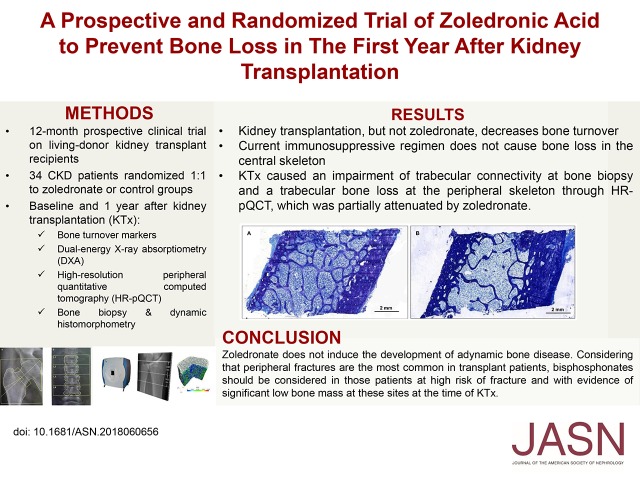
Significance Statement
How bisphosphonates affect bone quality in kidney transplant recipients is unclear. This study of 34 patients with kidney transplants randomized to receive zoledronate or no treatment for 12 months found that zoledronate did not induce adynamic bone disease; decreased bone turnover experienced by both groups was unaffected by zoledronate. Total hip and lumbar spine bone mineral density increased for both groups, especially at the lumbar spine and femoral neck in the zoledronate group. However, bone biopsies from both groups showed impaired trabecular connectivity, and high-resolution imaging detected trabecular bone loss at peripheral skeleton sites, which zoledronate partially attenuated. Because fractures in patients with transplants are most commonly peripheral fractures, bisphosphonates may be considered for patients with high fracture risk and evidence of bone loss in the peripheral skeleton at the time of transplant.
Keywords: mineral metabolism, kidney disease, kidney transplantation, bone biopsy, zoledronic acid, renal osteodystrophy
Visual Abstract
Abstract
Background
Bone and mineral disorders commonly affect kidney transplant (KTx) recipients and have been associated with a high risk of fracture. Bisphosphonates may prevent or treat bone loss in such patients, but there is concern that these drugs might induce adynamic bone disease (ABD).
Methods
In an open label, randomized trial to assess the safety and efficacy of zoledronate for preventing bone loss in the first year after kidney transplant, we randomized 34 patients before transplant to receive zoledronate or no treatment. We used dual-energy x-ray absorptiometry (DXA), high-resolution peripheral quantitative computed tomography (HR-pQCT), and bone biopsies to evaluate changes in bone in the 32 evaluable participants between the time of KTx and 12 months post-transplant.
Results
Both groups of patients experienced decreased bone turnover after KTx, but zoledronate itself did not affect this outcome. Unlike previous studies, DXA showed no post-transplant bone loss in either group; we instead observed an increase of bone mineral density in both lumbar spine and total hip sites, with a significant positive effect of zoledronate. However, bone biopsies showed post-transplant impairment of trabecular connectivity (and no benefit from zoledronate); HR-pQCT detected trabecular bone loss at the peripheral skeleton, which zoledronate partially attenuated.
Conclusions
Current immunosuppressive regimens do not contribute to post-transplant central skeleton trabecular bone loss, and zoledronate does not induce ABD. Because fractures in transplant recipients are most commonly peripheral fractures, clinicians should consider bisphosphonate use in patients at high fracture risk who have evidence of significantly low bone mass at these sites at the time of KTx.
Kidney transplantation (KTx) is considered the best option for RRT, because it promotes better survival and quality of life. However, a successful KTx does not completely restore renal function, as the eGFR stays around 50–60 ml/around 50-60 ml/min and patients are... patients are still considered to have CKD. In this scenario and because of the risk of bone disease associated with glucocorticoid use, bone loss is an expected event.1 Indeed, bone mass loss after KTx is a well described phenomenon that starts early after transplantation and may persist for several years.2
A cross-sectional analysis 2 years after KTx reported changes in bone turnover, mineralization, and volume in 95% of patients.3 Another study showed that bone mineral density (BMD) declines by 4%–10% in the first 6 months, with a further decrease of 0.4%–4.5% between 6 and 12 months after KTx.4 However, less severe bone loss in the lumbar spine has been reported by recent prospective trials that included patients managed with contemporary immunosuppression protocols.5,6
Most of the studies that evaluate bone loss after KTx were on the basis of BMD obtained by dual-energy x-ray absorptiometry (DXA). BMD can also be evaluated by high-resolution peripheral quantitative computed tomography (HR-pQCT). It is the state-of-the art image technique to assess microarchitectural aspects of bone quality at the distal radius and tibia in vivo, giving information on the volumetric density of cortical and trabecular regions separately. However, both DXA and HR-pQCT give no information on bone turnover and mineralization, which are obtained only through bone biopsy. Indeed, few studies have prospectively evaluated bone biopsy after KTx, and even in these cases, dynamic analysis has not been usually performed.7–9 This is partially explained by the difficulty in performing tetracycline labeling, which can only be done in the case of live donor KTx.
Despite the risk of development of adynamic bone disease (ABD) and infection, antiresorptive drugs, such as bisphosphonates 7,10 and more recently, denosumab,5 were tested in an attempt to prevent bone loss after KTx. However, most studies that have shown the benefit of these drugs in preventing bone loss were on the basis of BMD.5,10,11 In one of these studies, Bonani et al.5 also showed, in a subset of patients, that denosumab increased volumetric bone mineral density (vBMD) in the distal radius and tibia through HR-pQCT. Because of the paucity of bone histomorphometric data compared before and after KTx, we designed this study in patients under the new immunosuppressive era on the basis of tacrolimus, mycophenolate, and lower doses of glucocorticoids.
Here, we present the results of a 12-month prospective trial to evaluate the effects of zoledronate treatment in living donor kidney transplant recipients on bone histomorphometry, DXA, HR-pQCT, and biochemical measurements. The hypothesis of this study was that zoledronate acid administered to KTx recipients at the time of the surgery would not be associated with an increased prevalence of ABD diagnosticated by bone biopsy and that it would simultaneously prevent bone loss evaluated by DXA.
Methods
Study Design
A prospective and randomized trial was designed to evaluate the effects of zoledronate treatment on bone histomorphometry, DXA, HR-pQCT, and bone biochemical measures in adult patients who received a living donor kidney transplant at our center. This study was approved by the institutional review board, and it was registered at Clinicaltrials.gov (identifier NCT01675089).
Participants
Participants were recruited from eligible transplant recipients during 2012–2013. Eligibility criteria included adult age (≥18 years old at transplant), ability to give informed consent, undergoing a living donor kidney transplant, and an eGFR>30 ml/min per 1.73 m2 the first week after KTx. Exclusion criteria included the inability to return for regular follow-up, participation in another clinical trial, transplant of other solid organs, previous parathyroidectomy or ABD diagnosed by bone biopsy, pretransplant parathyroid hormone (PTH) <130 pg/ml, or previous use of bisphosphonates. Participants were not different from those patients who were not eligible for the study in terms of age, sex, ESRD etiology, or dialysis months (data not shown).
