Abstract
Cell therapy has great promise for treating gastrointestinal motility disorders caused by intestinal nervous system (ENS) diseases. However, appropriate sources, other than enteric neural stem cells and human embryonic stem cells, are seldom reported. Here, we show that neural progenitors derived from the dorsal root ganglion (DRG) of EGFP mice survived, differentiated into enteric neurons and glia cells, migrated widely from the site of injection, and established neuron-muscle connections following transplantation into the distal colon of postnatal mice. The exogenous EGFP+ neurons were physiologically functional as shown by the activity of calcium imaging. This study shows that that other tissues besides the postnatal bowel harbor neural crest stem cells or neural progenitors that have the potential to differentiate into functional enteric neurons in vivo and can potentially be used for intestinal nerve regeneration. These DRG-derived neural progenitor cells may be a choice for cell therapy of ENS disease as an allograft. The new knowledge provided by our study is important for the development of neural crest stem cell and cell therapy for the treatment of intestinal neuropathy.
Keywords: neural progenitor cells, enteric neuron, differentiation, transplantation, Hirschsprung disease
Introduction
The enteric nervous system (ENS) plays a significant role in regulating motility, secretion, and other gut functions1. The most common identifiable disorder of the ENS is Hirschsprung disease (HD), which occurs in 1 of every 5000 live births as a result of neural crest-derived precursor cells failing to colonize the gut during embryonic development2. Current therapy for HD is surgical resection of the aganglionic bowel or the region of the bowel affected by complications3, but few effective treatments are available for the acquired enteric neuropathies4,5.
Based on tremendous advances in regenerative medicine, stem cell-based replacement of defective or missing enteric neurons for HD treatment is a promising therapeutic tool to avoid surgery, optimize outcomes, and restore gut motility. Several candidate cells have been explored for ENS reconstruction, including the ENS itself, skin-derived neural crest stem cells (NCSC), human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs)6–13.
The fetal and postnatal intestine from rodent and human is an attractive source from which to isolate neural progenitor cells (NPs)14–22. Notably, these neonatal enteric neural stem cells (ENSC) showed migration ability, and differentiate into neurons and glia cells following transplantation into the postnatal rodent gut6,23–25. ENSC-expressing channelrhodopsin showed functional integration and innervation of the smooth muscle of the bowel wall following the transplantation into the bowel26.
Fattahi et al. reported vagal neural crest lineage induction from hESCs, and further differentiation into ENS in vivo13. Although the question of bowel motility remained to be addressed27, the HD model mice had extended lifespans after cell transplantation.
To date, no other source of cells has been reported for ENS formation in the gut. Previous reports indicated that, according to the development principle, NCSCs were the source of ENS. Various sources of NCSCs have been identified; NCSCs can be derived from the embryonic sciatic nerve28 and boundary cap29, the gut, skin30,31, bone marrow32, and the dorsal root ganglia (DRG)32,33 of adult rodents. The NCSCs from different tissues share a common developmental origin with ENS. Considering the roles of NCSCs during embryonic development and neuropathies of the intestine, other NCSC-containing tissues might be potential cell sources for intestinal ENS regeneration. In the present study, we explored the differentiation potential of NCSC isolated from DRG in ENS in the postnatal colon. We also studied whether NCSC coming from DRG-derived NCSC exhibit a similar potential for differentiation in the gut as the NCSC from intestine described in previous reports23,25.
Following transplantation into the postnatal colon of mice, the DRG-NPs survived, migrated, and differentiated into functional enteric neurons. The DRG-NPs also expressed mature presynaptic protein synaptophysin and closely associated with endogenous neurons and muscle. This study showed for the first time that NCSC from sources other than ENSCs and hESCs/iPSCs could generate functional enteric neuron in the postnatal gut, thus broadening the range of cell candidates for the anatomic and functional replacement of ENS cells. Our results also suggest that NCSCs from postnatal tissues besides the gut might still maintain their multipotency for ENS differentiation, independent of tissue source.
Materials and Methods
Experimental Animals
Wild-type C57BL/6 J and C57BL/6-Tg (CAG-EGFP) (average body weight 25 ± 1.2 g for both male and female) were purchased from Model Animal Center of Nanjing University (Nanjing, China). Animal experiments were conducted according to the Guidelines of the Zhejiang University Laboratory Animal Center for the care and use of laboratory animals, and were approved by the Animal Care and Use Committee of the Medical School, Zhejiang University.
DRG NP Cell Generation, Derivation and Identification
Lumbar dorsal roots at the levels between L1 and L5 were carefully removed from C57BL/6-Tg (CAG-EGFP) mice at postnatal day 7 and placed in cold Dulbecco’s modified eagle’s medium (DMEM) with 1% penicillin/streptomycin (Gibco, Grand Island, NY, USA). DRG-NCSCs were generated as previously described with some modifications34.
Each dorsal root was minced with scissors into small pieces for explant culture. The explants were harvested and seeded in 24-well plates coated with collagen (Gibco, 5 µg/cm2) in primary medium of DMEM: F12 (Gibco), containing N2 (100×, Gibco), B27(50×, Gibco), basic fibroblast growth factor (bFGF, 20 ng/ml, Peprotech, Rocky Hill, NJ, USA) and epidermal growth factor (EGF, 20 ng/ml, Peprotech). In DRG explant cultures, cells with a triangular shape migrated out of the explants and formed a layer on the collagen after the first 1–2 days. The explant pieces were then picked up and attached to a fresh plate for another 1–2 days. Each explant was cultured on a fresh plate two or three times. Cells migrating out from the explants were proliferated in neural progenitor proliferation medium, which was made up in 500 ml batches containing 432 ml of DMEM: F12, 50 ml of Probumin (20% vol/vol stock solution, Millipore, Bedford, MA, USA), 5 ml of penicillin/streptomycin (Gibco), 5 ml of l-alanyl-l-glutamine (Cellgro, Lincoln, NE, USA), 5 ml of MEM non-essential amino acids (Cellgro), 0.5 ml of trace elements A (Cellgro), 0.5 ml of trace elements B (Cellgro), 0.5 ml of trace elements C (Cellgro), 0.9 ml of 2-mercaptoethanol (Invitrogen), transferrin (10 mg/ml, Invitrogen), (+) sodium l-ascorbate (50 mg/ml, Sigma), Heregulin B-1 (10 ng/ml, Peprotech), LONGR3 IGF-I (200 ng/ml, Sigma) and bFGF (8 ng/ml, Peprotech)35. Cells were detached and formed spherical aggregates after they were transferred to low-attachment plates. Half of the medium was changed every 3 days.
Differentiation
To assess the ability of cells to differentiate into a neural lineage, the differentiation method from Lee et.al was used36. Briefly, DRG-NCSCs at passage 2 were chosen for neuron and glia differentiation analysis and seeded at 5000 cells/cm2 on coverslips coated with poly-ornithine (0.01%, 1:5 dilutions, Sigma, St. Louis, MO, USA)/fibronectin (25 µg/ml) in 24-well plates. For neuron differentiation, the medium consisted of DMEM: F12 medium (1:1, Gibco), 2% B27 medium (Gibco), brain-derived neurotrophic factor (10 ng/ml, Peprotech), glial cell line-derived neurotrophic factor (10 ng/ml, Peprotech), nerve growth factor (10 ng/ml, Peprotech), neurotrophin-3 (10 ng/ml, Peprotech), ascorbic acid (200 µM, Sigma) and cAMP (0.5 mM, Sigma). For glia differentiation, the medium consisted of DMEM: F12 medium (Gibco), N2, B27 medium (Gibco), bFGF (10 ng/ml, Peprotech), EGF (10 ng/ml, Peprotech) and 5% fetal bovine serum (FBS, Gibco). DRG-NPs were cultured for 2–3 weeks, and the medium was changed every 2–3 days. Differentiated cells were analyzed by assessing the expression of neural and glia markers by immunocytochemistry.
