Significance Statement
Considerable evidence indicates that basolateral inwardly rectifying potassium channel Kir4.1/Kir5.1 is essential for membrane transport in the distal convoluted tubule (DCT), and that dietary sodium and potassium are important in regulating activity of the thiazide-sensitive Na-Cl cotransporter (NCC). In mouse studies, the authors found that stimulation of NCC induced by sodium restriction was associated with increasing Kir4.1/Kir5.1 activity in the DCT and membrane hyperpolarization; NCC inhibition induced by high sodium intake was associated with decreasing Kir4.1/Kir5.1 activity in the DCT and membrane depolarization. In kidney-specific Kir4.1 knockout mice, the effect of dietary sodium on NCC activity was largely abolished, as were its effects on DCT membrane conductance and potential. The findings indicate that Kir4.1/Kir5.1 is essential for mediating dietary sodium intake–induced modulation of NCC function.
Keywords: potassium channels, epithelial sodium transport, hypertension
Visual Abstract
Abstract
Background
Dietary sodium intake regulates the thiazide-sensitive Na-Cl cotransporter (NCC) in the distal convoluted tubule (DCT). Whether the basolateral, inwardly rectifying potassium channel Kir4.1/Kir5.1 (a heterotetramer of Kir4.1/Kir5.1) in the DCT is essential for mediating the effect of dietary sodium intake on NCC activity is unknown.
Methods
We used electrophysiology, renal clearance techniques, and immunoblotting to examine effects of Kir4.1/Kir5.1 in the DCT and NCC in wild-type and kidney-specific Kir4.1 knockout mice.
Results
Low sodium intake stimulated basolateral Kir4.1/Kir5.1 activity, increased basolateral K+ conductance, and hyperpolarized the membrane. Conversely, high sodium intake inhibited the potassium channel, decreased basolateral K+ currents, and depolarized the membrane. Low sodium intake increased total and phosphorylated NCC expression and augmented hydrochlorothiazide-induced natriuresis; high sodium intake had opposite effects. Thus, elevated NCC activity induced by low sodium intake was associated with upregulation of Kir4.1/Kir5.1 activity in the DCT, whereas inhibition of NCC activity by high sodium intake was associated with diminished Kir4.1/Kir5.1 activity. In contrast, dietary sodium intake did not affect NCC activity in knockout mice. Further, Kir4.1 deletion not only abolished basolateral K+ conductance and depolarized the DCT membrane, but also abrogated the stimulating effects induced by low sodium intake on basolateral K+ conductance and hyperpolarization. Finally, dietary sodium intake did not alter urinary potassium excretion rate in hypokalemic knockout and wild-type mice.
Conclusions
Stimulation of Kir4.1/Kir5.1 by low intake of dietary sodium is essential for NCC upregulation, and inhibition of Kir4.1/Kir5.1 induced by high sodium intake is a key step for downregulation of NCC.
The distal convoluted tubule (DCT) is responsible for the reabsorption of 5% of filtered Na+ load and is the target for thiazide diuretics.1–4 The reabsorption of NaCl in the DCT is a two-step process (Supplemental Figure 1A): Na+ and Cl− enter the cells across the apical membrane through the Na-Cl cotransporter (NCC) and Na+ is then pumped out of the cell through the basolateral Na-K-ATPase, whereas Cl− exits the cell along its electrochemical gradient by basolateral Cl− channels (ClC-kb) or the KCl cotransporter.5–7 In the late part of the DCT (DCT2), Na+ enters the cell across the apical membrane through both NCC and ENaC.8–10 Although NCC is expressed in the apical membrane, the inwardly rectifying potassium channel (Kir) 4.1 is expressed in the basolateral membrane of the DCT.11,12 Moreover, Kir4.1 is the only K+ channel providing the basolateral K+ conductance in the DCT.13 A large body of evidence indicates that the basolateral Kir4.1 in the DCT is essential for the membrane transport in the DCT.13–16 Loss-of-function mutations of Kir4.1 in the kidney mainly impair the membrane transport in the DCT.17–19 The role of Kir4.1 in mediating membrane transport in the DCT is recapitulated in Ks-Kir4.1 knockout mice with mild salt wasting, metabolic alkalosis, and hypokalemia due to the inhibition of NCC activity.15
A large body of evidence has convincingly demonstrated that dietary Na+ and K+ intake play an important role in the regulation of NCC activity: low potassium or low sodium (LS) intake stimulates whereas high potassium or high sodium (HS) intake inhibits NCC activity.14–16,20–23 Furthermore, our previous experiments have demonstrated that Kir4.1 plays a key role in mediating the effect of dietary K+ intake on NCC because the deletion of Kir4.1 completely abolished the effect of dietary K+ intake on NCC.16 Thus, it raises the possibility whether Kir4.1 in the DCT is also involved in mediating the effect of dietary Na+ intake on the thiazide-sensitive NCC. Therefore, the aim of this study is to test the hypothesis that LS intake–induced stimulation of NCC requires the activation of basolateral Kir4.1 in the DCT, whereas HS intake–induced inhibition of NCC requires the suppression of Kir4.1 activity.
Methods
Animals
All animal studies were approved by the Institutional Animal Care and Use Committees of New York Medical College. Mice expressing Pax8-rtTA and tet-on LC-1 transgene were crossed with Kcnj10flox/flox mice to generate inducible kidney-specific Kir4.1 knockout mice (Ks-Kir4.1 KO) (detailed information is provided in Supplemental Material). Kcnj10 deletion was carried out in 8- to 10-week-old male and/or female mice homozygous for floxed Kcnj10 gene and hemizygous for Pax8-rtTA/LC-1 transgene by providing doxycycline (5 mg/ml, 5% sucrose) in the drinking water for 2 weeks. This was followed by at least two additional weeks without doxycycline treatment before performing experiments, and mice were kept under a 12-hour light/dark cycle, and were fed with normal rodent diet and plain drinking water. Littermate mice (male and female Kcnj10flox/flox) of the same age and genetic background drinking 5% sucrose were used as wild-type controls (WT). After 2 weeks on a normal sodium diet (NS; 0.4%), the mice were then fed with LS (TD90228, 0.01%–0.02%) or HS (TD92034, 4.0%) diet for an additional 7 days before experiments. The method of mouse genotyping and DCT preparation has been described previously and is included in the Supplemental Material.15
Patch-Clamp Experiment
A Narishige electrode puller (Narishige, Tokyo, Japan) was used to manufacture the patch-clamp pipettes from borosilicate glass (1.7-mm OD). The resistance of pipette was 5 MΩ (for single-channel recording) or 2 MΩ (for whole-cell recording) when it was filled with solution containing 140 mM KCl, 1.8 mM MgCl2, and 10 mM HEPES (titrated with KOH to pH 7.4). For the measurement of K+ currents (IK) reversal potential, we used either high Cl− pipette solution (140 mM KCl) or low Cl− pipette solution containing 125 mM K+-gluconate, 15 mM KCl, 2 mM MgATP, 1 mM EGTA, and 10 mM HEPES (titrated with KOH to pH 7.4). For single-channel recording, the bath solution contained 140 mM NaCl, 5 mM KCl, 2 mM MgCl2, 1.8 mM CaCl2, and 10 mM HEPES (pH 7.4), and the pipette was filled with 140 mM KCl solution. For measurement of ENaC activity, the tip of the pipette was filled with pipette solution containing 125 mM K+-gluconate, 15 mM KCl, 2 mM MgATP, 1 mM EGTA, and 10 mM HEPES (pH 7.4). The pipette was then back-filled with amphotericin B (20 μg/0.1 ml) containing the pipette solution. The bath solution contained 130 mM Na+-gluconate, 10 mM NaCl, 5 mM KCl, 2 mM CaCl2, 2 mM MgCl2, and 10 mM HEPES (pH 7.4) for Na+ currents. Details for the single-channel and whole-cell recordings have been described previously and also in the Supplemental Material.15
Renal Clearance and Urinary/Plasma Na+/K+ Analysis
The detailed method for renal clearance is described in the Supplemental Material. After surgery, the mice were perfused with isotonic saline intravenously for 4 hours (0.25–0.3 ml/h and total 1.0–1.2 ml) to maintain hemodynamics. Urine collections started 1 hour after infusion of 0.3 ml saline and a total of six collections (every 30 minutes) were performed (two for controls and four for experiments).