Protocol
Subjects were randomized via a computer-generated number system to one of two groups. The treatment group received a 15-minute intravenous administration of zoledronic acid (5 mg) in a single dose at the time of KTx and cholecalciferol supplementation. The control group received only cholecalciferol. Patients were admitted 2 days before KTx when DXA, HR-pQCT, and bone biopsy were done. The same procedures were repeated 12 months after.
Participants received standard immunosuppression with glucocorticoids, tacrolimus, and enteric-coated mycophenolate sodium. Cyclosporin was used in one participant instead of tacrolimus. Participants received induction immunosuppression with basiliximab (77%) or thymoglobulin (23%), 500 mg of intraoperative intravenous methylprednisolone followed by maintenance prednisone (0.5 mg/kg tapered to 5 mg/d by 60 days postoperatively), tacrolimus (adjusted to trough levels of 8–12 ng/ml for 3 months and then, 5–8 ng/ml), and mycophenolate sodium (1440 mg orally twice daily, with dose adjustments to manage adverse events). The acute rejection episodes, the total dosage of glucocorticoids, and tacrolimus levels were all recorded. All participants received 50,000 UI of cholecalciferol monthly to maintain 25-hydroxyvitamin D levels above 30 ng/ml.
Measurement of BMD by DXA
Areal BMD was measured by DXA using Hologic QDR 4500A densitometry equipment (Discovery model; Hologic Inc., Bedford, MA) at regions representing the central skeleton (lumbar spine, femoral neck, and total hip) on the basis of standard International Society for Clinical Densitometry protocols. The same experienced technologist performed DXA measurements. The least significant change for BMD measurement was 0.033 g/cm2 at the lumbar spine, 0.047 g/cm2 at the femoral neck, and 0.039 g/cm2 at the total hip.
Bone density was expressed in grams per centimeter squared and in terms of T score (for comparisons of subjects with the young normal population). Osteoporosis and osteopenia were defined as T scores ≤−2.5 and between −1 and −2.4, respectively.
HR-pQCT Imaging of the Radius and Tibia
vBMD and microarchitecture were measured at the distal radius of the nondominant forearm and the distal tibia of the nondominant leg (peripheral skeleton) using a three-dimensional HR-pQCT system with a resolution of 82 μm (XtremeCT; Scanco Medical AG, Bruttisellen, Switzerland) by a specialized densitometry technologist in the Bone Laboratory Metabolism of the Rheumatology Division. HR-pQCT of the dominant limb was performed when there was a previous fracture or an arteriovenous fistula or graft in the nondominant limb. The forearm or leg of the subject was positioned in the scanner, and it was immobilized during the examination in an anatomically formed carbon fiber shell. Briefly, the method consists of tomography slices at distances of 9.5 and 22.5 mm from a reference line, which was set up at the endplate of the radius and tibia. The volume of interest is separated into cortical and trabecular using a threshold-based algorithm that discriminates cortical from the trabecular bone at one third of the apparent cortical vBMD. We defined mean cortical thickness as the mean cortical volume divided by the outer bone surface. The outcome variables used in our analyses included the following: (1) vBMD parameters (milligrams HA per centimeter cubed): total vBMD, trabecular vBMD, and cortical vBMD; (2) bone structure parameters: trabecular number (1 per millimeter), trabecular thickness (millimeters), trabecular separation (millimeters), and cortical thickness (millimeters); and (3) cortical porosity parameters: cortical porosity (1).12 The precision of HR-pQCT showed coefficients of variation of 0.93%–1.41% at the distal radius and 0.25%–1.16% at the tibia for density measurements and 1.49%–7.59% at the distal radius and 0.78%–6.35% at the tibia for morphometric measurements, which are similar to the literature.13 Segmentation of the cortex was obtained through an automatic segmentation algorithm. The common regions between the baseline and 1-year scan were identified and analyzed using a three-dimensional image registration framework using a baseline-indexed analysis.14
Microfinite Element Analysis
Linear microfinite element models of the distal radius and tibia were created directly from the HR-pQCT images using software-specific finite element. It estimates bone strength and correlates strongly with ex vivo strength testing. The software uses the so-called voxel conversion technique to create finite element models (Finite Element software v. 1.13; Scanco Medical AG; January 2009) to assess biomechanical bone strength. The following biomechanical properties were analyzed using microfinite element analysis: stiffness (kilonewton per millimeter) and estimated failure load (newton).13
Biochemical and Hormonal Determinations
Blood samples were obtained at baseline and months 6 and 12 of the protocol. The samples were collected in a fasting state, and they were centrifuged in cryovials aliquots and stored at −80°C. Serum levels of calcium (reference range [RR]: 8.6–10.2 mg/dl), phosphate (RR: 2.7–4.5 mg/dl), and alkaline phosphatase (RR: 35–129 IU/L) were determined using routine laboratory techniques. Serum total PTH (RR: 10–65 pg/ml) was measured using a chemiluminescence assay (DPC; Medlab, San Antonio, TX). Serum 25(OH) vitamin D (RR: 30–100 ng/dl; insufficiency <30 ng/dl and deficiency <15 ng/dl) was measured using a chemiluminescent immunoassay (DiaSorin, Stillwater, MN). Bone-specific alkaline phosphatase (BAP; RR: 11.6–42.7 U/L) and tartrate-resistant acid phosphatase isoform 5b (TRAP5b; RR: 1.5–5.8 U/L) were measured using an enzyme immunoassay (Metra Biosystem, Mountain View, CA). Serum sclerostin (RR: 0.42–0.80 ng/ml) was measured using an enzyme immunoassay (Quidel Corporation–TECO Medical Group). eGFR was calculated according to the Modification of Diet in Renal Disease 4 equation that has been validated to transplant recipients at our center against the EDTA-Cr51 plasmatic clearance.15
Transiliac Bone Biopsy and Histomorphometry
Bone biopsies extracted from the anterior iliac crest using a 7-mm Bordier trephine were performed at the time of KTx and after 1 year. All patients received a course of double-labeling tetracycline (20 mg/kg per day) for 3 days separated by an interval of 10 days. This labeling gives the information on the dynamic parameters, making bone biopsy the gold standard for the diagnostic of renal osteodystrophy. The biopsy was performed 2–5 days after the last dose of antibiotics. Undecalcified bone fragments were submitted to standard processing for histologic studies. Bone histomorphometry was analyzed using a semiautomatic technique in the Osteomeasure software (Osteometrics, Atlanta, GA). The static and dynamic parameters were examined according to the standards established by the American Society of Bone and Mineral Research.16 Cortical porosity higher than 10% was considered as abnormal.17 Renal osteodystrophy was classified into one of the classic types according to the following criteria: (1) osteitis fibrosa defined as bone formation rate as well as either osteoblast surface or osteoclast surface >1 SD above the normal range, osteoid volume/bone volume (OV/BV) within or above the normal range, and marrow fibrosis (Fb.V) >0.5%; (2) ABD defined as bone formation rate/bone surface (BFR/BS) and OV/BV>1 SD below the normal range and Fb.V <0.5%; (3) osteomalacia defined as BFR/BS>1 SD below the normal range and OV/BV>1 SD above the normal range; and (4) mixed uremic osteodystrophy defined as BFR/BS and OV/BV>1 SD above the normal range, Fb.V>0.5%, and mineralization lag time >50 days. Thereafter, bone histology was categorized according to the turnover, mineralization, and volume classification.18 Osteitis fibrosa and mixed uremic osteodystrophy were considered high-turnover diseases, whereas osteomalacia and ABD were considered low-turnover diseases.
