Abstract
Background
The aim of this study was to investigate whether and how sulforaphane (SFN), a novel promising nuclear factor-E2-related factor 2 (Nrf2) activator, exerted antioxidative stress through activating Nrf2 signaling.
Material/Methods
Cultured human trabecular meshwork cells (HTMCs) were treated with SFN for 6 hours after establishing the oxidative stress model by hydrogen peroxide (H2O2). The cell viability, the level of intercellular reactive oxygen species (ROS), and the apoptosis rate were observed using various kits. In addition, the gene and protein expression of Nrf2 and the phase II antioxidative enzymes were determined by performing qRT-PCR and western blotting.
Results
In H2O2-treated HTMCs, SFN protected HTMCs from oxidative stress damage and decreased the intracellular ROS accumulation, thus inhibiting cell apoptosis. SFN also increased the gene and protein expression of phase II antioxidative enzymes such as NAD(P)H: quinone oxidoreductase 1 (NQO-1), heme oxygenase-1 (HO-1), glutamate-cysteine ligase catalytic subunit (GCLC), and glutamate-cysteine ligase modifier subunit (GCLM) by Nrf2-dependent pathway. Furthermore, investigations of the pathway showed that HTMCs pretreated with LY294002, an inhibitor of phosphatidylinositol 3-kinase (PI3K), downregulated the expression of phase II antioxidative enzymes, partly.
Conclusions
These results indicated a novel application for SFN in attenuating H2O2-induced oxidative stress in HTMCs through activating PI3K/Akt/Nrf2 signaling pathway.
MeSH Keywords: Oxidative Stress, Phosphatidylinositol 3-Kinases, Proto-Oncogene Proteins c-akt
Background
Glaucoma, the leading cause of irreversible blindness, is considered a progressive optic neuropathy, the most common form of which is primary open-angle glaucoma (POAG) [1]. Although the exact pathogenesis of POAG remains elusive, recent studies showed that oxidative stress played an important role in the progression of POAG [2,3]. On the one hand, oxidative stress promotes the production of reactive oxygen species (ROS), causing toxic reactions and oxidative damage [4]. On the other hand, oxidative stress is crucial to the degradation, dysfunction, and loss of trabecular meshwork cells (TMCs), leading to an elevated intraocular pressure (IOP), which is considered to be one of the important risk factors for POAG [3]. Therefore, recent studies have focused on the pathogenic of oxidative stress and potential anti-oxidative agents.
A number of protective mechanisms, including antioxidants and endogenous antioxidative enzymes, are initiated by the cells themselves to respond to antioxidative stress [5]. The antioxidative enzymes such as phase II antioxidative enzymes NAD(P)H: quinone oxidoreductase 1 (NQO-1), heme oxygenase-1 (HO-1), glutamate-cysteine ligase catalytic subunit (GCLC), and glutamate-cysteine ligase modifier subunit (GCLM) can be regulated by nuclear factor-E2-related factor 2 (Nrf2) signaling pathway [6]. Nrf2 is an essential transcription factor, and a specific receptor of which is Kelch-like associated protein 1 (Keap1) [6]. Activation of Nrf2 signaling pathway was shown to play an essential role in protecting against oxidative stress in many tissues including lung, liver, and brain [7,8] and alleviated oxidative damage in many ocular diseases, such as retinal ischemia-reperfusion injury [9], diabetic retinopathy [10], retinopathy of prematurity (ROP) [11], and age-related macular degeneration (AMD) [12].
Sulforaphane (SFN) is a natural dietary isothiocyanate found in cruciferous vegetables such as cabbage, Brussel sprouts, and broccoli [13]. SFN, as a novel promising Nrf2 activator, has attracted much attention because of its antioxidant effects. By sulfhydryl reaction with Keap1, SFN forms thioacyl adducts and advances the destruction of Nrf2-Keap1 interaction [14]. Sohel et al. reported that SFN protects granulosa cells against oxidative stress via activation of Nrf2/ARE (antioxidant response elements) pathway [15].
In this study, we investigated whether SFN might alleviate the oxidative stress damage induced by hydrogen peroxide (H2O2) in human trabecular meshwork cells (HTMCs) by activating Nrf2 signaling, and we explored the possible underlying mechanisms.
Material and Methods
Reagents
SFN and H2O2 were purchased from Sigma-Aldrich (St. Louis, MO, USA). All antibodies were obtained from Cell Signaling Technology (Danvers, MA, USA). Cell culture reagents were offered from Gibco (Grand Island, NY, USA). Inhibitor of phosphatidylinositol 3-kinase (PI3k), LY294002, was purchased from PeproTech (NJ, USA).
Cell cultures
HTMCs were obtained from Cell Bank of the Chinese Academy of Sciences (Shanghai, China) and cultured in 8 mL Dulbecco’s modified Eagle’s medium (DMEM, Gibco) containing 20% fetal bovine serum (FBS, Gibco). The medium was replaced every 3 days, and the cells were passaged when the cells reached 80% to 90% confluency. The cells of passage 3–5 were prepared for later experiments.
