Abstract
The transcriptional factor Nrf2, a master regulator of oxidative stress and inflammation that are tightly linked to the development and progression of cerebral ischemia pathology, plays a vital role in inducing the endogenous neuroprotective process. Here, hypoxic-ischemia (HI) was performed in adult Nrf2 knockout and wildtype mice that were orally pretreated either with standardized Korean red ginseng extract (Ginseng) or dimethyl fumarate (DMF), two candidate Nrf2 inducers, to determine whether the putative protection was through an Nrf2-dependent mechanism involving the attenuation of reactive gliosis. Results show that Nrf2 target cytoprotective genes were distinctly elevated following HI. Pretreatment with Ginseng or DMF elicited robust neuroprotection against the deterioration of acute cerebral ischemia damage in an Nrf2-dependent manner as revealed by the reductions of neurological deficits score, infarct volume and brain edema, as well as enhanced expression levels of Nrf2 target antioxidant proteins and anti-inflammation mediators. In both ischemic striatum and cortex, the dynamic pattern of attenuated reactive gliosis in astrocytes and microglia, including affected astrocytic dysfunction in glutamate metabolism and water homeostasis, correlated well with the Nrf2-dependent neuroprotection by Ginseng or DMF. Furthermore, such neuroprotective benefits extended to the late phase of ischemic brain damage after HI, as evidenced by improvements in neurobehavioral outcomes, infarct volume and brain edema. Overall, pretreatment with Ginseng or DMF identically attenuates reactive gliosis and confers long-lasting neuroprotective efficacy against ischemic brain damage through an Nrf2-dependent mechanism. This study also provides new insight into the profitable contribution of reactive gliosis in the Nrf2-dependent neuroprotection in acute brain injury.
Keywords: aquaporin 4, astrocyte, glutamine synthetase, ischemia, microglia, stroke
Graphical abstract
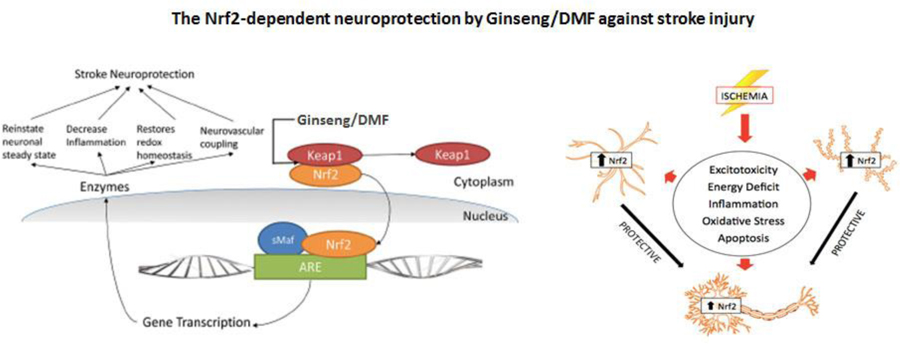
1. Introduction
Cerebral ischemia initiates a series of pathophysiological changes that eventually lead to neuronal death, involving the mobilization of various brain cell types, the accumulation of oxidants and the production inflammatory mediators [1]. Endogenous neuroprotective mechanism by which the brain prevents itself from noxious stimuli and promotes recovery from severe ischemic damage is the focus of stroke research, ultimately facilitating functional recovery [2]. Investigations of signaling pathways underlying endogenous neuroprotection have identified a number of therapeutic targets for pharmacological intervention [3]. It is widely known that oxidative stress and inflammation following cerebral ischemia are tightly related to the pathogenesis and the extent of brain injury, contributing significantly to the development and progression of ischemic pathology. Over the past decade, we and others have identified that the transcriptional factor Nrf2, a master regulator of oxidative stress and inflammation through diverse cytoprotective and detoxification genes, plays a vital role in inducing various endogenous neuroprotective process [4–9]. Consequently, targeting Nrf2 has emerged as a promising strategy for disease prevention, therapy or reversal. However, the precise underlying mechanisms remain limited [7, 8].
