Abstract
Background/Aims
An altered haemodynamic profile for various ocular posterior segment capillary beds has been documented in primary open-angle glaucoma (POAG). POAG may also involve abnormal non-ocular blood flow, and the nailfold capillaries, which are not affected by elevated intraocular pressure (IOP), are readily assessable.
Methods
We measured resting nailfold capillary blood flow in 67 POAG and 63 control subjects using video capillaroscopy. Masked readers tracked blood column voids between consecutive, registered image sequence frames, measured vessel diameter and calculated blood flow. We used multiple logistic regression to investigate the relation between nailfold capillary blood flow and POAG. In secondary analyses, we stratified cases by maximum IOP and concurrent topical beta-blocker use.
Results
Mean (±SD) blood flow in picolitres per second was 26.8±17.6 for POAG cases and 50.1±24.2 for controls (p<0.0001). After adjustment for demographic and clinical factors including blood pressure and pulse, every picolitre per second increase in resting nailfold blood flow was associated with a 6% (95% CI 0.92 to 0.96) reduced odds of POAG (p<0.0001). Similar relations between nailfold capillary blood flow and POAG were found for cases stratified by maximum known IOP and for cases stratified by concurrent topical beta-blocker use.
Conclusion
Reduced resting nailfold capillary blood flow is present in POAG independent of covariates such as blood pressure, pulse and IOP.
Keywords: nailfold video capillaroscopy, primary open-angle glaucoma, capillaries, blood flow
Introduction
Primary open-angle glaucoma (POAG) is characterised by irreversible vision loss due to death of retinal ganglion cells. Numerous investigators have reported abnormal resting blood flow in the retina, choroid and extraocular vessels in POAG.1–3 Blood flow studies have also characterised a form of vasospasm or impaired autoregulation in POAG that is apparent in both retinal vessels and non-ocular vascular beds in response to stress.4–6 However, the unperturbed state of non-ocular capillary beds has not been well studied,7 although cold hands are associated with normal tension glaucoma (NTG)8 and finger blood flow has been shown to correlate with ocular blood flow.9 Understanding resting non-ocular blood flow in POAG represents an opportunity to characterise novel disease features that may serve as therapeutic targets.
Nailfold video capillaroscopy is a convenient, non-invasive approach to visualise the capillaries at the base of the fingernail. Prior work has shown that patients with NTG demonstrate reduced nailfold capillary blood speed that is exacerbated in response to local cooling.10 Patients with NTG also exhibit significantly lower ocular pulse amplitudes (diastolic-systolic intraocular pressure (IOP) differential in the choroid) than controls independent of a history of nailfold capillary vasospasm, indicating that complex haemodynamic abnormalities exist in POAG.11 It is unknown if reduced resting nailfold capillary blood flow exists in POAG across a range of IOPs and independent of important covariates such as pulse rate, blood pressure and diabetes. We investigated the relation between resting nailfold capillary blood flow and presence of POAG.
Materials and methods
Nailfold capillary and covariate data collection
Informed consent was obtained from all subjects. Study procedures adhered to the Declaration of Helsinki.
We recruited 67 POAG and 63 control subjects aged 35–80 years from the Glaucoma and Comprehensive Ophthalmology Services at Massachusetts Eye and Ear from April 2016–June 2017 to undergo nailfold video capillaroscopy following eye examinations. Subjects with POAG demonstrated reproducible visual field (VF) loss in at least one eye on reliable 24–2 Humphrey Swedish Interactive Threshold Algorithm (SITA) standard VF testing (Humphrey Field Analyzer, Carl Zeiss Meditec, Dublin, California, USA). Reliable perimetry was defined by tests with fixation loss rate ≤33% and false positive/negative rates ≤20%. Subjects with POAG had no known secondary cause of elevated IOP and no suspected alternative condition producing an optic neuropathy. Control subjects had no family history of glaucoma, maximum IOP ≤21 mm Hg, cup:disc ratio ≤0.6, cup:disc ratio asymmetry ≤0.1, normal slit lamp examinations and full confrontational VFs. We excluded subjects with autoimmune connective tissue diseases, including systemic lupus erythematosus, systemic sclerosis, dermatomyositis and rheumatoid arthritis, which have well-defined nailfold capillary abnormalities (morphological and haemodynamic).12
Each subject’s weight, height, seated brachial artery blood pressure and resting pulse rate were measured. Brachial artery blood pressure was used to calculate the mean arterial pressure. From the medical record, IOP, central corneal thickness, cup:disc ratio, 24–2 Humphrey VF mean deviation and pattern standard deviation of the worse eye, glaucoma medications, race, cigarette smoking status, diabetes, connective tissue disease (eg, osteoarthritis) not listed as exclusion criteria, and use of blood pressure and blood thinning medication at recruitment were obtained.
