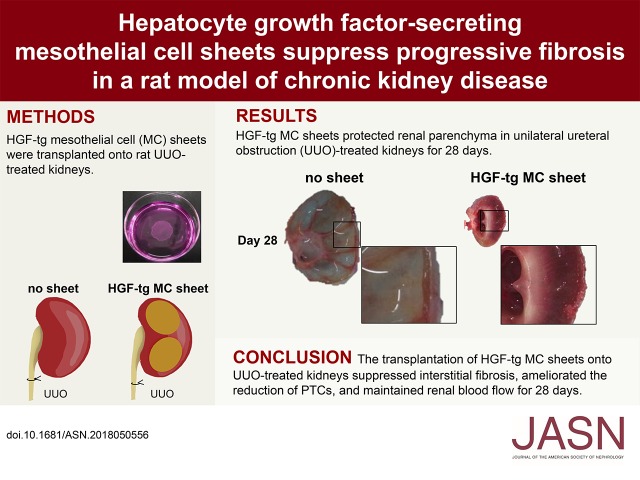
Significance Statement
No effective therapy is currently available to directly address a key feature of CKD progression: interstitial fibrosis leading to a loss of peritubular capillaries (PTCs). One potential antifibrotic candidate, hepatocyte growth factor (HGF), cannot be applied in clinical settings owing to its short t1/2 in blood and elimination by other organs, such as the liver. The authors describe use of a novel HGF therapy using cell sheet technology: transplantation of transgenic HGF-secreting mesothelial cell sheets to the kidney in a CKD rat model. The HGF-secreting cell sheet directly suppresses interstitial fibrosis for 28 days and prevents loss of peritubular capillaries. Inhibition of CKD progression requires long-term suppression of fibrosis, and cell sheet therapy represents a promising strategy for achieving that goal.
Keywords: fibrosis, chronic kidney disease, HGF, cell sheet, obstructive nephropathy
Visual Abstract
Abstract
Background
Although hepatocyte growth factor (HGF) has antifibrotic effects and is involved in angiogenesis and vasodilation, systemic administration of HGF to prevent kidney fibrosis is not a feasible strategy for suppressing interstitial fibrosis in patients with CKD.
Methods
We investigated a novel therapy involving HGF transgenic cell sheets grown in culture from human mesothelial cells and administered to rats with unilateral ureteral obstruction (UUO). We compared progression of fibrosis in rats with UUO that received one of five interventions: HGF-transgenic mesothelial cell sheets transplanted to the kidney surface, HGF-transgenic mesothelial cell sheets transplanted to thigh, mesotherial cell sheets transplanted to kidney, no sheets, or HGF injections.
Results
HGF transgenic cell sheets transplanted to the kidney strongly suppressed the induction of myofibroblasts and collagen in the kidney for 28 days; other interventions did not. Additionally, the HGF-secreting cell sheets ameliorated loss of peritubular capillaries and maintained renal blood flow.
Conclusions
These findings suggest that cell sheet therapy is a novel and promising strategy for inhibiting progressive fibrosis in CKD.
CKD involves the progressive deterioration of renal function. The health effect and economic burden of CKD have increased worldwide along with increases in CKD-attributable deaths and complications, such as cardiovascular disease.1 Several reports have shown that functional impairment of the kidneys directly correlates with the degree of tubulointerstitial fibrosis2,3 and that tubulointerstitial fibrosis is associated with a reduction in peritubular capillaries (PTCs; i.e., rarefaction).4,5
In CKD, kidney hypoxia increases6 and leads to fibrogenic responses in tubular epithelial cells and renal fibroblasts7 followed by a loss of PTCs.8 The loss of PTCs subsequently worsens hypoxia, and eventually, chronic hypoxia leads to progressive fibrosis. Many researchers have used the term “vicious cycle” to describe progressive renal fibrosis due to chronic hypoxia,9,10 and there is no specific therapy that directly blocks this cycle of progressive fibrosis.
Previous data showed that the administration of exogenous cytokines, including EGF,11 bone morphogenetic protein 7,12 and hepatocyte growth factor (HGF),13 exhibited renoprotective effects on injured kidneys. In particular, HGF causes antifibrotic effects and is involved in angiogenesis14–16 and vasodilation.17,18 HGF prevents increases in myofibroblasts by blocking TGF-β signals.19 Recent studies indicate that myofibroblasts were derived from endothelial cells, fibroblasts, fibrocytes, and epithelial cells20 and that HGF might affect them.19,21,22 Moreover, HGF might contribute to renal vasculature protection in injured kidneys lacking angiogenic factors.23,24 HGF also contributes to improved blood flow via nitric oxide synthase–mediated vasodilation.17,18 These findings suggest that HGF suppresses fibrosis in addition to maintaining PTCs and blood flow in injured kidneys.
Although systemic administration of HGF suppresses fibrosis in animal models,13,25 it is difficult to apply this factor in clinical settings due to its short t1/2 in blood (approximately 5 minutes).26,27 Furthermore, systemically administered HGF is eliminated by other organs, such as the liver, and only trace amounts reach the kidney.27 These problems impede the treatment of CKD, which requires long-term suppression of fibrosis.
To address these problems, we focused on cell sheet therapy. Cell sheet engineering technology uses temperature-responsive polymers to enable the nondisruptive detachment of intact cell sheets from the culture dish. Using such technology, cell-to-cell contacts are maintained, and bioactive extracellular matrix (ECM) components are retained in the cell sheets.28,29 Because the ECM promotes adhesion to the target organ, cell sheets are applied to treat various organs, such as the heart30 and cornea.31 There are no reports of direct treatment of kidney disease using cell sheet therapy, although several reports have shown that cell sheet therapy treated complications associated with renal failure.32,33 The findings of one study indicated that mesothelial cell (MC) sheets prevented intra-abdominal adhesion formation, which is a complication of peritoneal dialysis.33 This outcome is due to the effect of MCs possessing anti-inflammatory and immunomodulatory properties. To ameliorate progressive kidney deterioration, we show for the first time in this study a significant therapeutic effect of transplanting HGF-secreting cell sheets to the kidney on renal fibrosis. The grafted cell sheets are expected to remain on the kidney for a long duration and drive continuous HGF secretion. A previous study showed that HGF-overexpressing myoblast sheets enhanced angiogenesis in a rat model of chronic heart failure.34
In this study, we used a common model for CKD, namely unilateral ureteral obstruction (UUO). In UUO-treated kidneys, renal fibrosis was caused by chronic ischemia and inflammation as well as increased intratubular pressure due to stored urine.35 We transplanted HGF-secreting cell sheets onto the kidney during the UUO procedure, evaluated its efficacy, and verified that the HGF-secreting cell sheets functioned as a long-acting inhibitor of progressive fibrosis in the rat UUO model.
Methods
Human HGF Transfection
Met5A cells (American Type Culture Collection, Manassas, VA) are nonmalignant human pleural MCs immortalized by simian virus 40. The HGF plasmid (kindly provided by Kunio Matsumoto, Kanazawa University), with conferred resistance to neomycin, was mixed with Lipofectamine 2000 (Invitrogen) and Opti-MEM (Invitrogen); subsequently, this mixture was added to Met5A cells on the culture dishes. At 6 hours post-transfection, the medium was replaced with complete medium (Medium 199; Thermo Fisher Scientific) containing 10% FBS (Japan Bio Serum), 3.3 nM EGF (Wako), 400 nM hydrocortisone (Sigma-Aldrich), 870 nM zinc-free bovine insulin (Wako), 1% penicillin/streptomycin (Sigma-Aldrich), and 500 μg/ml Geneticin G418 (Sigma-Aldrich). Continuous exposure to G418 resulted in the selection of drug-resistant cells.
