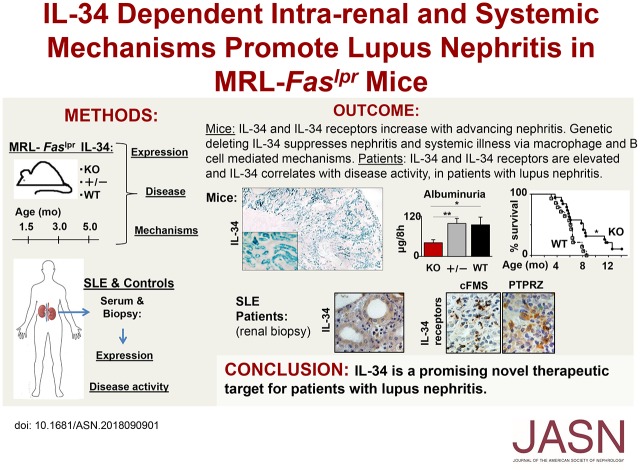
Significance Statement
Macrophages and autoantibodies play a central role in the pathology of lupus nephritis in patients with lupus and in the MRL-Faslpr mouse model. The authors demonstrate that IL-34 and its two receptors, cFMS and PTPRZ, are upregulated in the kidney with advancing nephritis in MRL-Faslpr mice. Genetically deleting IL-34 in these mice suppresses nephritis and the systemic illness via macrophage- and autoantibody-mediated mechanisms within and outside of the kidney. The authors also found that patients with lupus nephritis have elevated IL-34 in serum and urine; intrarenal and systemic expression of IL-34, cFMS, and PTPRZ similar to that displayed in MRL-Faslpr mice; and IL-34 expression that correlates with histopathologic index of disease activity. These findings suggest that IL-34 is a promising novel therapeutic target for patients with lupus nephritis.
Keywords: lupus nephritis, macrophages, IL-34, MRL-<i>Fas<sup>lpr</sup></i>, mice
Visual Abstract
Abstract
Background
In people with SLE and in the MRL-Faslpr lupus mouse model, macrophages and autoantibodies are central to lupus nephritis. IL-34 mediates macrophage survival and proliferation, is expressed by tubular epithelial cells (TECs), and binds to the cFMS receptor on macrophages and to a newly identified second receptor, PTPRZ.
Methods
To investigate whether IL-34–dependent intrarenal and systemic mechanisms promote lupus nephritis, we compared lupus nephritis and systemic illness in MRL-Faslpr mice expressing IL-34 and IL-34 knockout (KO) MRL-Faslpr mice. We also assessed expression of IL-34 and the cFMS and PTPRZ receptors in patients with lupus nephritis.
Results
Intrarenal IL-34 and its two receptors increase during lupus nephritis in MRL-Faslpr mice. In knockout mice lacking IL-34, nephritis and systemic illness are suppressed. IL-34 fosters intrarenal macrophage accumulation via monocyte proliferation in bone marrow (which increases circulating monocytes that are recruited by chemokines into the kidney) and via intrarenal macrophage proliferation. This accumulation leads to macrophage-mediated TEC apoptosis. We also found suppression of circulating autoantibodies and glomerular antibody deposits in the knockout mice. This is consistent with fewer activated and proliferating intrarenal and splenic B cells in mice lacking IL-34, and with our novel discovery that PTPRZ is expressed by macrophages, B and T cells. These findings appear translatable to human patients with lupus nephritis, whose expression of IL-34, cFMS, and PTPRZ is similar to that seen in the MRL-Faslpr lupus mouse model. Moreover, expression of IL-34 in TECs correlates with disease activity.
Conclusions
IL-34 is a promising novel therapeutic target for patients with lupus nephritis.
Nephritis is common in patients with lupus.1,2 Even with optimal therapy, up to 25% of these patients progress to ESRD.2–4 Moreover, a new therapeutic for lupus nephritis has not been approved in over five decades. Therefore, the need for a novel therapeutic target for lupus nephritis is pressing and timely.
Myeloid cells, most notably Mø, regulate the inflammatory response to kidney injury and repair. Activated Mø are broadly conceptually divided into M1 “destroyers” and M2 “healers.” Mø are integral in AKI that resolves in normal mice,5–7 but trigger CKD in lupus-prone mice.7 For example, after a transient kidney insult (ischemia), unlike normal mice, Mø in lupus-prone MRL-Faslpr mice are defective in shifting from M1 to M2 and hyperproliferate to Mø growth factors,8 thereby promoting an accumulation of Mø that escalate inflammation in the renal tubular-interstitium. Moreover, MRL-Faslpr Mø are defective in removing apoptotic cells, leading to the induction of autoantibodies that circulate, lodge in glomeruli, and thereby compromise glomerular filtration.7 Thus, the accumulation of Mø in lupus-prone mice is central to initiating and driving lupus nephritis.
IL-34 and colony stimulating factor 1 (CSF-1) are the principle Mø growth factors that regulate the accumulation of Mø in inflamed tissues. CSF-1 functions by engaging a high-affinity receptor tyrosine kinase encoded by the cFMS proto-oncogene, CSF-1R (cFMS, CD115).9,10 cFMS is principally expressed on mononuclear phagocytes, including progenitor cells,11 monoblasts, promonocytes, monocytes,12 Mø, dendritic cells,13 and some epithelial cells.14,15 The discovery that cFMS null mice were not viable, and that CSF-1–deficient mice survived, led to the identification of a second cFMS ligand, known as IL-34.16
IL-34 and CSF-1 have shared and differing properties. Both cytokines promote the growth and survival of monocytes and formation of Mø colonies from BM,17 but differ in spatiotemporal expression in some adult and developing tissues,17 as well as during disease. Although IL-34 and CSF-1 both signal through cFMS, a second IL-34 receptor, PTPRZ, was identified in brain.18 We elucidated a role for IL-34 using ischemia/reperfusion renal injury (I/R),19 an acute model of tubular injury. However, unlike I/R, lupus nephritis is a systemic illness involving cell- and antibody-mediated mechanisms driving chronic tubulointerstitial and glomerular disease. Given the dissimilarities between IL-34 and CSF-1, and I/R and lupus nephritis, it is unclear whether IL-34–mediated mechanisms lead to lupus nephritis.
To test the hypothesis that IL-34 is a potential therapeutic target for lupus nephritis, we compared IL-34 KO, wild-type (WT), and heterozygous (+/-) mice on the MRL-Faslpr background during age-related advancing lupus nephritis. The central questions are: (1) Do IL-34 and IL-34 receptors increase with progressive lupus nephritis in MRL-Faslpr mice? (2) Does deleting IL-34 suppress renal disease along with the systemic illness in MRL-Faslpr mice? (3) Is the accumulation of Mø in lupus nephritis a result of IL-34–mediated mechanisms within or outside of the kidney? (4) Are the IL-34 receptors, cFMS and PTPRZ, expressed by different cells within and outside of the kidney? And (5) are the IL-34–dependent findings in lupus-prone mice translatable to patients with lupus nephritis?
Methods
Mice
MRL-Faslpr, C57BL/6J, and B6.129S7-Rag1tm1Mom/J mice were purchased from The Jackson Laboratory (JAX), Bar Harbor, ME. The following mutant mice were backcrossed onto the MRL-Faslpr background for more than ten generations: (1) (Fms-eGFP) mice expressing eGFP under the control of Fms promoter and first intron, referred to as MacGreen, provided by David Hume (Roslin Institute, University of Edinburgh, Edinburgh, Scotland)20; (2) (TgN[Csf1-Z]Ers7/+) mice expressing lacZ under the control of Csf1 promoter and the first intron,21 referred to as TgZ mice, provided by Richard Stanley (Albert Einstein College of Medicine, New York, NY); and (3) B6.129S7-Rag1tm1Mom/J (JAX). IL-34 KO mice deleted of Il34 exons 3–5 and intercrossed with Il34 LacZ/+ offspring,22 provided by Marco Colonna (Washington University, St. Louis, MO), were backcrossed onto the MRL-Faslpr background (backcrossed eight generations, then using “speed congenics” [JAX] at generations 4 and 6 we selected breeders with maximal MRL-Faslpr genes). We bred and housed mice in the animal facility at Harvard Medical School, Boston, MA. Use of mice in this study was reviewed and approved by the Standing Committee on Animals in the Harvard Medical School (Protocol #2016N000161), in adherence to standards set in the Guide for the Care and Use of Laboratory Animals (eighth edition, The National Academies Press, revised 2011).
