Abstract
Bone non-union after fracture, considered a therapeutic challenge for orthopedics, always needs a reversion surgery, including autograft transplantation (AGT). However, adverse events related to autograft harvest cannot be ignored. Our group designed a novel system called the bone marrow stem cell Screen-Enrich-Combine Circulating System (SECCS) by seeding mesenchymal stem cells (MSCs) into β-tricalcium phosphate (β-TCP) during surgery to thereafter rapidly process bioactive bone implantation. In this retrospective case-control study, 30 non-union patients who accepted SECCS therapy and 20 non-union patients who accepted AGT were enrolled. By SECCS therapy, the MSC-enriched β-TCP particles were implanted into the non-union gap. During the enrichment procedure, a significant proportion of MSCs were screened and enriched from bone marrow into porous β-TCP particles, and the cells possessed the capacity for three-line differentiation and were CD90+/CD105+/CD34-/CD45-. Approximately 82.0±10.7% of MSCs were enriched from 60 mL bone marrow without damaging cell viability, and approximately 11,444.0±6,018 MSCs were transplanted per patient. No implant-related infections occurred in any case. After 9 months of follow-up, 27 patients (90%) in the SECCS group acquired clinical union, compared with 18 patients (90%) in the AGT group (clinical union time, P = 0.064), and postoperative radiographic union score at 9 months post-operation was similar between the two groups. In conclusion, the SECCS could concentrate a large proportion of MSCs from bone marrow to acquire enough effective cells for therapy without in vitro cell culture. Bone substitutes processed by SECCS demonstrated encouraging promotion of bone regeneration and showed a satisfactory clinical curative effect for diaphyseal bone non-union, which was non-inferior to AGT.
Keywords: bone mesenchymal stem cells, enrichment, β-tricalcium phosphate, diaphyseal bone, non-union, autograft transplantation
Introduction
Bone non-union or delayed union represents impaired bone regeneration resulting from various factors, such as the size of the fracture gap, bone loss of open fractures or during primary surgery, and other relevant internal and external factors and unhealthy lifestyles1,2. Approximately 5–10% of fractures can lead to delayed union or non-union, and the incidence can be more than 13% after tibia fracture3,4. Reversion surgery is mandatory to correct the non-union fractures, but this causes immense burden to patients, surgeons, and health care systems. Many surgical strategies are followed to prevent bone non-union. A number of invasive or non-invasive methods have been applied for the treatment of bone non-union, including bone implantation, bone transport methods, cell therapy, cytokine therapy, distraction osteogenesis, and physical therapy to promote bone regeneration. Some of these methods have yielded satisfactory results, especially autograft bone implantation, which reached a consensus as the “gold standard” for bone regeneration5. However, many adverse events related to the conventional autograft transplantation (AGT), such as damage to the harvest site, insufficient donors, chronic pain, local hematoma, and infection, may restrict its clinical application6–9.
Remarkable progress in materialogy has successfully increased the potential of seeing widespread use of bone marrow stem cells combined with an artificial bone, instead of performing a bone autograft10,11. However, an in vitro cell culture is necessary for cell-based therapy, which may prolong the waiting time and increase the risk of contamination. To overcome this problem, our research group has designed a novel proprietary system called bone mesenchymal stem cell Screen-Enrich-Combine Circulating System (SECCS) for the enrichment of mesenchymal stem cells (MSCs), and we have performed a series of clinical cell therapies based on in vitro and in vivo experiments over the past few years12,13. We confirmed that MSCs could be screened and enriched by SECCS and that β-tricalcium phosphate (β-TCP) particles as the scaffold combined with MSCs can be used for bone repair13,14. Moreover, recently we have reported the application of SECCS for critical-size bone defect (3 cm defect) in six goat tibias with extremely satisfactory results15. As this novel SECCS technique does not require ex vivo cell expansion, bone substitutes can be manufactured by performing a 10-min filtration through β-TCP. These bone implants can be successfully transplanted during surgery, cutting down the pre-operative waiting time and avoiding cell culture-related limitations. Here, we report a retrospective study using SECCS to prepare a bone substitute combined with MSCs for diaphyseal bone non-union, and observe the clinical outcome compared with a historical matched group treated with AGT.
Materials and Methods
Patients
A retrospective case-control study was performed. From June 2013 to September 2017, in-patients who were diagnosed with bone non-union and underwent SECCS or AGT were enrolled in this study. The inclusion criteria were: (1) age no more than 65 years, male or female; (2) diaphyseal bone non-union; and (3) X-ray exam at 3, 6, and 9 months post-operation. Exclusion criteria were: (1) myelodysplastic syndromes; (2) infection; and (3) post high-dose radiotherapy and chemotherapy. Eventually, 30 patients for the SECCS group and 20 patients for the ABT group were recruited to the study. The baseline data are given in Table 1. There was high consistency between the two groups on age, gender, and non-union site.
Table 1.
Baseline data of the two groups.
| SECCS | AGT | P | |
|---|---|---|---|
| N | 30 | 20 | |
| Age(years) | 36.0±14.3 | 43.1±13.9 | 0.09 |
| Gender | 0.892 | ||
| Male | 23 | 15 | |
| Female | 7 | 5 | |
| Non-union site | 0.752 | ||
| Humerus | 3 | 2 | |
| Radius and ulna | 2 | 1 | |
| Femur | 12 | 11 | |
| Tibiofibula | 13 | 6 |
Autologous Bone Graft Harvest
For AGT patients, after receiving general anesthesia or continuous epidural anesthesia, a 2–4 cm incision was made on the surface of the ilium; then the surgeon exposed the bone cortex with careful dissection, cut a specific section of the iliac crest for making strips or granules, sterilized the excision site, and covered it with the dressing.
