Abstract
Background:
Delirium, defined as an acute disorder of attention and cognition with high morbidity and mortality, can be prevented by multicomponent nonpharmacological interventions. The Hospital Elder Life Program is the original evidence-based approach targeted to delirium risk factors, which has been widely disseminated.
Objective:
To summarize the current state of the evidence regarding the Hospital Elder Life Program (HELP) and to highlight its effectiveness and cost-savings.
Method:
Systematic review of Ovid MEDLINE, EMBASE and the Cochrane Central Register of Controlled Trials from 1999 to 2017, using a combination of controlled vocabulary and keyword terms.
Results:
Of 44 final articles included, 14 were included in the meta-analysis for effectiveness, and 30 were included for examining cost-savings, adherence and adaptations, role of volunteers, successes and barriers, and issues in sustainability. The results for delirium incidence, falls, length of stay, and institutionalization were pooled for meta-analyses. Overall, 14 studies demonstrated significant reductions in delirium incidence (odds ratio [OR], 0.47; 95% CI, 0.37–0.59). The rate of falls was reduced by 42% among intervention patients in three comparative studies (OR, 0.58; 95% CI, 0.35–0.95). In 9 studies on cost-savings, the program saved $1600–3800 (2018 US dollars) per patient for hospital costs and over $16,000 (2018 US dollars) per person-year for long-term care costs in the year following delirium. The systematic review revealed that programs were generally successful in adhering to or appropriately adapting HELP (n=13 studies), and in finding the volunteer role to be valuable (n=6 studies). Successes and barriers to implementation were examined in 6 studies, including ensuring effective clinician leadership, finding senior administrative champions, and shifting organizational culture. Sustainability factors were examined in 10 studies, including adapting to local circumstances, documenting positive impact and outcomes, and securing long-term funding.
Conclusion:
The Hospital Elder Life Program is effective in reducing incidence of delirium and rate of falls, with a trend toward decreasing length of stay and preventing institutionalization. With ongoing efforts in continuous program improvement, implementation, adaptations, and sustainability, HELP has emerged as a reference standard model to improve the quality and effectiveness of hospital care for older persons worldwide.
Keywords: Delirium prevention, Hospital Elder Life Program, Multicomponent nonpharmacological intervention
Introduction
Delirium is one of the most common complications for hospitalized older persons, with occurrence rates as high as 50%1 in hospitalized persons. It is consistently associated with increased rates of morbidity, mortality, poorer long-term outcomes, longer hospitalizations and costlier treatment.2–4 Recent meta-analyses demonstrated that delirium in older patients is associated with poor outcomes independent of important confounders, such as age, sex, comorbid diseases or disease severity, and dementia.5 Delirium is serious and often fatal, and once present, no treatment strategy has been found to improve long-term mortality or need for institutional care.6,7 The mortality of patients with delirium is significantly higher compared with patients without delirium (38% vs. 27.5%, HR 1.95, 95% CI, 1.51–2.52) after 22.7 months’ follow-up.5 With its adverse impact, delirium accounts for more than $183 billion (2018 US dollars) in annual health care expenditures in the U.S.,8 rivaling the expenditures for diabetes.9 Importantly, delirium is preventable in 30–40% of cases.1 Currently, multicomponent nonpharmacologic approaches have been consistently demonstrated as the most effective strategies for delirium prevention.1 Among these approaches, the Hospital Elder Life Program (HELP) is the original evidence-based model which has been the most widely implemented worldwide.10–12
HELP, developed in 1993 as a targeted, multicomponent strategy to prevent functional and cognitive decline in hospitalized older persons, has now been disseminated to more than 200 hospitals worldwide. The original goals of HELP for older hospitalized patients were to preserve physical and cognitive functioning, maximize independence at discharge, help with the transition from hospital to home, and prevent unplanned readmission.11 The HELP model of care was originally described in 2 studies10,11 and a follow-up study examining the first 13 sites.12
To achieve its goals, the HELP program involves patients, caregivers, and HELP staff members – including an Elder Life Specialist, an Elder Life Nurse Specialist, a geriatrician and specially trained volunteers – to work together to implement a coordinated program centered around the patient. All admissions are screened for eligibility, and interventions are assigned based on the presence of risk factors for delirium, including baseline cognitive impairment, sleep deprivation, immobility, visual or hearing impairment, and dehydration.10,13 HELP assists patients in multiple ways to prevent both delirium and functional decline.14,15 While the intervention protocols are standardized, the assigned interventions are individualized and tailored to each patient in accordance with their abilities and preferences. Once interventions are assigned, adherence is tracked daily, and quality assurance measures are incorporated at each step of the program from admission to discharge. Skilled interdisciplinary teams assisted by trained volunteers conduct interventions.
The program includes core intervention protocols for daily visits, orientation, therapeutic activities, sleep enhancement, early mobilization, vision and hearing adaptation, fluid repletion, and feeding assistance. Other program interventions include geriatric nursing assessment and intervention, interdisciplinary rounds, ongoing staff educational programs, post-discharge community linkages, and telephone follow-up. Table 1 describes the interventions and designated staff from the original description of the HELP program and from recent adaptations.11,52 In 2013, HELP protocols were adapted to ensure fulfillment with the National Institute for Health and Care Excellence (NICE) guidelines.52 Interventions added based on the NICE guidelines included prevention of infection, and management of constipation, pain, and hypoxia (Table 1). In the past 15 years, the program has been implemented on all types of hospital services (medical, medical subspecialty, neurological, surgical, surgical subspecialty, mixed medical-surgical, orthopedic, palliative care, intensive care, rehabilitation, and emergency department), as well as post-acute and long-term care settings.
Table 1: HELP program interventions and staff.
| Interventions | Staff | Description |
|---|---|---|
| Core interventions | ||
| Orientation | ELS, volunteers | Daily orientation, orientation board with names of care team members and daily schedule |
| Therapeutic activities | ELS, volunteers | Cognitive stimulation activities three times daily |
| Sleep enhancement | ELNS, ELS, volunteers | At bedtime, warm milk or herbal tea, relaxation tapes or music, and back massage. Ward-wide noise reduction and schedule adjustments to allow uninterrupted sleep |
| Early mobilization | ELNS, ELS, volunteers | Ambulation or active range-of-motion exercises three times daily. Minimizing use of immobilizing equipment |
| Vision protocol & Vision protocol - Blindness | ELS, volunteers | Visual aids (e.g., glasses, magnifying lenses) and adaptive equipment (e.g., large illuminated telephone keypads, large print books, fluorescent tape on call bell), with daily reinforcement |
| Hearing protocol | ELNS, ELS, volunteers | Portable amplifying devices and special communication techniques, with daily reinforcement. Ear wax clearing by ELNS as needed |
| Fluid repletion/constipation | ELNS, ELS, volunteers | Encourage fluids. Encourage mobility and regular toileting. Added fiber to diet. Laxatives if needed |
| Feeding assistance | ELS, volunteers | Feeding assistance and encouragement during meals |
| Additional interventions based on the NICE | ||
| Hand Hygiene | ELNS, ELS, Volunteers | Hand washing protocol. Generalized infection control measures |
| Aspiration Prevention | ELNS | Regular oral care. Head of bed at 60 degrees during meals. Monitor for signs of pneumonia |
| CAUTI Prevention | ELNS | Sterile insertion technique. Early catheter removal |
| Constipation management | ELNS, ELS. Volunteers | Encourage fluids. Encourage mobility and regular toileting. Added fiber to diet. Laxatives if needed |
| Pain management | ELNS | Pain management plan and modify as needed. Non-pharmacological and pharmacological management |
| Hypoxia management | ELNS | Seek advice regarding oxygen administration. Check oxygen flow. Elevate head of bed to 45 degrees |
| Other interventions | ||
| Geriatric nursing assessment and interventions | ||
| Delirium protocol | ELNS | Create calm, orienting environment. Regular communication with patient; family involvement. Geriatric consult if needed |
| Dementia protocol | ELNS | Collaborate with medical staff and patient family. Avoid psychoactive medications |
| Psychoactive medications | ELNS, interdisciplinary group | Screen medication list daily. Interdisciplinary group discussions about potential adverse medication outcomes |
| Discharge planning | ELNS | Assessing home environment and social supports for possible discharge needs |
| Optimizing length of stay | ELNS | Identify risk factors for need of intensive discharge planning and anticipate discharge needs |
| Additional areas | ELNS | Nursing assessment and interventions for emotional health, nutrition, function, skin care, incontinence and elimination problems, social issues |
| Interdisciplinary rounds | ||
| Geriatric consultation | Geriatrician | Targeted consultation on Elder Life issues, as referred by program staff. Formal geriatric consultation as needed |
| Interdisciplinary rounds | ELNS, ELS, geriatrician, primary nurses, physical therapist, dietitian, pharmacist, chaplain, and consultants. | Twice-weekly rounds to discuss each Elder Life patient, set goals and review all Elder Life issues with interdisciplinary input. Interventions are recommended and tracked |
| Ongoing educational programs | ELNS, geriatrician, and nurse practitioner | Formal didactic sessions, one-on-one interactions, resource materials to educate about Elder Life issues |
| Community linkages & Telephone follow-up | ELNS, ELS | Referrals and communication with community agencies to optimize transition home. Telephone follow-up phone call within 7 days after discharge. |
ELS: Elder life specialist, ELNS: Elder life nurse specialist, CAUTI: catheter association urinary tract infection
The purpose of this systematic review is to provide a comprehensive examination of the HELP model of care and adaptations, evidence of its efficacy and cost-savings, adherence and adaptations to enhance adherence, unique role of the volunteer, successes and barriers to implementation, and steps to guide long-term sustainability.
