Abstract
Overdosing is the major cause of acute methotrexate toxicity in psoriasis patients. There are no published data regarding the acute cumulative dose causing acute toxicity, duration to achieve acute cumulative toxic dose and various reasons for wrong dosing of methotrexate in Indian patients. We are presenting a series of seven cases of toxicity due to overdosing of methotrexate in psoriasis. The acute cumulative dose of methotrexate ranging from 35 mg to 150 mg, taken over 3–7 days was responsible for acute toxicity in the psoriasis cases. Lack of counselling regarding the disease course, drug dosing, schedule and awareness about possible outcome of high and daily dose were found to be the causes of overdosing and toxicity in our patients. All cases presented with ulceration, bleeding and pain in skin lesions and five cases had oral mucosal ulceration and genital mucosa was involved in two cases. All cases were given injectable folinic acid. Five cases recovered and two cases expired. Authors postulate counselling about the course of disease, regarding dosing schedule of methotrexate and consequences of methotrexate overdosing is mandatory for all patients of psoriasis in country like India where drug regulation is not strict to prevent methotrexate toxicity and its dreaded consequences.
Keywords: Factors, methotrexate toxicity, overdosing, psoriasis, skin and mucosal lesion
Introduction
Methotrexate is the commonly used cytotoxic drug in wide spread and recalcitrant psoriasis with dose ranging from 7.5 mg–30 mg per week.[1] In psoriasis, it acts by inhibiting the DNA synthesis of T lymphocytes and epidermal keratinocytes.[2] Higher dose can often have toxic effect on different system causing lowering blood counts, deranging liver function by increasing enzyme level and skin and mucosal necrosis. The mechanism of acute toxicity due to methotreaxate is by inhibiting DNA synthesis in rapidly proliferating cells i.e., gastrointestinal (GI) tract, haematopoietic cells and cells on psoriatic lesion. Hence acute methotrexate toxicity causes decrease blood counts, nausea, vomiting, black stool, skin and mucosal erosion and ulceration.[3] There are no published data regarding the acute cumulative dose causing acute toxicity, duration to achieve acute cumulative toxic dose and various reasons for wrong dosing of methotrexate in Indian patients.
The present case series highlights the above aspects and in addition clinical presentations, systemic complications, risk factors of methotrexate toxicity and outcome of the cases have been described.
Case Report
Six males and one female patient of psoriasis of age ranging from 35 years to 57 years presented with sudden ulceration, bleeding and pain on the psoriatic plaques [Figures 1 and 2]. Five cases had oral mucosal ulceration (case 2, 3, 5, 6 and 7) and two had genital mucosa ulceration (case 5 and 6) [Figure 3]. Skin and mucosa findings, various reasons for overdosing, acute cumulative dose resulting in toxicity and duration to achieve acute toxic cumulative dose of all cases have been illustrated in Table 1. Various associated risk factors for toxicity, systemic complications and outcome of the cases have been described in Table 2. Demographic details, clinical manifestations, systemic complications, risk factors and treatment outcome have been illustrated in Table 1. None of our patients presented at day 1 with skin manifestations. None of the cases had any history of pre-existing renal and hepatic disease. All had deranged blood counts and serum methotrexate level could be done case in 2,3,4,5 and 7 which ranged from0.21 μmol/L to 97 μmol/L. Daily monitoring of serum methotrexate level could not be done due to cost factor and unavailability of the investigation at our institute. Hence all patients were treated with intravenous folinic acid (leucovorin) 20 mg 6 hourly on day 1 followed by 10 mg 6 hourly till counts returned to normal. Skin erosions were managed with aseptic barrier dressing using vaselinated gauze. Out of seven cases two cases died due to grossly deranged liver function tests, renal function tests, pancytopenia leading to septicaemia. Rest cases of skin and mucosal erosions and ulcerations improved with treatment. All patients received counselling regarding the disease course, dosing schedule of methotrexate and possible consequences of methotrexate overdosing.
Figure 1.
