Abstract
Spectinamides are a novel series of spectinomycin analogs being developed for the treatment of tuberculosis. Intrapulmonary aerosol (IPA) administration of lead spectinamide 1599 has previously been shown to be more efficacious than subcutaneous (SC) administration at comparable doses. The objective of the current study was to characterize the disposition of 1599 in plasma and lungs in mice in order to provide a potential rationale for the observed efficacy differences. 200 mg/kg of 1599 was administered to healthy BALB/c mice by SC injection or by IPA delivery. Plasma and major organs were collected at specified time points until 8 hours after dosing. Drug concentrations were measured by LC-MS/MS and analyzed by noncompartmental pharmacokinetic analysis. 1599 demonstrated rapid absorption into plasma after IPA and SC administration, resulting in very similar plasma exposure for both routes. In contrast, drug exposure in the lungs was 48 times higher following IPA as compared to SC administration, which is highly desirable as the lungs are the main site of infection in pulmonary TB. The higher local exposure in the lungs is likely the basis for the increased efficacy after IPA compared to SC administration. Overall, this study supports the pulmonary route as a potential pathway for the treatment of tuberculosis with 1599.
Keywords: Tuberculosis, Inhalation, Pharmacokinetics, Tissue Exposure, Antibiotics
1. Introduction
Spectinamides are semisynthetic spectinomycin derivatives with excellent narrow-spectrum anti-tubercular activity. They exhibit selective inhibition of the bacterial ribosome, are not cross-resistant with the existing tuberculosis therapeutics, and have shown good activity against multi-drug resistant (MDR) and extensively drug resistant (XDR) strains of Mycobacterium tuberculosis (Mtb) [1]. Physicochemically, spectinamides are hydrophilic in nature making them highly water soluble, with low plasma protein binding. However, these analogs have poor permeability across the intestinal barrier, resulting in neligible oral bioavailability. Consequently, a parenteral route such as subcutaneous (SC) injection was selected for dosing in initial preclinical efficacy studies [2]. SC administration in a mouse model of Mtb infection at a dose of 200 mg/kg once daily for 5 days a week for a period of four weeks resulted for spectinamide 1599 in a 1.57 log CFU (colony forming unit) reduction in the lungs. This excellent anti-tubercular activity of 1599 was similar to streptomycin at similar doses [1].
Historically, orally and parenterally administered antibiotics have been used to treat patients with lung infections, but the downside is that they do not directly target the lungs [3]. The targeted delivery of antibiotics via the pulmonary route potentially leads to much higher concentrations of antibiotic in the lungs, thereby resulting in less overall dose requirements without compromising the availability of high local concentrations to prevent amplification of less sensitive pathogens, especially when located intracellularly [4]. Inhalational delivery with antibiotics has revolutionized the treatment of chronic P. aeruginosa infections in cystic fibrosis since the approval of inhaled tobramycin and aztreonam.
Based on the experiences described above, the efficacy of 1599 had been evaluated after intrapulmonary aerosol (IPA) administration in the same mouse model of Mtb infection as used in our previous studies [5]. A dose of 200 mg/kg once daily for 3 days a week (Mondays, Wednesdays and Fridays) for 4 weeks demonstrated excellent efficacy with a 2.24 log CFU reduction in the lungs. This anti-tubercular activity was comparable to that of rifampin (administered orally at 10 mg/kg, 5 days per week) [1]. It was hypothesized that the excellent efficacy following IPA delivery of 1599 may be a result of extensive deposition and distribution of the drug within the lungs. In this short communication, we test this hypothesis by performing a comparative pharmacokinetic study of 1599 in plasma and lungs in BALB/c mice following SC and IPA administration.
