Abstract
In healthy women, the cervicovaginal microbiota is mostly populated by Lactobacillus spp., the main host defense factor of the female genital tract. In addition to Lactobacilli, other microorganisms populate the cervicovaginal microbiota, like Candida spp. and Gardnerella vaginalis. The overgrowth of Candida spp. or G. vaginalis, known as biofilm-producing microorganisms in the genital ecosystem, may lead to microbial dysbiosis that increases the risk of acquiring sexually transmitted infections, like Chlamydia trachomatis. C. trachomatis, the leading cause of bacterial sexually transmitted diseases, is still considered an important public health problem worldwide because of the impact of asymptomatic infections on long-term reproductive sequelae, including pelvic inflammatory disease and infertility. The aim of our study was to investigate the interaction between C. trachomatis and the biofilm produced by Candida albicans or Gardnerella vaginalis, evaluating whether the biofilm can harbor C. trachomatis and influence its survival as well as its infectious properties. In order to do so, we developed an in vitro coculture transwell-based biofilm model. Our findings proved, for the first time, that C. trachomatis, an intracellular obligate pathogen, survived, for up to 72 hours after exposure, inside the biofilm produced by C. albicans or G. vaginalis, retaining its infectious properties, as evidenced by the typical chlamydial inclusions observed in the cell monolayer (chlamydial inclusion-forming units at 72 h: 9255 ± 1139 and 9873 ± 1015, respectively). In conclusion, our results suggest that the biofilm related to Candida or Gardnerella genital infections may act as a reservoir of C. trachomatis and, thus, contribute to the transmission of the infection in the population as well as to its dissemination into the upper genital tract, increasing the risk of developing severe reproductive sequelae.
1. Introduction
In healthy women, the cervicovaginal microbiota is known to play a fundamental role in the defense of the female genital tract against potential infectious threats. A microbiota dominated by Lactobacillus spp. is classically associated with a healthy genital ecosystem [1, 2], since it has been shown to inhibit the growth of potential pathogens by competing for nutrients, releasing antimicrobial compounds, activating immune system pathways, and maintaining a low vaginal pH through the production of lactic acid [3–5].
In addition to Lactobacilli, other microorganisms populate the cervicovaginal microbiota, including Candida spp. and Gardnerella vaginalis. The overgrowth of Candida spp. or G. vaginalis and the depletion of Lactobacillus spp. may lead to a microbial imbalance that can precipitate in a genital infection [2].
Amongst genital infections, vulvovaginal candidiasis and bacterial vaginosis are the most frequent disorders in reproductive women, contributing to 90% of all cases of vaginitis [6]. Candida albicans is known as the most common cause of vulvovaginal candidiasis; in fact, almost 75% of the female population experience an episode at least once in their lifetime, 50% of whom experience at least a second episode, and 5–10% of all women experience recurrent vulvovaginal candidiasis [7]. Also, bacterial vaginosis, responsible for more than 60% of all cases of vaginitis in women of childbearing age, where Gardnerella vaginalis is the predominant bacterial species [8], has a 60% recurrence rate in the 12 months after metronidazole treatment [9].
The main virulence trait associated with recurrent C. albicans or G. vaginalis genital infections is the formation of a biofilm [8, 10], characterized by complex microbial communities attached to a substrate and surrounded by an extracellular matrix [11].
Importantly, biofilm-related infections have a unique clinical significance due to the tendency of embedded pathogens to harbor resistance against host defense factors and antimicrobial agents [12]. Indeed, it is well known that the biofilm may be responsible for treatment failure as well as recurrence of genital infections [13, 14]. In this regard, the observation of the presence of Candida on intrauterine devices (IUDs) removed from patients with genital infections is particularly interesting, suggesting that biofilm formation on IUDs might be an important risk factor for recurrent candidiasis [15]. Furthermore, the use of IUDs seems to also increase the risk for bacterial vaginosis [16].