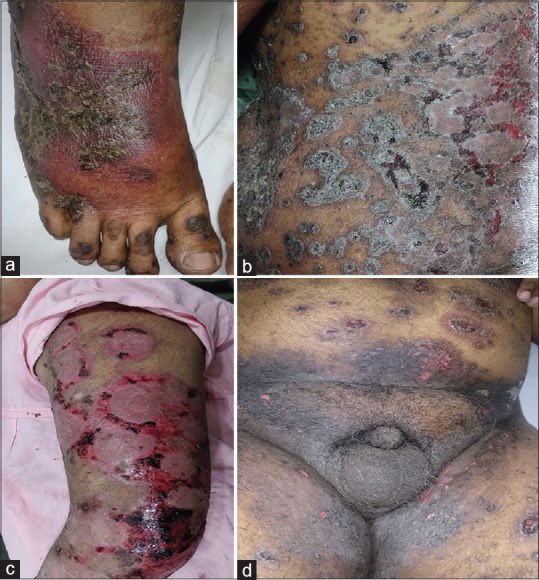
(a) Bright red lesional and perilesional erythema along with ulceration of psoriatic plaque. (b) Ulceration on psoriatic plaques. (c) Ulceration and bleeding on psoriatic plaques. (d) Erythema and ulceration on folds along with ulceration on psoriatic plaques
Figure 2.
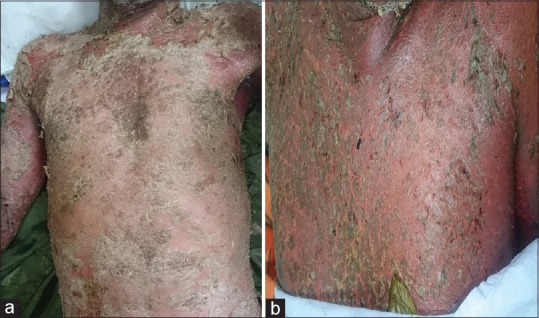
(a) Fissuring and erosions on erythrodermic psoriatic lesions. (b) Extensive erythema and erosions on psoriatic plaques
Figure 3.
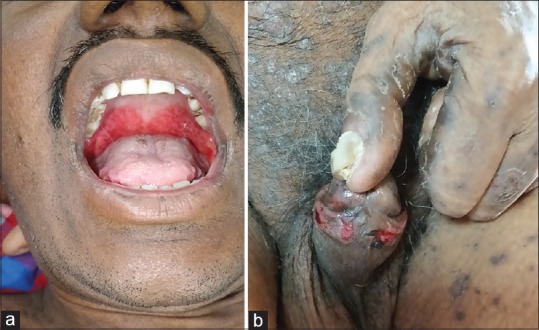
(a) Ulceration on oral mucosa. (b) Ulceration on genital mucosa
Table 1.
Demographic details, Acute cumulative toxic dose, duration, skin and mucosal signs and reasons for consuming toxic dose of Methotrexate
| Case number | Age/Sex | Acute cumulative dose | Duration of intake of acute cumulative dose | Skin and mucosa findings | Reasons for overdosing |
|---|---|---|---|---|---|
| 1 | 54year/male | 52.5 mg | 5 days | Skin - erythema, tenderness and ulceration over both older and new lesions of psoriasis Mucosa - nil |
Aggravation of disease and joint pain |
| 2 | 45year/male | 60 mg | 3 days | Skin - sudden ulceration and bleeding from the psoriatic lesions Mucosa - oral ulcerations |
Aggravation of disease |
| 3 | 48year/male | 60 mg | 4 days | Skin - tenderness, erythema, erosion and fissuring on the folds Mucosa - haemorrhagic spots on conjunctiva and oral mucosa |
Did not understand the dosing schedule |
| 4 | 50year/male | 150 mg | 5 days | Skin - tenderness, erythema, ulceration and bleeding from psoriatic plaques Mucosa - nil |
Aggravation of disease |
| 5 | 57year/male | 90 mg | 4 days | Skin - extensive erosions, ulceration and bleeding from psoriatic plaques Mucosa - oral and genital mucosal ulceration |
Out of frustration because of prolonged disease course |
| 6 | 53year/male | 90 mg | 3 days | Skin - extensive ulceration and bleeding from the pre-existing psoriatic lesions along with haematuria, jaundice, bloody diarrhoea Mucosa - oral and genital ulceration |
Did not understand dosing schedule |
| 7 | 35year/male | 35 mg | 7 days | Skin - severe pain and ulceration on her psoriatic plaques on hand, thighs and buttocks Mucosa - erosion on upper lip |
Aggravation of disease |
Table 2.
