Abstract
Milk and dairy products are integral part of human nutrition and they are considered as the carriers of higher biological value proteins, calcium, essential fatty acids, amino acids, fat, water soluble vitamins and several bioactive compounds that are highly significant for several biochemical and physiological functions. In recent years, foods containing natural antioxidants are becoming popular all over the world as antioxidants can neutralize and scavenge the free radicals and their harmful effects, which are continuously produced in the biological body. Uncontrolled free radicals activity can lead to oxidative stresses, which have been implicated in breakdown of vital biochemical compounds such as lipids, protein, DNA which may lead to diabetes, accelerated ageing, carcinogenesis and cardiovascular diseases. Antioxidant capacity of milk and milk products is mainly due to sulfur containing amino acids, such as cysteine, phosphate, vitamins A, E, carotenoids, zinc, selenium, enzyme systems, superoxide dismutase, catalase, glutathione peroxidase, milk oligosaccharides and peptides that are produced during fermentation and cheese ripening. Antioxidant activity of milk and dairy products can be enhanced by phytochemicals supplementation while fermented dairy products have been reported contained higher antioxidant capacity as compared to the non-fermented dairy products. Literature review has shown that milk and dairy products have antioxidant capacity, however, information regarding the antioxidant capacity of milk and dairy products has not been previously compiled. This review briefly describes the nutritional and antioxidant capacity of milk and dairy products.
Keywords: Antioxidants activity, Milk, Carotenoids, Enzymatic antioxidants, Phytochemicals
Background
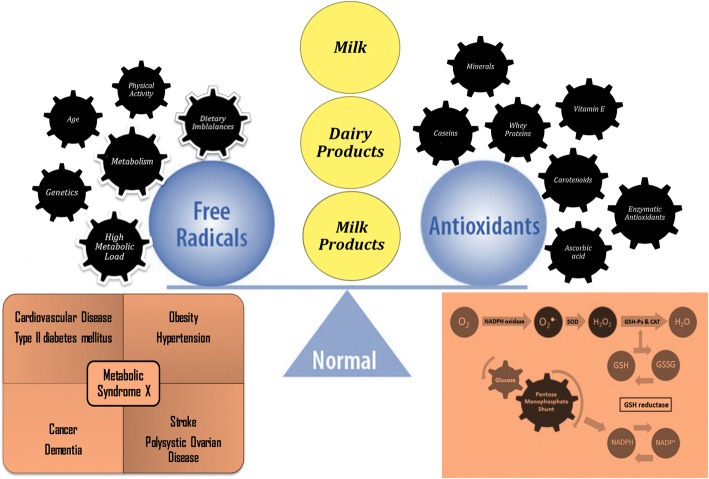
Dairy products constitute about 25–30% of the average diet of an individual [1] (Fig. 1). Milk and milk products are nutritious food items containing numerous essential nutrients such as, oleic acid, conjugated linoleic acid, omega-3 fatty acids, vitamins, minerals and bioactive compounds such as antioxidants [2]. Antioxidants are chemical substances that can neutralize and scavenge the free radicals, which are continuously produced in the body [3]. For the generation of energy, oxidation is indispensable to living organism for biological processes. However, oxidative stress can cause serious damage to biological systems. It is scientifically established that reactive oxygen species are unceasingly produced in human body. Uncontrolled free radicals in body can lead to oxidative stresses, which may consequence in atherosclerosis, diabetes, accelerated ageing, cardiovascular diseases and break down of vital biochemical compounds [4]. Intake of antioxidants in the form of antioxidative supplements of foods rich in antioxidants may protect the body from oxidative stress and damage [5]. Metabolic diseases are closely correlated with life style and changes in life style has a great deal of impact on disease patterns, about 20–30 years ago, infectious disease were more than non-communicable diseases, but now the non-communicable/metabolic diseases are on the higher side. In current scenario, healthy/functional foods should be selected to avoid or minimize non-communicable diseases, such as diabetes, cancers and cardiovascular diseases [6]. Demand for foods containing natural antioxidants is increasing across the globe. Large number of foods and dairy products are being supplemented with natural antioxidants [7]. Antioxidant capacity of milk and dairy products is due to sulfur containing amino acids cysteine, vitamins A, E, carotenoids, enzyme systems, superoxide dismutase, catalase and glutathione peroxidase [8]. Milk also contains appreciable amounts of equol, a polyphenolic metabolite of daidzein, antioxidant activity of this equol is scientifically established [9]. Superoxide radicals, hydroxyl radicals, and peroxide radicals can be inhibited by the antioxidant systems of milk [10]. Human body has mechanisms for the neutralization and scavenging of reactive oxygen species. Significant line of defense against reactive oxygen species are comprised of enzymes such as, glutathione peroxide, catalase and superoxide dismutase, ubiquinol and uric acid [11]. Lipid oxidation is the main reason for the chemical spoilage of food and dairy products and it leads to the production of objectionable changes in nutritional value, flavor and texture of foods [12]. Literature review has shown that milk and dairy products have antioxidant capacity, however, information regarding the antioxidant capacity of milk and dairy products has not been previously compiled. This paper briefly describes the antioxidant capacity of milk and dairy products.
Fig. 1.
Representative figure for antioxidant properties of milk and dairy products
Antioxidant properties of caseins
Caseins are the major protein of bovine and ovine milks present in the form of macro-molecular aggregates. Due to the difference in phosphate content, various casein fractions are present in milk, for example, phosphate content of α, β and κ caseins are 10, 5 and 1 mol per casein mole and phosphate can provide antioxidant activity to the casein micelles [13]. Milk proteins have shown antioxidant activity for the scavenging of reactive oxygen species. Studies have shown that casein inhibited the lipoxygenase-catalyzed lipid autoxidation. Free amino acids cannot quench the free radicals and for the scavenging of free radicals, primary structure of casein molecules acts as scavenger [14]. Phosphoserine residues associated with casein molecules and inorganic phosphate present in casein and serum can bind the non-heme iron. In a previous investigation, it was observed that 72 and 21% of the supplemented non-heme iron in skim milk was recovered from αs- and β-caseins and phosphoseryl rich peptides of casein phosphatides can bind the divalent metal and casein derived peptides inhibited lipoxygenase activity [15]. Casein derived phosphopeptides revealed the capability of sequestering iron in lipid and aqueous food systems [16]. Browning is a serious problem in many foods and casein based coatings are commercially used to prevent oxidation induced browning of fruits and vegetables. Efficacy of calcium caseinate and whey powder in delaying the enzymatic browning in sliced potatoes and apples were investigated and results showed that milk protein based edible coating efficiently postponed the enzymatic browning. Whey protein powder revealed better antioxidant activity than calcium caseinate and the differences in antioxidant activity of whey protein and caseinate was attributed to the difference in amino acid profile [17]. Antioxidant activities of superoxide dismutase, catalase and glutathione peroxidase, casein and certain peptides are well established [18].
Antioxidant properties of whey proteins
In recent years, utilization of whey in food and nonfood applications is mounting across the globe. Whey protein has higher biological value and despite the fact that about 30–35% of the whey is still discarded [19]. In food industries, whey proteins are used as emulsifying, gelling and bulking agent. Antioxidant activity of whey protein is scientifically established and antioxidants of whey can efficiently inhibit the lipid oxidation [20]. Antioxidant activity of whey protein is due to the chelation of transition metals by lactoferrin and scavenging of free radicals by sulphur containing amino acids [21]. Whey proteins boost the level of glutathione peroxidase which is regarded as one of the most significant water soluble antioxidant system [22]. Whey proteins have antioxidant activity and addition of whey protein in soybean oil emulsion increased the oxidative stability [12]. Antioxidant characteristics of salmon oil emulsion increased as a function of addition of whey protein [20]. Food containing whey proteins have better antioxidant activity. Lactoferrin and casein can inhibit lipid peroxidation, generation of peroxide radicals, thiobarbituric acid reacting substances, uptake of oxygen and iron oxide free radicals [23]. Casein fraction, composition of whey proteins and amino acids profile of cow, buffalo, sheep and goat milk have been presented in Tables 1, 2 and 3, respectively.
Table 1.
Casein fraction of cow, buffalo, goat and sheep milk
| Parameters | Cow | Buffalo | Sheep | Goat |
|---|---|---|---|---|
| TPC (g/L) | 27.8 | 49.2 | 59.4 | 33.4 |
| αS1-casein (%) | 37 | 90 | 33 | 99 |
| αS2-casein (%) | 7 | 13 | 14 | 8.52 |
| β-casein (%) | 42 | 28 | 30 | 63 |
| γ-casein (%) | 6 | 22 | 9 | 18 |
| κ- casein (%) | 9 | 7 | 14 | 8 |
Table 2.
Composition of whey proteins in cow, buffalo, goat and sheep milk
| Parameters | Cow Milk | Buffalo Milk | Sheep Milk | Goat Milk |
|---|---|---|---|---|
| Whey proteins (g/L) | 6.46 | 6.46 | 10.76 | 6.14 |
| β- Lactoglobulin (%) | 59.3 | 59.3 | 61.1 | 54.2 |
| α- lactalbumin (%) | 16.2 | 16.2 | 10.8 | 21.4 |
| Immunoglobulin’s (%) | 15.0 | 15.0 | 20.0 | 11.5 |
| Serum albumin/lactoferrin (%) | 9.5 | 9.5 | 8.1 | 12.8 |
Table 3.