In recent years, evidence that the C. albicans biofilm is capable of retaining herpes simplex virus type-1 without altering its infectivity led to the compelling hypothesis that the biofilm could potentially be a reservoir of sexually transmitted pathogens [17]. Consequently, it is tempting to speculate that the biofilm produced by C. albicans or G. vaginalis may also increase the risk of acquiring Chlamydia trachomatis, known as the leading cause of bacterial sexually transmitted diseases.
C. trachomatis is an obligate intracellular pathogen with a distinctive developmental cycle characterized by the extracellular infectious elementary body (EB), that invades the host cell, and the intracellular replicative reticulate body (RB), responsible for the multiplication within the host [18].
C. trachomatis is still considered an important public health problem worldwide because of the impact of asymptomatic infections (90%) on long-term reproductive sequelae, including pelvic inflammatory disease (PID) [19]. Particularly important, an increased risk of PID has also been observed in IUD users affected by C. trachomatis infection [20, 21].
Therefore, the aim of our study was to investigate the interaction between C. trachomatis and the biofilm produced by C. albicans or G. vaginalis. Specifically, we analyzed whether the biofilm can harbor C. trachomatis and influence its survival as well as its infectious properties.
2. Materials and Methods
2.1. Microbial Strains
The strains of Candida albicans (ATCC 10231), Gardnerella vaginalis (ATCC 4944), and Chlamydia trachomatis serovar D/UW-3Cx (ATCC VR-885), used in this study, were obtained from the American Type Culture Collection (ATCC), USA.
2.2. Cell Culture
The human epithelial HeLa-229 cell line (ATCC CCL-2.1) from the cervix adenocarcinoma was cultured at 37°C in Dulbecco's modified Eagle's medium (DMEM, EuroClone), supplemented with 10% (v/v) heat-inactivated fetal calf serum (FCS), in a humidified atmosphere with 5% CO2.
2.3. Propagation and Titration of Chlamydia trachomatis
C. trachomatis was propagated in HeLa-229 cells and grown in DMEM supplemented with 10% FCS, as previously described [22]. The infectious titer (inclusion-forming units per mL (IFUs/mL)) was assessed by the immunofluorescence assay. In brief, subconfluent HeLa-229 cell monolayers grown on glass coverslips in 24-well plates were infected with 10-fold serial dilutions of bacterial stock, incubated for 48 hours at 37°C, fixed with methanol, and stained with fluorescein isothiocyanate-conjugated monoclonal antibody anti-C. trachomatis LPS (IMAGEN Chlamydia kit, Oxoid). The total number of C. trachomatis IFUs was enumerated by counting all microscope fields using a fluorescence microscope (400x magnification).
2.4. Biofilm Formation
Transwell coculture systems (0.4 µm pore size, polyester membrane, Corning) were used for C. albicans or G. vaginalis biofilm formation (Figure 1). In brief, C. albicans was grown overnight at 37°C in yeast peptone dextrose, harvested, washed twice with phosphate-buffered saline (PBS), and then resuspended at a concentration of 1 × 106 cells/mL in RPMI supplemented with 10% FCS. Next, 500 µL of C. albicans cell suspension was seeded in triplicate on the insert membranes placed in a 24-well tissue plate and incubated at 37°C to allow the biofilm production.
Figure 1.

Experimental setup. C. albicans or G. vaginalis biofilms were produced in the upper chamber of transwell coculture systems. After 24 hours, C. trachomatis (as indicated by the arrow) was inoculated onto the biofilm, and epithelial cell monolayers, grown on glass coverslips, were seeded in the lower chamber.
G. vaginalis was grown in brain heart infusion supplemented with 2% (w/w) gelatin, 0.5% (w/w) yeast extract, and 0.1% (w/w) starch for 24 hours at 37°C with 10% CO2 and, then, diluted to a final concentration of approximately 107 colony-forming units (CFUs)/mL. Next, 500 µL of G. vaginalis suspension was seeded in triplicate on the insert membranes and incubated as described above to allow the biofilm production.
The next day, the culture medium was removed, and the inserts were washed once with PBS to eliminate nonadherent microbial cells. Biofilm formation was assessed by the crystal violet assay.