Bone Marrow Harvest
For SECCS patients, after receiving general anesthesia or continuous epidural anesthesia and proper aseptic measures, they were positioned in a supine position. The aspiration began with a 12-gauge bevel medullo-puncture needle puncturing the anterior superior iliac spine. Once the needle advanced into marrow cavity with the sense of breakthrough, a 20 mL syringe rinsed with heparinized saline in case of coagulation was used for aspirating bone marrow. To avoid mixing with too much peripheral blood, 10 mL aspiration was limited at every turn, and the depth or location was also changed every four times. Eventually, 80 mL bone marrow was collected into a blood collection bag containing 5000 U heparin sodium, of which 60 mL was used for MSC enrichment and 20 mL for enrichment efficiency testing and safety evaluation. The puncture site was then sterilized and covered with the dressing.
Enrichment of BMCs
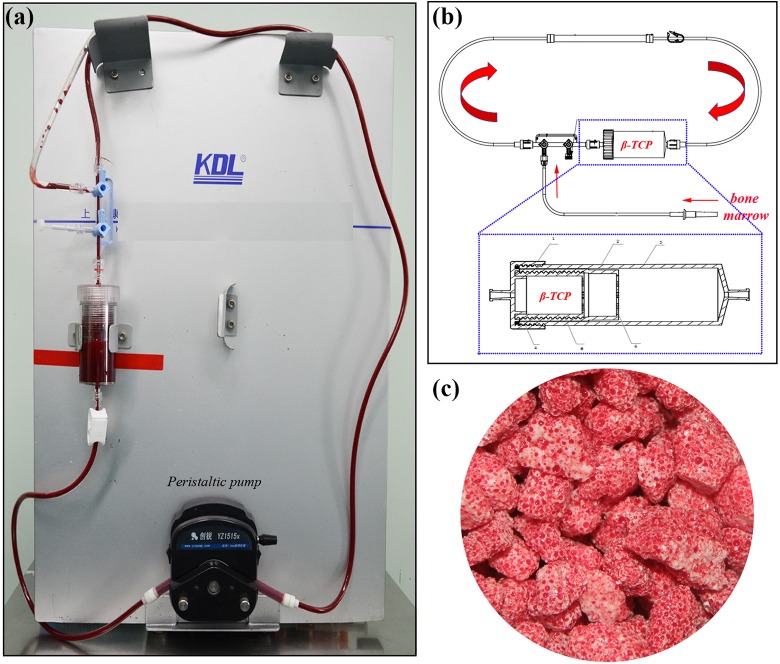
The proprietary SECCS system was composed of a peristaltic pump, a closed pipe, and a double storage box for bone substitute (Fig. 1a, b). First, approximately 5 g β-TCP (Bio-Lu, Shanghai, China, porosity of 75%±10%, diameter of 3–5 mm, mean pore size of 500±200 μm) was placed in the inner box, and the closed pipe was assembled with aseptic techniques. Subsequently, the collected 60 mL of bone marrow was introduced into the SECCS, and then the peristaltic pump was operated to circulate bone marrow through the pipe and filter through the porous β-TCP. After 10 min the MSCs-enriched β-TCP (MSCs/β-TCP) was manufactured and was ready for bone transplantation (Fig. 1c).
Figure 1.
The device and its schematic diagram of SECCS. (a) The device was composed of a peristaltic pump, a closed pipe, and a double storage box for bone substitute. (b) Schematic diagram of SECCS; porous β-tricalcium phosphate particles were placed in the inner box and bone marrow was driven by peristaltic pump to filtered through β-tricalcium phosphate particles when the device worked. 1: cap; 2: middle box; 3: outer box; 4: seal ring; 5: inner box; 6: filter hole. (c) Photograph of β-tricalcium phosphate particles manufactured after SECCS.
In vitro assay
Enrichment efficiency evaluation
Ten milliliters of pre- and post- enrichment bone marrow was collected for MSC adherent culture as reported by other researchers16,17. In brief, samples were cultured in six-well plates with osteogenic induction solution [alpha-minimum essential medium (Sigma, Santa Clara, California, USA) suspended in 10% fetal bovine serum (Hyclone, Logan, Utah, USA), 50 mg/mL sodium ascorbate (Sigma), 1% antibiotic/antimycotic (Sigma), 10 mM glycerophosphate (Sigma), and 10-8 M dexamethasone (Sigma)]. The medium was changed every 2 days, and at 10–14 days, the cultures were washed by phosphate-buffered saline (PBS), fixed by 4% paraformaldehyde and then stained with an alkaline phosphatase (ALP) staining kit (Lexiang, Shanghai, China), which as reported before was used for calculating colony-forming units (CFUs). Each CFU that was 2 mm in diameter or larger was manually quantified as one count. The mean count of ALP-positive colonies from six individual wells was used for each sample, and the presence of ALP+CFU represented an MSC colony18. Enrichment efficiency was formulated as (PREALP+CFUs – POSTALP+CFUs)/ PREALP+CFUs×100%. Another 1 mL bone marrow pre- and post-enrichment was sent for routine blood examination by a hematology analyzer (Mindray, China) to evaluate the adhesion of other cells in bone marrow, including leukocytes, erythrocytes, and platelets.
Bone Marrow Karyocyte Count and Cell Viability Test
One milliliter of pre- and post-enrichment samples from every patient was treated with red blood cell lysis buffer (BioTime, Shanghai, China) and was loaded on to the cell-counting machine (Beckman Coulter, Brea, California, USA) for karyocyte counting and cell viability testing.
MSC Identification by Flow Cytometry
To verify the presence of MSCs on the scaffold, the MSC-seeded β-TCP granules from a patient with femur non-union were collected and cultured as described above for 14 days. Then the cells adhering to the scaffold were digested by pancreatin (Sigma) and suspended and washed by PBS. The cell suspension was divided and labeled for CD34, CD45, CD90, and CD105 (Becton, Dickinson and Company, USA) and then analyzed by flow cytometry (Becton, Dickinson and Company).
MSC Identification by Osteogenic, Adipogenic, and Chondrogenic Induction
The MSC-seeded β-TCP granules from a patient with humerus non-union were cultured for 4 days, and cells were digested by pancreatin (Sigma) and suspended. Then, the cells were passaged, and the first-passage cells were collected.