Amino acids profile of cow, buffalo, sheep and goat milk
| Amino Acid (g/100 g) | Cow Milk | Goat Milk | Buffalo Milk | Sheep Milk |
|---|---|---|---|---|
| Aspartic acid | 7.8 | 7.4 | 7.13 | 6.5 |
| Threonine | 4.5 | 5.7 | 5.714 | 4.4 |
| Serine | 4.8 | 5.2 | 4.65 | 3.4 |
| aGlutamic acid | 23.2 | 19.3 | 21.4 | 14.5 |
| Proline | 9.6 | 14.6 | 12.0 | 16.2 |
| aCystine | 0.6 | 0.6 | 0.586 | 0.9 |
| Glycine | 1.8 | 2.1 | 1.93 | 3.5 |
| Alanine | 3.0 | 3.6 | 3.03 | 2.4 |
| aValine | 4.8 | 5.7 | 6.760 | 6.4 |
| aMethionine | 1.8 | 3.5 | 0.928 | 2.7 |
| aIsoleucine | 4.2 | 7.1 | 5.714 | 4.6 |
| aLeucine | 8.7 | 8.2 | 9.792 | 9.9 |
| aTyrosine | 4.5 | 4.8 | 3.858 | 3.8 |
| Phenylalanine | 4.8 | 6.0 | 4.713 | 4.3 |
| aHistidine | 3.0 | 5.0 | 2.73 | 6.7 |
| Lysine | 8.1 | 8.2 | 7.497 | 7.8 |
Antioxidant characteristics of carotenoids
Carotenoids are lipophilic molecules with a tendency to accrue in membrane or lipoproteins [24]. Milk fat globule membrane is considered as the most volatile site for auto-oxidation [24]. β-carotene is regarded as preventive antioxidant, it can quench singlet oxygen and one molecule of β-carotene can quench 250 to 1000 molecules of singlet oxygen [26]. Carotenoids act as scavengers of singlet oxygen and other reactive oxygen species [25]. Among the various antioxidant systems in milk, carotenoids act a scavenger of singlet oxygen and peroxyl radicals [27]. Dairy lipids may suffer from oxidation, which leads to the negative impact on quality and sensory characteristics of finished products. Auto-oxidation and light induced oxidation is affected by a complex interaction of pro and antioxidants. Photo-oxidation is predominantly inhibited by β-carotene, it absorbs light that would otherwise be absorbed by riboflavin, which may give rise to quality related issues. β-carotene absorbs light in a concentration dependent manner [28]. Results of an earlier investigation regarding the migration of carotenoids from milk to cheese and butter have shown that concentration of carotenoids was intensified in cheese and butter [29].
Antioxidant characteristics of ascorbic acid, vitamin E and minerals
Nutraceuticals and functional food ingredients that are beneficial to vascular health may represent useful compounds that are able to reduce the overall cardiovascular risks [30]. Ascorbic acid is one of the most strong and least toxic natural antioxidant. It is the main water soluble antioxidant present in milk and free radical scavenging activity of ascorbic acid is due to low oxidation-reduction potential (330 mV). Ascorbic acid is the major water-soluble antioxidant in milk and can act as strong free radical scavenger [31]. Ascorbic acid can scavenge superoxide anion radicals, alkoxyl radicals and singlet oxygen [31]. Ascorbic acid can scavenge superoxide, iron oxide, nitric oxide and alkoxyl radicals [32]. Ascorbic acid significantly inhibited the degradation of riboflavin in cream in presence of 1000 Lux light for four days [33]. Ascorbic acid and tocopherol were added in milk to enhance the flavor and photo-oxidative stability. Ascorbic acid and tocopherol supplemented samples revealed better flavor and photo-oxidative stability as compared to non-supplemented samples [34]. Ascorbic acid significantly inhibited the degradation of riboflavin in light exposed milk, antioxidant activities were mainly attributed to the scavenging effect on singlet oxygen [35]. A study was conducted to determine the effect of tocopherol and vitamin C against the development of atopy in infants. Increased concentration of vitamin C in breast milk reduced the risk of atopy in infants [36]. Ascorbic acid is extremely helpful for the infants as it plays a pivotal role in the formation of neuro transmitters, synthesis of carnitine and improves the absorption of iron. Human and cow milk contains about 40 and 20 mg/Liter [37, 38]. Oxidation of ascorbic acid depends upon temperature, light, oxygen and amount of catalysts. Vitamin A and E are regarded as primary lipid soluble antioxidants and main job of these vitamins is to protect the polyunsaturated fatty acids and associated bio-chemical compounds from peroxidation (Table 4). α-tocopherol can be considered one of the most important lipid-soluble antioxidants in milk, due to it is presence in milk fat globule membrane [39]. It can act as a preventative, chain breaking antioxidant and quencher of singlet oxygen in milk [40]. Milk can develop off flavor as a result of photo-oxidation and contamination with copper. The existence of antioxidants in milk can inhibit the free radical mechanism by donating the proton and thus inhibit the onset of auto-oxidation. Vitamin E can inhibit the activity of plasmin; a proteolytic enzyme and secondly it can directly scavenge the free radicals [41]. Among the tocopherols, α-tocopherol is regarded as more powerful scavenger of free radicals and antioxidant activity of β-, γ- and δ-tocopherol is about 80–90% less than α-tocopherol [42]. γ-tocopherol is of high functional value as it can trap the nitrogen oxide species. It helps the body to prevent cardiovascular diseases and cancers. The concentration of vitamin E in cow milk has been reported about 0.9 mg/mL while summer milk possessed higher concentration than winter milk. Concentration of vitamin in human milk ranges from 3 to 13 mg/mL [43]. Addition of 100 mg α-tocopherol/kg milk fat and 100 mg ascorbyl palmitate/kg milk fat to UHT milk decreased the concentration of hexanal during the storage period of 4 weeks [34]. Khan et al. [44] studied the effect of vitamin E supplementation on oxidative stability of sheep butter and supplementation of sheep butter with 60 mg/kg efficiently inhibited the lipid peroxidation and raised the shelf stability. Antioxidant activity of zinc and selenium for the inhibition of superoxide dismutase is scientifically proven [45]. Glutathione and selenium enhanced the functional value and antioxidant capacity of milk [46]. Mineral content of cow, buffalo, goat and sheep milk have been presented in Table 5.
Table 4.
Vitamin content of cow, buffalo, goat and sheep milk
| Vitamins | Cow Milk (mg/100 g) |
Buffalo Milk (mg/100 g) |
Goat Milk (mg/100 g) |
Sheep Milk (mg/100 g) |
|---|---|---|---|---|
| Vitamin Aa | 46 | 69 | 185 | 146 |
| Vitamin Ea | 0.21 | 0.19 | 0.03 | – |
| Thiamine | 0.05 | 0.05 | 0.068 | 0.08 |
| Riboflavin | 0.17 | 0.11 | 0.21 | 0.37 |
| Niacin | 0.09 | 0.17 | 0.27 | 0.416 |
| Pantothenic acid | 0.37 | 0.15 | 0.31 | 0.408 |
| Vitamin B6 | 0.04 | 0.33 | 0.046 | 0.08 |
| Vitamin B12 | 0.45 | 0.40 | 0.665 | 0.712 |
| Biotin | 2.0 | 13 | 1.5 | 0.93 |
| Vitamin Ca | 0.09 | 2.5 | 1.29 | 4.16 |
| Vitamin D | 2.0 | 2.0 | 1.33 | 1.18 |
Table 5.
Mineral content of cow, buffalo, goat and sheep milk
| Minerals | Cow Milk | Buffalo Milk | Goat Milk | Sheep Milk |
|---|---|---|---|---|
| Calcium | 122 (mg/100 g) | 112 (mg/100 g) | 134 (mg/100 g) | 195–200 (mg/100 g) |
| Phosphorusa | 119 (mg/100 g) | 99 (mg/100 g) | 121 (mg/100 g) | 124–158 (mg/100 g) |
| Potassium | 152 (mg/100 g) | 92 (mg/100 g) | 181 (mg/100 g) | 136–140 (mg/100 g) |
| Magnesium | 12 (mg/100 g) | 8 (mg/100 g) | 16 (mg/100 g) | 18–21 (mg/100 g) |
| Sodium | 58 (mg/100 g) | 35 (mg/100 g) | 41 (mg/100 g) | 44–58 (mg/100 g) |
| Zinca | 530 (μg/100 g) | 410 (μg/100 g) | 56 (μg/100 g) | 520–747 (μg/100 g) |
| Ironb | 80 (μg/100 g) | 161 (μg/100 g) | 7.22 (μg/100 g) | 72–122 (μg/100 g) |
| Copperb | 60.58 (μg/100 g) | 35 (μg/100 g) | 5.13 (μg/100 g) | 40–68 (μg/100 g) |
| Manganese | 20 (μg/100 g) | 27 (μg/100 g) | 3.2 (μg/100 g) | 5.39 (μg/100 g) |
| Iodine | 2.1 (μg/100 g) | 4 (μg/100 g) | 2.2 (μg/100 g) | 10.41 (μg/100 g) |
| Seleniuma | 0.96 (μg/100 g) | 6 (μg/100 g) | 1.33 (μg/100 g) | 3.14 (μg/100 g) |
Enzymatic antioxidants
Superoxide dismutase
Superoxide dismutase (SOD) catalyzes the removal of superoxide free radicals (O2−) and safeguards the cells from harmful effects by the following reaction.
Catalase, glutathione peroxidase or other reducing agents converts H2O2 to H2O, hydrogen peroxide formed from O2− and oxidases is eliminated by catalases and peroxidases [47]. Cytosolic Cu/Zn-SOD, mitochondrial Mn-SOD and extracellular EC-SOD are the major forms of SOD [48]. SOD can inhibit lipid peroxidation. In cow milk SOD is exclusively present in skim milk fraction, with a concentration of 0.15 mg to 2.4 mg/L [49]. Human milk has 2.0 to 2.3 time higher concentration of SOD than cow milk.
Glutathione peroxidase (GSHPx)
GSHPx is a selenium encompassing enzyme that provides protection against lipid peroxidation. It catalysis the breakdown of H2O2 and organic hydroperoxides (R-OOH) by glutathione (γGlu.Cys.Gly) as per following chemical reaction [50].