Transplantation
The surgery of transplantation was performed according to a previous report23. Briefly, DRG-derived neurospheres at passage 2 were transplanted into the distal intestine of 6- to 8-week-old C57BL/6 J wild-type mice. Animals were anesthetized by the injection of 200 µl 5% chloral hydrate solution (RWD Life Science, San Diego, CA, USA). A small abdominal incision was made, and the distal colon was exposed and exteriorized. A total of 6 µl of neurospheres (1.0 × 105 cells/µl) with a mean diameter of about 40 µm for transplantation in DMEM: F12 medium with 50% Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) were injected into the external muscle layer of the distal colon with a glass needle at three sites, and the injection sites were marked with 7/0 Nylon. After 10 min, the distal colon was returned, and then the abdominal wall and skin was seamed with 7-0 suture. Animals were injected with cefoperazone and sulbactam sodium 0.5 mg per gram of body weight (0.5 mg/g) postoperatively. The recipient mice were immunosuppressed by daily injections of cyclosporine 10 µg per gram of body weight (10 µg/g, Sigma). At 1, 2, 3, 4, 6, 7 or 8 weeks after surgery, the mice were sacrificed and the tissue was collected for histological analysis.
Tissue Preparation
For cryosections, the distal colon was opened along the mesenteric border, pinned, stretched on Sylgard coated dishes, fixed in 4% paraformaldehyde (PFA), and dehydrated in 30% sucrose with phosphate buffer saline (PBS), and then transferred to an O.C.T. cryomold (Tissue-Tek, Torrance, CA, USA). Frozen sections (12 μm thick) were cut transversely or longitudinally on a cryostat.
Immunohistochemistry
Immunostaining was performed on primary monolayer cells, spheres, differentiated cells and cryosections of gut samples. Cultured cells or tissue sections were fixed with 4% PFA for 15 min at room temperature, rinsed three times with PBS, and then exposed to blocking solution (PBS containing 5% serum of the same origin as the secondary antibody and 0.2% Triton X-100) for 30 min at room temperature followed by overnight incubation at 4°C with the primary antibodies in blocking solution, and subsequent incubation for 1 h with secondary antibodies at room temperature. Primary and second antibody information is listed in Table 1.
Table 1.
Antibodies Used in this Study.
| Primary antibodies used in this study | ||||
|---|---|---|---|---|
| Antigen | Supplier | Dilution | Cat. NO | Host |
| P75 | Abcam | 1:200 | ab8875 | Rabbit |
| Nestin | Millipore | 1:200 | MAB353 | mouse |
| Sox10 | Abcam | 1:250 | ab155279 | Rabbit |
| TuJ1 | Covance | 1:200 | mrb-435p | Rabbit |
| PGP9.5 | Abcam | 1:200 | ab8189 | mouse |
| NF200 | sigma | 1:200 | N4142 | Rabbit |
| Peripherin | Millipore | 1:200 | MAB1527 | mouse |
| S100β | Abcam | 1:200 | ab52642 | Rabbit |
| nNOS | Abcam | 1:500 | ab76067 | Rabbit |
| VIP | Santa Cruz | 1:20 | sc-207 | Rabbit |
| Tyrosine hydroxylase | Millipore | 1:300 | MAB318 | mouse |
| Synaptophysin | Abcam | 1:50 | ab8049 | mouse |
| Alpha smooth muscle Actin | Abcam | 1:1500 | ab5694 | Rabbit |
| Ki67 | Abcam | 1:500 | ab15580 | Rabbit |
| Secondary antibodies used in this study | ||||
| Secondary Antibody | Supplier | Dillution | Cat. NO | |
| Donkey anti-Mouse IgG (H+L), Alexa Fluor 488 | Thermo Fisher Scientific | 1:500 | A-21202 | |
| Donkey anti-Rabbit IgG (H+L), Alexa Fluor 488 | Thermo Fisher Scientific | 1:500 | A-21206 | |
| Donkey anti-Rabbit IgG (H+L), Alexa Fluor 594 | Thermo Fisher Scientific | 1:500 | A-21207 | |
| Donkey anti-Mouse IgG (H+L), Alexa Fluor 594 | Thermo Fisher Scientific | 1:500 | A-21203 | |
| Donkey anti-Rabbit IgG (H+L), Alexa Fluor 647 | Thermo Fisher Scientific | 1:500 | A-31573 | |
Measurement of Cell Fiber Length, Differentiation and Statistical Analysis
To determine the fiber length of cells derived from DRG-NCSC-derived neurospheres implanted in the distal colon, a z-series of images was obtained on a Nikon A1 confocal microscope. The images were projected, and the fiber length was measured using the ImageJ software program. GraphPad Prism was used for statistical analysis of cell numbers in the culture, proliferation and immunostaining experiments.
Ca2+ Imaging
Transplanted animals were euthanized by 200 µl chloral hydrate (10%). For Ca2+ imaging, the protocol used was that of Fried et al. with minor revision37. Intestinal segments were removed and immediately placed in the medium prepared as DMEM: F12 with 3 μM nicardipine hydrochloride and 1 μM scopolamine hydrochloride (hereafter referred to as “Medium”) in a dish on ice. The region of the distal colon owning EGFP+ transplanted cells was incised along the mesenteric border and pinned flat under light tension with the mucosa side up in medium with a silicone elastomer (Sylgard 184, Dow Corning, Midland, MI, USA). The mucosal and submucosal layers were carefully dissected using fine forceps. After washing with medium three times, tissue preparations were incubated with 4 μM Rhod-2 (Life Technologies, Thermo Fisher Scientific, Waltham, MA, USA) in loading solution (3 ml medium with 2.4 μl of a 250 mM probenecid stock and 1.5 μl of 4 mM Rhod-2 stock) for 30 min in a dark incubator at 37°C. After loading, tissues were transferred to a recording chamber and mounted on an inverted confocal laser-scanning microscope. Rhod-2 was excited at 552 nm, and its fluorescence emission was measured at 581 nm using a 10× objective. Recordings were made at room temperature under constant local perfusion (2 ml/min) with modified Krebs buffer (121 mM NaCl, 5.9 mM KCl, 2.5 mM CaCl2, 1.2 mM MgCl2, 1.2 mM NaH2PO4, 10 mM HEPES, 21.2 mM NaHCO3, 1 mM pyruvic acid, and 8 mM glucose with pH adjusted to 7.4 with NaOH). High K+ (70 mM) was used for stimulating the cells and image sequences were captured at a frequency of one image every 2s. After recording, the time-lapse movie of the experiment was viewed and regions of interest (ROIs) were selected. The fluorescence intensities (F) of the ROIs were compared with the initial baseline fluorescence value (F0). Changes in normalized fluorescence intensity (ΔF/F0) were directly proportional to changes in Ca2+ concentration.
Results
In Vitro Derivation and Characterization of Transplantable NCSC Progenitors from the Postnatal DRG
It has been demonstrated that adult DRGs harbor NCSCs and that these NCSCs could be derived from DRG explant culture33,38. Here, we isolated these NCSCs from postnatal C57BL/6-Tg (CAG-EGFP+) mice using a previously published protocol with some modifications38.
