Abstract
Background
Radiologic assessment of tumor size is an integral part of the work-up for breast carcinoma. With improved radiologic equipment, surgical decision relies profoundly upon radiologic/clinical stage. We wanted to see the concordance between radiologic and pathologic tumor size to infer how accurate radiologic/clinical staging is.
Materials and methods
The surgical pathology and ultrasonography reports of patients with breast carcinoma were reviewed. Data were collected for 406 cases. Concordance was defined as a size difference within ±2 mm.
Results
The difference between radiologic and pathologic tumor size was within ±2 mm in 40.4% cases. The mean radiologic size was 1.73 ± 1.06 cm. The mean pathologic size was 1.84 ± 1.24 cm. A paired t-test showed a significant mean difference between radiologic and pathologic measurements (0.12 ± 1.03 cm, p = 0.03). Despite the size difference, stage classification was the same in 59.9% of cases. Radiologic size overestimated stage in 14.5% of cases and underestimated stage in 25.6% of cases. The concordance rate was significantly higher for tumors ≤2 cm (pT1) (51.1%) as compared to those greater than 2 cm (≥pT2) (19.7%) (p < 0.0001). Significantly more lumpectomy specimens (47.5%) had concordance when compared to mastectomy specimens (29.8%) (p < 0.0001). Invasive ductal carcinoma had better concordance compared to other tumors (p = 0.02).
Conclusion
Mean pathologic tumor size was significantly different from mean radiologic tumor size. Concordance was in just over 40% of cases and the stage classification was the same in about 60% of cases only. Therefore, surgical decision of lumpectomy versus mastectomy based on radiologic tumor size may not always be accurate.
Keywords: Breast carcinoma, tumor size, pathologic staging, breast ultrasound, concordance
Introduction
The staging of breast carcinoma is mainly dependent on tumor size and lymph node status. Treatment modality, including choice of surgical procedure (lumpectomy versus mastectomy), is affected by tumor size and stage. As per the American Joint Committee on Cancer (AJCC) guidelines, small increments in tumor size upstage the patient.1 Although the final staging is based on pathologic measurement, radiologic measurements and thus clinical staging dictate the surgical options in a significant proportion of cases. National comprehensive cancer network (NCCN) has precise guidelines for management of each stage of breast carcinoma.2 A mismatch between pathologic and radiologic tumor size can lead to potential over or under treatment of the patient. A high concordance between pathologic and radiologic measurements is thus desired as it implies accurate management decision for the patient. We investigated the concordance and correlation between radiologic and pathologic tumor size in invasive breast carcinomas, in order to assess how reliable and accurate radiologic size and thus clinical staging is. Literature review reveals quite a few studies on radiologic-pathologic concordance; however, most of these studies discuss concordance with respect to nature/phenotype of the diagnosis, i.e. benign versus malignant.3–9 Some authors looked into tumor size concordance with respect to different imaging modalities, but many of these studies were limited, either by small sample size; because only a specific type of tumor was considered based on the exclusion criteria; or because only highly specialized techniques such as magnetic resonance imaging (MRI) was used, which is not as widely available or used as ultrasonography (USG) in the assessment of the breast carcinomas.10–21 Moreover, most studies considered a size difference of ±5 mm to be concordant,11,13 which is significant enough to result in a change of stage classification in a considerable number of cases. We compared pathologic tumor size with ultra-sonographic (USG) size. We preferred USG over other imaging modalities because despite the precise nature of CT scan and MRI, USG is still most widely used imaging modality in the assessment of breast carcinomas because of availability and insurance coverage issues. We used ±2 mm size as a cut-off for concordance. Further, we assessed the relationship of some of the factors that can cause discrepancy among these measurements and have not been studied extensively, such as neoadjuvant therapy, tumor location, specimen weight, formalin fixation time and radiologic circumscription and homogeneity of the tumor.
Materials and methods
Following institutional review board (IRB) approval, we conducted a retrospective search of the surgical pathology specimens received in the pathology department of St. John Hospital and Medical Center, in Detroit, MI, over a three-year period from 1 July 2014 to 30 June 2017. We included surgical pathology breast specimens (lumpectomy and mastectomy) for which USG reports within six months prior to the surgery were available. After confirming that the specimens met the inclusion criteria, we reviewed the pathologic and breast USG reports. The most recent USG report was used. The size mentioned in the reports was considered accurate and no repeat measurements were taken.
Data were collected for a total of 406 cases. The data collected included patients’ age and gender, date of USG, radiologic tumor size, tumor location (quadrant of breast), radiographic characteristics of the mass including homogeneity versus heterogeneity, well circumscribed versus ill-defined/speculated and presence and absence of calcifications, date of surgery, surgery type (lumpectomy versus mastectomy), total formalin fixation time, specimen weight, the pathologic tumor size, tumor stage and neoadjuvant therapy.
The mean difference between radiologic and pathologic tumor size, and their concordance and correlation were determined. For statistical analysis, we considered a size difference of ±2 mm concordant, as compared to most other studies, which considered ±5 mm to be concordant. We used a ±2 mm cutoff limit because a size difference of ±5 mm is much more likely to cause a stage migration, while still being considered concordant, as compared to a difference of ±2 mm. A 5 mm size difference being called concordant will definitively give a higher concordance rate but at the expense of much more cases with different stage classification being called concordant. This gives a false impression of lesser proportion of cases being dis-concordant when in reality much higher number of cases are either over or under staged radiologically. Although this problem is not completely eliminated by using a cutoff of 2 mm, yet it is greatly reduced. Using 2 mm cutoff gives a better idea of how accurate radiologic size and thus clinical staging is. A 2 mm cutoff better illustrates the proportion of cases that are not correctly staged radiologically/clinically and thus are prone to overtreatment or undertreatment.