More than 90% of GSHPx exists in milk as extra cellular enzyme and it is only enzyme which fixes selenium (about 30% of the total. Its concentration varies among the mammals and concentration is in the order of human > caprine > bovine [51]. Concentration of GSHPx in cow milk ranges from 12 to 30 U/mL and its activity is mainly dependent upon the concentration of selenium. Antioxidant activity and selenium content decreases with the progression of lactation [52].
Catalase
Milk catalase is a heme protein and molecular weight of catalase is 200 kDa with isoelectric pH of 5.5. This enzyme is stable in a wide range of pH 5–10 and however, it rapidly loses activity out this pH range [53]. Most of the catalases contain heme and catalase causes the dismutation of H2O2 (a chemical reaction in which H2O2 causes oxidation of the other H2O2 molecules, consequently, one is converted to O2 and the other two are converted to tow molecules of H2O) [54]. A polarographic method showed that average catalase activity in cow milk was 1.95 U/mL [55]. Concentration of catalase in human milk is approximately ten times greater than cow milk [56].
Oxidative stability of milk and milk products
The oxidative stability of milk and dairy products is of concern to the dairy industry. Oxidation in milk can result in strong off-flavors and in deterioration of the nutritional quality of milk. The oxidative stability of milk and dairy products is the result of a delicate balance between the anti- and pro-oxidative processes in milk. Oxidative stability of milk and dairy products depends upon fatty acid composition (Tables 6 and 7), contamination with metal ions, concentration of tocopherols and carotenoids [57]. Processing, packaging, storage conditions and period have a pronounced effect on the extent of natural antioxidants, which is directly connected with oxidative stability of pasteurized milk and dairy products [58]. It is extremely important to determine the antioxidant capacity of milk and milk products, as oxidation can only occur in case of an imbalance between the presence of reactive oxidants and the antioxidant defense mechanism [59]. Sensitivity to oxidation can also be monitored by measuring the antioxidative capacity of a product.
Table 6.
Fatty acid profile of cow, buffalo, goat and sheep milk
| Fatty acid | Cow Milk (g/100 g) |
Buffalo Milk (g/100 g) |
Goat Milk (g/100 g) |
Sheep Milk (g/100 g) |
|---|---|---|---|---|
| C4:0 | 3.5 | 3.90 | 2.46 | 4.06 |
| C6:0 | 2.3 | 2.33 | 2.40 | 2.78 |
| C8:0 | 1.2 | 2.41 | 2.53 | 3.13 |
| C10:0 | 2.6 | 2.40 | 9.38 | 4.97 |
| C12:0 | 2.7 | 3.09 | 4.45 | 3.35 |
| C14:0 | 9.3 | 10.64 | 10.16 | 10.16 |
| C16:0 | 25.9 | 28.02 | 24.20 | 23.11 |
| C18:0 | 14.3 | 12.58 | 12.51 | 12.88 |
| aC18:1 | 27.6 | 24.10 | 23.01 | 26.01 |
| aC18:2 | 2.1 | 2.04 | 2.72 | 1.61 |
| aC18:3 | 0.7 | 0.68 | 0.53 | 0.92 |
Table 7.
Relative rates (M− 1 S− 1) of oxidation by triplet (autoxidation) and singlet (photo-oxidation) oxygen
| Fatty acid | Triplet O3 | Singlet O2 |
|---|---|---|
| Oleic acid | 1 | 3 × 104 |
| Linoleic acid | 27 | 4 × 104 |
| Linolenic acid | 77 | 7 × 104 |
Measuring antioxidant capacity and oxidative stability
Antioxidant capacity assays are useful in measuring the overall antioxidant activity in foods. Antioxidant capacity assays can be categorized into hydrogen atom transfer based assays and electron transfer based assays [33]. Zulueta et al. [60] reported that hydrogen atom transfer based assays measured antioxidant activity from amino acids in milk that can act as hydrogen donors. Determination of nitric oxide free radicals, total phenolic contents, flavonoid contents, DPPH free radicals, inhibition of oxidation of linolenic acid and total reducing capacity can be used for the characterization of antioxidant capacity in milk and dairy products [61]. Lipid oxidation in milk can be measured by several methods which include instrumental methods, such as transition in fatty acid profile, concentration of vitamin A, E and C and total antioxidant assays. Peroxide value measures the primary stages of auto-oxidation and it a useful parameter to determine the oxidation status of milk, cheese, butter and ice cream [28, 62]. Thiobarbituric acid test (TBA) has been used for the determination of secondary oxidation products [63]. Lim et al. [64] used gas chromatography for the determination of oxidation status of ice cream. Sensory techniques are also commonly used for the assessment of oxidized flavor in milk and milk products [65]. Antioxidant characteristics of some dairy products have been illustrated in Table 8.
Table 8.
Antioxidant characteristics of some dairy products
| Study design | Conclusions | Reference |
|---|---|---|
| Effect of grazing on antioxidant characteristics of sheep milk was investigated | Grazing improved the total antioxidant capacity of sheep milk | [65] |
| Zingiber officinale and Beta vulgaris were added in yoghurt milk to improve the antioxidant capacity of herbal yoghurt of buffalo, cow and goat milk yoghurt | Supplementation of yoghurt milk with Zingiber officinale and beta vulgaris improved the 2,2 diphenyl-1 picrylhydrazyl and ferric reducing antioxidant power in yoghurts | [113] |
| 2,2 diphenyl-1 picrylhydrazyl and ferric reducing antioxidant power assays were used to determine the antioxidant capacity of milk along with conventional methods such as peroxide value, thiobarbituric acid value, loss of vitamins A & E | 2,2 diphenyl-1 picrylhydrazyl and ferric reducing antioxidant power assays provided useful information regarding antioxidant capacity of milk | [72] |
| A study was to analyze the antioxidant capacity of yoghurts, acidophillus milks, butter milk and vegetable flavored fermented milk were analyzed for their antioxidant potential | Yoghurt and kefir were characterized by the highest antioxidant activity. The presence of probiotic Lactobacillus casei strains in the product positively improved the ferric reducing antioxidant power. | [114] |
| A study was conducted to estimate the effect of cow feed supplementation by carrots on the βcarotene and α-tocopherol concentration in butter oil | At the same time it contributed in more stable β-carotene, as well as 30% higher α-tocopherol concentration (P < 0.05) | [115] |
| A study was undertaken to assess the effect of betel leaves (Piper betel Linn) extract on the physico-chemical, sensory and antioxidant properties of khoa made from cow milk and stored under room temperature | Free fatty acids levels were well within the prescribed limit because of antioxidant properties exhibited by the aqueous extract of betel leaves. From the study, it was concluded that khoa with 0.5 aqueous extract of betel leaves restricted the production of free fatty acid compared to control due to antioxidant property of betel leaves | [116] |
| the antioxidant properties of kefir produced from goat milk with kefir grains were investigated using total phenolic contents,,2-Diphenyl-1-picrylhydrazyl assays | Antioxidant capacity of kefir was more than parent milk. | [117] |
| Antioxidant properties of milk oligosaccharides from various ruminants were studied | The result suggests that milk oligosaccharides derived from certain ruminant species could be used as natural antioxidants and further studies can be done to elucidate the role of milk oligosaccharides as a functional food and potential drug | [118] |
| The effect of Pediococcus pentosaceus on antioxidant characteristics of probiotic yoghurt was studied in cow, goat and camel milk | Results evidence that antioxidant of goat milk yoghurt was 93% as compared to 86 in camel milk. These results suggested that antioxidant characteristics of yogurt can be enhanced by probiotic bacteria | [119] |
| Cow milk was fermented by Lactobacillus lactis and Lactobacillus delbeurkii | Antioxidant capacity of milk fermented with Lactobacillus, Lactobacillus lactis and Lactobacillus delbeurkii were 21.91 and 29.7% | [120] |
| A study examined the effect of fish oil, Opal linseed and Szafir linseed on the antioxidants of Polish Holstein Friesian cow’s milk | The highest level of α-tocopherol was found in fish oil + Opal linseed group at the 21st day of supplementation. Total antioxidative status increased in all experimental groups; however, the highest peak was recorded in fish oil + Szafir linseed and Szafir linseed group | [121] |
| Impact of Lactobacillus delbrueckii sp.bulgaricus, Lactobacillus rhamnosus, Streptococcus thermophilus or Lactobacillus delbrueckii and Lactobacillus fermentum on antioxidant capacity of bovine milk and whey were investigated | Bacterial strains improved the DPPH free radical scavenging activity, Inhibition of superoxide anions, lipid oxidation and reduces the atherogenesis in humans | [122] |
| Effect of supplementation of Pirotski Kachkaval by ethanolic extract of Kitaibelia vitifolia on antioxidant characteristics were investigated | supplementation of Pirotski Kachkaval cheese by ethanolic extract of Kitaibelia vitifolia raised the antioxidant capacity of cheese | [123] |
| Antioxidant characteristics of ice cream was increased by partially replacing the sucrose with sugarcane juice | Addition of sugarcane juice in ice cream increased the total phenolic contents, DPPH free radical scavenging activity, nitric oxide free radical scavenging activity and total antioxidant capacity of ice cream | [124] |
| Interesterified blends of butter oil and Moringa oleifera oil were characterized for antioxidant capacity and storage stability | Phenolic compounds of Moringa oleifera oil enhanced the antioxidant perspectives and storage stability of butter oil in long term storage | [125] |
| Impact of supplementation of ethanolic leaf extract of Moringa oleifera on storage stability of butter in refrigeration condition was investigated | Leaf extract of Moringa oleifera at 600 ppm may be used for reasonable storage stability of butter at refrigeration temperature with acceptable sensory characteristics | [126] |
| Effect of almond (Prunis dulcis) peel extract was determined on antioxidant characteristics of whey butter | Addition of 400 ppm ethanolic extract of almond peel increased the total phenolic contents and DPPH free radical scavenging activity | [127] |
| Gouda cheese was supplemented with mango (Mangifera indica L.) oil to improve the antioxidant characteristics | Supplementation of mango kernel oil increased the total phenolic contents, DPPH free radical scavenging activity, nitric oxide free radical scavenging activity and inhibited the lipid oxidation | [128] |
| Influence of intereterified Moringa oleiefera oil on oxidative stability of ice cream was studied | Addition of interesterified Moringa oleifera oil significantly improved the oxidative stability of ice cream | [129] |
| The main objective of this study was to raise the antioxidant characteristics of cheddar cheese of chia oil. Cheddar was supplemented with chia (Salvia hispanica L.) oil from 2.5 to 10% | Supplementation of cheddar cheese with chia oil increased the antioxidant capacity of cheddar cheese | [130] |
| Antioxidant characteristics of milk were enhanced by Hypotrigona squamuligera honey | Fortification of milk with Hypotrigona squamuligera honey inhibited 2,2-diphenyl- 1picrylhydrazyl free radicals with lower peroxide value | [131] |
Ripening effect on antioxidant characteristics of cheese
Cheese is one of the major fermented dairy products. Dairy products are an excellent source of high quality protein and milk fat. It is a rich source of fat soluble vitamins and also an important source of minerals such as calcium, phosphorous and concentrated source of energy [66]. A study was performed in traditional Mexican cheese (Cotiaj) to investigate the antioxidant activities of peptides produced during the 6 months ripening period. Peptides were characterized by HPLC and results showed that peptides with antioxidant activity were produced during the ripening period of 6 months. 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging activity of six month old cheese was 98% [67]. Cheddar cheese was prepared using lactobacillus para casei as starter culture and changes in antioxidant characteristics of cheddar was monitored for six months. Different antioxidant assays were used as indicators of antioxidant activity and it was noted that DPPH and superoxide free radical scavenging activities of cheese increased as up to four months of ripening. The increase in antioxidant activities was attributed to the production of water soluble peptides. Antioxidant activity and extent of water soluble peptides were strongly correlated [68]. Antioxidant activity of white brined cheese prepared from overheated milk (90 °C, 10 min) was investigated. Antioxidant activity of water soluble and water insoluble fraction of cheese increased during the ripening period and antioxidant activities were correlated with degree of proteolysis [69]. Effect of phytochemicals on antioxidant characteristics of cheese have been summarized in Table 9.