A BK-XW880 nailfold video capillaroscope at 300× magnification (Biobase Meihua Trading, Jinan, Shandong, China) was used to image nailfold capillaries in the non-dominant hand’s fourth and fifth digits. Cedarwood oil was applied to the nailfold to achieve adequate focus. The capillaroscope did not physically contact the patient’s skin. Moving laterally across the nailfold, the capillaroscope imaged capillaries consecutively, focusing on each until blood flow was visualised. If no blood flow was visualised in a capillary after 10 s in focus, the capillary immediately adjacent was assessed. Blood flow for 10 s was captured on video for each of the first five visible capillaries in each of the two fingers assessed.
Blood flow measurement
Nailfold videos were converted to image sequences and imported into ImageJ (National Institutes of Health, Bethesda, Maryland, USA) to measure blood speed, vessel diameter and flow. Each capillary was isolated from the image sequence spanning the duration of visible blood flow for the given capillary. The cropped image sequence was registered using StackReg, an open-source ImageJ plugin. Because morphological abnormalities such as tortuosity and haemorrhages in the nailfold capillary bed are present in POAG13 14 and morphology may affect blood flow within the vessel, only straight capillary segments were used for analysis. A reader masked to case status tracked the first visible void in the blood column (figure 1) between the first pair of consecutive frames in which the blood column void was clearly defined in both frames as previously described.15 16 Tortuous capillaries were measured as long as they exhibited linear vessel segments in which a blood column void’s path length could be assessed as the Euclidean distance between two points. The time elapsed between a consecutive set of frames was calculated based on the video frame per second rate (25 frames/s). The distance in microns covered by the blood column void was divided by the reciprocal of the frames per second rate to generate blood speed in microns per second. The reader also measured vessel diameter in the vicinity of the blood column void. Assuming a circular geometry for the lumen on the vessel, the cross-sectional area of the vessel was calculated. Capillary blood flow was calculated by multiplying blood speed by cross-sectional area of the vessel. The same reader assessed all videos and a second reader graded a subset of videos to assess inter-rater reliability. Both readers analysed another subset of videos to assess intrarater reliability.
Figure 1.
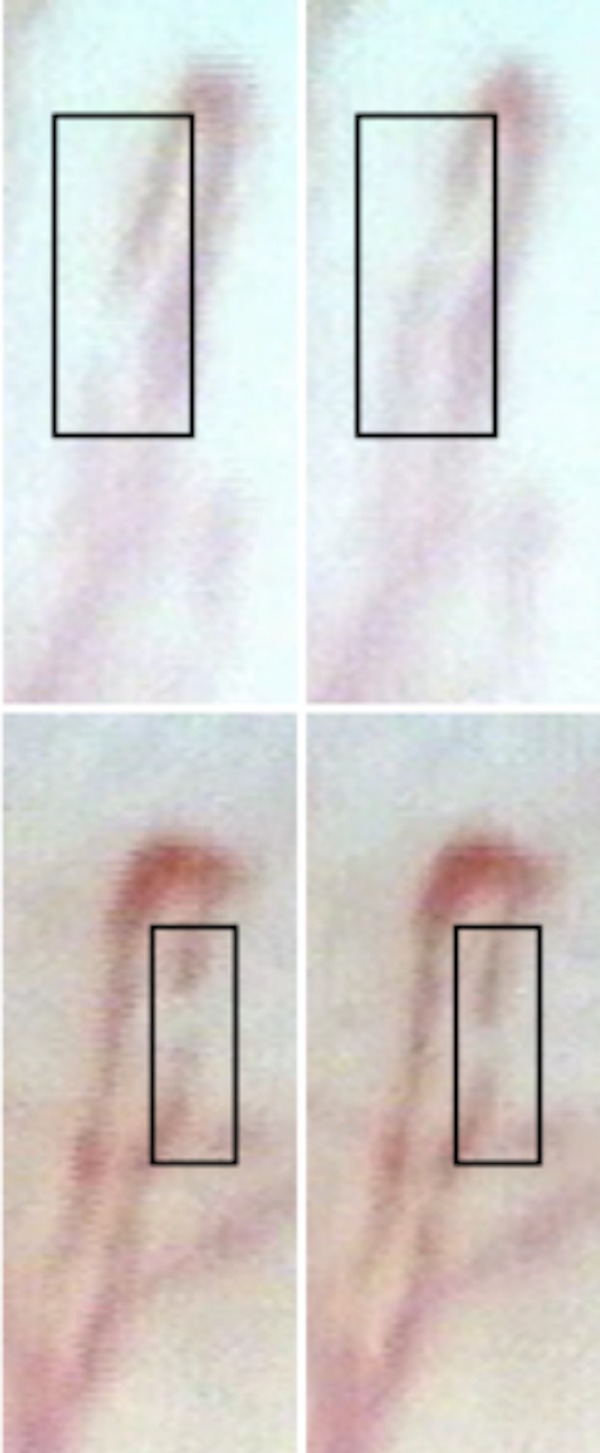
Sample nailfold capillaroscopic images of a control (top) and a patient with primary open-angle glaucoma (POAG) (bottom). The blood column voids are located inside the black boxes and the consecutive image sequence frames (0.04 s later) are shown to the right. Blood speed, vessel diameter and blood flow are 709.1 μm/s, 10.1 μm and 57.1 pL/s, respectively, in the given capillary of the control, and 234.4 μm/s, 8.0 μm and 11.6 pL/s, respectively, in the given capillary of the patient with POAG.