Serum and Renal Biopsy Specimens
Human renal biopsy specimens with the diagnosis of lupus nephritis (as defined by the ISN/RPS 2004 classification and histopathology activity and chronicity indices) and of healthy controls (patients with normal serum creatinine, no proteinuria but dysmorphic erythrocytes in the urine, and no evidence of kidney disease in the kidney biopsy specimen) were provided by the Department of Pathology, Friedrich-Alexander University Erlangen-Nuermberg, Germany.23 Renal pathologists, without access to the patient’s clinical data, evaluated these biopsy specimens. After informed consent, serum specimens were taken from patients who fulfilled the classification of SLE and diagnosis of lupus nephritis.24 Volunteers (age range, 18–70 years) were screened for health by exclusion of any prior kidney diseases, diabetes, hypertension, and autoimmune diseases. Healthy controls had normal serum creatinine levels and no proteinuria in spot urine (assessed by proteinuria and albuminuria strip). Freshly voided urine and drawn blood samples were collected, centrifuged, aliquoted, and stored at −80°C before analysis as previously described.23
Survival
We assessed survival in IL-34 KO and WT MRL-Faslpr mice dying with renal disease (proteinuria).
Gross Pathology
Lymphadenopathy (cervical, brachial, and inguinal) was graded as 0–4 (0=none; 1=small, one site; 2=moderate, two sites; 3=large, three sites; and 4=very large, four sites or more) and splenomegaly was analyzed by spleen weight upon euthanasia. Skin lesions were scored every 2 weeks from 1.5 months of age as detailed in the Supplemental Material.
β-Galactosidase
β-Galactosidase staining was performed, as previously described25 and detailed in the Supplemental Material.
Immunoblotting
We homogenized mouse kidney tissues and performed western blotting for PTPRZ, α-Tubulin, ERK2, and GAPDH as previously described.26 We performed SDS–PAGE (10% acryl-amide) and western blotting of freshly isolated (bead isolation) human cell populations, granulocytes (CD66b+; catalog no. 130–104–913), B cells (CD19+; catalog no. 130–050–301), T cells (CD3+; catalog no. 130–050–101), and monocytes (CD14+; catalog no. 130–050–201) from Miltenyi Biotech, as previously described.27 After isolation, PBL’s were stimulated for 4 hours with Poly I:C (catalog no. Tlrl-pic, 10 μg/ml; Invitrogen) and LPS (catalog no. L2143; Sigma-Aldrich) and CD14+ monocytes with CSF-1 (catalog no. 300–25, 10 ng/ml; Prepro Tec).
We stimulated monocytes to Mø for 7 days with CSF-1 (catalog no. 300–25, 10 ng/ml) and GM-CSF-1 (catalog no. 315–03, 10 ng/ml; Prepro Tec) in a humified chamber at 37°C.
Histopathology
We scored kidney pathology in periodic acid–Schiff–stained paraffin sections, as previously detailed.28 Paraffin sections of the submandibular salivary gland stained with H&E were evaluated as previously described29 and detailed in the Supplemental Material.
Renal Function
We analyzed albuminuria levels30 from urine collected over 8 hours by SDS–PAGE and BUN and serum creatinine levels as previously described28,31 and detailed in the Supplemental Material. Proteinuria was screened using Albustix.
Immunostaining
Mouse
To identify intrarenal cFMS-bearing cells, we analyzed eGFP+ cells per high-power field (HPF) using MacGreen MRL-Faslpr as previously described.20 As previously detailed, we analyzed the numbers of: glomerular IgG and C3 deposits,7,32 intrarenal proliferating Mø and B cells,19 Mø, CD4 T cells, B220 unique double-negative T cells,29 apoptotic tubular epithelial cells (TECs),7 and cells expressing PTPRZ.19
Human
Kidney formalin-fixed tissue sections were stained for the presence of IL-34, cFMS, PTPRZ, Mø (CD68), B cells (CD19), and T cells (CD3) in 20 randomly selected HPFs as previously detailed.23 Antibodies used for immunostaining are listed in Supplemental Table 2.
Immunofluorescence and immunoperoxidase methods are detailed in the Supplemental Material.
Generating BMMø
Mø were generated from mouse BM as previously detailed.7 Briefly BM cells were isolated by flushing the mouse bone with cold PBS, followed by culturing the BM cells in the presence of CSF-1 (10 ng/ml) in macrophage medium to separate adherent differentiated cells.
Cocultures: Hypoxic or TNF-α–Stimulated TECs with BMMø
We isolated and expanded TECs from IL-34 KO and WT MRL-Faslpr mouse kidneys, as previously reported.27 To isolate TECs, the renal cortex was diced into small pieces (<1 mm) and incubated in HBSS containing 0.2% collagenase II for 1 hour at 37°C in an oxygen-saturated atmosphere. The sample was then mashed through sieves of descending pore size (smallest<40 µm). The filtrated cells were resuspended and cultured in modified K1 medium containing 5% FBS (catalog no. 26–140–079; Gibco), 10 µg/ml EGF, 10 mM HEPES, 0.5 mg/ml PGE2, 180 µg/ml hydrocortisone, 100 U/ml penicillin, and 100 µg/ml streptomycin.
We cocultured TECs with BMMø for 48 hours under hypoxic conditions (1% O2) with 5% CO2-balanced nitrogen at 37°C in a hypoxic chamber. The number of apoptotic TECs was analyzed by FACS gating on CD45− annexin-V+ cells. Cell supernatants from hypoxic or TNF-α–stimulated TECs were collected and analyzed for IL-34 and CSF-1 expression by ELISA. To neutralize IL-34 and CSF-1 activity in the supernatants of TNF-α–stimulated TECs (WT), we added anti–IL-34 antibody (10 μg/ml, catalog no. AF5195; R&D Systems) and anti–CSF-1 antibody (2 μg/ml, 552513; BD Biosciences). We incubated TECs with TNF-α (25 ng/ml for 18 hours) before analyzing TEC apoptosis using the Toxilight assay.
ELISA
To quantify intrarenal IL-34 and CSF-1 levels in mice we used mouse IL-34 ELISA kit (R&D Systems), another IL-34 ELISA (detailed in the Supplemental Material), and CSF-1 ELISA, as previously detailed,33 and the human IL-34 and CSF-1 DuoSet ELISA kits (R&D Systems). We measured serum total IgG and ds-DNA antibody levels by ELISA as detailed in the Supplemental Material.
Monocytes, T, and B Cell Purification (Mouse)
Monocytes (catalog no. 130–100–629; Miltenyi) were isolated from BM. CD3+ T cells (catalog no. 8802–6840–74; MagniSort), CD4+ T cells (catalog no. 130–104–454; Miltenyi), and B cells (catalog no. 130–090–862; Miltenyi) were isolated from 3-month MRL-Faslpr spleen suspensions according to manufacturer instructions.
qPCR
qPCR was performed as previously described.29 We detected CSF-1, IL-34, cFMS, PTPRZ, and GAPDH using QuantiTect Primer Assays (QIAGEN) or using primers purchased from Invitrogen and Integrated DNA Technologies. The data were analyzed by the ΔΔ-CT method. Primers are listed in Supplemental Table 3. Further details are given in the Supplemental Material.
BrdU Incorporation
We injected mice (ip) with BrdU (2 mg/mouse; EMD Millipore) 3 hours before euthanasia. BrdU+ cells were analyzed with an anti-BrdU antibody by flow cytometry.
FACS
We prepared and stained single-cell suspensions from kidneys, RBC-lysed BM, and blood cells for intracellular and extracellular antigens as previously described.30 Briefly, after removing the capsule, kidneys were digested in collagenase IV for 1 hour at 37°C, mashed through a 40-µm sieve, and washed with PBS. Cells were collected by centrifugation. RBCs were lysed using ACK lysing buffer (BioSource International, Camarillo, CA) and the remaining cells were washed in PBS. FACS buffer (PBS, 5% BSA) was used to wash the cells and dilute antibodies. Antibodies used for FACS are listed in Supplemental Table 4.
Statistical Analyses
Data represent the mean±SEM prepared using Graph-Pad Prism software, version 5.0, or Excel. We used the Mann–Whitney U test to evaluate P values, the Cox proportional hazards model with a single variable run for the IL-34 group using PROC PHREG of SAS for survival, and Spearman correlation coefficient for correlation.
Human Study Approval
Specimens for human study were taken after informed consent and their use was approved by the Standing Committee for Clinical Studies of the Johannes-Gutenberg University, Mainz, Germany, in adherence to the Declaration of Helsinki; specimens were analyzed retrospectively. The protocol number is 837.467.13 (9152-F).