Table 9.
Effect of phytochemicals on antioxidant characteristics of cheese
| Study design | Conclusions | Reference |
|---|---|---|
| Green tea catechins were added in full fat cheeses at 250, 500, and 1000 ppm. Cheeses were ripened for 90 days at 8 °C. Total phenolic content and antioxidant activity of the cheeses were determined | The results showed that addition of GTE significantly decreased the pH of whey and curd during cheese manufacture and ripening, however there was no significant effect on moisture, protein, or fat contents. The addition of gate tea extract increased TPC and AA at all concentrations | [69] |
| Effect of rosemary leaf supplementation on the antioxidant activities and total phenolic content of Pecorino cheese was studied. Three hundred and twenty-four sheep were randomly assigned to two dietary groups. The concentrate of the rosemary supplemented group contained 2.50% dried rosemary leaves | Results showed that rosemary supplementation increased the total phenolic content, also enhanced the antioxidant properties and decreased the lipid oxidation in cheese | [132] |
| Effect of catechin on total phenolic content and antioxidant properties in low-fat hard cheese was examined over a 90-day ripening period at 8 °C | Total phenolic content and antioxidant activities were increased during the 90-day ripening period | [133] |
| Low fat Kalari cheese was treated with different concentrations of pine needle extract (0, 2.5 and 5%), aerobically packaged with polyethylene pouches and kept at 4 °C | Lipid oxidative stability of treated cheese was improved | [134] |
| The effect of oregano and rosemary essential oils on the oxidative and stability of cream cheese. Peroxide and anisidine values of treated cheese were determined | Supplementation of cream cheese with essentials oil improved the oxidative stability | [135] |
| Impact of rosemary extract (1.5%) on antioxidant characteristics of soft cheese was studied | Rosemary extract enhanced the antioxidant characteristics of soft cheese | [136] |
| Extract of fennel (Foeniculum vulgare) on antioxidant capacity of cottage cheese was studied | Addition of fennel extract enhanced the shelf life of cottage cheese | [137] |
| Soft cheeses were supplemented with bay, cinnamon, clove and thyme oils | Phenolic compounds of bay, cinnamon, clove and thyme oils inhibited the Listeria monocytogenes and Salmonella in soft cheeses | [138] |
| Impact of Matricaria recutita extract on antioxidant activity of cottage cheese was examined | Cottage cheese functionalized with chamomile extract showed the higher value of antioxidant activity for seven days | [139] |
| The study was conducted to check the antimicrobial effect of phenolic compounds of Moringa oleifera leaf extract in West African soft cheese at 1, 2 and 3% concentration | Phenolic compounds of Moringa oleifera lea extact efficiently inhibited the undesirable bacteria in West African Cheese | [140] |
Antioxidant characteristics of yoghurt
The effect of Pediococcus pentosaceus on antioxidant characteristics of probiotic yoghurt was studied in cow, goat and camel milk and results evidence that antioxidant of goat milk yoghurt was 93% as compared to 86% in camel milk. These results suggested that antioxidant characteristics of yogurt can be enhanced by probiotic bacteria [70]. Yoghurt is a fermented milk product with distinctive therapeutic value and presented in diversified forms and flavors. Yoghurt was added with carrots, pumpkin, broccoli and red sweet pepper at 10% concentration and ferric reducing antioxidant power (FRAP) and DPPH assays were used for antioxidant activity during the storage period of 14 days. Yoghurt added with broccoli and red sweet pepper revealed higher DPPH free radical scavenging activity and FRAP. However, antioxidant activity decreased during the storage period of 14 days [71]. Similarly, cow, buffalo and goat milk yoghurts were supplemented with aqueous extracts of Zingiber officinale and Beta vulgaris DPPH free radical scavenging activity and FRAP of goat milk yoghurt was greater than other cow and buffalo milk [72]. In another study, antioxidant capacity of yoghurt was increased by supplementing the yoghurt milk 60 mg vitamin C, 12 mg vitamin E and 3 mg beta-carotene. Antioxidant characteristics of supplemented yoghurts were higher than non-supplemented yoghurts with no effects on sensory properties [73]. Yoghurt was supplemented with fruit pulp of papaya and cactus pear using Lactobacillus bulgaricus and Streptococcus thermphillus as starter cultures and total phenolic contents, ascorbic acid and total antioxidant activity were analyzed. Yoghurt added with papaya fruit pulp had higher total phenolic contents, antioxidant activity and vitamin C concentration [74]. Typical yoghurt starter bacteria Lactobacillus delbrueckii ssp. bulgaricus and Streptococcus thermophiles inhibited the lipid peroxidation through the scavenging of reactive oxygen species such as hydrogen perode and hydroxyl radicals [75]. Antioxidant activity of milk fermented with Lactobacillus fermentum ME-3 was significantly increased higher than milk [76]. The antioxidant activity in fermented dairy products is mainly due to the bioactive peptides released from α-lactalbumin, β-lactoglobulin and α-casein [77].
In-vivo studies
In-vivo study was performed to assess the antioxidant capacity of the peptides produced in a fermented dairy product similar to yoghurt by the proteolytic strains of Lb. bulgaricus. Results revealed that reactive oxygen speices decreased in the live yeast cells [78]. In another in-vivo study, therapeutic perspectives of camel milk were established [79]. The effect of fermented dairy products was observed on the antioxidant enzymes in liver of Swiss mice. Feeding fermented dairy product considerably increased the level of Catalase, Superoxide dismutase (SOD), Glutathione peroxidase and superoxide dismutase [80]. The effect of antioxidant peptides of cow and buffalo cheddar cheese was evaluated against tert-butylhydroperoxide-induced colon cancer. Antioxidant peptides of cow and buffalo milk can protect intestinal epithelium from oxidative damage [81].
Therapeutic Perspectives of Milk and Dairy Products
Cultured milk has higher antioxidant properties as compared to the normal milk and intake of two servings of cultured milk on daily basis reduced the risk of bladder cancer up to 38% as compared to the people who do not use cultured milk [82]. Intake of milk fermented with E. faecium RM11 and L. fermentum RM28 had 21 and 29% less chances of colon cancer [83]. In another investigation, it was observed that men and women consuming milk on daily basis had 53% lower risk of bladder cancer while Swedish women containing four servings of high fat milk and dairy products showed 13% less risk of colorectal cancer [84]. Reyes et al. [85] found that milk fermented with Lactobacillus spp. and Bifidobacterium spp. had a protective effect against liver cancer. Consuming more than 500 ml milk/day significantly decreased the risk of colorectal cancer [86]. Individuals consuming lower amount of milk had higher chances of colorectal cancer [87]. Intake of half liter milk and ricotta cheese on daily basis reduced the risk of colon cancer to 12 and 17% [88]. Intake of 25 g white cheese on daily basis decreased the chances of premenopausal cancer up to 50% as compared to the women consuming less than 6 g white cheese on daily basis [89]. Milk proteins and peptides have shown anti-carcinogenic properties [90]. For instance, lactoferrin is well known for anti-cell proliferation, antioxidant and anti-inflammatory activities [91]. Oral administration of lactoferrin derived from bovine milk considerably reduced the risk of several types of cancers [92]. Casein and whey proteins may protect from colon, breast and prostate gland cancer [93]. The anticancer capability of casein and whey protein may be attributed to the presence of higher concentration of glutathione, which is well known for its antioxidant activity [91]. Immunoglobulins such as IgG1, IgM, IgA and IgG2 has antimicrobial and glutathione enhancing activities which is the important antioxidant of the cell [94]. The association between the dietary intake of milk and dairy products was evaluated and no association was found between the intake of dairy products and cardiovascular diseases [95]. An inverse relation was found between the intake of dairy products and non-fatal cardiovascular disease [96]. No significant association was recorded between the intake of cheese and cardiovascular disease [97]. Intake of low fat, medium fat and high fat cheese had no correlation with cardiovascular disease [98]. Intake of 54 g whey protein on daily basis for a period of 12 weeks significantly reduced the systolic and diastolic blood pressure [99]. Xu et al. [100] observed as strong correlation in the concentration of bioactive peptides produced by the activities of microbiota and gastrointestinal enzymes which are abundantly present in fermented dairy products [101]. A study was conducted on 3435 Parisians for the duration of three days, it was found that higher intake of dairy products led to a lower risk of types 2 diabetes up to 14% [102]. Components of milk and dairy products are industrially used to increase the functional value of the foods. For example, phosphopeptides of casein are used as supplement for several dietary and pharmaceutical applications [103].