2. Material and Methods
2.1. Animal Study
Spectinamide 1599 was synthesized as previously described [6] and provided by Dr. Richard Lee (St. Jude Children’s Research Hospital, Memphis TN). Healthy female BALB/c mice (n= 15) were administered a dose of 200 mg/kg of 1599 dihydrochloride in pyrogen-free saline solution by IPA delivery with a PennCentury MicroSprayer (PennCentury Inc., Wyndmoor, PA) as previously described [5]. The tip of the device was inserted up to the first bronchial bifurcation of the trachea of the anesthetized animal, bypassing the nose and throat and making it easier to precisely control the delivered dose. This is in contrast to metered-dose inhalers and dry powder inhalers and/or nebulizers in animal studies, which filter the aerosol through the nasopharynx, are prone to between-subject variability and are not capable of targeting a desired, predetermined area of the lungs. At pre-dose and 0.25, 1, 3, and 8 h after administration, mice (n=3) were sacrificed humanely. Lungs, spleen, liver, and kidneys were immediately harvested and frozen in liquid nitrogen. Blood obtained by cardiac puncture was collected in heparinized collection tubes, and plasma was separated immediately by centrifugation (6,000 g for 10 min at 4°C). All plasma and tissue samples were stored at −80°C until analysis.
For comparison of plasma and tissues pharmacokinetics of 1599, healthy female mice (n=15) were dosed via the SC route at 200 mg/kg of 1599 dihyrochloride in pyrogen-free saline solution. Blood samples were collected and processed as described for IPA administration.
All animal experiments were conducted in accordance with the Animal Welfare Act and the Public Health Service Policy on Humane Care and Use of Laboratory Animals. The study protocols were approved by the Institutional Animal Care and Use Committees of Colorado State University and the University of Tennessee Health Science Center, respectively.
2.2. Bioanalysis
Concentrations of 1599 in plasma and tissues were determined by a validated liquid chromatography tandem mass spectrometry assay. The frozen tissues were thawed at room temperature and weighed. For every 1 g of tissue 4 mL of phosphate buffered saline was added for homogenization using an UltraTurrax homogenizer (IKA, Wilmington, NC). To prevent cross contamination between two samples, the plunger of the homogenizer was washed with methanol and water after each sample. Samples were further prepared by protein precipitation with methanol (spiked with the internal standard 3′-dihydro-3′-deoxy-3′(R)-isopropylacetylamino spectinomycin) followed by centrifugation at 10,000 g for 10 min at 4°C. Chromatographic separation of the supernatant was carried out on a Luna 3 μm hydrophobic interaction liquid chromatography (HILIC) 100 × 4.6 mm column (Phenomenex, Torrance, CA) using a gradient mobile phase of methanol and 5 mM ammonium formate at a flow rate of 0.4 mL/min. Detection was performed with an API 5500 triple-quadruple mass spectrometer (ABSciex, Foster City, CA) with electrospray ionization in multiple reaction monitoring mode using the mass transitions of m/z 487.2/207.1 for 1599 and m/z 418.3/207.1 for the internal standard. Calibration curves were constructed for each test compound and validated with spiked samples of mouse plasma or respective homogenized tissue.
2.3. Pharmacokinetic Analysis
Pharmacokinetic profiles of 1599 were analyzed by standard non-compartmental procedures using Phoenix WinNonlin 7.0 (Certara, Princeton, NJ) in plasma and tissues. Penetration of drug in tissues was estimated from the ratios of the area under the concentration-time curve from time 0 to the last measured data point (AUC0-last) for tissues compared to the AUC0–last in plasma.