We assessed whether concordance was influenced by consistency (homogeneous versus heterogeneous) of the mass, circumscription of the mass, calcifications, gap between radiologic and pathologic measurement (date of surgery minus date of USG), type of surgery (lumpectomy versus mastectomy), formalin fixation time of the specimen, specimen weight, pathologic tumor (pT) stage, and neoadjuvant therapy.
Data were analyzed using paired samples t-test, chi-squared analysis, Spearman’s rho correlation and Pearson correlation. All data were analyzed using SPSS v. 22.0 and a p-value of 0.05 or less denoted statistical significance.
Results
Patients’ age ranged from 30 to 91 with a mean age of 61.70 ± 11.75 years. Among the 406 specimens, 162 (39.9%) were mastectomy and 244 (60.1%) were lumpectomy. Overall, 42 (10.3%) patients received neoadjuvant therapy, 364 (89.7%) did not. In 251 (62.1%) cases, the tumor was in the upper outer quadrant. The upper inner quadrant was the second most common location with 64 (15.8%) cases. Invasive ductal carcinoma was the most common diagnosis, accounting for 327 (80.5%) cases. Radiologically, the tumor was well circumscribed in 23 (5.7%) cases and ill-defined or speculated in 231 (56.9%) cases. In 152 (37.4%) cases, USG reports did not comment on tumor circumscription. The median specimen weight for mastectomy specimens was 638 g (IQR = 566.3) and the median weight for lumpectomy specimens was 41 g (IQR = 30). Formalin fixation time varied between 9 and 67 hours with a mean of 26.28 ± 6 hours.
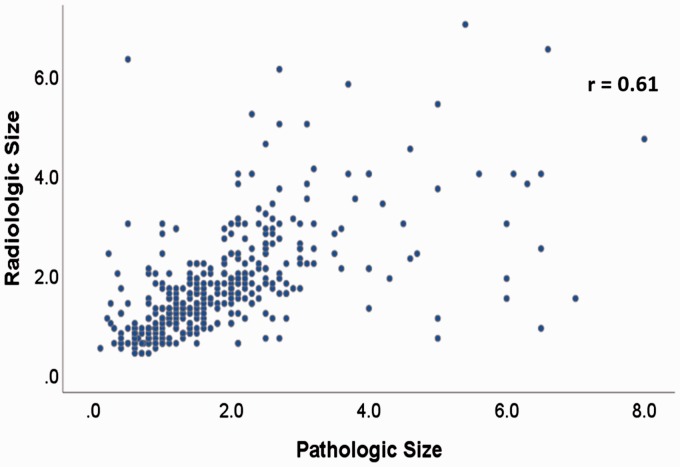
The mean radiologic size was 1.73 ± 1.06 cm and the mean pathologic size was 1.84 ± 1.24 cm. The Pearson’s correlation coefficient (r) for radiologic and pathologic sizes was 0.61, p < 0.0001 (Figure 1). The mean difference between individually paired pathologic and radiologic tumor sizes was 0.12 ± 1.03 cm, which was statistically significant at a p-value of 0.03 (paired samples t-test). Of the total 406 cases, 39 (9.6%) had the same size for both radiologic and pathologic measurements. Radiologic and pathologic size difference was within ±2 mm in 164 (40.4%) of the 406 cases. The difference was within ±5 mm in 270 (66.5%) of the cases.
Figure 1.
Correlation of radiologic and pathologic tumor size.
Despite the size difference, stage classification was the same in 243 (59.9%) cases. Radiologic measurement overestimated stage in 59 (14.5%) cases and underestimated it in 104 (25.6%) cases. Of the total 163 cases in which radiologic measurement-based stage was different, only 25 (15.3%) received neoadjuvant therapy.
The concordance rate was significantly higher for those tumors in which final pathologic tumor size was ≤2 cm (pT1) as compared to those greater than 2 cm (≥pT2) (≤2 cm tumors: 137 (51.1%) vs. > 2 cm tumors: 27 (19.7%), p < 0.0001) (Figure 2).
Figure 2.
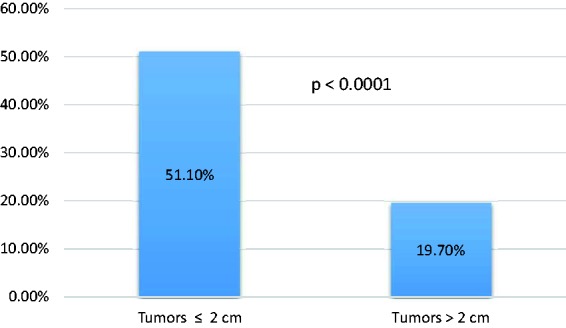
Concordance by tumor size.
A significantly higher proportion of lumpectomy specimens was concordant as compared to mastectomy specimens (lumpectomy: 116 (47.5%) vs. mastectomy: 48 (29.8%), p < 0.0001). Comparing the different tumor types, a significantly higher proportion of invasive ductal carcinoma had radiologic and pathologic size concordance as compared to other tumor types (Table 1).
Table 1.
Concordance among different tumor types
| Tumor type | Invasive ductal carcinoma | Other carcinomasa | p-Value |
|---|---|---|---|
| Concordant cases | 141 (43.3%) | 23 (29.1%) | 0.02 |
Other carcinomas included lobular, mucinous, metaplastic, adenoidcystic and mixed ductal/lobular carcinomas.
