Abstract
Study Design:
Broad narrative review.
Objectives:
To review and summarize the current literature on the outcomes, techniques, and indications of lumbar interbody fusion in degenerative spondylolisthesis.
Methods:
A thorough review of peer-reviewed literature was performed on the outcomes, techniques, and indications of lumbar interbody fusions in degenerative spondylolisthesis.
Results:
A number of studies have found similar results between interbody fusions and posterolateral fusion in the setting of degenerative spondylolisthesis. There is some evidence that suggests that interbody fusion may be a useful adjunct in the setting of unstable degenerative spondylolisthesis. The number of options for interbody fusions has quickly expanded. Initially, interbody fusions were accomplished via an anterior approach. Posterior and transforaminal interbody fusions are 2 options that accomplish an interbody fusion without the morbidity of an anterior approach. Over the past decade, minimally invasive options including extreme lateral, oblique, and minimally invasive transforaminal interbody fusions have gained popularity.
Conclusions:
Lumbar interbody fusion can be a useful tool in the setting of unstable degenerative spondylolisthesis. A number of technique options, both open and minimally invasive, are available to accomplish an interbody fusion. The literature to this date does not support a clear benefit of one technique over others in the setting of degenerative spondylolisthesis.
Keywords: lumbar interbody fusions, degenerative spondylolisthesis, review
Introduction
Lumbar degenerative spondylolisthesis (DS) was first described in the German literature in the early to mid-20th century by Junghanns.1 Newman described the present-day concept of DS in 1955.2 DS with spinal stenosis is one of the most thoroughly studied topics in spine literature to date. The surgical treatment for this condition has been rapidly evolving as the body of literature has grown.
Initially, decompression without fusion was the surgical option of choice. This changed when a randomized controlled trial by Herkowitz and Kurz in 1991 provided strong evidence for the addition of a fusion to the decompression.3 Patients in this trial underwent a noninstrumented intertransverse process fusion with iliac crest bone graft. A follow-up study evaluating instrumented versus noninstrumented fusion showed that instrumented fusion leads to an 82% fusion rate compared to 45% in the noninstrumented group, with no difference in clinical outcomes.4
Kornblum et al in a study with 7-year follow-up demonstrated that pseudarthrosis was associated with lower pain and functional outcomes.5 This was a key study in establishing the importance of attaining a solid fusion in patients with DS.
The benefits of augmenting a fusion construct with anterior column instrumentation were initially demonstrated in isthmic spondylolisthesis. Suk et al showed that interbody fusions were associated with higher fusion rates, better correction of deformity, better maintenance of correction, and improved clinical outcomes compared with posterolateral fusion alone.6 The literature is mixed on whether the addition of an interbody fusion in the setting of DS has clinical benefit.
Gottschalk et al demonstrated no clinical benefit, and an increase in cost, with the addition of an interbody fusion to posterolateral instrumentation compared with posterolateral fusion alone.7 However, there may be a benefit in the setting of unstable DS. Ha et al investigated the benefit of interbody fusion in stable and unstable DS, with stability defined as degree of slip <4 mm and slip angle <10°. The addition of an interbody fusion had no effect on clinical outcomes in the stable group. In the unstable group, addition of an interbody fusion was associated with improvements in function and greater pain relief.8
The literature on lumbar interbody fusions in the setting of DS is widely varied. An additional layer of complexity is that there are a number of techniques described that achieve an interbody fusion in the lumbar spine. The aim of this article is to provide a review of lumbar interbody fusions including the techniques and outcomes of anterior, posterior, transforaminal, and interbody fusions as well minimally invasive techniques including far lateral and oblique interbody fusions.
Techniques
Anterior Lumbar Interbody Fusion (ALIF)
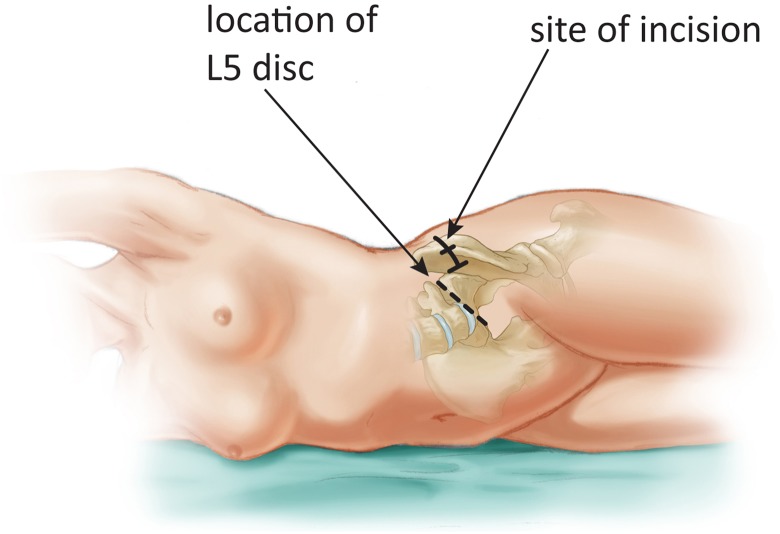
The patient is positioned supine, and an inflatable bag is placed underneath the lumbar spine in order to exaggerate the lumbar lordosis and open the anterior disc space. A number of skin incisions have been described for the anterior approach to the lumbar spine; these include the transverse, midline, and paramedian incisions. Transverse incisions have the advantage of better cosmesis, while the paramedian incisions require a smaller skin flap and create less dead-space. Transverse incisions are more appropriate to visualize the L4-5 and L5-S1 levels, while a paramedian incision may be useful for the higher lumbar levels. For the L4-5 level, the incision is placed just below the umbilicus, and for L5-S1 the incision is approximately two thirds the distance from the umbilicus to the pubic symphysis. The incisions are adjusted based on the angle of the target disc space in order to ensure that instruments can be inserted parallel to the disc space.
