Short abstract
Although much is understood about the proinflammatory cytokine TNF-α, very limited data are available about TNF-β (lymphotoxin). Whether TNF-β can induce the proliferation of tumor cells, how TNF-β-induced proliferation of tumor cells is affected by natural products such as resveratrol and the role of NF-κB in this process, is not understood. In the present study, we used clonogenic and cytotoxic methods to show the effect of TNF-β on cell proliferation. We also examined the impact of resveratrol on TNF-β-promoted proliferation and on NF-κB activation in HCT116 colorectal cancer (CRC). Our findings showed that TNF-β induced the proliferation and invasion in CRC cells and this was comparable with that of TNF-α. TNF-β-stimulated proliferation of CRC cells was blocked via anti-TNF-β-receptor. We found that resveratrol reversed the TNF-β-induced proliferation and invasion of CRC cells, and this correlated with the suppression of TNF-β-stimulated NF-κB signaling. Like resveratrol, IκB-kinase (IKK) inhibitor (BMS-345541), also reversed TNF-β-stimulated proliferation, NF-κB activation and these were mediated through inhibition of IκB-kinase, phosphorylation of IκBα, suppression of phosphorylation, and nuclear translocation of the p65 subunit of NF-κB. Furthermore, resveratrol similar to BMS-345541 suppressed TNF-β-promoted NF-κB-mediated gene biomarkers linked with proliferation, apoptosis, and invasion. Overall, our findings indicate for the first time that TNF-β/TNF-β-receptor signaling is involved in proliferation of CRC cells in parallel to TNF-α, and that resveratrol down-modulates TNF-β/TNF-β-receptor-mediated inflammatory response, at least in part through down modulating NF-κB activation, thereby regulating tumor cell growth.
Impact statement
The mechanism by which natural products such as resveratrol suppresses TNF-β-promoted tumor cell proliferation, invasion, and colony formation is unknown. In this study, we explored for the first time the effect of resveratrol on the proinflammatory cytokine TNF-β-, compared to TNF-α-stimulated proliferative and pro-inflammatory signaling in HCT116 cells. Our findings suggest that expression of TNF-β and TNF-β-receptor, like TNF-α, can lead to activation of inflammatory transcription factor (NF-κB) and NF-κB-regulated gene biomarkers, which are involved in the promotion of cancer proliferation, invasion, metastasis, and cell survival of tumor. Resveratrol can block TNF-β/TNF-β-receptor-induced activation of NF-κB, NF-κB-modulated gene products, and inhibition of caspase-3 cleavage. These results highlight the therapeutic effect of resveratrol-mediated anti-tumor activity by multitargeting cellular signaling pathways.
Keywords: Resveratrol, colorectal cancer, TNF-β, NF-κB, proliferation
Introduction
Comprising approximately 10% of estimated cancer death worldwide, colon cancer is the third most common cancer. Although the incidence of this cancer has stabilized through the use of improved screening methods, five-year overall survival rates in industrial countries still range around 65%, as a majority of patients’ tumors develop chemoresistance to standard treatments allowing for the tumor to metastasize.1,2
Colorectal cancer development is a multi-step process modulated by cellular and non-cellular interactions in the tumor microenvironment which subsequently trigger tumor progression and invasion.3,4 One of the known risk factors for colon cancer includes chronic inflammatory bowel diseases such as Crohn’s disease and ulcerative colitis.5 Indeed, chronic inflammation plays a major role as a mediator for a number of diseases, including cancer6 and it is now widely recognized that chronic inflammation drives cancer development and progression.7,8
Inflammatory cells and inflammatory mediators (e.g. pro-inflammatory cytokines and chemokines, carcinogens, and chemotherapeutic agents, radiation, physical, and chemical stress) in the tumor microenvironment have been shown to lead to abnormal activation of specific transcription factors, such as nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), and thereby stimulate and promote proliferation and survival of malignant cells, angiogenesis, and metastasis.6,9–11 Under physiological conditions, a group of inhibitory proteins (e.g. IκBα, Bcl-3, p100, and others), all belonging to the IκB family, tightly control activation of NF-κB.12,13 However, in the case of carcinogenesis, the NF-κB transcription factor was shown to be constitutively active and its gene end-products shown to markedly stimulate tumor progression and metastasis.6,11 Further, the activation of NF-κB in tumors modulates the expression of diverse genes involved in blocking apoptosis and thus stimulates the tumor cell proliferation.14,15
Several reports indicate that among other pro-inflammatory cytokines, tumor necrosis factor-alpha (TNF-α) is crucial in cancer development by regulating communication in the tumor microenvironment. It does this primarily by its activation of the NF-κB-pathway which up-regulates inflammatory cytokines, cell-adhesion molecules, and invasive factors.6,16–19 Intriguingly, TNF-α is activated by a large number of tumors, which induces its own expression and that of other cytokines demonstrating the capacity of the tumor for autocrine and paracrine signaling.19,20 Indeed, TNF-α has been suggested to play an important function in stimulating proliferation of tumors.21
Although much is known about the importance of TNF-α in carcinogenesis, much less is known about its closest structural homologue TNF-β, also called Lymphotoxin-α because it was primarily derived from lymphocytes.22,23 TNF-β belongs to the TNF superfamily and activates apoptosis and inflammatory signals similar to TNF-α.24 Alike to TNF-α, TNF-β signals via the lymphotoxin-β receptor (LTβR)23 and activates canonical and non-canonical NF-κB signal.25–28 The LTβR is established to play essential function in inflammation, lymphoid organogenesis, and retaining architecture and compartmentalization of B and T lymphocytes in the adult spleen through stimulating the NF-κB pathway.25 Interestingly, induction of LTβR via Helicobacter pylori in gastric cancer has been suggested to stimulate the non-canonical NF-κB pathway.29 In bladder tumor, LTβR activation by lymphotoxin promoted an inflammatory microenvironment through upregulation of endogenous TNF-α and IL-1β expression; however, no significant effect on cell proliferation was observed although the cyclin D levels increased.30
Natural compounds have been shown to directly and indirectly regulate inflammatory pathways in the colon, thus influencing the tumor growth, progression, and tumor relapse.31,32 The natural stilbene and non-flavonoid polyphenol resveratrol possesses anti-oncogenic and anti-inflammatory features.33 Multiple reports have suggested that in various tumors, as well as in colorectal carcinoma cells, resveratrol blocks NF-κB activation, inhibits the cell cycle, and induces apoptosis.34–36
This investigation was designed to elucidate the role of TNF-β in colorectal cancer cell proliferation, invasion, apoptosis, and to examine the capability of resveratrol in suppressing TNF-β-promoted tumorigenesis in alginate cultures in vitro. Furthermore, we researched potential reciprocity among the TNF-β and the NF-κB signaling pathways, together with the role of resveratrol or BMS-345541 in suppressing TNF-β-stimulated NF-κB-dependent gene products. These gene products lead to inflammation, tumor cell growth, invasion, and metastasis.
Materials and methods
Antibodies and chemicals
Monoclonal antibodies to MMP-9, to phospho-IκBα, to phospho p65 (NF-κB), to cyclin D1, and antibody to cleaved caspase-3 were from R&D (Heidelberg, Germany). Polyclonal antibody against CXCR4 was from Abcam (Cambridge, UK). Monoclonal anti-β-Actin was from Merck-Sigma-Aldrich (Munich, Germany). Monoclonal antibodies to Ki-67 and secondary antibodies for immunofluorescence were from Dianova (Hamburg, Germany). TNF-β, TNF-α, monoclonal antibodies to TNF-β, to TNF-α, to TNF-β-receptor (LTβR), and to TNF-α-receptor were from eBiosciences (Frankfurt, Germany). TNF-β and TNF-α were a kind gift from Genetech, Inc. (San Francisco, CA, USA).37 Secondary antibodies for western blotting were from Millipore (Schwalbach, Germany) and for immunoelectron microscopy from Amersham (Braunschweig, Germany). Alginate, IKK inhibitor (BMS-345541) and resveratrol were purchased from Sigma. Epon was purchased from Plano (Marburg, Germany).