Results
IL-34 and CSF-1 Are Expressed by TECs and Increase in the Kidney with Advancing Lupus Nephritis in MRL-Faslpr Mice
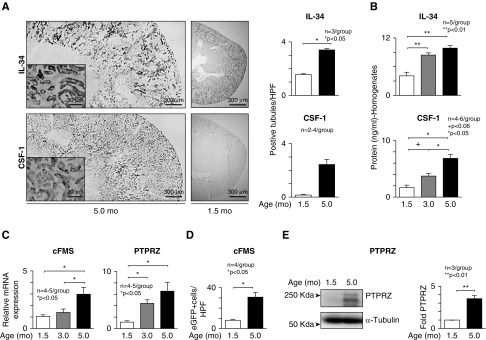
Previously, we established that CSF-1 is expressed by TECs and that this expression rises with advancing lupus nephritis.14,34 To determine whether IL-34 and CSF-1, which share the cFMS receptor, are similarly expressed, we compared the locale and magnitude of these ligands in the kidney using IL-34lacZ+/−MRL-Faslpr and TgZ MRL-Faslpr (CSF-1) reporter mice during the age-related progression of renal pathology: at 1.5 (before), 3.0 (mild), and 5.0 (severe) months of age. IL-34, similar to CSF-1, is robustly expressed in TECs in MRL-Faslpr mice with severe lupus nephritis (protein Figure 1A). Expression of both intrarenal IL-34 and CSF-1 rises (transcripts Supplemental Figure 1A, protein Figure 1B) and is higher in the medulla than the cortex (Supplemental Figure 1B) during the age-related progression of lupus nephritis. Moreover, IL-34 is elevated in the serum of MRL-Faslpr mice compared with non–lupus-prone C57BL/6 (B6) mice (Supplemental Figure 1C). Thus, the expression of IL-34 and CSF-1 similarly increases in TECs with advancing lupus nephritis in MRL-Faslpr mice.
Figure 1.
IL-34 ligand and receptors rise during advancing lupus nephritis in MRL-Faslpr mice. (A) IL-34 and CSF-1 expression in kidney of MRL-Faslpr mice identified using IL-34LacZ/+ MRL-Faslpr mice and TgZ MRL-Faslpr mice stained for β-galactosidase activity (X-gal) (original maginification, ×4; inset, ×40). (B) Intrarenal IL-34 and CSF-1 in kidney homogenates analyzed by ELISA. (C) cFMS and PTPRZ transcripts in kidney analyzed by qPCR. Values are normalized to GAPDH transcript and expressed as relative ratio. (D) Intrarenal cFMS expression identified using MacGreen MRL-Faslpr mice (eGFP under control of the cFMS). (E) Intrarenal PTPRZ protein levels analyzed by western blotting. Graph of PTPRZ relative to α-Tubulin. Data are mean±SEM. Mann–Whitney U test was used for statistical analysis.
IL-34 Receptors Both Rise in the Kidney with Advancing Lupus Nephritis in MRL-Faslpr Mice
Because IL-34 engages with two cognate receptors, cFMS and PTPRZ,18,35 we determined whether both receptors increase during the progression of lupus nephritis in MRL-Faslpr mice. cFMS and PTPRZ transcripts rise with advancing lupus nephritis (Figure 1C). By tracking a cFMS reporter gene (eGFP [enhanced green fluorescent protein] identifies cFMS in MacGreen MRL-Faslpr mice), we found that intrarenal cFMS expression increases during lupus nephritis (Figure 1D). Similarly, intrarenal PTPRZ protein increases during lupus nephritis (western blot, Figure 1E). Collectively, along with IL-34, intrarenal cFMS and PTPRZ expression rises with advancing lupus nephritis.
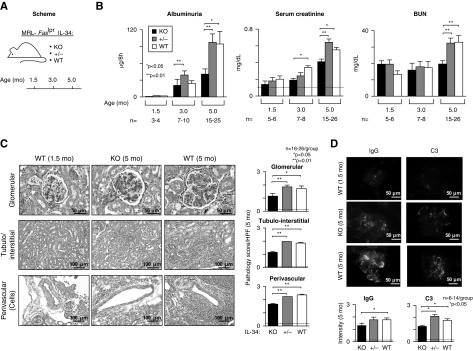
IL-34 Promotes Pathology and Loss of Renal Function in MRL-Faslpr Mice with Lupus Nephritis
To determine whether IL-34 drives lupus nephritis, we probed for the age-related loss of renal function and magnitude of renal pathology in IL-34 KO MRL-Faslpr mice compared with WT and +/- mice (Figure 2A). Albuminuria, serum creatinine, and BUN levels are suppressed in IL-34 MRL-Faslpr KO compared with WT and +/- mice (Figure 2B). Consistent with improved renal function, glomerular, tubular-interstitial, and peri-vascular pathology (Figure 2C) and IgG and C3 within glomerular peripheral loops (Figure 2D) decrease in the absence of IL-34. Of note, IL-34 levels in the serum (Supplemental Figure 1D) and kidney (data not shown) were similar in IL-34 WT and +/- MRL-Faslpr mice. Thus, IL-34 promotes renal pathology and the loss of renal function in MRL-Faslpr mice.
Figure 2.
Lupus nephritis is suppressed in IL-34 KO MRL-Faslpr mice. The following were compared: IL-34 KO, WT, and +/- MRL-Faslpr mice. (A) Schematic of experimental protocol. (B) Renal function including albuminuria, serum creatinine, and BUN levels. Dotted line in serum creatinine indicates the mean for B6 mice. (C) Representative photos of kidney stained with periodic acid–Schiff (original magnification, tubulo-interstitial and perivascular, ×20; glomeruli ×40). Quantification of glomerular, tubulo-interstitial, and perivascular pathology (5 months of age). Dotted lines in (C and D) indicate mean at 1.5 months of age (n=3). (D) Photos and graph of IgG and C3 deposition in MRL-Faslpr glomeruli at 5 months of age (original magnification, ×40). Data are mean±SEM. Mann–Whitney U test was used for statistical analysis.
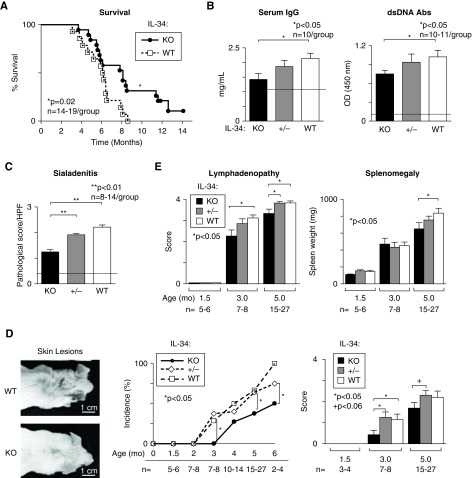
IL-34 Shortens Survival and Promotes Systemic Illness in MRL-Faslpr Mice
Because IL-34 promotes lupus nephritis, we determined whether IL-34 contributed to mortality in MRL-Faslpr WT mice. IL-34 KO MRL-Faslpr mice survive longer than WT mice (P<0.02, Figure 3A). These mice died with renal disease because proteinuria was substantially elevated before death (not shown). Thus, IL-34 shortens survival in MRL-Faslpr mice.
Figure 3.
Systemic illness is suppressed in IL-34 KO MRL-Faslpr mice. The following were compared: IL-34 KO, WT, and +/- MRL-Faslpr mice. (A) Survival. (B) Serum IgG and dsDNA antibody levels evaluated by ELISA. (C) Submandibular pathology (5 months of age). (D) Representative photos, incidence (left panel) and magnitude (right panel), of skin lesions. (E) Lymphadenopathy and splenomegaly. Dotted lines in (B and C) represent MRL-Faslpr mice at 1.5 months of age (n=3). Data are mean±SEM. Mann–Whitney U test was used for statistical analysis.
Multiorgan systemic disease is characteristic of lupus in patients and MRL-Faslpr mice.36–38 We detected suppression of the hallmarks of systemic illness in IL-34 KO MRL-Faslpr mice, including: circulating IgG and double-stranded DNA (dsDNA) (Figure 3B), sialadenitis (Figure 3C and Supplemental Figure 2), skin lesions (Figure 3D), lymphadenopathy, and splenomegaly (Figure 3E). Collectively, deleting IL-34 suppresses renal disease and systemic illness in MRL-Faslpr mice.