Statistical methods
All statistical analyses were performed in R V.0.98.1091 (R Foundation for Statistical Computing, Vienna, Austria). Tests were two-sided, and the significance level was set to p<0.05. Unpaired t-tests and χ2 tests were used to assess group differences in univariate continuous and categorical data, respectively. Relations between subject type and blood speed, vessel diameter or blood flow were assessed with multivariable logistic regression. These regression models adjusted for body mass index (BMI), mean arterial pressure, heart rate, sex, age, race, connective tissue disease not listed as exclusion criteria, diabetes, use of blood thinning or blood pressure medication, and smoking history (ever/never). Diabetes17 and cigarette smoking16 may contribute to reduced nailfold capillary blood speed, while osteoarthritis may involve morphological abnormalities.18 For the rest of the covariates included in the model, no prior study had been conducted to investigate their relation with nailfold capillary blood flow, but we suspected a potential relation a priori and adjusted for them.
Subjects with any missing covariate data were excluded from the appropriate analyses. Multivariable logistic regression was applied in the following analyses using controls as the reference group: all POAG; POAG with known IOP >21 mm Hg (high tension glaucoma (HTG)); POAG with no known IOP >21 mm Hg (NTG); POAG using topical beta-blocker; and POAG not using topical beta-blocker. The latter two case stratifications were pursued because topical beta-blocker use reduces pulse rate,19 which may impact nailfold capillary haemodynamics.
Power and reliability
Post-hoc sample size calculations indicate 80% power to detect a significant univariate difference in blood speed between POAG and controls with 12 subjects in each group, and in blood flow with 18 subjects in each group, assuming a 1:1 sampling ratio and a type 1 error rate of 5%. The study would require 304 subjects in each group to achieve 80% power in evaluating differences in vessel diameter between cases and controls. Intraclass correlation coefficients (ICC; two-way mixed-effect model for a single rater) between the two examiners for nailfold capillary measurements showed good to excellent inter-rater and intrarater reliability (inter-rater ICC ≥0.70 and intrarater ICC ≥0.81 for all haemodynamic measurements).
Results
Univariate analyses
Subjects with POAG had lower BMI (p=0.011; table 1), less diabetes (p=0.0098) and larger cup:disc ratio (p<0.0001) than controls. No other demographic or clinical features differed between these groups.
Table 1.