Methods
Search strategy and selection criteria
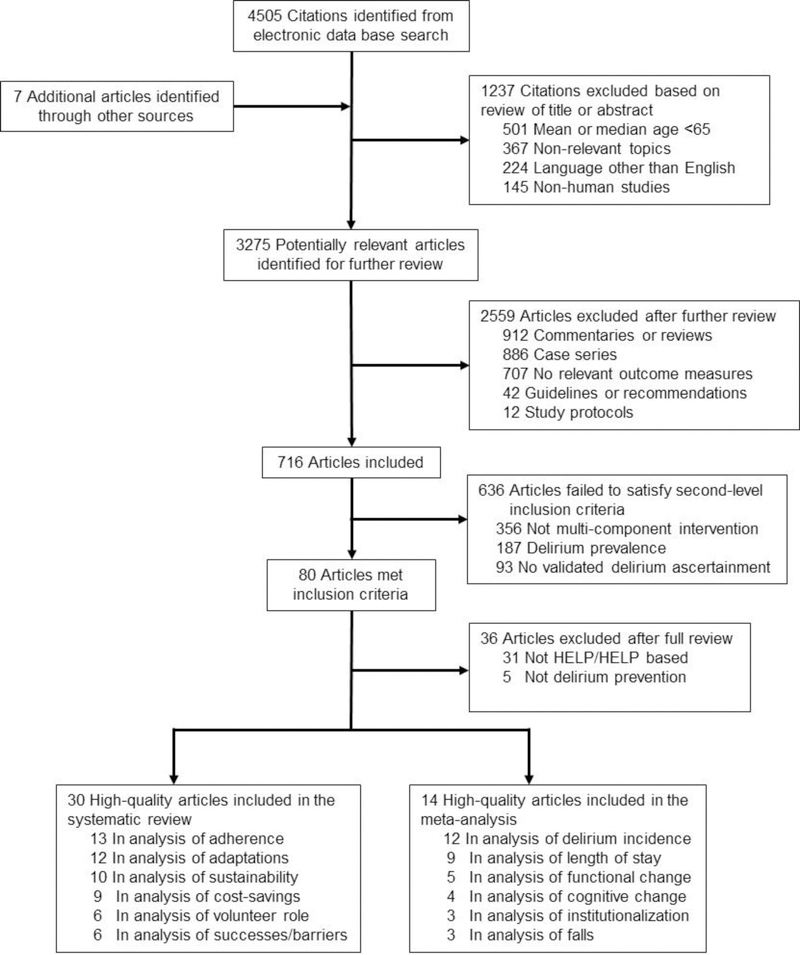
Articles for this review were identified by comprehensive searches in Ovid MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials, and the Cochrane Database of Systematic Reviews. The Ovid search strategy is available in Appendix Box 1. Reference lists from published narrative review articles and systematic reviews were further reviewed to identify additional studies. The following MeSH terms and free words were used: “delirium prevention,” “multicomponent intervention,” “non-pharmacological intervention” and “Hospital Elder Life Program.” Articles published in English between 1999 and 2017 were included. The start date of 1999 was chosen which marked the date of the first publication of the HELP model,10 which was developed and implemented in 1993. The initial search yielded 4505 articles and after exclusion by 2 independent reviewers (J.Y. and T.Y.) based on screening criteria (language, age range, relevance, nonhuman study), the number of articles was narrowed to 3275 (Figure 1). Upon further review, 2559 articles were excluded (case series, commentaries or reviews, guidelines or recommendations, no relevant outcome measures, or study protocols). The 2 independent clinical reviewers then reviewed these 716 articles and applied second-level inclusion criteria, which required that studies apply multicomponent approaches, focus on delirium prevention and use validated delirium instruments – eliminating 636 articles. Thus, 80 articles met all inclusion and exclusion criteria. After full review for HELP program and HELP adaptations only, 14 were selected for inclusion in the meta-analysis and 30 for the systematic review. All data presented are taken from the original articles. For the effectiveness studies, a previous meta-analysis was updated with data from new studies.16 Two reviewers (J.Y. and T.Y.) reviewed each of these effectiveness studies and reached consensus on all elements. Since the goal of this manuscript was to provide a comprehensive review of primary articles, systematic reviews and meta-analyses were not routinely included; however, all of their reference lists were checked to insure the comprehensive inclusion of primary articles in our review process.
Figure 1. Literature Identification, Review and Selection for Inclusion.
Databases searched included Ovid MEDLINE, EMBASE, The Cochrane Central Register of Controlled Trials, and The Cochrane Database of Systematic Reviews from January 1999 and December 2017. Reference lists from published narrative review articles and systematic reviews were further reviewed to identify additional studies. The following MeSH terms and free words were used: “delirium prevention,” “multicomponent intervention,” “non-pharmacological intervention” and “Hospital Elder Life Program.” Utilizing our systematic literature search strategy, 3275 articles were found. Of these, 3202 were excluded based on our screening criteria for relevance, language, age range, or non-human study subjects; full-text articles of the remaining 73 studies were retrieved for further assessment according to the inclusion criteria. A total of 14 studies were included in meta-analysis and an additional 30 were included as cost-savings studies, methodological papers, and qualitative studies.
Data extraction and data analyses
Two authors (J.Y. and T.Y.) independently extracted information on outcome measures and quality from the included studies. For effectiveness studies, we conducted a quantitative meta-analysis using accepted approaches. Data for meta-analyses were compiled using Review Manager Version 5.3. Intervention studies that used formalized methods for balanced allocation between treatment and control groups via randomization or prospective individual matching designs (blinded) were categorized into the group we described as randomized-matched trials (RMTs). We combined randomized clinical trials (RCTs) with prospective matched (blinded) trials because the few RCTs precluded separate meta-analyses. We analyzed both types of studies together, and separated from other sorts of comparative intervention studies (non-RMTs). The robust methods and balanced allocation with prospective matching and blinded outcome assessment created comparable study quality to RCTs and thus, were combinable without introducing excessive heterogeneity. We made the decision to include both RMTs and non-RMTs in our meta-analysis because both study designs provide robust comparisons of the HELP model. Moreover, some of the non-RMTs were of high quality, including matching or well-controlled analyses. We had previously used this methodology in 2015 for our systematic review and meta-analysis of multicomponent, nonpharmacologic delirium interventions;16 and we found this to be an effective approach to control for heterogeneity among studies.
Dichotomous outcomes (e.g. delirium incidence, falls, and institutionalization) were presented as odds ratios (OR) with 95% confidence intervals (CIs). The number needed to treat (NNT) was also calculated if p < 0.05. Continuous outcomes were combined by using the mean difference (MD) or standardized mean difference (SMD) when different scales were used across studies. Because the trials were not carried out according to a common protocol, there were understandably variations in patient groups, clinical settings, concomitant care, etc. We, therefore, assessed heterogeneity between trial results. Trial data were considered heterogeneous where the I2 statistic was > 50%. For analysis, we used the fixed-effect method unless data were heterogeneous in which case we used the random-effects model. Where significant heterogeneity was present, we further examined patient clinical characteristics and interventions of the included studies for explanatory purposes.
Quality and Risk of Bias
For the studies included in the meta-analysis, we examined each for quality by determining how many of the 6 domains of the Cochrane Collaboration’s risk of bias tool were incorporated. These domains include random or balanced allocation method, allocation concealment, blinding, completeness of outcome data, non-selective outcome reporting, and absence of other bias.
For additional HELP-related studies, one author (T.H.) abstracted descriptive information and text, and categorized each article into 4 major study categories, as follows: (1) Cost-savings – these studies examined the economic value of the HELP model in terms of healthcare costs (all cost figures were translated into 2018 US dollars); (2) Adherence and adaptation – these studies examined adherence rates with interventions or adaptations to improve program adherence; (3) Role of volunteers – these studies examined the unique role of volunteers in the HELP program, including their value, characteristics, and motivations; (4) Successes and barriers to implementation and sustainability – studies examining successes and barriers to setting up and maintaining a HELP site across diverse sites. Individual studies may have been used for more than one of these 4 categories.
Results
Study selection and study characteristics
A total of 14 studies were included in the meta-analysis10,17–29 and an additional 30 were included as cost-savings studies, methodological papers, and qualitative studies.9,11–15,27,29–51 A flow chart of identification, screening, review, and selection of studies is presented in Figure 1. The meta-analyses presented in Figures 2 and 3 categorized studies as RMTs and non-RMTs. More detailed categorizations of the study design, including randomized clinical trials, nonrandomized controlled, or historically controlled are also provided on Table 2 under the “Study Design” column.