Material and Statistical Analyses
HCTZ and doxycycline were purchased from Sigma-Aldrich (St. Louis, MO) and LS or HS diets were obtained from Harlan Laboratories (Madison, WI). All diets contained the same amount K+ and nutrition components. Data were analyzed using t test for comparisons between two groups, or one-way/two-way ANOVA followed by Holm–Sidak test for comparisons among more than two groups. P values <0.05 were considered statistically significant. Data are presented as mean±SEM.
Results
Dietary Na+ Intake Regulates Kir4.1/Kir5.1
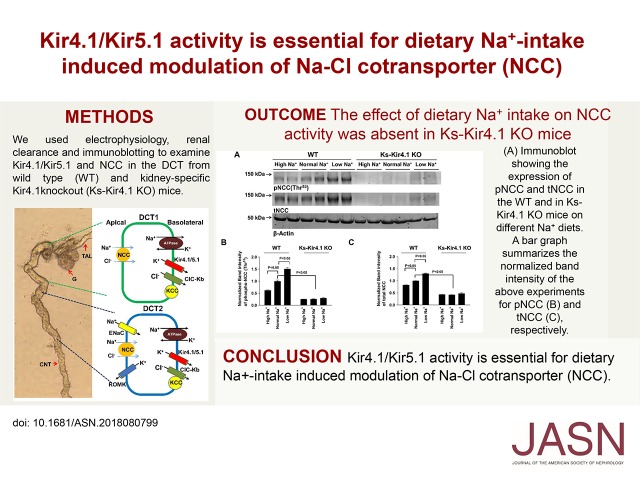
We first used the single-channel patch-clamp technique to examine the effect of dietary Na+ intake on the basolateral 40 pS K+ channel (comprising Kir4.1 and Kir5.1) in the DCT of both male and female Kcnj10flox/flox mice (referred as WT in the following section). Kir5.1 in the heterotetramer is solely responsible for providing K+ permeability and the basolateral K+ conductance in the DCT,13 the 40 pS K+ channel activity is exactly equal to the Kir4.1 activity. Figure 1A is a set of single-channel recordings showing the K+ channel activity in the DCT of the WT mice on LS, NS, and HS diets for 7 days, respectively. We have found the 40 pS K+ channels in 65 patches of 89 experiments (73%) from mice on the LS diet and in 31 patches of 58 experiments (53%) from mice on the NS diet (Figure 1B). The probability of finding K+ channels further decreased to 41% (22 patches with K+ channels of 54 experiments) from mice on the HS diet. Furthermore, LS intake significantly increased mean channel activity (defined by NPo) per patch (LS, 1.91±0.08; NS, 1.58±0.10; HS, 1.14±0.13). The calculated mean open probability (Po) of the 40 pS K+ channel in mice on the LS diet was significantly higher (0.49±0.05) than in mice on the HS diet (0.38±0.03). Thus, LS intake increased whereas HS intake decreased 40 pS K+ channel activity in the DCT. We also examined the expression of Kir4.1 in the WT mice on NS, LS, and HS diets for 7 days (Figure 1C). LS diet significantly increased (138%±6% of the control) whereas HS diet significantly decreased the expression of Kir4.1 (68%±5% of the control) compared with the NS diet (n=4 mice). In addition, we examined the Kir4.1 immunostaining images in the mice on different Na+ diets for 7 days (Supplemental Figure 2). Although no obvious changes in Kir4.1 staining were observed in the renal cortex of the mice on LS, NS, and HS diets (Supplemental Figure 2A), it is apparent that Kir4.1 staining in the basolateral membrane of mice on the LS diet was much sharper (indicated by an arrow) than those on NS and HS diets (Supplemental Figure 2B). In contrast, Kir4.1 immunostaining in mice on the HS diet became diffused (indicated by an arrow).
Figure 1.
Dietary Na+ intake regulates the basolateral 40 pS K+ channel in the DCT. (A) Representative single-channel recordings showing the basolateral 40 pS K+ channel activity in the DCT of WT mice on a low Na+, normal Na+, or high Na+ diet for 7 days, respectively. (B) A table showing probability of finding 40 pS K+ channel, channel activity defined by NPo (a product of channel Number and open Probability) and Po in the DCT of WT mice on different Na+ diets. * indicates significant difference (P<0.05, determined by one-way ANOVA compared with normal Na+ diet. # indicates the significant difference of Po between low Na+ and high Na+. The experiments were performed in cell-attached patches with bath solution containing 140 mM NaCl/5 mM KCl, and pipette solution containing 140 mM KCl. The channel closed level is indicated by “C” and the holding potential was 0 mV. (C) Western blot showing Kir4.1 expression in WT mice on different Na+ diets for 7 days. The results are summarized in a bar graph. * indicates the significant difference (P<0.05, determined by one-way ANOVA).
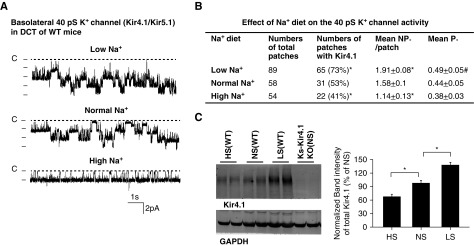
Because the 40 pS K+ channel is the only type of K+ channel in the basolateral membrane of the DCT, we expect that LS intake should increase and HS intake should decrease the basolateral K+ conductance in the DCT. Thus, we next used whole-cell recording to measure the Ba2+-sensitive K+ currents in the DCT1. The reason for conducting the whole-cell recording in the DCT1 is because of lack of ROMK (Kir1.1) activity (Supplemental Figure 1B). Thus, the whole-cell K+ currents measured in the DCT1 are equal to the basolateral K+ conductance. Figure 2A is a set of traces showing the whole-cell K+ currents measured with step protocol (−60 to 60 mV with 20 mV step) and Figure 2B is a set of traces demonstrating K+ currents measured with ramp protocol (−100 to 100 mV) in the DCT of mice on LS, NS, and HS diets for 7 days, respectively. It is apparent that LS diet increased whereas HS diet decreased K+ currents in the DCT compared with the control (NS diet). The whole-cell K+ currents measured at −60 mV from eight experiments (tubules) are summarized in the bar graph in Figure 2B and in the scatterplot in Supplemental Figure 3A, showing that LS intake increased K+ currents from 1374±69 pA to 1961±121 pA whereas HS intake decreased the K+ currents to 608±35 pA. We have also measured the whole-cell K+ currents with step protocol (Figure 2C) and with ramp protocol (Figure 2D) in the DCT of Ks-Kir4.1 KO mice on LS, NS, and HS diets for 7 days, respectively. We confirmed that the basolateral K+ conductance in the DCT was almost completely abolished in Ks-Kir4.1 KO mice (130±11 pA). Western blotting also confirmed that the expression of Kir4.1 was almost absent in Ks-Kir4.1 KO mice (Figure 1C). Furthermore, neither LS intake (150±20 pA) nor HS intake (120±10 pA) had a significant effect on DCT K+ currents (Figure 2D, Supplemental Figure 3A), suggesting that Kir4.1 is solely responsible for dietary Na+ intake–induced changes in the basolateral K+ conductance of the DCT.