Generation of Cell Sheets
MCs and hepatocyte growth factor transgenic (HGF-tg) MCs were seeded onto 35-mm temperature-responsive culture dishes (CellSeed, Inc., Tokyo, Japan) at a density of 1.2×106 cells per dish. Cells were cultured at 37°C for 4 days. Cells were detached as sheets from temperature-responsive dishes without enzyme treatment by reducing the temperature to 20°C for 30 minutes. The prepared cell sheets were transplanted into rats in the animal study described later.
Animal Study
All experimental protocols were approved by the Animal Welfare Committee of Tokyo Women’s Medical University School of Medicine (permit number 14–87). F344/NJcl-rnu/rnu rats (5–8 weeks of age; CLEA Japan, Tokyo, Japan) were divided into five groups: the no sheet (n=10), MC sheet (n=10), HGF-tg MC sheet (n=10), HGF injection (n=5), and HGF sheet on thigh (n=5) groups.
UUO Procedure
The left ureters of rats were ligated as described previously.13,25 The rats were incised from the back to expose the left kidney and ureter. After the left ureter was ligated near the renal pelvis, rats were subjected to various treatments. On day 7 after UUO and treatment, five rats were euthanized from each group. On day 28, the remaining rats were euthanized in the no sheet, MC sheet, and HGF-tg MC sheet groups (n=5 in each case). The vasculature was perfused from the left ventricle with 50 ml of 4% paraformaldehyde (Muto Pure Chemicals). After perfusion, the UUO-treated kidneys were harvested. The parenchymal thickness and the length of the kidney pelvis were then measured.
Transplantation of Cell Sheets onto the Kidney
After UUO in the MC sheet and HGF-tg MC sheet groups, the left renal capsule on the dorsal surface of the kidney was peeled away with tweezers. Two cell sheets were gently placed on the site where the capsule was removed. After standing for 5 minutes, the cell sheets were adhered. In the no sheet group, rats were not given any therapy. In the sham group, rats were not subjected to UUO or given any therapy.
Cell Sheet Transplantation on the Thigh Vascular Bed
In the HGF sheet on thigh group, the left femoral artery and vein were ligated 7 days before UUO. New blood vessels formed an immature vessel network (vascular bed) after 7 days. After UUO, two HGF-tg MC sheets were placed on the vascular bed. After standing for 5 minutes, the cell sheets were adhered.
HGF Injection
After UUO, rats in the HGF injection group received 200 pg recombinant human hepatocyte growth factor (hHGF; R&D Systems, Minneapolis, MN) via the tail vein every 24 hours for 7 days.
ELISA
HGF-tg MCs and nontransgenic MCs were cultured onto temperature-responsive culture dishes at a density of 1.2×106 cells per dish for 4 days. After replacing the medium and culturing for 24 hours, the supernatants were collected, and HGF in the supernatants was detected using ELISA kits (R&D Systems).
Microcomputed Tomography
Rats in three groups (no sheet, MC sheet, and HGF-tg MC sheet) were injected with 100 μl/g of a nonionic iodine-based contrast agent (Omnipark 350) in the tail vein and scanned using contrast-enhanced microcomputed tomography (micro-CT; R_mCT2; Rigaku, Tokyo, Japan) on days 7, 14, 21, and 28. Renal dimensions, parenchyma thickness, and pelvis lengths were measured from the CT images of UUO-treated kidneys. Kidney volumes were estimated from the linear dimensions using the ellipsoid formula (Equation 1)36:
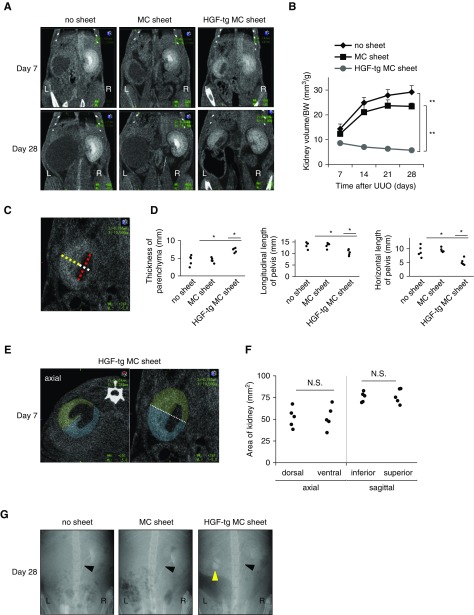
The ratio of the kidney volume to the body weight was compared. With the axial and sagittal CT images, two section areas divided by the center line were measured on day 7 in the HGF-tg MC sheet group.
Intravenous Urography
Intravenous urography in the live x-ray moving image mode of the micro-CT device was performed to evaluate urinary flow into the renal pelvis and ureter from days 7 to 28.
Histologic and Immunohistochemical Analyses
Paraffin-embedded kidney sections were stained with periodic acid–Schiff or Sirius red followed by immunolabeling using antibodies to α-smooth muscle actin (αSMA; M0851; Dako), SV40 T antigen (ab16879; Abcam), rat endothelial cell antigen-1 (ab9774; Abcam), or hHGF (provided by Kunio Matsumoto) and detected using a peroxidase-conjugated polymer reagent (REAL EnVision; Dako; Agilent Technologies, Santa Clara, CA). For phosphor-cMet staining, the sections were stained by Pathology Institute Corp. (Toyama, Japan) using an antibody against phospho-cMet (ab5662; Abcam). The area fraction of Sirius red and αSMA staining was analyzed using ImageJ software (National Institutes of Health, Bethesda, MD).
Capillary Quantification
The numbers of capillaries and tubules were counted in RECA-1–stained sections. The filled RECA-1 area fraction was determined as described previously.37
Ultrasonography
The left UUO-treated kidney was observed using an ultrasound imaging system (Vevo2100; Fujifilm VisualSonics, Toronto, ON, Canada) on day 7. Left renal artery (RA) blood flow was estimated from velocity time integral (VTI), and left renal artery diameter (RAD) was calculated by PW-Doppler echo using Equation 238:
 |
Statistical Analyses
Two-group differences were analyzed by an unpaired t test. Multigroup differences were analyzed by one-way ANOVA followed by the Tukey–Kramer post hoc comparison test using JMP Pro 13.0.0 software (SAS, Cary, NC). Time-dependent data were analyzed by one-way repeated measures ANOVA. P<0.05 was considered statistically significant, and all tests were two sided.
Results
Fabrication of hHGF-Secreting Cell Sheets
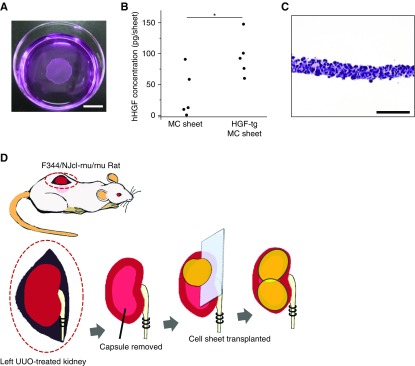
hHGF-tg MCs were harvested as cell sheets on temperature-responsive culture dishes (Figure 1A). After 24 hours of cultivation, the hHGF concentration from the HGF-tg MC sheet was 94.7 pg in 2 ml of culture medium, which was significantly higher than that of the nontransgenic control (P<0.05) (Figure 1B). On the basis of this finding, the amount of HGF produced by two HGF-tg MC sheets was approximately 200 pg at 24 hours.
Figure 1.