2.5. Exposure of Biofilms to C. trachomatis
After 24 hours, 100 µL of C. trachomatis (2.5 × 106 EBs) in DMEM with 10% FCS was added onto Candida or Gardnerella biofilms, and at the same time, subconfluent HeLa-229 cell monolayers, grown on glass coverslips, were seeded into the lower chamber (Figure 1). The transwell systems were, then, incubated at 37°C and 5% CO2. Twelve, 24, 48, and 72 hours later, the cell monolayers were removed from the lower chamber, and the presence of C. trachomatis was determined by the immunofluorescence assay, as previously described [22].
In other experiments, the inoculum of C. trachomatis and the cell suspensions of C. albicans or G. vaginalis were simultaneously added on the insert membranes, and after 48 hours, the amount of biofilm was quantified by the crystal violet assay.
2.6. Crystal Violet Assay
Inserts containing Candida or Gardnerella biofilms or controls (medium only) were washed 3 times with PBS and then air-dried for 5 min. Following fixation by 96% methanol for 20 min, the samples were stained with 1% crystal violet (CV) solution for 5 min. After three washes with distilled water, 1 mL of 33% acetic acid was added to each well. The optical densities (ODs) were measured at 594 nm 10 min later by using a microplate reader (Biotek).
2.7. Statistical Analysis
All values are expressed as mean ± standard deviation (SD) of three replicates from three independent experiments. Comparison of means was performed by using a two-tailed Student's t-test for independent samples. A value of P ≤ 0.05 was considered statistically significant.
3. Results
As expected, C. albicans and G. vaginalis produced biofilms on the transwell membranes 24 hours after the inoculation, as evidenced by the crystal violet assay (OD595 nm values: 1.1 ± 0.3 and 2.2 ± 0.9, respectively).
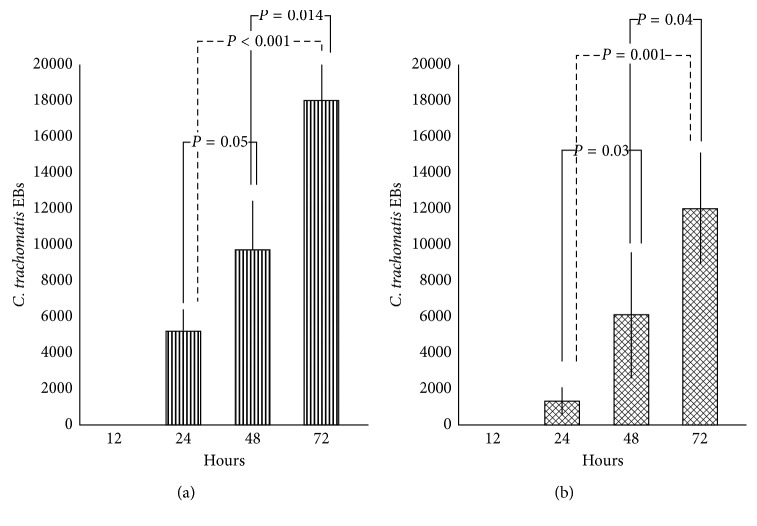
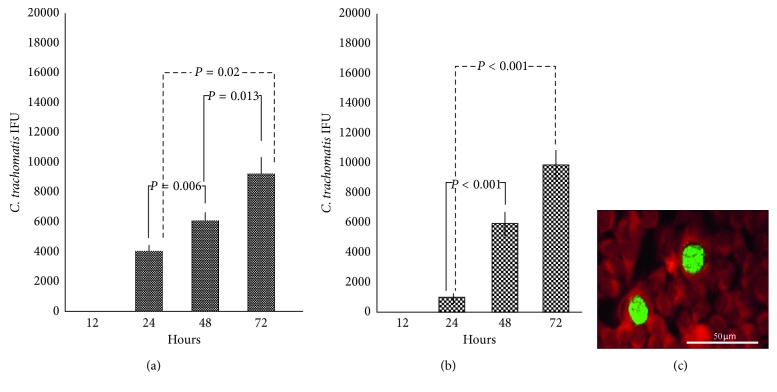
Then, we evidenced that the biofilm produced by C. albicans or G. vaginalis was able to retain C. trachomatis for up to 12 hours, as evidenced by the absence of EBs in cell monolayers removed from the lower chamber of the transwell system. Interestingly, the number of chlamydial EBs released from the Candida or Gardnerella biofilm increased significantly in a time-dependent manner (Table 1; Figures 2(a) and 2(b)), and more importantly, C. trachomatis was still able to infect and replicate within host cells (Table 2; Figures 3(a) and 3(b)). In fact, typical inclusions were found in cell monolayers removed from the lower chamber of transwell systems, confirming the ability of C. trachomatis to complete its developmental cycle (Figure 3(c)).