Growth media and chemicals
Culture growth medium (Dulbecco’s modified Eagle’s medium/Ham’s F-12 (1:1)), supplemented with as described before, was obtained from Biochrom (Berlin, Germany).36
Cell culture
The human colon cancer cell line (HCT116) was derived from the European Collection of Cell Cultures (91091005, Salisbury, UK) and cultured as described previously.36 Before starting the treatment, cells were washed with medium containing 3% FBS (serum-starved medium). All investigations were performed in serum-starved medium.
Alginate culture
Three-dimensional cultivation of CRC cells in alginate beads has been previously described in detail by our group.35,36,38,39
Light microscopy of alginate cultures
The effect of TNF-α, TNF-β, and/or resveratrol on the proliferation and colonosphere formation of HCT116 cells in alginate bead culture (cells were left untreated, and treated with 10 ng/mL TNF-α, 10 ng/mL TNF-β, or resveratrol (5 µM) by itself or co-treated with either 10 ng/mL TNF-α or 10 ng/mL TNF-β) was visualized at day 10 under a light microscope (Zeiss, Jena, Germany).
Cell proliferation assay
Effect of TNF-α, TNF-β, resveratrol, and anti-TNF-β-receptor alone or in combination on cell proliferation of HCT116 cells was probed using MTT-Assay as described previously.36,40 Briefly, monolayer cultures were left untreated, and treated with TNF-α (1, 5, 10 ng/mL), or TNF-β (1, 5, 10 ng/mL), or resveratrol (5 µM) by itself, or co-treated with either TNF-α (1, 5, 10 ng/mL) or TNF-β (1, 5, 10 ng/mL) for 24 h. In complementary experiments, cells were left untreated, and pre-treated in suspension with anti-TNF-β-receptor (0, 1, 10, 100, 200 µg/mL) for 10 min and then incubated with or without 10 ng/mL TNF-β for 10 min and cultivated for three days in alginate cultures. For subsequent MTT investigation, cells were retrieved from the alginate with 55 mM sodium citrate solution.39
Invasion assay
Invasive ability of HCT116 cells was investigated in 3D-alginate culture. HCT116 cells were left untreated, and treated with TNF-α (1, 5, 10, 20 ng/mL), or TNF-β (1, 5, 10, 20 ng/mL). In complementary experiments, HCT116 cells were left untreated, and treated with resveratrol (5 µM) by itself or co-treated with either TNF-α (1, 5, 10, 20 ng/mL) or TNF-β (1, 5, 10, 20 ng/mL) for 10 days. Cells migrated from the alginate and formed new colonies in the Petri dish. They were visualized and quantified as described before.38
Immunoelectron microscopy
Demonstration of TNF-β-receptor and TNF-α-receptor on tumor cells by immunogold labeling was performed as previously described.35 Sections were investigated under a Jeol 1200 EXII, Akishima Tokyo, Japan.
Immunofluorescence
Investigation of location of phosphorylated p65 in HCT116 cells was performed using monolayer cultures, as previously described.36 Tumor cells were left untreated, and treated with 10 ng/mL TNF-α, or 10 ng/mL TNF-β, or resveratrol (5 µM) by itself or co-treated with either 10 ng/mL TNF-α or 10 ng/mL TNF-β. In complementary experiments, tumor cells were either left untreated, and treated with BMS-345541 (5 µM) by itself or co-treated with either 10 ng/mL TNF-α or 10 ng/mL TNF-β for 24 h and immunofluorescence staining performed as previously described.36 Primary p65 antibody was diluted 1:80 in 1% BSA/PBS and secondary antibodies were diluted 1:100 in 1% BSA/PBS and co-stained with DAPI.36 Percentage of NF-κB positive cells and apoptotic cells was quantified by scoring 800–1000 cells from 10 different microscopic fields.
Western blot analysis
Dose- and time-dependent experiments of HCT116 cells in monolayer culture were investigated using immunoblotting as described previously.41 To study the effect of dose of TNF-β, tumor cells were left untreated, and treated with TNF-β (0, 1, 5, 10 ng/mL), or resveratrol (5 µM) by itself, or pre-treated with resveratrol (5 µM) for 12 h, followed by incubation with increasing concentrations of TNF-β (0, 1, 5, 10 ng/mL) for additional 12 h. In complementary experiments, cells were left untreated, and treated with TNF-β (0, 1, 5, 10 ng/mL), or BMS-345541 (5 µM) by itself, or co-treated with BMS-345541 (5 µM) for 4 h, followed by co-incubation with increasing concentrations of TNF-β (0, 1, 5, 10 ng/mL) for 12 h. To examine the effect of time, HCT116 cells were left untreated, and treated with TNF-β (10 ng/mL), TNF-α (10 ng/mL), or resveratrol (5 µM) by itself, or pre-treated with resveratrol (5 µM) for 12 h followed by co-treatment with 10 ng/mL TNF-β or TNF-α for 10, 20, and 40 min. In complementary experiments, cells were left untreated, and treated with TNF-β (10 ng/mL), TNF-α (10 ng/mL), or BMS-345541 (5 µM) by itself, or pre-treated with BMS-345541 (5 µM) for 4 h followed by co-treatment with 10 ng/mL TNF-β or TNF-α for 10, 20, and 40 min.
Statistics
The data were analyzed by Wilcoxon–Mann–Whitney test. The results are shown as mean ± standard deviation or SEM and were compared by one-way or a two-way ANOVA using SPSS Statistics, if the normality test passed (Kolmogorov–Smirnov test). Each study was repeated at least three times. P-value of < 0.05 was considered statistically significant.
Results
Resveratrol suppresses proliferation promoted by TNF-β or TNF-α in CRC cells in 3D-alginate tumor cultures
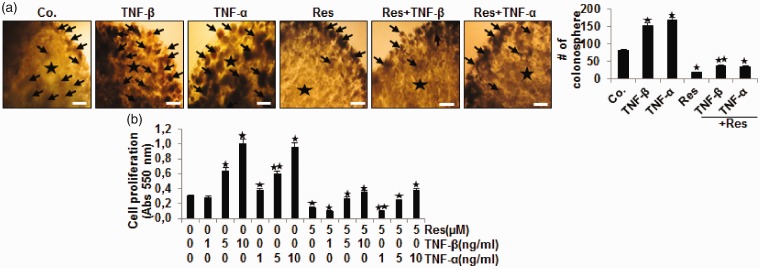
We compared TNF-β with TNF-α to see if it would affect the proliferation of CRC cells in a 3D alginate culture model which mimicked approximately the tumor microenvironment. The effects of TNF-β or TNF-α and/or resveratrol on cell proliferation were examined by performing a morphological investigation and MTT assay in HCT116 cells as indicated in Material and Methods (Figure 1(a) and (b)). Encapsulated tumor cells grew and formed typical colonosphere in the alginate beads (Figure 1(a)). TNF-β similar to TNF-α increased proliferation and colonosphere development of cells compared to control (Figure 1(a)). In opposite, resveratrol blocked proliferation and colonosphere development of tumor cells. It was noted that TNF-β or TNF-α and resveratrol significantly reduced cell proliferation compared to respective TNF treatment alone (Figure 1(a)). These results demonstrate that TNF-β, like TNF-α, markedly stimulates HCT116 proliferation in CRC. Further, as demonstrated by MTT results (Figure 1(b)), TNF-β or TNF-α by itself increased significantly cell proliferation dose-dependently, compared to control, by 11%, 110%, 267% and by 30%, 103%, and 223%, for TNF-β and TNF-α, respectively. Resveratrol alone reduced significantly proliferation of CRC cells, compared to control cultures, by ∼57%. In contrast, combination treatment of TNFs with resveratrol significantly induced cytotoxicity in HCT116 cells reducing cell proliferation compared to respective TNF treatment alone by about 66%, 55%, 65% and by about 74%, 61%, and 59%, TNF-β or TNF-α, respectively (P < 0.05) (Figure 1(b)). Taken together, these findings suggest that resveratrol inhibited cytokine-induced cell proliferation (Figure 1).