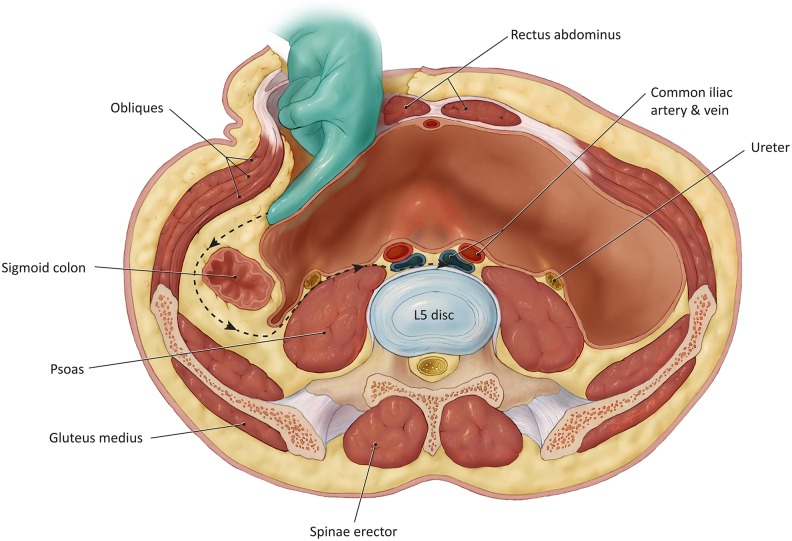
The transverse incision is used more commonly for cases of DS since the condition typically affects the L4-5 level. A transverse skin incision to the left of midline avoids the more prominent right iliac vein that can hinder the deeper dissection. A skin incision is carried out from midline to the lateral border of the rectus abdominus. Dissection is carried down to the anterior rectus (Figure 1). Subcutaneous flaps are raised exposing the rectus sheath from 1 cm to the right of midline medially to the external oblique aponeurosis laterally. The anterior rectus sheath is then incised and dissected away from the rectus muscle belly. The dissection is carried 4 to 6 cm superiorly and 4 to 6 cm inferiorly. The muscle is then dissected off of its sheath circumferentially to allow for easy mobilization; caution must be used on the posterior aspect of the rectus muscle where the inferior epigastric vessels reside.
Figure 1.
Dissection of the anterior approach to the lumbar spine.
With the rectus muscle mobilized medially, the posterior rectus sheath is then incised carefully to reveal the peritoneum. The rectus sheath is then lifted anteriorly, and blunt dissection is used to develop a plane between the peritoneum and the internal oblique and transversus abdominis muscles and fascia. The peritoneum is quite thin, and small perforations may be encountered and must be repaired with absorbable suture. The blunt dissection is carried posteriorly and then medially around the anterior aspect of the psoas muscle. The left ureter and genitofemoral nerve are identified as the peritoneum is lifted away from the psoas.
At this point the intervertebral disc, vertebral body, and iliac artery can be palpated medially. Blunt dissection is used to develop the retroperitoneal space and visualize the left iliac artery and vein. Self-retaining retractors, such as the Balfour or the Omni, are useful to both complete the exposure and maintain visualization. Iliolumbar veins tether the common iliac vein laterally and must be ligated in order for the common iliac vein to be retracted medially. The L5 nerve often runs in close proximity to the iliolumbar vein and must be identified. Once the iliolumbar veins are ligated, the iliac artery and vein are mobilized, and any segmental vessels overlying the anterior vertebral body are ligated.
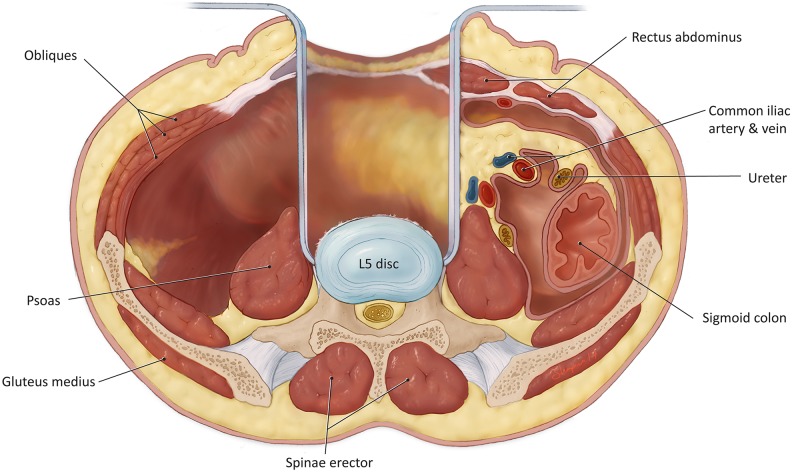
A variation of this approach has been described by Brau et al, which involves a similar initial exposure with dissection lateral to the rectus, followed by placement of self-retaining retractors medial to the rectus (as opposed to keeping the self-retaining retractors lateral).9 Brau et al advocate the use of reverse lip retractors that attached to the table-held system (Figure 2); the reverse lip is placed around the lateral and medial lumbar sides of the vertebral body and provides improved exposure.
Figure 2.
Anterior lumbar interbody fusion (ALIF) retractor positioning.