Table 1.
C. trachomatis EBs released from C. albicans or G. vaginalis biofilms 24, 48, and 72 hours after exposure.
| Biofilm | Number of C. trachomatis EBs | ||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| C. albicans | 5200 ± 1210 | 9700 ± 2750 | 18000 ± 2234 |
| G. vaginalis | 1300 ± 797 | 6100 ± 3513 | 12000 ± 3137 |
EB: elementary body.
Figure 2.
Release of C. trachomatis EBs from biofilms. Number of C. trachomatis EBs released from C. albicans (a) or G. vaginalis (b) biofilms detected at 24-hour intervals up to 72 hours from initial exposure.
Table 2.
Number of C. trachomatis IFUs observed on HeLa cell monolayers 24, 48, and 72 hours after exposure.
| Biofilm | Number of C. trachomatis IFUs | ||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| C. albicans | 4100 ± 375 | 6122 ± 557 | 9255 ± 1139 |
| G. vaginalis | 982 ± 298 | 5925 ± 807 | 9873 ± 1015 |
IFU: inclusion-forming unit.
Figure 3.
Infection of HeLa cell monolayers following C. trachomatis release from biofilms. Infectivity of C. trachomatis EBs released from C. albicans (a) or G. vaginalis (b) biofilms detected at 24-hour intervals up to 72 hours from initial exposure. (c) Immunohistological staining of C. trachomatis inclusions visualized in HeLa cell monolayers by fluorescence microscopy (400x magnification).
Lastly, we observed that the biofilm produced by C. albicans or G. vaginalis was unaffected by the presence of C. trachomatis. Indeed, no significant differences in the biofilm OD595 nm values were observed between the combinations of C. trachomatis with C. albicans (1.8 ± 0.5) or G. vaginalis (2.4 ± 0.9) and the microbial species alone (controls) (C. albicans: 1.5 ± 0.7, P = 0.58; G. vaginalis: 2.7 ± 1.1, P = 0.73) (Figure 4).
Figure 4.
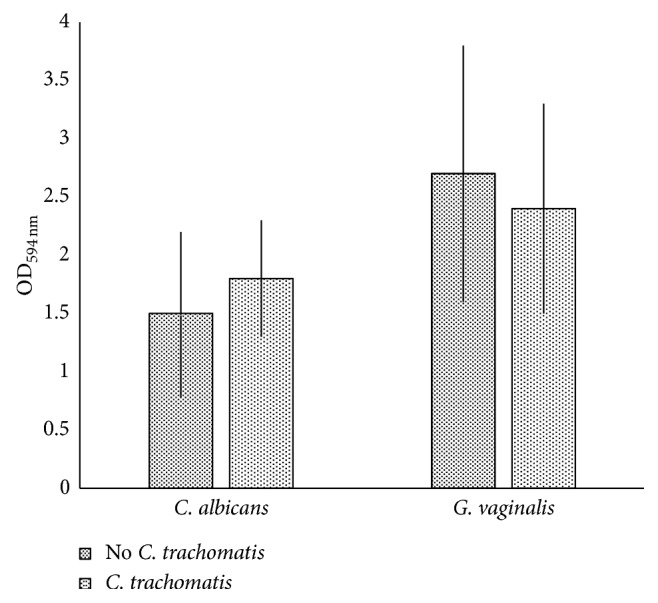
Candida and Gardnerella biofilm formation in the presence or absence of C. trachomatis. OD594 nm values were measured 48 hours after incubation of C. albicans and G. vaginalis in the presence or absence of C. trachomatis.