For osteogenic induction, the cells were seeded into a six-well plate and cultured with osteogenic induction solution. At day 21, the cells were washed by PBS and fixed with 95% ethyl alcohol for 30 minutes. Then alizarin red staining (Solarbio, USA) was performed for 30 min at 37°C.
For adipogenic induction, the cells were seeded into a six-well plate and cultured with adipogenic induction solution A (Cyagen, USA) for 3 days, which was then changed to adipogenic induction solution B (Cyagen) for 1 day. At day 21, the cells were washed by PBS, fixed with 4% paraformaldehyde for 15 min, and then stained by oil red (Sigma).
Chondrogenic induction was performed as before19. In brief, aliquots of 250,000 cells were suspended and centrifuged for 5 min at 600 g in a 15 mL tube. Then, 0.5 mL chondrogenic medium (Cyagen) was added into the tube. After 48 h incubation, the cell pellets were placed into the tube. Medium was changed twice a week. At 28 days, the pellets were fixed with 4% paraformaldehyde for 15 min, followed by alcian blue staining (Solarbio, China).
Cell Morphology Observation
Some β-TCP particles filtered after SECCS were collected and separated into two parts, which were cultured for 2 h and 2 weeks, respectively. Samples were then fixed in 2% glutaraldehyde, rinsed in PBS, and post-fixed in 1% osmium tetroxide and dehydrated in a graded series of alcohols, to critical-point dryness. Finally the specimens were sputter-coated with a layer of gold for scanning electron microscopy observation.
Adherence Assay of MSCs to β-TCP
Ten patients authorized us to harvest another 20 mL bone marrow for examination. The 20 mL of bone marrow was filtered through 5 g β-TCP layered in a syringe simulating liquid flow in SECCS. The filtration speed was controlled at 5 mL per minute. Samples after each filtration were collected for ALP staining and ALP+CFU counting as described above.
Bacterium Examination
To guarantee the asepsis of pre- and post-enrichment samples and to trace the source once contaminated, 2 mL bone marrow pre- and post-enrichment was injected into an automatic blood culture bottle (Biomerieux, Inc., Durham), incubated at 30°C and detected by an automated blood culture system (Biomerieux, Inc) at 3 d and 7 d.
Implantation in vivo and Clinical Observation
Bone Graft Implantation
After preparing the implantation material, the surgeon started the main operation, eliminating the granulation tissue or fiber texture between fracture gaps as much as possible, and thereby the prepared autologous bone graft or MSCs/β-TCP was implanted to fill the fracture gap. Reliable stable internal fixation was guaranteed at the same time.
Bone Union Evaluation
All patients underwent outpatient follow-up at 3, 6, and 9 months after surgery and were followed for a maximum of 2 years. The X-ray images were assessed by two orthopedic surgeons, who had not attended the surgical procedure. Fracture union condition at 9 months was assessed by the Radiographic Union Score for Tibial Fractures (RUST). As there was no uniform method to evaluate long bone union in radiographic images, we chose RUST, as the tibia is regarded as a long bone. The score system evaluated the bone callus at four sides, providing a comprehensive description of union condition, and there were similar utilizations except tibial union20,21. For RUST scoring, each cortex received 1 point for no callus, 2 points for callus present but a fracture line still visible, and 3 points for bridging callus formation with no visible fracture line. The sum of anterior, posterior, medial, and lateral cortex indicated the final RUST score. Clinical union time was determined when two surgeons reached a consensus and by following the criteria: (1) disappearance of the fracture line and bone callus bridge in the operative site; (2) no tenderness or vertical percussion pain; (3) no abnormal movement in the operative site22.
Statistics
All parameters are expressed as the mean ± standard deviation. Statistical analysis was performed using SPSS 22.0 software (SPSS Inc, Chicago, America). P < 0.05 was defined as reaching statistical significance. Data with a normal distribution were compared by paired t-test or Students’ t-test, and non-normally distributed data were compare by the Mann–Whitney U test.
Results
Enrichment Evaluation
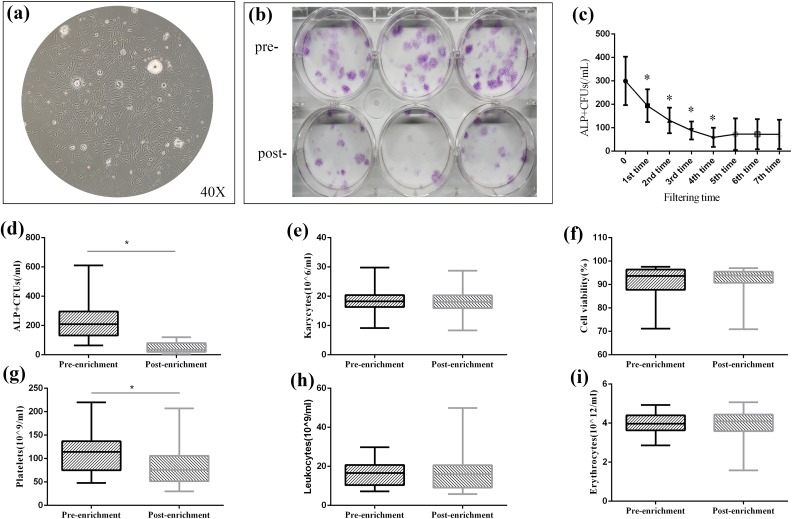
After approximately 2 weeks’ cell culture of bone marrow, cells proliferated and formed units. When observed under microscope, cells comprising CFUs appeared as long spindle-shaped or fibroblast-like cells (Fig. 2a). After ALP staining and calculations, the ALP+CFU number was 235.2±123.3/mL versus 44.5±33.1/mL for pre-enrichment versus post-enrichment, showing a significant decrease (P < 0.01), indicating that a large proportion of MSCs were adhered to β-TCP through filtration (Fig. 2b, d). In fact, the enrichment efficiency reached 82.0±10.7%. In other words, about 11,444.0±6,018.5 MSCs were transplanted per patient (calculated with 60 mL bone marrow blood).
