Abstract
Background.
Colonic functions (i.e., absorption of fluids and electrolytes, digestion of selected nutrients, harbor for microbes, and elimination of excreta) necessitate complex patterns of storage and transit. Indeed, colonic transit accounts for a major part of the mouth-to-anus transit time. Colonic transit assessments are useful for understanding the pathophysiology of disease, the pharmacodynamic effects of new medications and to diagnose slow transit constipation. Currently, radiopaque markers, scintigraphy, and a colonic pH-pressure capsule are used to measure overall colonic transit. Radioopaque markers, scintigraphy, and the electromagnetic capsule, which is a newer technique, also evaluate regional colonic transit. The pH-pressure capsule also measures colonic pressures. Magnetic resonance imaging and a radio-frequency identification-based device are evolving methods for assessing colonic transit.
Purpose.
This mini-review, which accompanies a study evaluating the assessment of colon transit with the electromagnetic capsule, evaluates and compares existing and evolving methods for evaluating colonic transit in humans.(1) In addition to overall and regional colonic transit, the electromagnetic capsule evaluates colonic motor patterns without radiation exposure. These patterns are summarized by analyzing the characteristics (i.e., distance and velocity) of discrete antegrade and retrograde capsule movements as they travel in the colon. However, the electromagnetic capsule does not measure pressure or colonic wall movement (i.e., contractions). The motor patterns identified by this capsule should be compared with motor patterns identified with manometry. The next challenge is to harness different techniques to evaluate the relationships between colonic pressures and transit or, even better, the trifecta of colonic contractions, pressure events, and transit.
Abbreviated abstract:
This mini-review, which accompanies a study evaluating the assessment of colon transit with the electromagnetic capsule, evaluates and compares existing and evolving methods for evaluating colonic transit in humans. In addition to overall and regional colonic transit, the electromagnetic capsule evaluates colonic motor patterns without radiation exposure.
Introduction
The colon is a versatile organ that maintains fluid and electrolyte balance, absorbs nutrients, stores, then eliminates excreta, and provides a harbor for billions of microorganisms, whose contributions to health and disease are increasingly recognized.(2–5) These diverse functions necessitate complex patterns of colonic storage and transit. Indeed, the colon accounts for a major part of the mouth-to-anus transit time. Assessments of colonic transit enable a diagnosis of slow transit constipation in patients who do not have a defecatory disorder, followed by colectomy for carefully-selected in patients whose symptoms are refractory to laxatives.(6, 7)1 Colonic transit assessments are also useful to understanding the pathophysiology of disease and the pharmacodynamic effects of new medications.(8, 9)
Three methods - radiopaque markers, scintigraphy, and a colonic pH-pressure capsule – are currently used to measure colonic transit in clinical practice. All these techniques assess overall colonic transit; radioopaque markers, scintigraphy and electromagnetic capsules also evaluate regional colonic transit. The pH-pressure capsule also measures colonic pressures. Accompanying an article on the 3D electromagnetic capsule in this issue of the journal,(1) this mini-review compares the methods for assessing colonic transit. While magnetic resonance imaging can evaluate colonic wall motion, volumes, and transit for short durations, logistical constraints likely preclude the assessment of pan-colonic transit with magnetic resonance imaging.(10)
Radioopaque markers
Radioopaque markers are an accurate, inexpensive, and widely used technique to assess colonic transit in constipated patients. Patients ingest gelatin capsules that contain radioopaque markers. Abdominal X’Rays are used to count the number of ingested radioopaque markers in the abdomen. In the original report, patients swallowed a capsule containing 24 radio-opaque markers on day 1.(11) As summarized in Table 1, the schedule for ingestion of markers and X’Rays has evolved over time.(12)
Table 1.