3. Results and Discussion
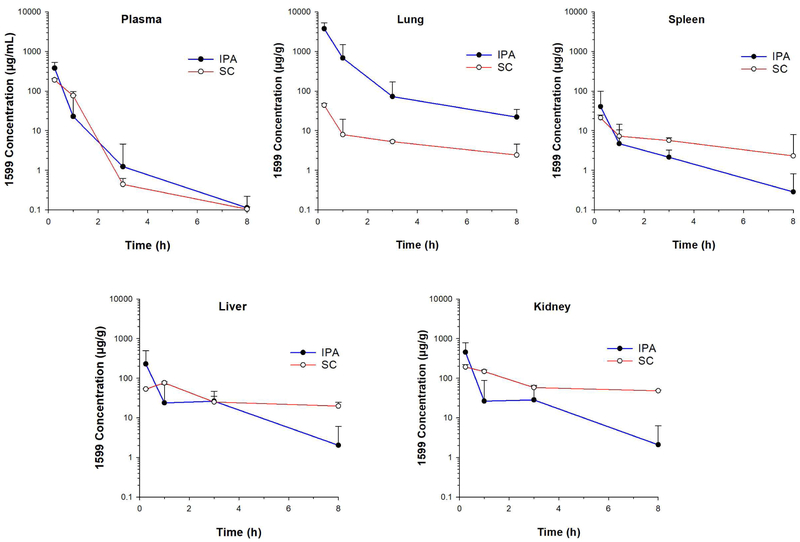
After IPA and SC administration 1599 demonstrated rapid absorption into plasma (Figure 1). The maximum plasma concentration Cmax was two times higher after IPA than SC administration. This is in line with the fact that the lungs are well known to be naturally permeable to most small molecule drugs [7]. Although the mechanism of absorption of molecules after pulmonary deposition is still being researched, it seems that the lungs’ tremendous surface area, very low surface fluid volume, thin diffusion layer and paracellular transport are some of the factors which contribute towards the rapid absorption of small molecules such as 1599 into the systemic circulation. For small molecule drugs which are not dissolution rate-limited, including 1599, the intrinsic absorption rates across the lungs are usually fast with absorption half-lives of ≤1 h, and are independent of lipophilicity or involvement of transporters [8]. The absorption rate also varies within the respiratory tract between the epithelial cells and alveolar cells. In this pilot study for 1599, the disposition pattern of the formulation in the lungs was not evaluated. The limited sampling schedule in this pilot study does not allow for estimation of pharmacokinetic parameters related to the rate of absorption of 1599 from the lungs and will be addressed in more detailed studies in the future. However, we could recently show that 1599 is readily taken up into murine lung derived dendritic cells and macrophage cell lines [9]. Based on the generally more rapid absorption into alveolar compared to epithelial cells and the overall ability of 1599 to be taken up into cells, the rapid pulmonary absorption rate of 1599 suggests that the drug is being delivered to the peripheral region of the lungs [10]. This is desirable as Mtb usually resides in the deeper regions of the lungs.
Figure 1:
Comparison of concentration-time profiles (mean + SD) in different tissues/plasma after intrapulmonary aerosol (IPA) and subcutaneous (SC) administration of a dose of 200 mg/kg of 1599 in saline solution in mice (n=3 at each time point).
Based on the observed plasma exposure (Table 1), the relative systemic bioavailability after IPA versus SC administration was 101%. The comparable exposure after both administration pathways indicates that the drug is permeable across the lung barrier to a similar extent compared to absorption from the SC route. Since 1599 has been shown to have negligible oral bioavailability [1], any fraction of the dose that may have been swallowed did not contribute to this systemic bioavailability. The high systemic plasma exposure of 1599 after IPA administration (Figure 1) also suggests that the majority of the administered dose was actually deposited in the lungs and that there was negligible pulmonary metabolism. This was expected based on the previously described high in vitro metabolic stability of 1599, that is matched by the in vivo observation that approximately 90% of the dose is renally excreted in unchanged from after intravenous administration to rats [1].
Table 1:
Plasma and tissues exposure expressed as area-under-the-concentration-time-curve (AUClast) and maximum concentration (Cmax) after intrapulmonary aerosol (IPA) or subcutaneous (SC) administration of 1599 in mice as a single dose of 200 mg/kg (expressed as mean (%CV)).
| Tissue | AUClast [μg h/mL or μg h/g]* |
Cmax [μg/mL or μg/g]* |
||
|---|---|---|---|---|
| IPA | SC | IPA | SC | |
| 159 (56.2) | 158 (6.1) | 378 (39.3) | 191 (8.4) | |
| Lung | 2576 (38.0) | 52.9 (22.6) | 750 (40.9) | 8.82 (11.6) |
| Spleen | 28.7 (75.2) | 44.1 (28.7) | 8.11 (144) | 4.24 (17.2) |
| Liver | 194 (59.5) | 258 (10.8) | 45.6 (115) | 10.6 (1.9) |
| Kidney | 273 (57.8) | 607 (4.9) | 90.0 (74.5) | 38.3 (15.0) |
Parameters are expressed as /mL for plasma and /g tissue for other organs
As expected, the highest tissue exposure of 1599 after IPA administration was attained in the lungs which was nearly 50-fold higher for AUC and 85-fold higher for Cmax compared to SC administration of the same dose (Table 1 and Figure 1). Thus, the penetration ratio for lung tissue relative to plasma was 16.2 after IPA compared to 0.33 after SC administration. This targeted delivery to the lungs is highly desirable as the lungs are the main site of infection in pulmonary tuberculosis. In addition, the high drug concentration in the lungs also allows easier accessibility of 1599 to alveolar macrophages [11], which are the predominant form of infected cells in pulmonary tuberculosis. Although macrophage uptake of 1599 was found to be significantly higher than spectinomycin, the parent compound of 1599, and streptomycin, a second line anti-tuberculosis agent [9], creating high localized concentrations of the drug that can kill the intracellular Mtb is considered highly desirable in tuberculosis therapy [12].