Sample Size Calculation and Outcomes
The primary end point was designed to assure that zoledronic acid administration would not increase the risk of ABD, which was found in 80% of patients in a previous study.7 These authors observed that pamidronate was associated with 100% prevalence of ABD, whereas the control group exhibited 50% prevalence of ABD. Assuming a similar bisphosphonate effect, 14 participants per group would provide statistical power of 80% to detect a 50% difference in the prevalence of ABD between groups. Because the 1-year graft survival is around 90%, we calculated the sample size of 15 patients per group.
Secondary end points included changes in bone microarchitecture and BMD induced by KTx as well as bisphosphonate treatment evaluated by HR-pQCT and DXA. For the changes in BMD, the study by Coco et al.7 described BMD changes in the control and pamidronate groups as −5.81%±0.09% and −0.39%±0.05%, respectively. To find similar results with zoledronate, the minimum number of patients would be eight per group, with a 90% power to detect a significant difference. Because this number is lower than that calculated for the primary end point, we maintained the plan to include at least 15 patients per group. Therefore, with the chosen sample size, we also had power to detect differences in this secondary end point.
At the time that this study was conceived, there were no previous data on the effects of KTx on HR-pQCT measurements. We decided to keep the calculation for the bone turnover effect of zoledonate (15 patients per group), recognizing the challenge of recruiting more patients for a bone biopsy study. During our protocol, a prospective, observational study was published19 showing that KTx was associated with 4.4% and 2.4% trabecular and cortical density losses, respectively. Assuming that zoledronate would attenuate this loss in 50%, a minimum number of 14 patients would be necessary, with an 80% power to detect this effect. However, because this calculation was performed after the study has begun, we recognize that the data provided by HR-pQCT should be interpreted with caution.
Statistical Analyses
Continuous variables are expressed as means and SDs or medians and interquartile ranges. Discrete variables are expressed as percentages. We used the unpaired t test or Mann–Whitney U test to compare continuous variables between zoledronate and control group as appropriate. The chi-squared or Fisher exact test was applied to compare proportions between these groups. A mixed linear model with maximum likelihood estimation of variance components was used to evaluate the effects of the kidney transplant (treated as a repeated measure on baseline and 1 year) according to a condition control versus zoledronate (treated as a fixed factor) and the interaction between treatment condition and kidney transplant. Repeated covariance type was treated as AR(1) heterogeneous. All statistical tests were two tailed, and the threshold of statistical significance was established at P<0.05. We performed statistical analyses with the Statistical Package for the Social Sciences, version 21.0 (SPSS Inc., Chicago, IL).
Results
Baseline Demographics
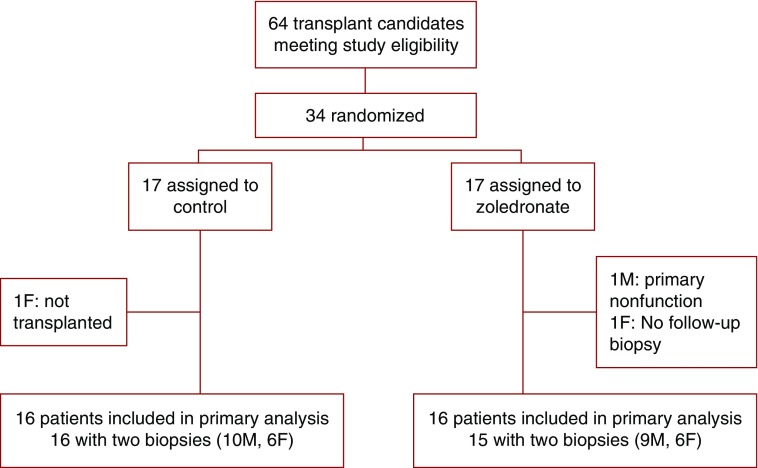
Between July 1, 2012 and July 1, 2013, 64 adult patients underwent living donor renal transplantation at our center, and none of them refused to participate in the study. Thirty-four patients met the eligibility criteria and were randomized to undergo baseline bone biopsy, DXA, HR-pQCT, and blood analyses. Thirty-two participants were included in the study, and 31 had a second biopsy at 12 months as shown in the trial flowchart (Figure 1). As shown in Tables 1 and 2, control and zoledronate groups were similar at baseline. Most patients were nonobese white men who were relatively young and on dialysis for a median of 2 years.
Figure 1.
Participant randomization flow chart. F, women; M, men.
Table 1.
Baseline demographics
| Characteristics | Zoledronate, n=16 | Control, n=16 | P Value |
|---|---|---|---|
| Men, n (%) | 9 (56) | 10 (62) | 0.72 |
| White, n (%) | 10 (62) | 11 (69) | 0.71 |
| Age, yr | 43±11 | 39±11 | 0.30 |
| Time on dialysis, mo | 25 (13–48) | 27 (14–56) | 0.80 |
| RRT | |||
| Hemodialysis | 14 (88) | 14 (88) | 0.51 |
| Peritoneal dialysis | 2 (12) | 1 (6) | |
| Preemptive transplantation | 0 | 1 (6) | |
| ESRD etiology | 0.23 | ||
| Chronic GN | 8 | 12 | |
| Hypertensive nephrosclerosis | 3 | 0 | |
| Diabetic nephropathy | 0 | 1 | |
| Polycystic kidney disease | 1 | 1 | |
| Other | 2 | 0 | |
| Undetermined | 2 | 2 | |
| BMI, kg/m2 | 22.7±3.8 | 25.5±5.7 | 0.12 |
Continuous variables are presented as mean ± SD if normally distributed or median (interquartile range) if not normally distributed. BMI, body mass index.
Table 2.