Cell viability assays
HTMC cells were added at 1×103 HTMCs/well to 96-well plates and cultured overnight. Then cells were treated with multiple concentrations of H2O2 (0, 1 μM, 10 μM, 100 μM, and 200 μM) for 24 hours with or without the pretreatment of SFN (0, 10 μM, 20 μM, and 30 μM). A Cell Counting Kit-8 (CCK-8, Dojindo, Kumamoto, Japan) was used to determine the toxic effects of H2O2 and SFN on HTMCs according the manufacture’s protocols. The absorbance value was recorded at 450 nm.
Intracellular ROS assays
HTMCs were inoculated into 6-well plates after the H2O2 induced oxidative stress model, and treated with SFN for 6 hours, washed 3 times with phosphate-buffered saline (PBS), then, the production of intracellular ROS was detected by Reactive Oxygen Species Assay Kit (Beyotime, Shanghai, China) and assayed by a microplate reader (Tecan Spark 10M, Switzerland) at 488 nm according to the manufacture’s protocols.
Annexin V/FITC assay
HTMCs were inoculated into 6-well plates, after H2O2 and SFN treatment, according to the instruction of a FITC Annexin V apoptosis detection kit (BD Biosciences, Franklin, NJ, USA). After collected and resuspended in 100 μL buffer, cells were stained with Annexin V-FITC and propidium iodide (PI) for 15 minutes on ice. Then, cells were suspended in precooled buffer and analyzed by CytExpert software (Beckman-Coulter, Miami, FL, USA).
Quantitative real-time reverse transcriptase (qRT)-PCR
Total RNAs were isolated from the HTMCs using TRIzol reagent (Invitrogen Life Technology, Carlsbad, CA, USA). RNA was reversed transcribed into cDNA by the PrimeScript RT Reagent kit (Takara, Shiga, Japan) which was used to perform the qRT-PCR analyses using the SYBR Premix ExTaqII (TliRNaseHPlus) kit (Takara) according to the manufacture’s instruction. The primers used were as follows:
-
NOQ-1: forward,5′-AACCAACAGAGCCAATC-3′,
reverse, 5′-CCTCCATCCTTTCCTC-3′;
-
HO-1: forward,5′-CTGGCTTCCTTCCCTTGAG-3′,
reverse, 5′-CTTTGGGTTGGAGATGT-3′;
-
GCLM: forward,5′-AATCTTGCCTCCTGCTGTGT-3′,
reverse, 5′-CTCGTGTGCTCGAATGTCAG-3′;
-
GCLC: forward, 5′-AAGCCTCCTCCTCCAAACTC-3′,
reverse, 5′-AGCACCACGAACACCACATA-3′;
-
β-actin: forward, 5′-CTGTCCACCTTCCAGCAGA -3′;
reverse, 5′-AGCCATGCCAATCTCATCTC-3′.
Quantitative and analysis of mRNA was performed by using Vii7 System (Applied Biosystems, Waltham, MA, USA).
Western blotting
Briefly, after treatment, the protein of the cytoplasm and nuclei was extracted by using a Nuclear and Cytoplasmic Protein Extraction Kit (Beyotime, Shanghai, China) and concentrations were quantified by a BCA protein assay kit (Beyotime). Proteins were separated by sodium dodecyl sulfate-polyacrylamide gels electrophoresis (SDS-PAGE) for 80 minutes, and then transferred onto a polyvinylidene fluoride (PVDF) membrane. The PDVF membranes were blocked in 5% bovine serum albumin (BSA) for 1 hour at room temperature, and incubated with rabbit anti-NQO-1, anti-HO-1, anti-GCLM, anti-GCLC, and anti-Nrf2, and mouse anti-GAPDH and anti-Lamin B primary antibodies at 4°C overnight, followed by secondary antibody goat anti-rabbit IgG and anti-mouse IgG for 1 hour at room temperature. The protein signals were observed using ECL reagent (Thermo Fisher, Rockford, IL, USA).
Statistical analysis
The data are presented as the mean ± standard deviation (SD). Difference between 2 groups were analyzed by the Student’s t-test, multiple group were assessed by one-way ANOVA using SPSS 22.0 (IBM Inc., Chicago, IL, USA). Statistical significance was established at P<0.05. The experiments were repeated 3 times.