Conclusions
Milk and dairy products, which are basic foods for human development, can be beneficial for the oxidative defence of consumers by several mechanisms. Milk and dairy products with protective properties have the potential to act as coadjuvants in conventional therapies, addressing cardiovascular diseases, metabolic disorders, intestinal health and chemopreventive properties.
Acknowledgements
The authors are highly obliged to the Library Department, University of Veterinary and Animal Sciences (UVAS), Government College University Faisalabad (GCUF), and IT Department, Higher Education Commission (HEC, Islamabad) for access to journals, books and valuable database.
Availability of data materials
The dataset supporting the conclusions of this article is included within the article.
Funding
Not Applicable.
Abbreviations
- DNA
Deoxyribo Nucleic Acid
- DPPH
2,2-Diphenyl-1-Picrylhydrazyl
- FRAP
Ferric Reducing Antioxidant Power
- GSHPx
Glutathione Peroxidase
- HPLC
High Performance Liquid Chromatography
- SOD
Super Oxide Dismutase
- TBA
Thiobarbituric Acid
- TPC
Total Protein Content; GTE: Green Tea Catechins
Authors’ contributions
ITK conceptualized the idea, MN provided the technical assistance and MHJ guided in the data collection. MI, RU and MA helped for drafting the manuscript. “It’s also confirmed that all the authors read and approved the final manuscript”.
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Imran Taj Khan, Email: imran.taj96@gmail.com.
Muhammad Nadeem, Phone: +92.334.4190542, Email: muhammad.nadeem@uvas.edu.pk.
Muhammad Imran, Email: imran@gcuf.edu.pk.
Rahman Ullah, Email: rahmanmohmand99@yahoo.com.
Muhammad Ajmal, Email: m.ajmaluvas290@gmail.com.
Muhammad Hayat Jaspal, Email: hayat.jaspal@uvas.edu.pk.
References
- 1.Richmond HD. Dairy chemistry: a practical handbook for dairy chemists and others having control of dairies. USA: Cole Press. 2007.
- 2.Saxelin M, Korpela R, Mayra-Makinen A. Introduction: classifying functional dairy products. In: Mattila-Sandholm T, Saarela M, editors. Functional dairy foods. Boca Raton, FL, USA.: CRC Press; 2003. p. 1–16.
- 3.Yazdanparast R, Ardestani A. In vitro antioxidant and free radical scavenging activity of Cyperus rotundus. J Med Food. 2007;10:667–74. [DOI] [PubMed]
- 4.Kris-Etherton PM, Hecker KD, Bonanome A, Coval SM, Binkoski AE, Hilpert KF, Griel AE, Etherton TD. Bioactive compounds in foods: their role in prevention of cardiovascular disease and cancer. Am J Med. 2002;113:71–88. [DOI] [PubMed]
- 5.Wang Y, Yu R, Chou C. Antioxidative activities of soymilk fermented with lactic acid bacteria and bifidobacteria. Food Microbiol. 2006;23:128–135. doi: 10.1016/j.fm.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 6.Jiang P, Burczynski F, Campbell C, Pierce G, Austria J, Briggs C. Rutin and flavonoid contents in three buckwheat species Fagopyrum esculentum, F. Tataricum, and F. Homotropicum and their protective effects against lipid peroxidation. Food Res Int. 2007;40:356–364. doi: 10.1016/j.foodres.2006.10.009. [DOI] [Google Scholar]
- 7.Santillo A. Role of indigenous enzymes in proteolysis of casein in caprine milk. Inter Dairy J. 2009:655–60.
- 8.Usta B, Yilmaz-Ersan L. Antioxidant enzymes of milk and their biological effects. J Agric Faculty of Uludag University. 2013;2:123–30.
- 9.Mustonen EA, Tuori M, Saastamoinen I, Taponen J, Wahala K, Saloniemi H, Vanhatalo A. Equol in milk of dairy cows is derived from forage legumes such as red clover. Br J Nutr. 2009;102:1552–1556. doi: 10.1017/S0007114509990857. [DOI] [PubMed] [Google Scholar]
- 10.Tong LM, Sasaki S, McClements DJ, Decker EA. Mechanisms of the antioxidant activity of a high molecular weight fraction of whey. J Agri Food Chem. 2000;48:1473–1478. doi: 10.1021/jf991342v. [DOI] [PubMed] [Google Scholar]
- 11.Karakaya S, El SN, Tas AA. Antioxidant activity of some foods containing phenolic compounds. Int J Sci Nutr. 2001;52:501–508. doi: 10.1080/713671810. [DOI] [PubMed] [Google Scholar]
- 12.Erel Ö. A novel automated method to measure total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004;7:277–285. doi: 10.1016/j.clinbiochem.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 13.Cervato G, Cazzola R, Cestaro B. Studies on the antioxidant activity of milk caseins. Int J Food Sci Nutr. 1999;50:291–6. [DOI] [PubMed]
- 14.Suetsuna K, Ukeda H, Ochi H. Isolation and characterization of free radical scavenging activities peptides derived from casein. J Nutr Biochem. 2000;11:128–131. doi: 10.1016/S0955-2863(99)00083-2. [DOI] [PubMed] [Google Scholar]
- 15.Rival SG, Fornaroli S, Boeriu CG, Wichers HJ. Caseins and casein hydrolysates. Lipoxygenase inhibitory properties. J Agric Food Chem. 2001;49:287–294. doi: 10.1021/jf000392t. [DOI] [PubMed] [Google Scholar]
- 16.Kitts DD. Antioxidant properties of casein phosphopeptides. Trends Food Sci Tech. 2005;16:549–554. doi: 10.1016/j.tifs.2005.08.009. [DOI] [Google Scholar]
- 17.Le Tien C, Vachon C, Mateescu MA, Lacroix M. Milk protein coatings prevent oxidative browning of apples and potatoes. J Food Sci. 2001;4:512–516. doi: 10.1111/j.1365-2621.2001.tb04594.x. [DOI] [Google Scholar]
- 18.Boor KJ. Fluid dairy product quality and safety: looking to the future. J Dairy Sci. 2001:1–11. [DOI] [PubMed]
- 19.Colbert LB, Decker EA. Antioxidant activity of an ultrafiltration permeate from acid whey. J Food Sci. 1991;5:1248–1250. doi: 10.1111/j.1365-2621.1991.tb04744.x. [DOI] [Google Scholar]
- 20.Tong LM, Sasaki S, McClements DJ, Decker EA. Antioxidant activity of whey in a salmon oil emulsion. J Food Sci. 2001;8:1325–1329. [Google Scholar]
- 21.Carthy TLM, Kerry JP, Kerry JF, Lynch PB, Buckley DJX. Evaluation of the antioxidant potential of natural food/plant extracts as compared with synthetic antioxidants and vitamin E in raw and cooked pork patties. Meat Sci. 2015;1:45–52. doi: 10.1016/s0309-1740(00)00129-7. [DOI] [PubMed] [Google Scholar]
- 22.Clouatre D. The whey to health. Total Health. 1999;2:65–66. [Google Scholar]
- 23.Chen J, Lindmark-Mansson H, Akesson B. Optimisation of a coupled enzymatic assay of glutathione peroxidase activity in bovine milk and whey. Int Dairy J. 2000:347–51.
- 24.Stahl W, Sies H. Antioxidant activity of carotenoids. Mol Asp Med. 2003;24:345–51. [DOI] [PubMed]
- 25.Lindmark-Mansson H, Akesson B. Antioxidative factors in milk. Br J Nutr. 2000;84:103–110. doi: 10.1017/S0007114500002324. [DOI] [PubMed] [Google Scholar]
- 26.Kim HJ, Min DB. Chemistry and reactions of singlet and triplet oxygen in lipid oxidation. In: Kamal-Eldin A and Min DB. Lipid Oxidation Pathways. Urbana, IL: AOCS Press. 2003;1–30.