With respect to neoadjuvant therapy, 160 (44%) cases without neoadjuvant therapy were concordant as compared to only four (9.5%) cases with neoadjuvant therapy (p < 0.0001). Correlation was also stronger for cases without therapy as compared to those with neoadjuvant therapy (no therapy: r = 0.65, p < 0.0001) vs. neoadjuvant therapy: r = 0.47, p = 0.002) (Figure 3).
Figure 3.
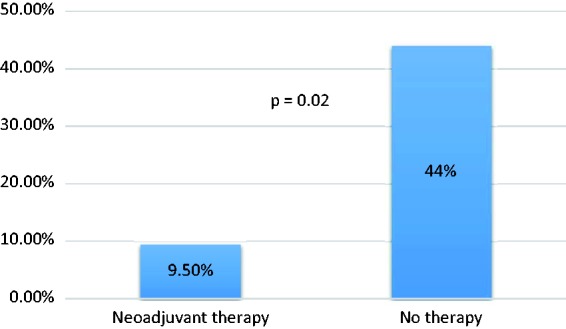
Concordance with and without neoadjuvant therapy.
We also assessed the impact of time between USG and surgery on concordance. Cases with a time gap of no more than two months had better concordance as compared to those where the time gap was more than two months (≤2 months: 138 (43.1%) vs. > 2 months: 25 (29.4%), p = 0.02).
A multivariable analysis showed that tumor size and neoadjuvant therapy are the only independent factors in concordance with radiologic and pathologic tumor size. Tumors ≤ 2 cm were 3.9 times more likely to be concordant as compared to those >2 cm, and cases that did not receive neoadjuvant therapy were eight times more likely to be concordant as compared with those that received therapy. These results are summarized in Table 2.
Table 2.
Results of multivariable analysis
| Factor | Odds ratio | p-Value | 95% CI |
|---|---|---|---|
| Tumor size ≤ 2 cm | 3.9 | <0.0001 | 2.3, 6.5 |
| No neoadjuvant therapy | 8.0 | <0.0001 | 2.5, 25.7 |
Tumor site (quadrant of breast) and radiologic characteristics of the tumor (circumscription, homogeneity versus heterogeneity and presence of calcifications) had no significant effect on concordance (Table 3).
Table 3.
Factors that did not have a significant effect on concordance
| Factor | p-Value |
|---|---|
| Tumor site | 0.65 |
| Radiologic circumscription | 0.31 |
| Radiologic homogeneity/heterogeneity | 0.80 |
| Calcifications | 0.38 |
Specimen weight had no significant correlation with concordance for lumpectomy (p = 0.98) or mastectomy specimens (p = 0.72). Formalin fixation time also had no significant correlation with concordance for lumpectomy (p = 0.62) as well as mastectomy specimens (p = 0.28).
Discussion
As with other malignancies, tumor size is an important staging element and prognostic factor in breast carcinomas. According to the American Joint Committee on Cancer (AJCC) guidelines, small increments in tumor size upstage the tumor.1 Pathologic tumor size is the gold standard; however, clinical decision usually relies on radiologic size. Radiologic tumor size is one of the factors that determine the clinical decision of lumpectomy versus mastectomy. Other factors that contribute include the tumor location, the ability to achieve margin control, the ease to deliver radiotherapy, anticipated cosmetic result and patient choice.
Multiple studies have correlated imaging and histopathology in breast lesions.3–9 Few studies compared pathologic tumor size to radiologic size using different radiologic modalities.11–18
In a recent study, Yoo et al.11 studied the correlation of MRI and pathologic size in breast carcinomas. However, not every breast carcinoma patient gets an MRI. Moreover, MRI is not widely available and not covered by all insurances.
Lai et al.14 interestingly found that USG had better concordance as compared to MRI (54.3% vs. 44.1%). They concluded that MRI frequently overestimates the tumor size, while USG tends to underestimate it, and combined USG and MRI increase the accuracy of tumor size prediction.
Ramirez et al.17 compared mammography (MG), USG, and MRI in assessment of breast carcinoma size and concluded that MG measurements most closely correlate with pathologic measurements. Their correlation coefficients for MG, USG, and MRI were 0.76, 0.67, and 0.75, respectively. The correlation coefficient for USG in different studies ranged from 0.45 to 0.92.19–41 In our study, the correlation coefficient for radiologic size and pathologic size was 0.61, p < 0.0001.
Overall concordance in our study was 40.4%. This appears low when compared to many other studies, but the main reason for this discrepancy is that we used a cutoff ±2 mm for concordance as compared to other studies that used a ±5 mm cutoff. As mentioned before, we used ±2 mm as the cutoff limit because a size difference of ±5 mm is much more likely to cause a change in stage classification, while still being considered concordant, as compared to a difference of ±2 mm. The 2 mm size cutoff therefore gives better estimate of potential patient miss-management caused by dis-concordance between radiologic/clinical stage and pathologic stage. Despite the size difference, however, we found that stage classification ended up being the same in 59.9% cases. Radiologic measurement overestimated stage in 59 (14.5%) cases and underestimated it in 104 (25.6%) cases. Establishing the management on radiologic tumor size and thus clinical stage in these cases can result either in a procedure that would otherwise not be necessary or on the other hand conservative and thus inadequate treatment.