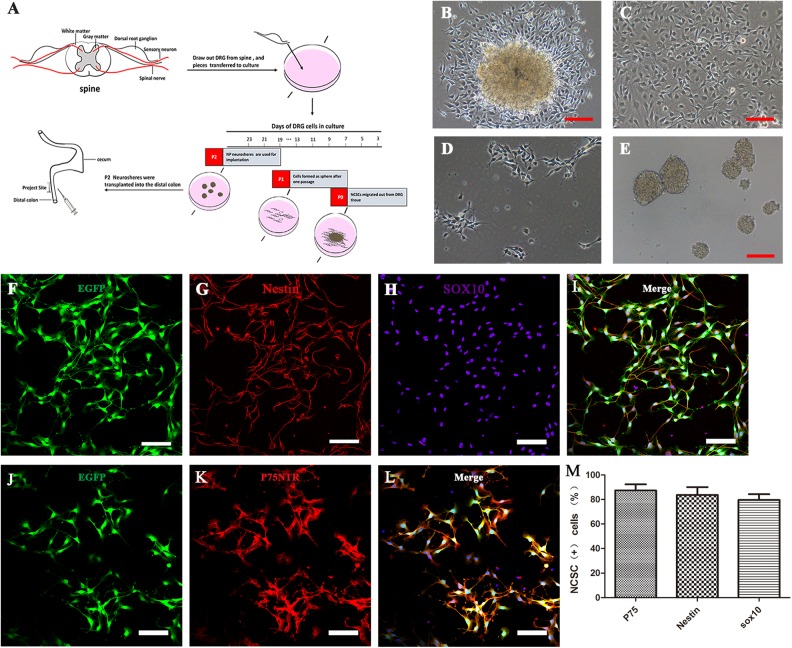
Because of the high migratory ability of NCSCs39, NCSCs were the first type of cells to migrate out of DRG explants after the explants had attached to the plate. Cells migrating out from the DRG formed a dense layer on the collagen after 1 week. Other kinds of cells also eventually migrated out the explants during the 1-week process of explant culture; thus, to optimize the purity and reduce the proportion of non-NCSCs within the emigrated cells, every piece of DRG was transferred to another well or plate after 1–2 days of attachment (Fig 1 B, C). Each piece of explant was used for attachment no more than three times, and cells migrating out from the DRG were used for further proliferation in NP proliferation medium. The selected cells were then continuously cultured as a monolayer, or as spheres that were spontaneously formed in passage 2 on low-attachment plates prior to transplantation (Fig 1D, E). The whole process is illustrated in Fig 1A. The next step was to confirm the identity of cells migrating out from the DRG. The low-affinity neurotrophin receptor (P75)—a transmembrane glycoprotein—is expressed in enteric NCSCs and glial cells40 and has been used as a selection marker for successfully isolating NCSCs from the gut tissue of mice and humans41–43. In combination with the NCSCs marker Sox10 and neural stem cell (NSC) marker Nestin, P75 was used to identify the NCSCs from the DRG. Cells migrating out from the DRG were cultured on fibronectin-coated coverslips for 2 days, fixed, and analyzed by immunocytochemistry. To monitor the behavior in vivo, these cells were derived from mice expressing enhanced green fluorescent protein (EGFP) (Fig 1F, J), and most of them were positive for the three NCSC markers, P75 (87.4% ± 6.9%, n = 3), Sox10 (79.7% ± 6.8%, n = 3) and Nestin (83.6% ± 1.4%, n = 3) (Fig 1F–L and M). To ensure a sufficient number of cells for transplantation, migrated cells at passage 2 or passage 3 were allowed to proliferate before the transplantation. To demonstrate that these cells still maintained NCSC markers after a period of time in proliferation medium, immunostaining of DRG-derived cells in passage 2 spheres showed that most of these cells expressed the NSC marker Nestin (79.3% ± 10.7%, n = 3), the NCSC markers Sox10 (85.4% ± 8.6%, n = 3) and P75 (87.6% ± 6.4%, n = 3) (Fig S1 A-C, E). The proliferative characteristics of the cells were demonstrated by the positive immunostaining of Ki67 (passage 5 as a monolayer, Ki67+ cells accounted for 67.7% ± 8.7% of cells, n = 3) (Fig S1 C–E) and by the growth curves of cells at passage 1 and passage 5 (Fig S1 F). The growth curves indicated that the proliferative ability slightly decreased after four passages.
Fig 1.
DRG-derived neural crest stem cells isolation, proliferation, and characterization in vitro. (A) Schematic representation of the workflow of DRG-NCSC derivation, proliferation, and transplantation into the distal intestine of postnatal mice. Pieces of mouse lumbar DRGs were placed on the 24-well plate in the primary medium. Cells migrated out after 1–2 days and were designated as passage 0 cells. Spheres formed after cells were transferred onto low-attachment plates. The spheres about 40 µm in diameter were harvested for transplantation into the distal colon. The cell culture in vitro took roughly 1520 days prior to transplantation. (B–E) Transplantable NCSC cell derivation from DRG explants, proliferation, passage and sphere formation. Phase contrast micrographs taken at different time points in the cell culture. (B) Cells migrated out of the DRG explants and formed a dense layer on the collagen after 1–2 days. (C) Cells that migrated out of the DRG explant proliferated almost to confluence until the next passage. (D) Cells in the primary culture were dissociated into single cells and re-seeded at 1 × 104 cells/cm2 as a monolayer on 6-well plates. (E) Cells were passaged and formed spherical aggregates on low-attachment plates in NPPM. These spheres were used for transplantation into the distal colon of mice. Scale bars, 100 μm. (F–L) DRG-derived NCSCs with EGFP fluorescence (F, J) cultured as primary culture passage were immunostained with the NSC marker Nestin (G) and the NCSC markers Sox10 (H) and P75 (K). Scale bars, 100 μm. (M) Most cells had immunoreactivity to the NCSC markers P75, Sox10 and the NSC marker Nestin as the expression proportion showed in the histogram.
NPs Can Be Induced from DRG-Derived NCSCs
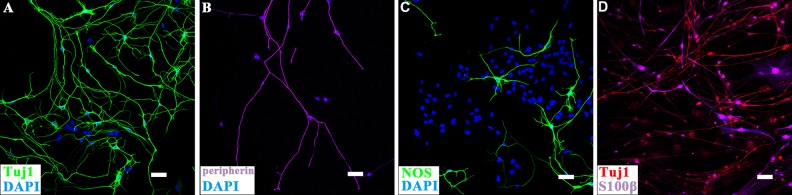
To explore the differentiation ability of DRG-NCSCs into peripheral neurons and glia cells, DRG-derived cells at passage 2 were transferred onto poly-ornithine/fibronectin-coated coverslips in neural differentiation medium for 10–14 days. Besides the pan-neuronal markers TuJ1 (β-tubulin), NF200, and PGP9.5, the peripheral neuron marker Peripherin and the enteric neural marker NOS (neuronal nitric oxide synthase, nNOS) were also detected on the DRG-derived cells (Fig 2 and Fig S2). In glia differentiation medium, which consisted of N2, B27, bFGF, EGF, and 5% serum, most of the cells were TuJ1+ (77.8% ± 9.7%, n = 3) and a smaller proportion showed the expression of the glia cell marker S100β (36.7% ± 11.2%, n = 3) (Fig 2D), which indicated that the DRG-derived NCSCs were able to differentiate into peripheral neurons and glia cells, and could be induced to become NPs after two passages.