Once adequate exposure is obtained, an annulotomy is made in the intervertebral disc. An AP fluoroscopy image can be used to ensure the annulotomy is midline. Enough disc and anterior longitudinal ligament are removed to fit the ALIF spacer, and incomplete discectomy can result in retropulsion of disc fragments into the canal. The disc space is distracted, and a trial spacer is placed with the goal of a tight fit at desired distraction. A lateral fluoroscopy image is used to confirm the trial placement, with the goal of being just posterior to the anterior vertebral body margin. Once the size of the implant is established the final implant is opened, packed with bone graft, and inserted.
Posterior Lumbar Interbody Fusion (PLIF)
The patient is positioned prone on a Jackson table to decrease intraabdominal pressure. Upper extremities are well padded and placed on arm-boards in a “90-90” position. A metal stylus is used to confirm the operative level using a lateral image. The iliac crests can be used as a reference point marking the L4-5 disc space. A midline longitudinal incision is carried down to the spinous processes.
Paraspinous muscles are elevated off of the spinous processes and lamina in the subperiosteal plane. Dissection is carried out laterally to the facet joints at the operative level, and care must be taken not to violate the facet joints above or below the operative level. At this point laminotomies and partial bilateral facetectomy are performed. Curettes are used to detach the ligamentum flavum from each of the adjacent lamina and the superior articular process of the lamina below. Next, the medial half of the superior and inferior articular processes is removed bilaterally using Kerrison rongeurs. The original technique described by Cloward advocated for spinous process removal and complete facetectomies; however, this has been modified in favor of preserving the spinous process and interspinous ligaments in order to provide additional stability whenever possible.10 Next, the operative level is distracted using pedicle screws, the lamina, or the spinous processes.
The posterolateral aspect of the annulus fibrosus can now be visualized by gentle medial retraction of the traversing nerve root as well as the dural sac. A more extensive facetectomy will allow for less retraction of the nerve roots. All work should now be done through a triangular safe zone: cephalad to pedicle of inferior vertebra, medial to exiting nerve root, and lateral to the traversing nerve root and dural sac.
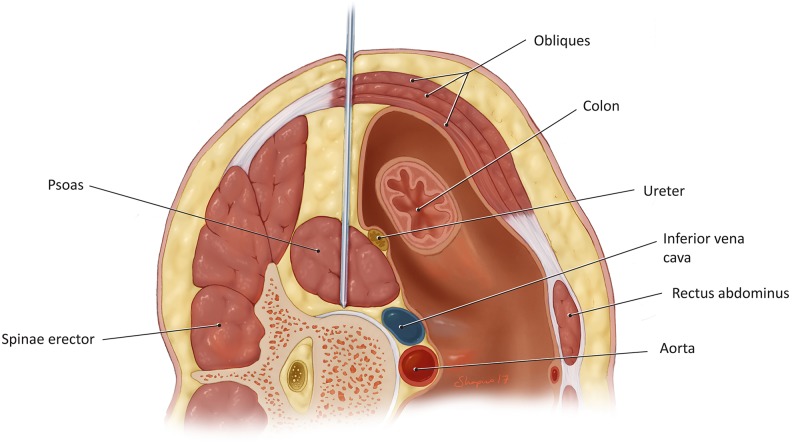
A thorough discectomy is performed using rongeurs, disc shavers, disc excisors, and curettes. A complete discectomy and removal of the cartilaginous endplates are paramount to successful fusion. After adequate preparation of the disc space, the implants are trialed and inserted. An advantage of PLIF is the ability to insert large bilateral cages that accommodate a significant amount of bone grafting material (Figure 4).
Figure 4.
Insertion of subsequent dilators over initial probe during the far lateral lumbar interbody fusion (XLIF) approach.
Transforaminal Lumbar Interbody Fusion (TLIF) and Minimally Invasive TLIF (MI-TLIF)
The positioning, incision, and approach for a TLIF are the same as that of a PLIF. The difference is primarily in the exposure. The TLIF involves a unilateral facetectomy, as well as resection of the pars interarticularis and a laminotomy to develop the triangular working zone described above. In a minimally invasive TLIF, this working zone is achieved with the use of a slightly smaller lateral incision and a minimally invasive retractor system. The incision for an MI-TLIF is placed 1 cm lateral to the midpedicular line, over the facet joint of the target level, and this is done with the assistance of fluoroscopy.
Regardless of approach, a unilateral annulotomy is then performed. A discectomy and endplate preparation is carried out using curved curettes. Endplates are prepared with the use of angled paddle rasps. Following this preparation, a trial is inserted unilaterally and turned and rotated across midline. The final implant is then packed with bone graft and inserted into the intervertebral space. The advantage of the TLIF is that it is a unilateral approach and allows for less traction on the roots and thecal sac. The disadvantage is that a TLIF accommodates a smaller graft compared to a PLIF and often provides less total surface area for fusion.
Far Lateral (Transpsoas) Interbody Fusion
Minimally invasive spine surgery was first described by Obenchain in 1991 with the laparoscopic lumbar discectomy. The field quickly evolved to laparoscopic-assisted ALIFs.11 Minimally invasive spine surgery promised the potential benefits of less morbidity, shorter hospital stays, and faster recovery. The early laparoscopic attempts at minimally invasive spine surgery were hampered by anesthetic complications, damage to intraabdominal viscera, damage to the great vessels, sexual dysfunction, in addition to the steep learning curve of laparoscopic techniques that were new for orthopedic surgeons.
Ozgur et al first introduced the far lateral interbody fusion (XLIF) via a transpsoas approach in 2006.12 The goal of this approach was to offer the benefits of minimally invasive surgery (MIS), while avoiding the morbidity of a transperitoneal approach. The patient is placed in a right lateral decubitus position, left side elevated, and AP fluoroscopic image is used to confirm true 90° position. The table and patient are flexed to open the space between the iliac crest and the 12th rib. This approach is limited in that it can be used to provide access to the disc spaces from L1-2 through L4-5, as the most proximal and most distal of those disc spaces may be difficult to enter. The XLIF approach in general cannot be used to access the L5-S1 space.