Figure 2.
In vitro cell culture and examination of bone marrow samples. (a, b) After monolayer culture, mesenchymal stem cells (MSCs) demonstrated fibroblast-like morphology and formed colony-forming units (CFUs) under inverted microscope (40×); the CFUs can be stained by alkaline phosphatase (ALP) staining after osteogenic induction and the ALP+CFUs decreased after filtration. (c) Adherence assay of MSCs to β-TCP; MSCs significantly decreased after each filtration until the fifth time. (d) ALP+CFUs counting for pre-enrichment and post-enrichment. (e, f) Karyocytes and cell viability showed no significant decrease after enrichment. (g–i) In routine blood examination results, a significant number of platelets were left in β-TCP while leukocytes and erythrocytes remained with no significant decrease.
From the routine blood examination, we evaluated the main cell composition and found leukocytes and erythrocytes remained similar after enrichment (15.5±6.7×109/L versus 16.4±9.3×109/L, P = 0.373, and 3.8±0.9×1019/L versus 3.8±1.0×1019/L, P = 0.595, respectively) (Fig. 2h, i). Only the platelets showed a significant difference between pre- and post-enrichment, with 108.8±49.9×109/L pre-enrichment versus 80.3±33.7×109/L post-enrichment (P < 0.01), and the enrichment rate reached 25.5±23.9% (Fig. 2g).These results indicate that a large number of platelets also adhered to β-TCP through filtration, in addition to MSCs.
Bone Marrow Karyocyte (KC) Count and Cell Viability Test
The average KC number pre-enrichment was 18.0±4.1×106/mL versus 17.9±3.9×106/mL post-enrichment (P = 0.759) (Fig. 2e). Cell viability remained similar after enrichment (91.2±7.6% versus 90.9±7.8%, P = 0.265) (Fig. 2f).
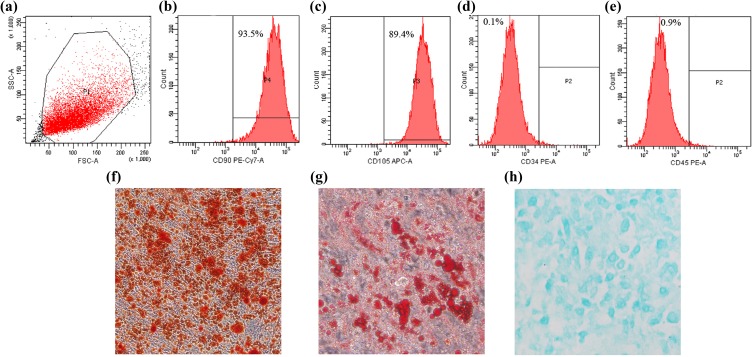
MSC Identification
In the immunophenotyping by flow cytometry, the cells collected from the scaffold demonstrated high expression of CD90 (93.5%) and CD105 (89.4%) and low expression of CD34 (0.1%) and CD45 (0.9%) (Fig. 3a–e). In addition, the cells could differentiate to osteoblasts, adipocytes, and chondroblasts in vitro (Fig. 3f–h). The ability for three-line differentiation and characteristics of surface markers confirmed the cells that adhered to scaffold were MSCs.
Figure 3.
Identification of cells adhering to β-TCP. (a–e) Immunophenotyping by flow cytometry; the cells demonstrated high expression of CD90 and CD105 and low expression of CD34 and CD45. (f) Alizarin red staining after osteogenic induction. (g) Oil red staining after adipogenic induction. (h) Alcian blue staining after chondrogenic induction.
Surface Morphology of MSCs/β-TCP Particles
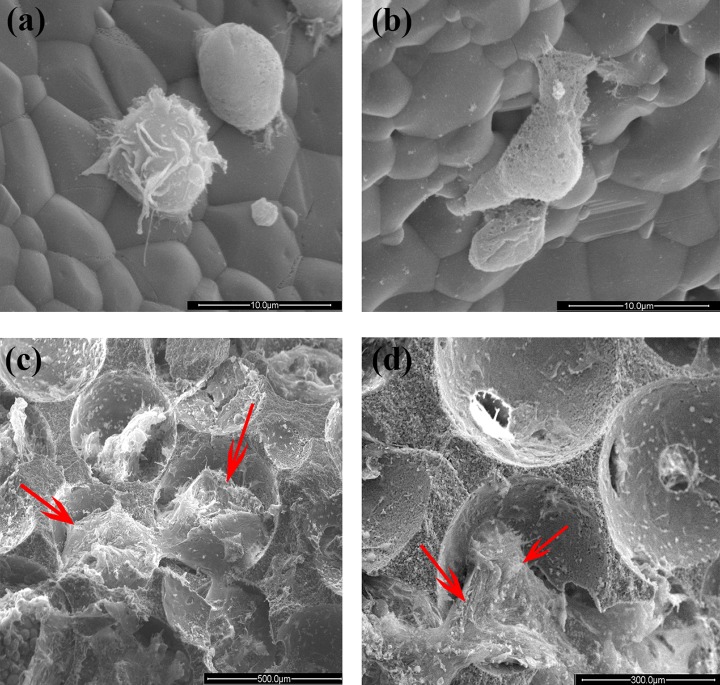
As shown in Fig. 4a and 4b, different cells with spheroidal and polygonal morphology were attached to the surface β-TCP after rapid filtration. When cultured for 2 weeks, the MSCs spread with a polygonal morphology and adhered tightly to the inner and outer surfaces of the granule (Fig. 4c, d).
Figure 4.
Photograph of MSCs/β-TCP particles under scanning electron microscopy. (a, b) β-TCP particles rapidly filtered and cultured for 2 h at 37°C. A lot of cells with different morphologies were seen attaching to the surface of β-TCP. (c, d) β-TCP particles rapidly filtered and cultured for 2 weeks at 37°C. The MSCs (red arrow) spread as polygon morphology and adhered tightly to the inner and outer surface of the granules.