Figure 2.
Low Na+ stimulates whereas high Na+ inhibits K+ currents of DCT cells. (A) A set of recordings showing Ba2+-sensitive K+ currents measured with step protocol from −60 to 60 mV, or (B) measured with ramp protocol from −100 to 100 mV in the DCT of WT mice on low Na+, normal Na+, and high Na+ diets for 7 days, respectively. The bottom panel is the bar graph summarizing the values measured at −60 mV with whole-cell recording in WT mice. For the whole-cell recording, a symmetric 140 mM K+ solution was used for the bath and the pipette. (C) Ba2+-sensitive K+ currents measured with step protocol from −100 to 60 mV, or (D) measured with ramp protocol from −100 to 100 mV in the DCT of Ks-Kir4.1 KO mice on low Na+ (red), normal Na+ (black), and high Na+ diets (green) for 7 days, respectively. The bottom panel is the bar graph summarizing the values measured at −60 mV with whole-cell recording in Ks-Kir4.1 KO mice. One-way ANOVA was used for determination of significance.
Dietary Na+ Intake Affects DCT Membrane Potential and Cl− Conductance
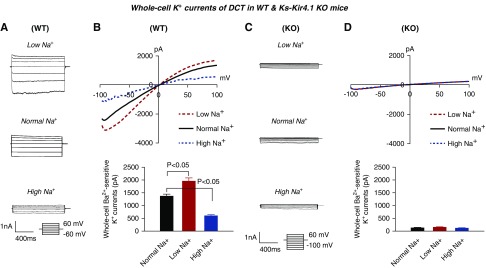
Kir5.1/Kir5.1 heterotetramer participates in generating negative membrane potential in the DCT, LS intake–induced stimulation of Kir4.1/Ki5.1 should increase and HS intake–induced inhibition of Kir4.1/Kir5.1 should decrease the negativity of the DCT membrane. Thus, we used the whole-cell recording to measure K+ current (IK) reversal potential (an index of the membrane potential) in the DCT of WT (Figure 3A) and Ks-Kir4.1 KO mice on LS, NS, and HS diets (Figure 3B), respectively. It is apparent that LS intake shifted IK reversal potential to the left (hyperpolarization) whereas HS intake shifted IK reversal potential to the right (depolarization). The results of eight experiments (tubules) are summarized in the bar graph in Figure 3C and in the scatterplot in Supplemental Figure 3B, showing that IK reversal potentials of the DCT are −73±3 mV (LS diet), −63±2 mV (NS diet), and −55±2 mV (HS diet). The deletion of Kir4.1 not only decreased the negativity of IK reversal potential (−40±2 mV), but also completely abolished the effect of either LS or HS intake on IK reversal potential. From six experiments, the mean value of IK reversal potential was −41±2 mV (LS diet) and −39±2 mV (HS diet) in the DCT of Ks-Kir4.1 KO mice (Figure 3C). In addition, we have also confirmed the previous observation that the basolateral Cl− conductance was closely related to the K+ conductance.15 We observed that LS intake increased and HS intake decreased the basolateral Cl− conductance. Figure 3D and Supplemental Figure 3C summarize the results of seven experiments in which NPPB (10 μM)-sensitive Cl− currents in the DCT were measured at −60 mV (a physiologic-relevant membrane potential) with the whole-cell recording in WT or in Ks-Kir4.1 KO mice on different Na+ diets for 7 days. We observed that LS intake increased Cl− current from 1110±50 to 1405±90 pA, whereas HS intake decreased it to 905±60 pA. However, the basolateral Cl− conductance was decreased to 165±10 pA in Ks-Kir4.1 KO mice on the NS diet. Also, neither LS nor HS intake had a significant effect on the basolateral Cl−conductance in the DCT of Ks-Kir4.1 KO mice (LS, 180±20 pA; HS, 155±15 pA).
Figure 3.
Low Na+ hyperpolarizes while high Na+ depolarizes DCT membrane. A recording showing IK reversal potential in the DCT cells of (A) WT and (B) Ks-Kir4.1 KO mice on a normal Na+ (black), low Na+ (red), or high Na+ (green) diet for 7 days. (C) A bar graph summarizing IK reversal potential in WT and Ks-Kir4.1 KO mice on different Na+ diets. For the measurement of IK reversal potential, the bath solution contained 140 mM NaCl and 5 mM KCl, and the pipette solution contained 140 mM KCl. (D) A bar graph summarizing the results of experiments in which NPPB-sensitive Cl− currents in the DCT were measured at −60 mV with the whole-cell recording in the WT or in Ks-Kir4.1 KO mice on a normal Na+ (black), low Na+ (red), or high Na+ (green) diet for 7 days. For the measurement of NPPB-sensitive Cl− current, the bath solution contained 140mM KCl, 2 mM MgCl2, 1.8 mM CaCl2, and 10 mM HEPES (pH 7.4). The significance was determined by one-way ANOVA (for more than two groups) or by t test (for two groups).