A recent study by Tseng et al.10 assessed the accuracy of MRI alone versus a combination of USG plus MG in accurate estimation of tumor size. Interestingly, instead of using an absolute value of 2 mm or 5 mm, they used a size difference of less than 33% as concordant. This is very logical and thoughtful but in our view 33% is a very high cutoff, as using this cutoff implies that for a 2 cm tumor, a size difference of about 7 mm is still considered concordant.
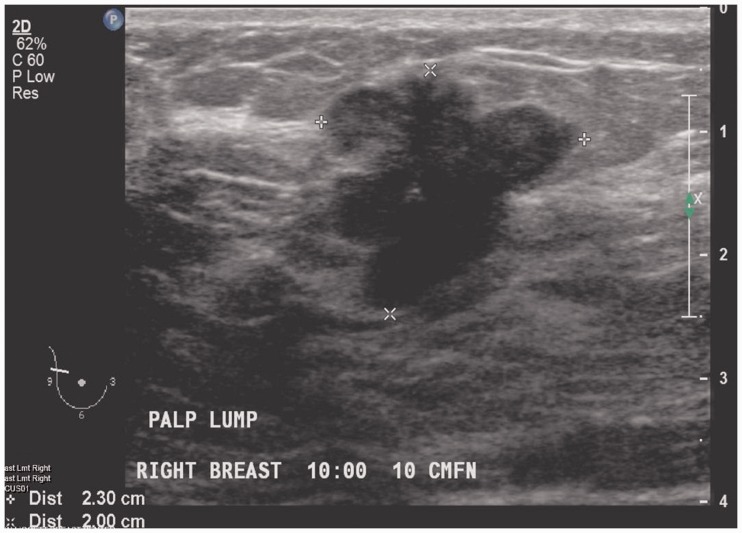
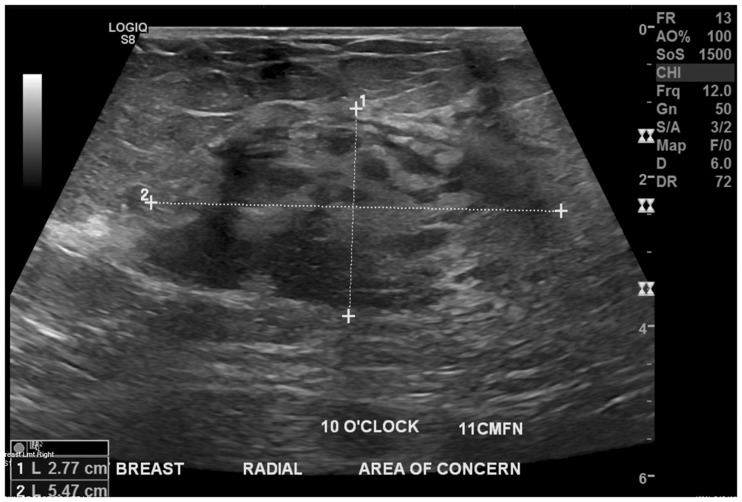
Like Ahn et al.,13 we found that smaller tumors (≤2 cm/pT1) had a higher concordance rate as compared to larger tumors (>2 cm/≥ pT2). Our correlation coefficient was also higher for smaller tumors as compared to larger ones. A possible explanation is that smaller tumors tend to be better circumscribed and can be measured more accurately in all three dimensions as compared to larger tumors, as illustrated in Figures 4 and 5. Other reasons why sizing can be difficult in larger lesions include shadowing on USG and posterior location. From a pathologic stand point however, a higher concordance in larger tumors is desired because it is indeed the larger tumors where it is most needed by the pathologists. Although final staging is based on microscopic tumor size, pathologists rely on gross measurement as well as radiologic measurement of tumor size in quite a few cases, especially the ones where tumors are large and accurate size cannot be confirmed microscopically.42–44 Additional tissue sections may be required is these cases and radiologic tumor size becomes critical.45
Figure 4.
Ultra-sonography demonstrating a lobulated, well-defined 2.3 cm mass that can be accurately measured in all three dimensions.
Figure 5.
Ultra-sonography demonstrating an ill-defined, 5.5 cm mass with vague margins precluding an accurate measurement.
Ahn et al.13 assessed the value of chest CT in determination of breast carcinoma size and found that mean tumor size determined by chest CT and pathology were not significantly different (p = 0.059). Yoo et al.11 came to a similar conclusion (p = 0.199) with respect to MRI. Our results, however, were different. Using paired samples t-test, we found that the mean difference between individually paired pathologic and radiologic sizes was significant (p = 0.03).