Techniques to Assess Colonic Transit with Radioopaque Markers
| Option | Number of healthy people/constipated patients | Schedule for ingestion of markers | Timing of X’rays | Comments |
|---|---|---|---|---|
| Option 1 (11) | 25 healthy adults | 20 markers on day one | Days 3 and 5 | All subjects passed the first marker within 66 hours (3 days) and all except one passed 80% of the markers within 114 hours (5 days). |
| Option 2 (38, 39) | 23 pediatric patients and 38 healthy adults (38) 62 adult patients (39) |
20 markers on day one | Days 2–8 | Several X’rays, hence greater radiation exposure. May underestimate transit if all markers are not passed on day 8 |
| Option 3 (14) | 65 healthy adults and 109 adult patients | 20 or 24 markers on day one | Day 5 | If more than 80% markers are passed by day 5, then transit is normal. Less accurate than option 1 because transit is not quantified, rather characterized as normal or slow |
| Option 4 (40, 41) | 10 healthy adults and 10 adult patients (40) 73 adult patients (41) |
20 markers, 3 types, on each of days 1–3 (40) 24 markers, 3 types, on each of days 1–3 (41) |
Days 4, 7, and 10 (40) Days 4 and 7 (41) |
Extensively validated in (41), including comparison of transit times by counting markers in stool and the abdomen. |
| Option 5 (14, 15, 42) | 56 healthy adults and X adult patients (42); 65 healthy adults and 109 adult patients (14) 148 healthy adults and 1309 adult patients (15) |
10 markers on days 1–6 (42) 12 markers on days 1–6 (14, 15) |
Day 7 (14, 15, 42) | Less radiation and can estimate characterize colonic transit time with reasonable accuracy subject to limitations in text. |
As long as 10 or more markers are ingested every day, the estimated colonic transit time (CTT) is reasonably accurate.(13) The “single marker type – single ingestion – single X’Ray” technique provides a qualitative (i.e., normal or slow) rather than a quantitative assessment of colonic transit (Figure 1).(11) Techniques that require several X’Rays (eg, option 2 in table 1), entail considerable radiation exposure, and are not widely used in clinical practice. By comparison, option 5 entails one X’Ray after markers are ingested on 6 days. Assuming that markers are ingested continuously and the movement of markers has reached steady state on the date of the X’Ray,(14) the estimated mean colonic transit time (CTT) of a single marker in a segment i is represented by CTTi, which is ni ΔT/N, where where ni is the number of markers seen on the X-ray in segment i, N is the number of markers ingested each day and ΔT is the time interval between consecutive ingestions of markers. If 12 markers were ingested daily for 6 days, the maximum possible CTT, which would occur if all 72 markers were present in the abdomen, is (24*72/12) = 144 hours.
Figure 1. Assessment of colonic transit with radioopaque markers.
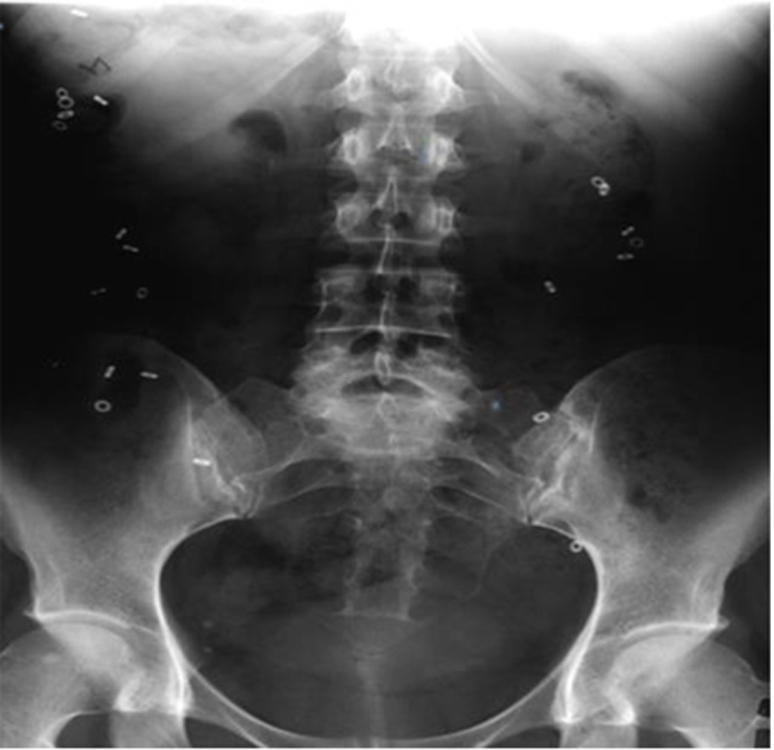
After ingestion of 24 markers, 21 markers were retained in the abdomen on day 5, indicating delayed colonic transit. This corresponds to option 3 in Table 1.
