Abstract
Context:
Pure neuritic leprosy is a risk factor for grade 2 disability owing to the early nerve damage.
Aims:
To study the clinical patterns of neuritic leprosy, to determine the percentage of patients manifesting grade 2 disability at the time of diagnosis and to identify any risk factors for the same.
Settings and Design:
Retrospective descriptive study from previous case records of pure neuritic leprosy patients who attended a tertiary centre from 1st July 2007 to 30th June 2017.
Subjects and Methods:
Data on patients who satisfied the World Health Organization (WHO) cardinal criteria for diagnosis of leprosy, who had no skin lesion of leprosy and had acid-fast bacilli negative status on skin smears were collected using a pre-set proforma.
Statistical Analysis Used:
The Chi-square test was used to assess statistical significance and logistic regression model was applied to avoid the effects of confounding factors.
Results:
A diagnostic delay of >1 year was observed in 44% patients. At the time of diagnosis, grade 2 disability was documented in 60 (80%) of patients. No statistically significant risk factor was identified for grade 2 disability.
Limitations:
Retrospective nature and the study conducted in a tertiary care centre not reflecting the status in the community were the limitations.
Conclusions:
Grade 2 disability noted in 80% of patients points to the inherent nature of disease to cause early nerve damage. Diagnostic delay of >1 year documented in 44% of patients underscores the diagnostic challenges in the absence of skin lesions.
Keywords: Grade 2 disability, neuritic leprosy, risk factor
Introduction
Among the various clinical presentations of leprosy, the most challenging to diagnose is the pure neuritic type where the disease manifests with involvement of peripheral nerves alone. Only the Indian Association of Leprologists has recognized 'pure neuritic leprosy' as a separate entity.[1] The widely used classification system of Ridley Jopling does not include pure neuritic leprosy.[2]
Pure neuritic leprosy may remain undiagnosed for a longer time compared to leprosy with skin lesions since those with skin lesions often get an early dermatology referral and a patient presenting with neuritic symptoms alone often seeks treatment in the neurology or medicine departments. Reduced awareness regarding leprosy leads to considerable delay in diagnosing this variant. Most of the neuritic leprosy cases already develop grade 2 disability by the time of diagnosis itself.[3] The World Health Organization (WHO) has urged the countries to bring down the prevalence of grade 2 disability at the time of diagnosis by an early diagnosis and treatment of the affected in its strive to abolish the stigma associated with the disease.[4]
Hence, we thought it was worthwhile to carry out a 10-year retrospective study on neuritic leprosy among patients who were diagnosed to have leprosy from our tertiary care institution aimed to understand the variable clinical presentations of this variant of leprosy, to determine the percentage of patients manifesting grade 2 disability at the time of diagnosis and to study the role of age, sex, occupation, smoking, alcohol intake, involvement of multiple nerves and diagnostic delay as risk factors for grade 2 disability.
Subjects and Methods
Study design
Retrospective descriptive Study.
Study subjects
Inclusion criteria
All leprosy patients diagnosed to have pure neuritic leprosy from our institution from 1st July 2007 to 30th June 2017 were included in the study after getting the ethical clearance and permission from Superintendent of our hospital to use the records.
Patients who satisfied the cardinal criteria for leprosy proposed by the WHO, who had no skin lesions of leprosy and who had negativity for acid-fast bacilli in skin smears were diagnosed as having pure neuritic leprosy.[5] As per the institutional policy in clinically doubtful cases, nerve twig biopsy was performed to confirm the diagnosis if superficial sensory nerve was found to be enlarged. When patients with thickened nerves had doubtful impairment of sensory or motor function of respective nerves, nerve conduction study was carried out for confirmation.
Exclusion criteria
Patients who were initially diagnosed to have neuritic leprosy from other centres and who were referred to us while on treatment for complications were excluded from the study.