Hepatocyte growth factor transgenic (HGF-tg) mesothelial cell (MC) sheets for transplantation onto kidneys secreted HGF. (A) Macroscopic image of an HGF-tg MC sheet detached from a temperature-responsive culture dish. Scale bar, 10 mm. (B) Levels of human hepatocyte growth factor (hHGF) measured by ELISA in the supernatant of MC and HGF-tg MC sheets. (C) Hematoxylin and eosin staining of an HGF-tg MC sheet section. Scale bar, 100 μm. (D) Schematic representation of the experimental procedure used to transplant HGF-tg MC sheets in a rat obstructive nephropathy model. The left kidney was exposed from the back of the immunodeficient rat, and the ureter was ligated. The renal capsule on the dorsal side was removed from the surface of the rat kidney with tweezers. In the HGF-tg MC sheet group, two HGF-tg MC sheets were placed on the site where the renal capsule was removed. In the MC sheet group, two MC sheets (lacking the HGF gene) were inserted. *P<0.05 (n=5 for all groups).
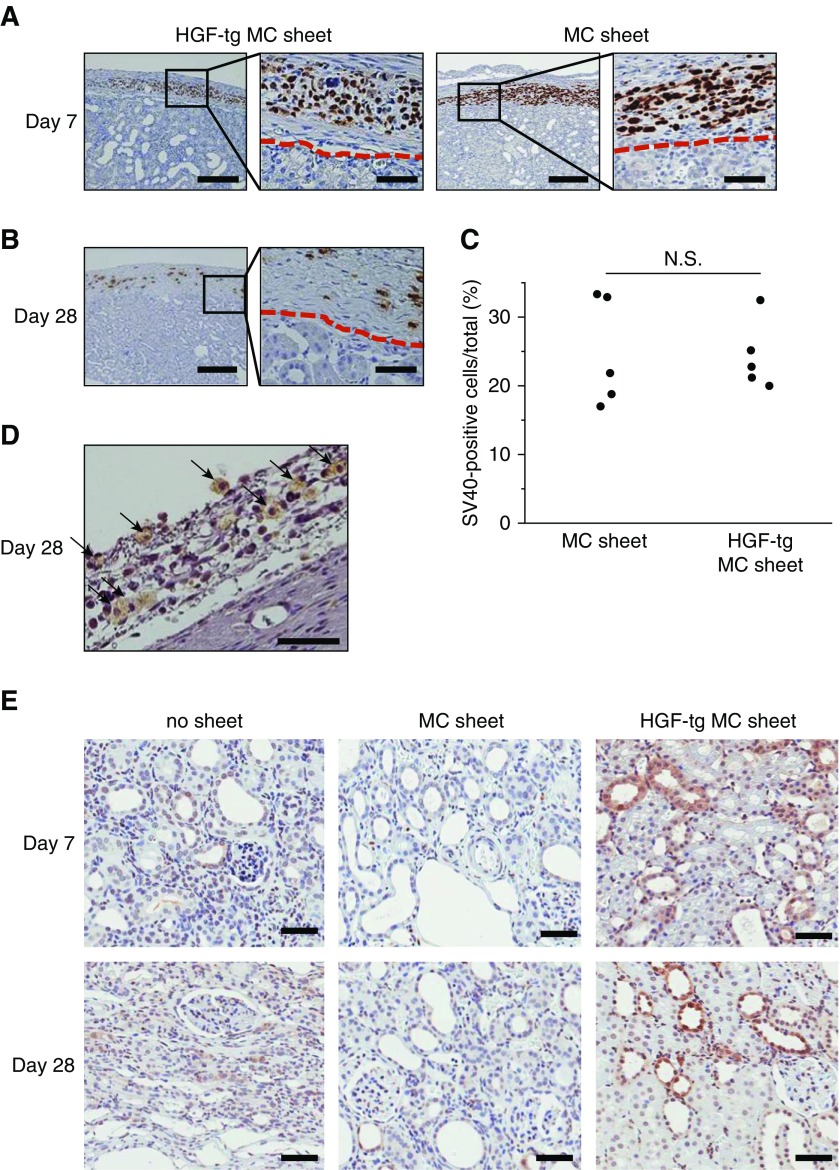
Histologic staining of an HGF-tg MC sheet showed intact cell-to-cell junctions (Figure 1C). Because SV40-T is a marker for Met5A cells, it was used for cell tracing in vivo. Surviving SV40-T–positive cells on the kidney surface were observed on days 7 and 28 without invasion into the parenchyma (Figure 2, A and B). Additionally, no significant difference in the number of SV40-T–positive cells was found between in the HGF-tg MC sheet and the MC sheet groups (Figure 2C). Moreover, hHGF-positive cells remained on the kidney surface on day 28 (Figure 2D). Phospho-cMet staining, which indicates the activated form of the HGF receptor, was positive in some tubular cells, with a greater number of phospho-cMET–positive cells in the HGF-tg MC sheet group versus that in the no sheet and MC sheet groups on days 7 and 28 after UUO (Figure 2E).
Figure 2.
Survival of cell sheets transplanted onto an immunodeficient rat kidney for 28 days. (A) SV40-T–immunostained image of hepatocyte growth factor transgenic (HGF-tg) mesothelial cell (MC) sheets (left panel) and MC sheets (right panel) on day 7 after transplantation onto an immunodeficient rat kidney. Higher-magnification photomicrographs of the border (red dashed line) between the capsule and cortex are shown on the right side of each panel, and they correspond to the boxed areas. Scale bars, 200 μm in the left side of each panel; 20 μm in the right side of each panel. (B) SV40-T–immunostained image of HGF-tg MC sheets on day 28 after transplantation onto an immunodeficient rat kidney. Lower (right panel) and higher (left panel) magnification images. Scale bars, 200 μm in the left panel; 20 μm in the right panel. (C) Fractions of simian virus 40 (SV40)–positive cells on the renal surface on day 7 after transplanting MC sheets or HGF-tg MC sheets. (D) Human hepatocyte growth factor (hHGF)–immunostained image of an HGF-tg MC sheet on day 28 after transplantation onto an immunodeficient rat kidney. Black arrows indicate hHGF-positive cells in the renal capsule. Scale bar, 50 μm. (E) Phospho-cMET–immunostained images of unilateral ureteral obstruction (UUO)–treated kidneys on days 7 (upper panels) and 28 (lower panels) after UUO in the no transplantation (no sheet), MC sheet, or HGF-tg MC sheet groups. P<0.05 (n=5 for all groups). Scale bar, 50 μm.
HGF-tg MC Sheets Suppressed Myofibroblasts and Interstitial Fibrosis in UUO Rats on Day 7
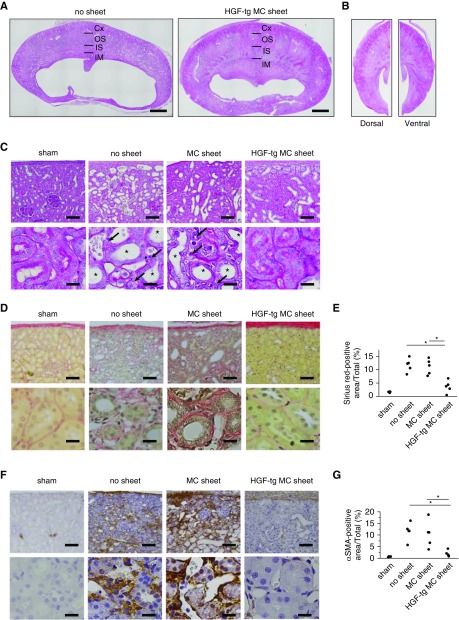
On whole-kidney cross-sections on day 7, the no sheet group showed an expanded renal pelvis and substantially atrophied parenchyma (especially the inner stripe and inner medulla) (Figure 3A, Supplemental Figure 1), whereas the HGF-tg MC sheet decreased the level of atrophy (Figure 3A). Comparison of the dorsal and ventral sides of axial sections revealed no differences in morphology and pathology (Figure 3B, Supplemental Figure 2), suggesting no difference in effect on the basis of the distance from the cell sheet.