Baseline characteristics of the study subjects
| Demographic and clinical features | Controls (n=63) | POAG (n=67) | P values* |
| Age in years, mean (SD) | 67.2 (9.9) | 66.0 (8.6) | 0.46 |
| Female sex, n (%) | 36 (57.1) | 41 (61.2) | 0.64 |
| Caucasian race,† n (%) | 54 (91.5) | 56 (84.8) | 0.25 |
| Pulse in beats/min,‡ mean (SD) | 65.3 (11.2) | 66.4 (10.8) | 0.56 |
| Mean arterial pressure in mm Hg,§ mean (SD) | 92.8 (11.4) | 93.2 (12.6) | 0.85 |
| Body mass index in kg/m2, mean (SD) | 27.3 (5.7) | 25.1 (3.7) | 0.011 |
| Ever smoker (vs never), n (%) | 26 (41.9) | 31 (46.3) | 0.57 |
| Diabetes mellitus, n (%) | 15 (23.8) | 5 (7.5) | 0.0098 |
| Use of blood pressure medication, n (%) | 28 (44.4) | 20 (30.0) | 0.085 |
| Use of systemic beta-blocker medication, n (%) | 13 (20.6) | 8 (11.9) | 0.18 |
| Use of blood thinning medication, n (%) | 29 (46.0) | 23 (34.3) | 0.17 |
| Connective tissue disease,¶ n (%) | 14 (22.2) | 14 (21.0) | 0.85 |
| Family history of glaucoma,** n (%) | 0 | 38 (57.6) | – |
| Intraocular pressure at recruitment in mm Hg, mean (SD) | 14.6 (2.0) | 14.0 (4.7) | 0.29 |
| Maximum intraocular pressure in mm Hg,†† mean (SD) | NA | 23.9 (7.9) | – |
| Central corneal thickness in μm,‡‡ mean (SD) | NA | 538.0 (35.7) | – |
| Cup:disc ratio for worse eye, mean (SD) | 0.29 (0.10) | 0.82 (0.12) | <0.0001 |
| Number of glaucoma medication drops used in the worse eye plus oral glaucoma medication, mean (SD) | 0 | 2.1 (1.4) | – |
| Using topical beta-blocker medication in either eye, n (%) | 0 | 40 (59.7) | – |
| Humphrey visual field mean deviation of the worse eye in decibels, mean (SD) | NA | −9.37 (7.12) | – |
| Humphrey visual field pattern SD of the worse eye in decibels, mean (SD) | NA | 8.12 (3.81) | – |
*P values for continuous variables are from unpaired t-tests and for categorical variables are from χ2 tests.
†Categorised as Caucasian or other race. Race unknown on 4 controls and 1 patient with POAG.
‡Pulse unknown on 2 controls and 4 patients with POAG.
§Mean arterial pressure unknown on 1 control and 1 patient with POAG.
¶Includes osteoarthritis, gout, Sjogren's syndrome and unspecified types of arthritis. Patients with systemic lupus erythematosus, systemic sclerosis, dermatomyositis and rheumatoid arthritis were not included.
**Family history unknown on 1 patient with POAG.
††Maximum intraocular pressure unknown on 6 patients with POAG.
‡‡Central corneal thickness of the eye with highest recorded intraocular pressure. Central corneal thickness unknown on 4 patients with POAG.
NA, not available; POAG, primary open-angle glaucoma.
The mean (±SD) blood speed and flow in subjects with POAG (328±201 μm/s and 26.8±17.6 pL/s, respectively) were significantly reduced compared with those in the controls (574±211 µm/s and 50.1±24.2 pL/s, respectively) in univariate analysis using an average of 9.4 capillaries per patient (p<0.0001 for both; table 2). Capillary diameter did not differ significantly between POAG and controls (p=0.20). Differences in blood speed and flow between cases and controls remained in stratified analyses (table 2). There was a small, insignificant increase in mean (±SD) pulse rate for subjects with POAG not using topical beta-blockers (n=26 cases) compared with current users (n=37 cases) in univariate analysis (68.5±11.2 vs 65.8±10.3 beats/min; p=0.21).
Table 2.
Descriptive analyses of nailfold capillary haemodynamic features in controls, all patients with POAG and the following subgroups: patients with the high tension variant of POAG (HTG), patients with the normal tension variant of POAG (NTG), patients with POAG concurrently using topical beta-blocker medication and patients with POAG not concurrently using topical beta-blocker medication
| Blood speed in μm/s, mean (SD) | Capillary diameter in μm, mean (SD) | Blood flow in pL/s, mean (SD) | P values* for blood speed (controls as referent) | P values* for capillary diameter (controls as referent) | P values* for blood flow (controls as referent) | |
| Controls (n=63) | 574 (211) | 10.0 (1.5) | 50.1 (24.2) | – | – | – |
| All POAG (n=67) | 328 (201) | 9.7 (1.5) | 26.8 (17.6) | <0.0001 | 0.20 | <0.0001 |
| HTG (n=34) | 303 (172) | 9.9 (1.5) | 26.8 (18.8) | <0.0001 | 0.78 | <0.0001 |
| NTG (n=27) | 353 (240) | 9.5 (1.5) | 26.2 (16.7) | 0.0002 | 0.15 | <0.0001 |
| POAG using topical beta-blockers (n=27) | 322 (187) | 9.8 (1.4) | 26.4 (15.0) | <0.0001 | 0.49 | <0.0001 |
| POAG not using topical beta-blockers (n=40) | 328 (223) | 9.5 (1.6) | 27.3 (21.2) | <0.0001 | 0.17 | <0.0001 |
*Unpaired t-test.