Fig 2.
Immunocytochemical analyses of the differentiation ability of DRG-derived NCSCs. (A,B) Immunostaining of DRG-derived NSCs cultured in neural differentiation condition for 2 weeks. The differentiation into neurons was confirmed by immunostaining of neuron markers TuJ1 and Peripherin. Scale bars, 100 μm. (C) Some cells expressed the enteric subtype neuron marker nNOS. Nuclei were counter-stained with DAPI. Scale bars, 100μm. Terminal differentiation into neurons and glial cells as recognized by antibodies of neuron markers TuJ1 and glia marker S100β. Scale bars, 100 μm. (D). Immunostaining of DRG-derived NSCs which were cultured in glia differentiation condition for 2 weeks. Scale bars, 100 μm.
DRG-Derived EGFP-Expressing NPs Migrated and Projected Nerve Fibers Following Transplantation into the Postnatal Mouse Hindgut
To determine whether DRG-NPs populate in the postnatal mouse colon, DRG-NPs harvested from EGFP+ mice were surgically implanted into the external muscle layer of the distal colon of 6- to 8-week-old C57BL/6 J mice. The cells used for transplantation were in short-term culture for no more than three passages, because cells in mid-term and long-term culture have been reported to often differentiate into smooth muscle cells after growth factors are withdrawn38. The recipient colons were examined at 1, 2, 3, 4, 7, and 8 weeks after surgery with 6–7 mice at each time point. Exogenous cells and neurites survived in 39 of 40 transplanted mouse colons, in which 6 of 6 were found available for 8 weeks following transplantation.
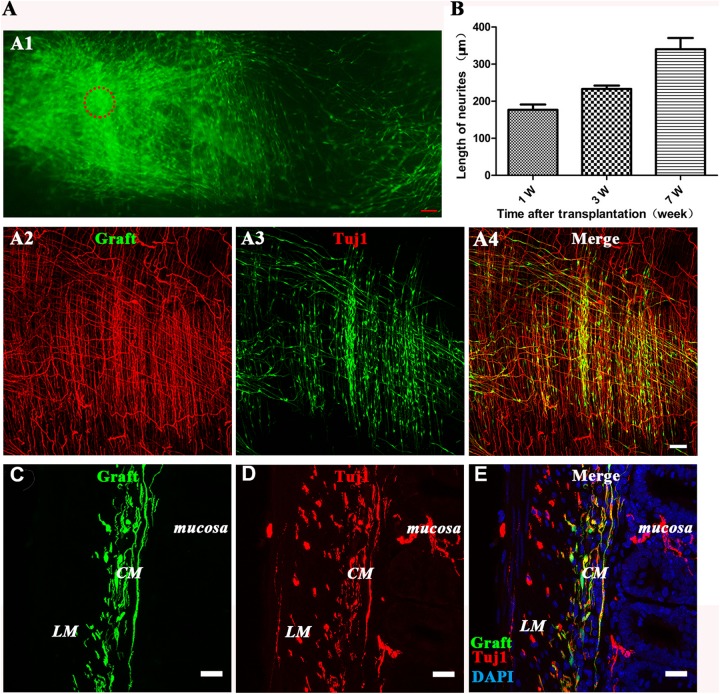
We first explored the ability of DRG-NPs to colonize in the wild type postnatal mouse hindgut. EGFP+ cells existed in the cryosections of the host gut 1–8 weeks after transplantation. This proved that exogenous DRG-NPs survived and engrafted in the host gut (Fig 3A1–E). Following the cell injection, numerous EGFP+ cells migrated away from the injection sites without specific direction. The migration distance exceeded 2.0 mm as shown in the figure, which was photographed and stitched together in the same plane (Fig. 3A2–A4), and the maximum distance of the observed migration in different planes in the colon was >3.5 mm. We observed EGFP+ distribution throughout the distal gut with many cells exhibiting TuJ1 staining (Fig 3A2–E, Fig 4 A–D, Fig S3 A–D). These results indicated that DRG-derived EGFP+ NPs were competent for colonization of the gut tube and contributed to enteric neurogenesis.
Fig 3.
DRG-derived GFP-expressing neural progenitors migrated mainly to the muscle layer with their neurites extending from exogenous neural spheres after implantation into the postnatal mouse hindgut. (A1) Composite image of low-magnification views of a whole-mount preparation of the distal colon showing the colonization in the distal intestine at 6 weeks after transplantation of the DRG-NPs (generated from the explants). Migration of graft-derived cells away from the original transplantation sites (circle) was observed. Fig 3A1 are stitched together with six continuous picture. Scale bars, 100 µm. (A2–A4) The immunostaining of the longitudinal section showed the distribution of neural cell body and its fiber elongation of exogenous DRG-NPs. Scale bars, 100 μm. (B) The average neurite length for elongation increased with time after transplantation into the distal hindgut. (C–E) immunostaining of the transverse section showed the distribution in the muscle layer of exogenous DRG-NPs. EGFP+ cells were observed in the mouse colon wall where they were mainly localized between the longitudinal muscle (LM) and circular muscle (CM) layers. Sections of mouse colon were counterstained in blue with DAPI to identify the cell nuclei. The colocalization of EGFP+ with TuJ1 staining (A4, E) indicated that most of the grafted cells had differentiated into neurons. To help identify the location of the grafted cells, fluorescent images of three channels were superimposed together. Scale bars, 20 µm.
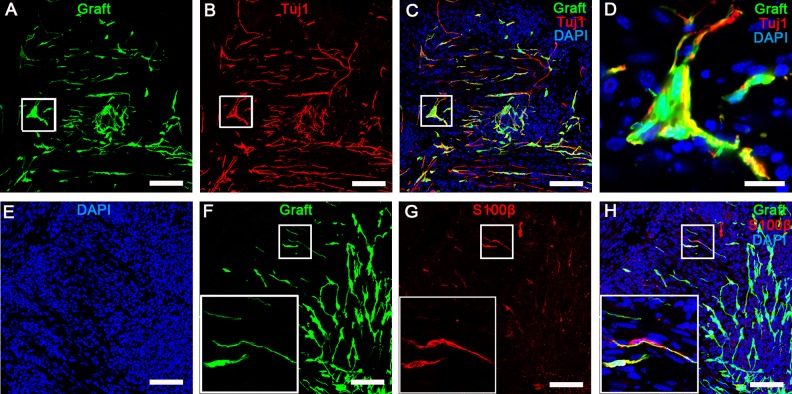
Fig 4.
Grafted EGFP+ cells showed that DRG-NPs were capable of differentiating into neurons and glia cells after implantation into the distal colon. (A–D) The longitude sections staining showed that most of graft-derived EGFP cells had immunoreactivity to pan-neural marker TuJ1 in the distal colon of mice. Ganglion-like clusters from graft-derived cells formed with cells expressing the neuronal marker TuJ1 in longitude sections of the distal colon 3 weeks after transplantation. (D) GFP was co-localized with TuJ1 staining in the ganglion-like cluster. Scale bars, A–C, 100 µm; D, 20 µm. (E–G) The longitudinal sections staining showed that a small part of the graft-derived EGFP cells had immunoreactivity to glia marker S100β in the distal colon. The box in (F–H) shows the exact match of EGFP and S100β staining in the cell morphology. Transections of mouse colon were counterstained in blue with DAPI to identify cell nuclei. Scale bars, 100 µm.
