Abstract
Pigs have traditionally been used for preclinical experiments, and body size-matching is important for cell therapy in animal models used for preclinical trials. It has been shown that the efficacy of the transplanted cells is dependent on the response of the host heart and the age of experimental pigs.
Keywords: Stem cell, cell transplantation, pig, myocardial infarction, age
Translational research is important for clinical applications, and this has been scientifically established. Large animals used in clinical trials, such as primates, dogs, and pigs, differ significantly in size as compared with humans. It has been shown that body size-matching in animal models is essential for medical devices. Pigs have especially been considered essential for cell therapy tests in cardiovascular diseases. However, most investigators tend to use young pigs, because it is hard to handle adult miniature pigs weighing more than 40 kg. To investigate the profile of the pigs used in recent studies, we performed a literature review of publications from the past 3 years using three key words: cell transplantation, heart, and pig (Table 1)1–16. As expected, there were only a few reports describing the use of adult pigs older than 1 year of age14, and most investigators used young domestic pigs in preclinical studies.
Table 1.
Profile of Pigs Used in Recent Preclinical Studies of Cell Transplantation.
| Author | Year | Cell Type | Pigs Used in Experiments | Duration of Observation | Cardiac Function | Reference |
|---|---|---|---|---|---|---|
| Blázquez R, et al. | 2016 | CDCs | Large white (3–4 months, 30–35 kg) | 1 month | Not improved | PLOS One (2016) |
| Cai M, et al. | 2016 | MSCs from bone marrow | Chinese mini (10 months, 25 ± 5 kg) | 1 month | Improved | Sci. Rep. (2016) |
| Chang MY, et al. | 2016 | Cord blood mononuclear cells | Lanyu mini (∼5 months, 22.26 ± 0.78 kg) | 2 months | Improved | Stem Cells Transl. Med. (2016) |
| Gómez-Mauricio G, et al. | 2016 | Modified MSCs from adipose tissue | Large white (3–4 months, 39 ± 9.72 kg) | 1 month | Not improved | Stem Cell Res. Ther. (2016) |
| Kanazawa H, et al. | 2016 | CDCs | Yucatan mini (- months, 42–55 kg) | 2 months | Improved | J. Am. Heart Assoc. (2016) |
| Kulandavelu S, et al. | 2016 | Modified CSCs | Yorkshire (- months, 20–30 kg) | 2 months | Improved | J. Am. Coll. Cardiol. (2016) |
| Tseliou E, et al. | 2016 | CDCs | Yucatan mini (- months, 40–45 kg) | 1 month | Improved | PLOS One (2016) |
| Bobi J, et al. | 2017 | MSCs from adipose tissue | Large white (3–4 months, 35.1 ± 2.7 kg) | 2 months | Not improved | J. Am. Heart. Assoc. (2017) |
| Dariolli R, et al. | 2017 | MSCs from adipose tissue | MS60 EMBRAPA (- months, 15–20 kg) | 3 months | Not improved | PLOS One (2017) |
| Gálvez-Montón C, et al. | 2017 | Progenitor cells from adipose tissue | Landrace × Large white (- months, 30.2 ± 3.6 kg) | 1 month | Improved | Stem Cells Transl. Med. (2017) |
| Gálvez-Montón C, et al. | 2017 | Porcine iPSCs | Landrace × Large white (- months, 28.5 ± 3.3 kg) | 3 months | Not improved | Tissue Eng. Part C Methods (2017) |
| Kim MC, et al. | 2017 | MSCs from adipose tissue | Yorkshire × Landrace (- months, 25 kg) | 1 month | Improved | J. Korean Med. Sci. (2017) |
| Natsumeda M, et al. | 2017 | MSCs and/or CSCs | Göttingen mini (12–15 months, 25–30 kg) | 3 months | Improved | J. Am. Coll. Cardiol. (2017) |
| Alvino VV, et al. | 2018 | Adventitial pericytes | Large white (- months, 34.8 ± 0.7 kg) | 1.5 months | Not improved | J. Am. Heart Assoc. (2018) |
| Gao L, et al. | 2018 | Human iPSC-cardiac patch | Yorkshire (- months, 20–30 kg) | 2 months | Improved | Circulation (2018) |
| Ishigami M, et al. | 2018 | Human iPSC-cardiac patch | Micro-mini (- months, 15–25 kg) | 1 month | Improved | PLOS One (2018) |
CDCs: cardiosphere-derived cells; CSCs: cardiac stem cells; iPSCs: induced pluripotent stem cells; MSCs: mesenchymal stem cells.