HTG, high tension glaucoma; NTG, normal tension glaucoma; POAG, primary open-angle glaucoma.
Multivariable analyses
In multivariate analyses of nailfold capillary haemodynamic parameters in relation to POAG (n=62) compared with controls (n=57), we detected little confounding by the 11 covariates captured (p≥0.054; table 3). Every micron per second increase in nailfold capillary blood speed was associated with a 0.5% reduced odds of POAG (95% CI 0.992 to 0.997; p<0.0001). Although there was no significant difference in vessel diameter between POAG and controls in univariate analysis, in multivariable analysis the difference was marginally significant (p=0.048), with every micron increase in vessel diameter being associated with a 27% reduced odds of POAG (95% CI 0.53 to 0.99). Every picolitre per second increase in blood flow was associated with a 6% decreased odds of POAG (95% CI 0.92 to 0.96; p<0.0001).
Table 3.
Multivariable logistic regression of mean nailfold capillary blood speed, diameter and flow in relation to primary open-angle glaucoma case (n=62) versus control (n=57) status
| Exposure (covariates indented) | OR (95% CI) | P values |
| Mean speed in μm/s | 0.995 (0.992 to 0.997) | <0.0001 |
| Body mass index in kg/m2 | 0.98 (0.87 to 1.09) | 0.69 |
| Mean arterial pressure in mm Hg | 0.99 (0.94 to 1.03) | 0.54 |
| Pulse in beats/min | 1.04 (1.00 to 1.09) | 0.091 |
| Female sex | 0.73 (0.25 to 2.03) | 0.55 |
| Age in years | 1.00 (0.94 to 1.06) | 0.91 |
| Caucasian race | 2.42 (0.58 to 12.1) | 0.25 |
| Connective tissue disease | 0.79 (0.24 to 2.64) | 0.70 |
| Diabetes mellitus | 0.19 (0.022 to 1.22) | 0.10 |
| Use of blood thinning medication | 0.73 (0.23 to 2.35) | 0.59 |
| Use of blood pressure medication | 0.66 (0.19 to 2.30) | 0.51 |
| Ever smoker (vs never) | 1.58 (0.56 to 4.62) | 0.39 |
| Mean diameter in μm | 0.73 (0.53 to 0.99) | 0.048 |
| Body mass index in kg/m2 | 0.91 (0.82 to 1.00) | 0.069 |
| Mean arterial pressure in mm Hg | 1.00 (0.97 to 1.04) | 0.86 |
| Pulse in beats/min | 1.03 (0.99 to 1.07) | 0.14 |
| Female sex | 0.75 (0.29 to 1.88) | 0.54 |
| Age in years | 0.98 (0.92 to 1.03) | 0.36 |
| Caucasian race | 2.95 (0.75 to 13.9) | 0.14 |
| Connective tissue disease | 0.87 (0.28 to 2.63) | 0.80 |
| Diabetes mellitus | 0.22 (0.032 to 1.17) | 0.094 |
| Use of blood thinning medication | 0.74 (0.26 to 2.06) | 0.56 |
| Use of blood pressure medication | 1.16 (0.39 to 3.60) | 0.79 |
| Ever smoker (vs never) | 1.88 (0.75 to 4.86) | 0.18 |
| Mean flow in pL/s | 0.94 (0.92 to 0.96) | <0.0001 |
| Body mass index in kg/m2 | 0.97 (0.86 to 1.08) | 0.55 |
| Mean arterial pressure in mm Hg | 0.99 (0.94 to 1.03) | 0.57 |
| Pulse in beats/min | 1.05 (1.00 to 1.10) | 0.058 |
| Female sex | 0.55 (0.18 to 1.57) | 0.28 |
| Age in years | 0.98 (0.93 to 1.04) | 0.59 |
| Caucasian race | 2.79 (0.64 to 14.9) | 0.19 |
| Connective tissue disease | 0.74 (0.21 to 2.53) | 0.63 |
| Diabetes mellitus | 0.12 (0.012 to 0.89) | 0.054 |
| Use of blood thinning medication | 0.68 (0.27 to 2.23) | 0.52 |
| Use of blood pressure medication | 0.92 (0.27 to 3.27) | 0.90 |
| Ever smoker (vs never) | 1.99 (0.69 to 6.10) | 0.21 |
Stratification of POAG cases by maximum known IOP (online supplementary table 1) revealed similar inverse relations between blood flow and glaucoma for HTG (n=30 cases) versus controls (n=57) (OR (95% CI): 0.93 (0.89 to 0.96); p=0.00010), and for NTG (n=26 cases) versus controls (n=57) (OR (95% CI): 0.93 (0.89 to 0.97); p=0.00072). Stratification of POAG cases by concurrent use of topical beta-blocker (online supplementary table 2) showed that the inverse association between nailfold capillary blood flow and glaucoma was essentially unchanged.