Many EGFP+ projections diffused from exogenous cell bodies in different directions. Fluorescence imaging showed that most of the EGFP+ cells exhibited neuron-like morphologies and showed fiber extension (Fig 3A1–A4). The fibers elongated over time after transplantation, and the average fiber length was 0.17mm + 0.018 mm at 1 week, 0.23 mm + 0.01 mm at 3 weeks, and 0.34 mm + 0.04 mm at 7 weeks after transplantation (n = 3 experiments). These results showed that the DRG-NPs survived, migrated, and fibers extended from the transplantation site following transplantation into the postnatal distal gut.
DRG-Derived NPs Differentiated into Neurons and Glial Cells in the Distal Gut
We next examined whether exogenous neurons and glial cells were present after the transplantation of EGFP+ DRG-NPs. We found that transplanted DRG-NPs differentiated into neurons based on the immuno-staining of the pan-neuronal cell marker TuJ1 in the longitudinal and transverse section, and glial cells indicated by the staining of glial cell marker S100β in the longitudinal section. Double immunofluorescence staining of grafted EGFP+ cells showed the co-localization of EGFP with TuJ1 and S100β individually (Fig 4 A–H, Fig S3 A–D). Four weeks after transplantation Tuj1-expressing cells accounted for 86% ± 5% of all cells (n = 4 experiments). The result showed that most of the grafted cells differentiated into neurons, while some DRG-derived cells differentiated into glia cells (8% ± 1.5%, n = 3 experiments) (Fig S3 E). These results suggested that engrafted DRG-NPs survived and differentiated in the distal colon of recipient mice.
Some exogenous clusters similar to enteric ganglia were found a distance away from the transplantation site 4 weeks after transplantation (Fig 4A–D). Most of cells in the ganglion-like clusters are TuJ1+ cells (Fig 4A–C). It showed that the transplanted DRG-NPs were capable of forming the ganglion-like clusters in the colon.
DRG-Derived NPs Differentiated into Neurons with Appropriate Enteric Subtypes
There are numerous types of neurons in the ENS in mice1. We used published markers (nNOS, VIP and TH) to identify the neurons and enteric subtype neurons in the mice 4 weeks after transplantation23. Some EGFP+ neurons and fibers showed positive immunostaining to nNOS (Fig 5A–D), vasoactive intestinal polypeptide (VIP) (Fig 5E–H), and tyrosine hydroxylase (TH) (Fig 5I–K). Grafted cells differentiated into different proportions of enteric subtypes as indicated by the presence of nNOS (26% ± 6%, n = 4 experiments), TH (12% ± 3%, n = 4 experiments) and VIP (21% ± 5%, n = 3 experiments) (Fig 5 L). These data showed that the niche in the postnatal colon supported the differentiation of enteric subtype neuron with relative neurochemical marker.
Fig 5.
Subtypes of neurons were determined in the section staining after the transplantation of DRG-NPs into the distal colon of mice. The colocalization of EGFP+ with NOS (A–D), VIP (E–H), and TH (I–K) staining indicated that a subpopulation of grafted cells differentiated into enteric subtypes. (D, H) The two pictures showed the inset part of (C) and (G) in higher magnification. Immunostaining showed the co-localization of exogenous EGFP+ cells with nNOS and VIP individually. (A–H) Immunostaining of the longitudinal section. Scale bars are 100 µm in (A, B, C, E, F, G) and 20 µm in (D, H). (I–K) Immunostaining of the transverse section. Scale bars, 20 µm. (L) The proportions of cells positive for the enteric subtype neuron markers nNOS, TH, and VIP. Sections of mouse colon were counterstained in blue with DAPI to identify the cell nuclei. Fluorescent images of three channels were superimposed together.
DRG-NPs colonized the appropriate gut layers, and the mature presynaptic protein synaptophysin expressed in conjunction of exogenous and endogenous cells.
To explore the location in the gut wall where EGFP+ cells and fibers migrated, we examined the longitudinal and transverse frozen sections from post-surgery mice using confocal microscopy. The staining of the transverse section at 4 weeks after transplantation showed that transplanted EGFP+ cells were present mainly in the inter-myenteric layer of the intestine (Fig 3 C–E, Fig 5 I–K, Fig 6 A–D). Graft-derived TuJ1+ cells were present in the group of cells that remained in the longitudinal muscle, circular muscle, and mucosa of the intestine (Fig 3 C–E, Fig S3 A–D). Furthermore, exogenous TH+ cells were detected in the longitudinal muscle and circular muscle layer of the intestine (Fig 5 I-K).
Fig 6.
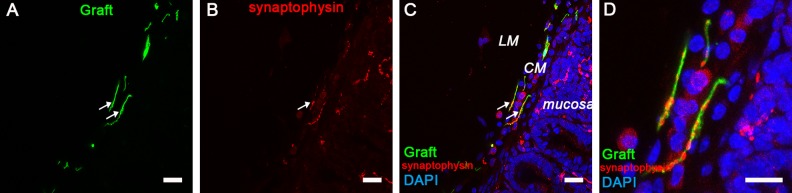
Exogenous DRG-NCSCs expressed synaptophysin in the circular muscle (CM) in the intestine 5 weeks after transplantation. (A) Transplanted EGFP+ cells migrated into the CM of the gut. Scale bar, 100 µm. (B-C) Synaptophysin (SYP) expression is identified in the CM area and mucosa area (as indicated by the arrows). Scale bars, 100 µm. (D) The merged image in high resolution from A, B, and C shows the presence of synaptophysin in the EGFP+ cells which migrated into the CM area. Scale bars, 20 µm.
Graft-derived neurites were found to exist in the longitudinal and circular muscle layers, and these neurites closely associated with endogenous neurons and muscles of the recipient animal as shown by synaptophysin staining (Fig 6 A–D; Fig S4 A–H). The layers where exogenous cells located were alike to the endogenous neurons’ location. It showed that EGFP+ neurons localized in the submucosa, and neurites were similarly found in the mucosa as endogenous fibers. These results indicated that DRG-derived cells and neurites expressed the mature presynaptic protein synaptophysin with endogenous neurons and muscles in these layers.
Transplanted DRG-NPs Differentiated into Neurons with Active Electrophysiology
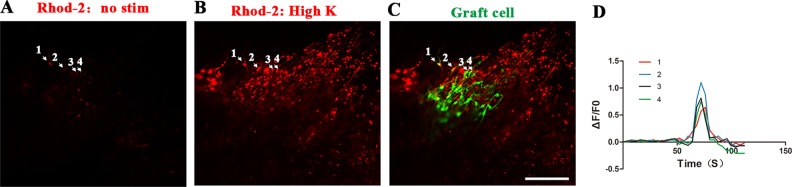
In order to test whether transplanted neurons were electrophysiologically functional, in situ Ca2+ imaging was performed 4–6 weeks after DRG-NP implantation. We determined the changes in Ca2+ concentration upon a brief high K+ exposure (70 µM, 15 s) through a continuous perfusion system (2 ml min–1). Grafted neurons responded with a rise in Ca2+ concentration (2 ± 0.55 F/F0, cells from the region of interest) (Fig 7A–D). These results showed that the surroundings of the postnatal colon support that DRG-NPs differentiate into functional neurons with active electrophysiology.
Fig 7.