Our group has been interested in actively screening various Nrf2 inducers and elucidating the underlying mechanisms for the benefit processes against stroke and other neurological disorders. Recently, both Korean red ginseng (Ginseng) and dimethyl fumarate (DMF), based on their antioxidative and anti-inflammatory attributes, have been associated with neuroprotective potentials for several CNS diseases [10–13]. Panax ginseng, a popular herb distributes in East Asia, has been used in traditional oriental medicine for thousands of years and is now one of the most extensively used herbs for medicinal and nutritional supplement worldwide [12, 13]. Standardized Korean red ginseng extracts, derived from the root of Panax ginseng C.A. Meyer, have exhibited an encouraging protective efficacy with antioxidant and anti-inflammatory properties against various neurological conditions in animal and human studies [13]. DMF, the dimethyl ester derivative of fumaric acid, exhibits both neuroprotective and immunomodulatory effects and was granted indication for Multiple Sclerosis by U.S. Food and Drug Administration in 2013 [10, 14] and Psoriasis by European Medicines Agency in 2017[15]. However, the respective effects of pretreatment with Ginseng or DMF on cerebral ischemia under HI are still unknown.
The pharmacological pretreatment on ischemia was expected to, similar as ischemic preconditioning, improve the resistance of brain tissue from a subsequent severe ischemic insult [16, 17]. Such stimulus induced neuroprotection comprises a rapid phase and a delayed phase [18–20]. The former occurs immediately after stimulus and lasts several hours through acting on interfering RNA, phosphorylation targets and transporter regulation, after which the effect wind down and disappears, and the later follows after a delay of 1–3 days, which is relying on gene activation and de novo protein synthesis.
Reactive gliosis, mainly refers to astrocytes and microglia, undergo varying gene expression, morphology and proliferation changes, having diverse and vital functions in the ischemia injury onset and progression [21–24]. In response to ischemic insult, reactive astrocytes assist in buffering changes in extracellular ion homeostasis like glutamate, altering the osmoregulation, counteracting the development of brain edema, and repairing the integrity of the blood–brain barrier [25, 26]. Studies have suggested that Nrf2 downstream target genes that encode for phase II defense enzymes and antioxidant proteins like heme oxygenase-1 (HO1), NAD(P)H dehydrogenase [quinone] 1 (NQO1), etc. are particularly enriched in glia cells, suggesting the important role of Nrf2 signals in reactive gliosis process that may confer sufficient neuroprotection in the pathogenesis and progression of neurological diseases including ischemic brain injury. However, the supporting experiment data remain limited.
In the present study, we hypothesized that the pretreatment with the standardized Korean red ginseng extract (Ginseng) or DMF, two potent candidate Nrf2 inducers, could elicit robust neuroprotective efficacy against cerebral ischemic damage by an Nrf2-dependent mechanism involving anti-oxidative and anti-inflammatory response and attenuation of reactive gliosis. Accordingly, we had six specific goals in this study by using a cerebral hypoxia-ischemia mouse model and Nrf2−/− mice: (1) to examine whether pretreatment with Ginseng or DMF protects against acute cerebral ischemia outcomes in an Nrf2-dependent manner; and if yes, (2) to address whether pretreatment with Ginseng or DMF could protect brain from ischemia triggered oxidative and inflammatory damage through Nrf2 activation by contrasting the Nrf2 and its target genes expression pattern between ischemic WT and Nrf2−/− mice; (3) to assess whether dynamics pattern of reactive gliosis process contributes to the Nrf2-dependent neuroprotection by Ginseng or DMF; (4) to evaluate whether such Nrf2-dependent neuroprotection can extend to late phase after HI; (5) to compare the differences in efficacy and associated mechanism between Ginseng and DMF at doses tested; and (6) to provide in vivo evidence for the crucial role of Nrf2 during the initiation and progression of cerebral ischemia under HI by transgenic loss-of-function of Nrf2 experiments.
2. Materials and methods
2.1. Animals
All animal experiments were performed according to the NIH Guide for the Care and Use of Laboratory Animals and approved by the University of Florida Institutional Animal Care and Use Committee. The Nrf2−/− mice were generated as described previously [5]. Adult (10–18 weeks) Nrf2−/− and matched WT C57BL/6 male mice were used for this study. The number of animals per group for each assay was calculated based on power analysis. All mice were subjected to neurological behavioral assessment and sacrificed at 6 h, 24 h and 7 day post-ischemia for different measurement. All experiments and analyses were performed in a blinded, randomized, and controlled design to genotype, group assignment and treatment.