However, this estimated CTT is subject to several kinds of systematic error, namely, 1) in patients with delayed colonic transit the steady-state assumption may not be valid on a X-ray obtained 7 days after the first markers were ingested, 2) in patients with very fast transit, the time interval between two ingestions is too large, and 3) this equation is only strictly applicable for continuous ingestion of markers, whereas markers are actually ingested in a bolus once a day.(15) When the CTT is estimated by a different (i.e., three-compartment) mathematical model instead of the classical approach indicated by the formula above, the normal values of total CTT were 44.3 ± 29.3 instead of 30.1 ± 23.6 h for males and 68.2 ± 54.4 instead of 47.1 ± 28.2 h for females.(15) However, the classical approach is used to estimate transit in clinical practice.
When it is necessary to identify rapid colonic transit, the markers should be ingested at shorter intervals, i.e., for example, every 8 to 12 hours during the day.(16) Regional colonic transit is estimated by dividing the colon is divided into three regions, i.e., right colon (from cecum to right part of transverse colon), left colon (from left part of transverse colon to descending colon) and sigmoid colon/rectum.(12) The spinal column separates the right from the left colon; the pelvic inlet separates the rectosigmoid area from the left colon. In addition to colonic transit, the contractile response to a meal can also be evaluated with radioopaue markers.(17)
Despite difference in the size and density of markers among these techniques, colonic transit measured by radioopaque markers markers, scintigraphy, and the SmartPill capsule are reasonably comparable.(18) However, transit through the ascending and transverse colon is considerably shorter when measured with radioopaque markers than with scintigraphy, probably because particle size influences regional colonic transit.(19) Similar to the electromagnetic capsule, a radio-frequency identification (RFID)-based markers for measuring colonic transit time has been developed.(20) These RFID markers do not require batteries and are ingested in a gelatin capsule that dissolves in the stomach. Antennas outside the body emit a magnetic field that induces power in the marker. The marker emits radio waves that are detected by the antenna and the reader. The number of markers in the different parts of the abdomen can be detected and counted with external antennas. Each marker has an identifier, which allows transit to be tracked over time.
Scintigraphy
The initial studies tracked isotope movement from cecal instillation to defecation.(21) Adapting a technique that was designed to deliver drugs to the proximal colon,(22) ion exchange resin pellets which measured 0.5–1.8 mm in diameter were radiolabeled with indium (111In) and coated with methacrylate, that is designed to dissolve at a pH of 6.8–7.4, resembling the terminal ileum.(23) Because ion exchange resin pellets require an investigational drug permit, they were replaced with activated charcoal.(24)
The overall and regional colonic distribution of radioisotope is studied over 24–48 hours (Figure 2).(8) Overall colonic transit is evaluated by the geometric center-that is, the weighted mean of the proportions of counts in each colonic region at 4, 12, and 24 hours. A low geometric center implies that most radiolabel is close to the cecum (i.e., slower colonic transit) whereas a high geometric center indicates that most radiolabel is close to the stool. The rate of proximal colonic emptying can also be plotted as an activity-time curve.
Figure 2. Assessment of colonic transit with scintigraphy.