These results suggest that despite its relatively rapid absorption into the systemic circulations, a substantial fraction of the administered dose is retained in the lungs for a prolonged time period. One potential mechanism for this pulmonary retention could be intracellular lysosomal trapping [13]. Drugs with ionizalbe amino groups with a pKa >6 such as 1599 can accumulate in the acidic environment of these organelles, especially in lysosome-rich organs such as the lungs and the liver [14]. This phenomenon has been observed for numerous clinically used drugs [15]. Further studies will need to address whether 1599 undergoes lysosomal sequestration and the frequently associated binding to membrane phospholipids.
Following IPA administration, 1599 distributes to a similar extent in the kidneys and liver with penetration ratios of 1.71 and 1.22 relative to plasma, respectively (Figure 1), although the maximum exposure in the liver was approximately two thirds of that in the kidneys. This is comparable to the exposure after SC administration, with penetration ratios of 1.63 and 3.84, respectively.
Passive diffusion across the cell membrane is highly unlikely for 1599 as it is very polar (ClogP = −2.52) and based on its pKa of 8.69, it is expected to be largely in ionized form at physiological pH. This strongly suggests an active role of transporters involved in the uptake into the kidneys and liver. The Biopharmaceutics Drug Disposition Classification System (BDDCS) classifies compounds into four types based on the solubility and permeability and is useful for predicting the role of transporters in the disposition of novel entities during the early stages of drug discovery. As 1599 exhibits high solubility and poor permeability, it can be categorized as a BDDCS Class 3 compound. BDDCS predicts that for class 3 drugs uptake transporters are important for their entry into the liver and other organs [16]. Likewise, high tissue levels in the liver were also found for trospectinomycin, another structurally similar aminocyclitol antibiotic [17]. The mechanism behind selective hepatic sequestration was not clear; however it is likely that hepatic uptake transporters are associated with it. In the kidneys, the endocytic receptor complex formed by megalin and cubulin is known to transport aminoglycosides, another group of aminocyclitols, into the renal tubular epithelial cells [18]. Megalin associated uptake transport has also been associated with accumulation of colistin in the kidneys [19].
The lowest penetration ratio was observed in the spleen with both SC and IT administration indicating that 1599 may not be successful in treating Mtb infections that have disseminated into the spleen (Figure 1). This was supported by the relatively lower reduction in bacterial load in the spleen as compared to the lungs observed after IPA and SC administration of 1599 in mouse models of Mtb infection [1]. This limitation, however, can likely be addressed by using 1599 in combination therapy with other anti-TB agents which have better penetration and efficacy in the spleen.
In conclusion, IPA administration of 1599 provides higher exposure in the lungs compared to the SC route. As the lungs are the main site of infection in tuberculosis, this could potentially explain the improvement in efficacy with lower weekly doses following IPA compared to SC administration in mouse models of Mtb infection. In spite of being highly hydrophilic, higher exposures in kidneys and liver relative to plasma suggest a potential role of uptake transporters for 1599 in these organs. Overall, these results are encouraging to further pursue pulmonary delivery as an administration route for spectinamides in general, and 1599 in specific, in the treatment of tuberculosis.