Bacterial Examination
All 30 samples, except one case, demonstrated sterile reports pre- and post-enrichment after 3 and 7 days’ bacterial culture, suggesting a higher safety level for the SECCS if sterile precautionary measures are followed rigorously. In addition, a single case of positive culture with Staphylococcus epidermidis was reported in the sample but did not show any adverse symptoms of infection after surgery and was negative for any signs of implant-related infection.
Adherence Assay of MSCs to β-TCP
The ALP+CFU number decreased after each filtration (P < 0.05) and reached a plateau after the fifth filtration (P > 0.05), indicating that the MSCs could be easily screened by the scaffold, and there was a saturation condition for MSCs adhering to the porous β-TCP particles (Fig. 2c).
Bone Union Evaluation
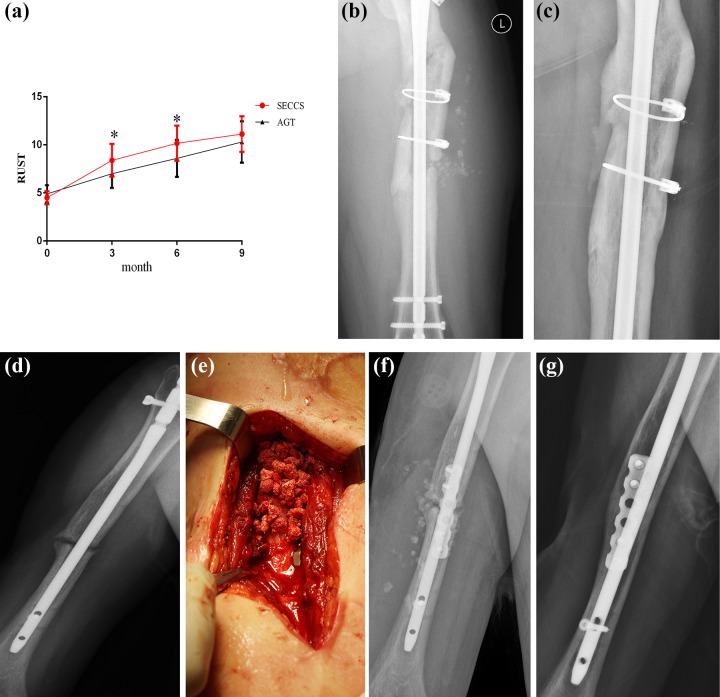
All patients underwent complete follow-up for at least 9 months (9–24 months). The average RUST score before SECCS and at 3 months, 6 months, and 9 months post-SECCS was 4.5±0.7, 8.4±1.7, 10.2±1.8, and 11.1±1.9 in the SECCS group and 4.9±0.9, 7.0±1.5, 8.6±1.9, and 10.3±2.2 in the AGT group, respectively. The score of SECCS was higher than AGT at 3 months and 6 months post-operation, but there was no difference at 9 months (Fig. 5a).
Figure 5.
Clinical observation after SECCS. (a) RUST scores of the two groups of pre-operation and 3 m,6 m,9 m post-operation. *:P < 0.05. (b, c) a 34-year-old male patient suffering from femora non-union. (b) X-ray at 7 days after SECCS. (c) X-ray at 2 years after SECCS. (d–g) a 28-year-old male patient with humeral shaft non-union (d). He accepted SECCS treatment, the MSCs/β-TCP particles were implanted inside and at the outer of the fracture gap (e), and the particles could be seen in the post-surgery X-ray (f). (g) X-ray indicated the patient acquired complete union after 9 months.
In the clinical follow-up, at 9 months post-operation, 27 patients had achieved clinical bone union compared with 18 in the AGT group. The success rate was 90% in each group, and clinical union time was 5.8±1.4 months (3–9 months) in SECCS and 6.8±2.0 months in AGT. There was no significant difference in clinical union time between the two groups (Mann–Whitney U test, P = 0.064). Follow-up of two patients who accepted SECCS treatment is shown in Fig. 5b–g.
Discussion
The current study presented a novel stem cell-based therapy for diaphyseal bone non-union by seeding MSCs into porous β-TCP. Diaphyseal bone healing is different from metaphyseal bone healing: the former mainly comprises cortical bone and engages marrow limited to MSCs, while the latter mainly comprises cancellous bone and is rich in stromal cells or MSCs23. Thereafter, fractured diaphyseal bone is more likely to develop into non-union bone and relies more on cells drawn from the periosteum, surrounding tissue, or blood. The MSCs inhabiting the diaphyseal bone also exhibit lower activity than those inhabiting the metaphyseal bone24. In our study, the bone substitute manufactured by SECCS not only provided a bone implant but also significantly increased the number of resident MSCs to promote bone regeneration.
In terms of clinical outcome, the ultimate union rate was similar in SECCS compared with the “gold standard.” However, even though the mean clinical union time was quicker in SECCS than AGT (5.8 months vs. 6.8 months), we cannot claim the SECCS was superior to AGT, and in fact, this difference did not reach statistical significance. We think this was because of the small sample size, and if the sample size were enlarged, the difference in clinical union time would narrow and show more similar promotion of bone regeneration. According to radiographic evaluation, patients acquired satisfactory bone callus formation by SECCS. Based on our radiographic observation, we believe the implanted MSCs-seeded β-TCP can survive well around the defect area, be degraded and be absorbed in a short time (many disappeared by the end of our follow-up), whereas the degradation time reported in other studies was quite long, up to 3 years, indicating that introducing MSCs can accelerate the degradation of β-TCP, thus facilitating new bone formation. In addition, we believe the degradation of calcium ion and phosphonium ion from the degradation of ß-TCP could provide materials and improve the microenvironment for bone regeneration. The similar clinical outcome between SECCS and AGT, and the reduced pain for patients, demonstrated that SECCS has potential for clinical applications and may be a substitute for autologous bone graft.