2.2. Cerebral hypoxia-ischemia model
Cerebral hypoxia-ischemia (HI) was induced in mice as Koh et al. described [27], and this transient unilateral cerebral ischemia model generates reproducible ischemic lesion in the ipsilateral hemisphere. Each mouse was anesthetized with isoflurane (4% for induction, 1.5% for maintenance) in an oxygen/air mixture during surgery. Artificial tear ointment was applied to each mouse for protection and lubrication. The right common carotid artery (CCA) was carefully separated and occluded permanently by a ligature using a 6/0 nylon suture. After the incision was closed, mouse was returned to its cage and allowed to recover for 2 h with free access to food and water. The mouse was then exposed to systemic hypoxia (8% O2/balance N2) for 1 h in a plexiglas chamber [28], and then was returned to its home cage.
2.3. Pretreatment with Ginseng or DMF
The standardized Korean red ginseng extract (Ginseng) is a water soluble red powder that is prepared and standardized as previous described [29, 30]. Dimethyl fumarate (DMF) was purchased from Sigma (242926). Vehicle (double-distilled water), Ginseng (100 mg/kg/day, dissolved in double-distilled water), or DMF (100 mg/kg/day, suspended in 0.08% methylcellulose) were administered orally for 7 days to C57BL/6 WT and Nrf2−/− mice prior to HI as previous reports [11, 29–32].
2.4. Measurement of infarct volume and edema
At 6 h, 24 h and 7 days after HI, the brain infarct volume and edema were measured by cresyl violet staining [33, 34]. Mice brains were sliced to 30-μm-thick coronal sections on a Leica rotary microtome cryostat, and every tenth section throughout the infarct-containing region was stained. The infarct area of each section was delineated and analyzed using image analysis software (ImageJ, National Institutes of Health, Rockville, MD). To minimize error induced by edema, an indirect method was used for calculating infarct volume. Corrected infarct volume (%) = [volume of contralateral hemisphere – (volume of ipsilateral hemisphere – volume of infarct)] / volume of contralateral hemisphere × 100. Edema volume (%) = [(volume of ipsilateral hemisphere – volume of contralateral hemisphere) / volume of contralateral hemisphere] × 100.
2.5. Behavioral testing
A battery of behavior tests, including neurological deficits score, open field test, and cylinder test, were designed to assess neurological behavioral deficits of mice at indicated time points. Experimenters for tests and analyses were blind to genotype, group assignment and treatment.
2.5.1. Neurological deficits Score
At 6 h, 24 h, 3 days and 7 days after HI, the neurological deficits score (NDS) of mice was assessed using six individual tests, including body symmetry, gait, climbing, circling behavior, front limb symmetry, and compulsory circling, as we have previously described [35, 36]. Scoring for each test was performed by 2 trained investigators in a blinded fashion using a 4-point scoring system (0, no deficits; 4, severe deficits), and the average of the summed scores from the six tests was reported as the final NDS of each mouse.
2.5.2. Open field test
At 3 and 7 days after HI, spontaneous locomotor activity of mice was assessed in an open field with an automated video tracking system (MED Associates, St. Albans, VT) as we described previously [37]. A mouse was placed in a plastic chamber and allowed to explore for 30 min. The total distance travelled for each mouse was used as the indices of motor/exploratory behavior.
2.5.3. Cylinder test
At 7 days after HI, the optimized cylinder test was used for assessing the sensorimotor impairment of mice by measuring the unilateral deficits in voluntary forelimb use as our previous report [38]. A transparent acrylic glass cylinder (8 cm in diameter and 25 cm in height) was placed on a glass-supporting frame. A mirror was placed beneath the glass at a 45-degree angle. Each mouse was video-recorded for 6 min to determine forelimb preference. At least 10 times of full rearings and 20 times of forelimb contacts are needed for analyzing using slow motion or frame-by-frame function of the Video Lan Client (VLC) freeware software. The following parameters were analyzed: (1) Total forelimb use (%, contralateral side to ischemic lesion), which is the total number of all forelimb contacts on the cylinder wall during individual full rearing period; (2) First contact events (%, with both forelimbs), which is when the first contact of a mouse on the cylinder wall happened with both forelimbs during individual full rearing period. The definitions of related parameters: (1) Full rearing, which is when mouse stand up on two hind legs only; (2) Forelimb use, which is the placement of the whole palm on the cylinder wall to support the body in full rearing instances; (3) The contact with both forelimbs, which is when the mouse contacts the cylinder wall with both forelimbs simultaneously (the interval between both forelimbs contacts is no more than 3 frames at 29 frames per second). The calculations for above parameters: (1) Total forelimb use (left, %) = total number of contacts with the left forelimb / total number of contacts with each side of forelimb × 100; (2) First contact events with both forelimbs (%) = total number of first contact events with both forelimbs / total number of full rearings with any forelimb contact (s) × 100.