Figure 1.
Resveratrol blocks and down-modulates TNF-β- and TNF-α-induced proliferation of CRC cells. HCT116 cells in alginate bead cultures were treated as described in Materials and Methods. (a) Light microscopic demonstration of HCT116 cells grown in alginate beads culture (*) after 10 days. The number of colonospheres (arrows) was quantified by counting 15 different microscopic fields. Magnification ×24, bar = 0.2 mm in all cases. (b) The MTT method was used to investigate cell proliferation of HCT116 cells as described in detail in Materials and methods. All experiments were performed at least three times. P < 0.05 (*) and P < 0.01 (**) indicate a significant difference compared to the control group. (A color version of this figure is available in the online journal.)
TNF-β and TNF-α receptors are expressed on the surface of human CRC cells and functionality markedly influences CRC cell proliferation
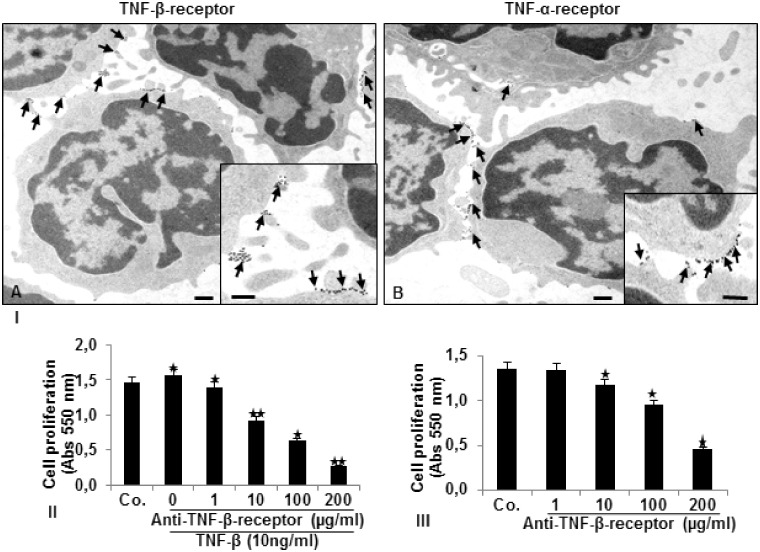
To demonstrate the localization and expression of TNF-β and TNF-α receptors on an ultrastructural level, we performed immunoelectron microscopy. Immunoelectron microscopic pictures in Figure 2(I) demonstrate basal expression and diffusely distributed TNF-β (A) and TNF-α (B) receptors predominantly on the round and planar cell surface. To elucidate further the importance of TNF-β receptors functional role for CRC cell proliferation, we performed an MTT assay. HCT116 were either left untreated, and pre-incubated in suspension with anti-TNF-β-receptor (0, 1, 10, 100, 200 µg/mL) for 10 min, then incubated with or without TNF-β for 10 min and cultured in alginate beads as indicated in Materials and Methods. Treatment with TNF-β alone led to significant enhance in cell proliferation compared to control cells by 8%. Pre-treatment with increasing amounts of anti-TNF-β-receptor to HCT116 cells stimulated with TNF-β, led to significant decrease in cell proliferation by 12%, 41%, 62%, and 86%, respectively (Figure 2(II)). More interestingly, anti-TNF-β-receptor treatment alone (Figure 2(III)) markedly decreased CRC cell proliferation compared to control by 3%, 10%, 30%, and 62%, respectively, with increasing dosage of antibody. These data not only demonstrate clearly the expression of TNF-β and TNF-α receptors on the tumor cell surface, but they show the important role of TNF-β receptors as signaling receptors on CRC cells for tumor cell survival.
Figure 2.
Expression of TNF-β- and TNF-α-receptors on the surface of HCT116 cells and the effect of a TNF-β-receptor antibody on HCT116 cell proliferation. I. Detection of TNF-β- (a) and TNF-α-receptor (b) expression (arrows) on HCT116 cells in suspension by pre-embedding immunogold labeling was performed as described in Materials and Methods. Ultrathin sections were examined under a transmission electron microscope. Scale bar = 0.2 µm; Insets: Scale bar = 0.09 µm. MTT-assay demonstrating the effect on proliferation of HCT116 cells by blocking the TNF-β-receptor co-treated with TNF-β (II) or without TNF-β (III) as described in Materials and Methods. *P < 0.05, **P < 0.01.
Resveratrol, as a multitargeted agent suppresses invasion and colony formation, stimulated by TNF-β or TNF-α in CRC cells
Figure 3 demonstrates, TNF-β (a), like TNF-α (b) promoted the invasion and colony development of CRC cells dose-dependently. These findings demonstrate that TNF-β, as TNF-α, markedly stimulates tumor cells migration in CRC. Next, we investigated whether TNF-β-promoted invasion and colony formation of HCT116 cells is affected by resveratrol treatment (Figure 3(c) and (d)). Therefore, CRC cells were left untreated, treated with resveratrol by itself or in combination with the TNFs as indicated in Material and Methods. Resveratrol alone inhibited clearly cell invasion in comparison to the control. Moreover, even the addition of 20 ng/mL of TNF-β or TNF-α could not totally block the inhibitory effect of resveratrol on invasion of HCT116 cells through the alginate-based matrix and suppressed colony formation (Figure 3(c) and (d)). The fact that neither TNF-α or TNF-β were able to totally overcome the effects of resveratrol highlights the multitarget potential of resveratrol suggesting that resveratrol may act through additional pathways independent of the TNFs.
Figure 3.
Resveratrol blocks and down-modulates TNF-β- and TNF-α-induced invasion and colony formation of CRC cells. HCT116 cells in alginate bead cultures were treated with TNF-β (a), or TNF-α (b), or resveratrol and TNF-β (c) or resveratrol and TNF-α (d) as described in Materials and Methods. Invasive colonies were stained with Toluidine blue after 10 days. The number of invasive and adhered colonies was visualized and quantified by counting all colonies under a light microscope (Zeiss, Germany). *P < 0.05, **P < 0.01. Invasion cell numbers were significantly lower in the resveratrol+TNF groups than in the TNF groups. (A color version of this figure is available in the online journal.)
Resveratrol blocks nuclear translocation of p65 promoted by TNF-β or TNF-α in HCT-116 cell as revealed by immunofluorescence microscopy
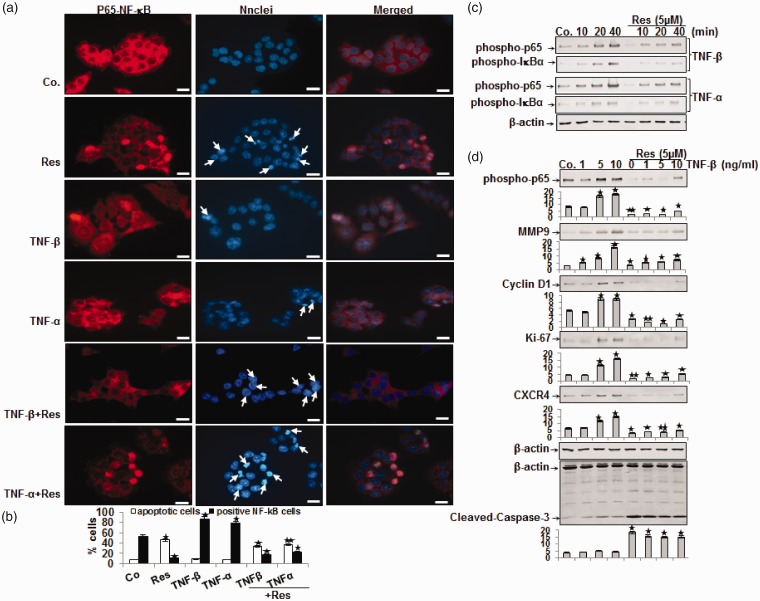
We further investigated whether TNF-β or TNF-α induced proliferation, invasion, and colony formation of CRC via activation of NF-κB.42 The p65 subunit is found in the cytoplasm of cells; however, after activation, p65 is translocated to the nucleus where it can modulate gene expression.43 Therefore, we investigated the effect of resveratrol on the TNF-β- or TNF-α-induced activation of p65.44 In control cells, immunofluorescence microscopy revealed intense cytoplasmic but weak nuclear staining (52%) for phospho-p65. TNF-β like TNF-α, induced comparable percentages of phospho-p65 to translocate to the nucleus (88% and 78%, respectively; Figure 4(a) and (b)). In contrast, pre-incubation with resveratrol before TNF-β or TNF-α treatment led to reduction of nuclear labeling of p65 (20% and 23%, respectively) (Figure 4(a) and (b)). There was marginal nuclear labeling of p65 in HCT116 cells treated with resveratrol by itself (10%) (Figure 4(a) and (b)). To confirm these results and the MTT assay, we investigated the extent of cell death using DAPI staining and fluorescence microscopy to reveal the nuclear morphological changes. We show that untreated, TNF-β, and TNF-α-treated HCT116 cells display normal nuclear size and minimal morphological changes resulting in 8%, 10%, and 7% apoptosis for control and TNF treatments, respectively. In contrast, significant increases in fragmented nuclei and apoptotic morphological changes were seen in HCT116 cells treated with resveratrol by itself (45%) or co-treated with TNF-β (33%) or TNF-α (37%) (Figure 4(a) and (b)). These results are consistent with results from the MTT study and support the fact that TNF-β and TNF-α have little or no effect on apoptosis in CRC cells (Figure 4(a) and (b)).