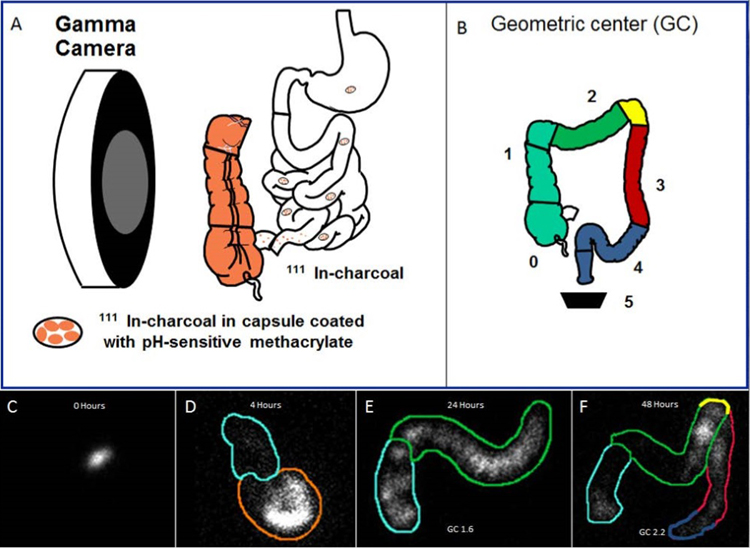
Panel A. 111 In-labeled charcoal pellets are filled in a capsule that is coated with pH-sensitive methacrylate, which dissolves in the terminal ileum, releasing the 111In particles, which are tracked by gamma camera imaging at defined intervals (i.e., at 4, 24 and 48 hours). Panel B. The weighted distribution of the isotope in the colon is summarized by multiplying the counts in each of 4 regions (i.e., ascending colon, transverse colon, descending colon, and rectosigmoid) and stool by the weighting factors indicated in the figure. Panels C-F. Anterior images of the abdomen demonstrate an undissolved capsule at 0 hours (C), dissolving capsule in the terminal ileum at 4 hours (D), and movement of the isotope thereafter at 24 (panel E) and 48 hours (panel F).
Radiopaque marker techniques and scintigraphy assess overall and regional colonic transit. Scintigraphy takes less time than radiopaque markers and can detect not only slow but also rapid colonic transit.(8) A large study comparing scintigraphy with the pH-pressure capsule and a smaller study comparing scintigraphy with radiopaque markers observed greater than 80% agreement between these techniques for discriminating between normal and slow colonic transit.(19, 25) Ingestible capsules – SmartPill™ and Motilis 3D-Transit System
By recording pH, the SmartPill™ capsule can determine when the capsule empties into the small intestine and thereafter into the colon, hence measure gastric, small intestinal, and colonic transit. In addition to discriminating between normal and slow colon transit, and identifying delayed gastric emptying and small intestinal transit in constipated patients, the colonic pressure measurements are useful for discriminating among subtypes of chronic constipation, being greater in IBS (or “painful”) than isolated slow transit (or “painless” constipation).(26–28) However, because the precise location of the Smart Pill™ capsule in the colon is unknown, regional colonic transit cannot be assessed.
The Motilis Transit System (MTS), which incorporates an electromagnetic capsule, was developed by Kucera in 2005.(29) By contrast to the Smart Pill™ capsule, it does not measure pressures or pH. Hence, it does not measure gastrointestinal transit or colonic pressures. However, its location in the colon can be tracked over time. Therefore, it measures not only overall but also regional colonic transit. The electromagnetic capsule measures 21 mm x 8 mm, which is slightly smaller than the Smart Pill capsule (26 × 13 mm). It emits an electromagnetic field that is detected by external sensors in a detector plate and used to track its location in the colon. The original version (MTS-1) employed a stationary magnet that required patients to be supine and at the research facility for the entire study. With the newer version (Motilis 3D-Transit System), participants they wear an external detector weighing 756 gm and can ambulate during studies (personal communication, Mr. Esben Mark). The battery is changed three times daily. Three prior pilot studies evaluated regional colonic transit with Motilis 3D-Transit System in 45 healthy volunteers and 7 patients with chronic diarrhea.(1) The paper by Mark that accompanies this review used a more sophisticated version of the software to reanalyze the data from these 3 studies.