The gold standard for bone non-union is bone implantation on the basis of stable internal fixation. The most common material for implantation includes autologous bone graft, allogenic bone graft, and artificial bone. Autologous bone grafts from the iliac crest are the most widely used materials for orthopedic surgery owing to their osteoconduction, osteoinduction, and osteogenesis characteristics25. Nonetheless, as mentioned before, the morbidity, chronic pain, and other adverse events at the graft donor site might restrict its usage. Other grafts, such as artificial bone, are often employed only as a supplement. However, the lack of potential of osteogenesis of artificial bone cannot be ignored26,27. During bone regeneration, MSCs play an important role and are recruited to the fracture site from perivascular tissue and differentiate into osteogenic cells28. In this way, bone osteogenesis initiated by MSCs with artificial bone may create a perfect combination, and make artificial bone a better scaffold29. These features were the inspiration to develop the SECCS technique by seeding MSCs into a β-TCP scaffold.
β-TCP, a popular kind of artificial bone, has a mineral composition similar to that of native bone mineral [Ca3(PO4)2 and a calcium-to-phosphate ratio of 1.5:1]30. It is a bioactive ceramic bone substitute that has high bioconductivity. In a study conducted by Orii et al.31, β-TCP was soaked in the MSC suspension-containing medium for only 1 min and cultured for 3 h at 37°C, and subsequent electron microscopy confirmed that MSCs adhered to the pore surface of β-TCP scaffolds, suggesting high compatibility between MSCs and β-TCP and providing a theoretical basis for SECCS. As reported in previous literature, MSCs are considered as “plastic-adherent”; here, according to the immunophenotyping results and the ability for three-line differentiation, we confirm the presence of MSCs adhering to β-TCP, indicating that MSCs can be selectively screened by β-TCP also. Porous β-TCP has interconnected pores to facilitate MSCs adhesion and new bone formation as well as vascular generation. Indeed, according to the adherence assay, MSCs were “trapped” in the β-TCP scaffolds after each filtration, reaching a plateau at the fifth filtration, and most of the MSCs were “enriched.” The saturation of filtration indicated that the benefit from repeated filtration was limited, and we believe 10 min was sufficient to acquire enough enrichment, thus making SECCS more efficient.
The primary concern in applying bone marrow cell therapy is how to acquire enough MSCs. The number of implanted MSCs is correlated with new bone formation, even though the exact concentration required remains controversial16. However, MSCs occupy a tiny proportion of bone marrow, making up only 0.001–0.01% of mononuclear cells17. Thus, the MSCs must be concentrated when considering clinical applications. To date, two main methods have been commonly used: density gradient centrifugation and in vitro cell culture expansion, but they are expensive, time-consuming, and may lead to cell viability impairment and ethical concerns32,33. However, the SECCS utilizes circulatory filtration within 10 min, thus greatly shortening the time in cell culture. In addition, there was no significant decrease in cell viability during the process, suggesting little mechanical damage to MSCs when cells go through β-TCP. Moreover, the SECCS can reach an enrichment efficiency of 82%, with approximately 11,420 MSCs transplanted. We have noticed that the number of enriched MSCs was correlated with their initial concentration, which means a higher concentration could result in greater MSC enrichment (Pearson correlation coefficient = 0.972, P < 0.01, not demonstrated above). Thus, patients who possess low MSC reserves may have limited benefit from SECCS.
Another question of concern is whether to manage the MSCs after culture or centrifugation through systemic infusion or direct injection. Systemic infusion of the MSC suspension would largely result in trapping MSCs in the lungs, leaving only a few to migrate to the injury site34. We believe that the direct injection of MSCs would result in cell loss and thereafter may impair bone regeneration, as observed in some previous reports. For example, Gross et al. observed 45 cases of bone non-union and reported a 62.2% union rate after treating with percutaneous autologous bone marrow, with an absolute failure on the humerus35. The SECCS takes advantage of the high compatibility between MSCs and β-TCP and ultimately transforms the liquid cell suspension to a solid-state implant without any cell depletion.
More interestingly, we noticed that the β-TCP treated by SECCS was not only MSC enriched but also platelet enriched. We found a prominent decrease in platelet count in post-enrichment samples, indicating that a majority of them were eluted from the surface of β-TCP. The platelets are capable of releasing platelet growth factors and cytokines, which can stimulate cell recruitment, differentiation, and communication. Thus, platelet-rich plasma (PRP) is usually applied for healing musculoskeletal soft tissue injuries36. Moreover, some recent studies have reported that PRP could enhance bone healing37,38. Undoubtedly, the SECCS enriches both MSCs and platelets simultaneously to promote bone regeneration. However, whether SECCS enriched other cells or cytokines, such as monocytes and macrophages that play important roles in bone healing and participate in the remodeling of bone, is unknown and needs further study. In conclusion, SECCS is an ideal stem cell therapy with features of easy accessibility, reduced invasiveness, and rapidly expandable or concentrated methodology39.
There were some limitations of this study. First, the sample size was not large, especially in the AGT group, and this made the results less persuasive. This was because of the low morbidity of non-union in our hospital. Second, the method we followed for MSC enumeration used CFUs, which, even though it widely adopted, is not precise. Third, the most common reason for non-union in our patients was instability of internal fixation. Thus, these patients acquired SECCS or AGT and changed internal fixation at the same time, making the effect of SECCS possibly not as direct. A randomized controlled trial with mono-therapy would yield more accurate and persuasive conclusions.
Conclusions
The SECCS is a novel system of MSC-based therapy. By screening and enrichment of MSCs from bone marrow, without in vitro cell culture, surgeons can rapidly manufacture bone substitutes for seeding with MSCs without damaging cell viability and implant them into fracture gaps during surgery, thus avoiding adverse events of autograft bone harvest. The bioactive substitutes processed by SECCS enhance the local number of MSCs and improve bone regeneration even when treating diaphyseal bone non-union. The SECCS represents a rapid, low-damage, safe, and promising system for bone regeneration.