Engrafted EGFP+ cells in the colon showed transient calcium response to glutamate in situ. Four regions of interest (ROI) are shown as arrows. (A) Engrafted EGFP+ cells showing Rhod-2 fluorescence under basal conditions. Scale bar, 100 µm. (B) Following stimulation with high K+ in 70 nm, the engrafted EGFP+ cells responded with increased Ca2+ concentrations as shown by increased Rhod-2 fluorescence. Scale bar, 100 µm. (C) Engrafted EGFP+ cells in the colon showed the co-localization with TuJ1 staining in some exogenous cells. Scale bar, 100 µm. (D) Traces corresponding to each ROI shown in A–C.
Discussion
The neural crest originates from the neural folds during vertebrate development. Neural crest cells migrate from the dorsal neural tube to different locations including the gut, dorsal root ganglion, heart, face, skin, etc., where they differentiate into different kinds of cells, including peripheral nervous systems including ENS and non-peripheral nervous systems containing smooth muscle cells, bone, and cartilage cells. Based on the developmental principle, NCSCs might be cell sources for gut neuropathy therapy. We thus hypothesized that those postnatal tissue-derived NCSCs from tissues other than the gut have the ability to reconstruct ENS tissues in vivo. DRG-derived NCSCs were chosen to explore this possibility based on the potential for multilineage differentiation into neurons, glial cells, myofibroblasts, and pericytes32,38.
In spite of the possibility of DRG-NPs for ENS formation, considering previous reports on the low proportion of neuron differentiation for DRG-derived NCSC33,38, we optimized the generation of NPs from the isolated postnatal DRG, and these cells exhibited high rates of differentiation into peripheral neurons in vitro and in vivo. We were able to direct a high proportion of neuron differentiation as opposed to glia cells. The in vitro expression pattern of DRG-NPs was different from previous studies, which showed the inclined differentiation into Schwann cells33,38. At 3 weeks after transplantation, the majority of graft cells were TuJ1+ and a small portion were S100β+ cells. The colon circumstances redirect adult DRG-NPs into more subtypes of enteric neurons following transplantation compared with the environment in vitro. These findings showed that most DRG-NPs differentiated into neurons and to a lesser extent into glial cells. In general, our data indicated that DRG-NPs effectively differentiated into neurons both in vitro and in vivo.
The neural stem cells/progenitors used for HD disease treatment should meet the following requirements, including migration, differentiation into enteric subtype neurons, integration into the local neural circuitry with the appropriate neurochemical and electrophysiological characteristics following transplantation into the postnatal colon23. In the present study, we showed that NPs, which were generated from DRG-NCSCs, survived, migrated, integrated, and differentiated into glial cells as well as diverse subtype neurons with similar neurochemical and electrophysiological properties to endogenous enteric neurons.
The gut contains a range of enteric subtype neurons, which are pivotal in controlling muscle contractile activity44,45. In this study, exogenous DRG-NPs differentiated into neurons alike to normal enteric neurons in terms of morphological and neurochemical markers such as nNOS, VIP, and TH23,46,47.
The neural circuitry in charge of motility requires accurate connection among different functional neurons and muscles. Our findings indicated that exogenous DRG-NP cells were distributed in layers similar to those of the NCSC derivatives that act during normal development. We observed that DRG-NP-derived EGFP+ neurons projected into the appropriate gut layer and expressed the mature presynaptic protein synaptophysin. It has been reported that enteric NPs are capable of differentiating into neurons with electrophysiological activity and are possibly functional in the gut23,25. Our results showed that exogenous neurons were also capable of electrophysiological activity after transplantation in vivo.
It was reported that some of the endogenous sensory neurons were found in the submucosal plexus48,49. Submucosal ganglia may be very important for the gut’s normal function. In our study, graft-derived cells and fibers were also observed in the submucosa. This was slightly different from previous work that used ENSCs for colon transplantation23.
One of the principle challenges for stem cell therapy in bowel motility is to decide which source of stem cells is most efficacious. However, it is currently difficult to define the best candidate. Each candidate of stem/progenitor cells possesses certain disadvantages and advantages, and the principles for utilizing each one depends on the desired applications and outcomes. The most attractive characteristics of enteric neural stem cells are the avoidance of immune suppression and ethical issues because the cells are patient-derived autologous cells. However, the limited quantity and limited ability in proliferation of these cells might be a major hurdle for clinical use16,17. Another issue is that the congenital defect gene in transplanted cells of HD patients has to be corrected prior to autologous cells transplantation, and gene correction is difficult because of the limited proliferation of enteric NCSC. Human ESCs are another promising cell source for bowel motility disease, and they could potentially provide the huge numbers of human enteric neurons needed for clinical use13. Nevertheless, some questions remain to be answered before human ESCs/iPSCs can be used for clinical therapy, especially questions related to bowel motility evaluation and safety concerns50,51. Future studies investigating these issues should not be limited to any single population of cells because information provided from the research of individual cell type will benefit the HD field as a whole27.
Considering the therapeutic purpose and their confined location, exploitation of DRG-NPs for autograft seems impractical. Much research has suggested that fetal stem cells have greater multilineage capacity than adult stem cells, and hence have greater potential for clinical use. Fetal grafts might be integrated into the host with lower immunogenicity by the host, or under HLA match52. Considering the limited proliferation of autologous cells and the huge demand for cell numbers in cell therapy of ENS disease, our present findings suggest that isolation of human DRG-NPCs from postmortem tissues, surgical specimens, or aborted fetus might be attempted as a way to produce neural progenitors for allograft53 for clinical use under conditions where HLAs match.
This study may broaden the range of candidates for cell replacement of ENS cells. Our data showed that NCSC from DRG, which harbor NCSCs, have similar differentiation potential as functional enteric neurons in vivo as enteric NCSC. It can be presumed that other tissues where NCSCs are maintained, such as hair follicles, skin, and teeth, might also be reasonable sources of cells for enteric regeneration. Thus, our study suggests important avenues for future studies into the development of cell therapies for treating intestine neuropathies.
Conclusion
On the whole, DRG-neural progenitor cells migrated and differentiated into enteric neuron subtypes and glia cells, established close connections with inherent enteric neurons and smooth muscles with physiological functions, and integrated into the neuronal circuitry in the postnatal gut. This suggested the potential for stem cells from allografts such as fetal or postmortem tissues to replace diseased or missing ENS in HD under HLA match. The function of the colon in the HD model mice following DRG-NP transplantation requires further investigation.
Supplemental Material
Supplementary_Material for DRG-Derived Neural Progenitors Differentiate into Functional Enteric Neurons Following Transplantation in the Postnatal Colon by Hui Hu, Yuanyuan Ding, Wenbo Mu, Ying Li, Yanpeng Wang, Weifang Jiang, Yong Fu, Jinfa Tou, and Wei Chen in Cell Transplantation
Acknowledgments
The authors thank Green Mountain Editing Services for the language editing of this manuscript. We thank members of the technical platform at the Institute of Translational Medicine, School of Medicine, Zhejiang University for helpful advice and comments.
Footnotes
Author Contribution: Hui Hu, Yuanyuan Ding These authors contributed equally to this work.
Ethical Approval: This study was approved by the Animal Care and Use Committee of the Medical School, Zhejiang University.