Method: Data on age, sex, occupation and personal habits including smoking, alcohol intake and substance abuse were collected from previous case records. Information on clinical features with special reference to nerve thickening and nerve function impairment were documented. Inability or reduced ability to appreciate temperature, pain and fine touch were considered as sensory function impairment. Water at 40°C and at 25°C in test tubes were used to assess temperature sensation, pin prick for evaluating ability to appreciate pain and a wisp of cotton was used to check fine touch.[6] Motor impairment was diagnosed when less than grade 5 power was recorded on voluntary muscle testing on the Medical Research Council scale.[7] Clinical evidence of involvement of single nerve due to leprosy (nerve thickening associated with nerve function impairment along the nerve supply) was categorised as mononeuritis and asymmetric involvement of multiple nerves as mononeuritis multiplex. Symmetric involvement of multiple nerves was considered as polyneuritis. Nerve tenderness and sudden onset or sudden worsening of nerve function impairment when documented, were noted. Grade 2 disability at the time of diagnosis was recorded as per the WHO grading of disability.[8] Laboratory information (complete hemogram, random blood sugar estimation, renal and liver function tests and serology for human immunodeficiency and hepatitis B and C viruses) was documented.
Patients were classified into paucibacillary and multibacillary cases as per the WHO operational guidelines.[5] Follow-up details when available were collected.
The data were entered in Microsoft excel and analysed with the SPSS for Windows, Version 16.0. (SPSS Inc., Chicago, USA). The association between age, sex, occupation, smoking, alcohol intake, multiple nerve thickening and diagnostic delay with grade 2 disability was assessed using the Chi-square test. A P value <0.05 was taken as significant. To avoid the effects of confounding factors, logistic regression model was applied.
Results
Among the 879 leprosy cases diagnosed from the Dermatology Department during the 10-year study period, 75 (8.5%) could be classified as pure neuritic leprosy [Table 1]. Nerve biopsy was needed to confirm diagnosis in three patients (ulnar cutaneous nerve in two patients and radial cutaneous nerve in the third) all of whom had borderline tuberculoid spectrum histologically. Twelve patients underwent nerve conduction study.
Table 1.
Year-wise distribution of neuritic leprosy cases
| Year | Neuritic leprosy (percentage of total) | Total |
|---|---|---|
| 1 July 2007-30 June 2008 | 4 (5.3) | 75 |
| 1 July 2008-30 June 2009 | 13 (10.8) | 120 |
| 1 July 2009-30 June 2010 | 6 (5.04) | 119 |
| 1 July 2010-30 June 2011 | 11 (15.9) | 69 |
| 1 July 2011-30 June 2012 | 9 (9.5) | 95 |
| 1 July 2012-30 June 2013 | 11 (12.9) | 85 |
| 1 July 2013-30 June 2014 | 5 (5.6) | 89 |
| 1 July 2014-30 June 2015 | 4 (5.3) | 76 |
| 1 July 2015-30 June 2016 | 6 (7.9) | 76 |
| 1 July 2016-30 June 2017 | 6 (8) | 75 |
| Total | 75 (8.5) | 879 |
There was a clear male predilection among leprosy cases (1.9:1) which was more pronounced in pure neuritic cases (59 males and 16 females; male to female ratio 3.7:1). Age of the study group ranged from 14 years to 75 years with most of the cases belonging to the 41–60 age group (36%) [Figure 1].
Figure 1.
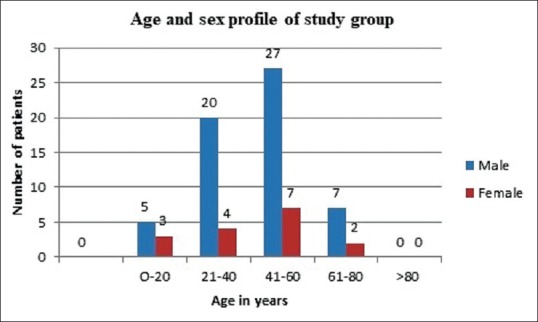
Age and sex distribution of the study population
Forty one study subjects were manual labourers (54.7%). Twelve of the 16 females were house wives (75%). Ten persons used to drink alcohol (13.3%) and 28 were smokers (37.3%).
Time interval between onset of symptoms and diagnosis was 1 year or less in 42 patients (56%), more than a year to 5 years in 23 patients (30.7%) and >5 years in 10 cases (13.3%). Thirty seven (49.3%) patients had thickening of less than three nerves and 38 (50.7%) had three or more thickened nerves. Mononeuritis multiplex predominated in the study group (66 patients, 88%). Mononeuritis was observed in nine patients (12%).
Most common nerves affected were ulnar nerve (50 cases, 66.7%), lateral popliteal nerve (49 cases, 65.3%), radial cutaneous nerve (20 cases, 26.7%) and sural nerve (13 cases, 17.3%) [Figures 2 and 3].