Figure 4.
Effect of resveratrol on 10 ng/mL TNF-β- or TNF-α-induced activation and nuclear translocation of p65 and NF-κB-regulated gene end-products in HCT116 cells. (a) HCT116 cells in monolayer culture were treated as described in Materials and Methods, labeled for p65 by immunofluorescence and counterstained with DAPI. The white arrows indicate apoptotic cells. Magnification 600×; bar = 30 µm. (b) Percentage of positively stained cells and apoptotic nuclei was quantified by counting 800–1000 cells from 10 different microscopic fields and values were compared to the control and statistically significant values with P < 0.05 are designated by an (*); P < 0.01 by two (**). Number of NF-κB-/apoptotic-positive cells was significantly lower/higher in the resveratrol+TNF groups than in the TNF groups. (c) Time- and (d) dose-dependent experiments of HCT116 cells in monolayer culture were performed as described in Materials and Methods. Immunoblotting of whole cell lysates was performed for anti-phospho-p65, anti-phospho-IκBα, anti-MMP-9, anti-cyclin D1, anti-Ki-67, anti-CXCR4, and anti-cleaved-caspase-3. The results are shown from at least three independent experiments and the housekeeping protein β-actin served as an internal loading control. Densitometric evaluation was performed for phospho-p65, MMP-9, cyclin D1, Ki-67, CXCR4 and cleaved-caspase-3. *P < 0.05, **P < 0.01.
Resveratrol down-modulates TNF-β- or TNF-α-induced p65 and IκBα phosphorylation in a time- and dose-dependent fashion in HCT116 cells
Because NF-κB activation is one of the first steps in the process of inflammation,42 we examined whether resveratrol modulates cytokine-promoted NF-κB activation time-dependently. Serum-starved HCT116 cells were examined for phosphorylated NF-κB after the tumor cells were treated as indicated in Material and Methods (Figure 4(c)). Western blotting results demonstrated that TNF-β, like TNF-α, induced phospho-p65 in a time-dependent fashion (Figure 4(c)). Interestingly, resveratrol inhibited both TNF-β- or TNF-α-promoted phosphorylation of p65 in a time-dependent fashion (Figure 4(c)). These Western blotting results were in accordance with the inhibition of phospho-p65 observed by immunomorphological methods. We wanted to know more about the mechanistic relationship of TNF-β and the NF-κB signaling in CRC cells. Phosphorylation and degradation of inhibitor of IκB-alpha, the natural suppressor of NF-κB, are required for the activation of NF-κB.45 We investigated this up-stream signaling of NF-κB activation by measuring p65 and IκBα phosphorylation by TNF-β or TNF-α in CRC cells.
TNF-β or TNF-α induced IκBα phosphorylation in a time-dependent fashion and phosphorylation was almost completely suppressed through addition of resveratrol (Figure 4(c)). These outcomes suggest that TNF-β/-α stimulates IκBα phosphorylation by acting up-stream to p65 phosphorylation and resveratrol inhibits TNF-β/-α-stimulated IκBα phosphorylation. They further indicate that the suppressing role of resveratrol on TNF-β signaling pathway, like TNF-α, in HCT116 cells is through blocking p65 phosphorylation. We probed whether the impact of resveratrol on TNF-β-promoted HCT116 cell proliferation and invasion in alginate was linked with the suppression of NF-κB signaling, as well as the down-regulation of p65-dependent gene products involved in tumor proliferation and metastasis. It is known that NF-κB modulates the expression of genes participating in proliferation (Ki-67, cyclin D1),44,45 invasion and metastasis (MMP-9, CXCR4),46 and apoptosis (caspase-3).47,48 HCT116 cells were treated with TNF-β (1, 5, 10 ng/mL), or with resveratrol, or resveratrol plus TNF-β for 12 h. As demonstrated in Figure 4(d), immunoblotting for phospho-p65 showed that TNF-β up-regulated phosphorylated p65 in a dose-dependent fashion, while resveratrol blocked this TNF-β-stimulated expression of phospho-p65 (Figure 4(d)). Similarly, TNF-β induced expression of MMP-9, cyclin D1, Ki-67, and CXCR4 (cancer-promoting proteins) but did not induce activity of caspase-3 (an anti-cancer protein). Conversely, resveratrol suppressed TNF-β-promoted expression of these cancer stimulating proteins and up-regulated the activity of caspase-3 in a dose-dependent fashion (Figure 4(d)). These results support the conclusion that resveratrol blocks TNF-β/-α-induced p65 phosphorylation and NF-κB-mediated inflammatory, proliferative, and invasive gene expression in tumor cells dose- and time-dependently.
Specific IKK inhibitor BMS-345541 blocks TNF-β- or TNF-α-promoted nuclear translocation of phospho-p65 to the cell nucleus in HCT116 cells
To further investigate the mechanisms involved in TNF-β-dependent activation of NF-κB, next we used immunofluorescence to monitor the effect of BMS-345541, a specific IKK (IκB kinase) inhibitor. Tumor cells were treated as indicated in Material and Methods and labeled with phospho-p65 antibody (Figure 5(a) and (b)). In untreated HCT116 cells, 50% of nuclear staining was observed. Treatment with TNF-β or TNF-α markedly increased nuclear staining (85% and 76%, respectively). BMS-345541 treatment alone markedly blocked nuclear translocation of phospho-p65 and only marginal nuclear staining was observed (8%). Interestingly, combining BMS-345541 with TNF-β or TNF-α blocked phospho-p65 nuclear translocation resulting in 20% and 12% positive stained cells, respectively. Additional investigation of apoptosis induction with DAPI revealed minimal apoptotic changes in untreated cells or treated with TNF-β or TNF-α (9%, 5%, 7% respectively). Consistent with the results found with phospho-p65 translocation, treatment with BMS-345541 by itself or co-treated with TNF-β or TNF-α increased apoptotic morphological changes in HCT116 cells with 38%, 30%, and 37%, respectively (Figure 5 (a) (b)). These results demonstrated that IKK is one of the kinases which is essential in TNF-β/-α-NF-κB-mediated signaling pathway. Taken together, these results further argue that phosphorylation of p65 nuclear translocation is necessary for TNF-β tumorigenic effects.
Figure 5.