A lateral image and 2 K-wires are used to mark the midpoint of the operative level. This marks the middle of the primary incision. If used, a second incision can be made posterior to the primary incision, at the junction of the erector spinae and abdominal obliques. This secondary incision can be used to access the retroperitoneal space and help direct the dilator to the psoas.
Through the primary incision an initial dialator is guided down to the psoas using the index finger through the secondary incision or through careful dissection. The dilator’s position on the psoas muscle is confirmed with AP and lateral images.
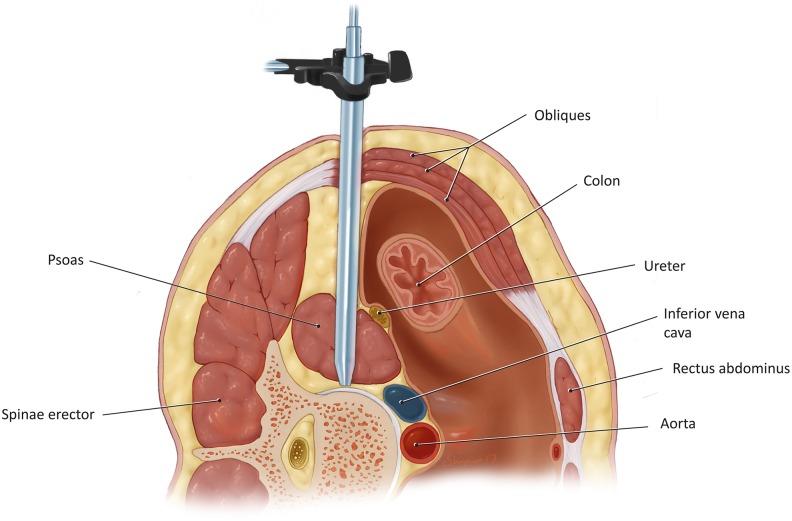
The initial probe is then gently passed through the psoas muscle, at the junction between anterior and middle third of the muscle in order to avoid the lumbar plexus, which rests in the posterior third of the muscle belly (Figure 3). The initial dilator is equipped with an EMG monitoring system that aids in avoiding the lumbar plexus and genitofemoral nerves. The trajectory of the initial dilator must be directly lateral, otherwise damage to the great vessels may occur. Once the surface of the intervertebral disc is reached, the position is confirmed with fluoroscopy, and subsequent dilators of increasing size are placed (Figure 4).
Figure 3.
Insertion of the initial probe via the far lateral lumbar interbody fusion (XLIF) approach.
An XLIF-specific retractor is then placed over the last dilator, and an articulating arm connects to the posterior handle of the retractor allowing for preferential dilation of the anterior arm in order to avoid pressure on the posterior aspect of the psoas muscle that contains the lumbar plexus. After adequate exposure, a standard discectomy, endplate preparation, and interbody fusion are performed similar to the ALIF technique.
Oblique Lateral Interbody Fusion (OLIF)
The OLIF was first described by Mayer et al in 1997, and the term was later coined by Silvestre et al in 2012.13,14 This approach aims to avoid the morbidity of the transpsoas approach by translating the incision anteriorly and dissecting around the psoas (Figure 5). The more anterior incision also makes the approach to the L4-5 disc space easier compared to the XLIF.
Figure 5.
Oblique lumbar interbody fusion (OLIF) incision.
The patient is placed in the right lateral decubitus position, and a 4-cm incision is made in the lateral abdominal region parallel to the fibers of the external abdominal oblique. The incision is centered over the operative level of interest using fluoroscopy as a guide, and is made perpendicular to the line joining the anterior superior iliac spine (ASIS) and the umbilicus. The incision is made one third the distance from the ASIS to the umbilicus. The external oblique, internal oblique, and transversus abdominus muscles are bluntly dissected. Next, the retroperitoneal space is accessed, the psoas muscle is identified and retracted posteriorly, while the ureter and sympathetic plexus are retracted anteriorly.
At this point the intervertebral space should be visible and 4 Steinman pins are used to secure the visual field surrounding the operative level of interest. This minimally invasive technique is suitable for exposure of L2-3, 3-4, and 4-5 levels. Exposure of the L4-L5 level requires ligation of the iliolumbar veins, which frequently traverse this disc space. Exposure of L1-2 is difficult secondary to obstruction by the 12th rib. Once the exposure is complete, disc preparation and cage insertion are the same as for an ALIF or XLIF.
Outcomes
ALIF, PLIF, and TLIF
High-quality studies comparing ALIF and PLIF have not been carried out in the DS population. A meta-analysis has been published on the outcomes of ALIF versus TLIF surgeries, and all patients that underwent these surgeries were included regardless of diagnosis; studies with stand-alone ALIF or TLIF were included as well as those involving posterior supplemental instrumentation.15 The meta-analysis found that the there was no statistically significant difference in the rate of fusions between the 2 approaches (ALIF = 88%, TLIF = 91.9%, P = .23). Rate of dural tears was higher in the TLIF group, while blood vessel injury occurred more frequently in the ALIF group.