2.6. Immunohistochemistry
Immunohistochemistry staining for mice brain slices (sham and post-ischemia at indicated time points) was performed as we described previously [35]. Mice were anesthetized and transcardially perfused with PBS (pH 7.4) followed by 4% paraformaldehyde (pH 7.4) in PBS. Brain samples were collected, post-fixed, and cryoprotected in 30% sucrose (w/v) and sliced to 30-μm-thick coronal sections on a Leica rotary microtome cryostat. Immunohistochemistry staining studies were performed using standard protocols. The primary antibodies were rabbit polyclonal ionized calcium-binding adapter protein 1 (Iba1; 1:5000, Wako Bioproducts, Richmond, VA), rabbit polyclonal glial fibrillary acidic protein (GFAP; 1:3000; DAKO, Carpinteria, CA), mouse monoclonal glutamine synthetase (GS; 1:3000; EMD Milipore, Billerica, MA), and rabbit polyclonal aquaporin 4 (AQP4; 1:3000; EMD Milipore, Billerica, MA). Sections were washed and incubated with appropriate secondary antibodies on the following day. The immunoreaction was visualized using 3,3-diaminobenzidine chromogen solution (DAB substrate kit; Vector Laboratories). Three consecutive coronal sections were quantitatively analyzed and provided a single value. We counted Iba1-positive cells area (%), the total number and the immunereactive intensity of GFAP-positive astrocytes, GS immunointensity and AQP4 immunointensity in indicated brain regions. The images were captured using ScanScope CS and analyzed using ImageScope Software (Aperio Technologies, Vista, CA) or ImageJ software.
2.7. Western blot
Western blot analyses were performed using standard protocols [5]. Mice brain samples from the peri-infarct regions of cortex or striatum were homogenized in lysis buffers, and total protein concentration was determined using a Bradford protein assay kit (Bio-Rad). An equal amount of protein was separated using 4–15% polyacrylamide Tris-HCl gradient gels (BioRad, Hercules, CA) and transferred to PVDF membranes. After blocking with 5% skim milk, the membranes were incubated with goat polyclonal NQO1 (1:1000; Abcam, Cambridge, MA), rabbit polyclonal HO1 (1:1000; Enzo Life Sciences, Farmingdale, NY), rabbit polyclonal superoxide dismutase 2 (SOD2; 1:2000; EMD Milipore, Billerica, MA), rabbit polyclonal glutathione peroxidase 1 (GPx1; 1:1000; Abcam, Cambridge, MA), and mouse anti-actin (1:5000, EMD Millipore, Billerica, MA). Quantification analysis was performed using ImageJ software.
2.8. Quantitative Real-Time PCR
RNA for tissue of peri-infarct brain regions of cortex or striatum was extracted using Trizol (Invitrogen) according to the protocol (Qiagen) [39]. Total RNA was purified using RNeasy kit (Qiagen). RNA (1.5 μg) per sample was reverse transcribed into cDNA using SuperScript III RT Reverse Transcriptase (Invitrogen) and then was stored at −20°C for further measurement. The mRNA levels were analyzed with a quantitative real-time PCR (qPCR) system (Bio-Rad). The relative expression value for each target gene was calculated using the ΔΔCt method after normalization to 18S. Each reaction was performed in triplicate and each experiment was repeated two times. The following primer sequences were used (5’ to 3’): 18S, Forward, CGATCCGAGGGCCTCACTA, Reverse, AGTCCCTGCCCTTTGTACACA; IL-10, Forward, GCTCTTACTGACTGGCATGAG, Reverse, CGCAGCTCTAGGAGCATGTG; iNOS, Forward, GTTCTCAGCCCAACAATACAAGA, Reverse, GTGGACGGGTCGATGTCAC; IL-1ß, Forward, GGATGAGGACATGAGCACCT, Reverse, TCCATTGAGGTGGAGAGCTT.