Figure 3.
Transplantation of hepatocyte growth factor transgenic (HGF-tg) mesothelial cell (MC) sheets on unilateral ureteral obstruction (UUO)–treated kidneys contributed to the suppression of fibrosis on day 7. (A) Periodic acid–Schiff-stained images of whole UUO–treated kidney cross-sections on day 7 after UUO in groups with no transplantation (left panel) or transplantation of HGF-tg MC sheets (right panel). Scale bar, 2 mm. (B) Periodic acid–Schiff-stained images of UUO-treated kidney axial sections in the HGF-tg MC sheet group on day 7. The dorsal surface (left panel) is the site of HGF-tg MC sheet transplantation, whereas the ventral surface (right panel) does not represent a transplantation site. (C) Each pair of representative periodic acid–Schiff-stained images shows low (upper panels) and high (lower panels) magnifications of the renal cortical interstitial area on day 7 after UUO. Treatments included sham operation, no transplantation (no sheet), or transplantation of an MC sheet or HGF-tg MC sheet onto the kidney. Black arrows indicate increased cells in the expanded interstitium. Scale bars, 100 μm in upper panels; 20 μm in lower panels. *Dilated tubule. (D) Each pair of representative Sirius red–stained images shows low (upper panel) and high (lower panel) magnification of the renal cortical interstitial area on day 7 after UUO. Scale bars, 100 μm in upper panels; 20 μm in lower panels. (E) Percentages of Sirius red–positive areas in each sample; quantification was performed using ImageJ software. (F) Each pair of representative α-smooth muscle actin (αSMA)–immunostained images shows low (upper panel) and high (lower panel) magnifications of the renal cortical interstitial area on day 7 after UUO. Scale bars, 100 μm in the upper panel; 20 μm in the lower panel. (G) Percentages of αSMA-positive areas. Quantification was performed using ImageJ software. Cx, cortex; IM, inner medulla; IS, inner stripe; OS, outer stripe. *P<0.01 (n=5 for all groups).
To evaluate the effect of the HGF-tg MC sheet, the HGF-tg MC sheet group was compared with the no sheet and MC sheet groups. In the HGF-tg MC sheet group, we observed fewer damaged and dilated tubules (Figure 3C). Sirius red staining, which enables collagen detection, was considerably lower in the HGF-tg MC sheet group than in the other two groups (3.6% versus approximately 11.1%–11.7%) (Figure 3, D and E). Positively stained areas for αSMA, a marker of myofibroblasts, were greatly reduced in the HGF-tg MC sheet group compared with those in the other two groups (1.9% versus approximately 10.3%–11.6%) (Figure 3, F and G).
To examine the necessity for the HGF-tg MC sheet to be in contact with the kidney, the HGF-tg MC sheet group was compared with two groups with different HGF supply routes: the HGF injection and HGF sheet on thigh groups. In both groups, we observed damaged and dilated tubules as well as expansion of the interstitial area (Supplemental Figure 3), similar to that observed in the no sheet group. Additionally, the areas positive for Sirius red and αSMA staining in the no sheet group were similar to those in both the HGF injection and HGF sheet on thigh groups (P<0.01) (Supplemental Figures 4 and 5).
HGF-tg MC Sheets Provided a Sustained Renal-Protective Effect
To assess the long-term effects of the HGF-tg MC sheet, the kidneys were analyzed until day 28 in the no sheet, MC sheet, and HGF-tg MC sheet groups. The parenchyma of UUO-treated kidneys without HGF-tg MC sheets became poorly distinguished and thin on the CT image (Figure 4A). In contrast, kidneys in the HGF-tg MC sheets group maintained a distinct parenchyma that was preserved until day 28 (Figure 4A). Additionally, the kidney volume per gram body weight was significantly lower in the HGF-tg MC sheet group than in the other two groups (P<0.01) (Figure 4B). On day 7 in the HGF-tg MC sheet group, we observed that the parenchyma was thicker and that the renal pelvis had expanded less compared with those parameters in the other two groups (P<0.05) (Figure 4, C and D). Additionally, the two cross-sections divided by the center line were highly similar in the sagittal and axial CT images (Figure 4, E and F), indicating no difference in the effect associated with the distance from the cell sheet.
Figure 4.
Transplantation of hepatocyte growth factor transgenic (HGF-tg) mesothelial cell (MC) sheets inhibits volumetric expansion of unilateral ureteral obstruction (UUO)–treated kidneys at 28 days. (A) Representative contrast-enhanced computed tomography (CT) images on days 7 and 28 after UUO in groups with no transplantation (no sheet), transplantation of an MC sheet, and transplantation of an HGF-tg MC sheet onto the kidney. (B) The kidney volume per gram body weight (BW) was estimated using the ellipsoid method (on the basis of CT images) at the indicated time points after UUO (kidney volume = length × width × thickness ×π/6). (C) Sagittal CT image on day 7 after UUO. The yellow line shows the thickness of the parenchyma. The white line shows the length of the pelvis. The red line shows the length of the pelvis. (D) Renal parenchyma thickness (left panel) and renal pelvis (center and right panels) lengths of the kidney at 7 days after UUO (n=5 for all groups). (E) Axial (left panel) and sagittal (right panel) CT images on day 7 after UUO in the HGF-tg MC sheet group. The axial image (green) represents the dorsal area, and the blue image represents the ventral area. The sagittal image (green) represents the superior area, and blue represents the inferior area. (F) For each area of the kidney on the CT images, quantification was performed using ImageJ software (n=5 for all groups). (G) Representative intravenous urography images 28 days after UUO from the no sheet, MC sheet, and HGF-tg MC sheet groups. The white arrowheads indicate the right ureters. The yellow arrowhead indicates the left renal pelvis. L, left; R, right. *P>0.05; **P>0.01.
In the intravenous urography images of all three groups, the contrast mediums flowed through the right (healthy) kidneys and ureters but did not flow through the left ureters, which were closed by ligation. In only the HGF-tg MC sheet group, the contrast mediums accumulated in the left renal pelvis, indicating that the produced urine accumulated before the closed site (Figure 4G).
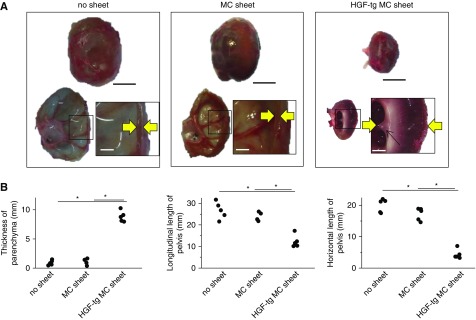
On day 28 and in both the no sheet and MC sheet groups, UUO-treated kidneys showed a bloated, balloon-like shape with fluid inside (Figure 5A), with histologic cross-sections of the bloated kidneys showing a large dilated pelvis and thin parenchyma (Figure 5A). By contrast, in the HGF-tg MC sheet group, UUO-treated kidneys showed a dilated pelvis and visible renal papillae (which form part of the medulla) in the thick parenchyma (Figure 5A).