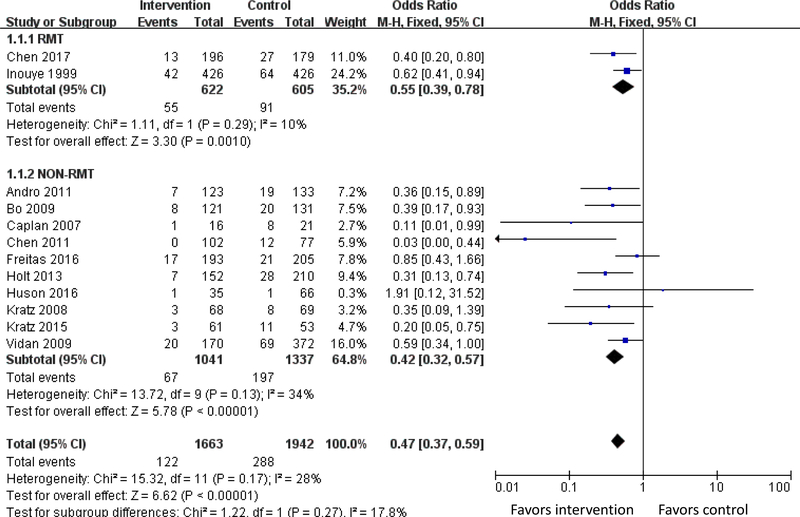
Figure 2. Meta-analysis of Outcome of Delirium Incidence.
Twelve studies of the HELP model measured delirium incidence. In total, the meta-analysis involved 3,605 patients and showed that the odds of delirium were 53% lower in the intervention group compared with controls (OR 0.47; 95% CI, 0.37–0.59, I2 = 28%). Stratified by study type (RMT versus non-RMT), HELP-based delirium interventions lowered the odds of delirium by 45% (OR 0.55; 95% CI, 0.39–0.78, I2 = 10%) among 1,267 patients included in 2 RMTs and by 58% (OR 0.42; 95% CI, 0.32–0.57, I2 = 34%) among 2,378 intervention patients included in 10 non-RMTs. The numbers needed to treat (NNTs) were 16.7 (95% CI, 10.0–33.3) among RMTs and 12.5 (95% CI, 10.0–20.0) among non-RMTs.
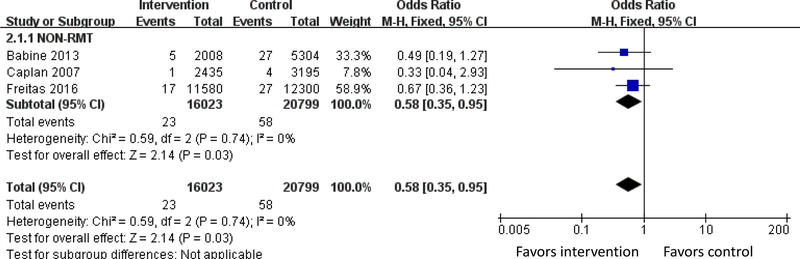
Figure 3. Meta-analysis of Outcome of Falls.
Three studies examined number of falls per patient-day. The meta-analysis showed that the odds of falling was 42% lower (OR 0.58, 95% CI 0.35–0.95, I2 =0%) among subjects in 3 non-RMT studies (Figure 3).
Table 2: Characteristics of studies included in effectiveness meta-analysis (n = 14).
| Study | Setting | Study Design | Sample Size | Mean Age (years) | Quality Measure | # HELP interventions | Outcomes |
|---|---|---|---|---|---|---|---|
| Andro 2011 (France) | Acute care geriatric ward, 1 site † | Historically controlled (non-RMT) | 256 | 84.7 | 1/6 (O) | 5/6 (C, E, H, V, W) | Delirium |
| Babine 2013 (USA) | Medical ward † | Historically controlled (non-RMT) | 516 | 70+ | 1/6 (O) | 6/6 (C, E, H, P, V, W) | Falls |
| Bo 2009 (Italy) | Medical ward, 1 site * | Nonrandomized controlled trial (non-RMT) | 252 | 82.4 | 3/6 (I, O, X) | 4/6 (C, E, P, W) | Delirium |
| Bogardus 2003 (USA) | Medical ward, 1 site * | Nonrandomized controlled trial - matched/blinded (RMT) | 705 | 80 | 5/6 (B, I, O, S, X) | 6/6 (C, E, H, P, V, W) | Delirium, function/cognition, institutionalization |
| Caplan 2007 (Australia) | Medical ward, 1 site † | Historically controlled (non-RMT) | 37 | 84.7 | 3/6 (I, O, S) | 4/6 (C, H, V, W) | Delirium (incidence, severity, duration), falls, LOS, institutionalization, readmission, function/cognition, cost-savings |
| Chen 2011 (Taiwan) | Surgical ward, 1 site † | Historically controlled (non-RMT) | 179 | 73.0 | 3/6 (B, I, O) | 2/6 (E, C) | Delirium, LOS, function/cognition, nutrition, depression |
| Chen 2017 (Taiwan) | Surgical ward, 1 site * | Randomized controlled trial (RMT) | 375 | 74.5 | 5/6 (A, B, I, O, S) | 3/6 (C, E, W) | Delirium, LOS |
| Freitas 2016 (USA) | Medical ward, 1 site * | Nonrandomized controlled (non-RMT) | 398 | >70 | 2/6 (I, O) | 2/6 (E, P) | Delirium (incidence, duration), falls |
| Holt 2013 (England) | Elderly care wards, 1 site † | Historically controlled (non-RMT) | 362 | 85.4 | 4/6 (B, I, O, X) | 5/6 (C, E, H, V, W) | Delirium (incidence, severity, duration), LOS, institutionalization, function/cognition, mortality |
| Huson 2016 (USA) | Post-acute rehabilitation settings, 1 site * | Nonrandomized controlled (non-RMT) | 100 | 82.7 | 4/6 (A, I, O, S) | 6/6 (C, E, H, P, V, W) | Delirium, LOS, institutionalization, function/cognition |
| Inouye 1999 (USA) | Medical ward, 1 site * | Nonrandomized controlled trial - matched/blinded (RMT) | 852 | 79.7 | 5/6 (B, I, O, S, X) | 6/6 (C, E, H, P, V, W) | Delirium (incidence, duration, recurrence, severity) |
| Kratz 2008 (USA) | Medical-surgical ward, 1 site * | Nonrandomized controlled trial (non-RMT) | 137 | 70+ | 1/6 (O) | 6/6 (C, E, H, P, V, W) | Delirium |
| Rubin 2017 (USA) | Medical/ surgical ward, 1 site * | Nonrandomized controlled (non-RMT) | 7628 | 79.4 | 3/6 (I, O, X) | 6/6 (C, E, H, P, V, W) | LOS, readmission |
|
Vidan 2009 (Spain)
|
Medical ward, 1 site * | Nonrandomized controlled (non-RMT) | 542 | 84.0 | 1/6 (O) | 6/6 (C, E, H, P, V, W) | Delirium (incidence, duration), function, mortality |
= control group is concurrent usual care
= control group is historical
LOS = length of stay. Quality Measures include allocation concealment (A), blinding of participants, personnel, assessors (B), completeness of outcome data (I), selective outcome reporting (O), random-sequence generation or balanced allocation (S), other biases (X). Adherence to evidence based interventions: Cognition/Orientation (C), Early mobility (E), Hearing (H), Sleep-wake cycle preservation (P), Vision (V), Hydration (W)
Effectiveness of HELP (Meta-analyses)
Meta-analysis results are presented for 5 major outcomes: delirium incidence, falls, length of hospital stay, institutionalization, and change in functional or cognitive status. The present analysis systematically updates a previous meta-analysis.
Delirium incidence.
Twelve studies of the HELP model10,17,18,20,22–25,28,29,53,54 measured delirium incidence. In total, the meta-analysis involved 3,605 patients and showed that the odds of delirium were 53% lower in the intervention group compared with controls (OR 0.47; 95% CI, 0.37–0.59, I2 = 28%). Stratified by study type (RMT versus non-RMT), HELP-based delirium interventions lowered the odds of delirium by 45% (OR 0.55; 95% CI, 0.39–0.78, I2 = 10%) among 1,267 patients included in 2 RMTs and by 58% (OR 0.42; 95% CI, 0.32–0.57, I2 = 34%) among 2,378 intervention patients included in 10 non-RMTs. The numbers needed to treat (NNTs) were 16.7 (95% CI, 10.0–33.3) among RMTs and 12.5 (95% CI, 10.0–20.0) among non-RMTs (Figure 2).
Falls.
Three studies examined number of falls per patient-day.21,22,54 The meta-analysis showed that the odds of falling was 42% lower (OR 0.58, 95% CI 0.35–0.95, I2 =0%) among subjects in 3 non-RMT studies (Figure 3).
Length of stay.
Nine studies measured length of stay.10,18,20,22–24,27–29 The meta-analysis indicated that the mean difference was −0.24 days favoring the intervention group (95% CI −0.95–0.48), but this did not achieve statistical significance and heterogeneity was high (I2=71%). Stratified by study type, the length of stay for the intervention group was also not statistically significant, mean difference −0.77 (−3.12–1.59, I2=91%) among 2 RMTs and −0.01 (95% CI, −0.83–0.80, I2=60%) among 7 non-RMTs.