BMS-345541 suppresses TNF-β- or TNF-α-induced activation and nuclear translocation of p65 and NF-κB-regulated gene end-products involved in proliferation, survival, invasion, and metastasis in HCT116 cells. (a) HCT116 cells were treated as described in Materials and Methods. They were labeled for p65 by immunofluorescence and counterstained with DAPI. Magnification 600×; bar = 30 µm. (b) All experiments were performed at least in triplicate and quantification of positively stained nuclei and apoptotic cells was performed by counting 800–1000 cells from 10 different microscopic fields. *P < 0.05, **P < 0.01. Number of NF-κB-/apoptotic-positive cells was significantly lower/higher in the BMS-345541+TNF groups than in the TNF groups. (c) Time- and (d) dose-dependent experiments of HCT116 cells in monolayer culture were performed as described in Materials and Methods. Immunoblotting of whole cell lysates was performed for anti-phospho-p65, anti-phospho-IκBα, anti-MMP-9, anti-cyclin D1, anti-Ki-67, anti- CXCR4, and anti-cleaved-caspase-3. The results are shown from at least three independent experiments and the housekeeping protein β-actin served as an internal loading control. Densitometric evaluation was performed for phospho-p65, MMP-9, cyclin D1, Ki-67, CXCR4 and cleaved-caspase-3. *P < 0.05, **P < 0.01.
BMS-345541 blocks TNF-β- or TNF-α-induced p-65 and IκBα phosphorylation in a time- and dose-dependent fashion
Additionally, we investigated up-stream in the NF-κB signaling pathway to elucidate the effect of TNF-β-induced IκBα phosphorylation as pre-requisite for p65 phosphorylation. Because IκBα phosphorylation and degradation require activation of IκB kinase (IKK), we investigated the impact of BMS-345541, a specific IKK inhibitor, on TNF-β- or TNF-α-promoted IKK activity. HCT116 cells were left untreated or treated as indicated in Material and Methods and whole cell lysates were probed for phosphorylated p65- and p-IκBα (Figure 5(c)). Western blotting results demonstrated that in HCT116 cells treated only with TNF-β, like TNF-α, time-dependent increases in phosphorylated p65 and IκBα were observed. In contrast, BMS-345541 pre-treatment markedly suppressed TNF-β- or TNF-α-promoted phosphorylation of p65 and IκBα in a time-dependent fashion (Figure 5(c)). These results highlight our previous findings and underline that resveratrol, like BMS-345541, suppresses TNF-β-induced IKK activity and IκBα phosphorylation thus blocking the NF-κB activation at an up-stream level. Next, HCT116 cells were treated with TNF-β (1, 5, 10 ng/mL), or BMS-345541 by itself or co-treated as indicated in Material and Methods, and subjected to immunoblotting to detect p65-NF-κB, MMP-9, cyclin D1, Ki-67, CXCR4, and cleaved-caspase-3. As shown in Figure 5(d), TNF-β up-regulated phospho-p65 in a dose-dependent fashion and this impact of TNF-β was suppressed by pre-treatment with BMS-345541. Furthermore, TNF-β induced NF-κB regulated gene products MMP-9, cyclin D1, Ki-67, CXCR4 and had no effect on caspase-3 activation, all factors relevant for promoting tumorigenesis. TNF-β-induced expression of these same factors was blocked by pre-treatment with BMS-345541. Addition of BMS-345541 induced cleavage of caspase-3. In sum, these data indicate that one way TNF-β promotes tumorigenesis in CRC cells is through the induction of the NF-κB pathway. Moreover, the anti-carcinogenic effects of resveratrol are, partially, mediated through up-stream inhibition of activation of this pathway, similar to BMS-345541 suppression of the NF-κB pathway.
Discussion
The purpose of this paper was to investigate whether the pro-inflammatory cytokine TNF-β (lymphotoxin α) can promote the proliferation and malignant potential of CRC cells. How TNF-β-stimulated proliferation of cancer cells is affected by natural products such as resveratrol and the role of the transcription factor NF-κB in this process is also not understood. In this study, therefore, we examined the impact of resveratrol on TNF-β-, compared to TNF-α-stimulated proliferative and pro-inflammatory signaling in HCT116 CRC cells.
In this study, we were able to demonstrate that TNF-β, as TNF-α, had a similar capability to induce proliferation, invasion, and colony development of tumor cells in an in vitro 3D-alginate tumor microenvironment. Interestingly, use of a TNF-β-receptor antibody blocked significantly TNF-β-induced cell proliferation. Pre-treatment of HCT116 cells with resveratrol or BMS-345541 (IKK-inhibitor) blocked cell proliferation, invasion, and colony formation stimulated by the TNFs, indicating that NF-κB signaling is involved in TNF-β-activated inflammatory tumor microenvironment. Further, resveratrol, similar to BMS-345541 inhibited the activation of NF-κB-specific biomarkers involved in tumorigenesis. TNF-β, like TNF-α promoted phosphorylation and translocation of p65 from the cytoplasm to the cell nucleus and these impacts were blocked by resveratrol or BMS-345541. Downregulation of NF-κB phosphorylation by resveratrol was mediated by the suppression of TNF-β/TNF-β-receptor-stimulated IKK activation, which resulted in suppressing IκBα and p65. Finally, to our knowledge, this is the first study showing that TNF-β like TNF-α acts as a potent inflammatory cytokine stimulating the cancer microenvironment. The suppressive impacts of resveratrol on TNF-β/TNF-β-receptor-stimulated tumor cell proliferation were found to be regulated, partially by blocking NF-κB signaling pathway.
Using this vitro model of HCT116 cells, after five days in culture, untreated cells proliferated, formed colonospheres, and migrated from the 3D culture matrix forming colonies on the bottom of the Petri dish. The proliferation, formation of colonosphere, and migration of HCT116 cells were clearly stimulated in the presence of TNF-β or TNF-α in a dose- and time-dependent fashion. These findings are in accordance with reports suggesting strong correlation between inflammation and tumor development in several cancers.46,48 Inflammation has been reported to promote a microenvironment that can lead to tumor formation and this is associated with tumorigenesis, including cellular transformation, promotion, proliferation, and metastasis.47,49–51
In support of the role of the TNFs in promoting inflammation and carcinogenesis, this study provides evidence of both TNF-β- and TNF-α-receptors in CRC cells, underlining that the TNFs receptor signaling may play a role in proliferation of CRC cells in response to these pro-inflammatory cytokines. Moreover, we surprisingly found that blocking of TNF-β-receptors (LTβR) significantly suppressed TNF-β-induced CRC cell proliferation and colonosphere development. This supports the idea that cancer cell survival is dependent on pro-inflammatory signaling in the tumor microenvironment and TNF-β/TNF-β-receptor play a major role as a mediator for inflammatory signaling. Furthermore, it underlines that TNF-β-receptor not only mediates cell–cell interaction but it shows the importance of functional role of TNF-β receptors as one of the major signaling receptors for communication of cancer microenvironment for cancer cell survival. Our data are in accordance with those studies, which showed the essential role of LTβR signaling in lymphoid organogenesis,52 tumorigenesis,30,53–55 and that it is involved in many inflammatory diseases.56 Indeed, it has been shown that the interaction of TNFs to their receptor induced stimulation of various intracellular pathways (such as NF-κB, JNK) and expression of TNF-α and IL-1β, and thus leads to cell proliferation, migration, and apoptosis.57–59
Because genetic biomarkers are regulated by transcription factors mediating the inflammatory process such as NF-κB, it is expected molecules that block NF-κB promotion could contribute to downregulation of these gene products. Indeed, previous studies have suggested that pro-inflammatory factors are linked with cancer growth through the activation of genes coding for NF-κB-mediated anti-apoptotic and pro-proliferation molecules.44,60,61 We demonstrated that TNF-β, like TNF-α, promoted p65 phosphorylation and nuclear translocation. Given that we are using phosphorylation of p65 as a marker for activation of NF-κB, this means that the TNFs increase the expression of pro-inflammatory gene products regulating cell survival, proliferation, and metastasis of tumor cells. In contrast to the tumor-promoting effect of the TNFs, resveratrol was potent in suppressing TNF-β-/-α-induced invasion, metastasis, and colony formation of CRC cells by blocking NF-κB signaling. Suppression of this pathway resulted in decreased tumorigenic gene products which play a role in tumor proliferation and survival. These data are in agreement with other investigations suggesting that downregulation of cytokine-induced NF-κB inhibits proliferation and colony formation of tumor cells.42,62–64
To further elucidate the mechanism by which the anti-tumorigenic effects of resveratrol are mediated by blocking of NF-κB, we used BMS-345541. We found that BMS-345541, similar to resveratrol, inhibits NF-κB specific activation in TNF-β- or TNF-α-stimulated HCT116 cells, preventing proliferation, invasion, and colony formation of CRC cells. Our findings correlate with another study showing that downregulation of NF-κB in cancer cells through expression of a dominant-negative mutant IκBα suppressed proliferation, survival, and cancer growth.65
Anti-tumorigenic effects of resveratrol were observed in the MTT and invasion assays in TNF-β- or TNF-α-stimulated CRC cells. We showed that treatment with resveratrol inhibited TNF-β- or TNF-α-induced NF-κB stimulation as well as NF-κB-mediated proteins through down-regulation of IKK, similar to BMS-345541. Indeed, it has been shown that resveratrol can inhibit IKK-NF-κB signaling.63 Furthermore, the anti-proliferative and anti-cancer impacts of resveratrol in TNF-β-stimulated HCT116 cells correlated with blocking of various cell proliferation, invasion, and survival gene products, e.g., cyclin D1, Ki-67, CXCR4, and MMP-9, all of which contain an NF-κB binding site in their promoters, regulating their transcription. Moreover, our data on morphological changes in response to treatment, for example pyknosis, chromatin condensation, apoptotic body formation and cleavage of caspase-3, demonstrated that resveratrol suppresses CRC proliferation by inducing apoptosis, which is consistent with earlier reports.35,36,66 The down-modulation of NF-κB and its downstream gene products leads to suppression of TNF-β-induced HCT116 cell proliferation, colony formation and invasion, which may be a mechanism for preventing the colorectal tumor cell metastasis. NF-κB is known to serve as a master transcription factor switch to turn on and off appropriate gene expression which plays major roles in carcinogenesis.44,62
TNF-β- or TNF-α-induced IKK activation, resulted in enhanced IκBα phosphorylation. Resveratrol not only was able to block this effect of TNF-β-/-α-induced IKK activation, but it suppressed TNF-β-/-α-promoted activation of p65, along with its nuclear translocation and enhanced production of NF-κB-regulated proteins.