Figure 5.
Unilateral ureteral obstruction (UUO) kidneys treated with hepatocyte growth factor transgenic (HGF-tg) mesothelial cell (MC) sheets retained their parenchyma for 28 days. (A) Representative photographs of a whole kidney (upper), transaxial cross-section (lower left), and parenchyma (lower right; corresponding to the boxed area) on day 28 after UUO using specimens obtained from the no sheet (left panel), MC sheet (center panel), and HGF-tg MC sheet (right panel) groups. The areas between the yellow arrows indicate the thickness of the parenchyma. Scale bars, 10 mm in upper panels; 2 mm in lower panels. (B) Renal parenchyma thickness (left panel) and renal pelvis longitudinal (center panel) and horizontal (right panel) lengths of the kidney at 28 days after UUO in the no sheet, MC sheet, and HGF-tg MC sheet groups. *P<0.01 (n=5 for all groups).
The parenchymal thickness in the HGF-tg MC sheet group was 8.8 mm, which was approximately eightfold thicker than that observed in the other two groups (P<0.01) (Figure 5B). Additionally, the longitudinal and horizontal lengths of the renal pelvis in the HGF-tg MC sheet group increased by significantly lower amounts compared with those in the other two groups (P<0.01) (Figure 5B).
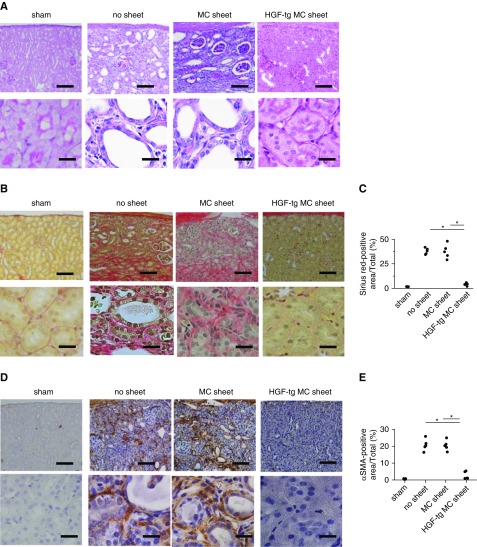
In both the no sheet and MC sheet groups, few glomeruli and dilated tubules remained in thin parenchyma. By contrast, in the HGF-tg MC sheet group, a thick parenchyma was preserved, and the tubules displayed a normal appearance (Figure 6A).
Figure 6.
Hepatocyte growth factor transgenic (HGF-tg) mesothelial cell (MC) sheet transplantation on unilateral ureteral obstruction (UUO)–treated kidneys contributed to the suppression of fibrosis on day 28 after UUO. (A) Each pair of representative periodic acid–Schiff-stained images shows low (upper panels) and high (lower panels) magnifications of the renal cortical interstitial area on day 28 after UUO. Treatments included sham operation, no transplantation (no sheet), and transplantation of an MC sheet or HGF-tg MC sheet onto the kidney. Scale bars, 100 μm in upper panels; 20 μm in lower panels. (B) Each pair of representative Sirius red–stained images shows low (upper panels) and high (lower panels) magnifications of the renal cortical interstitial area on day 28 after UUO. Scale bars, 100 μm in upper panels; 20 μm in lower panels. (C) Percentage of Sirius red–positive areas in each sample. Quantification was performed using ImageJ software. (D) Each pair of representative α-smooth muscle actin (αSMA)–immunostained images shows low (upper panels) and high (lower panels) magnifications of the renal cortical interstitial area on day 28 after UUO. Scale bars, 100 μm in upper panels; 20 μm in lower panels. (E) Percentage of αSMA-positive areas. Quantification was performed using ImageJ software. *P<0.01 (n=5 for all groups).
On day 28, the area positive for Sirius red staining in the HGF-tg MC sheet group was almost ninefold lower than in the other two groups (P<0.01) (Figure 6, B and C). Similarly, the amount of αSMA (a myofibroblast index) was significantly lower in the HGF-tg MC sheet group than in the other two groups (approximately 2.6% versus approximately 20.4%; P<0.01) (Figure 6, D and E). Overall, our findings indicate that direct HGF-tg MC sheet transplantation exerted a marked renal-protective effect until at least day 28.
HGF-tg MC Sheets Ameliorated the Reduction of PTCs
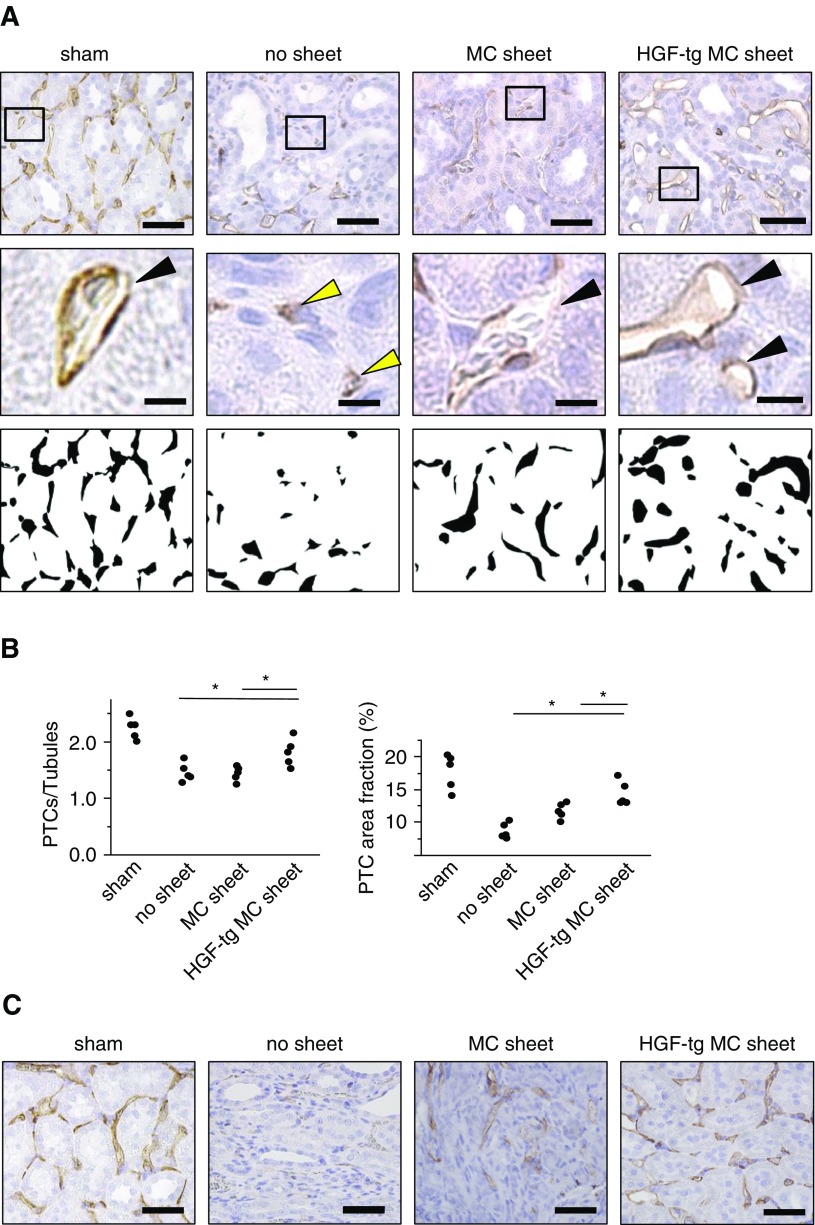
To assess its effect on PTCs, we evaluated the PTC status by staining for RECA-1. On day 7 in the no sheet group, many PTCs displayed an abnormal vasculature (i.e., a collapsed lumen structure) (Figure 7A). Moreover, the ratio of PTCs to tubules, which indicates the PTC density, was significantly higher in the HGF-tg MC sheet group than in the other two groups (P<0.05) (Figure 7B). Additionally, the filled RECA-1–stained area, which reflects the renal vascular volume,37,39 was 13.7% in the HGF-tg MC sheet group, significantly higher than those in the other two groups (Figure 7B).