Acknowledgments
We are grateful to LiangDong Ke, Dongqian Chu and Jiajia Feng for providing technical help.
Footnotes
Ethics Statement: This study was approved by the Translational Medicine Ethics Committee of Shanghai Ninth People’s Hospital, Shanghai JiaoTong University School of Medicine (Approval Number: 2017-385-T282).
Statement of Human Rights: All procedures in this study were conducted in accordance with the approved protocols of the Translational Medicine Ethics Committee of Shanghai Ninth People’s Hospital, Shanghai JiaoTong University School of Medicine (Approval Number: 2017-385-T282).
Statement of Informed Consent: Exemption of informed consent were approved by the Ethics Committee as it was a retrospective study and any identity information was anonymized in this article.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by funds of the Clinical Research Plan of SHDC [16CR3099B]; the Emerging Advanced Technology Joint Research Project [SHDC12016110]; and the Clinical Research Program of the 9th People’s Hospital, Shanghai Jiao Tong University School of Medicine [JYL1015], National Natural Science Foundation of China [30801175]; National Key Research and Development Program of China [2016YFC1102104].
ORCID iD: Xin Wang  https://orcid.org/0000-0001-5452-8597
https://orcid.org/0000-0001-5452-8597
References
- 1. Santolini E, West R, Giannoudis PV. Risk factors for long bone fracture non-union: a stratification approach based on the level of the existing scientific evidence. Injury. 2015;46(suppl 8):S8–S19. [DOI] [PubMed] [Google Scholar]
- 2. Neumann MV, Zwingmann J, Jaeger M, Hammer TO, Sudkamp NP. Non-union in upper limb fractures - clinical evaluation and treatment options. Acta Chir Orthop Traumatol Cech. 2016;83(4):223–230. [PubMed] [Google Scholar]
- 3. Heckman JD, Sarasohn-Kahn J. The economics of treating tibia fractures. The cost of delayed unions. Bull Hosp Jt Dis. 1997;56(1):63–72. [PubMed] [Google Scholar]
- 4. Audige L, Griffin D, Bhandari M, Kellam J, Ruedi TP. Path analysis of factors for delayed healing and nonunion in 416 operatively treated tibial shaft fractures. Clin Orthop Relat Res. 2005;438:221–232. [DOI] [PubMed] [Google Scholar]
- 5. Bose S, Roy M, Bandyopadhyay A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012;30(10):546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chimutengwende-Gordon M, Khan WS. Advances in the use of stem cells and tissue engineering applications in bone repair. Curr Stem Cell Res Ther. 2012;7(2):122–126. [DOI] [PubMed] [Google Scholar]
- 7. Li L, He ZY, Wei XW, Wei YQ. Recent advances of biomaterials in biotherapy. Regen Biomater. 2016;3(2):99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Al-Moraissi EA, Mounair RM, El-Sharkawy TM, El-Ghareeb TI. Comparison between three-dimensional and standard miniplates in the management of mandibular angle fractures: a prospective, randomized, double-blind, controlled clinical study. Int J Oral Maxillofac Surg. 2015;44(3):316–321. [DOI] [PubMed] [Google Scholar]
- 9. Shin SR, Tornetta P, 3rd Donor site morbidity after anterior iliac bone graft harvesting. J Orthop Trauma. 2016;30(6):340–343. [DOI] [PubMed] [Google Scholar]
- 10. Liebergall M, Schroeder J, Mosheiff R, Gazit Z, Yoram Z, Rasooly L, Daskal A, Khoury A, Weil Y, Beyth S. Stem cell-based therapy for prevention of delayed fracture union: a randomized and prospective preliminary study. Mol Ther 2013;21(8):1631–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Giannotti S, Trombi L, Bottai V, Ghilardi M, D’Alessandro D, Danti S, Dell’Osso G, Guido G, Petrini M. Use of autologous human mesenchymal stromal cell/fibrin clot constructs in upper limb non-unions: long-term assessment. Plos One. 2013;8(8):e73893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cao L, Liu G, Gan Y, Fan Q, Yang F, Zhang X, Tang T, Dai K. The use of autologous enriched bone marrow MSCs to enhance osteoporotic bone defect repair in long-term estrogen deficient goats. Biomaterials. 2012;33(20):5076–5084. [DOI] [PubMed] [Google Scholar]
- 13. Gan Y, Dai K, Zhang P, Tang T, Zhu Z, Lu J. The clinical use of enriched bone marrow stem cells combined with porous beta-tricalcium phosphate in posterior spinal fusion. Biomaterials. 2008;29(29):3973–3982. [DOI] [PubMed] [Google Scholar]
- 14. Stanovici J, Le Nail LR, Brennan MA, Vidal L, Trichet V, Rosset P, Layrolle P. Bone regeneration strategies with bone marrow stromal cells in orthopaedic surgery. Curr Res Transl Med. 2016;64(2):83–90. [DOI] [PubMed] [Google Scholar]
- 15. Chu W, Gan Y, Zhuang Y, Wang X, Zhao J, Tang T, Dai K. Mesenchymal stem cells and porous beta-tricalcium phosphate composites prepared through stem cell screen-enrich-combine(-biomaterials) circulating system for the repair of critical size bone defects in goat tibia. Stem Cell Res Ther. 2018;9(1):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hernigou P, Poignard A, Beaujean F, Rouard H. Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J Bone Joint Surg Am. 2005;87(7):1430–1437. [DOI] [PubMed] [Google Scholar]
- 17. Bianco P. “Mesenchymal” stem cells. Annu Rev Cell Dev Biol. 2014;30:677–704. [DOI] [PubMed] [Google Scholar]