bjophthalmol-2018-311846supp001.pdf (59.3KB, pdf)
bjophthalmol-2018-311846supp002.pdf (53.1KB, pdf)
Discussion
This is the first study to demonstrate that nailfold capillary blood speed and flow are markedly reduced in POAG compared with controls independent of demographic and clinical covariates and regardless of stratification by maximum IOP or topical beta-blocker medication use.
Blood flow through nailfold capillaries is controlled by upstream feeder arterioles via a local cell–cell signalling process termed conducted vasodilation.20 Conducted vasodilation begins with binding of acetylcholine to muscarinic receptors, which triggers the production of inositol 1,4,5 triphosphate that leads to potassium channel hyperpolarisation and ultimately smooth muscle relaxation in precapillary arterioles. While nitric oxide synthesis is not needed to initiate conducted vasodilation, it does contribute to maintaining this response.21 Interestingly, a pathway analysis that evaluated a gene panel involved in setting vascular tone in relation to POAG implicated three genes involved with conducted vasodilation: caveolin-1; inositol 1,4,5 triphosphate receptor type 3; and 5’ AMP-activated protein kinase.22 Our data are consistent with impaired conducted vasodilation in POAG, although other mechanisms could be involved, including reduced cardiac power and reduced cardiac beat-to-beat variability, both of which have been implicated in POAG.23 24 Furthermore, the lower BMI and frequency of diabetes in our POAG group suggests that the blood flow reduction we observed in POAG is unlikely a direct consequence of arteriosclerosis.25
Our study has several notable limitations. First, it is cross-sectional and thus we do not know whether reduced nailfold capillary blood flow precedes or postdates the onset of POAG. Most of our subjects were Caucasian (91.5% of controls and 84.8% of subjects with POAG), and it is unclear if the findings are generalisable to people of other ancestries. We had a higher rate of diabetes in the control compared with the POAG group, perhaps because patients with diabetes are more likely than non-diabetics to visit the Comprehensive Ophthalmology Services, the source of our controls. Furthermore, we were not able to randomise treatment by topical beta-blocker medication in order to investigate the effect of topical beta-blocker medication on nailfold capillary blood flow. Patients with POAG not using topical beta-blockers may be more likely than patients with POAG using topical beta-blockers to have contraindications to beta-blocker use, such as chronic bradycardia. Overall, while we adjusted for multiple covariates, we cannot eliminate the possibility that there was some sampling bias in generating these cohorts. Additionally, our assessments of nailfold capillary haemodynamic parameters were not automated. The videos contained too many artefacts and irregularities for us to design an algorithm for automated haemodynamic assessment. To mitigate the effects of grader subjectivity, graders remained masked to case status during all analyses and two readers validated the grading protocol. We also recognise that the restrictions of nailfold capillaroscopic technology are limitations to this study. Specifically, capillaries must not be obscured by other capillaries and must run parallel to the skin’s surface to be brought into focus (and thus assessed according to our protocol). Although we sought to achieve data from 10 capillaries per patient, we used data from an average of 9.4 capillaries per patient because we were not able to assess in ImageJ capillaries that were out of focus or did not exhibit a blood column void in a linear vascular segment. Moreover, we did not achieve 80% power in the evaluations of vessel diameter and our analyses of this parameter warrant replication with a larger sample. We did not control for the time of day when nailfold blood flow was assessed. We also did not control for the temperature of the finger during the video, although we conducted study procedures on every patient directly following their ophthalmology examinations.