2.9. Statistical analysis
PRISM software (GraphPad V.5) was used to perform the statistical analyses. The number of samples for each experiment is stated in each figure legend. Two-sample comparisons were analyzed using the two-tailed Student’s t test. Multiple comparisons were carried out using a two-way ANOVA followed by a Bonferroni post hoc test or using a one-way ANOVA followed by a Tukey-Kramer post hoc test. All data were expressed as group mean ± SEM and P ⩽ 0.05 was considered statistical significance.
3. Results
3.1. The expressions of Nrf2 and its downstream target genes are highly elevated in hypoxic-ischemic brain regions
To examine whether Nrf2 signals could play key roles in the pathophysiological oxidative and inflammatory events that follow cerebral hypoxia-ischemia (HI), we investigated the temporal pattern of HI lesion by cresyl violet staining and the expression levels of Nrf2 and its downstream genes in ischemic regions following HI. As shown in Fig. 1A, ischemic infarction size expanded rapidly from the ipsilateral striatum into nearby brain regions in the acute phase (within 24 h) followed by a tendency of spontaneous recovery in the late phase (7 days) after HI. The tissues from sham controls and ischemic striatum and cortex regions were used for the following measurements (Fig. 1 B-I). Since currently there is no reliable commercial Nrf2 antibody [40, 41], we measured the mRNA level of Nrf2. Compared to sham controls, Nrf2 mRNA expression had a 1.5–2.4 fold increase from 6 to 24 h after HI in both brain regions. In response to ischemia, the Nrf2 downstream antioxidant proteins NQO1 and HO1 in both brain regions were also significantly elevated compared to sham controls. No significant differences for the expressions of above markers were detected between indicated time points. These results suggest that ischemia insult triggered significant alteration of Nrf2 signals, implying the role of Nrf2 pathway in the pathophysiological events following cerebral ischemia.
Figure 1. Induction of Nrf2 signature in ischemic brain regions at various times following HI.
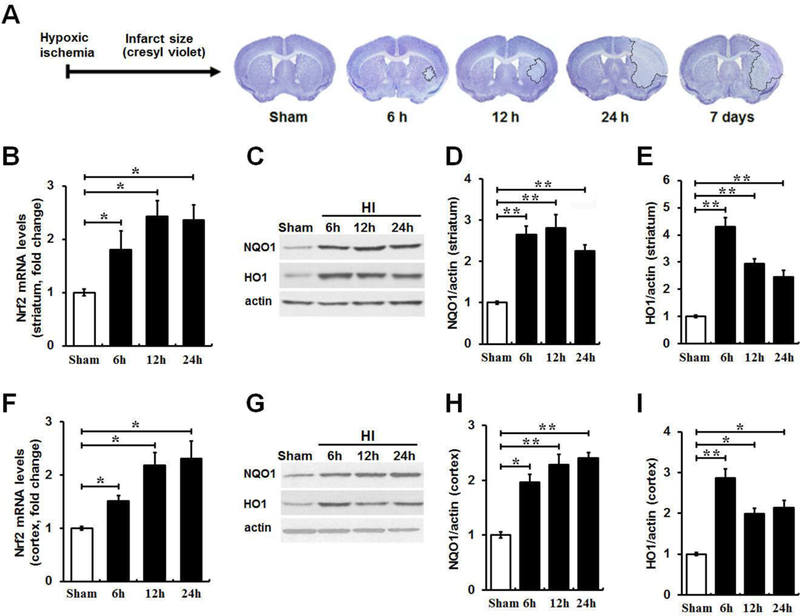
(A) The time-dependent ischemic brain damage over 7 days after HI was assessed by cresyl violet staining of coronal brain sections. HI resulted in a rapid increase in infarct size in striatum and cortex within 24 h followed with a gradual and spontaneous recovery on day 7. The initial ischemic damage occurred in striatum and then spread to nearby brain regions. (B and F) qRT-PCR was used to determine the Nrf2 mRNA level in the ischemic striatum and cortex at 6, 12 and 24 h following HI. Nrf2 mRNA levels had a 1.5–2.4 fold increase within 24 h after HI in both brain regions compared to sham controls, whereas there is no significant difference between indicated time points. (C, D, E, G, H and I) Western blot results were quantified for the protein levels of Nrf2 target markers NQO1 and HO1 in ipsilateral ischemic striatum and cortex at 6, 12 and 24 h following HI compared with sham controls. Protein levels of above markers at indicated times were also significantly elevated compared to sham controls, whereas no difference was detected between indicated time points. n = 3–4 per group. *P < 0.05, **P < 0.01.