Figure 7.
Hepatocyte growth factor transgenic (HGF-tg) mesothelial cell (MC) sheet transplantation on unilateral ureteral obstruction (UUO)–treated kidneys contributed to the amelioration of peritubular capillary injury. (A) Representative images of RECA-1–positive peritubular capillaries (PTCs; top panels) and RECA-1–positive PTC-filled areas transformed into binary images (bottom panels) in the kidney cortex of UUO-treated kidneys on day 7 from sham, no sheet, MC sheet, and HGF-tg MC sheet transplantation groups. The images in the middle panels show a higher magnification of the PTC lumen structure in the cortex (corresponding to the boxed areas in the upper images). Black arrowheads indicate maintained lumen structures of PTCs, and yellow arrowheads indicate collapsed lumen structures of PTCs. Scale bars, 50 μm in top panels; 10 μm in middle panels. (B) Ratios of the numbers of RECA-1–positive PTCs to tubules (left panel) and percentages of RECA-1–positive PTC luminal areas (right panel) in each RECA-1–stained section on day 7 after UUO. Quantification was performed using ImageJ software. (C) Representative images of RECA-1–positive PTCs in the cortex of UUO-treated kidneys on day 28. Scale bars, 50 μm. *P<0.05 (n=5 for all groups).
In both the no sheet and MC sheet groups, RECA-1–positive PTCs were widely distributed between injured tubules on day 28 (Figure 7C). In contrast, RECA-1–positive PTCs were almost normal and aligned in an orderly fashion surrounding the renal tubules on day 28 in the HGF-tg MC sheet group (Figure 7C). These results indicated that the HGF-tg MC sheets prevented the reduction of PTCs on days 7 and 28 after the UUO operation.
The HGF-tg MC Sheet Improved Renal Blood Flow
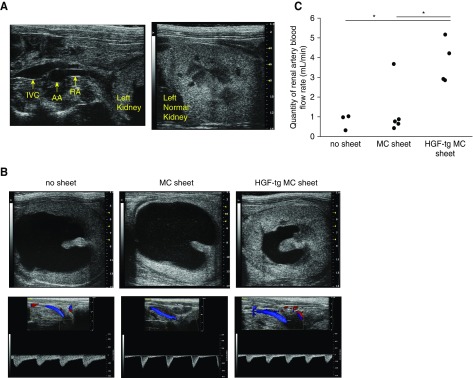
To evaluate renal blood flow (RBF) on day 7 after UUO, RA flow was analyzed in PW-Doppler mode (Figure 8, A and B). Although the no sheet and MC sheet groups showed an expanded renal pelvis and thinned parenchyma according to ultrasonography, the parenchymal thickness was maintained in the HGF-tg MC sheet group (Figure 8B). Additionally, in the HGF-tg MC sheet group, the left RA flow rate was 3.7 ml/min, which was significantly higher than that observed in the other two groups (P<0.05) (Figure 8C). These results indicated that the HGF-tg MC sheet attenuated reductions in RBF in UUO-treated kidneys.
Figure 8.
Transplantation of hepatocyte growth factor transgenic (HGF-tg) mesothelial cell (MC) sheets improved renal blood flow (RBF) in unilateral ureteral obstruction (UUO)–treated kidneys. (A) Echo images of the left renal artery (RA; left panel) and a healthy left kidney in the sham group (right panel). (B) Representative echo images of the left kidney (upper panels) on day 7 after UUO in groups with no transplantation, transplantation of MC sheets, or transplantation of HGF-tg MC sheets onto the kidney. Representative color Doppler images and Doppler waveforms of the RA flow (lower panels). (C) Quantity of renal artery blood flow estimated from the velocity time integral (VTI) and renal artery diameter (RAD) by PW-Doppler echo on day 7 after UUO, where the renal artery blood flow (milliliters per minute) = VTI (meters per minute) × cross-section area (CSA; millimeters squared) ×10−3: CSA (millimeters squared) =π/4× (RAD).2 AA, abdominal aorta; IVC, inferior vena cava. *P<0.05 for the sham (n=1), no sheet (n=3), MC sheet (n=5), and HGF-tg MC sheet (n=4) groups.
Discussion
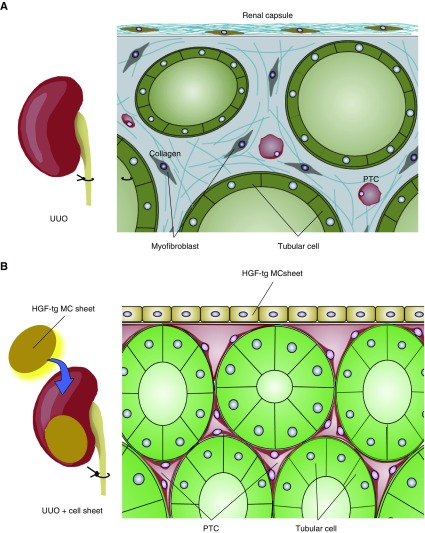
HGF-tg MC sheets increased HGF receptor phosphorylation in tubular cells in UUO-treated kidneys and suppressed the induction of myofibroblasts and interstitial fibrosis, whereas MC sheets lacking the hHGF gene did not promote phosphorylation of HGF receptor and did not suppress interstitial fibrosis. Because there were almost equal numbers of HGF-tg MCs and MCs after transplantation, this antifibrotic effect was on the basis of the release of HGF. Furthermore, the protection was dependent on the method used to supply HGF, and it was important for the HGF-tg MC sheet to be in contact with the kidney, because we observed no benefit from intravenous administration of HGF or transplantation HGF-tg MC sheets in a remote location, even with the same dose of HGF as that secreted by the HGF-tg MC sheets. Immunohistochemistry showed that grafted HGF-tg MC sheets remained and maintained their HGF secretion ability and increased levels of activated HGF receptor at 28 days after transplantation. As a result, HGF-tg MC sheets suppressed fibrosis for at least this time period. Furthermore, HGF-tg MC sheets prevented the reduction of PTCs and maintained RBF until day 28 in UUO-treated kidneys. We speculate that the maintenance of RBF contributed to the long-term inhibition of progressive fibrosis in UUO-treated kidneys. The model for cell sheet–mediated amelioration of UUO-treated kidney suggested by our findings is shown in Figure 9.
Figure 9.
Hepatocyte growth factor transgenic (HGF-tg) mesothelial cell (MC) sheet ameliorationed of damage to unilateral ureteral obstruction (UUO)–treated kidneys. (A) In UUO-treated kidney, peritubular capilaries (PTCs) reduced and RBF decreased. Myofibroblasts and collagen increased, and renal fibrosis progressed. (B) Two HGF-tg MC sheets were transplanted on the surface of the UUO-treated kidney. The transplanted cell sheets can adhere and survive on the surface of the kidney for a long duration. The HGF-tg MC sheets produce hepatocyte growth factor (HGF) at the site in contact with the kidney and activate the HGF receptor in the kidney. HGF prevents the reduction of peritubular capillaries (PTCs), maintains renal blood flow, and effectively suppresses the induction of myofibroblast progression and collagen synthesis for a long duration. As a result, transplantation of HGF-tg MC sheets can inhibit the progression of renal fibrosis.