Results
SFN protected HTMCs from H2O2-induced oxidative stress damage and decreased the intracellular ROS production
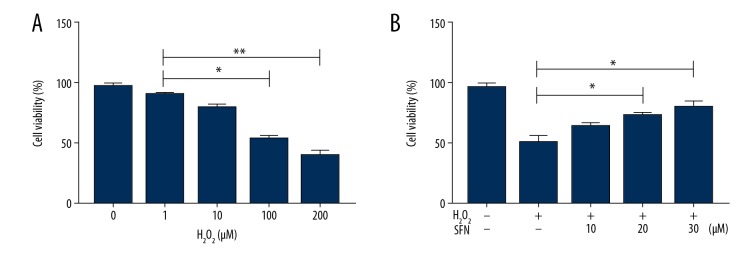
We exposed H2O2 to HTMCs to induce obvious oxidative injury, since this model has been widely used in previous studies [16,17]. First, HTMCs were treated with H2O2 at different concentrations (1 μM, 10 μM, 100 μM, and 200 μM) for 24 hours. The results of CCK-8 showed that H2O2 decreased the cell viability of HTMCs, and there was a significant reduction in cell viability at 100 μM of H2O2 (P<0.01). In addition, we found that H2O2 (100 μM) displayed about 50% cytotoxicity in HTMCs (Figure 1A). Thus, 100 μM was determined as the optimal concentration for follow-up experiments. Next, HTMCs were treated with various concentrations (10 μM, 20 μM, and 30 μM) of SFN for 6 hours, followed by the stimulation of H2O2 (100 μM) for 24 hours. As showed in Figure 1B, SFN increased cell viability in a dose-dependent manner and SFN (20 μM and 30 μM) suppressed H2O2-induced cytotoxicity significantly (P<0.05).
Figure 1.
Sulforaphane (SFN) protects human trabecular meshwork cells (HTMCs) from H2O2-induced oxidative damage. HTMCs were treated with H2O2 (1 μM, 10 μM, 100 μM, and 200 μM) for 24 hours with or without pretreatment of SFN (10 μM, 20 μM, and 30 μM) for 6 hours. Cell viability were determined by using a CCK-8 (A, B). Data were expressed as mean ± standard deviation. * P<0.05, ** P<0.01. CCK-8 – Cell Counting Kit 8; H2O2 – hydrogen peroxide.
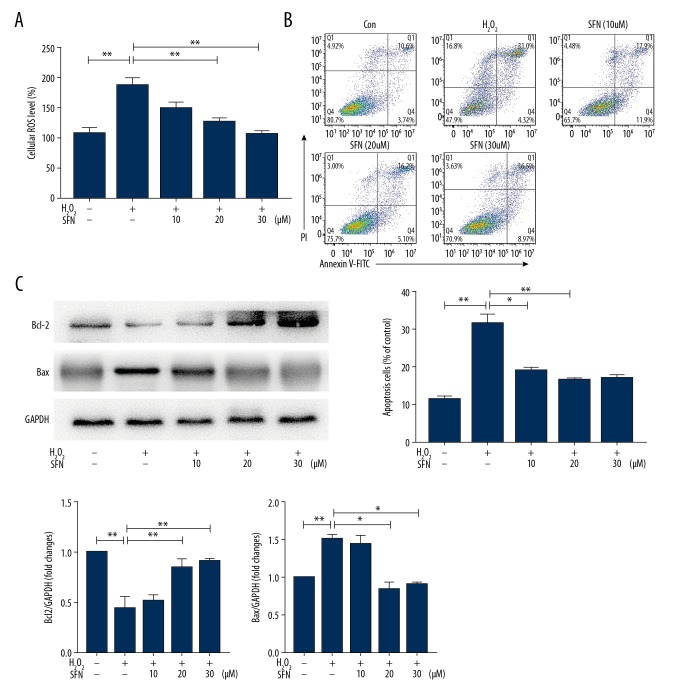
Then, we investigated the effects of H2O2 and SFN on the production of ROS, which was a marker of oxidative stress injury. Our findings suggested that treatment with H2O2 alone increased intracellular ROS levels obviously (P<0.01), but were blocked by pretreatment with SFN. Furthermore, SFN (20 μM and 30 μM) was significant for reducing the production of intracellular ROS (Figure 2A).
Figure 2.
Sulforaphane (SFN) inhibited H2O2-induced ROS over expression and apoptosis in human trabecular meshwork cells (HTMCs). HTMCs were treated with H2O2 (1 μM, 10 μM, 100 μM, and 200 μM) for 24 hours with or without pretreatment of SFN (10 μM, 20 μM, and 30 μM) for 6 hours. (A) The levels of intercellular ROS were detected by using a ROS assay kit. (B) Apoptotic cells were tested by a FITC Annexin V apoptosis detection kit. (C) Protein expression of PARP, Bcl2, and Bax. Data were expressed as mean ± standard deviation. * P<0.05, ** P<0.01. H2O2 – hydrogen peroxide; ROS – reactive oxygen species
It has been demonstrated that a high level of intracellular ROS in cells induces apoptosis. Thus, we examined the effect of SFN on apoptosis. As shown in Figure 2B, H2O2 increased the expression of apoptotic protein Bcl2 and PARP, which inhibited the expression of Bax, which significantly induced the occurrence of apoptosis. Compared with the H2O2 group, the apoptosis rate of cells with added SFN decreased, and the difference was significant when the concentration of SFN was 20 μM and 30 μM (Figure 2C). Therefore, we chose 20 μM as intervention concentration of SFN in subsequent experiments.