Biochemical parameters
| Parameter | Control Baseline | Control 12 mo | Changes,a Absolute/% | Zoledronate Baseline | Zoledronate 12 mo | Changes,a Absolute/% | P Value Transplant | P Value Zoledronate versus Control | P Value Interaction |
|---|---|---|---|---|---|---|---|---|---|
| Calcium, mg/dl | 8.3±0.7 | 9.6±0.5 | 1.3/15.7 | 8.5±0.5 | 9.5±0.6 | 1.0/11.8 | <0.001 | 0.64 | 0.43 |
| Phosphorus, mg/dl | 6.7±1.3 | 3.2±0.8 | −3.5/−52.2 | 7.9±2.0 | 3.2±0.8 | −3.8/−48.1 | <0.001 | 0.06 | 0.07 |
| AP, UI/L | 125±57 | 114±57 | −11/−8.8 | 114±85 | 70.6±21.6 | −43.4/−38 | 0.04 | 0.10 | 0.19 |
| PTH, pg/ml | 527±431 | 110±160 | −417/−79.1 | 437±324 | 91±62 | −346/−79.2 | <0.001 | 0.46 | 0.58 |
| 25OH vit.D, ng/ml | 28.5±7.6 | 30.2±14.4 | 1.7/6.0 | 23.9±20.5 | 28.4±8.0 | 4.5/18.8 | 0.18 | 0.33 | 0.54 |
| Sclerostin, ng/ml | 1.05±0.62 | 0.52±0.16 | −0.53/−50 | 1.47±1.0 | 0.55±0.14 | −0.92/−62.6 | <0.001 | 0.16 | 0.17 |
| BAP, UI/L | 100±62 | 45.3±28.7 | −54.7/−54.7 | 92±74 | 22.4±6.8 | −69.6/−75.6 | <0.001 | 0.23 | 0.54 |
| TRAP5b, UI/L | 10.2±4.4 | 4.9±2.6 | −5.3/−51.9 | 9.6±4.5 | 2.3±1.4 | −7.3/−76 | <0.001 | 0.07 | 0.20 |
Variables are presented as mean ± SD. P values are derived from a linear mixed model. AP, alkaline phosphatase; 25OH vit.D, 25 (OH) vitamin D; BAP, bone-specific alkaline phosphatase; TRAP5b, tartrate-resistant acid phosphatase.
Changes according to groups.
Renal Function and Immunosuppression
eGFR was similar between the control group and the zoledronate group at 12 months (62±25 versus 70±14 ml/min per 1.73 m2, respectively; P=0.24). There was no difference in episodes of acute rejection or adverse events between groups. The cumulative dose of glucocorticoids in the first year was not different between the groups (3590±759 mg in the zoledronate group versus 4038±1228 mg in the control group; P=0.49). Other than steroids, all patients were on tacrolimus and enteric-coated mycophenolate sodium, with no differences in doses and tacrolimus levels at 1, 3, 6, and 12 months (Supplemental Table 1).
Biochemical and Hormonal Markers
The main effects of KTx (baseline versus 12 months), intervention effects (control versus zoledronate), and interaction between factors are shown in Table 2.
KTx promoted an increase in serum calcium and a decrease in alkaline phosphatase, PTH, sclerostin, BAP, and TRAP5b from baseline to 12 months. However, we found no effect of zoledronate on these parameters.
Bone Histomorphometry
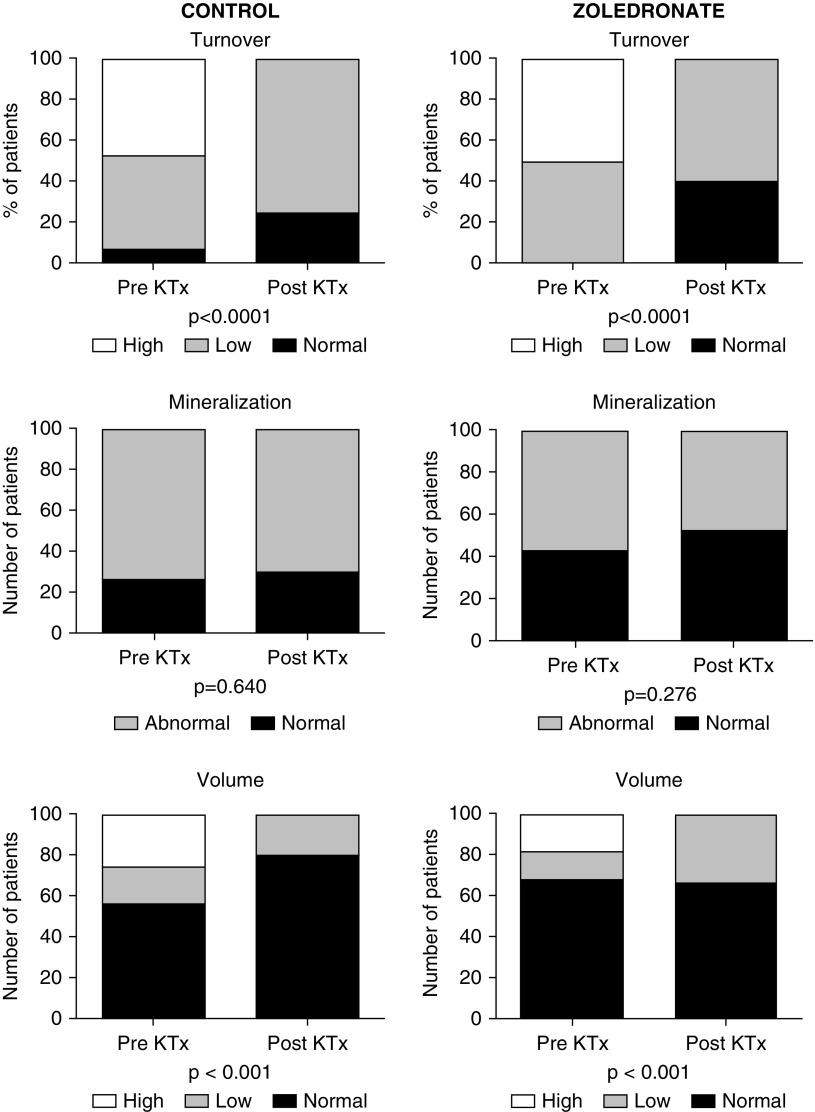
At baseline, by applying the turnover, mineralization, and volume classification, we found that one half of the patients presented high bone turnover (Figure 2, Supplemental Figure 1). KTx promoted a significant change in this status, such that ten patients normalized the bone turnover and 22 turned to a low bone turnover (P<0.001). The percentage of low bone turnover was similar when comparing patients from the control and zoledronate groups (63% versus 53%, respectively; P=0.74). KTx did not cause mineralization impairment, and the percentage of patients with increased mineralization lag time was 62.5% before and 61.3% after KTx (P=0.92). Also, the percentages of patients with ABD after KTx tended to be lower in the zoledronate groups, although they were not statistically significant (61.1% and 43.0%, respectively; P=0.48). The proportion of patients with abnormal mineralization after KTx was similar between control and zoledronate groups (50% versus 47%, respectively; P=0.99). The distribution among normal, low, and high bone trabecular volume groups before and after KTx changed from 61%, 15%, and 24% to 74%, 26%, and 0%, respectively (P=0.01). These changes did not differ between control and zoledronate groups, with 50% and 71% of patients presenting low bone trabecular volume, respectively (P=0.59). With respect to cortical compartment, few patients had abnormal cortical porosity before KTx (20% versus 13% of the control and zoledronate groups, respectively). After 1 year, only 6.7% of the patients from both groups remained with abnormal cortical porosity.