In this study, the HGF-tg MC sheets were still on the kidney surface on day 28 after transplantation. This is because the bioactive ECM in the bottom layer of the cell sheets enabled them to adhere and survive.40 The transplanted cell sheets survived a longer time in vivo than transplanted cell suspensions.41 Although the cell sheets were transplanted at the site where the renal capsule was removed, capsule cells were found under the grafted cells after transplantation. This finding suggests that capsule cells might proliferate under the grafted cells.
Because the HGF-tg MC sheets remained intact and produced HGF at the site in contact with the kidney, they suppressed fibrosis more effectively than other approaches that simply provided an internal supply of HGF. Transplantation of cell sheets onto organs also supplies cytokines directly. Adjacent HGF-secreting cells are reported to exert HGF-like effects through the organ.34,42 In this study, HGF-tg MC sheets affected both the area under the transplanted cell sheet and also, the whole kidney. Although the route whereby HGF enters from the cell sheet into the parenchyma is not clear, our findings suggest that HGF circulated in a manner similar to that of RBF.
HGF-tg MC sheets prevented PTC injury and maintained RA blood flow. The possibility exists that HGF from cell sheets affected the renal vascular. Considering that the HGF receptor was mainly activated in tubular cells, the effects might have been mediated by other cells. Previous findings showed that HGF stimulated neutrophils to induce the production of nitric oxide synthase,18 which is involved in vasodilation. Although it doesn't directly affect PTC blood flow, maintaining RA blood flow leads to improved renal ischemia. Thus, these effects might contribute to the suppression of fibrosis in UUO-treated kidneys, considering that hypoxia may lead to the development of interstitial fibrosis.7
Moreover, transplanted HGF-tg sheets may have helped to relax the increase of mechanical pressure on tubules caused by UUO stimulation. In this study, the renal pelvis and tubules suppressed dilation in only the HGF-tg MC sheet group. These results suggest that the effect of an HGF-tg sheet on the kidney is to alleviate the mechanical pressure on tubules induced by UUO.
To date, HGF therapy has failed to achieve long-term inhibition of fibrosis, because its effects are unsustainable,26,27 and it was reported to suppress renal fibrosis for only 7 days in animal models.13,25 A direct and continuous supply of HGF to the kidney using cell sheets avoids elimination in other organs and achieves long-term inhibition of fibrosis. This cell sheet therapy represents a promising approach to treating CKD requiring long-term therapy. Our study is the first report of using cell sheet therapy to treat kidney disease.
In our study, the effects of HGF-tg MC sheets may have been due to a combination of HGF effects and other effects from the MCs, which exert various functions, including anti-inflammatory effects,33 and they might contribute to suppressing disorders induced by UUO. Although our results comparing MCs with HGF-tg MC sheets showed that HGF secretion from transplanted cells was important, we did not investigate HGF concentrations in the blood, urine, and tissues in vivo or its protective mechanisms, especially the role of TGF-β signaling. Additionally, we did not exhaustively analyze other non-HGF effectors from MCs and HGF-tg MCs, and we did not analyze the effects of chronic inflammation and mechanical pressure induced by UUO. Another limitation of this study was the absence of experiments designed to investigate the molecular pathways used by HGF to induce the observed changes in kidney cells.
Overall, this study showed that transplantation of HGF-tg MC sheets onto UUO-treated kidneys suppressed interstitial fibrosis, ameliorated the reduction of PTCs, and maintained RBF for 28 days. These results suggested that cell sheet transplantation onto kidneys represents a useful strategy for long-term inhibition of the progression of renal fibrosis.
Disclosures
T.S. is a member of the scientific advisory board and a shareholder of CellSeed, Inc., Tokyo Women’s Medical University and received research funds from CellSeed, Inc.
Supplementary Material
Acknowledgments
We are grateful to Dr. Kunio Matsumoto (professor at the Cancer Research Institute at Kanazawa University) for kindly providing the hepatocyte growth factor plasmid vector and the anti-human hepatocyte growth factor antibody. We would like to thank Editage for English language editing.
This study was supported by the Creation of Innovation Centers for Advanced Interdisciplinary Research Areas Program of the Project for Developing Innovation Systems (“Cell Sheet Tissue Engineering Center”) funded by the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018050556/-/DCSupplemental.
Supplemental Figure 1. Periodic acid–Schiff-stained image of a whole-kidney cross-section on day 7 after the sham operation in the sham group.
Supplemental Figure 2. High-magnification, periodic acid–Schiff-stained images of an axial section of a UUO-treated kidney from the HGF-tg MC-sheet group on day 7.
Supplemental Figure 3. Each pair of representative periodic acid–Schiff-stained images show low (top) and high (bottom) magnifications of the renal cortical interstitial area on day 7 after UUO.
Supplemental Figure 4. Each pair of representative Sirius red-stained images show low (top) and high (bottom) magnifications of the renal cortical interstitial area on day 7 after UUO.
Supplemental Figure 5. Low (top) and high (bottom) magnifications of representative αSMA-immunostained images of the renal cortical interstitial area on day 7 after UUO.
References
- 1.Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, et al.: Chronic kidney disease: Global dimension and perspectives. Lancet 382: 260–272, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Eddy AA: Experimental insights into the tubulointerstitial disease accompanying primary glomerular lesions. J Am Soc Nephrol 5: 1273–1287, 1994 [DOI] [PubMed] [Google Scholar]
- 3.Nath KA: Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis 20: 1–17, 1992 [DOI] [PubMed] [Google Scholar]
- 4.Eardley KS, Kubal C, Zehnder D, Quinkler M, Lepenies J, Savage CO, et al.: The role of capillary density, macrophage infiltration and interstitial scarring in the pathogenesis of human chronic kidney disease. Kidney Int 74: 495–504, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Ohashi R, Shimizu A, Masuda Y, Kitamura H, Ishizaki M, Sugisaki Y, et al.: Peritubular capillary regression during the progression of experimental obstructive nephropathy. J Am Soc Nephrol 13: 1795–1805, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Inoue T, Kozawa E, Okada H, Inukai K, Watanabe S, Kikuta T, et al.: Noninvasive evaluation of kidney hypoxia and fibrosis using magnetic resonance imaging. J Am Soc Nephrol 22: 1429–1434, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, Hohenstein B, et al.: Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest 117: 3810–3820, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsumoto M, Tanaka T, Yamamoto T, Noiri E, Miyata T, Inagi R, et al.: Hypoperfusion of peritubular capillaries induces chronic hypoxia before progression of tubulointerstitial injury in a progressive model of rat glomerulonephritis. J Am Soc Nephrol 15: 1574–1581, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Tanaka S, Tanaka T, Nangaku M: Hypoxia as a key player in the AKI-to-CKD transition. Am J Physiol Renal Physiol 307: F1187–F1195, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Nangaku M: Chronic hypoxia and tubulointerstitial injury: A final common pathway to end-stage renal failure. J Am Soc Nephrol 17: 17–25, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Humes HD, Cieslinski DA, Coimbra TM, Messana JM, Galvao C: Epidermal growth factor enhances renal tubule cell