Risk factor, hematological changes and treatment out come in acute methotrexate toxicity
| Case number | Systemic abnormalities | Risk factors | Outcome |
|---|---|---|---|
| 1 | Haematological Haemoglobin level - 8 g/dl Total platelet count - 92,000/cmm Deranged liver function SGOT - 230 U/L (N-0-37 U/L) SGPT - 260 U/L (N-0-40 U/L) |
Intake of paracetamol and diclophenac along with methotrexate for joint pain | Recovered |
| 2 | Haematological Haemoglobin level - 9 g/dl Total platelet count - 90,000/cmm |
- | Recovered |
| 3 | Haematological Haemoglobin level - 9.2 g/dl Total platelet count - 80,000/cmm |
- | Recovered |
| 4 | Haematological Haemoglobin level - 9 g/dl Total platelet count - 95,000/cmm |
- | Recovered |
| 5 | Haematological Haemoglobin level - 6.5 g/dl Total platelet count - 43,000/cmm Total leucocyte count - 2500/cmm Deranged liver function tests SGOT - 510 U/L (N-0-37 U/L) SGPT - 420 U/L (N-0-40 U/L) Deranged renal function tests Serum urea - 90 mg/dl (N-15-40 mg/dl) Serum creatinine - 2.9 mg/dl (N-0.7-1.3 mg/dl) |
Alcoholic | Expired |
| 6 | Haematological Haemoglobin level - 7.2 g/dl Total leucocyte count - 1400/cmm Total platelet count - 80,200/cmm Deranged liver function tests SGOT - 460 U/L (N-0-37 U/L) SGPT - 370 U/L (N-0-40 U/L) Deranged renal function tests Serum urea - 80 mg/dl (N-15-40 mg/dl) Serum creatinine - 2.6 mg/dl (N-0.7-1.3 mg/dl) |
Alcoholic | Expired |
| 7 | Haematological Haemoglobin - 8.1 g/dl Total platelet count - 92,000/cmm |
- | Recovered |
SGPT = Serum glutamate pyruvate transaminase; SGOT = Serum glutamate oxaloacetate transferase
Discussion
Methotrexate is a cheap and most effective systemic drug prescribed for psoriasis. It inhibits mitosis of the cells by inhibiting dihydrofolate (DHF) reductase, an enzyme responsible for the conversion of dihydrofolate (DHF) to tetrahydrofolate (THF), leading to reduction in thymidylate and purine biosynthesis. It gets polyglutaminated to active form inside cell and thereby it stays in cell for a long period when taken daily or divided dose. The cells that effectively polyglutamate methotrexate includes leukemic myeloblasts, synovial macrophages, lymphoblasts and dividing epithelial cells.[1] Methotrexate toxicity is rare with low dose, correct scheduling of the dose and adherence to the recommended guidelines.[4] Acute methotrexate toxicity manifests itself in several forms including hepatotoxicity, pulmonary toxicity, acute renal failure, stomatitis, ulceration/erosion of the GI and pancytopenia.[5] In the present series we came across mucosal, skin and haematological toxicity predominantly. Hepatotoxicity and renal toxicity was found in case 2 and 3, respectively. All cases presented with ulceration on psoriatic lesions because of increased uptake of drug by hyperproliferative keratinocytes in lesional skin which was similar to the findings reported by Agarwal et al. and Pearce et al.[3,6]
Treatment of methotrexate toxicity is usually by folinic acid rescue therapy. Ideally, the dose of folinic acid is usually decided according to level of serum methotrexate and duration of overdosing.[7] However, facility of measuring serum methotrexate level is not available in resource poor settings. In present case series all patients had haematological toxicity at the time of presentation and serial methotrexate level could not be measured. Hence all patients were treated with intravenous folinic acid (leucovorin) 20 mg 6 hourly on day 1 followed by 10 mg 6 hourly till blood counts returned to normal.
The common reported precipitating factors for methotrexate toxicity are an alteration in methotrexate dosage and the concomitant use of non-steroidal anti-inflammatory drugs (NSAIDS).[6] Psoriasis patients commonly use NSAIDS for joint pain and co-administration of NSAIDS with methotrexate increases methotrexate level in blood by inhibition of renal tubular secretion of methotrexate. Other factors contributing to methotrexate toxicity include renal insufficiency, (the drug Methotrexate is excreted by renal system), infection, pustular psoriasis and age >55 years.[6] Drugs can increase the risk of methotrexate toxicity either by decreasing the renal elimination of methotrexate by competing with drugs like aminoglycosides, cyclosporine, non-steroidal anti-inflammatory agents, sulfonamides, probenecid, salicylates, penicillins, colchicines, cisplatin and other renotoxic drugs, or by displacing methotrexate from protein binding sites in the plasma by co-administration of drugs like salicylates, probenecid, sulfonamides, barbiturates, phenytoin, retinoids, sulfonylureas, tetracyclines.[3] One of our patients had a history of concomitant intake of excessive dose of methotrexate along with paracetamol and indomethacin for joint pain which could have increased the blood level of methotrexate by decreasing renal excretion which was already high because of overdosing which is similar to the case reported by Jariwala et al.[8] Four out of seven cases were >50 years of age which could be an additive risk factor for toxicity along with overdosing. None of our patients had a history of any pre-existing renal disease.