Recently, in a study using a swine myocardial infarction (MI) model, Gálvez-Montón et al. assessed myocardial function and scar evolution following the implantation of engineered bioactive impedance grafts made of a scaffold of decellularized human pericardium, porcine adipose tissue-derived progenitor cells, and a customized-design electrical impedance spectroscopy monitoring system8. In the above study, which used cross-bred Landrace × Large white pigs weighing 30.2 ± 3.6 kg, 1 month following the intervention, a significant improvement in left ventricular ejection fraction was detected via magnetic resonance imaging (MRI). Chang et al. also investigated whether injection of human cord blood mononuclear cells, when combined with hyaluronan hydrogel, could improve the efficacy of cell therapy in a miniature pig MI model15. The pigs were treated with cyclosporine and methylprednisolone to prevent rejection of human cell transplants. It was found that 2 months following the surgery, treatment with human mononuclear cells in hyaluronan hydrogel elicited the highest left ventricle ejection fraction. In this study, Lanyu minipigs (∼5 months old and weighing 22.26 ± 0.78 kg) were used. In contrast, Natsumeda et al. used adult Göttingen minipigs (older than 1 year of age and weighing 25–30 kg)14. They evaluated the efficacy of combination cell therapy using autologous mesenchymal stem cells (MSCs) and cardiac stem cells (CSCs) and revealed that combination cell therapy synergistically reduced scar size and improved cardiac function.
It is well known that domestic pigs can grow quickly in size; however, the optimal weight for experiments ranges between 20 and 30 kg. In contrast, some investigators use 3–4-month-old domestic pigs in preclinical studies. However, an age of 3–4 months for a pig is equivalent to 6–7 years for a human. Certainly, it is unreasonable to use domestic pigs when their body size reaches that of a man older than 60 years of age. Additionally, it is difficult to justify the costs and the demerits involved in using adult mature miniature pigs. In contrast, there are possibilities that the beneficial effects of transplanted cells may be dependent on the response of the host heart, such as through paracrine effects16,17, indicating that the immature host heart will respond positively to the paracrine factors secreted from transplanted cells. Therefore, it is important to use adult pig models for accurate evaluations, because inaccurate evaluations will lead to failure in future clinical trials.
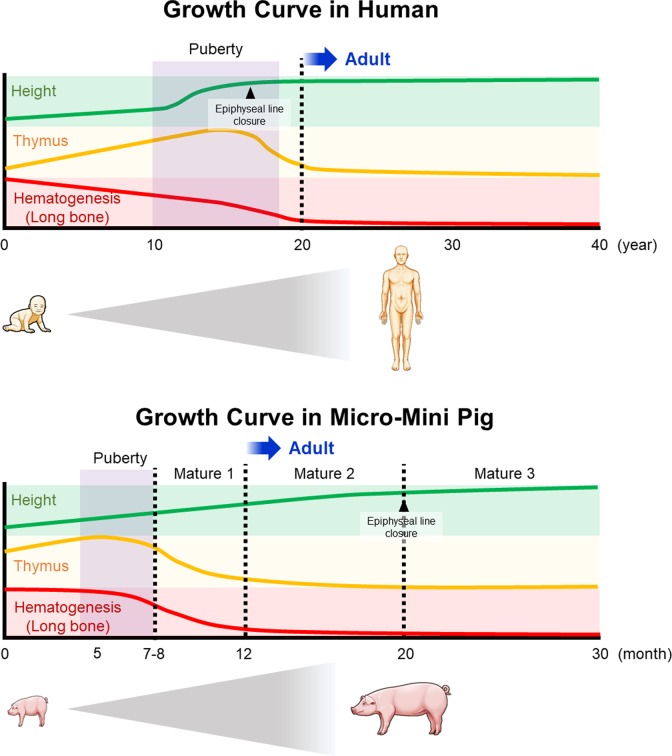
In our previous study, we investigated the smallest miniature pig, the micro-mini pig, as a preclinical model for cell therapy18. Based on the developmental profiles, we depicted the growth curve in humans and micro-mini pigs (Fig 1). Micro-mini pigs require approximately 12 months after birth to reach an adult mature body weight of 20 kg. In addition, epiphyseal lines close at 20 months of age in micro-mini pigs, while in humans, they usually close at 15–17 years of age. To establish an MI model in adult micro-mini pigs, cryoinjury-induced and ameroid constrictor-induced MI have been performed for preclinical studies19. Ishigami et al. also induced MI models in micro-mini pigs (weighing 15–25 kg) and demonstrated that the human pluripotent stem cell (hiPSC)-derived cardiac sheet transplantation significantly improved cardiac function as compared with that of the sham group in 1 month13. They also revealed that left ventricular (LV) remodeling was attenuated in the treatment group. However, even with the use of immunosuppressive drugs, it is very difficult to engraft the human cells in pig models. To solve this, we established an athymic micro-mini pig model, a kind of immunodeficiency model20. We achieved neonatal thymectomy in infantile micro-mini pigs born via cesarean section and demonstrated that engraftment of transplanted human cells tended to exhibit a longer retention in thymectomized micro-mini pigs. For accurate evaluation of safety and efficacy in human cell transplantation, a thymectomized micro-mini adult pig is expected to be a promising model in the fields of cardiovascular and stem cell research.