SFN induced phase II antioxidative enzymes expression by activating Nrf2 signaling
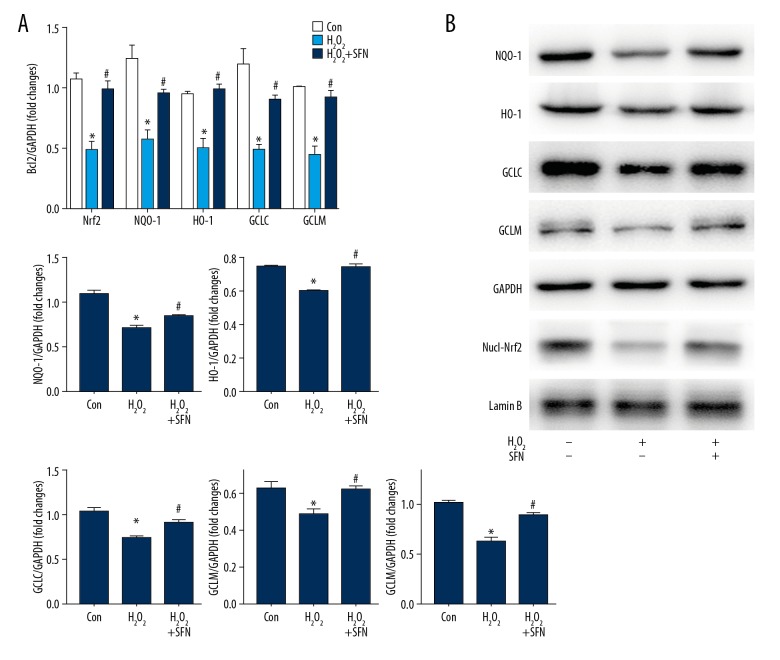
A previous study clarified that SFN induced phase II antioxidative enzymes expression in many cells, such as alveolar macrophages [18], primary rat hepatocytes [19], and retinal pigment epithelium [20]. To investigate whether SFN also induced the expression of phase II antioxidative enzymes in HTMCs, we determined the mRNA and protein expression of these enzymes by performing qRT-PCR and western blot analyses. As displayed in the Figure 3A and 3B, H2O2 alone inhibited the mRNA and protein expression of Nrf2, NQO-1, HO-1, GLCL, and GCLM, but pretreatment with SFN suppressed the decreasing effects of H2O2 on the secretion of phase II antioxidative enzymes in HTMCs.
Figure 3.
Sulforaphane (SFN) activates Nrf2 signaling in human trabecular meshwork cells (HTMCs). HTMCs were treated with SFN (20 μM) for 6 hours, followed by the stimulation of H2O2 (100 μM) for 24 hours. mRNA levels and protein expression of Nrf2 and phase II antioxidative enzymes NQO-1, HO-1, GCLC, and GCLM (A, B). Data were expressed as mean ± standard deviation. * P<0.05, Control versus H2O2, # P<0.05, H2O2 versus H2O2+SFN. Nrf2 – nuclearfactor-E2-related factor 2; NQO-1 – NAD(P)H: quinone oxidoreductase 1; HO-1 – heme oxygenase-1; GCLC – glutamate-cysteine ligase catalytic subunit; GCLM – glutamate-cysteine ligase modifier subunit; H2O2 – hydrogen peroxide.
SFN activated Nrf2 signaling via PI3K/Akt pathway
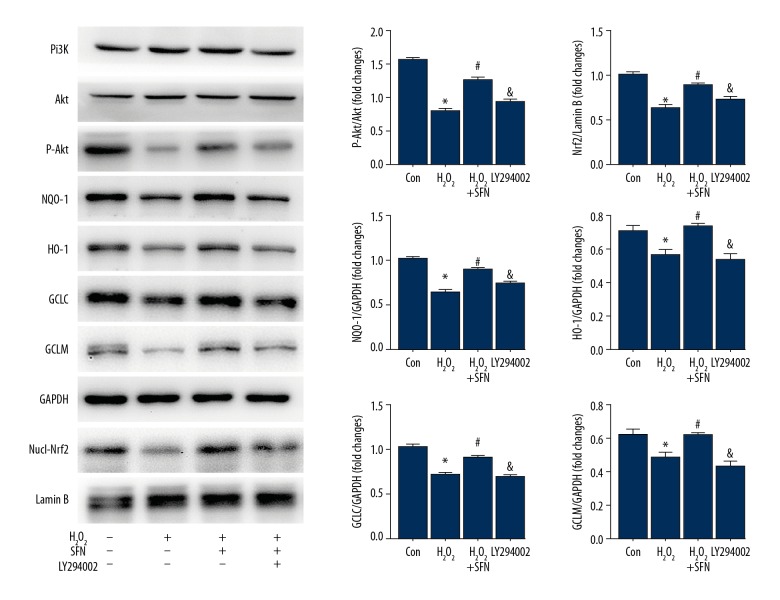
Next, we investigated the upstream signaling pathway involved in SFN-mediated Nrf2 signaling activation. Since the PI3K/Akt pathway has been reported to play a major role in modulating Nrf2 signaling activation, we examined the protein expression of the PI3K/Akt pathway and found that SFN remarkably promoted the phosphorylation of Akt (Figure 4). To verify that SFN activated Nrf2 signaling via the PI3K/Akt pathway, HTMCs were exposed to the inhibitor of PI3K, LY294002 (20 μM) for 2 hours. The results demonstrated that after the addition of LY294002, phosphorylation of Akt decreased, thereby reducing the protein expression of Nrf2 and phase II antioxidative enzymes, which reversed the activation of SFN to Nrf2 signaling.