Glassman et al compared the Short Form-36 (SF-36) and Oswestry Disability Index (ODI) scores for 497 patients undergoing single and double-level fusion with ALIF and PLIF/TLIF techniques.16 The ALIF subgroup had a greater reduction in both outcome scores at 2-year follow-up compared to the PLIF/TLIF group. The issue with this study is that it was a retrospective review, and the authors included all lumbar interbody fusions regardless surgical indication. The ALIF and PLIF/TLIF cohorts may have been significantly different in composition. There are no high-quality randomized clinical trials on the topic.
The advantage of the PLIF and TLIF approaches is that they avoid the morbidity of the transperitoneal dissection. Yan et al compared the outcomes of PLIFs versus TLIFs.17 Both approaches had similar reduction of spondylolisthesis, as well as similar ability to restore intervertebral height. No statistically significant difference in reduction of Japanese Orthopaedic Association scores was found. All 176 patients achieved fusion within the 2-year study period. Overall, this study suggests that TLIF and PLIF have similar outcomes in the DS population.
XLIF, OLIF, and MI-TLIF
XLIFs have been studied in a number of settings including DS as well as degenerative disc disease (DDD).12,18,19 They have been shown to reliably improve patients’ visual analogue scale (VAS) scores for both back and leg pain by approximately 70%.18 Knight et al published a series of 58 patients undergoing XLIF for DDD and found the overall complication rate to be 22.9%.20 The majority of the complications were related to L4 nerve root injury or anterior thigh pain. Rodgers et al reported on 100 patients that underwent XLIF with posterior instrumentation, and they found the overall complication rate to be 9% at 1 year.21 Complications included urinary retention and tibialis anterior weakness.
Marchi et al further investigated the outcomes of stand-alone XLIF in the setting of DS.22 They found a 60% reduction in back pain VAS and 57% in leg pain VAS at 2-year follow-up. Similar reductions were observed in the ODI. The fusion rate at 2 years was 86.5%; however, none of the nonunions were symptomatic and did not require revision. The revision rate was 13.5% at 2 years. Many of the revisions were secondary to graft subsidence.
The most common reported complication of XLIFs is neurologic deficits.18 Pumberger et al reported on the neurologic deficits following 235 XLIFs.23 Stand-alone XLIFs comprised 28% of the study sample. The authors note that the approach carries potential to injure the ilioinguinal, iliohypogastric, and lateral femoral cutaneous nerves. Anatomic studies suggest that the risk of injury to these nerves increases as the operative level moves caudal.24,25 Sensory deficits were found in 28.7% of patients at 6-week follow-up, which decreased to 5.7% at 6 months and remained at 1.6% at 1 year.
Groin and thigh pain occurred in 41% of patients at 6 weeks, decreased to 16% at 12 weeks, and to 0.8% at 1 year. Motor deficits from lumbar plexus injuries occurred in 4.9% at 6 weeks, which decreased to 2.9% at 1 year. While the OLIF approach may theoretically decrease the risk of injury to the lumbar plexus by avoiding dissection through the psoas muscle, studies with adequate follow-up that investigate the rate of neurologic deficits after this approach have not been carried out.
MI-TLIF has been shown to be effective in attaining fusion and providing pain relief in patients with spondylolisthesis. Kim et al published a study with 5-year follow-up that demonstrated 100% fusion rates in patients with DS and 96% fusion rate in patients with isthmic spondylolisthesis; all patients underwent XLIF with supplemental posterior instrumentation.26 The study demonstrated significant reduction in both VAS and ODI. Out of 44 patients that underwent an MI-TLIF, 5 perioperative complications were reported: 1 screw malpositioning leading to nerve root injury, 2 urinary tract infections, and 2 wound dehiscences. The improvements in VAS and ODI are comparable to those seen with PLIF or PLF.27,28
Open Versus Minimally Invasive
A number of retrospective trials have compared MIS and open interbody fusions and found similar improvements in both pain and function.29–32 Isaacs et al compared open PLIF and MI-TLIF found that the length of stay (LOS) was significantly shorter in the MIS group with 3.4 days compared to 5.1 days (P < .02).32 They also found less intraoperative blood loss, lower rates of transfusions, and less postoperative narcotic use. Similar results in terms of estimated blood loss and LOS are confirmed by other studies.31,33,34
Villavicencio et al reported on the complication rates of MI-TLIF versus open TLIF.29 While the 2 groups had similar overall complication rates of 31.6% for MI-TLIF and 31.7% for open, the major complication rate was almost double in the MI-TLIF group (18.4% vs 9.5%). The higher major complication rate is mostly attributable to the difference in the rate of neurologic deficits (10.5% in MI-TLIF, compared with 1.6%). The MIS group (76 patients in total) had 5 cases of neurologic deficits that lasted over 3 months compared with 1 case in the open group (63 patients). The MIS group contained an additional 3 cases that had neurologic deficits lasting less than 3 months.
The authors note that the learning curve of MIS surgery is likely a major contributor to this trend, as 6 of 8 neurologic deficits occurred in the first 15 MIS procedures performed. A study by Wang et al compares MI-TLIF with open TLIF and purposely excluded the first 100 MI-TLIFs that the authors performed to minimize the effects of the initial learning curve.30 This study found similar rates of perioperative radiculitis and nerve deficits between MIS and open cohorts. They also found the MIS group to have shorter operating room time (2.05 hours vs 3.75 hours), less blood loss (115 mL vs 485 mL), shorter LOS (2.75 days vs 4.40 days), and a lower 4-year reoperation rate (8.3% vs 20%).
The current literature, which is composed entirely of retrospective studies, suggests that MIS procedures are as effective at relieving pain and improving function as the open alternatives. MIS procedures are associated with less blood loss, shorter LOS, and less perioperative pain. The drawback of MIS interbody fusions is that in some studies they are associated with a higher rate of neurologic complications including hyperesthesia, numbness, and hip flexor weakness (Table 1). The great majority of these neurologic deficits resolve within 3 months. While it is possible that the increased rate of neurologic complications is secondary to a steep learning curve, further studies are necessary to investigate that hypothesis.