Fig 1.
Comparison of the growth curves in human and micro-mini pig. The growth curves with respect to height, development of thymus, and hematogenesis in humans and micro-mini pigs have been depicted. Micro-mini pigs take approximately 12 months after birth to reach an adult mature body weight of 20 kg. In addition, epiphyseal lines are closed at 20 months of age in micro-mini pigs, while in humans, they close at 15–17 years of age
Acknowledgement
We thank Akihisa Kangawa for excellent advice on micro-mini pig development (Fig. 1).
Footnotes
Ethical Approval: Ethical approval to report this case was obtained from the Keio University Institutional Animal Care and Use Committee.
Statement of Human and Animal Rights: All procedures in this study were conducted in accordance with the Institutional Guidelines on Animal Experimentation at Keio University.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: E.K. is a medical advisor of Fuji Micra Inc., Shizuoka, Japan.
Funding: The authors received no financial support for the research and/or authorship of this article.
References
- 1. Blázquez R, Sánchez-Margallo FM, Crisóstomo V, Báez C, Maestre J, Álvarez V, Casado JG. Intrapericardial delivery of cardiosphere-derived cells: an immunological study in a clinically relevant large animal model. PLOS One. 2016;11(2):e0149001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gómez-Mauricio G, Moscoso I, Martín-Cancho MF, Crisóstomo V, Prat-Vidal C, Báez-Díaz C, Sánchez-Margallo FM, Bernad A. Combined administration of mesenchymal stem cells overexpressing IGF-1 and HGF enhances neovascularization but moderately improves cardiac regeneration in a porcine model. Stem Cell Res Ther. 2016;7(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kanazawa H, Tseliou E, Dawkins JF, De Couto G, Gallet R, Malliaras K, Yee K, Kreke M, Valle I, Smith RR, Middleton RC, Ho CS, Dharmakumar R, Li D, Makkar RR, Fukuda K, Marbán L, Marbán E. Durable benefits of cellular postconditioning: long-term effects of allogeneic cardiosphere-derived cells infused after reperfusion in pigs with acute myocardial infarction. J Am Heart Assoc. 2016;5(2):e002796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kulandavelu S, Karantalis V, Fritsch J, Hatzistergos KE, Loescher VY, McCall F, Wang B, Bagno L, Golpanian S, Wolf A, Grenet J, Williams A, Kupin A, Rosenfeld A, Mohsin S, Sussman MA, Morales A, Balkan W, Hare JM. Pim1 kinase overexpression enhances ckit+ cardiac stem cell cardiac repair following myocardial infarction in swine. J Am Coll Cardiol. 2016;68(22):2454–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tseliou E, Kanazawa H, Dawkins J, Gallet R, Kreke M, Smith R, Middleton R, Valle J, Marbán L, Kar S, Makkar R, Marbán E. Widespread myocardial delivery of heart-derived stem cells by nonocclusive triple-vessel intracoronary infusion in porcine ischemic cardiomyopathy: superior attenuation of adverse remodeling documented by magnetic resonance imaging and histology. PLOS One. 2016;11(1):e0144523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bobi J, Solanes N, Fernández-Jiménez R, Galán-Arriola C, Dantas AP, Fernández-Friera L, Gálvez-Montón C, Rigol-Monzó E, Agüero J, Ramírez J, Roqué M, Bayés-Genís A, Sánchez-González J, García-Álvarez A, Sabaté M, Roura S, Ibáñez B, Rigol M. Intracoronary administration of allogeneic adipose tissue-derived mesenchymal stem cells improves myocardial perfusion but not left ventricle function, in a translational model of acute myocardial infarction. J Am Heart Assoc. 