Institutionalization.
Four studies of HELP-related models19,22–24 reported outcomes related to institutionalization after hospital discharge. The meta-analysis showed that the odds of being institutionalized was not significantly different (OR 1.00, 95% CI 0.72–1.27, I2=0%) among intervention subjects. Stratified by study type, the odds of institutionalization remained not significantly different, with an odds ratio for institutionalization in one RMT of 1.00 (95% CI, 0.74–1.35) and an odds ratio in 3 non-RMTs of 1.00 (95% CI, 0.55–1.82, I2=19%) favoring the intervention group.
Change in Functional or Cognitive status.
Five studies10,18,22,24,28 based on the HELP model examined functional or cognitive change from baseline. Based on the heterogeneity of the studies, it was not considered appropriate to combine the results; thus, conclusions could not be drawn about their combined effectiveness of HELP for function or cognition. However, the preponderance of studies showed stable or improved functional or cognitive status with HELP.18,22,31 It is important to note that the general goal of delirium prevention is to maintain stability of patients’ functional or cognitive status, not necessarily for improvements; thus, a lack of change from baseline may be considered a successful outcome.
Cost-savings studies
Cost-savings of the HELP program was examined directly in 9 studies (Caplan et al. in Table 2 plus Table 3).9,14,15,22,27,29,30,42,51 For all studies presented in this paper, costs are adjusted to 2018 US dollars. We summarize the impact of the HELP program on cost-savings in Table 4 with study description and a column detailing cost-savings calculations and methodology. Based on prior studies, the overall hospital cost savings of the HELP program range from $1661-$3779 (2018 US dollars) per person per hospitalization.14 In a large community hospital, Rubin et al. demonstrated total cost reductions of $769,987 (2018 US dollars) on one medical ward over 6 months, in a study involving 4763 patients.41 In a follow-up study, Rubin et al. were able to demonstrate savings of $8.48 million (2018 US dollars) per year with the care of approximately 7000 patients per year on 6 wards, based on delirium prevention and decreasing length of stay.42 Recently, in a study involving 7628 patients, Rubin et al. demonstrated that the HELP program is associated with lower risk for 30-day hospital readmission (adjusted relative risk 0.83, 95% confidence interval = 0.73–0.94, p = 0.003).27 Given the Medicare Hospital Readmission Reduction Program and its associated financial penalties, the decreased rate of 30-day rehospitalization provided by the HELP program yields significant cost-savings based on decreasing readmissions.
Table 3: Additional studies on Hospital Elder Life Program (HELP) and adapted HELP programs (n = 30).
| Study | Sample size/site | Study Description | Categories |
|---|---|---|---|
| Bakker 2013 (Netherlands) | 28 patients, 1 site * | Original: Determine feasibility of the HELP program and gather preliminary research data | Adherence, cost-savings, volunteers |
| Boockvar 2016 (USA) | 143 patients, 1 site * | Adaptation: In Long Term Care, delivered by nursing assistants | Adaptations, adherence |
| Bradley 2004 (USA) | 32 staff members, 9 sites ‡ | Original: Translating research into clinical practice is challenging. Six common challenges hospital staff addressed | Successes/barriers, adherence |
| Bradley 2005 (USA) | 42 staff members, 13 sites ‡ | Original: Sustained clinical leadership, funding, flexibility, modifications are key to sustain the diffusion of innovative programs such as HELP | Sustainability, adherence |
| Bradley 2006 (USA) | 63 sites † ‡ | Original: Diffusion and adoption of HELP. Realistic expectations about diffusion rates and understanding importance of senior management support crucial to effective adoption of HELP. | Successes/barriers, sustainability |
| Bradley 2006 (USA) | 63 sites ‡ | Original: Key roles and motivations of senior management, the perceived impact of HELP on patient and staff | Successes/barriers, sustainability |
| Chen, P 2015 (USA) | 73 sites ‡ | Original and Adaptations: HELP website resources were used for implementation of HELP and other delirium prevention programs, and were disseminated broadly in innovative educational efforts | Successes/barriers, sustainability, adherence, adaptations |
| Chen, C 2017 (Taiwan) | 179 patients, 1 site * | Adaptation: Modifying the HELP program to include only 3 key interventions was cost-effective and effective for surgical patients | Adherence, adaptations, cost-savings |
| Chong 2011 (Singapore) | 150 patients, 1 site * † | Adaptation: A specialized hospital ward for delirium management with the goal of improving clinical outcomes and staff knowledge, satisfaction. The model can be utilized in various locations and acute hospital settings | Adherence, adaptations |
| Heim 2017 (Netherlands) | 333 patients, 1 site * † | Original: Stepped wedge design for HELP program in daily practice requires attention to ethics, flexibility, time constraints, capacity of research team and data availability and quality | Sustainability |
| Inouye 2000 (USA) | 1507 patients, 1 site * | Original: HELP successfully prevents cognitive, functional decline in older patients. The program is unique in its hospital focus and teaching staff/volunteers skills to successfully implement interventions that target delirium risk factors | Adherence, volunteers |
| Inouye 2003 (USA) | 422 patients, 1 site * | Original: Increased adherence to HELP interventions results in reduced delirium rates | Adherence |
| Inouye 2006 (USA) | 11,344 patients, 13 sites * ‡ | Adaptations: Real world implementation of HELP is examined across 13 sites; its dissemination, local adaptations and successes are examined | Adaptations, adherence, volunteers, sustainability |
| Leslie 2005 (USA) | 801 patients, 1 sites * | Original: Long-term nursing home cost. This suggests the need for increased efforts to prevent and manage delirium among the geriatric population. | Cost-savings |
| Leslie 2008 (USA) | 835 patients, 1 site * | 1-year health care costs associated with delirium | Cost-savings |
| Macias 2017 (USA) | 1 site * | Adaptation: Collaboration between HELP and rehabilitation aides to improve early mobilization | Adaptations, adherence |
| Rizzo 2001 (USA) | 852 patients,1 site * | Original: HELP can prevent delirium without raising costs, supporting it as a cost-saving treatment option for patients at intermediate risk of developing delirium | Cost-savings |
| Rosenbloom- Brunton 2010 (USA) | 5 patients, 15 caregivers, 1 site * | Adaptation: The keys to successful implementation of Family-HELP | Adaptations |
| Rubin 2006`(USA) | 4763 patients, 38 staff nurses, 1 site † | Adaptation: Quality improvement; replicated in the community hospital setting | Adaptations, cost-savings |
| Rubin 2011 (USA) | 27,196 patients, 107 volunteers, 1 site ‡ | Adaptations: Sustainability and scalability, cost-savings of care | Cost-savings, adaptations, sustainability |
| Rubin 2017 (USA) | 7628 patients, 1 site * | Original: Cost-savings of care, 30-day readmission rates in community hospital setting | Cost-savings |
| Sandhaus 2009 (USA) | 122 nurses, 100 volunteers, 1 site † | Adaptation: HELP nursing staff at a community hospital were educated on dysphagia assessment and management. Improving clinical practice using evidence-based medicine requires stakeholder engagement and the use of multiple strategies to sustain change | Adaptations, adherence |
| Sandhaus 2010 (USA) | 110 nurses, 1 site † ‡ | Original: Enhanced participation of trained volunteers in HELP interventions found to improve nursing, patient and family satisfaction. The utilization of volunteers is cost-effective | Volunteers |
| Sandhaus 2010 (USA) | 110 nurses, 1 site † ‡ | Original: Enhanced participation of trained volunteers in HELP interventions found to improve nursing, patient and family satisfaction. The utilization of volunteers is cost-effective | Volunteers |
| Schoettinger 2017 (USA) | 2 medical wards, 1site‡ | Original: Why volunteers participate in HELP program at the University of Michigan Hospital is explored, including demographics, motivations, training, retention | Volunteers |
| Steelfisher 2011 (USA/Canada) | 62 interviews, 19 sites ‡ | Original: Sustaining clinical programs during difficult economic | Sustainability, successes/barriers |
| Steelfisher 2013 (USA/Canada) | 6 sites ‡ | Original: Learning from the closure of clinical programs | Successes/barriers, sustainability |
| Steunenberg 2016 (Netherlands) | 1 site † ‡ | Original: Using mixed-methods design, examined the added value of trained HELP volunteers. Patients appreciated the extra attention and volunteers appreciated their work | Volunteers |
| Zalon 2010 (USA) | 352 patients, 1 site * | Adaptation: Personal digital assistants were used in collecting assessment data for HELP | Adaptations, sustainability |
| Zalon 2017 (USA) | 34 patients, 1 site ‡ | Original: The purpose of the current study was to analyze delirium documentation and referral to HELP for hospitalized older adults | Adherence |
| Zaubler 2013 (USA) | 595 patients, 1 site † | Adaptation: Quality improvement and cost savings in a community hospital | Adaptations, cost-savings |
= control group is concurrent usual care
= control group is historical
other (e.g., study was a quality improvement project, or no comparison/control group)
Table 4. HELP Program impact on cost savings.
