohn M, Tang G, Goodyear G, Baldwin PR, Huang Z, Penczek PA, Yang C, Glaeser RM, Adams PD, and Ludtke SJ (2007). SPARX, a new environment for Cryo-EM image processing. Journal of structural biology 157, 47–55. [DOI] [PubMed] [Google Scholar]
- Homma N, Takei Y, Tanaka Y, Nakata T, Terada S, Kikkawa M, Noda Y, and Hirokawa N (2003). Kinesin superfamily protein 2A (KIF2A) functions in suppression of collateral branch extension. Cell 114, 229–239. [DOI] [PubMed] [Google Scholar]
- Honda Y, Tsuchiya K, Sumiyoshi E, Haruta N, and Sugimoto A (2017). Tubulin isotype substitution revealed that isotype combination modulates microtubule dynamics in C. elegans embryos. J Cell Sci 130, 1652–1661. [DOI] [PubMed] [Google Scholar]
- Hoyle HD, and Raff EC (1990). Two Drosophila beta tubulin isoforms are not functionally equivalent. J Cell Biol 111, 1009–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter AW, Caplow M, Coy DL, Hancock WO, Diez S, Wordeman L, and Howard J (2003). The kinesin-related protein MCAK is a microtubule depolymerase that forms an ATP-hydrolyzing complex at microtubule ends. Mol Cell 11, 445–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman A, Drechsel D, Kellogg D, Salser S, Sawin K, Steffen P, Wordeman L, and Mitchison T (1991). Preparation of modified tubulins. Methods Enzymol 196, 478–485. [DOI] [PubMed] [Google Scholar]
- Kellogg EH, Hejab NMA, Howes S, Northcote P, Miller JH, Diaz JF, Downing KH, and Nogales E (2017). Insights into the Distinct Mechanisms of Action of Taxane and Non-Taxane Microtubule Stabilizers from Cryo-EM Structures. J Mol Biol 429, 633–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Binder LI, and Rosenbaum JL (1979). The periodic association of MAP2 with brain microtubules in vitro. J Cell Biol 80, 266–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim LY, Thompson PM, Lee HT, Pershad M, Campbell SL, and Alushin GM (2016). The Structural Basis of Actin Organization by Vinculin and Metavinculin. J Mol Biol 428, 10–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita K, Arnal I, Desai A, Drechsel DN, and Hyman AA (2001). Reconstitution of physiological microtubule dynamics using purified components. Science 294, 1340–1343. [DOI] [PubMed] [Google Scholar]
- Kleiner RE, Ti SC, and Kapoor TM (2013). Site-specific chemistry on the microtubule polymer. J Am Chem Soc 135, 12520–12523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, DeRosier DJ, Nicholson WV, Nogales E, and Downing KH (2002). Microtubule structure at 8 A resolution. Structure 10, 1317–1328. [DOI] [PubMed] [Google Scholar]
- Ludueña RF, and Banerjee A (2008). The Isotypes of Tubulin In The Role of Microtubules in Cell Biology, Neurobiology, and Oncology, Fojo T, ed. (Humana Press; ), pp. 123–175. [Google Scholar]
- Lyumkis D, Brilot AF, Theobald DL, and Grigorieff N (2013). Likelihood-based classification of cryo-EM images using FREALIGN. Journal of structural biology 183, 377–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastronarde DN, and Held SR (2017). Automated tilt series alignment and tomographic reconstruction in IMOD. Journal of structural biology 197, 102–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memet E, Hilitsk F, Morris MA, Schwenger WJ, Dogic Z, and Mahadevan L (2018). Microtubules soften due to cross-sectional flattening. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MP, Asbury CL, and Biggins S (2016). A TOG Protein Confers Tension Sensitivity to Kinetochore-Microtubule Attachments. Cell 165, 1428–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz M, Braunfeld MB, Guenebaut V, Heuser J, and Agard DA (2000). Structure of the gamma-tubulin ring complex: a template for microtubule nucleation. Nat Cell Biol 2, 365–370. [DOI] [PubMed] [Google Scholar]
- Nogales E (2000). Structural insights into microtubule function. Annu Rev Biochem 69, 277–302. [DOI] [PubMed] [Google Scholar]
- Nogales E, Wolf SG, and Downing KH (1998). Structure of the alpha beta tubulin dimer by electron crystallography. Nature 391, 199–203. [DOI] [PubMed] [Google Scholar]
- Pamula MC, Ti SC, and Kapoor TM (2016). The structured core of human beta tubulin confers isotype-specific polymerization properties. J Cell Biol 213, 425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, and Ferrin TE (2004). UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem 25, 1605–1612. [DOI] [PubMed] [Google Scholar]
- Portran D, Schaedel L, Xu Z, Thery M, and Nachury MV (2017). Tubulin acetylation protects long-lived microtubules against mechanical ageing. Nat Cell Biol 19, 391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff EC, Fackenthal JD, Hutchens JA, Hoyle HD, and Turner FR (1997). Microtubule architecture specified by a beta-tubulin isoform. Science 275, 70–73. [DOI] [PubMed] [Google Scholar]
- Rice S, Lin AW, Safer D, Hart CL, Naber N, Carragher BO, Cain SM, Pechatnikova E, Wilson-Kubalek EM, Whittaker M, et al. (1999). A structural change in the kinesin motor protein that drives motility. Nature 402, 778–784. [DOI] [PubMed] [Google Scholar]
- Rohou A, and Grigorieff N (2015). CTFFIND4: Fast and accurate defocus estimation from electron micrographs. Journal of structural biology 192, 216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roostalu J, Cade NI, and Surrey T (2015). Complementary activities of TPX2 and chTOG constitute an efficient importin-regulated microtubule nucleation module. Nat Cell Biol 17, 1422–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein JL, and Brubaker MA (2015). Alignment of cryo-EM movies of individual particles by optimization of image translations. Journal of structural biology 192, 188–195. [DOI] [PubMed] [Google Scholar]