By contrast to manometry, the electromagnetic capsule measures motion but not pressures.(10) The analysis comprises several steps. First, fast movements (i.e., displacements longer than 4 cm with an average velocity of more than 4 cm/minute) are inspected to identify and subsequently eliminate artefact. Then, an automated algorithm categorized every movement into one of 5 patterns based on the velocity, distance, and direction of propagation. Movements slower than 4 cm h-1 or shorter than 4 cm were excluded from subsequent analysis and categorized as ‘non-movement’ periods.
Among 76% of healthy volunteers with satisfactory recordings, data were missing for only 6% of the time. It is striking that the capsule often “darted” between regions, then paused before resuming its motion (Figures 1 and 3 in (1)). The “pauses” are often prolonged. Indeed, for approximately 80% of the recording duration, there was no capsule motion. Simultaneous measurement of colonic pressures and motion may be useful to determine the extent to which this reflects true colonic motor quiescence (e.g., at night), non-propulsive motor activity, or propulsive contractions that do not move the capsule. Similar to colonic transit, these motor patterns may be influenced by particle size.(19)
Among 16 healthy volunteers, the average pan-colonic transit time for 3 capsules was 16.3, 18.2, and 24.9 hours (Table 2 in (1)), which is comparable to the pH pressure capsule (30) but shorter than the colonic transit time measured with radiopaque markers (Table 1), perhaps because radiopaque markers are much smaller. Consistent with the known regional differences; colonic transit time was shorter in the descending (average of 0.9 hours) than the cecum and ascending (average of 4.2 hours), transverse (average of 4 hours) and sigmoid colon/rectum (average of 6 hours). However, there are no differences between motor patterns in the right and left colon. Similar to manometry, which reveals pressure events that are propagated in an antegrade and retrograde manner,(31) the electromagnetic capsule detected antegrade and retrograde movements. The retrograde events may contribute to to-and-fro motion that delays transit and facilitates mixing of colonic contents. Motion increased upon awakening, which is consistent with the known diurnal variation in colonic motor activity.(32) A subset of long fast antegrade movements (i.e., 10 cm/minute, >10 cm) were rapidly propagated, with a velocity that is comparable to high-amplitude propagated contractions (i.e., 1.5 cm s-1).(33) Patients with chronic diarrhea had more antegrade movements. However, contrary to some previous studies propagated colonic contractions did not increase after a meal; capsule motion increased after breakfast but the effects were not statistically significant.(34–36) Perhaps the caloric intake at breakfast was insufficient to evoke a contractile response, especially against a background of increased activity upon awakening.
Hence, the electromagnetic capsule is a promising, noninvasive, and probably safe technique that can be used in ambulatory patients and evaluates overall and regional colonic transit and colonic motor patterns. Several findings in this paper confirm what we know about colonic motor patterns. It is a superb example of what can be accomplished by transdisciplinary collaboration between engineers and physicians. As with any technique, these interesting findings prompt further questions and highlight opportunities for improving the technique. In patients with slow colon transit, the battery life of 5 days may not suffice to assess transit in the entire colon.2 (30) One in five recordings were of poor quality and discarded. During physical activity or otherwise, the external detector may be displaced, causing artefact. The motor patterns identified by the magnetic capsule and high resolution manometry should be compared.(37) In addition, it is essential to integrate the electromagnetic capsule with other approaches to simultaneously measure colonic pressure events and transit, or, even better, the trifecta of colonic contractions, pressure events, and transit.(10)
Key Messages.
Radiopaque markers, scintigraphy, and a colonic pH-pressure capsule are currently used to measure colonic transit in humans.