The study has several strengths. The technique is convenient, inexpensive and non-invasive, making assessment of peripheral blood flow a possible surrogate for assessing vascular pathology in POAG, which may be particularly useful should systemic interventions be developed for this disease. This study was robustly powered for the key parameters (blood speed and flow) and used multivariable modelling to account for demographic and clinical covariates that may influence our outcomes of interest. We were able to stratify by maximum IOP and topical beta-blocker use to describe further the relation of nailfold capillary blood flow and POAG.
In conclusion, we report a non-ocular microvascular deviation in POAG that is unrelated to IOP level, local ocular treatment or systemic factors. Insufficient peripheral capillary perfusion in POAG deserves further study, including determining if this feature of POAG is modifiable and if correcting it to normal levels diminishes susceptibility to or progression of the disease. Longitudinal studies are needed to determine the relation between nailfold capillary blood flow and disease severity.
Acknowledgments
We thank the Massachusetts Eye and Ear Ophthalmology Clinical Research Operations.
Footnotes
Presented at: This work was submitted in part for the 2018 American Glaucoma Society Meeting in New York City.
Contributors: All authors contributed to drafting this work or revising it critically for important intellectual content and provided approval for it to be published. All authors are in agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. CCC, JCC, SHG, SCB, LQS, AVT, PH and LRP acquired the data. CCC, JCC, RR, JLW, PAK and LRP analysed the data. CCC, RR, JLW, PAK and LRP contributed to the conception and design of the work.
Funding: This work was supported by the National Eye Institute (grant number EY 015473) and the Harvard Glaucoma Center of Excellence (no grant number).
Competing interests: LRP is a consultant for Bausch+Lomb and is on the advisory board for Eyenovia. He has received an honorarium for an unrestricted CME event sponsored by Alcon. He has received travel support from The Glaucoma Foundation (New York, New York). LQS is a consultant for Genentech and has received research support from Topcon Research Foundation.
Patient consent: Obtained.
Ethics approval: The Massachusetts Institutional Review Board approved this study (approval no.
558105-28).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The raw data of nailfold capillary blood flow measurements are available upon request.
References
- 1. Logan JF, Rankin SJ, Jackson AJ. Retinal blood flow measurements and neuroretinal rim damage in glaucoma. Br J Ophthalmol 2004;88:1049–54. 10.1136/bjo.2003.034884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yin ZQ, Vaegan, Millar TJ, et al. Widespread choroidal insufficiency in primary open-angle glaucoma. J Glaucoma 1997;6:23???32–32. 10.1097/00061198-199702000-00006 [DOI] [PubMed] [Google Scholar]
- 3. Kaiser HJ, Schoetzau A, Stümpfig D, et al. Blood-flow velocities of the extraocular vessels in patients with high-tension and normal-tension primary open-angle glaucoma. Am J Ophthalmol 1997;123:320–7. 10.1016/S0002-9394(14)70127-8 [DOI] [PubMed] [Google Scholar]
- 4. Feke GT, Pasquale LR. Retinal blood flow response to posture change in glaucoma patients compared with healthy subjects. Ophthalmology 2008;115:246–52. 10.1016/j.ophtha.2007.04.055 [DOI] [PubMed] [Google Scholar]
- 5. Flammer J, Konieczka K, Flammer AJ. The primary vascular dysregulation syndrome: implications for eye diseases. Epma J 2013;4:14 10.1186/1878-5085-4-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Su WW, Cheng ST, Ho WJ, WW S, WJ H, et al. Glaucoma is associated with peripheral vascular endothelial dysfunction. Ophthalmology 2008;115:1173–8. 10.1016/j.ophtha.2007.10.026 [DOI] [PubMed] [Google Scholar]
- 7. Harris A, Zarfati D, Zalish M, et al. Reduced cerebrovascular blood flow velocities and vasoreactivity in open-angle glaucoma. Am J Ophthalmol 2003;135:144–7. 10.1016/S0002-9394(02)01927-X [DOI] [PubMed] [Google Scholar]