The Effect of LS or HS Intake on NCC Is Compromised in Ks-Kir4.1 KO Mice
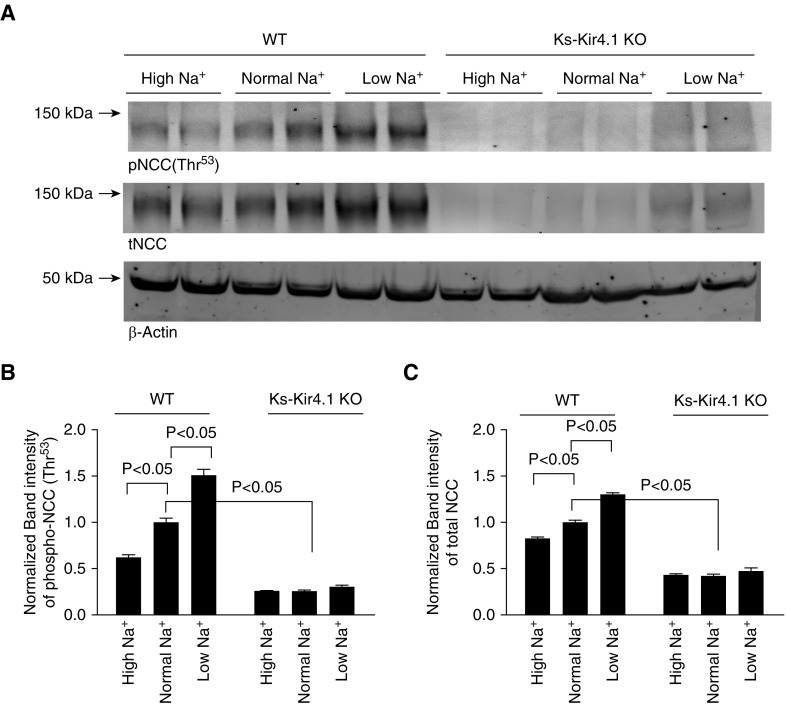
Previous studies have demonstrated that a hyperpolarization in the DCT is associated with an increase in NCC activity, whereas a depolarization of the DCT is associated with a decrease in NCC activity.14–16 After demonstrating that the deletion of Kir4.1 abolished the effect of dietary Na+ intake on the basolateral K+ conductance and DCT membrane potential, we suspect that the effect of dietary Na+ intake on NCC should be absent or attenuated in Ks-Kir4.1 KO mice. Thus, we next examined the effect of dietary Na+ intake on NCC in WT and Ks-Kir4.1 KO mice on LS, NS, and HS diets for 7 days (four male and three female mice for each group). Figure 4A shows that LS intake increased and HS intake decreased the expression of pNCC (Thr53) in WT mice compared with NS intake (Figure 4A; full-page gel of pNCC and tNCC Western blot is shown in Supplemental Figure 4, A and B). Although the expression of NCC in female mice has a tendency to be higher than in male mice (Figure 5A), the percentage changes of pNCC (Supplemental Figure 5B) and tNCC (Supplemental Figure 5C) induced by LS or HS intake were similar between male and female mice. Thus, we pooled the data and the results from five repeated experiments are summarized in Figure 4, B and C, showing the effect of dietary Na+ intake on the expression of tNCC and pNCC. Analysis of Figure 4B (pNCC) and Figure 4C (tNCC) shows that HS significantly decreased the expression of pNCC and tNCC to 62%±3% and 83%±2% of the control value (NS), respectively (individual data points are shown in Supplemental Figure 4, C and D). In contrast, LS significantly increased the expression of pNCC (by 51%±7%) and tNCC (by 30%±2%). Thus, our data confirmed the previous report that LS intake stimulated and HS intake suppressed NCC activity.23 As reported previously that the deletion of Kir4.1 inhibited NCC activity,15 we observed that the expression of pNCC and tNCC was decreased to 26%±2% and 42%±2% of the corresponding values in WT mice on an NS diet, respectively. Furthermore, the effect of dietary Na+ intake on the expression of pNCC and tNCC was largely abolished or attenuated in Ks-Kir4.1 KO mice. Results summarized in Figure 4, B and C show that compared with WT mice on an NS diet, the expression of pNCC was decreased to 26%±1% (HS) or 30%±2% (LS), whereas the expression of tNCC was decreased 43%±1% (HS) or 48%±5% (LS). These values were not significantly different compared with the NCC expression in Ks-Kir4.1 KO mice on an NS diet.
Figure 4.
Deletion of Kir4.1 abolishes the effect of dietary Na+ intake on NCC. (A) Immunoblot showing the expression of pNCC (at Thr53) and tNCC in the WT (left six lanes) and in Ks-Kir4.1 KO mice (right six lanes) on different Na+ diets for 7 days. A bar graph summarizes the normalized band intensity of the above experiments for (B) pNCC and (C) tNCC, respectively (seven mice). Western blots were generated from the tissue lysates harvested from renal cortex. Two-way ANOVA was used for determination of significance.
Figure 5.
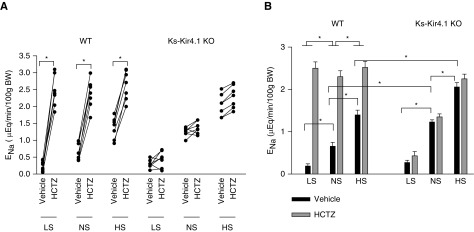
Deletion of Kir4.1 abolishes HCTZ-induced natriuresis. (A) A line graph showing the results of each experiment in which the effect of single-dose HCTZ (25 mg/kg body wt) on urinary Na+ excretion (ENa) within 120 minutes was measured with the renal clearance method in control (WT) or Ks-Kir4.1 KO mice on low Na+, normal Na+, and high Na+ diets for 7 days, respectively. t test was used to determine the significance. (B) A bar graph showing the mean value and statistical information for all above experiments (eight mice for each group). Two-way ANOVA was used for determination of significance. *P<0.05.
We next examined the effect of HCTZ (25 mg/1 kg body wt) on urinary Na+ excretion using renal clearance method in WT and Ks-Kir4.1 KO mice on an NS, HS, or LS diet for 7 days. Figure 5A summarizes results from each individual experiment (eight mice for each group, including males and females) and Figure 5B demonstrates the mean value and statistical information. From the inspection of Figure 5, it is apparent that the basal level (vehicle) of urinary Na+ excretion (ENa) in WT mice on an LS diet (0.19±0.05 μEq/min per 100 g body wt) was significantly lower than those on an NS diet (0.66±0.09 μEq/min per 100 g body wt). Conversely, the basal level of ENa on mice on an HS diet (1.40±0.11 μEq/min per 100 g body wt) was significantly higher than the animals on an NS diet. Moreover, the natriuretic effect of HCTZ was significantly larger in the mice on an LS diet for 7 days (from 0.19±0.05 to 2.50±0.15 μEq/min per 100 g body wt) than those on an NS diet (from 0.66±0.09 to 2.30±0.14 μEq/min per 100 g body wt), whereas it was smaller in the mice on an HS diet than those on an NS diet (from 1.40±0.11 to 2.52±0.14 μEq/min per 100 g body wt). Thus, data of the renal clearance study are consistent with Western blotting and suggest that LS intake enhanced and HS intake inhibited NCC function in WT mice. The deletion of Kir4.1 increased the urinary Na+ excretion under NS conditions compared with those of corresponding WT mice (1.23±0.05 μEq/min per 100 g body wt). Moreover, the HCTZ-induced natriuretic effect was almost absent in Ks-Kir4.1 KO mice on an NS diet (1.35±0.07 μEq/min per 100 g body wt), suggesting that NCC activity was completely inhibited. Although the NCC activity was inhibited in Ks-Kir4.1 KO mice, LS intake was still able to significantly decrease ENa (0.27±0.05 μEq/min per 100 g body wt) compared with NS. However, HCTZ also failed to produce a significant natriuretic effect (0.43±0.10 μEq/min per 100 g body wt) in Ks-Kir4.1 KO mice on LS intake, suggesting that LS intake did not affect NCC activity in Ks-Kir4.1 KO mice. Although HS intake further increased the basal level of ENa to 2.06±0.11 μEq/min per 100 g body wt in Ks-Kir4.1 KO mice, the application of HCTZ had also no significant effect on urinary Na+ excretion (2.25±0.11 μEq/min per 100 g body wt) in Ks-Kir4.1 KO mice on HS intake. Thus, the results strongly suggest that Kir4.1 activity is essential for mediating the dietary Na+ intake–induced modulation of NCC function.