Many studies22–24,26,27,29,31,32,36,46 compared MRI with other imaging modalities and physical examination in assessing residual tumor size after neoadjuvant therapy. These studies concluded that MRI was far superior in determining the extent of residual disease with a correlation coefficient ranging from 0.64 to 0.93. The correlation coefficient for USG in these studies ranged from 0.48 to 0.70. A recent study47 compared Contrast-Enhanced Spectral Mammography (CESM) to MRI in the assessment of tumor size following neoadjuvant therapy and found that CESM and MRI had comparable sensitivity, specificity PPV, and NPV. Cavallo et al.48 found that unenhanced MRI achieves similar results to contrast enhanced MRI for the assessment of residual tumor. We did not assess the reliability of USG in determining treatment response, instead we analyzed if neoadjuvant therapy is a significant factor affecting radiologic-pathologic concordance of tumor size. Our data showed that a significantly less proportion of cases with neoadjuvant therapy had size concordance as compared to those without any therapy (no therapy: 160 (44%) versus neoadjuvant therapy 4 (9.5%), p < 0.0001). The correlation coefficients for cases with and without neoadjuvant therapy were 0.47 (p = 0.002) and 0.65 (p < 0.0001), respectively. Fibrosis and background enhancement post therapy precludes to accurate assessment of tumor size on radiologic imaging leading to disconcordance with pathologic size of residual tumor.49
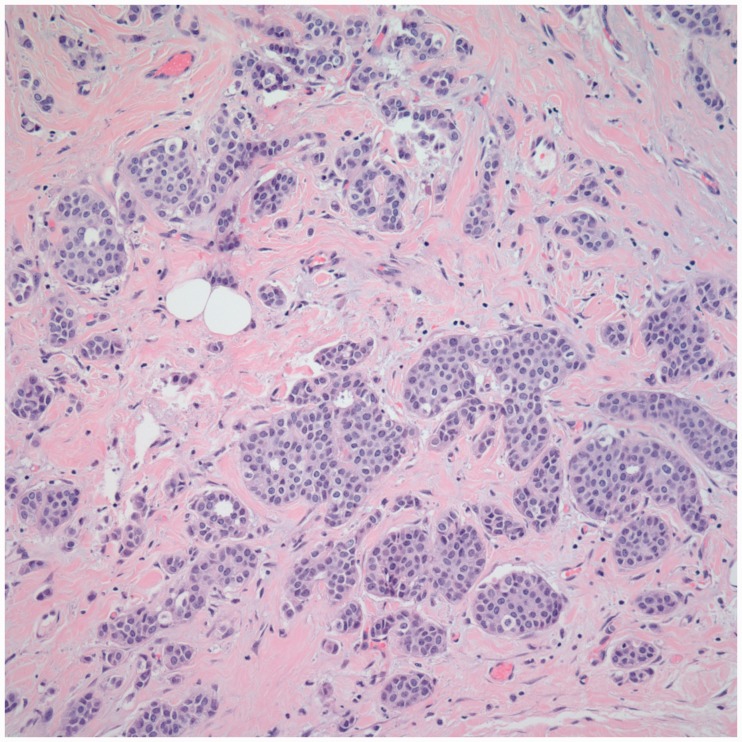
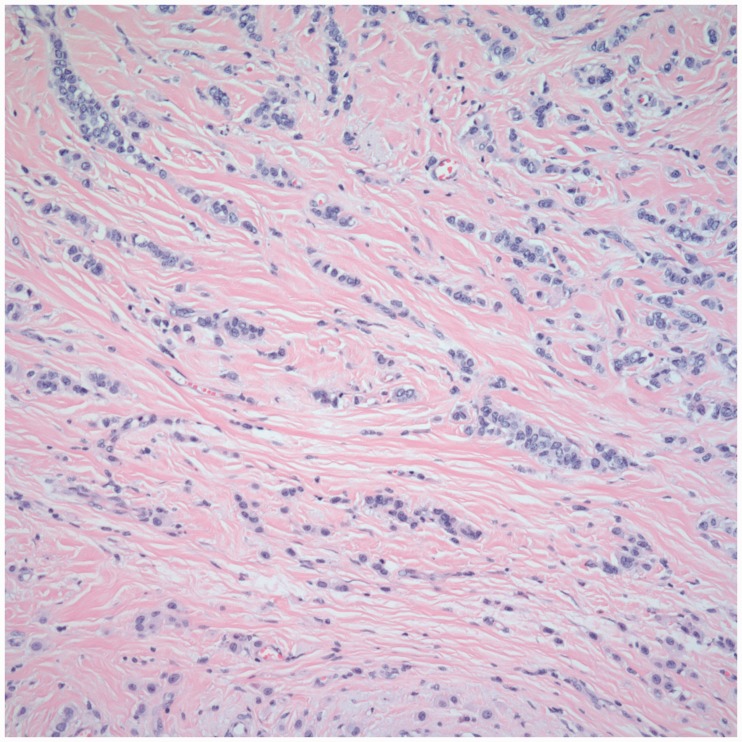
Not many studies have compared the concordance rate with respect to tumor site and histologic type. In our study, we found no significant effect of tumor site on the concordance (p = 0.65); however, with respect to histologic type, we found a greater concordance rate for invasive ductal carcinoma (IDC) as compared to other tumors (p = 0.02) (Table 1). A possible explanation for this difference is the morphologic differences in the tumors. Invasive lobular carcinomas tend to have more single filing and infiltrative morphology, leading to poor concordance compared to IDC as demonstrated in Figures 6 and 7. Ahn et al.13 also found increased concordance for IDC. In our study, correlation was also stronger for IDC as compared to all other tumors (IDC: r = 0.61, p < 0.0001 vs. all other tumor types: r = 0.56, p < 0.0001).
Figure 6.
Relatively circumscribed morphologic appearance of IDC (H&E, 400×).
Figure 7.
Infiltrative morphology of ILC leading to disconcordance between radiologic and pathologic tumor size (H&E, 400×).