Statement of Human and Animal Rights: Animal experiments were conducted according to the Guidelines of the Zhejiang University Laboratory Animal Center for the care and use of laboratory animals, and were approved by the Animal Care and Use Committee of the Medical School, Zhejiang University.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the National Key Research and Development Program of China (No. 2012CB967903 and No. 2014CB541705), the National Natural Science Foundation of China (No. 31171423, No. 30825040 and No. 81671531), Zhejiang Provincial Natural Science Foundation of China (No. Z18C090002, LY14H130001), Xiamen’s two hundred talent Program (10th).
ORCID iD: Wei Chen  https://orcid.org/0000-0001-7893-2092
https://orcid.org/0000-0001-7893-2092
Supplemental Material: Supplemental material for this article is available online
References
- 1. Sasselli V, Pachnis V, Burns AJ. The enteric nervous system. Dev Biol. 2012;366(1):64–73. [DOI] [PubMed] [Google Scholar]
- 2. Heanue TA, Pachnis V. Enteric nervous system development and Hirschsprung’s disease: advances in genetic and stem cell studies. Nat Rev Neurosci. 2007;8(6):466–479. [DOI] [PubMed] [Google Scholar]
- 3. McKeown SJ, Stamp L, Hao MM, Young HM. Hirschsprung disease: a developmental disorder of the enteric nervous system. Wiley Interdiscip Rev Dev Biol. 2013;2(1):113–129. [DOI] [PubMed] [Google Scholar]
- 4. Rohof WO, Boeckxstaens GE. Treatment of the patient with achalasia. Curr Opin Gastroenterol. 2012;28(4):389–394. [DOI] [PubMed] [Google Scholar]
- 5. Camilleri M, Grover M, Farrugia G. What are the important subsets of gastroparesis? Neurogastroenterol Motil. 2012;24(7):597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dettmann HM, Zhang Y, Wronna N, Kraushaar U, Guenther E, Mohr R, Neckel PH, Mack A, Fuchs J, Just L, Obermayr F. Isolation, expansion and transplantation of postnatal murine progenitor cells of the enteric nervous system. Plos One. 2014;9(5):e97792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hagl CI, Heumuller-Klug S, Wink E, Wessel L, Schafer KH. The human gastrointestinal tract, a potential autologous neural stem cell source. Plos One. 2013;8(9):e72948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hotta R, Natarajan D, Burns AJ, Thapar N. Stem cells for GI motility disorders. Curr Opin Pharmacol. 2011;11(6):617–623. [DOI] [PubMed] [Google Scholar]
- 9. Hotta R, Natarajan D, Thapar N. Potential of cell therapy to treat pediatric motility disorders. Semin Pediatr Surg. 2009;18(4):263–273. [DOI] [PubMed] [Google Scholar]
- 10. Kulkarni S, Becker L, Pasricha PJ. Stem cell transplantation in neurodegenerative disorders of the gastrointestinal tract: future or fiction? Gut. 2012;61(4):613–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kwok CK, Tam PK, Ngan ES. Potential use of skin-derived precursors (SKPs) in establishing a cell-based treatment model for Hirschsprung’s disease. J Pediatr Surg. 2013;48(3):619–628. [DOI] [PubMed] [Google Scholar]
- 12. Schafer KH, Micci MA, Pasricha PJ. Neural stem cell transplantation in the enteric nervous system: roadmaps and roadblocks. Neurogastroenterol Motil. 2009;21(2):103–112. [DOI] [PubMed] [Google Scholar]
- 13. Fattahi F, Steinbeck JA, Kriks S, Tchieu J, Zimmer B, Kishinevsky S, Zeltner N, Mica Y, El-Nachef W, Zhao H, de Stanchina E, Gershon MD, Grikscheit TC, Chen S, Studer L. Deriving human ENS lineages for cell therapy and drug discovery in Hirschsprung disease. Nature. 2016;531(7592):105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Almond S, Lindley RM, Kenny SE, Connell MG, Edgar DH. Characterisation and transplantation of enteric nervous system progenitor cells. Gut. 2007;56(4):489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bixby S, Kruger GM, Mosher JT, Joseph NM, Morrison SJ. Cell-intrinsic differences between stem cells from different regions of the peripheral nervous system regulate the generation of neural diversity. Neuron. 2002;35(4):643–656. [DOI] [PubMed] [Google Scholar]
- 16. Bondurand N, Natarajan D, Thapar N, Atkins C, Pachnis V. Neuron and glia generating progenitors of the mammalian enteric nervous system isolated from foetal and postnatal gut cultures. Development. 2003;130(25):6387–6400. [DOI] [PubMed] [Google Scholar]
- 17. Kruger GM, Mosher JT, Bixby S, Joseph N, Iwashita T, Morrison SJ. Neural crest stem cells persist in the adult gut but undergo changes in self-renewal, neuronal subtype potential, and factor responsiveness. Neuron. 2002;35(4):657–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Metzger M, Caldwell C, Barlow AJ, Burns AJ, Thapar N. Enteric nervous system stem cells derived from human gut mucosa for the treatment of aganglionic gut disorders. Gastroenterology. 2009;136(7):2214–25 e1-3. [DOI] [PubMed] [Google Scholar]
- 19. Tsai YH, Gariepy CE. Dynamic changes in the proximal gut neural crest stem cell population are associated with successful development of the distal enteric nervous system in rats. Pediatr Res. 2005;58(4):636–643. [DOI] [PubMed] [Google Scholar]
- 20. Walters LC, Cantrell VA, Weller KP, Mosher JT, Southard-Smith EM. Genetic background impacts developmental potential of enteric neural crest-derived progenitors in the Sox10Dom model of Hirschsprung disease. Hum Mol Genet. 2010;19(22):4353–4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mosher JT, Yeager KJ, Kruger GM, Joseph NM, Hutchin ME, Dlugosz AA, Morrison SJ. Intrinsic differences among spatially distinct neural crest stem cells in terms of migratory properties, fate determination, and ability to colonize the enteric nervous system. Dev Biol. 2007;303(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lindley RM, Hawcutt DB, Connell MG, Almond SL, Vannucchi MG, Faussone-Pellegrini MS, Edgar DH, Kenny SE. Human and mouse enteric nervous system neurosphere transplants regulate the function of aganglionic embryonic distal colon. Gastroenterology. 2008;135(1):205–216 e6. [DOI] [PubMed] [Google Scholar]
- 23. Hotta R, Stamp LA, Foong JP, McConnell SN, Bergner AJ, Anderson RB, Enomoto H, Newgreen DF, Obermayr F, Furness JB, Young HM. Transplanted progenitors generate functional enteric neurons in the postnatal colon. J Clin Invest. 2013;123(3):1182–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hetz S, Acikgoez A, Voss U, Nieber K, Holland H, Hegewald C, Till H, Metzger R, Metzger M. In vivo transplantation of neurosphere-like bodies derived from the human postnatal and adult enteric nervous system: a pilot study. Plos One. 2014;9(4):e93605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cooper JE, McCann CJ, Natarajan D, Choudhury S, Boesmans W, Delalande JM, Vanden Berghe P, Burns AJ, Thapar N. In vivo transplantation of enteric neural crest cells into mouse gut; engraftment, functional integration and long-term safety. Plos One. 2016;11(1):e0147989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stamp LA, Gwynne RM, Foong JPP, Lomax AE, Hao MM, Kaplan DI, Reid CA, Petrou S, Allen AM, Bornstein JC, Young HM. Optogenetic demonstration of functional innervation of mouse colon by neurons derived from transplanted neural cells. Gastroenterology. 2017;152(6):1407–1418. [DOI] [PubMed] [Google Scholar]