Figure 2.
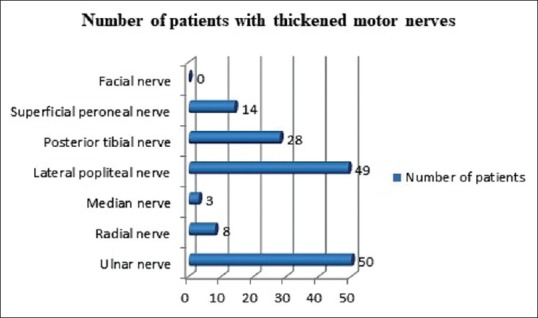
Pattern of motor nerve thickening in study population
Figure 3.
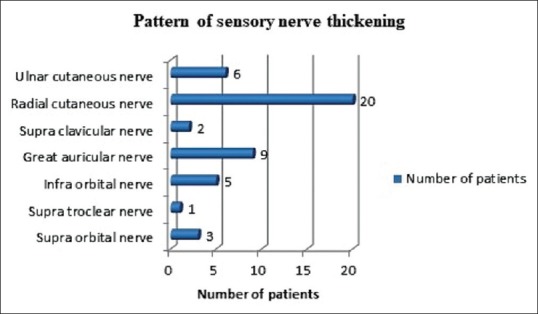
Pattern of sensory nerve thickening in study population
Nerve palsy was detected in 51 cases (68%) and the common nerves affected were ulnar nerve (28 cases, 37.3%) and median nerve (18 cases, 24%) [Figure 4]. None of the patients had nerve tenderness or nerve abscess. Trophic ulcers were observed in 16 (21.3%) cases. Grade 1 disability was noted in 15 (20%) and grade 2 in 60 (80%) patients at the time of diagnosis [Table 2]. Sixty six (88%) patients required multibacillary (MB) multidrug treatment (MDT) and nine needed (12%) paucibacillary treatment.
Figure 4.
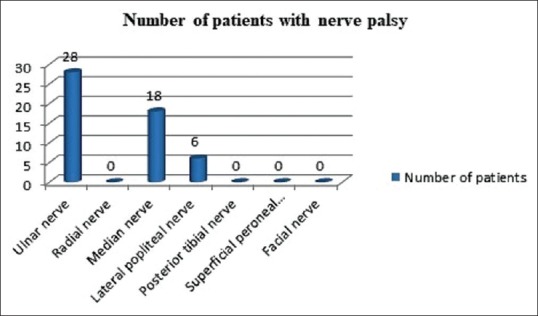
Pattern of nerve palsy in study population
Table 2.
Distribution of grade 2 disability in study group
| Grade 2 disability | Number of patients (%) |
|---|---|
| Ulnar clawing | 28 (37.3) |
| Clawing due to median nerve palsy | 18 (24) |
| Wrist drop | 0 |
| Foot drop | 6 (8) |
| Trophic ulcer - hands | 4 (5.3) |
| Trophic ulcer - foot | 12 (16) |
Twenty six (34.7%) patients required treatment with steroids either due to worsening of nerve function impairment while on treatment (3 patients, 4%) or for nerve palsy that appeared within 6 months preceding diagnosis (12 patients, 16%) or for incomplete nerve palsy (11, 14.7%). All the 26 patients showed improvement with physiotherapy and systemic steroids (30 mg prednisolone which was tapered at 2–4 weeks interval). Seventeen (65.4%) of the 26 needed steroid treatment for <6 months and >6 months treatment was required in 9 (34.6%) patients as tapering of steroids worsened the nerve palsy. They were managed with stepping up the dose of steroids and attempting a slower taper. None of them received steroid sparing agents. None of the study subjects had skin lesions of leprosy in the past. None of them developed skin lesions while on treatment. Follow up upto 1 year was available in 46 patients (61.3%) and skin lesions were not documented during this period either in any of the study population.
Advancing age, manual labour, alcohol intake and diagnostic delay of >5 years were found to be risk factors for grade 2 disability at the time of diagnosis in this study. But a statistically significant association was noted only between age of the affected and grade 2 disability on Chi-square analysis (P value 0.04) [Table 3]; but on applying logistic regression model for doing away with confounding variables, this also was found to be insignificant. No association was noted for smoking, sex of the affected and multiple nerve thickening (thickening involving three or more nerves) with grade 2 disability at the time of diagnosis.