IL-34 Fosters Intrarenal Mø, T, and B Cells in MRL-Faslpr Mice by Inducing Chemokines in Lupus Nephritis
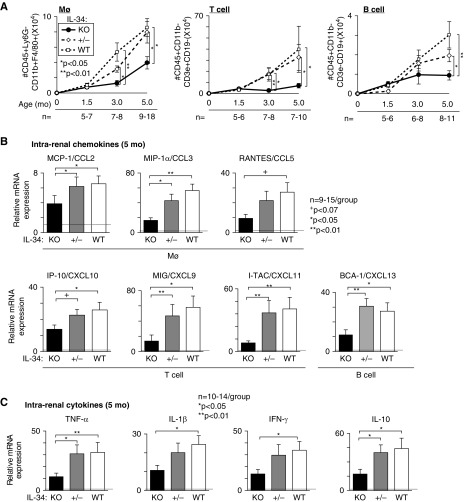
Because IL-34 promotes Mø proliferation, we probed for the accumulation of intrarenal Mø in IL-34 KO and WT MRL-Faslpr mice. We detected a suppression in Mø, T, and B cells in IL-34 KO compared with WT and +/- MRL-Faslpr kidneys during advancing lupus nephritis (FACS Figure 4A, immunostaining Supplemental Figure 3). Moreover, intrarenal B220+ cells which include B cells and the unique CD4−CD8− T cells that increase in inflamed tissues in MRL-Faslpr mice are decreased in the absence of IL-34 (Supplemental Figure 3). Note, there are few neutrophils among the intrarenal neutrophils (CD45+CD11b+Ly6G+F4/80−) in MRL-Faslpr mice with lupus nephritis and we did not detect a difference in these neutrophils with and without IL-34 (data not shown). Thus, deleting IL-34 suppresses intrarenal Mø, T, and B cells in lupus nephritis.
Figure 4.
Mø, T, and B cell accumulation and their respective chemokines and cytokines are suppressed in MRL-Faslpr mice. The following were compared: IL-34 KO, WT, and +/- MRL-Faslpr mice. (A) Age-related numbers of Mø, T, and B cells analyzed by FACS. (B) Intrarenal chemokine transcripts known to recruit Mø, T, and B cells. (C) Intrarenal cytokines transcripts. Mice in (B and C) are 5 months of age and dotted lines indicate mean levels at 1.5 months of age (n=4). Values are normalized to GAPDH or β-actin (for BCA-1/CXCL13) transcripts and expressed as relative ratio. Data are mean±SEM. Mann–Whitney U test was used for statistical analysis.
Does IL-34 induce intrarenal chemokines that attract Mø, T, and B cells into the kidney and thereby promote lupus nephritis? Intrarenal chemokines, known to recruit Mø, T, and B cells, are suppressed in IL-34 KO compared with WT and +/- MRL-Faslpr mice (Figure 4B). On the basis of our prior studies in I/R, IL-34 does not directly recruit BM-derived cells into the inflamed kidney.19 Moreover, multiple intrarenal cytokines that lead to chemokine induction are amplified in WT compared with IL-34 KO MRL-Faslpr mice (Figure 4C). Collectively, IL-34 induces intrarenal cytokines known to increase chemokines that recruit Mø, T, and B cells into the kidney, and, thereby, drive inflammation in MRL-Faslpr mice.
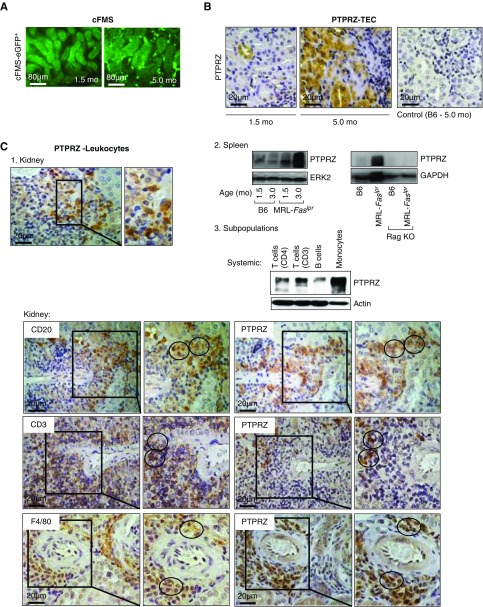
PTPRZ Is Expressed by TECs, T and B cells, and Monocytes/Mø during Lupus Nephritis
To probe for the function of IL-34 in lupus nephritis, it is imperative to identify the cell types expressing IL-34 receptors. It is well established that cFMS is expressed predominantly by monocytes/Mø (Figure 5A). We now report that PTPRZ is expressed by renal intrinsic and infiltrating cells. Similar to our data in I/R in nonautoimmune mice,19 PTPRZ protein is expressed by TECs in mice with lupus nephritis (Figure 5B). However, we discovered the novel finding that renal infiltrating leukocytes express PTPRZ (Figure 5C). To identify the leukocytes that express PTPRZ we first probed for expression in spleens from B6 and MRL-Faslpr WT and RAG KO (lack mature T and B cells) mice using western blotting (Figure 5C). Enlarged spleens from MRL-Faslpr mice with lupus (3.0 months of age) expressed more PTPRZ compared with age-matched B6 and MRL-Faslpr mice before disease (1.5 months) (Figure 5C, left graph). By comparison, because RAG KO MRL-Faslpr mice (3 months) do not express PTPRZ, this suggests that PTPRZ is expressed by T and/or B cells (Figure 5C, right graph). Probing further, we show for the first time that PTPRZ is expressed by splenic T and B cells and BM monocytes/Mø in mice with lupus (Figure 5C, upper panel). Moreover, using sequential immunostained sections, we identified PTPRZ-expressing T and B cells and Mø in the renal interstitium (Figure 5C, lower panel). Collectively, PTPRZ is robustly expressed in renal intrinsic TECs, T and B cells, and monocytes/Mø both systemically and in the kidney in MRL-Faslpr mice with lupus nephritis.
Figure 5.
PTPRZ is expressed by TECs and hematopoietic kidney infiltrates. (A) Intrarenal cFMS expression identified using MacGreen MRL-Faslpr mice (eGFP under control of the cFMS). Representative photos of cFMS+ cells (original magnification, ×40). (B) Intrarenal PTPRZ protein in MRL-Faslpr mice at 1.5 and 5.0 months of age compared with control (B6 mice, 5 months of age) using immunostaining; representative photos (original magnification, ×40). (C) 1. Intrarenal PTPRZ expression in infiltrates of MRL-Faslpr mice (5 months of age) using immunostaining (original magnification, ×40; insert, ×60). 2. Western blots of PTPRZ expression in spleens from: Left panel: B6 and MRL-Faslpr WT mice 1.5 and 3.0 months of age. Right panel: RAG KO and WT B6 and MRL-Faslpr mice 3.0 months of age. Both ERK2 and GAPDH were used to ensure equal protein loading, but only one displayed. 3. Upper panel: Western blot of T and B cells isolated from spleens and BM monocytes from MRL-Faslpr mice at 4 months of age (n=3 pooled samples). Representative of five experiments. Lower panel: Kidney sections (MRL-Faslpr, 3 months of age) sequentially stained for the presence of B (CD20) and T (CD3) cells, Mø (F4/80), and PTPRZ. Representative photos (original magnification, ×40; boxed enlargement, ×60). Circles encompass the same area stained for the presence of PTPRZ and leukocytic markers.
IL-34 Drives an Expansion of Mø within the Kidney and BM during Lupus Nephritis
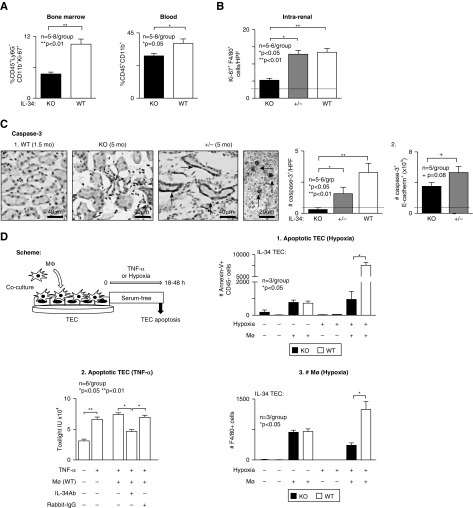
Because IL-34 promotes Mø proliferation, we tested the hypothesis that IL-34–dependent Mø proliferation outside of and within the kidney leads to lupus nephritis. BM is a rich source of Mø that traffic to the kidney during inflammation. We find that the number of monocytes proliferating in the IL-34 KO MRL-Faslpr BM is suppressed compared with WT (Figure 6A). Moreover, there are fewer circulating myeloid cells in IL-34 KO MRL-Faslpr mice. Thus, IL-34 increases BM monocytes and elevates circulating monocytes that are available for chemokine recruitment into the kidney. To test the hypothesis that IL-34 promotes intrarenal Mø proliferation, we analyzed the number of intrarenal proliferating (Ki67+) Mø (F4/80) by immunostaining. We detected fewer intrarenal proliferating Mø in the IL-34 KO compared with WT and +/- MRL-Faslpr mice with lupus nephritis (5 months of age) (Figure 6B). Because IL-34 and CSF-1 both promote Mø proliferation, we determined whether CSF-1 compensated for the absence of IL-34 by probing for expression of CSF-1 in IL-34 KO, WT, and +/- MRL-Faslpr kidneys (5 months of age) and primary cultured TECs (Supplemental Figure 4). We detected similar expression of CSF-1 transcripts and secreted protein in the kidney (Supplemental Figure 4A) and stimulated TECs (Supplemental Figure 4B) in IL-34 KO, WT, and +/- MRL-Faslpr mice. Thus, CSF-1 does not compensate for the absence of IL-34, a finding consistent with the deletion of IL-34 suppressing lupus nephritis in MRL-Faslpr mice. Collectively, nonredundant IL-34–dependent mechanisms within and outside of the kidney contribute to lupus nephritis.