4. Discussion
This is the first study investigating the interaction between the biofilm produced by C. albicans or G. vaginalis and C. trachomatis, since previously published reports on biofilms were exclusively focused on pathogens involved in infections of medical devices, such as catheters, prostheses, and heart valves [23, 24].
For reaching our goal, we developed an in vitro transwell-based model which allowed us to monitor the effects of biofilm exposure to C. trachomatis over time. The biofilm was produced in the upper chamber, while the epithelial cell monolayer was placed in the lower one; such a system closely resembles a more physiological microenvironment and allowed us to investigate C. trachomatis interaction with Candida or Gardnerella biofilm. Our in vitro model also mimics the biofilm growing on artificial surfaces, including IUDs, known risk factors for recurrent genital infections [15, 16]. More importantly, our in vitro model was essential to directly observe the ability of the biofilm to enclose C. trachomatis EBs while gradually releasing them, producing clear images of chlamydial inclusions visualized by fluorescence microscopy. By contrast, chlamydial inclusions were not easily visualized when the biofilm was produced on cell monolayers grown on traditional cell-culture microplates.
The main result of our study is the ability of C. trachomatis to survive inside the biofilm produced by C. albicans or G. vaginalis. Specifically, we demonstrated that C. trachomatis survived within the biofilm, retaining its infectious properties, for up to 72 hours after exposure, as evidenced by the numerous typical inclusions observed in the cell monolayers removed from the transwell system. As a result, both Candida and Gardnerella biofilms may provide a protective niche for the survival of C. trachomatis, reducing its antibiotic susceptibility as well as favoring the evasion of the host immune system. In fact, it is well known that microorganisms encased in biofilms are generally well protected against environmental stresses, antibiotics, and disinfectants, as well as the host immune system, and thus, they are extremely difficult to eradicate [25].
Several clinical treatment failures have been reported concerning C. trachomatis genital infections [4, 26, 27], and a possible explanation may lie in the presence of a potential biofilm protecting C. trachomatis. Such hypothesis may also be suggested by the evidence that microbial dysbiosis in a cervicovaginal ecosystem, usually characterized by the overgrowth of biofilm-producing microorganisms, may contribute to the acquisition of C. trachomatis infection [1, 3].
In our study, the observation that C. trachomatis, released from the biofilm, was able to infect and replicate within epithelial cells is extremely intriguing. In the literature, in fact, there is a plethora of studies demonstrating that biofilm dispersion/detachment is most likely to play a significant role in a long-term colonization, dissemination, and transmission of pathogens [28, 29].
Therefore, the continuous release of C. trachomatis infectious EBs following the biofilm dispersion/detachment is of particular pathological importance as this phenomenon may contribute to the dissemination of this pathogen to the upper genital tract, leading to severe reproductive sequelae; notably, up to 26% of women with C. trachomatis infection develop PID, nearly 10% of women with PID have ectopic pregnancy, and up to 38% of women with recurrent episodes of PID may become infertile [19, 30].
5. Conclusions
In conclusion, our findings could have important clinical implications since the biofilm associated with Candida or Gardnerella genital infections may act as a chlamydial reservoir contributing to the transmission of C. trachomatis in the population, alongside its dissemination in the female upper genital tract. The survival of C. trachomatis within the biofilm may also help to explain the increased risk of PID in C. trachomatis-infected women who also use IUDs, where the biofilm formation is more likely to happen.
In the future, it will be interesting to translate our results in clinical studies to confirm that the biofilm produced by microorganisms colonizing the genital ecosystem may be considered as a risk factor for C. trachomatis infection.
Acknowledgments
This work was partially supported by the Regione Lazio, Italy (Project “Education and transfer of innovative methodologies,” 2017).