To the best of our knowledge, no one has looked into radiology – pathology size correlation with respect to surgery type (i.e. lumpectomy versus mastectomy). We found that a significantly higher proportion of lumpectomy specimens had concordance in size as compared to mastectomy specimens (p < 0.0001). A possible explanation for this is that lumpectomy is usually done on smaller tumors and concordance rate for smaller tumors is usually high as depicted in our study and supported by Ahn et al.13
Yoo et al.11 investigated the effect of multiple factors, including lymphovascular invasion, immunohistochemical profile and molecular subtype of breast carcinoma on concordance. Their results showed that MRI pathology discordance was associated with larger tumor size (p < 0.001), estrogen receptor (ER) negativity (p = 0.006), and lymphovascular invasion (p = 0.003). They also concluded that the human epidermal growth factor receptor 2 (HER2) positive molecular subtype showed worse a correlation between the tumor size measured by MRI and pathology compared with luminal A and luminal B subtypes (p = 0.008 and 0.007, respectively). The data on these factors are, however, limited and needs to be supplemented by additional studies.
The interval between radiologic assessment and surgical intervention can also affect tumor size concordance. A larger time gap is likely to result in discordance because of two reasons. Tumors tend to increase in size with time. Conversely, there can be a decrease in size if a patient undergoes neoadjuvant therapy; however, in these cases, the patient usually has follow-up USG. We used the most recent USG reports that mentioned tumor size in our study and found a better concordance for a time gap of less than or equal to two months (p = 0.02).
Other factors that can affect the concordance rate include radiologic characteristics of the tumor (circumscription, homogeneity versus heterogeneity and presence of calcifications), specimen weight and formalin fixation time. Poorly circumscribed, heterogeneous tumors with calcifications should have worse concordance as these factors make accurate determination of radiologic size difficult. Bulkier specimen and longer formalin fixation time might result in discordance in size, though in our study, we did not find any impact of these factors on concordance rate.
Conclusion
We found a significant mean difference between radiology and pathology measurements. Radiology and pathology tumor measurements were concordant in only 40.4% of cases. Although the size difference stage classification was same in 59.9% cases, however, remaining 40.1% cases were at risk of undertreatment or overtreatment. Although different imaging modalities have gained attention in accurate determination of tumor size in past few years, USG is still the most widely available and routinely used in work-up of breast carcinoma patients. The large variability in the correlation coefficient between USG size and pathologic size in different studies means that USG measurements are highly dependent on the expertise of the person performing USG.50–54 Because radiologic size helps tailor surgery in some cases, a better correlation would mean accurate decision regarding surgical procedure for the patient. Also, knowledge of factors influencing this concordance can be useful in regards to surgical planning.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval
St. John Hospital and Medical Center Institutional Review Board. Study ID 1106801-2.
Guarantor
AH.
Contributors
AH wrote the manuscript after gathering all the data; AH, RS, AA, WI, JE, and SS collected data; SM ran statistical analysis; SK did literature review and wrote parts of discussion; DO was the mentor author; SM, SK and DO proof read and improved the language of the manuscript. All authors reviewed and approved the final version of the manuscript.
References
- 1.Amin MB, Edge S, Greene F, et al. (eds) AJCC Cancer Staging Manual. 8th ed. Springer International Publishing: American Joint Commission on Cancer, 2017. Available from: http://www.springer.com/us/book/9783319406176#aboutBook (accessed 11 February 2018).
- 2.https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed 11 February 2018).
- 3.Jahan AB, Ahmed MU, Begum M, et al. Ultrasonographic evaluation of palpable breast mass and correlation with histopathology. Mymensingh Med J 2017; 26: 223–229. [PubMed] [Google Scholar]
- 4.Catalano OA, Horn GL, Signore A, et al. PET/MR in invasive ductal breast cancer: correlation between imaging markers and histological phenotype. Br J Cancer 2017; 116: 893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gallagher R, Schafer G, Redick M, et al. Microcalcifications of the breast: a mammographic-histologic correlation study using a newly designed Path/Rad Tissue Tray. Ann Diagn Pathol 2012; 16: 196–201. [DOI] [PubMed] [Google Scholar]
- 6.Tot T, Tabár L. The role of radiological-pathological correlation in diagnosing early breast cancer: the pathologist's perspective. Virchows Arch 2011; 458: 125–131. [DOI] [PubMed] [Google Scholar]
- 7.Uematsu T, Kasami M, Yuen S. Triple-negative breast cancer: correlation between MRimaging and pathologic findings. Radiology 2009; 250: 638–647. [DOI] [PubMed] [Google Scholar]