Acknowledgments
Funding
This research was supported by the National Institute of Allergy and Infectious Diseases and the Office of the Director of the National Institutes of Health (grant numbers R01AI090810, R01AI120670, S10OD016226), and ALSAC, St. Jude Children’s Research Hospital. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Lee RE, Hurdle JG, Liu J, Bruhn DF, Matt T, Scherman MS, et al. Spectinamides: A new class of semisynthetic antituberculosis agents that overcome native drug efflux. Nat Med 2014;20(2): 152–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Robertson GT, Scherman MS, Bruhn DF, Liu J, Hastings C, McNeil MR, et al. Spectinamides are effective partner agents for the treatment of tuberculosis in multiple mouse infection models. J Antimicrob Chemother 2017;72(3): 770–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hoiby N Recent advances in the treatment of pseudomonas aeruginosa infections in cystic fibrosis. BMC Med 2011;9: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gonzalez-Juarrero M, O’Sullivan MP. Optimization of inhaled therapies for tuberculosis: The role of macrophages and dendritic cells. Tuberculosis (Edinb) 2011;91(1): 86–92. [DOI] [PubMed] [Google Scholar]
- [5].Gonzalez-Juarrero M, Woolhiser LK, Brooks E, DeGroote MA, Lenaerts AJ. Mouse model for efficacy testing of antituberculosis agents via intrapulmonary delivery. Antimicrob Agents Chemother 2012;56(7): 3957–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Liu J, Bruhn DF, Lee RB, Zheng Z, Janusic T, Scherbakov D, et al. Structure-activity relationships of spectinamide antituberculosis agents: A dissection of ribosomal inhibition and native efflux avoidance contributions. ACS Infect Dis 2017;3(1): 72–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cooper AE, Ferguson D, Grime K. Optimisation of dmpk by the inhaled route: Challenges and approaches. Curr Drug Metab 2012;13(4): 457–73. [DOI] [PubMed] [Google Scholar]
- [8].Hastedt JE, Bäckman P, Clark AR, W. D, Hickey A, Hochhaus G, et al. Scope and relevance of a pulmonary biopharmaceutical classification system AAPS/FDA/USP workshop march 16–17th, 2015 in Baltimore, MD. AAPS Open 2016;2(1). [Google Scholar]
- [9].Santos K, Lukka PB, Grzegorzewicz A, Jackson M, Trivedi A, Pavan F, et al. Primary lung dendritic cell cultures to assess efficacy of spectinamide-1599 against intracellular mycobacterium tuberculosis. Front Microbiol 2018;9: 1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mortensen NP, Hickey AJ. Targeting inhaled therapy beyond the lungs. Respiration 2014;88(5): 353–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dartois V, Barry CE 3rd., A medicinal chemists’ guide to the unique difficulties of lead optimization for tuberculosis. Bioorg Med Chem Lett 2013;23(17): 4741–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Madhura DB, Trivedi A, Liu J, Boyd VA, Jeffries C, Loveless V, et al. Tissue penetration of a novel spectinamide antibiotic for the treatment of tuberculosis. AAPS J 2016;18(3): 788–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hruban Z Pulmonary and generalized lysosomal storage induced by amphiphilic drugs. Environ Health Perspect 1984;55: 53–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Goldman SD, Funk RS, Rajewski RA, Krise JP. Mechanisms of amine accumulation in, and egress from, lysosomes. Bioanalysis 2009;1(8): 1445–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kaufmann AM, Krise JP. Lysosomal sequestration of amine-containing drugs: Analysis and therapeutic implications. J Pharm Sci 2007;96(4): 729–46. [DOI] [PubMed] [Google Scholar]
- [16].Benet LZ, Broccatelli F, Oprea TI. BDDCS applied to over 900 drugs. AAPS J 2011;13(4): 519–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cox JW, Dring LG, Ginsberg LC, Larson PG, Constable DA, Ulrich RG. Distribution and disposition of trospectomycin sulfate in the in vivo rat, perfused rat liver, and cultured rat hepatocytes. Drug Metab Dispos 1990;18(5): 726–31. [PubMed] [Google Scholar]
- [18].Lopez-Novoa JM, Quiros Y, Vicente L, Morales AI, Lopez-Hernandez FJ. New insights into the mechanism of aminoglycoside nephrotoxicity: An integrative point of view. Kidney Int 2011;79(1): 33–45. [DOI] [PubMed] [Google Scholar]
- [19].Suzuki T, Yamaguchi H, Ogura J, Kobayashi M, Yamada T, Iseki K. Megalin contributes to kidney accumulation and nephrotoxicity of colistin. Antimicrob Agents Chemother 2013;57(12): 6319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]