Figure 6.
Intrarenal Mø proliferation and Mø-mediated TEC apoptosis are suppressed in IL-34 KO MRL-Faslpr mice. (A) Proliferation of myeloid progenitors in BM (left panel) and myeloid cells in peripheral blood (right panel) of IL-34 KO and WT MRL-Faslpr mice at 5 months of age analyzed by FACS. (B) Intrarenal Mø proliferation quantified by dual staining for Ki-67 and F4/80 in IL-34 KO, WT, and +/-MRL-Faslpr mice (5 months of age). (C) Intrarenal apoptosis identified using caspase-3 in IL-34 KO, WT, and +/- MRL-Faslpr mice at 5 months of age. Arrows, caspase-3+ cells. 1. Representative photos of tubules and interstitial infiltrates. Graph, analysis of ten HPFs/sample. 2. TEC apoptosis. Quantitation of caspase-3+ e-cadherin+ cell number analyzed by FACS. Dotted line in (B and C) indicates mean at 1.5 months of age. (D) Scheme for in vitro analysis of TECs cocultured with Mø under hypoxic conditions (1% O2 for 48 hours) or with TNF-α (25 ng/ml for 18 hours). 1. Hypoxia: Apoptotic TECs (Annexin V+ CD45−) analyzed by FACS. 2. TNF-α (25 ng/ml for 18 hours): Apoptotic TECs analyzed by toxilight assay. 3. Hypoxia: Mø (F4/80+) number after coculture with TECs analyzed by FACS. Data are mean±SEM. Mann–Whitney U test was used for statistical analysis.
IL-34 Promotes Mø-Mediated TEC Apoptosis in MRL-Faslpr Mice
To test the hypothesis that IL-34 promotes TEC apoptosis during lupus nephritis, we did in situ and in vitro experiments. We detected fewer apoptotic cells (caspase-3+) in the IL-34 KO compared with WT and ± MRL-Faslpr mice (5 months of age) by immunostaining (Figure 6C). These apoptotic cells were TECs along with cells in the interstitium (Figure 6C) and glomeruli. This was confirmed by enumerating intrarenal caspase-3+E-cadherin+ cells by FACS analysis (Figure 6C). Note, these studies are limited, because we did not analyze nonapoptotic, caspase-independent forms of cell death. To determine whether IL-34 promotes Mø-mediated TEC apoptosis in MRL-Faslpr kidneys, we exposed BMMø cocultured with either IL-34 KO or WT TECs to hypoxia (see Figure 6D for scheme). There are fewer hypoxia-induced apoptotic TECs in IL-34 KO MRL-Faslpr compared with WT TECs (Figure 6D). This was verified by substituting TNF-α in place of hypoxia and neutralizing IL-34 with anti–IL-34 antibody instead of using IL-34 KO mice (Figure 6D). Note, there are fewer Mø when cocultured with IL-34 KO MRL-Faslpr compared with WT TECs under hypoxic conditions (Figure 6D). Moreover, as in MRL-Faslpr kidneys with lupus nephritis (Supplemental Figure 5A), BMMø skew toward a cyto-destructive M1 phenotype when cocultured with hypoxic TECs (Supplemental Figure 5B). However, IL-34 does not skew the Mø phenotype. The numbers of M1 and M2 Mø in the presence or absence of IL-34 broadly do not differ in MRL-Faslpr kidney (Supplemental Figure 5C). Collectively, these results suggest that IL-34 does not polarize, but, rather, increases the number of activated Mø in the inflamed kidney that, in turn, mediate TEC apoptosis.
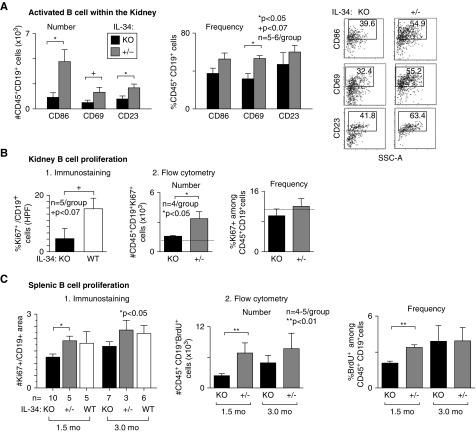
IL-34 Promotes Intrarenal B Cell Activation and Proliferation during Lupus Nephritis
Naïve B cells encountering antigen in the presence of CD4+ T cells become activated, proliferate, and differentiate into effector B cells that produce antibody. Because fewer antibodies are in the circulation and lodge in glomeruli of IL-34 KO MRL-Faslpr mice, we tested the hypothesis that IL-34 increases intrarenal B cell activation and proliferation during lupus nephritis. Using B cell activation markers and gating on CD45+ and CD19+ cells, we detected suppression of intrarenal activated B cell number and frequency in IL-34 KO compared with +/- MRL-Faslpr mice with lupus nephritis (Figure 7A). This could be the result of the increased infiltration or enhanced B cell activation within the ± MRL-Faslpr kidney. Moreover, intrarenal B cell proliferation is suppressed in IL-34 KO compared with ± or WT MRL-Faslpr mice (immunostaining, Figure 7B and Supplemental Figure 6, and FACS, Figure 7B). Because plasma cells develop in secondary lymphoid organs, we probed for the effect of IL-34 on splenic B cells. Similar to the kidney, we detected fewer proliferating B cells in the spleens of MRL-Faslpr IL-34 KO mice compared with WT (Figure 7C). Note, this change began before overt pathology, a finding consistent with prior reports.39 Collectively, these findings suggest that PTPRZ may have a yet-to-be-appreciated effect on B cell biology.
Figure 7.
Intrarenal activated B cells and proliferating B cells in the kidney and spleen are reduced in IL-34 KO MRL-Faslpr mice. (A) Activated B cell number and frequency in IL-34 KO and WT MRL-Faslpr mice (5 months of age) analyzed by FACS. (B) Proliferating B cells in IL-34 KO and WT MRL-Faslpr kidney (5 months of age): 1. Dual cryo-sections stained for CD19 and Ki67 (5 HPF/sample) and 2. gated on CD45+CD19+ Ki67+ cells in whole kidney; FACS analysis. Dotted lines indicate mean levels at 1.5 months of age (n=2). (C) Proliferating B cells in IL-34 KO and WT MRL-Faslpr spleens (1.5 and 3.0 months of age) quantified using: 1. Cryo-sections immunostained for CD19 and Ki67 (5 HPF/sample) and 2. FACS by gating on CD45+CD19+BrdU+ cells. Data are mean±SEM. Mann–Whitney U test was used for statistical analysis.
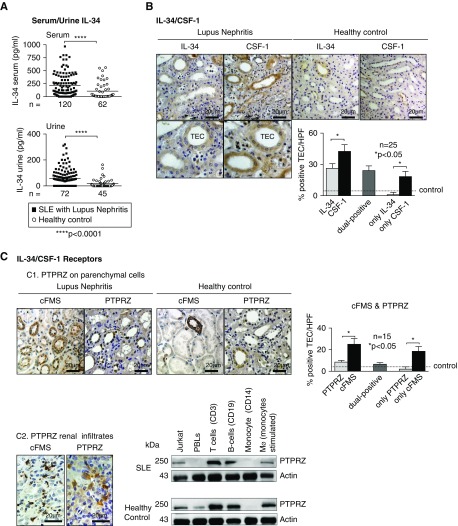
Elevated Serum and Urine IL-34 in Patients with Lupus Nephritis and Similar Cell Types in Mice and Humans with Lupus Nephritis Express IL-34
To determine whether our findings in mice are translational to human lupus, we tested the hypothesis that IL-34 is upregulated in patients with lupus nephritis. We detected elevated IL-34 levels in the serum and urine of patients with lupus nephritis compared with healthy volunteers (Figure 8A, and demographics, Supplemental Table 1). Using immunostaining in kidney sequential sections from patients with lupus nephritis versus healthy controls, we detected both IL-34 and CSF-1 in TECs, but not necessarily in the same TECs (Figure 8B, and demographics, Supplemental Table 1). IL-34 expression correlates with the number of intrarenal Mø and T cells in patients with type II–IV lupus nephritis (Supplemental Figure 7A) and histopathology disease activity (Supplemental Figure 7B). However, IL-34 does not correlate with chronicity (Supplemental Figure 7B), a finding consistent with the progressive loss of TECs, the main source of IL-34. Thus, IL-34 is a potential therapeutic target for lupus nephritis during escalating renal inflammation, but not end-stage disease.