| Study | Sample size/site | Study Description | Methodology/Cost Savings Calculations |
|---|---|---|---|
| Bakker 2013 | 28 patients, 1 site | Before-after study with preintervention control and postintervention groups. HELP program was implemented; feasibility was examined and preliminary research data gathered. | Mean cumulative costs per patient three months after discharge were $11,979 for intervention group vs. $14,743 for control = $2,764 total savings per patient |
| C. Chen 2017 | 179 patients, 1 site | Cluster-randomized controlled trial. Modified HELP program (3 key interventions) was implemented for abdominal surgery patients in Taiwan. | Cost savings calculated by applying cost savings from prior studies. For example, mHELP could have prevented delirium in approximately 674,576 surgical patients, resulting in a Medicare cost savings of approximately $10,000 per case or $6.7 billion for the year. By cutting 2 days from LOS, mHELP could have saved $1624 per hospital stay or $12.9 billion per year in Medicare costs for hospitalization. |
| Caplan 2007 | 37 patients, 1 site | Two historical controlled studies of HELP implementation. | Cost savings calculated by applying rates from hospitals in Australia. $67,876 per year saved by decreasing length of stay through delirium prevention. $91,678 per year saved on hospital sitter costs for patients with hyperactive delirium. |
| Leslie 2005 | 801 patients, 1 site | From controlled clinical trial, compared intervention and control groups for long-term nursing home costs when HELP was received during prior hospitalization. | HELP had significantly lower total nursing home costs, shorter length of stay and lower cost per survival day. Adjusted total costs were $50,881 per patient in HELP and $60,327 in control group = $9446 savings per patient (15.7%, p = 0.01). |
| Leslie 2008 | 835 patients, 1 site | From controlled clinical trial, determined additional costs in control group for delirium (compared with intervention group) for 1-year health care costs associated with delirium. | Total cost estimates attributable to delirium was $16,303-$64,421 per patient; thus, the national burden of delirium was estimated at $38–152 billion each year (after adjusting for pertinent demographic and clinical characteristics) |
| Rizzo 2001 | 852 patients, 1 site | From controlled clinical trial, compared intervention and control groups in true cost-effectiveness analysis. | Detailed formal cost-effectiveness analysis, accounting for all costs of intervention. Overall hospital cost savings of $1661–3779 per hospitalization. |
| Rubin 2011 | 27,196 patients, 107 volunteers, 1 site | Historical controlled analysis done in a large quality improvement study. | Financial return of the program was estimated at > $7.3 million per year (from delirium prevention, shorter length of stay, and revenue generated from freeing up hospital beds). |
| Rubin 2017 | 7628 patients, 1 site | Historical controlled analysis done in a large quality improvement study. | HELP lowers risk for 30-day hospital readmission (adjusted relative risk 0.83, 95% CI 0.73–0.94). This translates to 100 fewer readmissions due to HELP during the one year study period. A 2% reduction in the Medicare readmission rate would mean 40,000 fewer readmissions nationally per year, or cost savings of approximately $491 million per year. |
| Zaubler 2013 | 595 patients, 1 site | Historical controlled analysis done in a quality improvement project. HELP adapted to a community hospital results in cost savings when variable costs were compared between patients and potential increased revenue calculated based on shorter lengths of stay for intervention patients. | Interventions resulted in $841,000 cost savings over 9 months for the hospital. |
Note: All cost figures in 2018 US Dollars
In a follow-up study to the original HELP study led by Inouye et al. and involving 841 patients, Leslie et al. demonstrated savings in long-term nursing home costs of approximately $16,125 (2018 US dollars) per person-year in the year following discharge with implementation of HELP.9,15 In a study involving 37 patients, Caplan et al. found that $67,876 (2018 US dollars) per year could be saved by preventing delirium (and thus decreasing length of stay) using HELP interventions, plus $91,678 (2018 US dollars) per year on hospital sitter (companion) costs for patients with hyperactive delirium for a total annual saving of $159,554 (2018 US dollars).22 In a study involving 28 patients from a Dutch hospital, Bakker at al. found that mean cumulative costs per patient 3 months after discharge were $11,979 (2018 US dollars) for the intervention group, compared with $14,743 for controls.30
Our prior meta-analysis estimated that over $18 billion (2018 US dollars) could be saved in a year if just half of the US hospitals adopted multicomponent non-pharmacologic interventions like HELP to prevent delirium.16 On surgical services, previous studies have estimated that HELP could prevent more than half a million cases of delirium (674,576 cases) each year, resulting in a Medicare cost savings of approximately $12,000 per case15 or $7 billion per year (2018 US dollars).1,29
Adherence to interventions and adaptations
The effect of adhering to HELP interventions was examined in 13 studies,11–13,29–32,35,36,38,39,43,49 and adaptations to enhance implementation and adherence were presented in 12 studies (Table 2).12,13,29,31,36,39,40,41,42,44,50,51 Complete adherence is defined as a patient receiving all parts of the assigned protocol for the number of times it was intended to be given. The HELP program recommends target goals for each hospital to provide seven days per week coverage and to meet a minimum of 80% adherence with HELP interventions.10–12 In the original HELP study involving 422 patients, Inouye et al. found that adherence had a significant independent protective effect against delirium (adjusted OR=0.69, 95% CI 0.56–0.87).38 Higher adherence to HELP interventions was associated with lower delirium rates in an exposure-response fashion, the first such demonstration for a non-pharmacological intervention. In fact, for the highest adherence group, the delirium rate was 2.9% (89% risk reduction). Inouye et al. examined the major reasons for non-adherence in 2 separate studies (Inouye 2000: 1 site, 1507 patients; and Inouye 2006: 13 sites, 11,344 patients), which included: lack of availability of staff or volunteers (32%), patient refusal (26%), medical contraindication (22%), and patient unavailability (13%).11,12
Monitoring and enhancing adherence is key to successful implementation, and finding approaches to address obstacles to adherence may vary across patient populations and settings.29,37 Chen et al. examined the feasibility of adhering to HELP interventions in the abdominal surgery patient population in Taiwan. Adherence with individual interventions was rated on a Likert-type scale from 0 (no adherence) to 3 (full implementation and adherence).29 Good adherence was achieved to 3 selected HELP interventions, with 166 out of 197 participants (84.3%) receiving Likert mean scores of 2 (of 3) or higher.29 Chong et al. developed a hospital ward specialized for delirium management in Singapore36 and was able to achieve good adherence to the HELP intervention protocols for 150 patients. The study found 100% adherence with all interventions adhered to via semi-structured protocols involving trained geriatric nurses in a single specialized unit. As described above, Bakker et al. implemented HELP in the Netherlands, with good adherence – defined as adoption of 4 or more of the 6 HELP interventions; Bakker et al. noted that training and integrating volunteers into daily work flow was the most challenging component.30 One study examined adherence to HELP in long-term care.31 Boockvar et al. examined an adaptation of HELP (HELP-LTC), and demonstrated that the protocols could be delivered successfully with 75% adherence to 143 residents during and after an acute illness episode by nursing assistants.31
Strategies to improve adherence were examined in 2 recent studies.39 In one study, HELP staff trained two Rehabilitation Aides to implement the six core protocols, with particular focus on the mobility protocol. Full implementation of the mobility protocol increased from 9% to 57% with this strategy.39 A second study by Sandhaus et al. educated 22 nurses and 100 volunteers on dysphagia in their older HELP patients, and were able to improve the effectiveness and safety of their feeding protocol.44
Six studies examined adaptations to enhance implementation of the HELP model. In one study, 15 family members for 5 patients were recruited to address 4 risk factors for delirium (cognitive impairment, impairment of activities of daily living, vision impairment and hearing impairment) by having family members orient patients, implement cognitively stimulating activities, and bring in glasses/visual aides and hearing aides. The program was found to be both feasible and empowering for family members.40 Zalon et al. examined use of medical technology in HELP, specifically personal digital assistants (PDAs) to gather data on implementation and adherence with HELP protocols in 352 patients.50 The PDAs helped them to monitor delirium status and track HELP protocol implementation, as well as collect data on program outcomes. In another study (n=34), Zalon et al. examined delirium recognition and documentation in the electronic medical records and referral patterns to HELP. This study documented low rates of recognition of delirium and of referrals to HELP (5.9%). Physicians’ typical responses to delirium included prescribing medications (usually antipsychotics and benzodiazepines) and ordering tests for work-up of delirium. This study suggested a need to improve delirium recognition and management.49 Others have harnessed the resources of quality improvement research to translate HELP into practice.41,42,51 As described above, in 2006, Rubin et al. adapted and streamlined HELP protocols and procedures to the community hospital setting. Specifically, some intervention protocols were simplified or omitted (e.g., sleep protocol) due to staff/volunteer availability. For quality improvement, an educational campaign was incorporated and nursing and patient satisfaction were regularly assessed. Rubin et al. successfully enrolled 4763 patients, demonstrated substantial cost-savings, and found high rates of nursing and patient satisfaction with the modified HELP program.41 By 2011, Rubin et al. had sustained HELP for 5 years and successfully scaled up to 27,196 patients (6 medical wards), demonstrating ongoing program fidelity and cost-savings.42 Zaubler et al. also successfully implemented HELP in their community hospital setting.51 They initiated the HELP program as a quality improvement project and were able to secure two 3-year grants to support two full-time Elder Life Specialists, purchase HELP training materials and recruit and train volunteers. Zaubler et al. incorporated all 6 core HELP protocols into daily visits, therapeutic activities and assistance (with feeding, hydration, sleep and vision/hearing impairment). Among 595 patients, they demonstrated significant decreases in delirium episodes, total patient-days with delirium, length of stay, and hospital costs.51 P. Chen et al. surveyed 73 HELP sites, who reported many adaptations and uses of the HELP materials and website, described further below.13
Studies of volunteer role