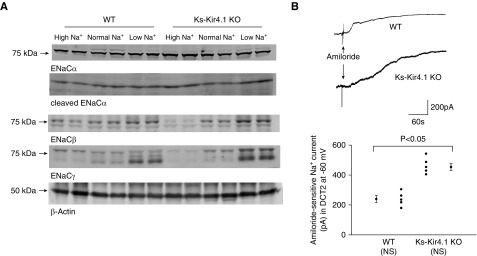
The observation that LS intake decreased whereas HS intake increased the basal level of ENa in Ks-Kir4.1 KO mice suggests that Na+ transport mechanisms other than NCC may compensate the function of NCC, thereby regulating renal Na+ absorption in response to different dietary Na+ intake. Because aldosterone levels in Ks-Kir4.1 KO mice were increased,15 it is possible that the upregulation of ENaC activity may be, at least partially, responsible for compensating the NCC function and regulating renal Na+ excretion. Thus, we next examined the expression of ENaCα-, β-, and γ-subunits in WT and Ks-Kir4.1KO mice on LS, NS, and HS diets for 7 days, respectively (Figure 6A, Supplemental Figure 6). Supplemental Figure 7 is a set of bar graphs showing the normalized band density of ENaCα-, β-, and γ-subunits of the mice on different Na+ diets for 7 days compared with the value obtained from WT mice on an NS diet. It is apparent that LS intake significantly increased the expression of full-length (by 49%±12%)/cleaved ENaCα-subunit (by 58%±12%) and cleaved ENaCγ- subunit (by 115%±10%) in WT mice compared with NS intake. The deletion of Kir4.1 significantly increased the expression of full-length ENaCα-subunit (by 32%±6%) compared with the WT mice. Moreover, LS intake further increased the expression of ENaCβ-subunit (by 120%±12%), full-length ENaCγ-subunit (by 49%±5%), and cleaved ENaCγ-subunit (by 500%±50%). In contrast, HS intake decreased the expression of ENaCβ-subunit (by 40%±6%), full-length ENaCγ-subunit (by 60%±4%), and cleaved ENaCγ-subunit (by 80%±5%). The notion that ENaC activity is upregulated in Ks-Kir4.1 KO mice was further confirmed by patch-clamp experiments in which amiloride-sensitive Na+ currents were measured at −60 mV using whole-cell recording in the DCT2 of WT and Ks-Kir4.1 KO mice (Figure 6B). It is apparent that amiloride-sensitive Na+ currents (454±24 pA, n=6) in Ks-Kir4.1 KO mice were larger than those of WT mice (240±23 pA, n=5). Thus, deletion of Kir4.1 stimulates ENaC activity in the DCT.
Figure 6.
Deletion of Kir4.1 increases ENaC. (A) A Western blot showing the expression of ENaC-α, -β and γ-subunits in WT and Ks-Kir4.1 KO mice on a low Na+, normal Na+, and high Na+ diet for 7 days, respectively (n=6 mice). (B) A whole-cell recording showing amiloride-sensitive Na+ currents in the DCT2 of the WT and Ks-Kir4.1 KO mice on a normal Na+ diet. A scatterplot graph shows the mean value and each data point measured at −60 mV in the WT (n=5 tubules) and Ks-Kir4.1 KO mice (n=6 tubules). Significance was determined by t test.
Dietary Na+ Intake Did Not Affect Urinary K+ Excretion
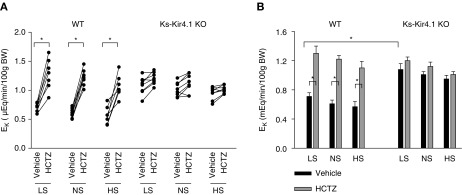
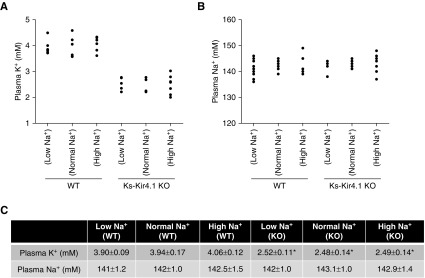
After demonstrating that the deletion of Kir4.1 inhibited NCC and stimulated ENaC activity, we examined whether dietary Na+ intake affected renal K+ excretion in WT and Ks-Kir4.1 KO mice, using the renal clearance method. As mentioned above, eight mice (male and female) for each group were used for the experiments. Figure 7A summarizes results from each individual experiment and Figure 7B demonstrates the mean value and statistical information. Although dietary Na+ intake affected the basal level of urinary Na+ excretion, it did not significantly alter the basal level of urinary K+ excretion (LS, 0.71±0.07μEq/min per 100 g body wt; NS, 0.61±0.05μEq/min per 100 g body wt; HS, 0.57±0.07 μEq/min per 100 g body wt). This notion was also supported by measuring plasma Na+ and K+ concentrations in WT mice on different Na+ diets. Results are summarized in Figure 8, showing that dietary Na+ intake did not change plasma K+ concentration (Figure 8A) or plasma Na+ concentrations (Figure 8B). The mean plasma K+ concentration is 3.90±0.09 mM (LS), 3.94±0.17 mM (NS), and 4.06±0.12 mM (HS) (Figure 8C). Thus, these data were consistent with previous report that dietary sodium intake did not affect the renal K+ excretion and plasma K+ concentrations.24 The application of HCTZ increased urinary K+ excretion to 1.30±0.10 μEq/min per 100 g body wt (LS), 1.22±0.05 μEq/min per 100 g body wt (NS), and 1.10±0.09 μEq/min per 100 g body wt (HS) . Thus, dietary Na+ intake did not significantly alter HCTZ-induced urinary K+ excretion.
Figure 7.
Deletion Of Kir4.1 increases urinary K+ excretion. (A) Line graph showing the results of each experiment in which the effect of single-dose HCTZ on urinary K+ excretion (EK) within 120 minutes was examined with the renal clearance method in the WT or Ks-Kir4.1 KO mice on normal Na+, high Na+, and low Na+ diets, respectively. t test was used to determine the significance. (B) A bar graph showing the mean value and statistical information for all of the above experiments (eight mice for each group). Two-way ANOVA was used for determination of significance. *P<0.05.
Figure 8.
Dietary K+ intake does not affect plasma K+ concentrations. A scatterplot graph showing plasma (A) K+ concentrations or (B) Na+ concentrations in WT and Ks-Kir4.1 KO mice on a low Na+, normal Na+, and high Na+ diets for 7 days, respectively (n=6–8 mice). The mean value and SEM of above measurements are shown in the table (C). * indicates a significant difference compared with the WT control (determined by two-way ANOVA).