3.2. Pretreatment with Ginseng or DMF identically reduces acute ischemic brain damage in an Nrf2-dependent manner
To examine whether pretreatment with Ginseng or DMF, two most potent Nrf2 inducers, can exert neuroprotection against acute ischemic brain damage under HI and if Nrf2 pathway contributes this effect, we assessed the neurological deficits score, infarct volume and brain edema of post-ischemia mice at 24 h after HI (Fig. 2). WT and Nrf2−/− mice received 7 days of pretreatment with Ginseng or DMF and then were subject to ischemic injury by HI. As shown in Fig. 2A, neurological deficits, indicated by neurological deficits score, were significantly improved in WT, but not Nrf2−/−, mice pretreated with Ginseng or DMF when compared with corresponding controls. Likewise, the infarct volume and brain edema were significantly reduced in WT (infarct volume: 23.11 ± 0.97% vs 10.60 ± 1.43% or 12.43 ± 0.85%, P < 0.05; brain edema: 11.47 ± 0.74% vs 4.21 ± 1.03% or 6.69 ± 0.91%, P < 0.05; Fig. 2B-D), but not Nrf2−/−(infarct volume: 34.63 ± 1.63 % vs 23.91 ± 2.67% or 27.34 ± 1.76%, P < 0.05; brain edema: 17.33 ± 1.13% vs 13.77 ± 1.63% or 12.61 ± 0.80%, P < 0.05; Fig. 2B-D), mice pretreated with Ginseng or DMF when compared to corresponding control mice. In contrast, Nrf2 deficiency resulted in significantly more severe neurological deficits score and larger infarct volume and brain edema than WT control mice after HI. No any significant difference was detected in above measures between Ginseng and DMF pretreated groups, indicating their similar efficacy on HI brain damage at indicated dose. These findings suggested that pretreatment with Ginseng or DMF identically protects against acute ischemic damage in an Nrf2-dependent manner and further supported the neuroprotection role of Nrf2 signals in the context of HI.
Figure 2. Pretreatment with Ginseng or DMF remarkably protects against acute stroke damage following HI, but not under Nrf2 deficiency.
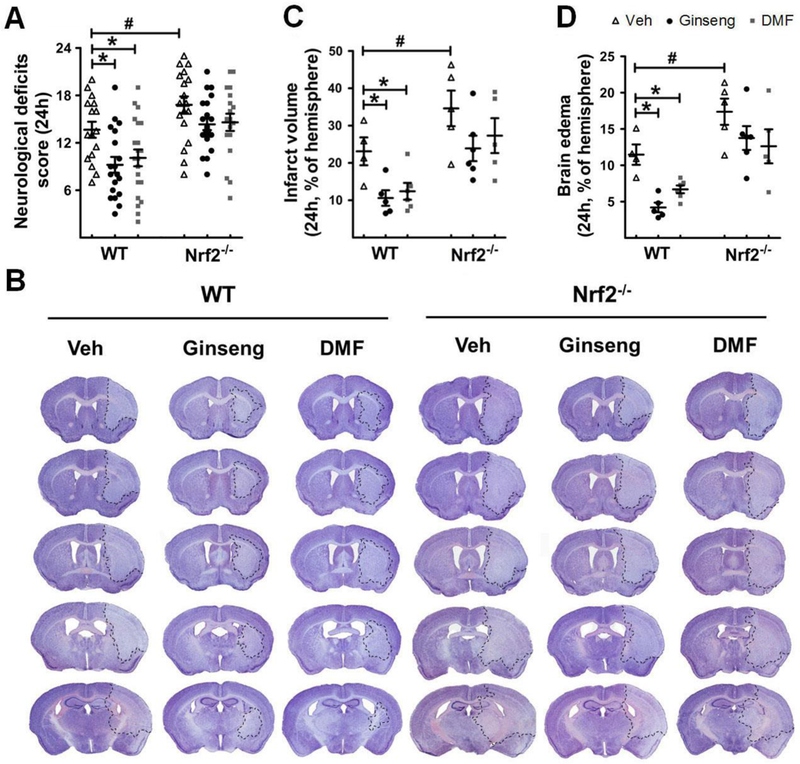
After 7 days of pretreatment with Ginseng or DMF, both WT and Nrf2−/− mice were subjected to HI. Quantitative analysis was performed to measure neurological deficits score (A), infarct volume (B and C) and brain edema (D) at 24 h after HI. (A) Neurological deficits scoring, where a lower value indicates a better function (n = 16–21 per group). (B) Representative images of cresyl violet stained brain slices (n = 4–6 per group). (C and D) Quantitative analyses of brain infarct volume and edema (n = 4–6 per group). Pretreatment with Ginseng or DMF significantly attenuated the neurological deficits score, infarct volume and brain edema in WT mice, but failed to do so in Nrf2−/− mice, while Nrf2 deficiency exacerbated these acute stroke outcomes. *P < 0.05, #P < 0.05.