Methotrexate is used at a dose: 5–7.5 mg/week in elderly or frail patients and 15 mg/week in healthy patients. Maximum 25 mg/week can be used in psoriasis safely.[9]
However, acute cumulative toxic dose and duration required to achieve the toxic cumulative dose of methotrexate is unclear. In the present case series we observed acute cumulative dose of methotrexate ranging from 35 mg to 150 mg, taken over 3 to 7 days was responsible for acute toxicity in the psoriasis cases. The most common cause of drug overdose was in the quest to achieve rapid response or achieve permanate cure from psoriasis by self-medication which is similar to the cases reported by Agarwal et al. and Jariwala et al. [Table 1].[3,8] All the patients had a poor understanding regarding use, dosing schedule and consequences of overdosing of the drug methotrexate along with the course of psoriasis. All had stopped medication out of frustration leading to flaring of disease following which they took the drug frequently within short span of time. Secondly, since the drug was available over-the-counter majority of patients resorted to self-medication. Following treatment skin lesions healed completely in five patients and two patients died due to septicaemia and grossly deranged liver and renal function tests.
From the present case series, our observation suggest that counselling about the course of disease, regarding dosing schedule of methotrexate and consequence of methotrexate overdosing should be mandatory for all patients of psoriasis in country like India where drug regulation is not strict and patients often buy medications over-the-counter and resort to self-medication. The cases are reported to make the physicians familiar with the different challenges of acute methotrexate toxicity due to overdosing in Indian patients where drug like methotrexate is easily availabile without prescription over-the-counter and also to create awareness regarding counseling the various aspects of disease and methotrexate while prescribing in psoriasis patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Roenigk HH, Jr, Auerbach R, Maibach HI, Weinstein GD. Methotrexate in psoriasis: Revised guidelines. J Am Acad Dermatol. 1988;19:145–56. doi: 10.1016/s0190-9622(88)80237-8. [DOI] [PubMed] [Google Scholar]
- 2.Czarnecka-Operacz M, Sadowska-Przytocka A. The possibilities and principles of methotrexate treatment of psoriasis – The updated knowledge. Postepy Dermatol Alergol. 2014;31:392–400. doi: 10.5114/pdia.2014.47121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agarwal KK, Nath AK, Thappa DM. Methotrexate toxicity presenting as ulceration of psoriatic plaques: A report of two cases. Indian J Dermatol Venereol Leprol. 2008;74:481–4. doi: 10.4103/0378-6323.44305. [DOI] [PubMed] [Google Scholar]
- 4.Morgan SL, Baggott JE, Vaughn WH, Austin JS, Veitch TA, Lee JY, et al. Supplementation with folic acid during methotrexate therapy for rheumatoid arthritis. A double-blind, placebo-controlled trial. Ann Intern Med. 1994;121:833–41. doi: 10.7326/0003-4819-121-11-199412010-00002. [DOI] [PubMed] [Google Scholar]
- 5.Olsen EA. The pharmacology of methotrexate. J Am Acad Dermatol. 1993;25:300–18. doi: 10.1016/0190-9622(91)70199-c. [DOI] [PubMed] [Google Scholar]
- 6.Pearce HP, Wilson BB. Erosion of psoriatic plaques: An early sign of methotrexate toxicity. J Am Acad Dermatol. 1996;35:835–8. doi: 10.1016/s0190-9622(96)90097-3. [DOI] [PubMed] [Google Scholar]
- 7.Madke B, Singh AL. Acute methotrexate toxicity. Indian J Drugs Dermatol. 2015;1:46–9. [Google Scholar]
- 8.Jariwala P, Kumar V, Kothari K, Thakkar S, Umrigar DD. Acute methotrexate toxicity: A fatal condition in two cases of psoriasis. Case Rep Dermatol Med. 2014;2014:946716. doi: 10.1155/2014/946716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menting SP, Dekker PM, Limpens J, Hooft L, Spuls PI. Methotrexate dosing regimen for plaque-type psoriasis: A systematic review of the use of test-dose, start-dose, dosing scheme, dose adjustments, maximum dose and folic acid supplementation. Acta Derm Venereol. 2016;96:23–8. doi: 10.2340/00015555-2081. [DOI] [PubMed] [Google Scholar]