Table 1.
Rates of Neurologic Complications With Minimally Invasive MI-TLIF, XLIF, OLIF, and Open TLIF.
| Study | Surgery | n | Complication | Rate |
|---|---|---|---|---|
| Wong et al (2014)31 | MI-TLIF | 144 | Radiculitis/deficits | 7.80% |
| Archavlis and Carvi (2013)35 | MI-TLIF | 24 | Radiculopathy | 8.30% |
| Deutsch and Musacchio (2006)36 | MI-TLIF | 20 | Radiculopathy | 10% |
| Lau et al (2013)37 | MI-TLIF | 15 | Radiculopathy | 6.25% |
| Lee and Fessler (2014)38 | MI-TLIF | 84 | Dysesthesia | 1.19% |
| Rosen et al (2008)39 | MI-TLIF | 110 | Radiculopathy | 4.55% |
| Schwender et al (2005)40 | MI-TLIF | 49 | Radiculopathy | 4.10% |
| Villavicencio et al (2010)2 | MI-TLIF | 76 | Nerve root deficit | 10.50% |
| Pumberger et al (2012)23 | XLIF | 235 | Sensory deficits | 28.7% |
| Groin/anterior thigh pain | 41% | |||
| Tohmeh et al (2012)41 | XLIF | 102 | Radiculopathy | 28% |
| Sensory loss | 18% | |||
| Tormenti et al (2010)42 | XLIF | 8 | Motor radiculopathy | 25% |
| Thigh paresthesia/dysesthesia | 75% | |||
| Silvestre et al (2012)14 | OLIF | 179 | Sympathetic chain injury | 1.68% |
| Neurologic deficit | 1.11% | |||
| Wong et al (2014)31 | Open TLIF | 54 | Radiculitis/deficit | 9.30% |
| Villavicencio et al (2010)29 | Open TLIF | 63 | Neurologic deficits | 1.60% |
| Wang et al (2010)30 | Open TLIF | 43 | Radiculopathy | 2.30% |
Abbreviations: TLIF, transforaminal lumbar interbody fusion; MI-TLIF, minimally invasive TLIF; XLIF, far lateral interbody fusion; OLIF, oblique lateral interbody fusion.
Cost Effectiveness
The current-day practice of medicine mandates a cost-conscious approach; interventions must be weighed in terms of both their outcomes and cost in order to provide the most cost-effective care possible. Comparing the costs of MIS lumbar interbody fusion techniques with that of open techniques is particularly important, since the outcomes through 2 years appear similar between the 2 groups. Lucio et al investigated the hospital costs of MI-TLIFs compared with open TLIFs from index hospitalization through 45 days postoperatively.43
They found that the total costs were $24 270 for the MIS group compared with $27 055 for the open group (P = .029). While the implants/instruments were more expensive for the MIS group, the operating room costs and room/board costs were significantly lower. The authors also looked at “residual events,” which included readmissions, emergency room visits, physical therapy costs, reoperations, and complication-related costs. Residual event costs were significantly less frequent in the MIS group (21%) compared with open (37%).
In this study, while the instruments and implants were more expensive in the MIS group, that difference was more than compensated for by the shorter length of stay and less resource utilization in the perioperative period. It is important to note that the cost of the 2 procedures is close enough where operative time becomes an important factor in which option is more cost-effective since operating room time tends to be one of the most expensive components of operative cost.
A second study by Parker et al investigated cost-effectiveness of MIS versus open TLIF from a payer perspective using Medicare reimbursement data.44 They found that the 2-year costs of the procedures was $35 996 and $44 727 in the MIS and open groups, respectively; however this difference is not statistically significant (P = .18). This study did not find a difference in cost-utility between the 2 groups at 2 years.
Summary
A number of open and minimally invasive interbody fusion options are available for the treatment of DS. The ALIF has the longest track record; however, it has been largely supplanted by PLIF and TLIF techniques, which avoid the morbidity of the anterior approach. Minimally invasive options including the XLIF, OLIF, and MI-TLIF appear to have similar fusion rates, and similar long-term outcomes in terms of improvements in pain and function.