The deletion of Kir4.1 increased the urinary K+ excretion in Ks-Kir4.1 KO mice on an NS diet compared with those of corresponding WT mice (1.01±0.04 μEq/min per 100 g body wt). Moreover, the HCTZ application had no additional effect on urinary K+ excretion in Ks-Kir4.1 KO mice on an NS diet (1.12±0.06 μEq/min per 100 g body wt). Also, neither LS nor HS intake significantly affected the basal level of urinary K+ excretion in Ks-Kir4.1 KO mice compared with those on an NS diet (LS, 1.08±0.08 μEq/min per 100 g body wt; HS, 0.95±0.05 μEq/min per 100 g body wt). Furthermore, HCTZ application also had no additional effect on urinary K+ excretion in Ks-Kir4.1 KO mice on an LS (1.20±0.05 μEq/min per 100 g body wt) or HS diet (1.01±0.04 μEq/min per 100 g body wt). The notion that dietary Na+ intake had no significant effect on urinary K+ excretion in Ks-Kir4.1 KO mice was also suggested by measurement of plasma K+ and Na+ concentrations (Figure 8, A and B). Although Ks-Kir4.1 mice were hypokalemic (2.48±0.14 mM), neither HS nor LS intake further altered the plasma K+ concentrations (LS, 2.52±0.11 mM; HS, 2.49±0.14 mM). Thus, dietary Na+ intake had no net effect on urinary K+ excretion or on plasma Na+/K+ concentrations in WT and Ks-Kir4.1 KO mice.
Discussion
The main finding of this study is that the basolateral Kir4.1 activity is required for the effect of dietary Na+ intake on NCC activity. Kir5.1 is also expressed in the TAL and CCD,25,26 the deletion of Kir4.1 in the kidney is expected to affect the basolateral K+ conductance in the TAL and in the CCD. Thus, it is possible that Kir4.1 in the TAL and CCD may also be involved in mediating the effect of Na+ intake on NCC. However, the finding that the deletion of Kir4.1 caused the larger depolarization of the membrane in the DCT than in the TAL and CCD strongly indicates that Kir4.1 plays a dominant role in determining the membrane potential in the DCT compared with the TAL and CCD.25,26 This is partially because K+ channels other than Kir4.1 have been shown to be expressed in the TAL and CCD but not in the DCT. Thus, Kir4.1 in the DCT should play a dominant role in mediating the effect of Na+ diets on NCC.
Previous studies showed that dietary Na+ intake regulated thiazide-sensitive NCC activity.23,27,28 In this study, we have also observed that LS intake increased the expression of pNCC and augmented thiazide-induced natriuretic effect, whereas HS intake had an opposite effect on pNCC expression and thiazide-induced natriuresis. Thus, our finding confirms that LS intake stimulates whereas HS intake inhibits NCC activity in the DCT. Two lines of evidence suggest that Kir4.1/Kir5.1 plays a key role in mediating dietary Na+ intake on NCC activity. First, LS-induced stimulation of NCC was associated with increasing Kir4.1/Kir5.1 activity in the DCT and membrane hyperpolarization, whereas HS-induced inhibition of NCC was associated with decreasing Kir4.1/Kir5.1 activity in the DCT and membrane depolarization. Second, the effect of dietary Na+ intake on NCC activity was largely, if not completely, abolished in Ks-Kir4.1 KO mice in which dietary Na+ intake also failed to affect the DCT membrane conductance and potential. We speculate that LS-induced hyperpolarization should decrease the intracellular Cl− concentrations whereas HS-induced depolarization should increase the intracellular Cl− concentration. This notion was also supported by the observation that LS intake increased whereas HS intake decreased the basolateral Cl− conductance. It is conceivable that alteration in the intracellular Cl− concentrations should affect the Cl−-sensitive with-no-lysine kinase (WNK) activity.29,30
A large body of evidence has convincingly demonstrated that WNK, ste20-proline-alanine-rick kinase (SPAK), and oxidative-sensitive responsive kinase (OSR) play key roles in stimulating NCC expression and activity.23,31–36 Gain-of-function mutations of WNK1 and WNK4 have been shown to stimulate NCC, thereby causing familial hyperkalemic hypertension.37,38 The mice expressing constitutively activated SPAK have high NCC activity, hyperkalemia, and hypertension, suggesting a role of SPAK in the modulation of NCC.34 It is now generally accepted that WNK phosphorylates and activates SPAK/OSR, which in turn stimulates NCC activity by phosphorylation.39,40 We have also shown that the deletion of Kir4.1-induced suppression of NCC was associated with a decrease in phosphorylated SPAK, indicating the inhibition of SPAK.15 Thus, it is conceivable that HS intake–induced inhibition of Kir4.1/Kir5.1 in the DCT should inhibit WNK/SPAK and NCC, whereas LS intake–induced stimulation of Kir4.1/Kir5.1 should activate WNK/SPAK and NCC. Because NCC is responsible for the reabsorption of NaCl in the DCT,1 Kir4.1/Kir5.1 should play a role in maintaining renal Na+ homeostasis in response to dietary Na+ intake changes. For instance, HS intake–induced inhibition of Kir4.1/Kir5.1 should be essential for facilitating renal Na+ excretion during volume expansion because the inhibition of Kir4.1/Kir5.1 is a necessary step for the downregulation of NCC. On the other hand, LS intake–induced stimulation of Kir4.1/Kir5.1 should enhance renal Na+ absorption in the ASDN during Na+ restriction.
This study has also shown that Ks-Kir4.1 KO mice were able to enhance renal Na+ absorption during Na+ restriction because the basal urinary Na+ excretion in the Ks-Kir4.1 KO mice on an LS diet was similar to those of WT mice. This indicates that Ks-Kir4.1 KO mice were able to enhance renal Na+ absorption and to maintain Na+ homeostasis even during dietary Na+ restriction despite NCC inhibition. Two possible mechanisms may be responsible for compensating the NCC function and enhancing Na+ reabsorption in Ks-Kir4.1 KO mice. First, because Ks-Kir4.1 mice had a high aldosterone level,15 high aldosterone should stimulate ENaC activity, thereby augmenting Na+ absorption in ASDN. This notion was supported by two lines of evidence: (1) the expression of ENaC was increased in Ks-Kir4.1 KO mice, and (2) ENaC activity in the DCT was significantly increased in Ks-Kir4.1 KO mice. Second, the deletion of Kir4.1 is expected to stimulate the electroneutral Na+ absorption through pendrin and Na+-dependent Cl−-bicarbonate exchange (NDCBE) in the intercalated cells because Ks-Kir4.1 KO mice were hypokalemic and had high aldosterone levels.15,41,42 Previous studies had shown that aldosterone stimulated pendrin expression during hypokalemia by activating mineralocorticoid receptor in intercalated cells, thereby stimulating pendrin/NDCBE-dependent electroneutral Na+ absorption in the ASDN.43,44 Moreover, we speculate that the pendrin/NDCBE-mediated Na+ absorption, rather than ENaC, may mainly be responsible for the regulation of renal Na+ absorption in Ks-Kir4.1 KO mice. This notion is supported by the fact that neither LS nor HS intake had any additional effect on renal K+ excretion in Ks-Kir4.1 KO. Otherwise, LS would further increase urinary K+ wasting and worsen hypokalemia if the increased Na+ absorption in Ks-Kir4.1 KO mice during Na+ restriction was mainly mediated by ENaC. Further experiments are needed to examine the role of ENaC and pendrin/NDCBE in mediating Na+ absorption in Ks-Kir4.1 KO mice.