- 27.Young AJ, Lowe GM. Antioxidant and prooxidant properties of carotenoids. Arch Biochem Biophys. 2001;385:20–27. doi: 10.1006/abbi.2000.2149. [DOI] [PubMed] [Google Scholar]
- 28.Mortensen G, Sorensen J, Stapelfeldt H. Comparison of peroxide value methods used for semihard cheeses. J Agric Food Chem. 2002;50:5007–5011. doi: 10.1021/jf0200220. [DOI] [PubMed] [Google Scholar]
- 29.Panfili G, Manzi P, Pizzoferrato L. Influence of thermal and other manufacturing stresses on retinol isomerization in milk and dairy products. J Dairy Res. 1998;65:253–260. doi: 10.1017/S0022029997002811. [DOI] [PubMed] [Google Scholar]
- 30.Scicchitano P, Cameli M, Maiello M, Modesti PA, Muiesan ML, Novo S, et al. Nutraceuticals and dyslipidaemia: beyond the common therapeutics. J Funct Foods. 2014;6:11–32. doi: 10.1016/j.jff.2013.12.006. [DOI] [Google Scholar]
- 31.Choe E, Min DB. Mechanisms of antioxidants in the oxidation of foods. Comp Rev Food Sci Food Safety. 2009;8:345–358. doi: 10.1111/j.1541-4337.2009.00085.x. [DOI] [Google Scholar]
- 32.Hosaka S, Obuki M, Nakajima J, Suzuki M. Comparative study of antioxidants as quenchers or scavengers of reactive oxygen species based on quenching of MCLA-dependent chemiluminescence. Luminescence. 2005;20:419–427. doi: 10.1002/bio.867. [DOI] [PubMed] [Google Scholar]
- 33.Huang D, OU B, Prior RL. The chemistry behind antioxidant capacity assay. J Agric Food Chem. 2005;53:1841–56. [DOI] [PubMed]
- 34.Van Aardt M, Duncan SE, Marcy JE, Long TE, O’Keefe SF, Nielsen-Sims SR. Aroma analysis of light-exposed milk stored with and without natural and synthetic antioxidants. J Dairy Sci. 2005;88:881–90. [DOI] [PubMed]
- 35.Whited LJ, Hammond BH, Chapman KW, Boor KJ. Vitamin a degradation and light oxidized flavor defects in milk. J Dairy Sci. 2002;85:351–354. doi: 10.3168/jds.S0022-0302(02)74080-0. [DOI] [PubMed] [Google Scholar]
- 36.Hoppu U, Isolauri E. Vitamin C in breast milk may reduce the risk of atopy in the infant. European J Clinical Nutri. 2004;59:123–128. doi: 10.1038/sj.ejcn.1602048. [DOI] [PubMed] [Google Scholar]
- 37.Singh M. Role of micronutrients for physical growth and mental development. Indian J Pediatr. 2004;71:59–62. doi: 10.1007/BF02725658. [DOI] [PubMed] [Google Scholar]
- 38.Humma N, Sameen A, Zahoor T, Anjum M. Composition and physico-chemical characteristics of buffalo milk with particular emphasis on lipids, proteins, minerals, enzymes and vitamins. J Animal and Plant Sci. 2013;23:62–74. [Google Scholar]
- 39.Jensen SK, Nielsen KN. Tocopherols, retinol, β-carotene and fatty acids in fat globule membrane and fat globule core in cow’s milk. J Dairy Res. 1996;63:565–574. doi: 10.1017/S0022029900032106. [DOI] [PubMed] [Google Scholar]
- 40.O’Connor TP, O’Brien NM. Lipid oxidation. In: Fox PF, McSweeney PLH, editors. Advanced dairy chemistry: volume 2: lipids. New York: Springer; 2006. p. 557–600.
- 41.Politis I. Reevaluation of vitamin E supplementation of dairy cows: bioavailability, animal health and milk quality. Animal. 2012;6:1427–1434. doi: 10.1017/S1751731112000225. [DOI] [PubMed] [Google Scholar]
- 42.Hosseinian FS, Li W, Tsopmo A, Friel JK, Beta T. Evaluation of antioxidant capacity and aroma quality of breast milk. Nutrition. 2009;25:105–114. doi: 10.1016/j.nut.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 43.Moltó Puigmartí C, Castellote AI, López-Sabater MC. Ultra-high-pressure liquid chromatographic method for the analysis of tocopherols in human colostrum and milk. J Chromatography A. 2009;1216:4388–94. [DOI] [PubMed]
- 44.Khan GA, Nadeem M, Abdullah M, Ilyas M. Effect of vitamin E on storage stability of sour cream butter made from sheep milk. Carpathian J Food Sci Tech. 2011;3:21–25. [Google Scholar]
- 45.Geissler C, Powers H. Human nutrition London. UK: Churchill Livingstone. 2011:509–32.
- 46.Sretenović LJ, Aleksić S, Petrović PM, Miščević B. Nutritional factors influecing improvement of milk and meat quality as well as productive and reproductive parameters of cattle. Biotechnol Anim. 2007;5–6:217–226.
- 47.Fox PF, McSweeney PLH. Dairy Chemistry and Biochemistry. First edition. Blackie Academic and Professional, Thomson Science, London. 1998;1:265–340.
- 48.Matés JM. Pérez-Gómez C, Núñez de Castro I. Antioxidant enzymes and human diseases. Clin Biochem. 1999;32:595–603. doi: 10.1016/S0009-9120(99)00075-2. [DOI] [PubMed] [Google Scholar]
- 49.Fang YZ, Yang S, Wu G. Free radicals, antioxidants and nutrition. Nutrition. 2002;18:872–879. doi: 10.1016/S0899-9007(02)00916-4. [DOI] [PubMed] [Google Scholar]
- 50.Torres A, Farré R, Lagarda MJ, Monleón J. Determination of glutathione peroxidase activity in human milk. Nahrung. 2003;47:430–433. doi: 10.1002/food.200390095. [DOI] [PubMed] [Google Scholar]
- 51.Fox PF, Kelly AL. Indigenous enzymes in milk: overview and historical aspects - part 2. Int Dairy J. 2006;16:517–532. doi: 10.1016/j.idairyj.2005.09.017. [DOI] [Google Scholar]
- 52.Quiles JL, Ochoa JJ, Ramirez-Tortosa MC, Linde J, Bompadre S, Battino M. Coenzyme Q concentration and total antioxidant capacity of human milk at different stage of lactation in mothers of preterm and full-term infants. Free Radic Res. 2006;40:199–206. doi: 10.1080/10715760500404805. [DOI] [PubMed] [Google Scholar]
- 53.Abbas H, Hassan F, Enab A, Gawad A. Physicochemical characteristics of goat’s milk. J Life Sci. 2014;11:307–317. [Google Scholar]
- 54.Silanikove N, Merin U, Leitner G. Physiological role of indigenous milk enzymes: an overview of an evolving picture. Int Dairy Sci. 2006;16:533–545. doi: 10.1016/j.idairyj.2005.08.015. [DOI] [Google Scholar]
- 55.Niklowitz P, Menke T, Giffei J, Andler W. Coenzyme Q10 in maternal plasma and milk throughout early lactation. Biofactors. 2005;25:67–72. doi: 10.1002/biof.5520250108. [DOI] [PubMed] [Google Scholar]
- 56.Tijerina-Sáenz A, Innis SM, Kitts DD. Antioxidant capacity of human milk and its association with vitamins a and E and fatty acid composition. Acta Paediatr. 2009;98:1793–1798. doi: 10.1111/j.1651-2227.2009.01437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nielsen JH. Lund-Nielsen T and Skibsted L. Higher antioxidant content in organic milk than in conventional milk due to feeding strategy. 2004:1–2.
- 58.Zygoura P, Moyssiadi T, Badeka A, Kondyli A, Savvaidis I, Kontominas MG. Shelf life of whole pasteurized milk in Greece: effect of packaging material. Food Chem. 2004;87:1–9. doi: 10.1016/j.foodchem.2003.10.009. [DOI] [Google Scholar]
- 59.Halliwell, B. Antioxidants in human health and diseases. Annu Rev Nutr. 1996;1–2;33–50. [DOI] [PubMed]
- 60.Zulueta A, Esteve MJ, Frigola A. ORAC and TEAC assays comparison to measure the antioxidant capacity of food products. Food Chem. 2009;114:310–316. doi: 10.1016/j.foodchem.2008.09.033. [DOI] [Google Scholar]
- 61.Chen J, Lindmark-Mansson H, Gorton L, Akesson B. Antioxidant capacity of bovine milk as assayed by spectrophotometric and amperometric methods. Int Dairy J. 2003;13:927–935. doi: 10.1016/S0958-6946(03)00139-0. [DOI] [Google Scholar]
- 62.Liang JH. Kinetics of fluorescence formation in whole milk powders during oxidation. Food Chem. 2000;71:459–463. doi: 10.1016/S0308-8146(00)00172-2. [DOI] [Google Scholar]
- 63.Fenaille F, Mottier P, Turesk RJ, Ali S, Guy PA. Comparison of analytical techniques to quantify malondialdehyde in milk powders. J Chromatogr A . 2001;921:237–245. [DOI] [PubMed]
- 64.Lim CW, Norziah MH, Lu HFS. Effect of flaxseed oil towards physicochemical and sensory characteristic of reduced fat ice creams and its stability in ice creams upon storage. Food Res Int. 2010;17:393–403. [Google Scholar]
- 65.Chapman KW. Sensory evaluation of milk. In Griffiths MW. Improving the safety and quality of milk. Cambridge, Uk: Woodhead Publishing Limited. 2010;159–180.