Table 3.
Risk factors for grade 2 disability at the time of diagnosis
| Variables | Grade 1 disability (no patients) (%) | Grade 2 disability (number of patients) (%) | P |
|---|---|---|---|
| Age group (years) | |||
| 0-20 (8) | 4 (50) | 4 (50) | 0.04 |
| 21-40 (24) | 5 (20.8) | 19 (79.2) | |
| 41-60 (34) | 5 (14.7) | 29 (85.3) | |
| 61-80 (9) | 1 (11.1) | 8 (88.9) | |
| Sex | |||
| Male (59) | 9 (15.3) | 50 (84.7) | 0.57 |
| Female (16) | 6 (37.5) | 10 (62.5) | |
| Occupation | |||
| Manual labourers (41) | 5 (12.2) | 36 (87.8) | 0.05 |
| Others (34) | 10 (29.4) | 24 (70.6) | |
| Alcohol intake | |||
| Yes (10) | 0 | 10 (100) | 0.09 |
| No (65) | 15 (23.1) | 50 (76.9) | |
| Smoking | |||
| Yes (28) | 5 (17.9) | 23 (82.1) | 0.48 |
| No (47) | 10 (21.3) | 37 (78.7) | |
| Nerve thickening (no of involved nerves) | |||
| 3 or more (38) | 10 (26.3) | 28 (73.7) | 0.13 |
| <3 (37) | 5 (13.5) | 32 (86.5) | |
| Delay in diagnosis (years) | |||
| 0-1 (42) | 11 (26.2) | 31 (73.8) | 0.29 |
| >1-2 (11) | 2 (18.2) | 9 (81.8) | |
| >2-3 (5) | 0 | 5 (100) | |
| >3-4 (5) | 1 (20) | 4 (80) | |
| >4-5 (2) | 1 (50) | 1 (50) | |
| >5 (10) | 0 | 10 (100) | |
Discussion
Neuritic leprosy constituting 8.5% of total case load in the current study was comparable to existing data which states that the incidence of pure neuritic leprosy in India varies from 5·5 to 18% of total leprosy cases.[9,10,11] The male predilection noted in pure neuritic leprosy fell between the findings in previous studies on neuritic leprosy [Table 4].[9,10,12] The most common age group (41–60 years) affected in this study was higher than that documented in previous studies.[9,10,12]
Table 4.
Studies on pure neuritic leprosy
| Studies | Number of pure neuritic leprosy cases | Percentage of pure neuritic cases | Most common age group (years) | Male to female ratio | Most common presentation | Percentage of patients with grade 2 disability at the time of presentation | Appearance of skin lesions while on treatment or during follow-up period |
|---|---|---|---|---|---|---|---|
| Narang et al. | 48 | 5.3 | Below 30 | 5.9:1 | Mononeuritis multiplex | 66.6 | - |
| *Kumar et al. | 65 | 4.2 | 15-35 | 2.6:1 | Mononeuritis multiplex | - | None |
| Mendiratta et al. | 32 | 4.6 | 15-30 | 9.7:1 | Mononeuritis multiplex | 50 | - |
| †Talwar et al. | 42 | 10.7 | - | - | Mononeuritis | - | Three patients |
| ‡Present study | 75 | 8.5 | 41-60 | 3.7:1 | Mononeuritis multiplex | 80 | None |
* Kumar et al.: Two-year follow up was available in only 32/65 patients (49.2%) and none of them developed skin lesions during this period.
† Talwar et al.: The study also included data on 20 cases of pure neuritic leprosy managed with dapsone monotherapy; the information on only those patients who received multidrug therapy (paucibacillary type) are shown in the table.