Figure 4.
Sulforaphane (SFN) exerts an anti-oxidative role by PI3K/Akt-mediated Nrf2 signaling pathway. Human trabecular meshwork cells (HTMCs) were pretreated with LY294002 (20 μM) for 6 hours prior to being exposed to SFN (20 μM) for 6 hours and H2O2 (100 μM) for 24 hours respectively. Protein expression of PI3K, Akt, P-Akt, Nrf2, and phase II antioxidative enzymes NQO-1, HO-1, GCLC, and GCLM. Data were expressed as mean ± standard deviation. * P<0.05, Control versus H2O2, # P<0.05, H2O2 versus H2O2+SFN, & P<0.05, H2O2+SFN versus LY294002. PI3K – phosphatidylinositol 3-kinase; Akt – serine/threonine kinase; Nrf2 – nuclearfactor-E2-related factor 2; H2O2 – hydrogen peroxide; NQO-1 – NAD(P)H: quinone oxidoreductase 1; HO-1 – heme oxygenase-1; GCLC – glutamate-cysteine ligase catalytic subunit; GCLM – glutamate-cysteine ligase modifier subunit.
Discussion
In the present study, we found that different concentrations of H2O2 could decrease cell viability of HTMCs to varying degrees, and SFN displayed a dose-dependent response in increasing cell viability of H2O2-treated HTMCs. In addition, SFN inhibited the production of intracellular ROS induced by H2O2 in HTMCs, thereby restraining apoptosis. SFN activated Nrf2 signaling, promoting the gene and protein expression of phase II antioxidative enzymes NOQ-1, HO-1, GCLC, and GCLM. Further studies suggested that the upregulating effects on the expression of phase II antioxidative enzymes were partially suppressed after pretreating with LY294002, an inhibitor of PI3k. These results indicated that SFN protected HTMCs from H2O2-induced oxidative damage by PI3K/Akt-mediated Nrf2 signaling pathway activation.
POAG is a chronic, irreversible optic neuropathy. Although the exact pathogenesis of POAG is still not clear, a variety of pathogenic theories have proposed for the development of this disease, such as elevated IOP, optic nerve susceptibility, vascular disorders, and oxidative stress [21]. Most studies focused on glaucoma retinopathy, which occurs at the terminal disease stage [22]. Sacca et al. [4] demonstrated that ROS over-production induced by oxidative stress contributed to retinal degeneration by inducing lipid peroxidation, calcium overload, and DNA damage, eventually causing retinal cell death and apoptosis. The relationship between increased IOP caused by trabecular meshwork dysfunction and POAG progression is attracting increasing attention. The TMCs is a complex tissue with different cell types, morphology and function of which contribute to maintaining normal IOP [23]. Any factors that affect the function of TMCs may lead to increasing IOP, promoting the progression of POAG. The oxidative denaturation of trabecular meshwork causes damage to the outflow tract and regional blood perfusion [24]. In early glaucoma, mitochondrial damage and endothelial dysfunction caused by oxidative stress promotes endogenous cell loss and defense function reduction, thus activating the apoptosis of TMCs [2].
Nrf2 is a transcription factor that controlling hundreds of detoxifying and antioxidant genes [25]. When exposed to oxidation or electrophilic stress, the Nrf2-Keap1 complex in the cytoplasm will be separated. Then Nrf2 translocates into the nucleus where it combines with antioxidant response elements to initiate the gene expression regulation of antioxidative enzymes system, thereby enhancing cell resistance to oxidative stress and nucleophilic compounds [26,27]. The activation of Nrf2 has been implicated as an important protective role in protecting against oxidative stress in multiple cells [28,29]. Harvey et al. [18] reported that activating Nrf2 signaling pathway inhibited oxidative stress caused by cigarette smoke, leading to attenuating chronic obstructive pulmonary disease in mice. Exerting antioxidation by activating Nrf2 signaling is mainly dependent on the release of antioxidant kinase, such as NQO-1, HO-1, GCLC, and GCLM. Ross et al. [30] found that NQO-1 protected cell membranes from oxidative damage by decreasing endogenous quinine compounds. HO-1 is a major antioxidant enzyme that catalyzes the speed limiting step of heme catabolism, leading to the formation of bilirubin, free iron and carbon monoxide, thus increasing the resistance of cells to oxidative damage [31]. Glutamate cysteine ligase (GCL), is a heterodimeric protein composed of catalytic (GCLC) and modifier (GCLM) subunits. Studies have shown that GCL is the main determinant of cellular GSH level, effecting the antioxidant capacity of cells [32]. This study is the first to clarify the activation of Nrf2 signaling pathways in HTMCs, which could also increase the expression of antioxidant kinase NQO-1, HO-1, and GCL, playing a role of antioxidant stress.