- 27. Burns AJ, Goldstein AM, Newgreen DF, et al. White paper on guidelines concerning enteric nervous system stem cell therapy for enteric neuropathies. Dev Biol. 2016;417(2):229–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Morrison SJ, White PM, Zock C, Anderson DJ. Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell. 1999;96(5):737–749. [DOI] [PubMed] [Google Scholar]
- 29. Hjerling-Leffler J, Marmigere F, Heglind M, Cederberg A, Koltzenburg M, Enerback S, Ernfors P. The boundary cap: a source of neural crest stem cells that generate multiple sensory neuron subtypes. Development. 2005;132(11):2623–2632. [DOI] [PubMed] [Google Scholar]
- 30. Fernandes KJ, McKenzie IA, Mill P, Smith KM, Akhavan M, Barnabé-Heider F, Biernaskie J, Junek A, Kobayashi NR, Toma JG, Kaplan DR, Labosky PA, Rafuse V, Hui CC, Miller FD. A dermal niche for multipotent adult skin-derived precursor cells. Nat Cell Biol. 2004;6(11):1082–1093. [DOI] [PubMed] [Google Scholar]
- 31. Sieber-Blum M, Grim M, Hu YF, Szeder V. Pluripotent neural crest stem cells in the adult hair follicle. Dev Dyn. 2004;231(2):258–269. [DOI] [PubMed] [Google Scholar]
- 32. Nagoshi N, Shibata S, Kubota Y, Nakamura M, Nagai Y, Satoh E, Morikawa S, Okada Y, Mabuchi Y, Katoh H, Okada S, Fukuda K, Suda T, Matsuzaki Y, Toyama Y, Okano H. Ontogeny and multipotency of neural crest-derived stem cells in mouse bone marrow, dorsal root ganglia, and whisker pad. Cell Stem Cell. 2008;2(4):392–403. [DOI] [PubMed] [Google Scholar]
- 33. Li HY, Say EH, Zhou XF. Isolation and characterization of neural crest progenitors from adult dorsal root ganglia. Stem Cells. 2007;25(8):2053–2065. [DOI] [PubMed] [Google Scholar]
- 34. Uesaka T, Nagashimada M, Enomoto H. Neuronal differentiation in Schwann cell lineage underlies postnatal neurogenesis in the enteric nervous system. J Neurosci. 2015;35(27):9879–9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Menendez L, Kulik MJ, Page AT, Park SS, Lauderdale JD, Cunningham ML, Dalton S. Directed differentiation of human pluripotent cells to neural crest stem cells. Nat Protoc. 2013;8(1):203–212. [DOI] [PubMed] [Google Scholar]
- 36. Lee G, Chambers SM, Tomishima MJ, Studer L. Derivation of neural crest cells from human pluripotent stem cells. Nat Protoc. 2010;5(4):688–701. [DOI] [PubMed] [Google Scholar]
- 37. Fried DE, Gulbransen BD. In situ Ca2+ imaging of the enteric nervous system. J Vis Exp. 2015;2015(95): e52506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vidal M, Maniglier M, Deboux C, Bachelin C, Zujovic V, Baron-Van Evercooren A. Adult DRG stem/progenitor cells generate pericytes in the presence of central nervous system (CNS) developmental cues, and schwann cells in response to CNS demyelination. Stem Cells. 2015;33(6):2011–2024. [DOI] [PubMed] [Google Scholar]
- 39. Le Douarin NM, Creuzet S, Couly G, Dupin E. Neural crest cell plasticity and its limits. Development. 2004;131(19):4637–4650. [DOI] [PubMed] [Google Scholar]
- 40. Young HM, Ciampoli D, Hsuan J, Canty AJ. Expression of Ret-, p75(NTR)-, Phox2a-, Phox2b-, and tyrosine hydroxylase-immunoreactivity by undifferentiated neural crest-derived cells and different classes of enteric neurons in the embryonic mouse gut. Developmental Dynamics. 1999;216(2):137–152. [DOI] [PubMed] [Google Scholar]
- 41. Li W, Huang L, Lin W, Ke Q, Chen R, Lai X, Wang X, Zhang J, Jiang M, Huang W, Wang T, Yang X, Chen Y, Song W, Xiang AP. Engraftable neural crest stem cells derived from cynomolgus monkey embryonic stem cells. Biomaterials. 2015;39:75–84. [DOI] [PubMed] [Google Scholar]
- 42. Binder E, Natarajan D, Cooper J, Kronfli R, Cananzi M, Delalande JM, McCann C, Burns AJ, Thapar N. Enteric neurospheres are not specific to neural crest cultures: implications for neural stem cell therapies. Plos One. 2015;10(3):e0119467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wilkinson DJ, Bethell GS, Shukla R, Kenny SE, Edgar DH. Isolation of enteric nervous system progenitor cells from the aganglionic gut of patients with Hirschsprung’s disease. Plos One. 2015;10(5):e0125724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9(5):286–294. [DOI] [PubMed] [Google Scholar]
- 45. Costa M, Furness JB, Pompolo S, Brookes SJ, Bornstein JC, Bredt DS, Snyder SH. Projections and chemical coding of neurons with immunoreactivity for nitric oxide synthase in the guinea-pig small intestine. Neurosci Lett. 1992;148(1-2):121–125. [DOI] [PubMed] [Google Scholar]
- 46. Qu ZD, Thacker M, Castelucci P, Bagyanszki M, Epstein ML, Furness JB. Immunohistochemical analysis of neuron types in the mouse small intestine. Cell Tissue Res. 2008;334(2):147–161. [DOI] [PubMed] [Google Scholar]
- 47. Porter AJ, Wattchow DA, Brookes SJ, Costa M. The neurochemical coding and projections of circular muscle motor neurons in the human colon. Gastroenterology. 1997;113(6):1916–1923. [DOI] [PubMed] [Google Scholar]
- 48. Kirchgessner AL, Gershon MD. Projections of submucosal neurons to the myenteric plexus of the guinea pig intestine: in vitro tracing of microcircuits by retrograde and anterograde transport. J Comp Neurol. 1988;277(4):487–498. [DOI] [PubMed] [Google Scholar]
- 49. Kirchgessner AL, Tamir H, Gershon MD. Identification and stimulation by serotonin of intrinsic sensory neurons of the submucosal plexus of the guinea pig gut: activity-induced expression of Fos immunoreactivity. J Neurosci. 1992;12(1):235–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Heuckeroth RO. Regeneration: stem cells make the bowel nervous. Nature. 2016;531(7592):44–45. [DOI] [PubMed] [Google Scholar]
- 51. Aoi T. 10th anniversary of iPS cells: the challenges that lie ahead. J Biochem. 2016;160(3):121–129. [DOI] [PubMed] [Google Scholar]
- 52. Ishii T, Eto K. Fetal stem cell transplantation: past, present, and future. World J Stem Cells. 2014;6(4):404–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Palmer TD, Schwartz PH, Taupin P, Kaspar B, Stein SA, Gage FH. Cell culture. Progenitor cells from human brain after death. Nature. 2001;411(6833):42–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary_Material for DRG-Derived Neural Progenitors Differentiate into Functional Enteric Neurons Following Transplantation in the Postnatal Colon by Hui Hu, Yuanyuan Ding, Wenbo Mu, Ying Li, Yanpeng Wang, Weifang Jiang, Yong Fu, Jinfa Tou, and Wei Chen in Cell Transplantation