- 18. Majors AK, Boehm CA, Nitto H, Midura RJ, Muschler GF. Characterization of human bone marrow stromal cells with respect to osteoblastic differentiation. J Orthop Res. 1997;15(4):546–557. [DOI] [PubMed] [Google Scholar]
- 19. Zavan B, Giorgi C, Bagnara GP, Vindigni V, Abatangelo G, Cortivo R. Osteogenic and chondrogenic differentiation: comparison of human and rat bone marrow mesenchymal stem cells cultured into polymeric scaffolds. Eur J Histochem. 2007;51(suppl 1):1–8. [PubMed] [Google Scholar]
- 20. Litrenta J, Tornetta P, 3rd, Mehta S, Jones C, O'Toole RV, Bhandari M, Kottmeier S, Ostrum R, Egol K, Ricci W, Schemitsch E, Horwitz D. Determination of radiographic healing: an assessment of consistency using rust and modified rust in metadiaphyseal fractures. J Orthop Trauma. 2015;29(11):516–520. [DOI] [PubMed] [Google Scholar]
- 21. Guimarães JA, Duarte ME, Fernandes MB, Vianna VF, Rocha TH, Bonfim DC, Casado PL, do Val Guimarães IC, Velarde LG, Dutra HS, Giannoudis PV. The effect of autologous concentrated bone-marrow grafting on the healing of femoral shaft non-unions after locked intramedullary nailing. Injury 2014;45(suppl 5):S7–S13. [DOI] [PubMed] [Google Scholar]
- 22. Wade R, Richardson J. Outcome in fracture healing: a review. Injury. 2001;32(2):109–114. [DOI] [PubMed] [Google Scholar]
- 23. Aspenberg P, Sandberg O. Distal radial fractures heal by direct woven bone formation. Acta Orthop. 2013;84(3):297–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Siclari VA, Zhu J, Akiyama K, Liu F, Zhang X, Chandra A, Nah HD, Shi S, Qin L. Mesenchymal progenitors residing close to the bone surface are functionally distinct from those in the central bone marrow. Bone. 2013;53(2):575–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Myeroff C, Archdeacon M. Autogenous bone graft: donor sites and techniques. J Bone Joint Surg Am. 2011;93(23):2227–2236. [DOI] [PubMed] [Google Scholar]
- 26. Zivadinovic M, Andric M, Milosevic V, Manojlovic-Stojanoski M, Prokic B, Prokic B, Dimic A, Calasan D, Brkovic B. Histomorphometric evaluation of bone regeneration using autogenous bone and beta-tricalcium phosphate in diabetic rabbits. Vojnosanit Pregl. 2016;73(12):1132–1138. [DOI] [PubMed] [Google Scholar]
- 27. Malhotra A, Habibovic P. Calcium phosphates and angiogenesis: implications and advances for bone regeneration. Trends Biotechnol. 2016;34(12):983–992. [DOI] [PubMed] [Google Scholar]
- 28. Westhauser F, Zimmermann G, Moghaddam S, Bruckner T, Schmidmaier G, Biglari B, Moghaddam A. Reaming in treatment of non-unions in long bones: cytokine expression course as a tool for evaluation of non-union therapy. Arch Orthop Trauma Surg. 2015;135(8):1107–1116. [DOI] [PubMed] [Google Scholar]
- 29. Hosseinpour S, Ghazizadeh Ahsaie M, Rezai Rad M, Baghani MT, Motamedian SR, Khojasteh A. Application of selected scaffolds for bone tissue engineering: a systematic review. Oral Maxillofac Surg. 2017;21(2):109–129. [DOI] [PubMed] [Google Scholar]
- 30. Szpalski C, Wetterau M, Barr J, Warren SM. Bone tissue engineering: current strategies and techniques--part I: scaffolds. Tissue Eng Part B Rev. 2012;18(4):246–257. [DOI] [PubMed] [Google Scholar]
- 31. Orii H, Sotome S, Chen J, Wang J, Shinomiya K. Beta-tricalcium phosphate (beta-TCP) graft combined with bone marrow stromal cells (MSCs) for posterolateral spine fusion. J Med Dent Sci. 2005;52(1):51–57. [PubMed] [Google Scholar]
- 32. Chang Y, Hsieh PH, Chao CC. The efficiency of Percoll and Ficoll density gradient media in the isolation of marrow derived human mesenchymal stem cells with osteogenic potential. Chang Gung Med J. 2009;32(3):264–275. [PubMed] [Google Scholar]
- 33. Zhang W, Zhang F, Shi H, Tan R, Han S, Ye G, Pan S, Sun F, Liu X. Comparisons of rabbit bone marrow mesenchymal stem cell isolation and culture methods in vitro. Plos One. 2014;9(2):e88794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Oryan A, Kamali A, Moshiri A, Baghaban Eslaminejad M. Role of mesenchymal stem cells in bone regenerative medicine: what is the evidence? Cells Tissues Organs. 2017;204(2):59–83. [DOI] [PubMed] [Google Scholar]
- 35. Gross JB, Diligent J, Bensoussan D, Galois L, Stoltz JF, Mainard D. Percutaneous autologous bone marrow injection for treatment of delayed and non-union of long bone: a retrospective study of 45 cases. Biomed Mater Eng. 2015;25(suppl 1):187–197. [DOI] [PubMed] [Google Scholar]
- 36. Wu PI, Diaz R, Borg-Stein J. Platelet-rich plasma. Phys Med Rehabil Clin N Am. 2016;27(4):825–853. [DOI] [PubMed] [Google Scholar]
- 37. Akcay S, Kazimoglu C. Bone marrow aspirate concentrate and platelet-rich plasma enhanced bone healing in distraction osteogenesis of the tibia. Clin Orthop Relat Res. 2014;472(7):2301–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yu T, Pan H, Hu Y, Tao H, Wang K, Zhang C. Autologous platelet-rich plasma induces bone formation of tissue-engineered bone with bone marrow mesenchymal stem cells on beta-tricalcium phosphate ceramics. J Orthop Surg Res. 2017;12(1):178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang X, Wang Y, Gou W, Lu Q, Peng J, Lu S. Role of mesenchymal stem cells in bone regeneration and fracture repair: a review. Int Orthop. 2013;37(12):2491–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]