- 66.Dzomba P, Ngoroyemoto N, Musarurwa R. Antioxidant capacity and microbial attributes of raw cow milk fortified with Hypotrigonasquamuligera honey. Global J Medi Res Microbio Patho. 2013;13:9–12. [Google Scholar]
- 67.Hаug M, Laubach C, Burke M, Harzer G. Vitamin E in human milk from mothers of preterm and term infants. J Pediatr Gastroenterol Nutr. 1987;6:605–609. doi: 10.1097/00005176-198707000-00020. [DOI] [PubMed] [Google Scholar]
- 68.Hernández Galán L, Cardador Martínez A, Picque D, Spinnler HE, López Del Castillo Lozano M, Martín Del Campo Barba ST. Angiotensin converting enzyme inhibitors and antioxidant peptides release during ripening of Mexican Cotija hard cheese. J Food Res. 2016;5(3):85–91. doi: 10.5539/jfr.v5n3p85. [DOI] [Google Scholar]
- 69.Gupta A, Mann B, Kumar R, Sangwan RB. Antioxidant activity of Cheddar cheeses at different stages of ripening. Inter J Dairy Tech. 2009;62:339–347. doi: 10.1111/j.1471-0307.2009.00509.x. [DOI] [Google Scholar]
- 70.Balakrishnan G, Agrawal R. Antioxidant activity and fatty acid profile of fermented milk prepared by Pediococcus pentosaceus. J Food Sci Technol. 2014;51:4138–4142. doi: 10.1007/s13197-012-0891-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Najgebauer-Lejko D, Grega T, Tabaszewsa M. Yoghurts with addition of selected vegetables: acidity, antioxidant properties and sensory quality. Acta Sci Pol Technol Aliment. 2014;13:35–42. doi: 10.17306/J.AFS.2014.1.3. [DOI] [PubMed] [Google Scholar]
- 72.Srivastava P, Prasad SGM, Ali MN, Prasad M. Analysis of antioxidant activity of herbal yoghurt prepared from different milk. The Pharma Innovation J. 2015;4:18–20. [Google Scholar]
- 73.Brignac B, Aryana KJ. Influence of various antioxidants on the characteristics of plain yogurt. Food Nutri Sci. 2012;3:1277–1280. [Google Scholar]
- 74.Matter AA, Mahmoud EAM, Zidan NS. Fruit flavored yoghurt: chemical, functional and rheological properties. Inter J Enviro Agric Res. 2016;2:57–66. [Google Scholar]
- 75.Lin MY, Yen CL. Reactive oxygen species and lipid peroxidation product-scavenging ability of yogurt organisms. J Dairy Sci. 1999;82:1629–1634. doi: 10.3168/jds.S0022-0302(99)75391-9. [DOI] [PubMed] [Google Scholar]
- 76.Songisepp E, Kals J, Kullisaar T, Mändar R, Hütt P, Zilmer M, Mikelsaar M. Evaluation of the functional efficacy of an antioxidative probiotic in healthy volunteers. Nutri J. 2005;4:22. doi: 10.1186/1475-2891-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Park YW, Nam MS. Bioactive peptides in milk and dairy products: a review. Korean J Food Sci Anim Resour. 2015;35:831–840. doi: 10.5851/kosfa.2015.35.6.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aleksandrova V, Chikov G, Velikova G, Dimitrov M, Dimov SG. In vivo antioxidant activity evaluation of peptides produced during the fermentation of yoghourt-like dairy products. Bulg J Agri Sci. 2013;19:97–100. [Google Scholar]
- 79.Alimi D, Abidi A, Sebai E, Rekik M, Maizels RM, Dhibi M, Akkari H. In vivo nematicidal potential of camel milk on Heligmosomoides polygyrus gastro-intestinal nematode of rodents. Institute of Parasitology, SAS, Košice. 2018;5:1–7. [DOI] [PMC free article] [PubMed]
- 80.Padghan PV, Mann B, Sharma R. In-vivo studies of antioxidant activity of fermented milk (Lassi) by Lactobacillus acidophilus and standard dahi culture. J Phar & Phytoche. 2018;7:25–30. [Google Scholar]
- 81.Huma N, Rafiq S, Sameen A, Pasha I, Khan MI. Antioxidant potential of buffalo and cow milk Cheddar cheeses to tackle human colon adenocarcinoma (Caco-2) cells. Asian-Australas J Anim Sci. 2018;31:287–292. doi: 10.5713/ajas.17.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Larsson SC, Andersson SO, Johansson JE, Wolk A. Cultured milk, yogurt, and dairy intake in relation to bladder cancer risk in a prospective study of Swedish women and men. Am J Clin Nutr. 2008;88:1083–1087. doi: 10.1093/ajcn/88.4.1083. [DOI] [PubMed] [Google Scholar]
- 83.Thirabunyanon M, Boonprasom P, Niamsup P. 2009. Probiotic potential of lactic acid bacteria isolated from fermented dairy milks on antiproliferation of colon cancer cells. Biotechnol Lett. 2009;31:571–576. doi: 10.1007/s10529-008-9902-3. [DOI] [PubMed] [Google Scholar]
- 84.Larsson SC, Bergkvist L, Wolk A. High-fat dairy food and conjugated linoleic acid intakes in relation to colorectal cancer incidence in the Swedish mammography cohort. Am J Clin Nutr. 2005;82:894–900. doi: 10.1093/ajcn/82.4.894. [DOI] [PubMed] [Google Scholar]
- 85.Rayes AAH, El-Naggar SMM, Mehanna NS. The effect of natural fermented milk in the protection of liver from cancer. Nutr Food Sci. 2008;38:578–592. doi: 10.1108/00346650810920196. [DOI] [Google Scholar]
- 86.Aune D, Lau R, Chan D, Vieira R, Greenwood D, Kampman E, Norat T. Dairy products and colorectal cancer risk: a systematic review and meta-analysis of cohort studies. Ann Oncol. 2012;23:37–45. doi: 10.1093/annonc/mdr269. [DOI] [PubMed] [Google Scholar]
- 87.Alvarez-León EE, Román-Vinas B, Serra-Majem L. Dairy products and health: a review of the epidemiological evidence. Br J Nutr. 2006;96:94–99. doi: 10.1079/BJN20061709. [DOI] [PubMed] [Google Scholar]
- 88.Cho E, Smith-Warner SA, Spiegelman D, Beeson WL, van den Brandt PA, Colditz GA, Folsom AR, Fraser GE, Freudenheim JL, Giovannucci E. Dairy foods, calcium, and colorectal cancer: a pooled analysis of 10 cohort studies. J Natl Cancer Inst. 2004;96:1015–1022. doi: 10.1093/jnci/djh185. [DOI] [PubMed] [Google Scholar]
- 89.Hjartåker A, Thoresen M, Engeset D, Lund E. Dairy consumption and calcium intake and risk of breast cancer in a prospective cohort: the Norwegian women and cancer study. Cancer Cause Control. 2010;21:1875–1885. doi: 10.1007/s10552-010-9615-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rodrigues L, Teixeira J, Schmitt F, Paulsson M, Månsson HL. Lactoferrin and cancer disease prevention. Crit Rev Food Sci. 2008;49:203–217. doi: 10.1080/10408390701856157. [DOI] [PubMed] [Google Scholar]
- 91.Tsuda H, Kozu T, Iinuma G, Ohashi Y, Saito Y, Saito D, Akasu T, Alexander DB, Futakuchi M, Fukamachi K, Xu J, Kakizoe T, Iigo M. Cancer prevention by bovine lactoferrin: from animal studies to human trial. Biometals. 2010;23:399–409. doi: 10.1007/s10534-010-9331-3. [DOI] [PubMed] [Google Scholar]
- 92.Tsuda H, Sekine K, Ki F, Iigo M. Cancer prevention by bovine lactoferrin and underlying mechanisms—a review of experimental and clinical studies. Biochem Cell Biol. 2002;80:131–136. doi: 10.1139/o01-239. [DOI] [PubMed] [Google Scholar]
- 93.Parodi P. A role for milk proteins and their peptides in cancer prevention. Curr Pharm Des. 2007;13:813–828. doi: 10.2174/138161207780363059. [DOI] [PubMed] [Google Scholar]
- 94.Mehra R, Marnila P, Korhonen M. Milk immunoglobulins for health promotion. Int Dairy J. 2006;16:1262–1272. doi: 10.1016/j.idairyj.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.O'Sullivan TA, Hafekost K, Mitrou F, Lawrence D. Food sources of saturated fat and the association with mortality: a meta-analysis. Am J Public Health. 2013;103:31–42. doi: 10.2105/AJPH.2013.301492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Qin LQ, Xu JY, Han SF, Zhang ZL, Zhao YY, Szeto IM. Dairy consumption and risk of cardiovascular disease: an updated meta-analysis of prospective cohort studies. Asia Pac J Clin Nutr. 2015;24:90–100. doi: 10.6133/apjcn.2015.24.1.09. [DOI] [PubMed] [Google Scholar]
- 97.Alexander DD, Bylsma LC, Vargas AJ, Cohen SS, Doucette A, Mohamed M, Irvin SR, Miller PE, Watson H, Fryzek JP. Dairy consumption and CVD: a systematic review and meta-analysis. Br J Nutr. 2016;115:737–750. doi: 10.1017/S0007114515005000. [DOI] [PubMed] [Google Scholar]
- 98.von Ruesten A, Feller S, Bergmann MM, Boeing H. Diet and risk of chronic diseases: results from the first 8 years of follow-up in the EPIC-Potsdam study. Eur J Clin Nutr. 2013;67:412–419. doi: 10.1038/ejcn.2013.7. [DOI] [PubMed] [Google Scholar]
- 99.Pal S, Ellis V. The chronic effects of whey proteins on blood pressure, vascular function, and inflammatory markers in overweight individuals. Obesity (Silver Spring) 2010;18:1354–1359. doi: 10.1038/oby.2009.397. [DOI] [PubMed] [Google Scholar]
- 100.Xu JY, Qin LQ, Wang PY, Li W, Chang C. Effect of milk tripeptides on blood pressure: a meta-analysis of randomized controlled trials. Nutr. 2008;24:933–940. doi: 10.1016/j.nut.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 101.Boelsma E, Kloek J. Lactotripeptides and antihypertensive effects: a critical review. Br J Nutr. 2009;101:776–786. doi: 10.1017/S0007114508137722. [DOI] [PubMed] [Google Scholar]
- 102.Tong X, Dong JY, Wu ZW, Li W, Qin LQ. Dairy consumption and risk of type 2 diabetes mellitus: a meta-analysis of cohort studies. Eur J Clin Nutr. 2011;65:1027–1031. doi: 10.1038/ejcn.2011.62. [DOI] [PubMed] [Google Scholar]
- 103.Aimutis WR. Bioactive properties of milk proteins with particular focus on anticariogenesis. J Nutr. 2004;134:989–995. doi: 10.1093/jn/134.4.989S. [DOI] [PubMed] [Google Scholar]
- 104.Borková M, Snášelová J. Possibilities of different animal milk detection in milk and dairy products – a review. Czech J Food Sci. 2005;2:41–50.