Nrf2 signaling can be activated by many agents, most of which are plant-chemical extracts or their derivatives, such as SFN [19], carotenoids [29], and flavonoids [33]. Unlike other antioxidants, SFN does not scavenge reactive oxygen species directly, but relies on the ability to induce the expression of antioxidant enzymes [34]. Gao et al. [14] showed that SFN protected retinal pigment epithelial cells (RPE) from photooxidative stress via Nrf2 signaling. Sohel et al. [15] reported that SFN promoted the release of NQO-1, HO-1 via Nrf2/antioxidant response elements pathway. The present study also revealed that SFN inhibited H2O2-induced intracellular ROS production and increased the HTMCs activity.
PI3K/Akt pathway signaling plays an important role in modulating Nrf2 signaling [29]. It has been reported that drugs such as astaxanthin activated Nrf2 signals in many cells, and the action was partly dependent on the activation of the PI3K/Akt pathway [28,35,36].
Conclusions
Consistent with previous studies, we found that HTMCs pretreated with LY294002, an inhibitor of PI3K. suppressed expression of antioxidant enzymes increased by SFN partially.
In summary, we suggest that SFN protects HTMCs from H2O2-induced oxidative stress via the PI3K/Akt-mediated Nrf2 pathway (Figure 5). Our study establishes a basis for further studies on SFN as a promising trabecular meshwork cytoprotective agent against oxidative stress.
Figure 5.
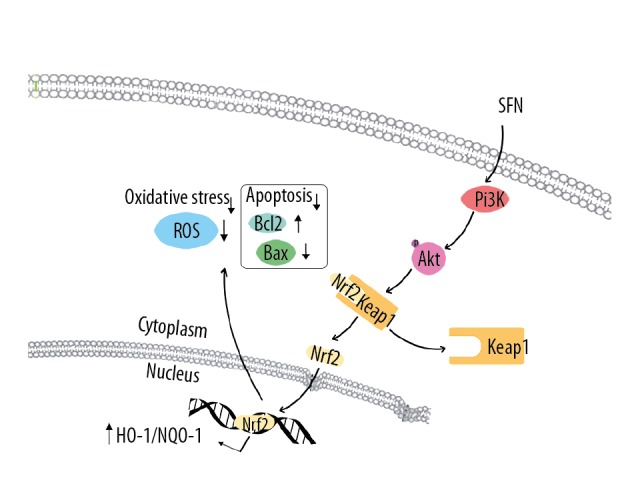
Sulforaphane (SFN) exerts antioxidant stress through PI3K/Akt and Nrf2 signaling pathways. SFN, as a new type of Nrf2 agonist, activate Nrf2 by promoting PI3K/Akt signaling pathways, mainly enhancing the phosphorylation of Akt. Nrf2 is translocated into nuclei after released from Keap1 to induce the transcription of phase II antioxidative enzymes NQO-1, HO-1, GCLC, and GCLM. However, the direct relationship between PI3K/Akt and Nrf2 requires future researches to elaborate. Nrf2 – nuclearfactor-E2-related factor 2; PI3K – phosphatidylinositol 3-kinase; Akt – serine/threonine kinase; NQO-1 – NAD(P)H: quinone oxidoreductase 1; HO-1 – heme oxygenase-1; GCLC – glutamate-cysteine ligase catalytic subunit; GCLM – glutamate-cysteine ligase modifier subunit.
Abbreviation
- Akt
serine/threonine kinase
- AMD
age-related macular degeneration
- ARE
antioxidant response elements
- BSA
bovine serum albumin
- DMEM
Dulbecco’s modified Eagle’s medium
- FBS
fetal bovine serum
- GCLC
glutamate-cysteine ligase catalytic subunit
- GCLM
glutamate-cysteine ligase modifier subunit
- HO-1
heme oxygenase-1
- H2O2
hydrogen peroxide
- HTMCs
human trabecular meshwork cells
- IOP
intraocular pressure
- Keap1
Kelch-like associated protein 1
- NQO-1
NAD(P)H: quinone oxidoreductase 1
- Nrf2
nuclear factor-E2-related factor 2
- PI3K
phosphatidylinositol 3-kinase
- POAG
primary open-angle glaucoma
- PVDF
polyvinylidene fluoride
- ROS
reactive oxygen species
- ROP
retinopathy of prematurity
- RPE
rigment epithelial cells
- SD
standard deviation
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gels for electrophoresis
- SFN
sulforaphane
Footnotes
Source of support: This work was supported by The Plan of Science and Technology Development of Shandong Province (Grant No. 2012YD18117)
References
- 1.Liton PB, Lin H, Gonzalez P, Epstein DL. Potential role of lysosomal dysfunction in the pathogenesis of primary open angle glaucoma. Autophagy. 2009;5(1):122–24. doi: 10.4161/auto.5.1.7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babizhayev MA. Biomarkers and special features of oxidative stress in the anterior segment of the eye linked to lens cataract and the trabecular meshwork injury in primary open-angle glaucoma: challenges of dual combination therapy with N-acetylcarnosine lubricant eye drops and oral formulation of nonhydrolyzed carnosine. Fundam Clin Pharmacol. 2012;26(1):86–117. doi: 10.1111/j.1472-8206.2011.00969.x. [DOI] [PubMed] [Google Scholar]
- 3.Sacca SC, Pascotto A, Camicione P, et al. Oxidative DNA damage in the human trabecular meshwork: Clinical correlation in patients with primary open-angle glaucoma. Arch Ophthalmol. 2005;123(4):458–63. doi: 10.1001/archopht.123.4.458. [DOI] [PubMed] [Google Scholar]