Figure 2.
Distribution of bone turnover, mineralization and volume at baseline and 12 months in the zoledronate and control study groups. One year after kidney transplant, we observed an increase in low turnover bone disease in both groups. The prevalence of high trabecular volume decreased in the control and zoledronate groups. No significant changes were seen in mineralization. KTx, kidney transplantation.
The analysis of bone histomorphometric parameters showed that KTx was associated with a significant decrease in bone formation and resorption as shown in Table 3. In general, KTx promoted a worsening of trabecular connectivity characterized by an increase in trabecular separation and a decrease in trabecular number (Figure 3), with no difference between control and zoledronate groups. Changes in cortical compartment revealed an improvement in cortical thickness and porosity after KTx, with no differences between groups.
Table 3.
Bone histomorphometric parameters in the zoledronate and control groups
| Parameter | Control Baseline | Control 12 mo | Changes,a Absolute/% | Zoledronate Baseline | Zoledronate 12 mo | Changes,a Absolute/% | P Value Transplant | P Value Zoledronate versus Control | P Value Interaction |
|---|---|---|---|---|---|---|---|---|---|
| BV/TV, % | 24.7±6.5 | 25.3±8.6 | 0.6/2.4 | 24.4±6.3 | 20.1±5.9 | −4.3/−17.6 | 0.42 | 0.30 | 0.22 |
| Tb.Sp, μm | 393±155 | 429±169 | 36/9.2 | 387±106 | 541±185 | 154/39.8 | 0.003 | 0.24 | 0.06 |
| Tb.Th, μm | 125±24 | 134±35 | 9/7.2 | 120±27 | 128±22 | 8/6.7 | 0.12 | 0.53 | 0.98 |
| Tb.N, per mm | 2.1±0.6 | 1.9±0.4 | −0.1/−4.8 | 2.0±0.4 | 1.5±0.3 | −0.5/−25 | <0.001 | 0.20 | 0.14 |
| OV/BV, % | 7.0±6.2 | 6.8±9.2 | −0.2/−2.9 | 9.9±13.3 | 2.9±3.1 | −7/−70.7 | 0.07 | 0.82 | 0.09 |
| OS/BS, % | 34.1±20.1 | 28.7±18.4 | −5.4/−15.8 | 39.1±19.5 | 19.2±14.4 | −19.9/−50.9 | 0.002 | 0.64 | 0.06 |
| O.Th, μm | 9.7±4.8 | 10.4±5.3 | 0.7/7.2 | 10.1±5.3 | 8.0±3.5 | −2.1/−20.8 | 0.56 | 0.36 | 0.24 |
| Ob.S/BS, % | 7.9±6.4 | 7.2±5.7 | −0.7/−8.9 | 12.4±10.2 | 5.0±4.6 | −7.4/−59.7 | 0.002 | 0.48 | 0.27 |
| Oc.S/BS, % | 1.5±1.2 | 0.5±0.5 | −1.0/−66.7 | 2.5±1.9 | 0.4±0.4 | −2.1/−84 | <0.001 | 0.18 | 0.06 |
| ES/BS, % | 11.1±6.1 | 4.6±2.4 | −6.5/−58.6 | 13.0±6.5 | 4.0±2.2 | −9/−69.2 | <0.001 | 0.57 | 0.23 |
| BFR, μm3/μm2 per day | 0.06±0.06 | 0.04±0.03 | −0.02/−33.3 | 0.07±0.09 | 0.04±0.03 | −0.03/−42.9 | 0.04 | 0.83 | 0.71 |
| Mlt, d | 225.2±245.8 | 198.1±228.7 | −27.1/−12 | 191.5±226.8 | 170.7±343.3 | −20.8/−10.9 | 0.66 | 0.66 | 0.99 |
| Ct.Th, μm | 735±183 | 1105±446 | 370/50.3 | 714±171 | 1234±385 | 520/72.8 | <0.001 | 0.62 | 0.24 |
| Ct.Po, % | 8.0±3.4 | 5.1±3.0 | −2.9/−36.2 | 7.5±2.8 | 5.8±3.2 | −1.7/−22.7 | 0.001 | >0.99 | 0.32 |
P values are derived from a linear mixed model. BV/TV, bone volume/tissue volume; Tb.Sp, trabecular separation; Tb.Th, trabecular thickness; Tb.N, trabecular number; OV/BV, osteoid volume/bone volume; OS/BS, osteoid surface; O.Th, osteoid thickness; Ob.S/BS, osteoblasts/bone surface; Oc.S/BS, osteoclasts/bone surface; ES/BS, erosion surface/bone surface; BFR, bone formation rate; Mlt, mineralization lag time; Ct.Th, cortical thickness; Ct.Po, cortical porosity.
Changes according to groups.
Figure 3.
Bone biopsies of a patient in the zoledronate group (A) at baseline and (B) after 12 months showing a worsening of trabecular connectivity, which was characterized by an increase in trabecular separation and a decrease in trabecular number.
Change in BMD on the Basis of DXA
Most of the participants had either normal BMD or osteopenia at baseline. In the control group, osteoporosis and osteopenia were found in 18.8% and 50% of the patients, respectively, whereas in the zoledronate group, osteoporosis and osteopenia were found 18.8% and 56.3%, respectively (P=0.92). At baseline, patients in the zoledronate group had significantly lower total hip BMD compared with those in the control group. KTx was associated with an increase in BMD at the lumbar spine and total hip, with a significant positive effect of zoledronate at the lumbar spine and femoral neck as shown in Table 4. Of note, patients from the zoledronate group had at least twice as much gain in percentage in BMD at the lumbar spine and femoral neck compared with patients from the control group.
Table 4.