Our study has also shown that dietary Na+ intake did not significantly change renal K+ excretion rate and plasma K+ concentrations. This finding is consistent with a previous report by Young et al.,24 showing that increasing Na+ intake in dogs did not change either urinary K+ excretion or plasma K+ levels. It has been suggested that the aldosterone-dependent feedback mechanism was responsible for maintaining renal K+ excretion constant in response to changing dietary Na+ intake because LS intake decreased whereas HS intake increased renal K+ excretion in adrenalectomized dogs.45 Also, although Ks-Kir4.1 KO mice were hypokalemic, dietary Na+ intake did not further alter the plasma K+ levels and urinary K+ excretion rate. As discussed above, this suggests strongly that pendrin/NDCBE-dependent electroneutral Na+ absorption was responsible for compensating NCC function in Ks-Kir4.1 KO mice, thereby preventing further K+ wasting.
The physiologic significance of our findings is to suggest the role of Kir4.1/Kir5.1 in the regulation of K+ and Na+ homeostasis. Both high K+ intake and LS intake are known to stimulate aldosterone synthesis. However, high K+ intake stimulates renal K+ excretion and enhances natriuresis despite high aldosterone levels, whereas Na+ restriction stimulates renal Na+ absorption in the ASDN without enhancing K+ excretion. The discriminatory effects of aldosterone on Na+ and K+ transport in response to hyperkalemia and Na+ depletion depend on the presence of Kir4.1 in the DCT. LS-induced activation of basolateral Kir4.1/Kir5.1 in the DCT switches off the stimulatory effect of aldosterone on renal K+ excretion by stimulating NCC and decreasing Na+ and volume delivery to the ASDN. On the other hand, high K+ intake–induced inhibition of Kir4.1/Kir5.1 plays a role in turning off NCC function and increasing Na+ and volume delivery to the ASDN, thereby stimulating ENaC-dependent K+ excretion. Thus, the inhibition of Kir4.1/Kir5.1 in the DCT during increased dietary K+ intake synergizes efficiency of ENaC-dependent K+ excretion. Thus, the regulation of Kir4.1/Kir5.1 by dietary K+ or Na+ intake is an essential step to control renal K+ and Na+ transport in ASDN. In summary, LS intake stimulates the basolateral K+ channels, hyperpolarizes DCT membrane, and increases NCC, whereas HS intake inhibits the basolateral K+ conductance, depolarizes the DCT membrane, and decreases NCC. We conclude that LS-induced stimulation of Kir4.1/Kir5.1 is essential for the activation of NCC in response to a Na+ restriction, whereas HS-induced inhibition of Kir4.1/Kir5.1 is important for the inhibition of NCC during increasing dietary Na+ consumption.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Ms. Gail Anderson for her assistance in editing the manuscript and Dr. Lars Bellner for the technical support in processing Kir4.1 images.
P.W., Z.-X.G., X.-T.S., and M.-X.W. performed experiments and analyzed the results. W.-H.W. worked as a consultant and edited the manuscript. D.-H.L. designed and conducted experiments, wrote and edited the manuscript. All authors have been involved in revising and approved the manuscript.
This work is supported by National Institutes of Health grants DK54983 (to W.-H.W.) and DK115366 (D.-H.L.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018080799/-/DCSupplemental.
Supplemental Figure 1. The cell model of DCT1 and DCT2.
Supplemental Figure 2. Representative immunostaining images of Kir4.1.
Supplemental Figure 3. K currents in WT (Kcnj10flox/flox ) and Ks-Kir4.1 KO mice.
Supplemental Figure 4. The effect of dietary Na+ intake on the expression of NCC.
Supplemental Figure 5. The effect of dietary Na+ intake on the expression of NCC.
Supplemental Figure 6. The effect of dietary Na+ intake on the expression of ENaC.
Supplemental Figure 7. A set of bar graphs shows normalized band density of full-length (F) or cleaved (C) ENaCα-, β- and γ-subunits.
References
- 1.Ellison DH, Velázquez H, Wright FS: Thiazide-sensitive sodium chloride cotransport in early distal tubule. Am J Physiol 253: F546–F554, 1987 [DOI] [PubMed] [Google Scholar]
- 2.Ellison DH, Velázquez H, Wright FS: Mechanisms of sodium, potassium and chloride transport by the renal distal tubule. Miner Electrolyte Metab 13: 422–432, 1987 [PubMed] [Google Scholar]
- 3.Kim GH, Masilamani S, Turner R, Mitchell C, Wade JB, Knepper MA: The thiazide-sensitive Na-Cl cotransporter is an aldosterone-induced protein. Proc Natl Acad Sci USA 95: 14552–14557, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Obermüller N, Bernstein P, Velázquez H, Reilly R, Moser D, Ellison DH, et al.: Expression of the thiazide-sensitive Na-Cl cotransporter in rat and human kidney. Am J Physiol 269: F900–F910, 1995 [DOI] [PubMed] [Google Scholar]
- 5.Mount DB, Mercado A, Song L, Xu J, George AL Jr., Delpire E, et al.: Cloning and characterization of KCC3 and KCC4, new members of the cation-chloride cotransporter gene family. J Biol Chem 274: 16355–16362, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Lourdel S, Paulais M, Marvao P, Nissant A, Teulon J: A chloride channel at the basolateral membrane of the distal-convoluted tubule: A candidate ClC-K channel. J Gen Physiol 121: 287–300, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vitzthum H, Castrop H, Meier-Meitinger M, Riegger GA, Kurtz A, Krämer BK, et al.: Nephron specific regulation of chloride channel CLC-K2 mRNA in the rat. Kidney Int 61: 547–554, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Schmitt R, Ellison DH, Farman N, Rossier BC, Reilly RF, Reeves WB, et al.: Developmental expression of sodium entry pathways in rat nephron. Am J Physiol 276: F367–F381, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Loffing J, Pietri L, Aregger F, Bloch-Faure M, Ziegler U, Meneton P, et al.: Differential subcellular localization of ENaC subunits in mouse kidney in response to high- and low-Na diets. Am J Physiol Renal Physiol 279: F252–F258, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Zhang C, Wang L, Su XT, Zhang J, Lin DH, Wang WH: ENaC and ROMK activity are inhibited in the DCT2/CNT of TgWnk4PHAII mice. Am J Physiol Renal Physiol 312: F682–F688, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lourdel S, Paulais M, Cluzeaud F, Bens M, Tanemoto M, Kurachi Y, et al.: An inward rectifier K(+) channel at the basolateral membrane of the mouse distal convoluted tubule: Similarities with Kir4-Kir5.1 heteromeric channels. J Physiol 538: 391–404, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang C, Wang L, Thomas S, Wang K, Lin DH, Rinehart J, et al.: Src family protein tyrosine kinase regulates the basolateral K channel in the distal convoluted tubule (DCT) by phosphorylation of KCNJ10 protein. J Biol Chem 288: 26135–26146, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang C, Wang L, Zhang J, Su X-T, Lin DH, Scholl UI, et al.: KCNJ10 determines the expression of the apical Na-Cl cotransporter (NCC) in the early distal convoluted tubule (DCT1). Proc Natl Acad Sci U S A 111: 11864–11869, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Terker A-S, Zhang C, McCormick J-A, Lazelle R-A, Zhang C, Meermeier N-P, et al.: Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab 21: 39–50, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cuevas CA, Su XT, Wang MX, Terker AS, Lin DH, McCormick JA, et al.: Potassium