The MIS procedures are associated with less intraoperative blood loss, shorter LOS, and less perioperative pain. A potential downside of these procedures is an alarming rate of neurologic deficits and radiculitis. This was seen specifically in studies of XLIFs. There is a wide disparity in the rate of these complications in the literature, and some studies would suggest that these complications are by-products of a steep learning curve. Further studies are necessary to delineate the complication profile of MIS surgery for DS.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Junghanns H. Spondylolisthesen ohne Spalt im Zwischengelenkstück. Archiv für orthopädische und Unfall-Chirurgie, mit besonderer Berücksichtigung der Frakturenlehre und der orthopädisch-chirurgischen Technik. 1931;29:118–127. [Google Scholar]
- 2. Newman P. Spondylolisthesis, its cause and effect: Hunterian Lecture delivered at the Royal College of Surgeons of England on 10th February 1955. Ann R Coll Surg Engl. 1955;16:305–323. [PMC free article] [PubMed] [Google Scholar]
- 3. Herkowitz HN, Kurz L. Degenerative lumbar spondylolisthesis with spinal stenosis. A prospective study comparing decompression with decompression and intertransverse process arthrodesis. J Bone Joint Surg Am. 1991;73:802–808. [PubMed] [Google Scholar]
- 4. Fischgrund JS, Mackay M, Herkowitz HN, Brower R, Montgomery DM, Kurz LT. 1997. Volvo award winner in clinical studies: degenerative lumbar spondylolisthesis with spinal stenosis: a prospective, randomized study comparing decompressive laminectomy and arthrodesis with and without spinal instrumentation. Spine (Phila Pa 1976). 1997;22:2807–2812. [DOI] [PubMed] [Google Scholar]
- 5. Kornblum MB, Fischgrund JS, Herkowitz HN, Abraham DA, Berkower DL, Ditkoff JS. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective long-term study comparing fusion and pseudarthrosis. Spine (Phila Pa 1976). 2004;29:726–733. [DOI] [PubMed] [Google Scholar]
- 6. Suk SI, Lee CK, Kim WJ, Lee JH, Cho KJ, Kim HG. Adding posterior lumbar interbody fusion to pedicle screw fixation and posterolateral fusion after decompression in spondylolytic spondylolisthesis. Spine (Phila Pa 1976). 1997;22:210–219. [DOI] [PubMed] [Google Scholar]
- 7. Gottschalk MB, Premkumar A, Sweeney K, et al. Posterolateral lumbar arthrodesis with and without interbody arthrodesis for L4-L5 degenerative spondylolisthesis: a comparative value analysis. Spine (Phila Pa 1976). 2015;40:917–925. [DOI] [PubMed] [Google Scholar]
- 8. Ha KY, Na KH, Shin JH, Kim KW. Comparison of posterolateral fusion with and without additional posterior lumbar interbody fusion for degenerative lumbar spondylolisthesis. J Spinal Disord Tech. 2008;21:229–234. [DOI] [PubMed] [Google Scholar]
- 9. Brau SA. Mini-open approach to the spine for anterior lumbar interbody fusion: description of the procedure, results and complications. Spine J. 2002;2:216–223. [DOI] [PubMed] [Google Scholar]
- 10. Cloward RB. The treatment of ruptured lumbar intervertebral discs by vertebral body fusion: I. Indications, operative technique, after care. J Neurosurg. 1953;10:154–168. [DOI] [PubMed] [Google Scholar]
- 11. Obenchain TG. Laparoscopic lumbar discectomy: case report. J Laparoendosc Surg. 1991;1:145–149. [DOI] [PubMed] [Google Scholar]
- 12. Ozgur BM, Aryan HE, Pimenta L, Taylor WR. Extreme lateral interbody fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006;6:435–443. [DOI] [PubMed] [Google Scholar]
- 13. Mayer MH. A new microsurgical technique for minimally invasive anterior lumbar interbody fusion. Spine (Phila Pa 1976). 1997;22:691–699. [DOI] [PubMed] [Google Scholar]
- 14. Silvestre C, Mac-Thiong JM, Hilmi R, Roussouly P. Complications and morbidities of mini-open anterior retroperitoneal lumbar interbody fusion: oblique lumbar interbody fusion in 179 patients. Asian Spine J. 2012;6:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Phan K, Thayaparan GK, Mobbs RJ. Anterior lumbar interbody fusion versus transforaminal lumbar interbody fusion—systematic review and meta-analysis. Br J Neurosurg. 2015;29:705–711. [DOI] [PubMed] [Google Scholar]
- 16. Glassman S, Gornet MF, Branch C, et al. MOS short form 36 and Oswestry Disability Index outcomes in lumbar fusion: a multicenter experience. Spine J. 2006;6:21–26. [DOI] [PubMed] [Google Scholar]
- 17. Yan DL, Pei FX, Li J, Soo CL. Comparative study of PILF and TLIF treatment in adult degenerative spondylolisthesis. Eur Spine J. 2008;17:1311–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Patel VC, Park DK, Herkowitz HN. Lateral transpsoas fusion: indications and outcomes. ScientificWorldJournal. 2012;2012:893608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Anand N, Baron EM, Thaiyananthan G, Khalsa K, Goldstein TB. Minimally invasive multilevel percutaneous correction and fusion for adult lumbar degenerative scoliosis: a technique and feasibility study. J Spinal Disord Tech. 2008;21:459–467. [DOI] [PubMed] [Google Scholar]
- 20. Knight RQ, Schwaegler P, Hanscom D, Roh J. Direct lateral lumbar interbody fusion for degenerative conditions: early complication profile. J Spinal Disord Tech. 2009;22:34–37. [DOI] [PubMed] [Google Scholar]
- 21. Rodgers WB, Cox C, Gerber E. Experience and early results with a minimally invasive technique for anterior column support through extreme lateral interbody fusion (XLIF). US Musculoskel Rev. 2007;2(1):28–32. [Google Scholar]