Dual-energy x-ray absorptiometry and high-resolution peripheral quantitative computed tomography parameters in the zoledronate and control groups
| Parameter | Control Baseline | Control 12 mo | Changes,a Absolute/% | Zoledronate Baseline | Zoledronate 12 mo | Changes,a Absolute/% | P Value Transplant | P Value Zoledronate versus Control | P Value Interaction |
|---|---|---|---|---|---|---|---|---|---|
| BMD lumbar spine, g/cm2 | 0.95±0.13 | 0.96±0.13 | 0.01/1.05 | 0.90±0.14 | 0.95±0.13 | 0.05/5.6 | <0.01 | 0.63 | 0.04 |
| BMD total hip, g/cm2 | 0.89±0.14b | 0.96±0.13 | 0.07/7.9 | 0.80±0.14 | 0.90±0.20 | 0.1/12.5 | <0.001 | 0.55 | 0.15 |
| BMD femoral neck, g/cm2 | 0.79±0.14 | 0.81±0.14 | 0.02/2.5 | 0.69±0.14 | 0.73±0.11 | 0.04/5.8 | 0.001 | 0.28 | 0.03 |
| HR-pQCT tibia | |||||||||
| Tt.vBMD, mg HA/cm3 | 282±48b | 278±42 | −4/−1.4 | 239±67 | 236±64 | −3/−1.3 | 0.03 | 0.14 | 0.35 |
| Tb.vBMD, mg HA/cm3 | 148±41b | 144±32 | −4/−2.7 | 117±32 | 115±33 | −2/−1.7 | 0.04 | 0.02 | 0.53 |
| Ct.vBMD, mg HA/cm3 | 895±86 | 898±95 | 3/0.3 | 870±101 | 865±95 | −5/−0.6 | 0.55 | 0.41 | 0.61 |
| BV/TV, % | 12.3±3.2 | 11.8±2.7 | −0.5/−4.1 | 9.8±2.7 | 9.5±2.7 | −0.3/−3.1 | 0.78 | <0.001 | 0.94 |
| Tb.N,1/mm | 1.58±0.25 | 1.60±0.29 | 0.02/1.3 | 1.34±0.32 | 1.45±0.40 | 0.11/8.2 | 0.10 | 0.02 | 0.38 |
| Tb.Th, mm | 0.08±0.01 | 0.07±0.01 | −0.01/−12.5 | 0.07±0.01 | 0.07±0.01 | 0/0 | 0.02 | 0.48 | 0.34 |
| Tb.Sp, mm | 0.57±0.11b | 0.57±0.11 | 0/0 | 0.72±0.20 | 0.69±0.26 | −0.03/−4.2 | 0.20 | 0.01 | 0.69 |
| Ct.Th, mm | 1.20±0.23 | 1.19±0.21 | −0.1/−8.3 | 1.02±0.33 | 1.03±0.32 | 0.1/9.8 | 0.98 | 0.16 | 0.73 |
| Ct.Po, % | 5.0±4.2 | 5.2±4.3 | 0.2/4 | 5.0±3.8 | 4.8±2.9 | −0.2/−4 | 0.87 | 0.91 | 0.29 |
| S, kN/mm | 222.5±65.7 | 217.7±60.9 | −7.8/−3.5 | 192.5±63.6 | 189.4±62.6 | −3.1/−1.6 | 0.20 | 0.19 | 0.78 |
| F.ult, kN | 58.8±39.3 | 58.1±39.1 | −0.7/−1.2 | 51.5±35.3 | 51.0±34.9 | −0.5/−1.0 | 0.88 | 0.56 | 0.98 |
| HR-pQCT radius | |||||||||
| Tt.vBMD, mg HA/cm3 | 322±69 | 317±69 | −5/−1.6 | 274±93 | 264±91 | −10/−3.6 | 0.08 | 0.07 | 0.62 |
| Tb.vBMD, mg HA/cm3 | 182±55 | 173±48 | −9/−4.9 | 153±49 | 145±48 | −8/−5.2 | 0.02 | 0.14 | 0.99 |
| Ct.vBMD, mg HA/cm3 | 837±121 | 839±118 | 2/0.2 | 799±122 | 798±107 | −1/−0.1 | 0.97 | 0.27 | 0.86 |
| BV/TV, % | 14.6±4.8 | 14.8±4.1 | 0.2/1.4 | 12.8±3.8 | 12.1±4.0 | −0.7/−5.5 | 0.01 | 0.13 | 0.98 |
| Tb.N, 1/mm | 1.95±0.40 | 2.05±0.29 | 0.1/5.1 | 1.83±0.37 | 1.92±0.35 | 0.09/4.9 | 0.06 | 0.36 | 0.80 |
| Tb.Th, mm | 0.08±0.02 | 0.07±0.01 | −0.01/−12.5 | 0.07±0.02 | 0.06±0.02 | −0.01/−14.2 | 0.001 | 0.18 | 0.67 |
| Tb.Sp, mm | 0.46±0.13 | 0.43±0.08 | −0.03/−6.5 | 0.50±0.14 | 0.48±0.13 | −0.02/−4 | 0.07 | 0.50 | 0.68 |
| Ct.Th, mm | 0.71±0.22 | 0.72±0.22 | 0.1/14 | 0.61±0.30 | 0.60±0.27 | −0.01/−1.6 | >0.99 | 0.20 | 0.69 |
| Ct.Po, % | 2.9±2.5 | 3.1±2.5 | 0.2/6.9 | 2.4±1.4 | 2.2±1.3 | −0.2/−8.3 | 0.94 | 0.32 | 0.25 |
| S, kN/mm | 87.6±25 | 86.0±25.5 | −1.6/−1.8 | 75.6±33.4 | 75.5±28.2 | −0.1/−0.3 | >0.99 | 0.29 | 0.34 |
| F.ult, kN | 42.4±14.4 | 43.5±13.1 | 1.1/2.6 | 35.3±12.5 | 34.7±10.7 | −0.6/−1.7 | 0.84 | 0.31 | 0.32 |
Continuous variables are presented as mean ± SD. P values are derived from a linear mixed model. BMD, bone mineral density; HR-pQCT, high-resolution peripheral quantitative computed tomography; Tt.vBMD, total volumetric bone mineral density; HA, hydroxyapatite; Tb.vBMD, trabecular volumetric bone mineral density; Ct.vBMD, cortical volumetric bone mineral density; BV/TV, trabecular bone volume/total volume; Tb.N, number of trabeculae; Tb.Th, trabecular thickness; Tb.Sp, trabecular separation; Ct.Th, cortical thickness; Ct.Po, cortical porosity; S, stiffness; F.ult, estimated failure load.
Changes according to groups.
P<0.05 control versus zoledronate at baseline (tested by unpaired t test or Mann–Whitney as appropriate).
Change in BMD on the Basis of HR-pQCT
Similar to what was found in DXA, patients in the zoledronate group had significantly lower total and trabecular vBMD and higher trabecular separation at the tibia compared with those in the control group at baseline (Table 4). Contrary to what was seen in DXA, HR-pQCT showed a significant bone loss as seen by a decrease in trabecular BMD at the tibia and the radius. Zoledronate partially prevented this bone loss at the tibia but not that at the radius. We found no changes in porosity at the tibia and the radius.
Correlations among Changes from Baseline to 1 Year after KTx Obtained from Serum Biomarkers, Histomorphometry, DXA, and HR-pQCT Methods
We found significant correlations between changes in PTH, BAP, and TRAP5b and changes in some bone histomorphometric parameters of bone formation and resorption (Supplemental Table 2). Changes in BAP and TRAP5b also correlated with changes in cortical density in tibia and radius. The greater the decrease in serum TRAP5b, the greater the percentage increases in lumbar and hip density measured by DXA (Supplemental Figure 2). No significant correlations were seen between changes in serum sclerostin and changes in DXA, HR-pQCT, and bone histomorphometry.