- 8.Barreau B, de Mascarel I, Feuga C, et al. Mammography of ductal carcinoma in situ of the breast: review of 909 cases with radiographic-pathologic correlations. Eur J Radiol 2005; 54: 55–61. [DOI] [PubMed] [Google Scholar]
- 9.Murphy TJ, Conant EF, Hanau CA, et al. Bilateral breast carcinoma: mammographic and histologic correlation. Radiology 1995; 195: 617–621. [DOI] [PubMed] [Google Scholar]
- 10.Tseng J, Kyrillos A, Liederbach E, et al. Clinical accuracy of preoperative breast MRI for breast cancer. J Surg Oncol 2017; 115: 924–931. [DOI] [PubMed] [Google Scholar]
- 11.Yoo EY, Nam SY, Choi HY, et al. Agreement between MRI and pathologic analyses for determination of tumor size and correlation with immunohistochemical factors of invasive breast carcinoma. Acta Radiol 2018; 59: 50–57. [DOI] [PubMed] [Google Scholar]
- 12.Berger N, Schwizer SD, Varga Z, et al. Assessment of the extent of microcalcifications to predict the size of a ductal carcinoma in situ: comparison between tomosynthesis and conventional mammography. Clin Imag 2016; 40: 1269–1273. [DOI] [PubMed] [Google Scholar]
- 13.Ahn SJ, Kim YS, Kim EY, et al. The value of chest CT for prediction of breast tumor size: comparison with pathology measurement. World J Surg Oncol 2013; 11: 130–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai HW, Chen DR, Wu YC, et al. Comparison of the diagnostic accuracy of magnetic resonance imaging with sonography in the prediction of breast cancer tumor size: a concordance analysis with histopathologically determined tumor size. Ann Surg Oncol 2015; 22: 3816–3823. [DOI] [PubMed] [Google Scholar]
- 15.Weatherall PT, Evans GF, Metzger GJ, et al. MRI vs. histologic measurement of breast cancer following chemotherapy: comparison with X-ray mammography and palpation. J Magn Reson Imag 2001; 13: 868–875. [DOI] [PubMed] [Google Scholar]
- 16.Carlson KL, Helvie MA, Roubidoux MA, et al. Relationship between mammographic screening intervals and size and histology of ductal carcinoma in situ. AJR Am J Roentgenol 1999; 172: 313–317. [DOI] [PubMed] [Google Scholar]
- 17.Ramirez SI, Scholle M, Buckmaster J, et al. Breast cancer tumor size assessment with mammography, ultrasonography, and magnetic resonance imaging at a community based multidisciplinary breast center. Am Surg 2012; 78: 440–446. [PubMed] [Google Scholar]
- 18.Mainiero MB, Philpotts LE, Lee CH, et al. Stereotaxic core needle biopsy of breast microcalcifications: correlation of target accuracy and diagnosis with lesion size. Radiology 1996; 198: 665–669. [DOI] [PubMed] [Google Scholar]
- 19.Fornage BD, Toubas O, Morel M. Clinical, mammographic, and sonographic determination of preoperative breast cancer size. Cancer 1987; 60: 765–771. [DOI] [PubMed] [Google Scholar]
- 20.Yang WT, Lam WW, Cheung H, et al. Sonographic, magnetic resonance imaging, and mammographic assessments of preoperative size of breast cancer. J Ultrasound Med 1997; 16: 791–797. [DOI] [PubMed] [Google Scholar]
- 21.Madjar H. Preoperative staging of breast cancer by palpation, mammography and high resolution ultrasound. Ultrasound Obstet Gynecol 1993; 3: 185–190. [DOI] [PubMed] [Google Scholar]
- 22.Kim HJ, Im YH, Han BK, et al. Accuracy of MRI for estimating residual tumor size after neo-adjuvant chemotherapy in locally advanced breast cancer: relation to response patterns on MRI. Acta Oncol 2007; 46: 996–1003. [DOI] [PubMed] [Google Scholar]
- 23.Partridge SC, Gibbs JE, Lu Y, et al. Accuracy of MR imaging for revealing residual breast cancer in patients who have undergone neoadjuvant chemotherapy. AJR Am J Roentgenol 2002; 179: 1193–1199. [DOI] [PubMed] [Google Scholar]
- 24.Bhattacharyya M, Ryan D, Carpenter R, et al. Using MRI to plan breast-conserving surgery following neo-adjuvant chemotherapy for early breast cancer. Br J Cancer 2008; 98: 289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hieken TJ, Harrison J, Herreros J, et al. Correlating sonography, mammography, and pathology in the assessment of breast cancer size. Am J Surg 2001; 182: 351–354. [DOI] [PubMed] [Google Scholar]
- 26.Segara D, Krop IE, Garber JE, et al. Does MRI predict pathologic tumor response in women with breast cancer undergoing preoperative chemotherapy? J Surg Oncol 2007; 96: 474–480. [DOI] [PubMed] [Google Scholar]
- 27.Akazawa K, Tamaki Y, Taguchi T, et al. Preoperative evaluation of residual tumor extent by three-dimensional magnetic resonance imaging in breast cancer patients treated with neoadjuvant chemotherapy. Breast J 2006; 12: 130–137. [DOI] [PubMed] [Google Scholar]
- 28.Blair S, McElroy M, Middleton MS, et al. The efficacy of breast MRI in predicting breast conservation therapy. J Surg Oncol 2006; 94: 220–225. [DOI] [PubMed] [Google Scholar]
- 29.Montemurro F, Martincich L, De Rosa G, et al. Dynamic contrast-enhanced MRI and sonography in patients receiving primary chemotherapy for breast cancer. Eur Radiol 2005; 15: 1224–1233. [DOI] [PubMed] [Google Scholar]
- 30.Bodini M, Berruti A, Bottini A, et al. Magnetic resonance imaging in comparison to clinical palpation in assessing the response of breast cancer to epirubicin primary chemotherapy. Breast Cancer Res Treat 2004; 85: 211–218. [DOI] [PubMed] [Google Scholar]
- 31.Londero V, Bazzocchi M, Del Frate C, et al. Locally advanced breast cancer: comparison of mammography, sonography and MR imaging in evaluation of residual disease in women receiving neoadjuvant chemotherapy. Eur Radiol 2004; 14: 1371–1379. [DOI] [PubMed] [Google Scholar]