How resveratrol suppresses NF-κB activation in TNF-β-stimulated HCT116 cells was also explored. We have shown that resveratrol, similar to the IKK-specific inhibitor, blocked IκBα phosphorylation and IKK activity, which is in agreement with earlier studies,67–70 suggesting direct suppression of this kinase activity. Indeed, resveratrol clearly suppressed NF-κB activation, as revealed by immunohistochemical and immunoblotting techniques and these results may be associated with its anti-tumor mechanism.71
We found that HCT116 cells express the proliferation markers cyclin D1 and Ki-67 proteins and resveratrol suppresses their expression. Furthermore, the CCND1 gene is a proto-oncogene, which is upregulated through gene enhancement or translocation in various tumor cells.72–74 Interestingly, cyclin D1 and Ki-67 activation were previously demonstrated to be regulated via NF-κB in cancer cells and their metastatic counterparts.75–77 Moreover, we found that HCT116 cells express MMP-9 and CXCR4 and TNF-β induced the expression of these proteins, which is consistent with previous studies.38,78 These genes are also modulated by NF-κB and we showed that pre-treatment of HCT116 with resveratrol or BMS-345541 decreased TNF-β-stimulated expression. Interestingly, the downregulation of gene products and thus suppression of proliferation of CRC cells through resveratrol may be by mechanisms other than NF-κB suppression and this cannot be entirely ruled out.
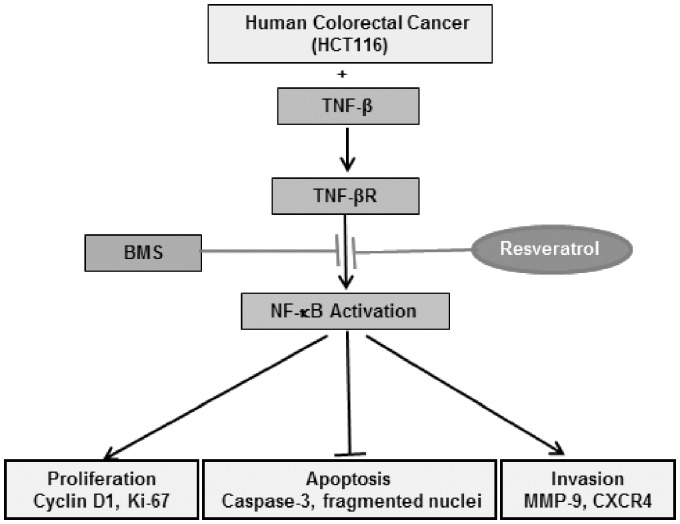
In conclusion, our findings are the first to suggest that expression of TNF-β and TNF-β-receptor, like TNF-α, can promote inflammatory transcription factor (NF-κB) and NF-κB-regulated gene biomarkers, which are involved in promotion of tumor cell proliferation (Cyclin D1, Ki-67), cell invasion (MMP-9), metastasis (CXCR4) and cell survival, all traits of cancer cells. Use of a natural product such as resveratrol can block TNF-β/TNF-β-receptor-stimulated activation of NF-κB, NF-κB-regulated gene products and inhibition of caspase-3 cleavage, suggesting that resveratrol activates caspase-dependent CRC cell death, rather than modulating NF-κB-regulated apoptotic gene biomarkers. These findings underline the therapeutic effect of resveratrol-mediated anti-tumor activity by multitargeting cellular signaling pathways (Figure 6).
Figure 6.
Schematic diagram showing resveratrol-mediated anti-tumorigenic activity by down-regulation of NF-κB signaling in TNF-β-stimulated colorectal cancer cells.
ACKNOWLEDGEMENTS
We thank Sabine Miech and Dr. Andreas Eimannsberger for excellent technical assistance and Margaret Hinshelwood, PhD, for editing. This study is a part of the doctoral thesis of Mina Yazdi to be submitted to Ludwig-Maximilian-University Munich, Germany.