sensing by renal distal tubules requires Kir4.1. J Am Soc Nephrol 28: 1814–1825, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang MX, Cuevas-Gallardo C, Su XT, Wu P, Gao Z-X, Lin DH, et al.: Potassium intake modulates the thiazide-sensitive sodium-chloride cotransporter (NCC) activity via the Kir4.1 potassium channel. Kidney Int 93: 893–902, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scholl UI, Choi M, Liu T, Ramaekers VT, Häusler MG, Grimmer J, et al.: Seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME syndrome) caused by mutations in KCNJ10. Proc Natl Acad Sci U S A 106: 5842–5847, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bockenhauer D, Feather S, Stanescu HC, Bandulik S, Zdebik AA, Reichold M, et al.: Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. N Engl J Med 360: 1960–1970, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reichold M, Zdebik AA, Lieberer E, Rapedius M, Schmidt K, Bandulik S, et al.: KCNJ10 gene mutations causing EAST syndrome (epilepsy, ataxia, sensorineural deafness, and tubulopathy) disrupt channel function. Proc Natl Acad Sci U S A 107: 14490–14495, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sorensen MV, Grossmann S, Roesinger M, Gresko N, Todkar AP, Barmettler G, et al.: Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int 83: 811–824, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Frindt G, Palmer LG: Effects of dietary K on cell-surface expression of renal ion channels and transporters. Am J Physiol Renal Physiol 299: F890–F897, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rengarajan S, Lee DH, Oh YT, Delpire E, Youn JH, McDonough AA: Increasing plasma [K+] by intravenous potassium infusion reduces NCC phosphorylation and drives kaliuresis and natriuresis. Am J Physiol Renal Physiol 306: F1059–F1068, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiga M, Rai T, Yang SS, Ohta A, Takizawa T, Sasaki S, et al.: Dietary salt regulates the phosphorylation of OSR1/SPAK kinases and the sodium chloride cotransporter through aldosterone. Kidney Int 74: 1403–1409, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Young DB, McCaa RE, Pan UJ, Guyton AC: Effectiveness of the aldosterone-sodium and -potassium feedback control system. Am J Physiol 231: 945–953, 1976 [DOI] [PubMed] [Google Scholar]
- 25.Su XT, Zhang C, Wang L, Gu R, Lin DH, Wang WH: Disruption of KCNJ10 (Kir4.1) stimulates the expression of ENaC in the collecting duct. Am J Physiol Renal Physiol 310: F985–F993, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang C, Wang L, Su XT, Lin DH, Wang WH: KCNJ10 (Kir4.1) is expressed in the basolateral membrane of the cortical thick ascending limb. Am J Physiol Renal Physiol 308: F1288–F1296, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frindt G, Yang L, Uchida S, Weinstein AM, Palmer LG: Responses of distal nephron Na+ transporters to acute volume depletion and hyperkalemia. Am J Physiol Renal Physiol 313: F62–F73, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vallon V, Schroth J, Lang F, Kuhl D, Uchida S: Expression and phosphorylation of the Na+-Cl- cotransporter NCC in vivo is regulated by dietary salt, potassium, and SGK1. Am J Physiol Renal Physiol 297: F704–F712, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piala AT, Moon TM, Akella R, He H, Cobb MH, Goldsmith EJ: Chloride sensing by WNK1 involves inhibition of autophosphorylation. Sci Signal 7: ra41, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bazúa-Valenti S, Chávez-Canales M, Rojas-Vega L, González-Rodríguez X, Vázquez N, Rodríguez-Gama A, et al.: The effect of WNK4 on the Na+-Cl- cotransporter is modulated by intracellular chloride. J Am Soc Nephrol 26: 1781–1786, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ponce-Coria J, Markadieu N, Austin TM, Flammang L, Rios K, Welling PA, et al.: A novel Ste20-related proline/alanine-rich kinase (SPAK)-independent pathway involving calcium-binding protein 39 (Cab39) and serine threonine kinase with no lysine member 4 (WNK4) in the activation of Na-K-Cl cotransporters. J Biol Chem 289: 17680–17688, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lalioti MD, Zhang J, Volkman HM, Kahle KT, Hoffmann KE, Toka HR, et al.: Wnk4 controls blood pressure and potassium homeostasis via regulation of mass and activity of the distal convoluted tubule. Nat Genet 38: 1124–1132, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Castañeda-Bueno M, Cervantes-Pérez LG, Vázquez N, Uribe N, Kantesaria S, Morla L, et al.: Activation of the renal Na+:Cl- cotransporter by angiotensin II is a WNK4-dependent process. Proc Natl Acad Sci U S A 109: 7929–7934, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grimm PR, Coleman R, Delpire E, Welling PA: Constitutively active SPAK causes hyperkalemia by activating NCC and remodeling distal tubules. J Am Soc Nephrol 28: 2597–2606, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Z, Xie J, Wu T, Truong T, Auchus RJ, Huang CL: Downregulation of NCC and NKCC2 cotransporters by kidney-specific WNK1 revealed by gene disruption and transgenic mouse models. Hum Mol Genet 20: 855–866, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCormick JA, Mutig K, Nelson JH, Saritas T, Hoorn EJ, Yang CL, et al.: A SPAK isoform switch modulates renal salt transport and blood pressure. Cell Metab 14: 352–364, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bazúa-Valenti S, Gamba G: Revisiting the NaCl cotransporter regulation by with-no-lysine kinases. Am J Physiol Cell Physiol 308: C779–C791, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kahle KT, Ring AM, Lifton RP: Molecular physiology of the WNK kinases. Annu Rev Physiol 70: 329–355, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Piechotta K, Lu J, Delpire E: Cation chloride cotransporters interact with the stress-related kinases Ste20-related proline-alanine-rich kinase (SPAK) and oxidative stress response 1 (OSR1). J Biol Chem 277: 50812–50819, 2002 [DOI] [PubMed] [Google Scholar]
- 40.San-Cristobal P, Pacheco-Alvarez D, Richardson C, Ring AM, Vazquez N, Rafiqi FH, et al.: Angiotensin II signaling increases activity of the renal Na-Cl cotransporter through a WNK4-SPAK-dependent pathway. Proc Natl Acad Sci U S A 106: 4384–4389, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wall SM, Lazo-Fernandez Y: The role of pendrin in renal physiology. Annu Rev Physiol 77: 363–378, 2015 [DOI] [PubMed] [Google Scholar]
- 42.Leviel F, Hübner CA, Houillier P, Morla L, El Moghrabi S, Brideau G, et al.: The Na+-dependent chloride-bicarbonate exchanger SLC4A8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice. J Clin Invest 120: 1627–1635, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu N, Hirohama D, Ishizawa K, Chang WX, Shimosawa T, Fujita T, et al.: Hypokalemia and pendrin induction by aldosterone. Hypertension 69: 855–862, 2017 [DOI] [PubMed] [Google Scholar]
- 44.Shibata S, Rinehart J, Zhang J, Moeckel G, Castañeda-Bueno M, Stiegler AL, et al.: Mineralocorticoid receptor phosphorylation regulates ligand binding and renal response to volume depletion and hyperkalemia. Cell Metab 18: 660–671, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Young DB, Jackson TE, Tipayamontri U, Scott RC: Effects of sodium intake on steady-state potassium excretion. Am J Physiol 246: F772–F778, 1984 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.