In addition to measuring overall colonic transit, radiopaque markers and scintigraphy also assess regional colonic transit; colonic pH-pressure capsules also measure colonic pressures, which provide an index of colonic motility.
An electromagnetic capsule-based system provides an integrated assessment of (overall and regional) transit and colonic motor patterns.
Acknowledgments
Funding: This study was supported by a grant from DK078924 from the National Institutes of Health (NIH). There are no competing interests.
Abbreviations
- CTT
Colonic transit time
- MTS
Motilis Transit System
Footnotes
Funding and Disclosures
In the early 1900s (i.e., before measurements of colonic transit were routinely available), Sir Arbuthnot Lane and others proposed the unfounded hypothesis that constipation led to systemic dysfunction, with symptoms ranging from lassitude to epilepsy, prompting colon bypass or colectomy. Later, this practice was recognized to be futile. Assessments of colonic transit facilitate the appropriate selection of patients for colectomy.
For the pH-pressure capsule, which has similar dimensions, the 95th percentile value for whole gut transit time is 72 hours 40 minutes. Hence a battery life of 5 days may be sufficient to detect prolonged transit in some patients.
References
- 1.Mark EB, Poulsen JL, Haase AM, et al. Ambulatory assessment of colonic motility using the electromagnetic capsule tracking system. Neurogastroenterology & Motility 2018; In press. [DOI] [PubMed]
- 2.Phillips SF. Functions of the large bowel: an overview. Scandinavian Journal of Gastroenterology - Supplement 1984; 93: 1–12. [PubMed] [Google Scholar]
- 3.Parthasarathy G, Chen J, Chen X, et al. Relationship Between Microbiota of the Colonic Mucosa vs Feces and Symptoms, Colonic Transit, and Methane Production in Female Patients With Chronic Constipation. Gastroenterology 2016; 150: 367–379 e361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolf PG, Parthasarathy G, Chen J, et al. Assessing the colonic microbiome, hydrogenogenic and hydrogenotrophic genes, transit and breath methane in constipation. Neurogastroenterology & Motility 2017; 29: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zmora N, Suez J, Elinav E. You are what you eat: diet, health and the gut microbiota. Nature Reviews Gastroenterology & Hepatology 2018; 27: 27. [DOI] [PubMed] [Google Scholar]
- 6.Smith JL. Sir Arbuthnot Lane, chronic intestinal stasis, and autointoxication. Ann Intern Med 1982; 96: 365–369. [DOI] [PubMed] [Google Scholar]
- 7.Wilkinson-Smith V, Bharucha AE, Emmanuel A, Knowles C, Yiannakou Y, Corsetti M. When all seems lost: management of refractory constipation-Surgery, rectal irrigation, percutaneous endoscopic colostomy, and more. Neurogastroenterology & Motility 2018; 30: e13352. [DOI] [PubMed] [Google Scholar]
- 8.Camilleri M Scintigraphic biomarkers for colonic dysmotility. Clinical Pharmacology & Therapeutics 2010; 87: 748–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bharucha AE, Locke GR, Pemberton JH. AGA Practice Guideline on Constipation:Technical Review. Gastroenterology 2013; 144: 218–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corsetti M, Costa M, Bassotti G, et al. First “translational” consensus on terminology and definition of colonic motility as studied in humans and animals by means of manometric and non-manometric techniques. Nature Reviews Gastroenterology & Hepatology 2018; In press. [DOI] [PMC free article] [PubMed]