Discussion
To our knowledge, this is the first randomized study assessing the 1-year effects of zoledronate treatment on dynamic bone histomorphometry in KTx recipients. We found that KTx itself, but not zoledronate, decreases bone turnover. Bone histomorphometry and DXA confirmed that the current immunosuppressive regimen does not cause bone loss in the central skeleton, which is represented by the spine and hip BMD. This finding challenges the classic concept that KTx is closely associated with a significant bone loss.1 However, bone histomorphometry showed a decrease in trabecular connectivity after KTx, with no benefits of zoledronate. In line with this finding, HR-pQCT also disclosed a loss of trabecular bone at the peripheral skeleton, but loss was partially prevented at the tibia by the zolendronate.
This study gave us the opportunity to evaluate the effect of KTx on different sites of the skeleton through different methods. Our primary end point was to assure that the zoledronate would not increase the risk of ABD. Therefore, our primary goal was to confirm that we would not see a common adverse effect of bisphosphonates: an increased prevalence of low-bone turnover disease. Although we found no effect of zoledronate on bone turnover, in general, KTx was capable of increasing the risk of ABD. This adverse effect as a result of bisphosphonate therapy is not been a consistently observed phenomenon. For example, in the study of Coco et al.,7 pamidronate was associated with preservation of vertebral BMD as well as an increased risk of low bone turnover. In contrast, more recent data from the same group showed that risedronate did not affect BMD or that it was not associated with an increased risk of developing ABD in kidney transplant recipients.9 However, dynamic parameters of histomorphometry were not studied in most of the patients. In the past, we have shown reversal of aluminum-related osteomalacia20 and improvement in ABD (mostly aluminum related)21 1 year after a successful kidney transplant.
Contrary to our initial hypothesis and previous studies,4,22 we could not observe clinically relevant bone loss after KTx in the central skeleton represented by lumbar spine and hip. In accordance, some other studies on the basis of BMD findings have also failed in showing bone loss.5,6 Other studies conducted in the past have shown a rapid bone loss, particularly during the first 6–12 months after KTx and mainly in trabecular bone.4,22 However, most recent studies as well as those in which corticosteroids were withdrawn rapidly after transplantation have shown stabilization or even an increase in BMD in the lumbar spine or hip.19 In the study by Bonani et al.,5 which evaluated the efficacy of denosumab in preventing bone loss in the first year after KTx, lumbar spine BMD increased by 4.6% in the intervention group and decreased by only 0.5% in the control group. A recent study by Smerud et al.6 showed no benefit with ibandronate compared with calcium and calcitriol supplementation alone on lumbar BMD, but ibandronate did modestly increase hip and forearm BMD and did cause suppression of bone turnover markers, including type 1 procollagen N terminus, osteocalcin, and BAP. This finding is in contrast to earlier studies, which seemed to show a beneficial effect of bisphosphonates on lumbar BMD. These conflicting results are possibly due to less glucocorticoid exposure in the study by Smerud et al.6 as well as due to the fact that their control group received calcitriol and calcium supplementation. Indeed, a number of studies in which vitamin D therapy was part of standard care in the control group failed to show a benefit of bisphosphonates on BMD after KTx. This seems to be our case, because our patients did not receive a high cumulative dose of glucocorticoids and were supplemented with vitamin D. However, we have observed that zoledronate enhanced the bone gain at the spine and femoral neck.
With respect to HR-pQCT, our data must be interpreted with caution, because our study was underpowered to detect significant changes through this methodology. Nevertheless, we observed a reduction in trabecular density in the peripheral skeleton that might be associated with glucocorticoids use. No significant change was seen in the cortical compartment. These findings are in line with the decrease in the trabecular connectivity that was detected by the bone histomorphometry. Zoledronate was able to reduce this loss significantly only at the tibia. Therefore, we should hypothesize that KTx is no longer associated with central skeleton losses but is associated with peripheral skeleton losses. Similar results have already been shown by Iyer et al.19 Significant deterioration of both cortical and trabecular bone was linked to reductions in estimated bone strength, and it was directly associated with the severity of post-transplant hyperparathyroidism and elevated bone turnover markers. In our case, we found bone loss only at the trabecular compartment. The discrepancy with our results might rely on the facts that our patients were younger, received a live donor KTx, ended up with a high eGFR, and received steroids, which commonly affect trabecular bone. In the study by Iyer et al.,19 patients had a steroid-free regimen and presented hyperparathyroidism. Nevertheless, we also found significant correlations between changes in bone turnover markers and changes in DXA, HR-pQCT, and bone biopsy such that the higher the change in BAP, the higher the decrease in bone formation rate. Recently, our group showed that HR-pQCT associated with BAP could also provide some clues on the turnover status. We showed that cortical density at radius was higher and that bone alkaline phosphatase was lower in patients with low turnover in a cross-sectional analysis. Combined, these parameters could identify the turnover status better than the parameters could individually.23 However, this was not confirmed in this prospective analysis (data not shown).
This study has some limitations. It was a single-center study with a relatively small number of patients. There were imbalances in the baseline BMD measurements because of chance randomization, with patients in the zoledronate group presenting significantly lower total femur BMD and total and trabecular BMD at the tibia than patients in the control group. Although no patient experienced a bone fracture, the small number of patients and the short follow-up time preclude evaluation of zoledronate on this outcome. The lack of bone loss might reflect the use of a single dose of zoledronate or a short-term observation period. However, because of the risk of ABD development, this limitation is unlikely to be overcome in future studies. Importantly, our study was not powered for changes in microarchitecture. Therefore, our findings for HR-pQCT must be interpreted with caution. Also, our patients should not be considered representative of all patients on dialysis, and our findings cannot be extended to a broader population, such as those patients with low bone density at the time of KTx. The strength of our study was using three methods to evaluate bone, including bone biopsy, randomized design, and paired design, which reduces the possibility of bias.
In summary, we have shown that zoledronate does not induce the development of ABD. In the contemporary immunosuppression era, with lower rates of acute rejection, reduced use of glucocorticoids, and widespread use of vitamin D and analogs, central skeleton trabecular bone loss in the early post-transplantation period does not seem to be as important as previously shown, and the use of bisphosphonates to prevent bone loss can be questioned in patients with normal BMD. However, KTx induces a loss of connectivity detected by bone biopsy, which is also seen in the peripheral skeleton through HR-pQCT. Considering that peripheral fractures are the most common ones in patients with transplants,24 bisphosphonates should only be reserved for those patients at high risk of fracture with evidence of significant bone loss at these sites at the time of KTx. Additional studies, especially evaluating the effects of fracture prevention in the long term, are necessary until prophylactic use of bisphosphonates can be broadly recommended after KTx.