Data Availability
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- 1.Filardo S., Di Pietro M., Porpora M. G., et al. Diversity of cervical microbiota in asymptomatic Chlamydia trachomatis genital infection: a pilot study. Frontiers in Cellular and Infection Microbiology. 2017;7:p. 321. doi: 10.3389/fcimb.2017.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van de Wijgert J. H., Borgdorff H., Verhelst R., et al. The vaginal microbiota: what have we learned after a decade of molecular characterization? PLoS One. 2014;9(8) doi: 10.1371/journal.pone.0105998.e105998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Pietro M., Filardo S., Porpora M. G., Recine N., Latino M. A., Sessa R. HPV/Chlamydia trachomatis co-infection: metagenomic analysis of cervical microbiota in asymptomatic women. New Microbiologica. 2018;41:34–41. [PubMed] [Google Scholar]
- 4.Sessa R., Di Pietro M., Filardo S., et al. Lactobacilli-lactoferrin interplay in Chlamydia trachomatis infection. Pathogens and Disease. 2017;75(5) doi: 10.1093/femspd/ftx054.ftx054 [DOI] [PubMed] [Google Scholar]
- 5.Mastromarino P., Di Pietro M., Schiavoni G., Nardis C., Gentile M., Sessa R. Effects of vaginal lactobacilli in Chlamydia trachomatis infection. International Journal of Medical Microbiology. 2014;304(5-6):654–661. doi: 10.1016/j.ijmm.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Eckert L. O. Acute vulvovaginitis. New England Journal of Medicine. 2006;355(12):1244–1252. doi: 10.1056/nejmcp053720. [DOI] [PubMed] [Google Scholar]
- 7.Bitew A., Abebaw Y. Vulvovaginal candidiasis: species distribution of Candida and their antifungal susceptibility pattern. BMC Womens Health. 2018;18(1):p. 94. doi: 10.1186/s12905-018-0607-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Machado A., Cerca N. Influence of biofilm formation by Gardnerella vaginalisand other anaerobes on bacterial vaginosis. Journal of Infectious Diseases. 2015;212(12):1856–1861. doi: 10.1093/infdis/jiv338. [DOI] [PubMed] [Google Scholar]
- 9.Bradshaw C. S., Morton A. N., Hocking J., et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. Journal of Infectious Diseases. 2006;193(11):1478–1486. doi: 10.1086/503780. [DOI] [PubMed] [Google Scholar]
- 10.Cauchie M., Desmet S., Lagrou K. Candida and its dual lifestyle as a commensal and a pathogen. Research in Microbiology. 2017;168(9-10):802–810. doi: 10.1016/j.resmic.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Costerton J., Cheng K. J., Geesey G. G., et al. Bacterial biofilms in nature and disease. Annual Review of Microbiology. 1987;41(1):435–464. doi: 10.1146/annurev.micro.41.1.435. [DOI] [PubMed] [Google Scholar]
- 12.Hu X., Huang Y. Y., Wang Y., Wang X., Hamblin M. R. Antimicrobial photodynamic therapy to control clinically relevant biofilm infections. Frontiers in Microbiology. 2018;9:p. 1299. doi: 10.3389/fmicb.2018.01299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muzny C. A., Schwebke J. R. Biofilms: an underappreciated mechanism of treatment failure and recurrence in vaginal infections: table 1. Clinical Infectious Diseases. 2015;61(4):601–606. doi: 10.1093/cid/civ353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swidsinski A., Mendling W., Loening-Baucke V., et al. An adherent Gardnerella vaginalis biofilm persists on the vaginal epithelium after standard therapy with oral metronidazole. American Journal of Obstetrics and Gynecology. 2008;198(1):97.e1–97.e6. doi: 10.1016/j.ajog.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 15.Auler M. E., Morreira D., Rodrigues F. F. O., et al. Biofilm formation on intrauterine devices in patients with recurrent vulvovaginal candidiasis. Medical Mycology. 2010;48(1):211–216. doi: 10.3109/13693780902856626. [DOI] [PubMed] [Google Scholar]