- 11.Hinton JM, Lennard-Jones JE, Young AC. A new method for studying gut transit times using radioopaque markers. Gut 1969; 10: 842–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bouchoucha M, Devroede G, Bon C, Raynaud J-J, Bejou B, Benamouzig R. How many segments are necessary to characterize delayed colonic transit time? International Journal of Colorectal Disease 2015; 30: 1381–1389. [DOI] [PubMed] [Google Scholar]
- 13.Abrahamsson H, Antov S. Accuracy in assessment of colonic transit time with particles: how many markers should be used? Neurogastroenterology & Motility 2010; 22: 1164–1169. [DOI] [PubMed] [Google Scholar]
- 14.Bouchoucha M, Devroede G, Arhan P, et al. What is the meaning of colorectal transit time measurement? Diseases of the Colon & Rectum 1992; 35: 773–782. [DOI] [PubMed] [Google Scholar]
- 15.Bouchoucha M, Thomas SR. Error analysis of classic colonic transit time estimates. American Journal of Physiology - Gastrointestinal & Liver Physiology 2000; 279: G520–527. [DOI] [PubMed] [Google Scholar]
- 16.Sadik R, Abrahamsson H, Ung K-AA, Stotzer P-OO. Accelerated regional bowel transit and overweight shown in idiopathic bile acid malabsorption. Am J Gastroenterol 2004; 99: 711–718. [DOI] [PubMed] [Google Scholar]
- 17.Bouchoucha M, Devroede G, Faye A, Le Toumelin P, Arhan P, Arsac Ml. Colonic response to food in constipation. Int J Colorectal Dis 2006; 21: 826–833. [DOI] [PubMed] [Google Scholar]
- 18.Tuleu C, Andrieux C, Boy P, Chaumeil JC. Gastrointestinal transit of pellets in rats: effect of size and density. International Journal of Pharmaceutics 1999; 180: 123–131. [DOI] [PubMed] [Google Scholar]
- 19.Stivland T, Camilleri M, Vassallo M, et al. Scintigraphic measurement of regional gut transit in idiopathic constipation. Gastroenterology 1991; 101: 107–115. [DOI] [PubMed] [Google Scholar]
- 20.Bouchoucha M, Amiel F, Benamouzig R. Su1579 RFIDTRANSIT - A New Non-Irradiant Method of Measure of Total and Segmental Colonic Transit Time. Gastroenterology 2016; 150: S531–S532. [Google Scholar]
- 21.Krevsky B, Malmud LS, D’Ercole F, Maurer AH, Fisher RS. Colonic transit scintigraphy. A physiologic approach to the quantitative measurement of colonic transit in humans. Gastroenterology 1986; 91: 1102–1112. [PubMed] [Google Scholar]
- 22.Hardy JG, Wilson CG, Wood E. Drug delivery to the proximal colon. Journal of Pharmacy & Pharmacology 1985; 37: 874–877. [DOI] [PubMed] [Google Scholar]
- 23.Proano M, Camilleri M, Phillips SF, Brown ML, Thomforde GM. Transit of solids through the human colon: regional quantification in the unprepared bowel. American Journal of Physiology 1990; 258: G856–862. [DOI] [PubMed] [Google Scholar]
- 24.Burton DD, Camilleri M, Mullan BP, Forstrom LA, Hung JC. Colonic transit scintigraphy labeled activated charcoal compared with ion exchange pellets. Journal of Nuclear Medicine 1997; 38: 1807–1810. [PubMed] [Google Scholar]
- 25.Camilleri M, Thorne NK, Ringel R, et al. Wireless pH-motility capsule for colonic transit: prospective comparison with radiopaque markers in chronic constipation. Neurogastroenterology & Motility 2010; 22: 874–e233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hasler WL, Saad RJ, Rao SS, et al. Heightened colon motor activity measured by a wireless capsule in patients with constipation: relation to colon transit and IBS. American Journal of Physiology - Gastrointestinal & Liver Physiology 2009; 297: G1107–1114. [DOI] [PubMed] [Google Scholar]