Figure 8.
Serum and urine IL-34 are elevated in patients with SLE and IL-34 receptors are expressed on distinct cell types in patients with lupus nephritis. (A) Serum and urine IL-34 in patients with lupus nephritis or healthy controls. (B) IL-34/CSF-1 expression in renal TEC of lupus nephritis biopsy specimens and healthy controls (kidney biopsy specimen without inflammatory kidney disease); representative photos (original magnification [×40], bottom part shows enlarged extracted part of the original image) and graph of expression. (C) 1. cFMS and PTPRZ expression in TECs of lupus nephritis biopsy specimens and healthy controls; representative photos (original magnification, ×40) and graph of expression. Data are mean±SEM. Mann–Whitney U test was used for statistical analysis. 2. Left panel: Intrarenal infiltrates expressing cFMS and PTPRZ by immunostaining. Right panel: PTPRZ expression in leukocyte subpopulations isolated from whole blood (T and B cells, monocytes/Mø). Jurkat cells served as positive control (n=3 SLE, n=4 healthy control). Volunteers (healthy controls) were screened for exclusion of prior kidney and autoimmune diseases, diabetes, and hypertension.
To determine the level of expression and locale of IL-34 receptors in patients with lupus nephritis, we stained sequential sections for cFMS and PTPRZ. Although cFMS and PTPRZ are both detected in TECs, cFMS is more robustly expressed (Figure 8C). Note, these receptors are often, but not always, expressed by the same TECs (Figure 8C). Are IL-34 receptors expressed by renal infiltrating cells? Although it is well established that cFMS is expressed by Mø, we now report the novel finding that PTPRZ is expressed by leukocytes. We detect PTPRZ protein in renal infiltrates (immunostaining, Figure 8C, left) and T and B cells and monocytes/Mø (cultured and stimulated) isolated from the buffy coat of patients with lupus nephritis and healthy controls (western blotting, Figure 8C, right, and demographics, Supplemental Table 1). Thus, IL-34, cFMS, and PTPRZ are similarly expressed in mice and patients with lupus nephritis. Collectively, our data suggest that IL-34 is a promising therapeutic target for lupus nephritis.
Discussion
We now report that targeting IL-34 is a potential therapeutic target for lupus nephritis. Using a reliable and reproducible model of lupus, MRL-Faslpr mice, we determined that: (1) intrarenal IL-34, and IL-34 receptors, cFMS and PTPRZ, increase during the progression of lupus nephritis; (2) IL-34 is largely expressed by TECs, cFMS is mainly expressed by Mø, whereas PTPRZ is expressed by systemic and intrarenal T and B cells and monocytes/Mø; (3) lupus nephritis, along with the systemic illness, is suppressed in IL-34 KO mice, and thereby extends survival; (4) fewer intrarenal Mø, T, and B cells accumulate in IL-34 KO mice, a finding congruent with a reduction in chemokines known to recruit Mø, T, and B cells; (5) intrarenal Mø accumulation is dependent on IL-34 fostering an expansion of monocytes in the BM and the subsequent rise in circulating monocytes that are recruited to the kidney; (6) within the kidney, TEC expression of IL-34 induces Mø proliferation which amplifies Mø-mediated TEC apoptosis; and (7) there are fewer proliferating intrarenal and splenic B cells in IL-34 KO mice, a finding consistent with reduced antibodies in the circulation and glomeruli. Moreover, our IL-34–dependent findings in mice and patients with lupus nephritis are translatable. Collectively, we suggest that IL-34 is a potential therapeutic target for patients with lupus nephritis and other systemic forms of this illness.
Intrarenal and circulating IL-34 promotes lupus. We show that systemically depleted IL-34 suppresses pathology in multiple tissues, including kidney, skin, and salivary glands, targeted for autoimmune destruction in MRL-Faslpr mice. Because IL-34 is expressed within tissues, for example by TECs in the kidney, keratinocytes in the mouse skin,40 and ductal epithelial cells in human salivary glands,41 and IL-34 fosters intrarenal proliferation of destructive Mø,19 eliminating IL-34 within tissues likely attenuates lupus. On the other hand, IL-34 in the circulation alone may contribute to renal disease. This concept is on the basis of the present and previous findings indicating that IL-34 promotes an increase in monocytes in the BM that are recruited to the inflamed kidney and subsequently mediate injury.19 Moreover, we show that serum IL-34 is elevated in MRL-Faslpr mice with lupus nephritis and that serum IL-34 is upregulated and tracks with histopathology disease activity in lupus patients with nephritis. Importantly, others previously reported that serum IL-34 is upregulated and tracks with disease activity in patients with lupus.42,43 Taken together, we suggest that blocking IL-34 within the kidney and/or the circulation will suppress lupus nephritis.
IL-34 induces intrarenal chemokines, known to recruit Mø, T, and B cells. Our prior data suggest that IL-34 indirectly recruits Mø into the inflamed kidney.19 In support of this, IL-34 upregulates cytokines such as IL-6,44 IL-1β, and TNF-α45 that induce MCP-1. Alternatively, because IL-34 stimulates the expression of chemokines, such as MCP-1, IP-10, and IL-8, in whole human blood,46 it is possible that IL-34 directly stimulates leukocytes in blood to express chemokines that within the kidney foster further leukocyte recruitment. This is compatible with IL-34–mediated events outside of the kidney contributing to intrarenal inflammation. Irrespective of the exact mechanism, eliminating IL-34 reduces intrarenal chemokines, leukocytes, and inflammation and, in turn, suppresses lupus nephritis in MRL-Faslpr mice.
Intriguingly, IL-34 induces antibodies that lodge in glomeruli and subsequently compromise renal function in MRL-Faslpr mice. We find that IgG/C3 and serum anti-dsDNA antibody levels are suppressed in IL-34 KO MRL-Faslpr mice. IL-34 may indirectly induce autoantibodies in lupus-prone mice by several mechanisms. IL-34, produced by follicular dendritic cells in germinal centers, may expand a monocyte subset in the spleen that stimulates activated B cell proliferation.47 Alternatively, IL-34 may foster Mø accumulation and thereby promote TEC apoptosis. Moreover, the defective MRL-Faslpr Mø clearance of apoptotic TECs (not published) may release neoantigens and thereby induce autoantibodies that deposit in glomeruli.7 With the novel discovery of PTPRZ expression on T and B cells along with monocytes/Mø, IL-34 may directly stimulate autoantibodies by binding to PTPRZ on B cells. Adding complexity to a potential role for PTPRZ in lupus nephritis, PTPRZ engages a multitude of other ligands,48 including heparin binding growth factors midkine and pleiotrophin,49 the cell surface protein contactin,50 and the extracellular matrix protein tenacin-R.51 Therefore, even though PTPRZ is expressed by T and B cells and monocytes/Mø in lupus nephritis, ligands other than IL-34 may activate these leukocytes. The biologic significance of the newly discovered PTPRZ expression on T and B cells and monocytes/Mø has yet to be elucidated and undoubtedly will seed future in-depth analyses in lupus nephritis and a broad range of other diseases.
Our studies indicate that IL-34 drives disease, at least in the kidney. However, one group reported that IL-34 inhibited allogenic rejection in a rat cardiac transplant model and showed that IL-34–stimulated human Mø expand Tregs.52 This finding is not consistent with a rise in serum IL-34 during kidney transplant rejection,19 nor a rise in serum IL-34 in inflammatory bowel disease53 in patients. On the other hand, CSF-1 promotes repair in an acute model of renal injury19 and drives CKD in MRL-Faslpr mice.14 Thus, the actions of IL-34 may differ depending on the species, tissue, model, mechanism, microenvironment etc. Clearly, unraveling the possible diverse actions of IL-34 in various diseases requires further clarification.