Authors’ contributions
MY, BP, PS and CB performed all the experiments and analyses. AG, CB, BBA and MS were responsible for the study design, data interpretation and writing.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The author(s) received no financial support for the research, authorship, and/or publication of this article
References
- 1.Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, Jemal A. Colorectal cancer statistics, 2017. CA Cancer J Clin 2017; 67:177–93 [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65:87–108 [DOI] [PubMed] [Google Scholar]
- 3.Sambi M, Haq S, Samuel V, Qorri B, Haxho F, Hill K, Harless W, Szewczuk MR. Alternative therapies for metastatic breast cancer: multimodal approach targeting tumor cell heterogeneity. Breast Cancer 2017; 9:85–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer 2006; 6:392–401 [DOI] [PubMed] [Google Scholar]
- 5.Brenner H, Stock C, Hoffmeister M. Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: systematic review and meta-analysis of randomised controlled trials and observational studies. BMJ 2014; 348:g2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: how hot is the link? Biochem Pharmacol 2006; 72:1605–21 [DOI] [PubMed] [Google Scholar]
- 7.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature 2008; 454:436–44 [DOI] [PubMed] [Google Scholar]
- 8.Gupta SC, Tyagi AK, Deshmukh-Taskar P, Hinojosa M, Prasad S, Aggarwal BB. Downregulation of tumor necrosis factor and other proinflammatory biomarkers by polyphenols. Arch Biochem Biophys 2014; 559:91–9 [DOI] [PubMed] [Google Scholar]
- 9.Mantovani A. Molecular pathways linking inflammation and cancer. Curr Mol Med 2010; 10:369–73 [DOI] [PubMed] [Google Scholar]
- 10.Candido J, Hagemann T. Cancer-related inflammation. J Clin Immunol 2013; 33:S79–84 [DOI] [PubMed] [Google Scholar]
- 11.Xia L, Tan S, Zhou Y, Lin J, Wang H, Oyang L, Tian Y, Liu L, Su M, Wang H, Cao D, Liao Q. Role of the NFkappaB-signaling pathway in cancer. Onco Targets Ther 2018; 11:2063–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-kappa B. Annu Rev Cell Biol 1994; 10:405–55 [DOI] [PubMed] [Google Scholar]
- 13.Durand JK, Baldwin AS. Targeting IKK and NF-kappaB for therapy. Adv Protein Chem Struct Biol 2017; 107:77–115 [DOI] [PubMed] [Google Scholar]
- 14.Bharti AC, Aggarwal BB. Chemopreventive agents induce suppression of nuclear factor-kappaB leading to chemosensitization. Ann N Y Acad Sci 2002; 973:392–5 [DOI] [PubMed] [Google Scholar]
- 15.Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer 2002; 2:301–10 [DOI] [PubMed] [Google Scholar]
- 16.Sethi G, Sung B, Aggarwal BB. TNF: a master switch for inflammation to cancer. Front Biosci 2008; 13:5094–107 [DOI] [PubMed] [Google Scholar]
- 17.Balkwill F. TNF-alpha in promotion and progression of cancer. Cancer Metastasis Rev 2006; 25:409–16 [DOI] [PubMed] [Google Scholar]
- 18.Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer 2009; 9:361–71 [DOI] [PubMed] [Google Scholar]
- 19.Balkwill F. Tumor necrosis factor or tumor promoting factor? Cytokine Growth Factor Rev 2002; 13:135–41 [DOI] [PubMed] [Google Scholar]
- 20.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001; 357:539–45 [DOI] [PubMed] [Google Scholar]
- 21.Sugarman BJ, Aggarwal BB, Hass PE, Figari IS, Palladino MA, Jr, Shepard HM. Recombinant human tumor necrosis factor-alpha: effects on proliferation of normal and transformed cells in vitro. Science 1985; 230:943–5 [DOI] [PubMed] [Google Scholar]
- 22.Aggarwal BB, Moffat B, Harkins RN. Human lymphotoxin. Production by a lymphoblastoid cell line, purification, and initial characterization. J Biol Chem 1984; 259:686–91 [PubMed] [Google Scholar]
- 23.Aggarwal BB, Eessalu TE, Hass PE. Characterization of receptors for human tumour necrosis factor and their regulation by gamma-interferon. Nature 1985; 318:665–7 [DOI] [PubMed] [Google Scholar]
- 24.Etemadi N, Holien JK, Chau D, Dewson G, Murphy JM, Alexander WS, Parker MW, Silke J, Nachbur U. Lymphotoxin alpha induces apoptosis, necroptosis and inflammatory signals with the same potency as tumour necrosis factor. FEBS J 2013; 280:5283–97 [DOI] [PubMed] [Google Scholar]
- 25.Dejardin E, Droin NM, Delhase M, Haas E, Cao Y, Makris C, Li ZW, Karin M, Ware CF, Green DR. The lymphotoxin-beta receptor induces different patterns of gene expression via two NF-kappaB pathways. Immunity 2002; 17:525–35 [DOI] [PubMed] [Google Scholar]
- 26.Muller JR, Siebenlist U. Lymphotoxin beta receptor induces sequential activation of distinct NF-kappa B factors via separate signaling pathways. J Biol Chem 2003; 278:12006–12 [DOI] [PubMed] [Google Scholar]
- 27.Cupedo T, Mebius RE. Cellular interactions in lymph node development. J Immunol 2005; 174:21–5 [DOI] [PubMed] [Google Scholar]
- 28.Schneider K, Potter KG, Ware CF. Lymphotoxin and LIGHT signaling pathways and target genes. Immunol Rev 2004; 202:49–66 [DOI] [PubMed] [Google Scholar]
- 29.Feige MH, Vieth M, Sokolova O, Tager C, Naumann M. Helicobacter pylori induces direct activation of the lymphotoxin beta receptor and non-canonical nuclear factor-kappa B signaling. Biochim Biophys Acta 2018; 1865:545–50 [DOI] [PubMed] [Google Scholar]
- 30.Shen M, Zhou L, Zhou P, Zhou W, Lin X. Lymphotoxin β receptor activation promotes mRNA expression of RelA and pro-inflammatory cytokines TNFalpha and IL-1beta in bladder cancer cells. Mol Med Rep 2017; 16:937–42 [DOI] [PubMed] [Google Scholar]
- 31.Kuppusamy P, Yusoff MM, Maniam GP, Ichwan SJ, Soundharrajan I, Govindan N. Nutraceuticals as potential therapeutic agents for colon cancer: a review. Acta Pharm Sin B 2014; 4:173–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Umesalma S, Sudhandiran G. Differential inhibitory effects of the polyphenol ellagic acid on inflammatory mediators NF-kappaB, iNOS, COX-2, TNF-alpha, and IL-6 in 1,2-dimethylhydrazine-induced rat colon carcinogenesis. Basic Clin Pharmacol Toxicol 2010; 107:650–5 [DOI] [PubMed] [Google Scholar]
- 33.Ko JH, Sethi G, Um JY, Shanmugam MK, Arfuso F, Kumar AP, Bishayee A, Ahn KS. The role of resveratrol in cancer therapy. Int J Mol Sci 2017; 18:2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cal C, Garban H, Jazirehi A, Yeh C, Mizutani Y, Bonavida B. Resveratrol cancer chemoprevention, apoptosis, and chemo-immunosensitizing activities. Curr Med Chem Anticancer Agents 2003; 3:77–93 [DOI] [PubMed] [Google Scholar]
- 35.Buhrmann C, Shayan P, Kraehe P, Popper B, Goel A, Shakibaei M. Resveratrol induces chemosensitization to 5-fluorouracil through up-regulation of intercellular junctions, epithelial-to-mesenchymal transition and apoptosis in colorectal cancer. Biochem Pharmacol 2015; 98:51–68 [DOI] [PubMed] [Google Scholar]
- 36.Buhrmann C, Shayan P, Goel A, Shakibaei M. Resveratrol regulates colorectal cancer cell invasion by modulation of focal adhesion molecules. Nutrients 2017; 9:1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bringman TS, Aggarwal BB. Monoclonal antibodies to human tumor necrosis factors alpha and beta: application for affinity purification, immunoassays, and as structural probes. Hybridoma 1987; 6:489–507 [DOI] [PubMed] [Google Scholar]
- 38.Shakibaei M, Kraehe P, Popper B, Shayan P, Goel A, Buhrmann C. Curcumin potentiates antitumor activity of 5-fluorouracil in a 3D alginate tumor microenvironment of colorectal cancer. BMC Cancer 2015; 15:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shakibaei M, De Souza P. Differentiation of mesenchymal limb bud cells to chondrocytes in alginate beads. Cell Biol Int 1997; 21:75–86 [DOI] [PubMed] [Google Scholar]
- 40.Shakibaei M, Mobasheri A, Lueders C, Busch F, Shayan P, Goel A. Curcumin enhances the effect of chemotherapy against colorectal cancer cells by inhibition of NF-kappaB and Src protein kinase signaling pathways. PLoS One 2013; 8:e57218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shakibaei M, John T, De Souza P, Rahmanzadeh R, Merker HJ. Signal transduction by beta1 integrin receptors in human chondrocytes in vitro: collaboration with the insulin-like growth factor-I receptor. Biochem J 1999; 342:615–23 [PMC free article] [PubMed] [Google Scholar]