- 4.Sacca SC, Izzotti A. Oxidative stress and glaucoma: injury in the anterior segment of the eye. Progr Brain Res. 2008;173:385–407. doi: 10.1016/S0079-6123(08)01127-8. [DOI] [PubMed] [Google Scholar]
- 5.Qi HY, Li L, Chen L, et al. Proteomic identification of Nrf2-mediated phase II enzymes critical for protection of Tao Hong Si Wu Decoction against oxygen glucose deprivation injury in PC12 Cells. Evid Based Complement Alternat Med. 2014;2014 doi: 10.1155/2014/945814. 945814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu R, Chen C, Mo YY, et al. Activation of mitogen-activated protein kinase pathways induces antioxidant response element-mediated gene expression via a Nrf2-dependent mechanism. J Biol Chem. 2000;275(51):39907–13. doi: 10.1074/jbc.M004037200. [DOI] [PubMed] [Google Scholar]
- 7.Ishaq M, Evans MD, Ostrikov KK. Atmospheric pressure gas plasma-induced colorectal cancer cell death is mediated by Nox2-ASK1 apoptosis pathways and oxidative stress is mitigated by Srx-Nrf2 anti-oxidant system. Biochim Biophys Acta. 2014;1843(12):2827–37. doi: 10.1016/j.bbamcr.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Jiang WD, Liu Y, Hu K, et al. Copper exposure induces oxidative injury, disturbs the antioxidant system and changes the Nrf2/ARE (CuZnSOD) signaling in the fish brain: Protective effects of myo-inositol. Aquat Toxicol. 2014;155:301–1. doi: 10.1016/j.aquatox.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Wei Y, Gong J, Yoshida T, et al. Nrf2 has a protective role against neuronal and capillary degeneration in retinal ischemia-reperfusion injury. Free Radic Biol Med. 2011;51(1):216–24. doi: 10.1016/j.freeradbiomed.2011.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu Z, Wei Y, Gong J, et al. NRF2 plays a protective role in diabetic retinopathy in mice. Diabetologia. 2014;57(1):204–13. doi: 10.1007/s00125-013-3093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gergely K, Gerinec A. Retinopathy of prematurity – epidemics, incidence, prevalence, blindness. Bratisl Lek Listy. 2010;111(9):514–17. [PubMed] [Google Scholar]
- 12.Gao P, Li L, Ji L, et al. Nrf2 ameliorates diabetic nephropathy progression by transcriptional repression of TGFbeta1 through interactions with c-Jun and SP1. Biochim Biophys Acta. 2014;1839(11):1110–20. doi: 10.1016/j.bbagrm.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 13.James D, Devaraj S, Bellur P, et al. Novel concepts of broccoli sulforaphanes and disease: Induction of phase II antioxidant and detoxification enzymes by enhanced-glucoraphanin broccoli. Nutr Rev. 2012;70(11):654–65. doi: 10.1111/j.1753-4887.2012.00532.x. [DOI] [PubMed] [Google Scholar]
- 14.Gao X, Dinkova-Kostova AT, Talalay P. Powerful and prolonged protection of human retinal pigment epithelial cells, keratinocytes, and mouse leukemia cells against oxidative damage: The indirect antioxidant effects of sulforaphane. Proc Natl Acad Sci USA. 2001;98(26):15221–26. doi: 10.1073/pnas.261572998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sohel M, Amin A, Prastowo S, et al. Sulforaphane protects granulosa cells against oxidative stress via activation of NRF2-ARE pathway. Cell Tissue Res. 2018;374(3):629–41. doi: 10.1007/s00441-018-2877-z. [DOI] [PubMed] [Google Scholar]
- 16.Li XM, Huang D, Yu Q, et al. Neuroligin-3 protects retinal cells from H2O2-induced cell death via activation of Nrf2 signaling. Biochem Biophys Res Commun. 2018;502(1):166–72. doi: 10.1016/j.bbrc.2018.05.141. [DOI] [PubMed] [Google Scholar]
- 17.Xiao Q, Piao R, Wang H, et al. Orientin-mediated Nrf2/HO-1 signal alleviates H2O2-induced oxidative damage via induction of JNK and PI3K/Akt activation. Int J Biol Macromol. 2018;118(Pt A):747–55. doi: 10.1016/j.ijbiomac.2018.06.130. [DOI] [PubMed] [Google Scholar]
- 18.Harvey CJ, Thimmulappa RK, Sethi S, et al. Targeting Nrf2 signaling improves bacterial clearance by alveolar macrophages in patients with COPD and in a mouse model. Sci Transl Med. 2011;3(78):78ra32. doi: 10.1126/scitranslmed.3002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.La Marca M, Beffy P, Della CC, et al. Structural influence of isothiocyanates on expression of cytochrome P450, phase II enzymes, and activation of Nrf2 in primary rat hepatocytes. Food Chem Toxicol. 2012;50(8):2822–30. doi: 10.1016/j.fct.2012.05.044. [DOI] [PubMed] [Google Scholar]