- 27.Kuo B, Maneerattanaporn M, Lee AA, et al. Generalized transit delay on wireless motility capsule testing in patients with clinical suspicion of gastroparesis, small intestinal dysmotility, or slow transit constipation. Digestive Diseases & Sciences 2011; 56: 2928–2938. [DOI] [PubMed] [Google Scholar]
- 28.Bharucha AE, Sharma M. Painful and Painless Constipation: All Roads Lead to (A Change in) Rome. Digestive Diseases & Sciences 2018; 63: 1671–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stathopoulos E, Schlageter V, Meyrat B, Ribaupierre Y, Kucera P. Magnetic pill tracking: a novel non-invasive tool for investigation of human digestive motility. Neurogastroenterology & Motility 2005; 17: 148–154. [DOI] [PubMed] [Google Scholar]
- 30.Wang YT, Mohammed SD, Farmer AD, et al. Regional gastrointestinal transit and pH studied in 215 healthy volunteers using the wireless motility capsule: influence of age, gender, study country and testing protocol. Alimentary Pharmacology & Therapeutics 2015; 42: 761–772. [DOI] [PubMed] [Google Scholar]
- 31.Dinning PG, Wiklendt L, Maslen L, et al. Colonic motor abnormalities in slow transit constipation defined by high resolution, fibre-optic manometry. Neurogastroenterology & Motility 2015; 27: 379–388. [DOI] [PubMed] [Google Scholar]
- 32.Rao SS, Sadeghi P, Beaty J, Kavlock R, Ackerson K. Ambulatory 24-h colonic manometry in healthy humans. American Journal of Physiology Gastrointestinal & Liver Physiology 2001; 280: 629–639. [DOI] [PubMed] [Google Scholar]
- 33.Bharucha AE. High amplitude propagated contractions. Neurogastroenterology & Motility 2012; 24: 977–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bazzocchi G, Ellis J, Villanueva-Meyer J, et al. Postprandial colonic transit and motor activity in chronic constipation. Gastroenterology 1990; 98: 686–693. [DOI] [PubMed] [Google Scholar]
- 35.Bazzocchi G, Ellis J, Villanueva-Meyer J, Reddy SN, Mena I, Snape WJ Jr. Effect of eating on colonic motility and transit in patients with functional diarrhea. Simultaneous scintigraphic and manometric evaluations.[see comment]. Gastroenterology 1991; 101: 1298–1306. [DOI] [PubMed] [Google Scholar]
- 36.Cook IJ, Furukawa Y, Panagopoulos V, Collins PJ, Dent J. Relationships between spatial patterns of colonic pressure and individual movements of content. American Journal of Physiology - Gastrointestinal & Liver Physiology 2000; 278: G329–341. [DOI] [PubMed] [Google Scholar]
- 37.Dinning PG. A new understanding of the physiology and pathophysiology of colonic motility? Neurogastroenterology & Motility 2018; 30: e13395. [DOI] [PubMed] [Google Scholar]
- 38.Arhan P, Devroede G, Jehannin B, et al. Segmental colonic transit time. Diseases of the Colon & Rectum 1981; 24: 625–629. [DOI] [PubMed] [Google Scholar]
- 39.Martelli H, Devroede G, Arhan P, Duguay C. Mechanisms of idiopathic constipation: outlet obstruction. Gastroenterology 1978; 75: 623–631. [PubMed] [Google Scholar]
- 40.Chaussade S, Roche H, Khyari A, Couturier D, Guerre J. [Measurement of colonic transit time: description and validation of a new method]. Gastroenterologie Clinique et Biologique 1986; 10: 385–389. [PubMed] [Google Scholar]
- 41.Metcalf AM, Phillips SF, Zinsmeister AR, MacCarty RL, Beart RW, Wolff BG. Simplified assessment of segmental colonic transit. Gastroenterology 1987; 92: 40–47. [DOI] [PubMed] [Google Scholar]
- 42.Abrahamsson H, Antov S, Bosaeus I. Gastrointestinal and colonic segmental transit time evaluated by a single abdominal x-ray in healthy subjects and constipated patients. Scandinavian Journal of Gastroenterology - Supplement 1988; 152: 72–80. [DOI] [PubMed] [Google Scholar]