- Sano K, Maeda K, Oki M, and Maeda Y (2002). Enhancement of protein expression in insect cells by a lobster tropomyosin cDNA leader sequence. FEBS Lett 532, 143–146. [DOI] [PubMed] [Google Scholar]
- Savage C, Hamelin M, Culotti JG, Coulson A, Albertson DG, and Chalfie M (1989). mec-7 is a beta-tubulin gene required for the production of 15-protofilament microtubules in Caenorhabditis elegans. Genes & development 3, 870–881. [DOI] [PubMed] [Google Scholar]
- Scheres SH (2012). RELION: implementation of a Bayesian approach to cryo-EM structure determination. Journal of structural biology 180, 519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheres SH, and Chen S (2012). Prevention of overfitting in cryo-EM structure determination. Nature methods 9, 853–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger A, and Rost B (2005). Protein flexibility and rigidity predicted from sequence. Proteins 61, 115–126. [DOI] [PubMed] [Google Scholar]
- Shaikh TR, Gao H, Baxter WT, Asturias FJ, Boisset N, Leith A, and Frank J (2008). SPIDER image processing for single-particle reconstruction of biological macromolecules from electron micrographs. Nature protocols 3, 1941–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirajuddin M, Rice LM, and Vale RD (2014). Regulation of microtubule motors by tubulin isotypes and post-translational modifications. Nat Cell Biol 16, 335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Kirkpatrick LL, Schilling AB, Helseth DL, Chabot N, Keillor JW, Johnson GV, and Brady ST (2013). Transglutaminase and polyamination of tubulin: posttranslational modification for stabilizing axonal microtubules. Neuron 78, 109–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srayko M, Kaya A, Stamford J, and Hyman AA (2005). Identification and characterization of factors required for microtubule growth and nucleation in the early C. elegans embryo. Dev Cell 9, 223–236. [DOI] [PubMed] [Google Scholar]
- Sui H, and Downing KH (2010). Structural basis of interprotofilament interaction and lateral deformation of microtubules. Structure 18, 1022–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka E, Ho T, and Kirschner MW (1995). The role of microtubule dynamics in growth cone motility and axonal growth. J Cell Biol 128, 139–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G, Peng L, Baldwin PR, Mann DS, Jiang W, Rees I, and Ludtke SJ (2007). EMAN2: an extensible image processing suite for electron microscopy. Journal of structural biology 157, 38–46. [DOI] [PubMed] [Google Scholar]
- Ti SC, Pamula MC, Howes SC, Duellberg C, Cade NI, Kleiner RE, Forth S, Surrey T, Nogales E, and Kapoor TM (2016). Mutations in Human Tubulin Proximal to the Kinesin-Binding Site Alter Dynamic Instability at Microtubule Plus- and Minus-Ends. Dev Cell 37, 72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney LG, Bryan J, Bush DJ, Fujiwara K, Mooseker MS, Murphy DB, and Snyder DH (1973). Microtubules: evidence for 13 protofilaments. J Cell Biol 59, 267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemu A, Atherton J, Spector JO, Szyk A, Moores CA, and Roll-Mecak A (2016). Structure and Dynamics of Single-isoform Recombinant Neuronal Human Tubulin. J Biol Chem 291, 12907–12915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitre B, Coquelle FM, Heichette C, Garnier C, Chretien D, and Arnal I (2008). EB1 regulates microtubule dynamics and tubulin sheet closure in vitro. Nat Cell Biol 10, 415–421. [DOI] [PubMed] [Google Scholar]
- von Loeffelholz O, Venables NA, Drummond DR, Katsuki M, Cross R, and Moores CA (2017). Nucleotide- and Mal3-dependent changes in fission yeast microtubules suggest a structural plasticity view of dynamics. Nature communications 8, 2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak CE, Gayek S, and Ohi R (2013). Microtubule-depolymerizing kinesins. Annual review of cell and developmental biology 29, 417–441. [DOI] [PubMed] [Google Scholar]
- Witte H, Neukirchen D, and Bradke F (2008). Microtubule stabilization specifies initial neuronal polarization. J Cell Biol 180, 619–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, and Afzelius BA (1988). Early changes in the substructure of the marginal bundle in human blood platelets responding to adenosine diphosphate. J Ultrastruct Mol Struct Res 99, 254–260. [DOI] [PubMed] [Google Scholar]
- Xu Z, Schaedel L, Portran D, Aguilar A, Gaillard J, Marinkovich MP, Thery M, and Nachury MV (2017). Microtubules acquire resistance from mechanical breakage through intralumenal acetylation. Science 356, 328–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Alushin GM, Brown A, and Nogales E (2015). Mechanistic Origin of Microtubule Dynamic Instability and Its Modulation by EB Proteins. Cell 162, 849–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Wong ML, Alberts B, and Mitchison T (1995). Nucleation of microtubule assembly by a gamma-tubulin-containing ring complex. Nature 378, 578–583. [DOI] [PubMed] [Google Scholar]
- Zhou H, and Zhou Y (2004). Quantifying the effect of burial of amino acid residues on protein stability. Proteins 54, 315–322. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