The role of volunteers in HELP was examined in 6 studies.11,12,30,43,45,48 HELP volunteers play a unique role, delivering core HELP interventions at the bedside for patients at risk of delirium.11 Two studies by Inouye et al. previously described, also examined the role of volunteers at the original HELP site and 13 dissemination sites. The two studies demonstrated that most hospital-based sites in the United States used trained volunteers,11,12 who were generally recruited from local colleges, hospital volunteer services, general community organizations, local churches or religious organizations, hospital employees, and other sources. Across the 13 HELP dissemination sites, the use of volunteers in implementation of multicomponent non-pharmacologic interventions was demonstrated to be feasible, safe and cost-saving.12
Steunenberg et al. in the Netherlands examined the added value of trained HELP volunteers.48 Using mixed-methods approaches to 94 participants, the study found that patients appreciated the extra attention and services provided by volunteers, with beneficial effects for loneliness. Volunteers reported enjoyment in their role, and many brought prior experience with delirium in family members.48 Steunenberg et al. characterized their typical volunteers as female, highly educated, possessing some medical knowledge and having prior volunteer experience in health care settings.48 Sandhaus et al. conducted qualitative interviews of 110 nurses at one HELP site and found that volunteers improved nursing, patient, and family satisfaction with care – and also improved the cost of care.43 Bakker et al., described above, interviewed hospital staff, patients, and family members, who consistently reported that volunteers added value to health care.30 In their study of 102 volunteers at a university hospital (serving 795 patients per year), Schoettinger et al. report that the sense of value and importance of the work imparted by the program attracts new volunteers, and the number of volunteers at the site has increased 250% in the past three years.45 Volunteer retention was aided by positive feedback from patients, family members, and the volunteers themselves.45
Successes and barriers to implementation and sustainability of HELP
Successes and barriers to implementation and sustainability of HELP have been evaluated in-depth in 6 qualitative and mixed-methods studies,13,32–34,47,46 augmented with 10 studies focusing on sustainability.12,13,33–35,37,42,46,47,50 Bradley et al. examined initial implementation of HELP at 9 sites, and conducted qualitative interviews with 32 staff members.32 Major challenges to initiating a new site were gaining internal support, ensuring effective clinician leadership, integrating with existing programs, maintaining program fidelity, documenting positive outcomes, and shifting organizational culture.32 To study patterns of diffusion, Bradley et al. studied 63 sites via an on-line survey and demonstrated the critical role of senior management support for successful implementation of a program.33 Key elements of success included gaining senior management support, demonstrating concordance with the hospital’s mission, and obtaining commitment of nursing and physician leaders.33,34 Studying sustainability of the HELP program, Bradley et al. conducted in-depth interviews with 42 staff members at 13 sites and found that the important elements for sustaining a program included ensuring effective clinician leadership, adapting the program to local circumstances, and obtaining long-term funding and resources.35 Steelfisher et al. conducted qualitative interviews (n=62) at 19 HELP sites which had been implemented for at least 2 years.47 Strategies for sustaining HELP identified by Steelfisher et al. included interacting meaningfully with decision-makers, documenting success with metrics that resonate with decision-makers, and garnering support from influential hospital staff.46,47 Inouye et al. 2006, as previously described, examined successful implementation and sustainability of the HELP model at 13 dissemination sites, involving 11,344 patients.12 All sites were able to implement the program with a high degree of fidelity and built-in quality assurance procedures. Moreover, sites identified at least 11 important benefits of their programs, including providing education and training for staff in geriatrics, increasing nursing retention, improving patient and family satisfaction, enhancing clinical outcomes, improving quality of care, increasing visibility for geriatrics, providing cost-effective care, improving public relations and community outreach, distinguishing volunteer services, contributing to commendations for the hospital, and providing research opportunities.12
In site-specific studies, Zalon et al. and Heim et al. examined data collection and documentation and their importance for sustainability for their HELP programs.37,50 Zalon et al., described above, examined the use of Personal Digital Assistants in a sample of 34 patients, to gather data on cognitive functioning and delirium, important outcomes for their HELP program.50 In their stepped wedge study of 333 patients, Heim et al. found that HELP programs needed to be flexible and realistic with time constraints and team capacity for HELP to be sustainable. This study also underscored the importance of data collection to demonstrate the efficacy and sustainability of the HELP model.37
P. Chen et al., mentioned previously, examined the HELP website and its role in implementation, adaptation and sustainability of the HELP program.13 In an on-line survey and follow-up interviews of 73 HELP sites, the study found that the HELP website was an efficient tool for the dissemination of the program, and importantly, provided detailed information about delirium prevention that was useful for training and education purposes. The survey demonstrated that the HELP website resources were used to plan for implementation of the HELP model and to support the program after launch.13 In order to sustain HELP-like interventions, the website resources were also used to develop delirium prevention programs and guidelines, and to educate healthcare professionals, patients, families, and volunteers.13
Discussion
This systematic meta-analysis and review provides the most comprehensive overview to date of the effectiveness, cost-savings, and other benefits of the Hospital Elder Life Program (HELP) based on published studies of the original model program as well as many independent follow-up studies from sites around the world. The quantitative meta-analysis of 14 HELP studies demonstrates the significant reduction in delirium (OR 0.47; 95% CI, 0.37–0.59, I2 = 28%) and falls (OR 0.58, 95% CI 0.35–0.95, I2 =0%), and non-significant trends towards reduced length of stay and institutionalization. These findings support the efficacy of HELP and HELP-related models, and are in keeping with a previous meta-analysis examining multicomponent, nonpharmacologic interventions for delirium prevention.16
HELP also provides cost-savings, with multiple studies demonstrating substantially reduced costs for both acute and long-term care services. The savings amount to an average $2700 per hospitalization (range $1661-$3779 in 2018 US dollars) and about $16,000 (2018 US dollars) per person-year in long-term care costs for the year following delirium. Based on 9 studies examining the cost savings of HELP and HELP-based programs, savings have been estimated at over $18 billion (2018 US dollars) per year if HELP could be implemented in just half of U.S. hospitals. In surgical patients alone, about 1 million cases of delirium in the hospital could be prevented by HELP each year, contributing to cost savings of nearly $12,000 per case prevented or $7 billion per year (2018 US dollars).1,9,16 Thirteen studies examined adherence to HELP interventions and 12 studies examined successful adaptations of HELP to enhance implementation. These adaptations have enabled HELP to be disseminated worldwide across many different clinical populations. Six studies examined the important role of volunteers, a unique component of HELP that has consistently been found to bring enrichment to the overall program, for medical staff, patients, families and volunteers. Six studies examined successes and barriers to implementation, and 10 studies identified factors to maximize sustainability of the HELP model. In total, these 30 studies represent a robust body of research supporting the effectiveness, cost savings, and value of the HELP program.
Since its creation in 1993, the HELP program has fulfilled the triple aim of improving healthcare quality, enhancing patient satisfaction, and reducing healthcare costs. The present study provides the first systematic review of the breadth of studies on the HELP model to date, and provides a rigorous examination of its impact. The strengths of this paper are in its meta-analysis of important clinical outcomes of HELP and HELP-based programs, including delirium incidence, falls, length of stay, and institutionalization. Another strength of this paper is in the comprehensive examination of additional quantitative and qualitative studies of the HELP model of care and its relevant adaptations. In particular, this paper focused on cost savings, adherence, adaptations, volunteer role, and successes and barriers to implementation and sustainability. Despite its strengths, important limitations are worthy of note. For the quantitative meta-analyses, there were a limited number of studies for meta-analysis of falls (n=3) and institutionalization (n=4), as well as functional or cognitive change, along with high heterogeneity, that precluded definitive conclusions about these outcomes. Moreover, some of the additional methodological and adaptation studies were single-site studies or quality improvement initiatives, some of which had limited sample size or lacked comparison groups, which may limit their internal and external validity. Despite these limitations, the accumulation of evidence supports the far-reaching contributions of the HELP model to improving the quality and outcomes of healthcare in vulnerable older populations.
The HELP Program <www.hospitalelderlifeprogram.org> provides information and guidance by the central HELP program and HELP Centers of Excellence for implementing and sustaining successful programs through on-line materials, on-line community networking, national conferences and interest groups, newsletters, and webinars. Practical advice, such as engaging volunteers, gaining administrative support, and finding clinical champions, is also provided through these resources. The studies examining sustainability and adaptability of the HELP program underscore the need to engage both clinician leaders and hospital administration from the start for successful implementation of HELP. While the demonstrated benefits of HELP for delirium and falls in the meta-analysis were highly significant, the findings for other outcomes (such as length of stay and institutionalization) were limited by a relatively smaller number of studies with limited sample sizes. Importantly, cost savings have been established across multiple studies. Future work is needed to examine the HELP model in larger, high-quality, multi-center studies, to compare the model with other nonpharmacologic delirium interventions, and to examine a range of outcomes including delirium, length of stay, falls, mortality, institutionalization, functional and cognitive decline, and development of dementia. Examining whether prevention of delirium can lead to prevention of dementia is a critically important area for future investigation.