- 105.Rafiq S, Huma N, Pasha I, Sameen A, Mukhtar O, Khan MI. Chemical composition, nitrogen fractions and amino acids profile of milk from different animal species. Asian-Australias J Anim Sci. 2016;29:1022–1028. doi: 10.5713/ajas.15.0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Medhammar E, Wijesinha-Bettoni R, Stadlmayr B, Nilsson E, Charrondiere UR, Burlingame B. Composition of milk from minor dairy animals and buffalo breeds: a biodiversity perspective. J Sci Food Agric. 2012;92:445–474. doi: 10.1002/jsfa.4690. [DOI] [PubMed] [Google Scholar]
- 107.Barlowaska J, Litwinczuk Z, Krol J. Nutritional value and technological suitability of milk from various animal species used for dairy production. Compr Rev Food Sci Food Saf. 2011;10:291–302. doi: 10.1111/j.1541-4337.2011.00163.x. [DOI] [Google Scholar]
- 108.Ren DX, Zou CX, Lin B, Chen YL, Liang XW, Li JX. A comparison of milk protein, amino acid and fatty acid profiles of river buffalo and their F1 and F2 hybrids with swamp buffalo in China. Pakistan J Zool. 2015;47:1459–1465. [Google Scholar]
- 109.Helaly L, Rashed S, Bdaiwi L. A comparative study of oxidant and antioxidant levels between human milk with other type of ruminant milk. J Iraqi National Chem. 2013;49:86–99. [Google Scholar]
- 110.Nadeem M, Abdullah M, Hussain I. Inayat. Modification of fatty acid profile of cow milk by calcium salts of fatty acids and its use in ice cream. J Food Sci Technol. 2015;52:1061–1067. doi: 10.1007/s13197-013-1050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Abdel M. Present status of the world goat populations and their productivity. King Faisal University, Al-Ahsa. Saudi Arabia Lohmann information. 2010;42:160–195. [Google Scholar]
- 112.Talpur FN, Memon NN, Bhangar MI. Comparison of fatty acid and cholesterol content of Pakisani buffalo breeds. Pak J Analyt Environ Chem. 2007;8:15–20. [Google Scholar]
- 113.Ruiz de Gordoa JC, Bustamante M, Arranz J, Virto M, Barrón LJR, Beltrán de Heredia I, Amores G, Abilleira E, Nájera AI, Ruiz R, Albisu M, Pérez-Elortondo FJ, Mandaluniz N. Increase in water-soluble total antioxidant capacity of sheep's milk as a result of increased grazing time. Options Méditerranéennes. 2011;99:267–271.
- 114.Smet K, Raes K, De Block J, Herman L, Dewettinck K, Coudijzer K. A change in antioxidative capacity as a measure of onset to oxidation in pasteurized milk. Int Dairy J. 2008;18:520–530. doi: 10.1016/j.idairyj.2007.11.012. [DOI] [Google Scholar]
- 115.Najgebauer-Lejko D, Sady M. Estimation of the antioxidant activity of the commercially available fermented milks. Acta Sci Pol Technol Aliment. 2015;14:387–396. doi: 10.17306/J.AFS.2015.4.38. [DOI] [PubMed] [Google Scholar]
- 116.Antone U, Zagorska J, Sterna V, Jemeljanovs A, Berzins A, Ikauniece D. Effects of dairy cow diet supplementation with carrots on milk composition, concentration of cow blood serum carotenes, and butter oil fat-soluble antioxidative substances. Agron Res. 2015;13:879–891. [Google Scholar]
- 117.Sivakumar GM, Dhanalakshmi B, Nareshkumar C, Pugazhenthi TR. Antioxidant activity of herbal extract on khoa. Ind J Vet Anim Sci Res. 2014;43:445–451. [Google Scholar]
- 118.Yilmaz-Ersan L, Ozcan T, Akpinar-Bayizit A, Sahin S. The antioxidative capacity of kefir produced from goat milk. Int J Chem Eng Appl. 2016;7:22–6.
- 119.Roy T, Deepak D. Antioxidant properties of milk oligosaccharides from various ruminants. Int J Pharm Bio Sci. 2014;5:400–8.
- 120.Balakrishnan G, Agrawal R. 2012. Antioxidant activity and fatty acid profile of fermented milk prepared by Pediococcus pentosaceus. J Food Sci Technol. 2012;51:4138–4142. doi: 10.1007/s13197-012-0891-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vankudre M, Balpande A, Athale M. Comparative analysis of α-amylase inhibition and antioxidant activity of whey from cow and buffalo milk fermented with lactobacillus species. Biosci Biotech Res Comm. 2015;8:25–28. [Google Scholar]
- 122.Puppel K, Kuczyńska B, Nałęcz-Tarwacka T, Grodzki H. Influence of linseed variety on fatty acid profile in cow’s milk. J Sci Food Agric. 2017;93:2276–80. [DOI] [PubMed]
- 123.Hernandez-Ledesma B, Amigo L, Ramos M, Recio I. Release of angiotensin converting enzyme-inhibitory peptides by simulated gastrointestinal digestion of infant formulas. Int Dairy J. 2004;14:889–898. doi: 10.1016/j.idairyj.2004.02.011. [DOI] [Google Scholar]
- 124.Kurćubić VS, Vujić JM, Iličić MD, Vranić D, Vesković-Moračanin SM, Mašković PZ. Effect of plant extracts of Kitaibelia vitifolia on antioxidant activity, chemical characteristics, microbiological status and sensory properties of Pirotski Kachkaval cheese. Hemi indust. 2015;69:85–93.
- 125.Ullah R, M. Nadeem, M. Imran, M. Tayyab and R. Sajid. Antioxidant characteristics of ice cream supplemented with sugarcane (Saccharum officinarum L.) juice. Food Sci Biotec. 2015;24:1227–1232.
- 126.Nadeem M, Abdullah M, Javid MT. Evaluation of functional fat from interesterified blends of butter oil and Moringa oleifera oil. Pak J Nutri. 2012;11:725–729. [Google Scholar]
- 127.Nadeem M, Abdullah M, Hussain I, Javid A, Zahoor Y. Antioxidant potential of Moringa oleifera leaf extract for the stabilization of butter at refrigeration temperature. Czech J Food Sci. 2013;31:332–339. doi: 10.17221/366/2012-CJFS. [DOI] [Google Scholar]
- 128.Nadeem M, Mahmud A, Imran M. Khalique. Enhancement of the oxidative stability of whey butter through almond (Prunis dulcis) peel extract. J Food Process Preserv. 2015;39:591–598. doi: 10.1111/jfpp.12265. [DOI] [Google Scholar]
- 129.Nadeem M, Abdullah M, Hussain I. Improvement of the oxidative stability of butter oil by blending with Moringa oleifera oil. J Food Process Preserv. 2014;38:1491–1500. doi: 10.1111/jfpp.12108. [DOI] [Google Scholar]
- 130.Khan IT, Nadeem M, Imran M, Ajmal M, Ali S. Antioxidant activity, fatty acids characterization and oxidative stability of Gouda cheese fortified with mango (Mangifera indica L.) kernel fat. J Food Sci Technol. 2018;55(3):992–1002. doi: 10.1007/s13197-017-3012-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nadeem M, Ullah R, Ullah A. Improvement of the physical and oxidative stability characteristics of ice cream through interesterified Moringa oleifera oil. Pakistan J Sci Indust res. Ser B: biol sci. 2016;59:38–43. [Google Scholar]
- 132.Rashidinejad A, Birch EJ, Everett DW. Antioxidant activity and recovery of green tea catechins in full-fat cheese following gastrointestinal simulated digestion. J Food Comp Anal. 2016;48:13–24. doi: 10.1016/j.jfca.2016.02.004. [DOI] [Google Scholar]
- 133.Branciari R, Ranucci D, Trabalza-Marinucci M, Codini M, Orru M, Ortenzi R, Forte C, Ceccarini MR, Valiani A. Evaluation of the antioxidant properties and oxidative stability of pecorino cheese made from the raw milk of ewes fed Rosmarinus officinalis L. leaves. Int J Food Sci Tech. 2015;50:558–565. doi: 10.1111/ijfs.12712. [DOI] [Google Scholar]
- 134.Rashidinejad A, Birch EJ, Sun Waterhouse D, Everet DW. Effects of catechin on the phenolic content and antioxidant properties of low-fat cheese. Int J Food Sci Technol. 2013;48:2448–2455. doi: 10.1111/ijfs.12234. [DOI] [Google Scholar]
- 135.Mahajan D, Bhat ZF, Kumar S. Pine needles (Cedrus deodara (Roxb.) loud.) extract as a novel preservative in cheese. Food Packag Shelf Life. 2016;7:20–25. doi: 10.1016/j.fpsl.2016.01.001. [DOI] [Google Scholar]
- 136.Olmedo RH, Nepote V, Grosso NR. Preservation of sensory and chemical properties in flavoured cheese prepared with cream cheese base using oregano and rosemary essential oils. LWT-Food Sci Tech. 2013;53:409–417. doi: 10.1016/j.lwt.2013.04.007. [DOI] [Google Scholar]
- 137.Hala M, Ebtisam E, Sanaa I, Badran M, Marwa A, Said M. Manufacture of low fat UF-soft cheese supplemented with rosemary extract (as natural antioxidant) J Amr Sci. 2010;6:570–579. [Google Scholar]
- 138.Caleja C, Barros L, Antonio AL, Ciric A, Soković M, Oliveira MBP, Santos-Buelga C, Ferreira IC. Foeniculum vulgare mill as natural conservation enhancer and health promoter by incorporation in cottage cheese. J Funct Food. 2015;12:428–438. doi: 10.1016/j.jff.2014.12.016. [DOI] [Google Scholar]
- 139.Smith-Palmer A, Stewart J, Fyfe L. The potential application of plant essential oils as natural food preservatives in soft cheese. Food Microbiol. 2001;18:463–470. doi: 10.1006/fmic.2001.0415. [DOI] [Google Scholar]
- 140.Caleja C, Ribeiro A, Barros L, Barreira JC, Antonio AL, Oliveira MBP, Barreiro MF, Ferreira IC. Cottage cheeses functionalized with fennel and chamomile extracts: comparative performance between free and microencapsulated forms. Food Chem. 2016;199:720–726. doi: 10.1016/j.foodchem.2015.12.085. [DOI] [PubMed] [Google Scholar]