Why target IL-34, rather than CSF-1, or IL-34 along with CSF-1? IL-34 is likely a safer therapeutic target for lupus nephritis than CSF-1. This is on the basis of several findings. Mice deficient in CSF-1 (osteopetrotic, op/op mice) are fraught with abnormalities, including infertility, altered estrogen and androgen regulation, low body weight, abnormal lipid metabolism, deafness, blindness, skeletal abnormalities, osteopetrosis, reduced B cell lymphopoiesis, and dermal and synovial defects.54,55 Moreover, cFMS KO mice do not survive.56 Because IL-34 and CSF-1 are the sole ligands for this receptor, blocking both cytokines undoubtedly will be problematic. By comparison, IL-34 KO mice do not express overt phenotypic abnormalities, other than a decline in Langerhans cells and microglia that occurs during development.22 However, even if IL-34 reduced Langerhans cells and microglia after development, blocking IL-34 in the adult is not a concern for several reasons: (1) cutaneous (discoid) lupus is suppressed in IL-34 KO MRL-Faslpr mice, thus eliminating some Langerhans cells is not apparently harmful; and (2) antibodies are unlikely to cross the blood-brain barrier. Thus, IL-34 is an appealing therapeutic target for lupus nephritis and by extension other inflamed tissues in patients with lupus.
Disclosures
V.R.K. is funded by Pfizer Centers for Therapeutic Innovation and has an equity interest in Biogen-Idec, a company with research and development interests in lupus.
Supplementary Material
Acknowledgments
We wish to acknowledge Anu Khanna and Lin Chen for proofreading the manuscript.
This work was supported by the Lupus Research Alliance (V.R.K.), Pfizer Centers for Therapeutic Innovation (V.R.K.), the National Council on Science and Technology (CONACyT, Fellowship No. 265757) (H.M.G.-S.), and the Deutsche Forschungsgemeinschaft (ME3194/2-1) (J.W.-M.).
V.R.K. designed the study; Y.W., H.M.G.-S., J.W.-M., Y.I., A.K.A., and M.M. carried out the experiments; Y.W., H.M.G.-S., J.W.-M., Y.I., A.K.A., and M.M. analyzed the data; Y.W., H.M.G.-S., J.W.-M., Y.I., A.K.A., and V.R.K. made the figures; V.R.K. drafted the manuscript; and H.M.G.-S., J.W.-M., and V.R.K. edited/revised the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
SUPPLEMENTAL MATERIAL
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018090901/-/DCSupplemental.
Supplemental Figure 1. IL-34 and CSF-1 are expressed with advancing lupus nephritis in MRL-Faslpr mice.
Supplemental Figure 2. Submandibular inflammation (sialadenitis) is suppressed in IL-34 KO MRL-Faslpr mice.
Supplemental Figure 3. Mø, T, and B cell accumulation is suppressed in IL-34 KO MRL-Faslpr mice.
Supplemental Figure 4. CSF-1 does not compensate for the absence of IL-34 in the kidney of IL-34 KO MRL-Faslpr mice.
Supplemental Figure 5. Mø skew toward cyto-destructive M1 phenotype during lupus nephritis in MRL-Faslpr mice and in BM Mø cocultured with hypoxic TECs.
Supplemental Figure 6. Proliferating B cells are suppressed in IL-34 KO MRL-Faslpr mice with lupus nephritis.
Supplemental Figure 7. IL-34 correlates with Mø and T cells and histopathology disease activity in type II–IV lupus nephritis.
Supplement Table 1. Study cohort demographic and clinical characteristics.
Supplement Table 2. Antibodies used for immunostaining.
Supplement Table 3. qPCR primers used to detect mRNAs.
Supplement Table 4. Antibodies used for FACS.
References
- 1.Klippel JH: Systemic lupus erythematosus: Demographics, prognosis, and outcome. J Rheumatol Suppl 48: 67–71, 1997 [PubMed] [Google Scholar]
- 2.Petri M: Long-term outcomes in lupus. Am J Manag Care 7[Suppl]: S480–S485, 2001 [PubMed] [Google Scholar]
- 3.Hill GS, Delahousse M, Nochy D, Thervet E, Vrtovsnik F, Rémy P, et al.: Outcome of relapse in lupus nephritis: Roles of reversal of renal fibrosis and response of inflammation to therapy. Kidney Int 61: 2176–2186, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Cortés-Hernández J, Ordi-Ros J, Labrador M, Segarra A, Tovar JL, Balada E, et al.: Predictors of poor renal outcome in patients with lupus nephritis treated with combined pulses of cyclophosphamide and methylprednisolone. Lupus 12: 287–296, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, et al.: Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol 22: 317–326, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lech M, Gröbmayr R, Ryu M, Lorenz G, Hartter I, Mulay SR, et al.: Macrophage phenotype controls long-term AKI outcomes--kidney regeneration versus atrophy. J Am Soc Nephrol 25: 292–304, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwata Y, Boström EA, Menke J, Rabacal WA, Morel L, Wada T, et al.: Aberrant macrophages mediate defective kidney repair that triggers nephritis in lupus-susceptible mice. J Immunol 188: 4568–4580, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore KJ, Naito T, Martin C, Kelley VR: Enhanced response of macrophages to CSF-1 in autoimmune mice: A gene transfer strategy. J Immunol 157: 433–440, 1996 [PubMed] [Google Scholar]
- 9.Guilbert LJ, Stanley ER: Specific interaction of murine colony-stimulating factor with mononuclear phagocytic cells. J Cell Biol 85: 153–159, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sherr CJ, Rettenmier CW, Sacca R, Roussel MF, Look AT, Stanley ER: The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell 41: 665–676, 1985 [DOI] [PubMed] [Google Scholar]
- 11.Tushinski RJ, Oliver IT, Guilbert LJ, Tynan PW, Warner JR, Stanley ER: Survival of mononuclear phagocytes depends on a lineage-specific growth factor that the differentiated cells selectively destroy. Cell 28: 71–81, 1982 [DOI] [PubMed] [Google Scholar]
- 12.Byrne PV, Guilbert LJ, Stanley ER: Distribution of cells bearing receptors for a colony-stimulating factor (CSF-1) in murine tissues. J Cell Biol 91: 848–853, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiktor-Jedrzejczak W, Bartocci A, Ferrante AW Jr, Ahmed-Ansari A, Sell KW, Pollard JW, et al.: Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc Natl Acad Sci U S A 87: 4828–4832, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menke J, Iwata Y, Rabacal WA, Basu R, Yeung YG, Humphreys BD, et al.: CSF-1 signals directly to renal tubular epithelial cells to mediate repair in mice. J Clin Invest 119: 2330–2342, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menke J, Kriegsmann J, Schimanski CC, Schwartz MM, Schwarting A, Kelley VR: Autocrine CSF-1 and CSF-1 receptor coexpression promotes renal cell carcinoma growth. Cancer Res 72: 187–200, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin H, Lee E, Hestir K, Leo C, Huang M, Bosch E, et al.: Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science 320: 807–811, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Wei S, Nandi S, Chitu V, Yeung YG, Yu W, Huang M, et al.: Functional overlap but differential expression of CSF-1 and IL-34 in their CSF-1 receptor-mediated regulation of myeloid cells. J Leukoc Biol 88: 495–505, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nandi S, Cioce M, Yeung YG, Nieves E, Tesfa L, Lin H, et al.: Receptor-type protein-tyrosine phosphatase ζ is a functional receptor for interleukin-34. J Biol Chem 288: 21972–21986, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baek JH, Zeng R, Weinmann-Menke J, Valerius MT, Wada Y, Ajay AK, et al.: IL-34 mediates acute kidney injury and worsens subsequent chronic kidney disease. J Clin Invest 125: 3198–3214, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sasmono RT, Oceandy D, Pollard JW, Tong W, Pavli P, Wainwright BJ, et al.: A macrophage colony-stimulating factor receptor-green fluorescent protein transgene is expressed throughout the mononuclear phagocyte system of the mouse. Blood 101: 1155–1163, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Ryan GR, Dai XM, Dominguez MG, Tong W, Chuan F, Chisholm O, et al.: Rescue of the colony-stimulating factor 1 (CSF-1)-nullizygous mouse (Csf1(op)/Csf1(op)) phenotype with a CSF-1 transgene and identification of sites of local CSF-1 synthesis. Blood 98: 74–84, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Szretter KJ, Vermi W, Gilfillan S, Rossini C, Cella M, et al.: IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat Immunol 13: 