HELP has been implemented in hundreds of hospitals around the world, and has been demonstrated to be sustainable even in difficult economic environments, improving geriatric care on a wide scale. It is adaptable and flexible, with many innovations (e.g., volunteer component, web-based training materials, on-line community) and website for dissemination that help to create an efficient and cost-saving program. Thus, the HELP model provides an effective and well-tested means to improve the quality and outcomes of hospital care for older persons. In a real way, the HELP model prepares our health care system to care for our rapidly aging society.
Acknowledgments
The authors extend thanks to the many HELP sites, staff, volunteers, Advisory Board, and Centers of Excellence around the world who have made this work possible. Your dedication is unparalleled and inspiring. This work is dedicated to the memory of Joshua Bryan Inouye Helfand and Mitsuo Inouye.
Funding sources: This work is supported in part by Grants No. R24AG054259 (Dr. Inouye), and K07AG041835 (Dr. Inouye) from the U.S. National Institute on Aging; and by the Milton and Shirley F. Levy Family Chair at Hebrew SeniorLife/Harvard Medical School (Dr. Inouye). No Disclosures to Report; no conflicts of interest.
Appendix 1. Ovid Search Strategy
| #1 “Hospital Elder Life Program”. ti. Ab |
| #2 “Multicomponent intervention”. ti. ab |
| #3 “Nonpharmacological intervention”. ti. ab |
| #4 or/1–3 |
| #5 delirium |
| #6 deliri*. ti, ab. |
| #7 “acute confusion”. ti, ab. |
| #8 “acute organic psychosyndrome”. ti, ab. |
| #9 “acute brain failure”. ti, ab. |
| #10 “organic mental disorders”. ti, ab. |
| #11 “acute brain syndrome” . ti, ab. |
| #12 “metabolic encephalopathy” . ti, ab. |
| #13 “ICU psychosis” . ti, ab. |
| #14 “acute psycho-organic syndrome” . ti, ab. |
| #15 “clouded state” . ti, ab. |
| #16 “clouding of consciousness” . ti, ab. |
| #17 “exogenous psychosis” . ti, ab. |
| #18 “toxic psychosis” . ti, ab. |
| #19 “toxic confusion” . ti, ab. |
| #20 or/5–19 |
| #21 Alcohol-Withdrawal-Delirium/ |
| #22 delirium tremens/ |
| #23 21 or 22 |
| #24 20 not 23 |
| #25 exp aged/ |
| #26 (elder$ or older$ or geriatrics).ti, ab. |
| #27 25 or 26 |
| #28 primary prevention/ |
| #29 prevent*.mp |
| #30 reduc*. ti, ab |
| #31 stop*. ti, ab |
| #32 taper*. ti, ab |
| #33 avoid*. ti, ab |
| #34 “cut* down”. ti, ab |
| #35 or/28–34 |
| #36 24 AND 27 AND 35 |
| #37 drag therapy. fs. |
| #38 36 not 37 |
| #39 4 and 38 |
Footnotes
Financial Disclosures: No Disclosures to Report; no conflicts of interest.
References
- 1.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet 2014;383(9920):911–922. doi: 10.1016/S0140-6736(13)60688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McAvay GJ, Van Ness PH, Bogardus ST, et al. Older Adults Discharged from the Hospital with Delirium: 1-Year Outcomes. J Am Geriatr Soc 2006;54(8):1245–1250. doi: 10.1111/j.1532-5415.2006.00815.x. [DOI] [PubMed] [Google Scholar]
- 3.Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing 2006;35(4):350–364. doi: 10.1093/ageing/afl005. [DOI] [PubMed] [Google Scholar]
- 4.Tabet N, Howard R. Non-pharmacological interventions in the prevention of delirium. Age Ageing 2009;38(4):374–379. doi: 10.1093/ageing/afp039. [DOI] [PubMed] [Google Scholar]
- 5.Witlox J, Eurelings LSM, de Jonghe JFM, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in Elderly Patients and the Risk of Postdischarge Mortality, Institutionalization, and Dementia. JAMA 2010;304(4):443. doi: 10.1001/jama.2010.1013. [DOI] [PubMed] [Google Scholar]
- 6.Fukata S, Kawabata Y, Fujisiro K, et al. Haloperidol prophylaxis does not prevent postoperative delirium in elderly patients: a randomized, open-label prospective trial. Surg Today 2014;44(12):2305–2313. doi: 10.1007/s00595-014-0859-7. [DOI] [PubMed] [Google Scholar]
- 7.Oh ES, Fong TG, Hshieh TT, Inouye SK. Delirium in Older Persons. JAMA 2017;318(12):1161. doi: 10.1001/jama.2017.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gross AL, Jones RN, Habtemariam DA, et al. Delirium and Long-term Cognitive Trajectory Among Persons With Dementia. Arch Intern Med 2012;172(17):1324. doi: 10.1001/archinternmed.2012.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leslie DL. One-Year Health Care Costs Associated With Delirium in the Elderly Population. Arch Intern Med 2008;168(1):27. doi: 10.1001/archinternmed.2007.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inouye SK, Bogardus ST, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med 1999;340(9):669–676. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 11.Inouye SK, Bogardus ST, Baker DI, Leo-Summers L, Cooney LM. The Hospital Elder Life Program: a model of care to prevent cognitive and functional decline in older hospitalized patients. Hospital Elder Life Program. J Am Geriatr Soc 2000;48(12):1697–1706. http://www.ncbi.nlm.nih.gov/pubmed/11129764. [DOI] [PubMed] [Google Scholar]
- 12.Inouye SK, Baker DI, Fugal P, Bradley EH. Dissemination of the Hospital Elder Life Program: Implementation, Adaptation, and Successes. J Am Geriatr Soc 2006;54(10):1492–1499. doi: 10.1111/j.1532-5415.2006.00869.x. [DOI] [PubMed] [Google Scholar]
- 13.Chen P, Dowal S, Schmitt E, et al. Hospital Elder Life Program in the Real World: The Many Uses of the Hospital Elder Life Program Website. J Am Geriatr Soc 2015;63(4):797–803. doi: 10.1111/jgs.13343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rizzo JA, Bogardus ST, Leo-Summers L, Williams CS, Acampora D, Inouye SK. Multicomponent targeted intervention to prevent delirium in hospitalized older patients: what is the economic value? Med Care 2001;39(7):740–752. http://www.ncbi.nlm.nih.gov/pubmed/11458138. [DOI] [PubMed] [Google Scholar]
- 15.Leslie DL, Zhang Y, Bogardus ST, Holford TR, Leo-Summers LS, Inouye SK. Consequences of preventing delirium in hospitalized older adults on nursing home costs. J Am Geriatr Soc 2005;53(3):405–409. doi: 10.1111/j.1532-5415.2005.53156.x. [DOI] [PubMed] [Google Scholar]
- 16.Hshieh TT, Yue J, Oh E, et al. Effectiveness of Multicomponent Nonpharmacological Delirium Interventions. JAMA Intern Med 2015;175(4):512. doi: 10.1001/jamainternmed.2014.7779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andro M, Comps E, Estivin S, Gentric A. Prevention of delirium in demented hospitalized patients. Eur J Intern Med 2012;23(2):124–125. doi: 10.1016/j.ejim.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 18.Chen CC-H, Lin M-T, Tien Y-W, Yen C-J, Huang G-H, Inouye SK. Modified Hospital Elder Life Program: Effects on Abdominal Surgery Patients. J Am Coll Surg 2011;213(2):245–252. doi: 10.1016/j.jamcollsurg.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Bogardus ST, Desai MM, Williams CS, Leo-Summers L, Acampora D, Inouye SK. The effects of a targeted multicomponent delirium intervention on postdischarge outcomes for hospitalized older adults. Am J Med 2003;114(5):383–390. http://www.ncbi.nlm.nih.gov/pubmed/12714128. [DOI] [PubMed] [Google Scholar]
- 20.Bo M, Martini B, Ruatta C, et al. Geriatric ward hospitalization reduced incidence delirium among older medical inpatients. Am J Geriatr Psychiatry 2009;17(9):760–768. http://www.ncbi.nlm.nih.gov/pubmed/19705520. [DOI] [PubMed] [Google Scholar]
- 21.Babine RL, Farrington S, Wierman HR. HELP© prevent falls by preventing delirium. Nursing (Lond) 2013;43(5):18–21. doi: 10.1097/01.NURSE.0000428710.81378.aa. [DOI] [PubMed] [Google Scholar]
- 22.Caplan GA, Harper EL. Recruitment of volunteers to improve vitality in the elderly: the REVIVE* study. Intern Med J 2007;37(2):95–100. doi: 10.1111/j.1445-5994.2007.01265.x. [DOI] [PubMed] [Google Scholar]
- 23.Holt R, Young J, Heseltine D. Effectiveness of a multi-component intervention to reduce delirium incidence in elderly care wards. Age Ageing 2013;42(6):721–727. doi: 10.1093/ageing/aft120. [DOI] [PubMed] [Google Scholar]