- 16.Pal Z., Urbán E., Dósa E., Pál A., Nagy E. Biofilm formation on intrauterine devices in relation to duration of use. Journal of Medical Microbiology. 2005;54(12):1199–1203. doi: 10.1099/jmm.0.46197-0. [DOI] [PubMed] [Google Scholar]
- 17.Mazaheritehrani E., Sala A., Orsi C. F., et al. Human pathogenic viruses are retained in and released by Candida albicans biofilm in vitro. Virus Research. 2014;179:153–160. doi: 10.1016/j.virusres.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 18.Mylonas I. Female genital Chlamydia trachomatis infection: where are we heading? Archives of Gynecology and Obstetrics. 2012;285(5):1271–1285. doi: 10.1007/s00404-012-2240-7. [DOI] [PubMed] [Google Scholar]
- 19.Haggerty C. L., Gottlieb S. L., Taylor B. D., et al. Risk of sequelae after Chlamydia trachomatis genital infection in women. Journal of Infectious Diseases. 2010;201(S2):S134–155. doi: 10.1086/652395. [DOI] [PubMed] [Google Scholar]
- 20.Grentzer J. M., Peipert J. F., Zhao Q., McNicholas C., Secura G. M., Madden T. Risk-based screening for Chlamydia trachomatis and Neisseria gonorrhoeae prior to intrauterine device insertion. Contraception. 2015;92(4):313–318. doi: 10.1016/j.contraception.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohllajee A. P., Curtis K. M., Peterson H. B. Does insertion and use of an intrauterine device increase the risk of pelvic inflammatory disease among women with sexually transmitted infection? A systematic review. Contraception. 2006;73(2):145–153. doi: 10.1016/j.contraception.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 22.Sessa R., Di Pietro M., De Santis, et al. Effects of Mentha suaveolens essential oil on Chlamydia trachomatis. BioMed Research International. 2015;2015:7. doi: 10.1155/2015/508071.508071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joo H.-S., Otto M. Molecular basis of in vivo biofilm formation by bacterial pathogens. Chemistry & Biology. 2012;19(12):1503–1513. doi: 10.1016/j.chembiol.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lebeaux D., Chauhan A., Rendueles O., Beloin C. From in vitro to in vivo models of bacterial biofilm-related infections. Pathogens. 2013;2(2):288–356. doi: 10.3390/pathogens2020288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macia M. D., Rojo-Molinero E., Oliver A. Antimicrobial susceptibility testing in biofilm-growing bacteria. Clinical Microbiology and Infection. 2014;20(10):981–990. doi: 10.1111/1469-0691.12651. [DOI] [PubMed] [Google Scholar]
- 26.de Vries H. J. C., Zingoni A., Kreuter A., Moi H., White J. A. 2013 European guideline on the management of lymphogranuloma venereum. Journal of the European Academy of Dermatology and Venereology. 2014;29(1):1–6. doi: 10.1111/jdv.12461. [DOI] [PubMed] [Google Scholar]
- 27.Kong F. Y., Hocking J. S. Treatment challenges for urogenital and anorectal Chlamydia trachomatis. BMC Infectious Diseases. 2015;15(1):p. 29. doi: 10.1186/s12879-015-1030-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaplan J. B. Biofilm dispersal: mechanisms, clinical implications, and potential therapeutic uses. Journal of Dental Research. 2010;89(3):205–218. doi: 10.1177/0022034509359403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Percival S. L., Suleman L., Vuotto C., Donelli G. Healthcare-associated infections, medical devices and biofilms: risk, tolerance and control. Journal of Medical Microbiology. 2015;64(4):323–334. doi: 10.1099/jmm.0.000032. [DOI] [PubMed] [Google Scholar]
- 30.Wiesenfeld H. C. Screening for Chlamydia trachomatis infections in women. New England Journal of Medicine. 2017;376(8):765–773. doi: 10.1056/nejmcp1412935. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.