- 20.Koskela A, Reinisalo M, Hyttinen J, et al. Pinosylvin-mediated protection against oxidative stress in human retinal pigment epithelial cells. Mol Vision. 2014;20:760–69. [PMC free article] [PubMed] [Google Scholar]
- 21.Ster AM, Popp RA, Petrisor F, et al. The role of oxidative stress and vascular insufficiency in primary open angle glaucoma. Clujul Med. 2014;87(3):143–46. doi: 10.15386/cjmed-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uno K, Prow TW, Bhutto IA, et al. Role of Nrf2 in retinal vascular development and the vaso-obliterative phase of oxygen-induced retinopathy. Exp Eye Res. 2010;90(4):493–500. doi: 10.1016/j.exer.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Izzotti A, Bagnis A, Sacca SC. The role of oxidative stress in glaucoma. Mutat Res. 2006;612(2):105–14. doi: 10.1016/j.mrrev.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Izzotti A, Di Marco B, De Flora S, Sacca S. [Open angle glaucoma: Epidemiology, pathogenesis and prevention]. Recenti Prog Med. 2006;97(1):37–45. [in Italian] [PubMed] [Google Scholar]
- 25.Simmons SO, Fan CY, Yeoman K, et al. NRF2 oxidative stress induced by heavy metals is cell type dependent. Curr Chem Genomics. 2011;5:1–12. doi: 10.2174/1875397301105010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Himori N, Yamamoto K, Maruyama K, et al. Critical role of Nrf2 in oxidative stress-induced retinal ganglion cell death. J Neurochem. 2013;127(5):669–80. doi: 10.1111/jnc.12325. [DOI] [PubMed] [Google Scholar]
- 27.Itoh K, Chiba T, Takahashi S, et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236(2):313–22. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 28.Kim KC, Kang KA, Zhang R, et al. Upregulation of Nrf2-mediated heme oxygenase-1 expression by Eckol, a phlorotannin compound, through activation of Erk and PI3K/Akt. Int J Biochem Cell Biol. 2010;42(2):297–305. doi: 10.1016/j.biocel.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Miyake S, Kobayashi S, Tsubota Ozawa Y. Phase II enzyme induction by a carotenoid, lutein, in a PC12D neuronal cell line. Biochem Biophys Res Commun. 2014;446(2):535–40. doi: 10.1016/j.bbrc.2014.02.135. [DOI] [PubMed] [Google Scholar]
- 30.Ross D, Kepa JK, Winshi SL, et al. NAD(P)H: quinone oxidoreductase 1 (NQO1): Chemoprotection, bioactivation, gene regulation and genetic polymorphisms. Chem Bol Interact. 2000;129(1–2):77–97. doi: 10.1016/s0009-2797(00)00199-x. [DOI] [PubMed] [Google Scholar]
- 31.He M, Pan H, Chang RC, et al. Activation of the Nrf2/HO-1 antioxidant pathway contributes to the protective effects of Lycium barbarum polysaccharides in the rodent retina after ischemia-reperfusion-induced damage. PLoS One. 2014;9(1):e84800. doi: 10.1371/journal.pone.0084800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franklin CC, Backos DS, Mohar I, et al. Structure, function, and post-translational regulation of the catalytic and modifier subunits of glutamate cysteine ligase. Mol Aspects Med. 2009;30(1–2):86–98. doi: 10.1016/j.mam.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsuji PA, Stephenson KK, Wade KL, et al. Structure-activity analysis of flavonoids: Direct and indirect antioxidant, and anti-inflammatory potencies and toxicities. Nutrit Cancer. 2013;65(7):1014–25. doi: 10.1080/01635581.2013.809127. [DOI] [PubMed] [Google Scholar]
- 34.Gao X, Talalay P. Induction of phase 2 genes by sulforaphane protects retinal pigment epithelial cells against photooxidative damage. Proc Natl Acad Sci USA. 2004;101(28):10446–51. doi: 10.1073/pnas.0403886101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pitha-Rowe I, Liby K, Royce D, Sporn M. Synthetic triterpenoids attenuate cytotoxic retinal injury: Cross-talk between Nrf2 and PI3K/Akt signaling through inhibition of the lipid phosphatase PTEN. Invest Ophthalmol Vis Sci. 2009;50(11):5339–47. doi: 10.1167/iovs.09-3648. [DOI] [PubMed] [Google Scholar]
- 36.Li Z, Dong X, Liu H, Chen X, et al. Astaxanthin protects ARPE-19 cells from oxidative stress via upregulation of Nrf2-regulated phase II enzymes through activation of PI3K/Akt. Mol Vis. 2013;19:1656–66. [PMC free article] [PubMed] [Google Scholar]