- 8. Konieczka K, Choi HJ, Koch S, et al. Relationship between normal tension glaucoma and Flammer syndrome. Epma J 2017;8:111–7. 10.1007/s13167-017-0097-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Flammer J, Konieczka K. The discovery of the Flammer syndrome: a historical and personal perspective. Epma J 2017;8:75–97. 10.1007/s13167-017-0090-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gasser P, Flammer J. Blood-cell velocity in the nailfold capillaries of patients with normal-tension and high-tension glaucoma. Am J Ophthalmol 1991;111:585–8. 10.1016/S0002-9394(14)73703-1 [DOI] [PubMed] [Google Scholar]
- 11. Schmidt KG, Rückmann AV, Mittag TW, et al. Reduced ocular pulse amplitude in low tension glaucoma is independent of vasospasm. Eye 1997;11(Pt 4):485–8. 10.1038/eye.1997.131 [DOI] [PubMed] [Google Scholar]
- 12. Cortes S, Cutolo M. Capillarosecopic patterns in rheumatic diseases. Acta Reumatol Port 2007;32:29–36. [PubMed] [Google Scholar]
- 13. Pasquale LR, Hanyuda A, Ren A, et al. Nailfold Capillary Abnormalities in Primary Open-Angle Glaucoma: A Multisite Study. Invest Ophthalmol Vis Sci 2015;56:7021–8. 10.1167/iovs.15-17860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cousins CC, Kang JH, Bovee C, et al. Nailfold capillary morphology in exfoliation syndrome. Eye 2017;31:698–707. 10.1038/eye.2016.312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mugii N, Hasegawa M, Hamaguchi Y, et al. Reduced red blood cell velocity in nail-fold capillaries as a sensitive and specific indicator of microcirculation injury in systemic sclerosis. Rheumatology 2009;48:696–703. 10.1093/rheumatology/kep066 [DOI] [PubMed] [Google Scholar]
- 16. Kim KM, Lee DJ, Joo NS. Reduction of the nailfold capillary blood velocity in cigarette smokers. Korean J Fam Med 2012;33:398–405. 10.4082/kjfm.2012.33.6.398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yi Y, Baoyu W, Shenyuan Y, et al. Changes of nailfold microcirculation in patients of type II diabetes mellitus with diabetic retinopathy. Chin Med Sci J 1999;14:233–6. [PubMed] [Google Scholar]
- 18. Day RO, Wacher T, Cairns D, et al. Nailfold capillary circulation in osteoarthritis. Br J Rheumatol 1993;32:1062–5. 10.1093/rheumatology/32.12.1062 [DOI] [PubMed] [Google Scholar]
- 19. Nordlund JR, Pasquale LR, Robin AL, et al. The cardiovascular, pulmonary, and ocular hypotensive effects of 0.2% brimonidine. Arch Ophthalmol 1995;113:77–83. 10.1001/archopht.1995.01100010079024 [DOI] [PubMed] [Google Scholar]
- 20. Bagher P, Segal SS. Regulation of blood flow in the microcirculation: role of conducted vasodilation. Acta Physiol 2011;202:271–84. 10.1111/j.1748-1716.2010.02244.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Doyle MP, Duling BR. Acetylcholine induces conducted vasodilation by nitric oxide-dependent and -independent mechanisms. Am J Physiol 1997;272(Pt 2):H1364–71. 10.1152/ajpheart.1997.272.3.H1364 [DOI] [PubMed] [Google Scholar]
- 22. Kang JH, Loomis SJ, Yaspan BL, et al. Vascular tone pathway polymorphisms in relation to primary open-angle glaucoma. Eye 2014;28:662–71. 10.1038/eye.2014.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Riccadonna M, Covi G, Pancera P, et al. Autonomic system activity and 24-hour blood pressure variations in subjects with normal- and high-tension glaucoma. J Glaucoma 2003;12:156–63. 10.1097/00061198-200304000-00011 [DOI] [PubMed] [Google Scholar]
- 24. Kashiwagi K, Tsumura T, Ishii H, et al. Circadian rhythm of autonomic nervous function in patients with normal-tension glaucoma compared with normal subjects using ambulatory electrocardiography. J Glaucoma 2000;9:239–46. 10.1097/00061198-200006000-00007 [DOI] [PubMed] [Google Scholar]
- 25. Flammer J, Konieczka K, Bruno RM, et al. The eye and the heart. Eur Heart J 2013;34:1270–8. 10.1093/eurheartj/eht023 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bjophthalmol-2018-311846supp001.pdf (59.3KB, pdf)
bjophthalmol-2018-311846supp002.pdf (53.1KB, pdf)