regeneration and repair and accelerates the recovery of renal function in postischemic acute renal failure. J Clin Invest 84: 1757–1761, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeisberg M, Hanai J, Sugimoto H, Mammoto T, Charytan D, Strutz F, et al.: BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med 9: 964–968, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Mizuno S, Matsumoto K, Nakamura T: Hepatocyte growth factor suppresses interstitial fibrosis in a mouse model of obstructive nephropathy. Kidney Int 59: 1304–1314, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Hayashi S, Morishita R, Nakamura S, Yamamoto K, Moriguchi A, Nagano T, et al.: Potential role of hepatocyte growth factor, a novel angiogenic growth factor, in peripheral arterial disease: Downregulation of HGF in response to hypoxia in vascular cells. Circulation 100[Suppl]: 11301–11308, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Morishita R, Aoki M, Hashiya N, Makino H, Yamasaki K, Azuma J, et al.: Safety evaluation of clinical gene therapy using hepatocyte growth factor to treat peripheral arterial disease. Hypertension 44: 203–209, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Kaga T, Kawano H, Sakaguchi M, Nakazawa T, Taniyama Y, Morishita R: Hepatocyte growth factor stimulated angiogenesis without inflammation: Differential actions between hepatocyte growth factor, vascular endothelial growth factor and basic fibroblast growth factor. Vascul Pharmacol 57: 3–9, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Makondo K, Kimura K, Kitamura N, Kitamura T, Yamaji D, Jung BD, et al.: Hepatocyte growth factor activates endothelial nitric oxide synthase by Ca(2+)- and phosphoinositide 3-kinase/Akt-dependent phosphorylation in aortic endothelial cells. Biochem J 374: 63–69, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finisguerra V, Di Conza G, Di Matteo M, Serneels J, Costa S, Thompson AA, et al.: MET is required for the recruitment of anti-tumoural neutrophils. Nature 522: 349–353, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang J, Liu Y: Blockage of tubular epithelial to myofibroblast transition by hepatocyte growth factor prevents renal interstitial fibrosis. J Am Soc Nephrol 13: 96–107, 2002 [DOI] [PubMed] [Google Scholar]
- 20.LeBleu VS, Taduri G, O’Connell J, Teng Y, Cooke VG, Woda C, et al.: Origin and function of myofibroblasts in kidney fibrosis. Nat Med 19: 1047–1053, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J, Qu X, Bertram JF: Endothelial-myofibroblast transition contributes to the early development of diabetic renal interstitial fibrosis in streptozotocin-induced diabetic mice. Am J Pathol 175: 1380–1388, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong KM, Belperio JA, Keane MP, Burdick MD, Strieter RM: Differentiation of human circulating fibrocytes as mediated by transforming growth factor-β and peroxisome proliferator-activated receptor γ. J Biol Chem 282: 22910–22920, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Yo Y, Morishita R, Nakamura S, Tomita N, Yamamoto K, Moriguchi A, et al.: Potential role of hepatocyte growth factor in the maintenance of renal structure: Anti-apoptotic action of HGF on epithelial cells. Kidney Int 54: 1128–1138, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Gong R, Rifai A, Dworkin LD: Anti-inflammatory effect of hepatocyte growth factor in chronic kidney disease: Targeting the inflamed vascular endothelium. J Am Soc Nephrol 17: 2464–2473, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Kawaida K, Matsumoto K, Shimazu H, Nakamura T: Hepatocyte growth factor prevents acute renal failure and accelerates renal regeneration in mice. Proc Natl Acad Sci U S A 91: 4357–4361, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ido A, Moriuchi A, Kim I, Numata M, Nagata-Tsubouchi Y, Hasuike S, et al.: Pharmacokinetic study of recombinant human hepatocyte growth factor administered in a bolus intravenously or via portal vein. Hepatol Res 30: 175–181, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Appasamy R, Tanabe M, Murase N, Zarnegar R, Venkataramanan R, Van Thiel DH, et al.: Hepatocyte growth factor, blood clearance, organ uptake, and biliary excretion in normal and partially hepatectomized rats. Lab Invest 68: 270–276, 1993 [PubMed] [Google Scholar]
- 28.Okano T, Yamada N, Okuhara M, Sakai H, Sakurai Y: Mechanism of cell detachment from temperature-modulated, hydrophilic-hydrophobic polymer surfaces. Biomaterials 16: 297–303, 1995 [DOI] [PubMed] [Google Scholar]
- 29.Shimizu T, Yamato M, Kikuchi A, Okano T: Cell sheet engineering for myocardial tissue reconstruction. Biomaterials 24: 2309–2316, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Sawa Y, Miyagawa S, Sakaguchi T, Fujita T, Matsuyama A, Saito A, et al.: Tissue engineered myoblast sheets improved cardiac function sufficiently to discontinue LVAS in a patient with DCM: Report of a case. Surg Today 42: 181–184, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Nishida K, Yamato M, Hayashida Y, Watanabe K, Yamamoto K, Adachi E, et al.: Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med 351: 1187–1196, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Sekiya S, Shimizu T, Yamato M, Okano T: Hormone supplying renal cell sheet in vivo produced by tissue engineering technology. Biores Open Access 2: 12–19, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawanishi K, Yamato M, Sakiyama R, Okano T, Nitta K: Peritoneal cell sheets composed of mesothelial cells and fibroblasts prevent intra-abdominal adhesion formation in a rat model. J Tissue Eng Regen Med 10: 855–866, 2016 [DOI] [PubMed] [Google Scholar]
- 34.Siltanen A, Kitabayashi K, Lakkisto P, Mäkelä J, Pätilä T, Ono M, et al.: hHGF overexpression in myoblast sheets enhances their angiogenic potential in rat chronic heart failure. PLoS One 6: e19161, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Misseri R, Meldrum DR, Dinarello CA, Dagher P, Hile KL, Rink RC, et al.: TNF-α mediates obstruction-induced renal tubular cell apoptosis and proapoptotic signaling. Am J Physiol Renal Physiol 288: F406–F411, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Breau RH, Clark E, Bruner B, Cervini P, Atwell T, Knoll G, et al.: A simple method to estimate renal volume from computed tomography. Can Urol Assoc J 7: 189–192, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bábíčková J, Klinkhammer BM, Buhl EM, Djudjaj S, Hoss M, Heymann F, et al.: Regardless of etiology, progressive renal disease causes ultrastructural and functional alterations of peritubular capillaries. Kidney Int 91: 70–85, 2017 [DOI] [PubMed] [Google Scholar]
- 38.Kawakami S, Yasuno T, Matsuda T, Fujimi K, Ito A, Yoshimura S, et al.: Association between exercise intensity and renal blood flow evaluated using ultrasound echo. Clin Exp Nephrol 22: 1061–1068, 2018 [DOI] [PubMed] [Google Scholar]
- 39.Ehling J, Bábíčková J, Gremse F, Klinkhammer BM, Baetke S, Knuechel R, et al.: Quantitative micro-computed tomography imaging of vascular dysfunction in progressive kidney diseases. J Am Soc Nephrol 27: 520–532, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kushida A, Yamato M, Konno C, Kikuchi A, Sakurai Y, Okano T: Decrease in culture temperature releases monolayer endothelial cell sheets together with deposited fibronectin matrix from temperature-responsive culture surfaces. J Biomed Mater Res 45: 355–362, 1999 [DOI] [PubMed] [Google Scholar]
- 41.Sekine H, Shimizu T, Dobashi I, Matsuura K, Hagiwara N, Takahashi M, et al.: Cardiac cell sheet transplantation improves damaged heart function via superior cell survival in comparison with dissociated cell injection. Tissue Eng Part A 17: 2973–2980, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Onitsuka I, Tanaka M, Miyajima A: Characterization and functional analyses of hepatic mesothelial cells in mouse liver development. Gastroenterology 138: 1525–1535, 2010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.