Disclosures
None.
Supplementary Material
Acknowledgments
Operating grant 2011/22962-3 provided by Fundação de Amparo à Pesquisa do Estado de São Paulo supported this work. V.J., R.M.A.M., and E.D.-N. were supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil.
This work was presented in part at the American Society of Nephrology meeting, October 31–November 5, 2017, New Orleans, Louisiana.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018060656/-/DCSupplemental.
Supplemental Figure 1. Distribution of bone histomorphometry at baseline and 12 months in the zoledronate and control study groups.
Supplemental Figure 2. Correlations between changes in TRAP5b and changes in lumbar spine and osteoclastic surface. Correlations between changes in BAP and changes in osteoblastic surface and bone formation rate.
Supplemental Table 1. Renal function and CNI levels: zoledronate versus control.
Supplemental Table 2. Correlation between changes in bone turnover markers and changes in DXA, HR-pQCT, and bone histomorphometry parameters.
References
- 1.Bouquegneau A, Salam S, Delanaye P, Eastell R, Khwaja A: Bone disease after kidney transplantation. Clin J Am Soc Nephrol 11: 1282–1296, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rojas E, Carlini RG, Clesca P, Arminio A, Suniaga O, De Elguezabal K, et al.: The pathogenesis of osteodystrophy after renal transplantation as detected by early alterations in bone remodeling. Kidney Int 63: 1915–1923, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Neves CL, dos Reis LM, Batista DG, Custodio MR, Graciolli FG, Martin RC, et al.: Persistence of bone and mineral disorders 2 years after successful kidney transplantation. Transplantation 96: 290–296, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Brandenburg VM, Politt D, Ketteler M, Fassbender WJ, Heussen N, Westenfeld R, et al.: Early rapid loss followed by long-term consolidation characterizes the development of lumbar bone mineral density after kidney transplantation. Transplantation 77: 1566–1571, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Bonani M, Frey D, Brockmann J, Fehr T, Mueller TF, Saleh L, et al.: Effect of twice-yearly denosumab on prevention of bone mineral density loss in de novo kidney transplant recipients: A randomized controlled trial. Am J Transplant 16: 1882–1891, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Smerud KT, Dolgos S, Olsen IC, Åsberg A, Sagedal S, Reisæter AV, et al.: A 1-year randomized, double-blind, placebo-controlled study of intravenous ibandronate on bone loss following renal transplantation. Am J Transplant 12: 3316–3325, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Coco M, Glicklich D, Faugere MC, Burris L, Bognar I, Durkin P, et al.: Prevention of bone loss in renal transplant recipients: A prospective, randomized trial of intravenous pamidronate. J Am Soc Nephrol 14: 2669–2676, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Haas M, Leko-Mohr Z, Roschger P, Kletzmayr J, Schwarz C, Mitterbauer C, et al.: Zoledronic acid to prevent bone loss in the first 6 months after renal transplantation. Kidney Int 63: 1130–1136, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Coco M, Pullman J, Cohen HW, Lee S, Shapiro C, Solorzano C, et al.: Effect of risedronate on bone in renal transplant recipients. J Am Soc Nephrol 23: 1426–1437, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeffery JR, Leslie WD, Karpinski ME, Nickerson PW, Rush DN: Prevalence and treatment of decreased bone density in renal transplant recipients: A randomized prospective trial of calcitriol versus alendronate. Transplantation 76: 1498–1502, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Walsh SB, Altmann P, Pattison J, Wilkie M, Yaqoob MM, Dudley C, et al.: Effect of pamidronate on bone loss after kidney transplantation: A randomized trial. Am J Kidney Dis 53: 856–865, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Alvarenga JC, Fuller H, Pasoto SG, Pereira RM: Age-related reference curves of volumetric bone density, structure, and biomechanical parameters adjusted for weight and height in a population of healthy women: An HR-pQCT study. Osteoporos Int 28: 1335–1346, 2017 [DOI] [PubMed] [Google Scholar]
- 13.Paupitz JA, Lima GL, Alvarenga JC, Oliveira RM, Bonfa E, Pereira RM: Bone impairment assessed by HR-pQCT in juvenile-onset systemic lupus erythematosus. Osteoporos Int 27: 1839–1848, 2016 [DOI] [PubMed] [Google Scholar]
- 14.Nishiyama KK, Pauchard Y, Nikkel LE, Iyer S, Zhang C, McMahon DJ, et al.: Longitudinal HR-pQCT and image registration detects endocortical bone loss in kidney transplantation patients. J Bone Miner Res 30: 554–561, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Medeiros FS, Sapienza MT, Prado ES, Agena F, Shimizu MH, Lemos FB, et al.: Validation of plasma clearance of 51Cr-EDTA in adult renal transplant recipients: Comparison with inulin renal clearance. Transpl Int 22: 323–331, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, et al.: Bone histomorphometry: Standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2: 595–610, 1987 [DOI] [PubMed] [Google Scholar]
- 17.Malluche HH, Mawad HW, Monier-Faugere MC: Renal osteodystrophy in the first decade of the new millennium: Analysis of 630 bone biopsies in black and white patients. J Bone Miner Res 26: 1368–1376, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moe S, Drüeke T, Cunningham J, Goodman W, Martin K, Olgaard K, et al. Kidney Disease: Improving Global Outcomes (KDIGO) : Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 69: 1945–1953, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Iyer SP, Nikkel LE, Nishiyama KK, Dworakowski E, Cremers S, Zhang C, et al.: Kidney transplantation with early corticosteroid withdrawal: Paradoxical effects at the central and peripheral skeleton. J Am Soc Nephrol 25: 1331–1341, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.David-Neto E, Jorgetti V, Soeiro NM, Pereira RC, Borelli A, Ianhez LE, et al.: Reversal of aluminum-related bone disease after renal transplantation. Am J Nephrol 13: 12–17, 1993 [DOI] [PubMed] [Google Scholar]
- 21.Abdallah KA, Jorgetti V, Pereira RC, Reis LM, Pereira LM, Corrêa PH, et al.: Improvement of adynamic bone disease after renal transplantation. Braz J Med Biol Res 39: 31–41, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Malluche HH, Monier-Faugere MC, Herberth J: Bone disease after renal transplantation. Nat Rev Nephrol 6: 32–40, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marques ID, Araújo MJ, Graciolli FG, Reis LM, Pereira RM, Custódio MR, et al.: Biopsy vs. peripheral computed tomography to assess bone disease in CKD patients on dialysis: Differences and similarities. Osteoporos Int 28: 1675–1683, 2017 [DOI] [PubMed] [Google Scholar]
- 24.Nikkel LE, Mohan S, Zhang A, McMahon DJ, Boutroy S, Dube G, et al.: Reduced fracture risk with early corticosteroid withdrawal after kidney transplant. Am J Transplant 12: 649–659, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.