‡ Present study = 1-year follow up was available in 46 patients and none of them developed skin lesions during this period
Our observation of manual labourers contributing >50% of study group underscores the fact that nerves more prone to trauma are likely to be affected in leprosy since manual labourers are more likely to sustain trauma during their profession. Another explanation for the same could be the patient population seeking treatment in a government institution usually belong to the low socioeconomic strata.[1] The diagnostic delay of 1–5 years observed in significant percentage of study subjects and a delay of >5 years observed in >10% of patients highlights the need to increase awareness regarding this not so uncommon manifestation of the disease in our country as recommended by others as well.[1]
Half of the study group manifesting mononeuritis multiplex was discordant to certain studies; but consistent with certain others.[1,9,10,12,13]
A slight preference for involvement of upper limb nerves was observed in our study which was supported by existing data but certain other studies have documented a predilection for lower limb nerves.[1,9,10,12]
One interesting observation in this study was the absence of patients with radial nerve palsy, though there were >10% patients manifesting thickened radial nerves. On the contrary, median nerve thickening was documented in only 4% cases; but 24% of study subjects had features of median nerve palsy. The disparity noted between nerve function impairment and nerve thickening with respect to median nerve in our study could be explained on the basis of pressure effect exerted by the unyielding carpal tunnel on even a slightly inflamed median nerve. A diagnosis of neuritic leprosy was considered since all of them had at least one other nerve with thickening and nerve function impairment along its course. This observation of ours was discordant to the findings of Nascimento who suggested that median nerve compromise is a rare finding in leprosy.[14]
Absence of features of acute neuritis (nerve tenderness) and hence absence of features of type 1 lepra reaction observed in our patients were unlike the findings of others.[1,15] But the acute worsening of symptoms following MDT in three of our cases, the nerve palsy setting within 6 months' time in 12 others and a good response observed for systemic steroids in 26 cases with nerve palsy indicates a role for subacute neuritis in at least some of our patients. The efficacy of systemic steroids in recent onset nerve function impairment noted by us were in concordance with the findings of Narang et al.[10]
Neuritic leprosy not progressing with appearance of skin lesions documented by us was similar to the observation of Mahajan et al.[16] Though Talwar et al. has documented appearance of skin lesion following completion of treatment in leprosy, most of them had received dapsone monotherapy.[13]
A high percentage of neuritic leprosy cases (80%) developing grade 2 disability by the time of diagnosis documented by us was consistent with existing data and is attributed to the inherent nature of disease with early nerve involvement and damage. The delay in diagnosis in the absence of skin lesions might have also contributed.[1,9,10,13,14] This statistics of grade 2 disability in 80% of 75 pure neuritic cases, cannot be used to describe the prevalence of grade 2 disability at the time of diagnosis in leprosy in the community since the patients attending a tertiary referral unit usually have more severe manifestations. To estimate the actual prevalence of grade 2 disability at the time of diagnosis in leprosy we need multicentre studies incorporating primary, secondary and tertiary care centres. Still 80% of new cases of pure neuritic leprosy attending a tertiary centre having grade 2 disability calls for urgent measures to improve the early case detection rate in leprosy as a whole and pure neuritic leprosy in particular.
Previous studies have documented multiple nerve thickening, pure neuritic spectrum of leprosy and delay in diagnosis to be major risk factors for grade 2 disability.[1,9,10] The risk factors noted for the same by us (though statistically insignificant) were advancing age, manual labour, alcohol intake and diagnostic delay of >5 years. The lack of association noted between multiple nerve thickening and grade 2 disability in the study may be reflection of the study done in a tertiary referral centre, since patients with more severe symptoms are likely to seek treatment in a referral centre. Hence those without grade 2 disability may either be detected during field surveys or in primary care centres. All 75 of our patients manifesting either grade 1 or 2 disability support this argument.
Limitations
The main limitation of the current study was its retrospective nature. The information is based on data collected by different dermatologists over a period of time and response to treatment with respect to nerve function impairment was recorded only in those who received systemic steroids as it was necessary for tapering steroids. Since the study was carried out in a tertiary referral centre, it does not reflect the status of the disease in the community. Lack of data on follow up of all treated cases to determine the chance of developing skin lesions in treated pure neuritic leprosy was another drawback.
Despite these limitations, we were able to gather information on the clinical patterns and the greater risk of disability associated with pure neuritic leprosy.
Conclusions
The clear male predilection noted for this disease and the high percentage of nerve function impairment observed in the study group involving the important sensory and motor nerves of upper and lower limbs indicates the burden, this disease inflicts on the society by often affecting the sole bread winner of a family. Our finding of mononeuritis multiplex as the most common presentation of pure neuritic leprosy (88%) points to the crippling potential of this disease if not diagnosed early. High percentage of study subjects manifesting grade 2 disability at the time of diagnosis highlights the need to train health care workers to detect nerve thickening and thus ensuring early detection of cases through field surveys since unlike in leprosy with skin lesions, diagnosis of pure neuritic leprosy requires clinical expertise to detect thickened nerves. Early referral of suspected neuritic leprosy cases can be confirmed by utilising sophisticated techniques like high-resolution ultrasound in higher centres.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The first author expresses sincere gratitude to the Indian Council of Medical Research for encouraging research projects by medical students.