- 42.Bharti AC, Aggarwal BB. Nuclear factor-kappa B and cancer: its role in prevention and therapy. Biochem Pharmacol 2002; 64:883–8 [DOI] [PubMed] [Google Scholar]
- 43.Ding GJ, Fischer PA, Boltz RC, Schmidt JA, Colaianne JJ, Gough A, Rubin RA, Miller DK. Characterization and quantitation of NF-kappaB nuclear translocation induced by interleukin-1 and tumor necrosis factor-alpha. Development and use of a high capacity fluorescence cytometric system. J Biol Chem 1998; 273:28897–905 [DOI] [PubMed] [Google Scholar]
- 44.Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell 2004; 6:203–8 [DOI] [PubMed] [Google Scholar]
- 45.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell 2002;April(109 Suppl):S81–96 [DOI] [PubMed]
- 46.Coussens LM, Werb Z. Inflammation and cancer. Nature 2002; 420:860–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 2005; 7:211–7 [DOI] [PubMed] [Google Scholar]
- 48.Busquets S, Ametller E, Fuster G, Olivan M, Raab V, Argiles JM, Lopez-Soriano FJ. Resveratrol, a natural diphenol, reduces metastatic growth in an experimental cancer model. Cancer Lett 2007; 245:144–8 [DOI] [PubMed] [Google Scholar]
- 49.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature 2006; 441:431–6 [DOI] [PubMed] [Google Scholar]
- 50.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol 2005; 5:749–59 [DOI] [PubMed] [Google Scholar]
- 51.Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol 2006; 71:1397–421 [DOI] [PubMed] [Google Scholar]
- 52.Weinstein AM, Storkus WJ. Therapeutic lymphoid organogenesis in the tumor microenvironment. Adv Cancer Res 2015; 128:197–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wolf MJ, Seleznik GM, Zeller N, Heikenwalder M. The unexpected role of lymphotoxin beta receptor signaling in carcinogenesis: from lymphoid tissue formation to liver and prostate cancer development. Oncogene 2010; 29:5006–18 [DOI] [PubMed] [Google Scholar]
- 54.Fernandes MT, Ghezzo MN, Silveira AB, Kalathur RK, Povoa V, Ribeiro AR, Brandalise SR, Dejardin E, Alves NL, Ghysdael J, Barata JT, Yunes JA, dos Santos NR. Lymphotoxin-beta receptor in microenvironmental cells promotes the development of T-cell acute lymphoblastic leukaemia with cortical/mature immunophenotype. Br J Haematol 2015; 171:736–51 [DOI] [PubMed] [Google Scholar]
- 55.Hu X, Zimmerman MA, Bardhan K, Yang D, Waller JL, Liles GB, Lee JR, Pollock R, Lev D, Ware CF, Garber E, Bailly V, Browning JL, Liu K. Lymphotoxin beta receptor mediates caspase-dependent tumor cell apoptosis in vitro and tumor suppression in vivo despite induction of NF-kappaB activation. Carcinogenesis 2013; 34:1105–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ruddle NH. High endothelial venules and lymphatic vessels in tertiary lymphoid organs: characteristics, functions, and regulation. Front Immunol 2016; 7:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aggarwal BB. Tumour necrosis factors receptor associated signalling molecules and their role in activation of apoptosis, JNK and NF-kappaB. Ann Rheum Dis 2000; 59:i6–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hsu H, Shu HB, Pan MG, Goeddel DV. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell 1996; 84:299–308 [DOI] [PubMed] [Google Scholar]
- 59.Hsu H, Huang J, Shu HB, Baichwal V, Goeddel DV. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity 1996; 4:387–96 [DOI] [PubMed] [Google Scholar]
- 60.Ahn KS, Aggarwal BB. Transcription factor NF-kappaB: a sensor for smoke and stress signals. Ann N Y Acad Sci 2005; 1056:218–33 [DOI] [PubMed] [Google Scholar]
- 61.Shishodia S. Aggarwal BB. Nuclear factor-kappaB activation mediates cellular transformation, proliferation, invasion angiogenesis and metastasis of cancer. Cancer Treat Res 2004; 119:139–73 [DOI] [PubMed] [Google Scholar]
- 62.Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol 2003; 3:745–56 [DOI] [PubMed] [Google Scholar]
- 63.Manna SK, Mukhopadhyay A, Aggarwal BB. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidation. J Immunol 2000; 164:6509–19 [DOI] [PubMed] [Google Scholar]
- 64.Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected]. J Biol Chem 1995; 270:24995–5000 [DOI] [PubMed] [Google Scholar]
- 65.Duffey DC, Chen Z, Dong G, Ondrey FG, Wolf JS, Brown K, Siebenlist U, Van Waes C. Expression of a dominant-negative mutant inhibitor-kappaBalpha of nuclear factor-kappaB in human head and neck squamous cell carcinoma inhibits survival, proinflammatory cytokine expression, and tumor growth in vivo. Cancer Res 1999; 59:3468–74 [PubMed] [Google Scholar]
- 66.Buhrmann C, Shayan P, Popper B, Goel A, Shakibaei M. Sirt1 is required for resveratrol-mediated chemopreventive effects in colorectal cancer cells. Nutrients 2016; 8:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Busch F, Mobasheri A, Shayan P, Lueders C, Stahlmann R, Shakibaei M. Resveratrol modulates interleukin-1beta-induced phosphatidylinositol 3-kinase and nuclear factor kappaB signaling pathways in human tenocytes. J Biol Chem 2012; 287:38050–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Busch F, Mobasheri A, Shayan P, Stahlmann R, Shakibaei M. Sirt-1 is required for the inhibition of apoptosis and inflammatory responses in human tenocytes. J Biol Chem 2012; 287:25770–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Montaseri A, Busch F, Mobasheri A, Buhrmann C, Aldinger C, Rad JS, Shakibaei M. IGF-1 and PDGF-bb suppress IL-1beta-induced cartilage degradation through down-regulation of NF-kappaB signaling: involvement of Src/PI-3K/AKT pathway. PLoS One 2011; 6:e28663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shakibaei M, Csaki C, Nebrich S, Mobasheri A. Resveratrol suppresses interleukin-1beta-induced inflammatory signaling and apoptosis in human articular chondrocytes: potential for use as a novel nutraceutical for the treatment of osteoarthritis. Biochem Pharmacol 2008; 76:1426–39 [DOI] [PubMed] [Google Scholar]
- 71.Chaturvedi MM, Sung B, Yadav VR, Kannappan R, Aggarwal BB. NF-kappaB addiction and its role in cancer: 'one size does not fit all'. Oncogene 2011; 30:1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bartkova J, Lukas J, Muller H, Strauss M, Gusterson B, Bartek J. Abnormal patterns of D-type cyclin expression and G1 regulation in human head and neck cancer. Cancer Res 1995; 55:949–56 [PubMed] [Google Scholar]
- 73.Park SB, Park GH, Song HM, Son HJ, Um Y, Kim HS, Jeong JB. Anticancer activity of calyx of Diospyros kaki Thunb. through downregulation of cyclin D1 via inducing proteasomal degradation and transcriptional inhibition in human colorectal cancer cells. BMC Complement Altern Med 2017; 17:445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Song HM, Park GH, Park SB, Kim HS, Son HJ, Um Y, Jeong JB. Vitex rotundifolia fruit suppresses the proliferation of human colorectal cancer cells through down-regulation of cyclin D1 and CDK4 via proteasomal-dependent degradation and transcriptional inhibition. Am J Chin Med 2018; 46:191–207 [DOI] [PubMed] [Google Scholar]
- 75.Dong G, Loukinova E, Chen Z, Gangi L, Chanturita TI, Liu ET, Van Waes C. Molecular profiling of transformed and metastatic murine squamous carcinoma cells by differential display and cDNA microarray reveals altered expression of multiple genes related to growth, apoptosis, angiogenesis, and the NF-kappaB signal pathway. Cancer Res 2001; 61:4797–808 [PubMed] [Google Scholar]
- 76.Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin AS., Jr. NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol 1999; 19:5785–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Joyce D, Bouzahzah B, Fu M, Albanese C, D'Amico M, Steer J, Klein JU, Lee RJ, Segall JE, Westwick JK, Der CJ, Pestell RG. Integration of Rac-dependent regulation of cyclin D1 transcription through a nuclear factor-kappaB-dependent pathway. J Biol Chem 1999; 274:25245–9 [DOI] [PubMed] [Google Scholar]
- 78.Franchi A, Santucci M, Masini E, Sardi I, Paglierani M, Gallo O. Expression of matrix metalloproteinase 1, matrix metalloproteinase 2, and matrix metalloproteinase 9 in carcinoma of the head and neck. Cancer 2002; 95:1902–10 [DOI] [PubMed] [Google Scholar]