- 24.Huson K, Stolee P, Pearce N, Bradfield C, Heckman GA. Examining the Hospital Elder Life Program in a rehabilitation setting: a pilot feasibility study. BMC Geriatr 2016;16(1):140. doi: 10.1186/s12877-016-0313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kratz A Use of the Acute Confusion Protocol. J Nurs Care Qual 2008;23(4):331–337. doi: 10.1097/01.NCQ.0000336673.02725.ec. [DOI] [PubMed] [Google Scholar]
- 26.Stenvall M, Olofsson B, Lundström M, et al. A multidisciplinary, multifactorial intervention program reduces postoperative falls and injuries after femoral neck fracture. Osteoporos Int 2007;18(2):167–175. doi: 10.1007/s00198-006-0226-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubin FH, Bellon J, Bilderback A, Urda K, Inouye SK. Effect of the Hospital Elder Life Program on Risk of 30-Day Readmission. J Am Geriatr Soc October 2017. doi: 10.1111/jgs.15132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.VidÃn MT, SÃnchez E, Alonso M, Montero B, Ortiz J, Serra JA. An Intervention Integrated into Daily Clinical Practice Reduces the Incidence of Delirium During Hospitalization in Elderly Patients. J Am Geriatr Soc 2009;57(11):2029–2036. doi: 10.1111/j.1532-5415.2009.02485.x. [DOI] [PubMed] [Google Scholar]
- 29.Chen CC-H, Li H-C, Liang J-T, et al. Effect of a Modified Hospital Elder Life Program on Delirium and Length of Hospital Stay in Patients Undergoing Abdominal Surgery. JAMA Surg 2017;152(9):827–834. doi: 10.1001/jamasurg.2017.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bakker FC, Persoon A, Schoon Y, Olde Rikkert MGM. Hospital Elder Life Program Integrated in Dutch Hospital Care: A Pilot. J Am Geriatr Soc 2013;61(4):641–642. doi: 10.1111/jgs.12173. [DOI] [PubMed] [Google Scholar]
- 31.Boockvar KS, Teresi JA, Inouye SK. Preliminary Data: An Adapted Hospital Elder Life Program to Prevent Delirium and Reduce Complications of Acute Illness in Long-Term Care Delivered by Certified Nursing Assistants. J Am Geriatr Soc 2016;64(5):1108–1113. doi: 10.1111/jgs.14091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bradley EH, Schlesinger M, Webster TR, Baker D, Inouye SK. Translating research into clinical practice: making change happen. J Am Geriatr Soc 2004;52(11):1875–1882. doi: 10.1111/j.1532-5415.2004.52510.x. [DOI] [PubMed] [Google Scholar]
- 33.Bradley EH, Webster TR, Schlesinger M, Baker D, Inouye SK. The roles of senior management in improving hospital experiences for frail older adults. J Healthc Manag 2006;51(5):323-36-7 http://www.ncbi.nlm.nih.gov/pubmed/17039691. [PubMed] [Google Scholar]
- 34.Bradley EH, Webster TR, Schlesinger M, Baker D, Inouye SK. Patterns of diffusion of evidence-based clinical programmes: a case study of the Hospital Elder Life Program. Qual Saf Health Care 2006;15(5):334–338. doi: 10.1136/qshc.2006.018820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bradley EH, Webster TR, Baker D, Schlesinger M, Inouye SK. After Adoption: Sustaining the Innovation A Case Study of Disseminating the Hospital Elder Life Program. J Am Geriatr Soc 2005;53(9):1455–1461. doi: 10.1111/j.1532-5415.2005.53451.x. [DOI] [PubMed] [Google Scholar]
- 36.Chong MS, Chan MP, Kang J, Han HC, Ding YY, Tan TL. A New Model of Delirium Care in the Acute Geriatric Setting: Geriatric Monitoring Unit. BMC Geriatr 2011;11(1):41. doi: 10.1186/1471-2318-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heim N, van Stel HF, Ettema RG, van der Mast RC, Inouye SK, Schuurmans MJ. HELP! Problems in executing a pragmatic, randomized, stepped wedge trial on the Hospital Elder Life Program to prevent delirium in older patients. Trials 2017;18(1):220. doi: 10.1186/s13063-017-1933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inouye SK, Bogardus ST, Williams CS, Leo-Summers L, Agostini J V. The Role of Adherence on the Effectiveness of Nonpharmacologic Interventions. Arch Intern Med 2003;163(8):958. doi: 10.1001/archinte.163.8.958. [DOI] [PubMed] [Google Scholar]
- 39.Macias JA, Malone ML, Tooke D, Wardynski M, Legros C. Collaboration between hospital elder life program (HELP) and rehab aides to improve early mobilization of older patients. J Am Geriatr Soc 2017;65:S175. [Google Scholar]
- 40.Rosenbloom-Brunton DA, Henneman EA, Inouye SK. Feasibility of family participation in a delirium prevention program for hospitalized older adults. J Gerontol Nurs 2010;36(9):22–33-5. doi: 10.3928/00989134-20100330-02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rubin FH, Williams JT, Lescisin DA, Mook WJ, Hassan S, Inouye SK. Replicating the Hospital Elder Life Program in a community hospital and demonstrating effectiveness using quality improvement methodology. J Am Geriatr Soc 2006;54(6):969–974. doi: 10.1111/j.1532-5415.2006.00744.x. [DOI] [PubMed] [Google Scholar]
- 42.Rubin FH, Neal K, Fenlon K, Hassan S, Inouye SK. Sustainability and Scalability of the Hospital Elder Life Program at a Community Hospital. J Am Geriatr Soc 2011;59(2):359–365. doi: 10.1111/j.1532-5415.2010.03243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sandhaus S, Zalon ML, Valenti D, Dzielak E, Smego RA, Arzamasova U. A Volunteer-Based Hospital Elder Life Program to Reduce Delirium. Health Care Manag (Frederick) 2010;29(2):150–156. doi: 10.1097/HCM.0b013e3181daa2a0. [DOI] [PubMed] [Google Scholar]
- 44.Sandhaus S, Zalon ML, Valenti D, Harrell F. Promoting Evidence-Based Dysphagia Assessment and Management by Nurses. J Gerontol Nurs 2009;35(6):20–27. doi: 10.3928/00989134-20090331-08. [DOI] [PubMed] [Google Scholar]
- 45.Schoettinger AR, Hickey K. Listening to our HELP volunteers: Why they stay. J Am Geriatr Soc 2017;65:S212. [Google Scholar]
- 46.SteelFisher GK, Martin LA, Dowal SL, Inouye SK. Learning from the Closure of Clinical Programs: A Case Series from the Hospital Elder Life Program. J Am Geriatr Soc 2013;61(6):999–1004. doi: 10.1111/jgs.12274. [DOI] [PubMed] [Google Scholar]
- 47.SteelFisher GK, Martin LA, Dowal SL, Inouye SK. Sustaining Clinical Programs During Difficult Economic Times: A Case Series from the Hospital Elder Life Program. J Am Geriatr Soc 2011;59(10):1873–1882. doi: 10.1111/j.1532-5415.2011.03585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steunenberg B, van der Mast R, Strijbos MJ, Inouye SK, Schuurmans MJ. How trained volunteers can improve the quality of hospital care for older patients. A qualitative evaluation within the Hospital Elder Life Program (HELP). Geriatr Nurs (Minneap) 2016;37(6):458–463. doi: 10.1016/j.gerinurse.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 49.Zalon ML, Sandhaus S, Kovaleski M, Roe-Prior P. Hospitalized Older Adults With Established Delirium: Recognition, Documentation, and Reporting. J Gerontol Nurs 2017;43(3):32–40. doi: 10.3928/00989134-20161109-01. [DOI] [PubMed] [Google Scholar]
- 50.Zalon ML, Sandhaus S, Valenti D, Arzamasova U. Using PDAs to detect cognitive change in the hospitalized elderly patient. Appl Nurs Res 2010;23(3):e21–e27. doi: 10.1016/j.apnr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 51.Zaubler TS, Murphy K, Rizzuto L, et al. Quality Improvement and Cost Savings with Multicomponent Delirium Interventions: Replication of the Hospital Elder Life Program in a Community Hospital. Psychosomatics 2013;54(3):219–226. doi: 10.1016/j.psym.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 52.Yue J, Tabloski P, Dowal SL, Puelle MR, Nandan R, Inouye SK. NICE to HELP: Operationalizing National Institute for Health and Clinical Excellence Guidelines to Improve Clinical Practice. J Am Geriatr Soc 2014;62(4):754–761. doi: 10.1111/jgs.12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kratz T, Heinrich M, Schlauß E, Diefenbacher A. Preventing postoperative delirium. Dtsch Arztebl Int 2015;112(17):289–296. doi: 10.3238/arztebl.2015.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Freitas C, Hussain SY, Fleischman R, et al. Delirium quality improvement (QI) project in a coronary care unit (CCU). J Am Geriatr Soc 2016;64:S271. [Google Scholar]