References
- 1.Rao PN, Suneetha S. Pure neuritic leprosy: Current status and relevance. Indian J Dermatol Venereol Leprol. 2016;82:252–61. doi: 10.4103/0378-6323.179086. [DOI] [PubMed] [Google Scholar]
- 2.Ridley DS, Jopling WH. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966;34:255–73. [PubMed] [Google Scholar]
- 3.Rao PN. Global leprosy strategy 2016-2020: Issues and concerns. Indian J Dermatol Venereol Leprol. 2017;83:4–6. doi: 10.4103/0378-6323.195075. [DOI] [PubMed] [Google Scholar]
- 4.Alberts CJ, Smith WC, Meima A, Wang L, Richardus JH. Potential effect of the World Health Organization's 2011-2015 global leprosy strategy on the prevalence of grade 2 disability: A trend analysis. Bull World Health Organ. 2011;89:487–95. doi: 10.2471/BLT.10.085662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organisation. Eighth Report. Geneva: World Health Organisation; 2010. WHO Expert Committee on Leprosy. (Technical Report Series 968). [PubMed] [Google Scholar]
- 6.Sasidharanpillai S, Binitha MP, Riyaz N, Ambooken B, Mariyath OK, George B, et al. Childhood leprosy: A retrospective descriptive study from government medical college, Kozhikode, Kerala, India. Lepr Rev. 2014;85:100–10. [PubMed] [Google Scholar]
- 7.Brandsma JW, van Brakel WH. Protocol for motor function assessment in leprosy and related research questions. Indian J Lepr. 2001;73:145–58. [PubMed] [Google Scholar]
- 8.Directorate of Health Services, Ministry of Health and Family Welfare. Classification and management of leprosy. Training Manual for Medical Officers: NLEP. Ch. 7. Directorate of Health Services, Ministry of Health and Family Welfare, New Delhi: Directorate of Health Services, Ministry of Health and Family Welfare. [Last accessed on 2013 Mar 14]. Available from: http://www.nlep.nic.in/training .
- 9.Kumar B, Kaur I, Dogra S, Kumaran MS. Pure polyneuritic leprosy in India: An appraisal. Int J Lepr. 2004;72:284–90. doi: 10.1489/0020-7349(2004)72<284:PNLIIA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 10.Narang T, Vinay K, Kumar S, Dogra S. A critical appraisal on pure neuritic leprosy from India after achieving WHO global target of leprosy elimination. Lepr Rev. 2016;87:456–63. [PubMed] [Google Scholar]
- 11.Sasidharanpillai S, Reena Mariyath OK, Riyaz N, Binitha MP, George B, Janardhanan AK, et al. Changing trends in leprosy among patients attending a tertiary care institution. Indian J Dermatol Venereol Leprol. 2014;80:338–40. doi: 10.4103/0378-6323.136909. [DOI] [PubMed] [Google Scholar]
- 12.Mendiratta V, Khan A, Jain A. Primary neuritic leprosy: A reappraisal at a tertiary care hospital. Indian J Lepr. 2006;78:261–7. [PubMed] [Google Scholar]
- 13.Talwar S, Jha PK, Tiwari VD. Neuritic leprosy: Epidemiology and therapeutic responsiveness. Lepr Rev. 1992;63:263–8. doi: 10.5935/0305-7518.19920031. [DOI] [PubMed] [Google Scholar]
- 14.Nascimento OJ. Leprosy neuropathy: Clinical presentations. Arq Neuropsiquiatr. 2013;71:661–6. doi: 10.1590/0004-282X20130146. [DOI] [PubMed] [Google Scholar]
- 15.Kaur G, Girdhar BK, Girdhar A, Malaviya GN, Mukherjee A, Sengupta U, et al. Aclinical, immunological, and histological study of neuritic leprosy patients. Int J Lepr Other Mycobact Dis. 1991;59:385–91. [PubMed] [Google Scholar]
- 16.Mahajan PM, Jogaikar DG, Mehta JM. A study of pure neuritic leprosy: Clinical experience. Indian J Lepr. 1996;68:137–41. [PubMed] [Google Scholar]