753–760, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menke J, Amann K, Cavagna L, Blettner M, Weinmann A, Schwarting A, et al.: Colony-stimulating factor-1: A potential biomarker for lupus nephritis. J Am Soc Nephrol 26: 379–389, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hochberg MC: Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 40: 1725, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, et al.: Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell 3: 169–181, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ajay AK, Kim TM, Ramirez-Gonzalez V, Park PJ, Frank DA, Vaidya VS: A bioinformatics approach identifies signal transducer and activator of transcription-3 and checkpoint kinase 1 as upstream regulators of kidney injury molecule-1 after kidney injury. J Am Soc Nephrol 25: 105–118, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faust J, Menke J, Kriegsmann J, Kelley VR, Mayet WJ, Galle PR, et al.: Correlation of renal tubular epithelial cell-derived interleukin-18 up-regulation with disease activity in MRL-Faslpr mice with autoimmune lupus nephritis. Arthritis Rheum 46: 3083–3095, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Lenda DM, Stanley ER, Kelley VR: Negative role of colony-stimulating factor-1 in macrophage, T cell, and B cell mediated autoimmune disease in MRL-Fas(lpr) mice. J Immunol 173: 4744–4754, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Kikawada E, Lenda DM, Kelley VR: IL-12 deficiency in MRL-Fas(lpr) mice delays nephritis and intrarenal IFN-gamma expression, and diminishes systemic pathology. J Immunol 170: 3915–3925, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Schaldecker T, Kim S, Tarabanis C, Tian D, Hakroush S, Castonguay P, et al.: Inhibition of the TRPC5 ion channel protects the kidney filter. J Clin Invest 123: 5298–5309, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menke J, Lucas JA, Zeller GC, Keir ME, Huang XR, Tsuboi N, et al.: Programmed death 1 ligand (PD-L) 1 and PD-L2 limit autoimmune kidney disease: Distinct roles. J Immunol 179: 7466–7477, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Menke J, Zeller GC, Kikawada E, Means TK, Huang XR, Lan HY, et al.: CXCL9, but not CXCL10, promotes CXCR3-dependent immune-mediated kidney disease. J Am Soc Nephrol 19: 1177–1189, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menke J, Rabacal WA, Byrne KT, Iwata Y, Schwartz MM, Stanley ER, et al.: Circulating CSF-1 promotes monocyte and macrophage phenotypes that enhance lupus nephritis. J Am Soc Nephrol 20: 2581–2592, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Menke J, Bork T, Kutska B, Byrne KT, Blanfeld M, Relle M, et al.: Targeting transcription factor Stat4 uncovers a role for interleukin-18 in the pathogenesis of severe lupus nephritis in mice. Kidney Int 79: 452–463, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanchez-Niño MD, Sanz AB, Ortiz A: Chronicity following ischaemia-reperfusion injury depends on tubular-macrophage crosstalk involving two tubular cell-derived CSF-1R activators: CSF-1 and IL-34. Nephrol Dial Transplant 31: 1409–1416, 2016 [DOI] [PubMed] [Google Scholar]
- 36.Elman SA, Joyce C, Nyberg F, Furukawa F, Goodfield M, Hasegawa M, et al.: Development of classification criteria for discoid lupus erythematosus: Results of a Delphi exercise. J Am Acad Dermatol 77: 261–267, 2017 [DOI] [PubMed] [Google Scholar]
- 37.Maloney KC, Ferguson TS, Stewart HD, Myers AA, De Ceulaer K: Clinical and immunological characteristics of 150 systemic lupus erythematosus patients in Jamaica: A comparative analysis. Lupus 26: 1448–1456, 2017 [DOI] [PubMed] [Google Scholar]
- 38.Lucas JA, Menke J, Rabacal WA, Schoen FJ, Sharpe AH, Kelley VR: Programmed death ligand 1 regulates a critical checkpoint for autoimmune myocarditis and pneumonitis in MRL mice. J Immunol 181: 2513–2521, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Theofilopoulos AN, Dixon FJ: Etiopathogenesis of murine SLE. Immunol Rev 55: 179–216, 1981 [DOI] [PubMed] [Google Scholar]
- 40.Greter M, Lelios I, Pelczar P, Hoeffel G, Price J, Leboeuf M, et al.: Stroma-derived interleukin-34 controls the development and maintenance of Langerhans cells and the maintenance of microglia. Immunity 37: 1050–1060, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ciccia F, Alessandro R, Rodolico V, Guggino G, Raimondo S, Guarnotta C, et al.: IL-34 is overexpressed in the inflamed salivary glands of patients with Sjogren’s syndrome and is associated with the local expansion of pro-inflammatory CD14(bright)CD16+ monocytes. Rheumatology (Oxford) 52: 1009–1017, 2013 [DOI] [PubMed] [Google Scholar]
- 42.Wang H, Cao J, Lai X: Serum interleukin-34 levels are elevated in patients with systemic lupus erythematosus. Molecules 22: E35, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xie HH, Shen H, Zhang L, Cui MY, Xia LP, Lu J: Elevated serum interleukin-34 level in patients with systemic lupus erythematosus is associated with disease activity. Sci Rep 8: 3462, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Biswas P, Delfanti F, Bernasconi S, Mengozzi M, Cota M, Polentarutti N, et al.: Interleukin-6 induces monocyte chemotactic protein-1 in peripheral blood mononuclear cells and in the U937 cell line. Blood 91: 258–265, 1998 [PubMed] [Google Scholar]
- 45.Parry GC, Martin T, Felts KA, Cobb RR: IL-1beta-induced monocyte chemoattractant protein-1 gene expression in endothelial cells is blocked by proteasome inhibitors. Arterioscler Thromb Vasc Biol 18: 934–940, 1998 [DOI] [PubMed] [Google Scholar]
- 46.Eda H, Zhang J, Keith RH, Michener M, Beidler DR, Monahan JB: Macrophage-colony stimulating factor and interleukin-34 induce chemokines in human whole blood. Cytokine 52: 215–220, 2010 [DOI] [PubMed] [Google Scholar]
- 47.Yamane F, Nishikawa Y, Matsui K, Asakura M, Iwasaki E, Watanabe K, et al.: CSF-1 receptor-mediated differentiation of a new type of monocytic cell with B cell-stimulating activity: Its selective dependence on IL-34. J Leukoc Biol 95: 19–31, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peles E, Schlessinger J, Grumet M: Multi-ligand interactions with receptor-like protein tyrosine phosphatase beta: Implications for intercellular signaling. Trends Biochem Sci 23: 121–124, 1998 [DOI] [PubMed] [Google Scholar]
- 49.Li YS, Milner PG, Chauhan AK, Watson MA, Hoffman RM, Kodner CM, et al.: Cloning and expression of a developmentally regulated protein that induces mitogenic and neurite outgrowth activity. Science 250: 1690–1694, 1990 [DOI] [PubMed] [Google Scholar]
- 50.Peles E, Nativ M, Campbell PL, Sakurai T, Martinez R, Lev S, et al.: The carbonic anhydrase domain of receptor tyrosine phosphatase beta is a functional ligand for the axonal cell recognition molecule contactin. Cell 82: 251–260, 1995 [DOI] [PubMed] [Google Scholar]
- 51.Milev P, Chiba A, Häring M, Rauvala H, Schachner M, Ranscht B, et al.: High affinity binding and overlapping localization of neurocan and phosphacan/protein-tyrosine phosphatase-zeta/beta with tenascin-R, amphoterin, and the heparin-binding growth-associated molecule. J Biol Chem 273: 6998–7005, 1998 [DOI] [PubMed] [Google Scholar]
- 52.Bézie S, Picarda E, Ossart J, Tesson L, Usal C, Renaudin K, et al.: IL-34 is a Treg-specific cytokine and mediates transplant tolerance. J Clin Invest 125: 3952–3964, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Franzè E, Monteleone I, Cupi ML, Mancia P, Caprioli F, Marafini I, et al.: Interleukin-34 sustains inflammatory pathways in the gut. Clin Sci (Lond) 129: 271–280, 2015 [DOI] [PubMed] [Google Scholar]
- 54.Dai XM, Zong XH, Sylvestre V, Stanley ER: Incomplete restoration of colony-stimulating factor 1 (CSF-1) function in CSF-1-deficient Csf1op/Csf1op mice by transgenic expression of cell surface CSF-1. Blood 103: 1114–1123, 2004 [DOI] [PubMed] [Google Scholar]
- 55.Alikhan MA, Jones CV, Williams TM, Beckhouse AG, Fletcher AL, Kett MM, et al.: Colony-stimulating factor-1 promotes kidney growth and repair via alteration of macrophage responses. Am J Pathol 179: 1243–1256, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dai XM, Ryan GR, Hapel AJ, Dominguez MG, Russell RG, Kapp S, et al.: Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood 99: 111–120, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.