- 32.Rosen EL, Blackwell KL, Baker JA, et al. Accuracy of MRI in the detection of residual breast cancer after neoadjuvant chemotherapy. AJR Am J Roentgenol 2003; 181: 1275–1282. [DOI] [PubMed] [Google Scholar]
- 33.Bosch AM, Kessels AG, Beets GL, et al. Preoperative estimation of the pathological breast tumor size by physical examination, mammography and ultrasound: a prospective study of 105 invasive tumors. Eur J Radiol 2003; 48: 285–292. [DOI] [PubMed] [Google Scholar]
- 34.Kuroki Y, Nawano S, Hasebe T, et al. Efficacy of MR mammography (MRM) in providing preoperative locoregional information on breast cancer: correlation between MRM and histological findings. Magn Reson Med Sci 2002; 1: 73–80. [DOI] [PubMed] [Google Scholar]
- 35.Esserman L, Kaplan E, Partridge S, et al. MRI phenotype is associated with the response to doxorubicin and cyclophosphamide neoadjuvant chemotherapy in stage III breast cancer. Ann Surg Oncol 2001; 8: 549–559. [DOI] [PubMed] [Google Scholar]
- 36.Weatherall PT, Evans GF, Metzger GJ, et al. MRI vs. histologic measurement of breast cancer following chemotherapy: comparison with X-ray mammography and palpation. J Magn Reson Imag 2001; 13: 868–875. [DOI] [PubMed] [Google Scholar]
- 37.Amano G, Ohuchi N, Ishibashi T, et al. Correlation of three dimensional magnetic resonance imaging with precise histopathological map concerning carcinoma extension in the breast. Breast Cancer Res Treat 2000; 60: 43–55. [DOI] [PubMed] [Google Scholar]
- 38.Davis PL, Staiger MJ, Harris KB, et al. Breast cancer measurements with magnetic resonance imaging, ultrsonography, and mammography. Breast Cancer Res Treat 1996; 37: 1–9. [DOI] [PubMed] [Google Scholar]
- 39.Wasif N, Garreau J, Terando A, et al. MRI versus ultrasonography and mammography for preoperative assessment of breast cancer. Am Surg 2009; 75: 970–975. [PubMed] [Google Scholar]
- 40.Vinnicombe SJ, MacVicar AD, Guy RL, et al. Primary breast cancer: mammographic changes after neoadjuvant chemotherapy, with pathologic correlation. Radiology 1996; 198: 333–340. [DOI] [PubMed] [Google Scholar]
- 41.Cheung YC, Wan YL, Lo YF, et al. Preoperative magnetic resonance imaging evaluation for breast cancers after sonographically guided core-needle biopsy: a comparison study. Ann Surg Oncol 2004; 11: 756–761. [DOI] [PubMed] [Google Scholar]
- 42.Varma S, Ozerdem U, Hoda SA. Complexities and challenges in the pathologic assessment of size (T) of invasive breast carcinoma. Adv Anat Pathol 2014; 21: 420–432. [DOI] [PubMed] [Google Scholar]
- 43.Hamza A, Sakhi R, Alrajjal A, et al. Tumor size in breast carcinoma: gross measurement is important!. Int J Surg Pathol 2018; 26: 494–499. [DOI] [PubMed] [Google Scholar]
- 44.Shin SJ, Osborne MP, Moore A, et al. Determination of size in invasive breast carcinoma: pathologic considerations and clinical implications. Am J Clin Pathol 2000; 113: S19–S29. [DOI] [PubMed] [Google Scholar]
- 45.Hamza A, Alrajjal A, Edens J, et al. Utility of additional tissue sections in surgical pathology. Int J Surg Pathol 2018; 26: 392–401. [DOI] [PubMed] [Google Scholar]
- 46.Scheel JR, Kim E, Partridge SC, et al. MRI, clinical examination, and mammography for preoperative assessment of residual disease and pathologic complete response after neoadjuvant chemotherapy for breast cancer: ACRIN 6657 Trial. Am J Roentgenol 2018; 210: 1376–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patel BK, Hilal T, Covington M, et al. Contrast-enhanced spectral mammography is comparable to MRI in the assessment of residual breast cancer following neoadjuvant systemic therapy. Ann Surg Oncol 2018; 25: 1350–1356. [DOI] [PubMed] [Google Scholar]
- 48.Cavallo Marincola B, Telesca M, Zaccagna F, et al. Can unenhanced MRI of the breast replace contrast-enhanced MRI in assessing response to neoadjuvant chemotherapy? Acta Radiol 2018. Jan: 284185118773512. doi: 10.1177/0284185118773512. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 49.Kim SY, Cho N, Shin SU, et al. Contrast-enhanced MRI after neoadjuvant chemotherapy of breast cancer: lesion-to-background parenchymal signal enhancement ratio for discriminating pathological complete response from minimal residual tumour. Eur Radiol 2018; 28: 2986–2995. [DOI] [PubMed] [Google Scholar]
- 50.Rella R, Belli P, Giuliani M, et al. Automated breast ultrasonography (ABUS) in the screening and diagnostic setting: indications and practical use. Acad Radiol 2018; 18: 100–104. [DOI] [PubMed] [Google Scholar]
- 51.Stasi G, Ruoti EM. A critical evaluation in the delivery of the ultrasound practice: the point of view of the radiologist. Italian J Med 2015; 9: 5–10. [Google Scholar]
- 52.Skaane P, Olsen JB, Sager EM, et al. Variability in the interpretation of ultrasonography in patients with palpable noncalcified breast tumors. Acta Radiol 1999; 40: 169–175. [DOI] [PubMed] [Google Scholar]
- 53.Lee YJ, Choi SY, Kim KS, et al. Variability in observer performance between faculty members and residents using breast imaging reporting and data system (BI-RADS)-ultrasound. Iranian J Radiol 2016; 13: e28281–e28281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hashimoto BE, Morgan GN, Kramer DJ, et al. Systematic approach to difficult problems in breast sonography. Ultrasound Quarter 2008; 24: 31–38. [DOI] [PubMed] [Google Scholar]