2017;6(5):e005771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dariolli R, Naghetini MV, Marques EF, Takimura CK, Jensen LS, Kiers B, Tsutsui JM, Mathias W, Jr, Lemos Neto PA, Krieger JE. Allogeneic pASC transplantation in humanized pigs attenuates cardiac remodeling post-myocardial infarction. PLOS One. 2017;12(4):e0176412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gálvez-Montón C, Bragós R, Soler-Botija C, Díaz-Güemes I, Prat-Vidal C, Crisóstomo V, Sánchez-Margallo FM, Llucià-Valldeperas A, Bogónez-Franco P, Perea-Gil I, Roura S, Bayes-Genis A. Noninvasive assessment of an engineered bioactive graft in myocardial infarction: impact on cardiac function and scar healing. Stem Cells Transl Med. 2017;6(2):647–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gálvez-Montón C, Soler-Botija C, Iborra-Egea O, Díaz-Güemes I, Martí M, Iglesias-García O, Prat-Vidal C, Crisóstomo V, Llucià-Valldeperas A, Perea-Gil I, Roura S, Sánchez-Margallo FM, Raya Á, Bayes-Genis A. Preclinical safety evaluation of allogeneic induced pluripotent stem cell-based therapy in a swine model of myocardial infarction. Tissue Eng Part C Methods. 2017;23(11):736–44. [DOI] [PubMed] [Google Scholar]
- 10. Kim MC, Kim YS, Kang WS, Lee KH, Cho M, Hong MH, Lim KS, Jeong MH, Ahn Y. Intramyocardial injection of stem cells in pig myocardial infarction model: the first trial in Korea. J Korean Med Sci. 2017;32(10):1708–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alvino VV, Fernández-Jiménez R, Rodriguez-Arabaolaza I, Slater S, Mangialardi G, Avolio E, Spencer H, Culliford L, Hassan S, Sueiro Ballesteros L, Herman A, Ayaon-Albarrán A, Galán-Arriola C, Sánchez-González J, Hennessey H, Delmege C, Ascione R, Emanueli C, Angelini GD, Ibanez B, Madeddu P. Transplantation of allogeneic pericytes improves myocardial vascularization and reduces interstitial fibrosis in a swine model of reperfused acute myocardial infarction. J Am Heart Assoc. 2018;7(2):e006727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gao L, Gregorich ZR, Zhu W, Mattapally S, Oduk Y, Lou X, Kannappan R, Borovjagin AV, Walcott GP, Pollard AE, Fast VG, Hu X, Lloyd SG, Ge Y, Zhang J. Large cardiac muscle patches engineered from human induced-pluripotent stem cell-derived cardiac cells improve recovery from myocardial infarction in swine. Circulation. 2018;137(16):1712–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ishigami M, Masumoto H, Ikuno T, Aoki T, Kawatou M, Minakata K, Ikeda T, Sakata R, Yamashita JK, Minatoya K. Human iPS cell-derived cardiac tissue sheets for functional restoration of infarcted porcine hearts. PLOS One. 2018;13(8):e0201650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Natsumeda M, Florea V, Rieger AC, et al. A combination of allogeneic stem cells promotes cardiac regeneration. J Am Coll Cardiol. 2017;70(20):2504–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chang MY, Huang TT, Chen CH, Cheng B, Hwang SM, Hsieh PC. Injection of human cord blood cells with hyaluronan improves postinfarction cardiac repair in pigs. Stem Cells Transl Med. 2016;5(1):56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cai M, Shen R, Song L, Lu M, Wang J, Zhao S, Tang Y, Meng X, Li Z, He ZX. Bone marrow mesenchymal stem cells (BM-MSCs) improve heart function in swine myocardial infarction model through paracrine effects. Sci Rep. 2016;6:28250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kawamura M, Miyagawa S, Fukushima S, Saito A, Toda K, Daimon T, Shimizu T, Okano T, Sawa Y. Xenotransplantation of bone marrow-derived human mesenchymal stem cell sheets attenuates left ventricular remodeling in a porcine ischemic cardiomyopathy model. Tissue Eng Part A. 2015;21(15–16):2272–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kobayashi E, Hanazono Y, Kunita S. Swine used in the medical university: overview of 20 years of experience. Exp Anim. 2018;67(1):7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hirano A, Fujita J, Kanazawa H, Kawaguchi S, Handa N, Yamada Y, Shigeo Okuda S, Hishikawa S, Teratani T, Kunita S, Tohyama S, Seki T, Tabei R, Nakajima K, Kishino Y, Okada M, Okamoto K, Shimizu H, Kobayashi E, Fukuda K. Cryoinjury-induced acute myocardial infarction model and ameroid constrictor-induced ischemic heart disease model in adult micro-mini pigs for preclinical studies. Translat Med Communicat. 2017;2(1). [Google Scholar]
- 20. Hsu HC, Enosawa S, Yamazaki T, Tohyama S, Fujita J, Fukuda K, Kobayashi E. Enhancing survival of human hepatocytes by neonatal thymectomy and partial hepatectomy in micro-miniature pigs. Transplant Proc. 2017;49(1):153–8. [DOI] [PubMed] [Google Scholar]