- 22. Marchi L, Abdala N, Oliveira L, Amaral R, Coutinho E, Pimenta L. Stand-alone lateral interbody fusion for the treatment of low-grade degenerative spondylolisthesis. ScientificWorldJournal. 2012;2012:456346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pumberger M, Hughes AP, Huang RR, Sama AA, Cammisa FP, Girardi FP. Neurologic deficit following lateral lumbar interbody fusion. Eur Spine J. 2012;21:1192–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Benglis DM, Jr, Vanni S, Levi AD. An anatomical study of the lumbosacral plexus as related to the minimally invasive transpsoas approach to the lumbar spine: laboratory investigation. J Neurosurg Spine. 2009;10:139–144. [DOI] [PubMed] [Google Scholar]
- 25. Regev GJ, Chen L, Dhawan M, Lee YP, Garfin SR, Kim CW. Morphometric analysis of the ventral nerve roots and retroperitoneal vessels with respect to the minimally invasive lateral approach in normal and deformed spines. Spine (Phila Pa 1976). 2009;34:1330–1335. [DOI] [PubMed] [Google Scholar]
- 26. Kim JS, Jung B, Lee SH. Instrumented minimally invasive spinal-transforaminal lumbar interbody fusion (MIS-TLIF); minimum 5-years follow-up with clinical and radiologic outcomes [published online September 28, 2017]. J Spinal Disord Tech. doi:10.1097/BSD.0b013e31827415cd. [DOI] [PubMed] [Google Scholar]
- 27. Dehoux E, Fourati E, Madi K, Reddy B, Segal P. Posterolateral versus interbody fusion in isthmic spondylolisthesis: functional results in 52 cases with a minimum follow-up of 6 years. Acta Orthop Belg. 2004;70:578–582. [PubMed] [Google Scholar]
- 28. Zhao J, Hou T, Wang X, Ma S. Posterior lumbar interbody fusion using one diagonal fusion cage with transpedicular screw/rod fixation. Eur Spine J. 2003;12:173–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Villavicencio AT, Burneikiene S, Roeca CM, Nelson EL, Mason A. Minimally invasive versus open transforaminal lumbar interbody fusion. Surg Neurol Int. 2010;1:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang J, Zhou Y, Zhang ZF, Li CQ, Zheng WJ, Liu J. Comparison of one-level minimally invasive and open transforaminal lumbar interbody fusion in degenerative and isthmic spondylolisthesis grades 1 and 2. Eur Spine J. 2010;19:1780–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wong AP, Smith ZA, Stadler JA, 3rd, et al. Minimally invasive transforaminal lumbar interbody fusion (MI-TLIF): surgical technique, long-term 4-year prospective outcomes, and complications compared with an open TLIF cohort. Neurosurg Clin N Am. 2014;25:279–304. [DOI] [PubMed] [Google Scholar]
- 32. Isaacs RE, Podichetty VK, Santiago P, et al. Minimally invasive microendoscopy-assisted transforaminal lumbar interbody fusion with instrumentation. J Neurosurg Spine. 2005;3:98–105. [DOI] [PubMed] [Google Scholar]
- 33. Adogwa O, Parker SL, Bydon A, Cheng J, McGirt MJ. Comparative effectiveness of minimally invasive versus open transforaminal lumbar interbody fusion: 2-year assessment of narcotic use, return to work, disability, and quality of life. J Spinal Disorde Tech. 2011;24:479–484. [DOI] [PubMed] [Google Scholar]
- 34. Dhall SS, Wang MY, Mummaneni PV. Clinical and radiographic comparison of mini-open transforaminal lumbar interbody fusion with open transforaminal lumbar interbody fusion in 42 patients with long-term follow-up. J Neurosurg Spine. 2008;9:560–565. [DOI] [PubMed] [Google Scholar]
- 35. Archavlis E, Carvi y, Nievas M, Nievas Carvi y. Comparison of minimally invasive fusion and instrumentation versus open surgery for severe stenotic spondylolisthesis with high-grade facet joint osteoarthritis. Eur Spine J. 2013;22:1731–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Deutsch H, Musacchio MJ., Jr Minimally invasive transforaminal lumbar interbody fusion with unilateral pedicle screw fixation. Neurosurg Focus. 2006;20:E10. [DOI] [PubMed] [Google Scholar]
- 37. Lau D, Ziewacz J, Park P. Minimally invasive transforaminal lumbar interbody fusion for spondylolisthesis in patients with significant obesity. J Clin Neurosci. 2013;20:80–83. [DOI] [PubMed] [Google Scholar]
- 38. Lee P, Fessler RG. Perioperative and postoperative complications of single-level minimally invasive transforaminal lumbar interbody fusion in elderly adults. J Clin Neurosci. 2012;19:111–4. [DOI] [PubMed] [Google Scholar]
- 39. Rosen DS, Ferguson SD, Ogden AT, Huo D, Fessler RG. Obesity and self-reported outcome after minimally invasive lumbar spinal fusion surgery. Neurosurgery. 2008;63:956–60. [DOI] [PubMed] [Google Scholar]
- 40. Schwender JD, Holly LT, Rouben DP, Foley KT. Minimally invasive transforaminal lumbar interbody fusion (TLIF): technical feasibility and initial results. J Spinal Disord Tech. 2005;18 Suppl:S1–6. [DOI] [PubMed] [Google Scholar]
- 41. Tohmeh AG, Rodgers WB, Peterson MD. Dynamically evoked, discrete-threshold electromyography in the extreme lateral interbody fusion approach. J Neurosurg Spine. 2011;14:31–7. [DOI] [PubMed] [Google Scholar]
- 42. Tormenti MJ, Maserati MB, Bonfield CM, Okonkwo DO, Kanter AS. Complications and radiographic correction in adult scoliosis following combined transpsoas extreme lateral interbody fusion and posterior pedicle screw instrumentation. Neurosurg Focus. 2010;28:E7. [DOI] [PubMed] [Google Scholar]
- 43. Lucio JC, Vanconia RB, Deluzio KJ, Lehmen JA, Rodgers JA, Rodgers W. Economics of less invasive spinal surgery: an analysis of hospital cost differences between open and minimally invasive instrumented spinal fusion procedures during the perioperative period. Risk Manag Healthc Policy. 2012;5:65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Parker SL, Adogwa O, Bydon A, Cheng J, McGirt MJ. Cost-effectiveness of minimally invasive versus open transforaminal lumbar interbody fusion for degenerative spondylolisthesis associated low-back and leg pain over two years. World Neurosurg. 2012;78:178–184. [DOI] [PubMed] [Google Scholar]