3.3. Ginseng or DMF associated neuroprotection is correlated with changes in Nrf2-related phase II proteins
Oxidative stress and inflammation are tightly related to the extent of injury following cerebral ischemia attack. To examine whether the activation of Nrf2 pathway though regulation of its pahse II proteins contributes to the beneficial effects of pretreatment with Ginseng or DMF against oxidative damage caused by ischemic insult, we measured the induction of four Nrf2-regulated proteins NQO1, HO1, GPx1, and SOD2 at 24 h after HI, which are well known as cytoprotective and antioxidative proteins [42]. As shown in Fig. 3, ipsilateral ischemic striatum and cortex extracts were subjected to Western blot analysis. The results revealed that post-ischemic WT animal pretreated with Ginseng or DMF had a significant increase of NQO1, HO1, GPx1, and SOD2 proteins levels when compared with WT controls, while such effects were absent in post-ischemic Nrf2−/− mice. In addition, Nrf2 deficiency significantly decreased the expression levels of above markers when compared with corresponding controls. No significant difference was detected between Ginseng and DMF pretreated groups at indicated dose. These results indicated the important role of Nrf2 pathway in acute ischemic brain damage in HI model mice, which may, at least in part, contribute to the identical neuroprotective effects of pretreatment with Ginseng or DMF against ischemic injury.
Figure 3. Pretreatment with Ginseng or DMF protects brain from oxidative damage and enhances Nrf2 downstream antioxidant proteins following HI in WT mice, but not in Nrf2−/− mice.
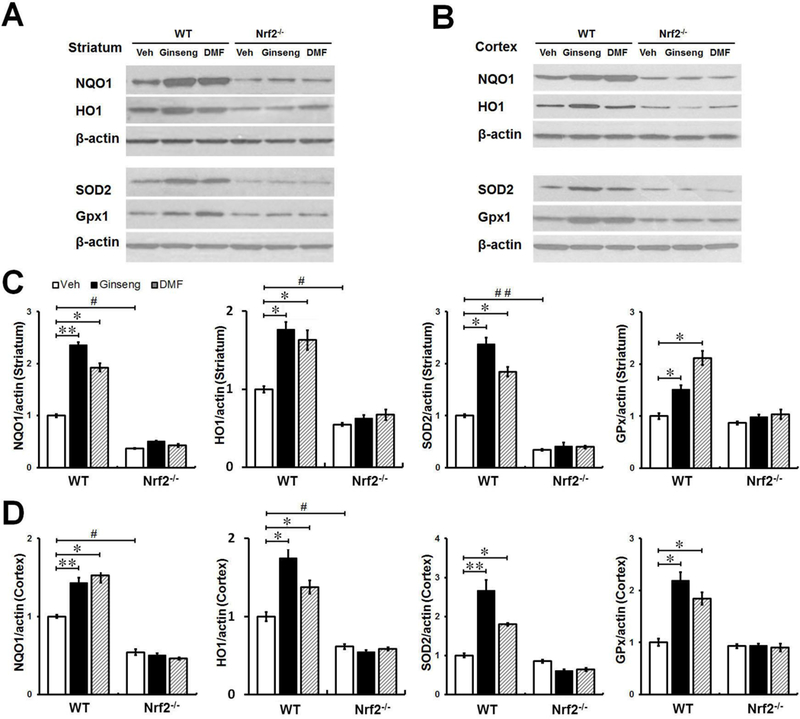
(A and B) Western blot showed the expression levels of Nrf2 target proteins NQO1, HO1, SOD2 and Gpx1 at 24 h after injury in peri-infarct tissue in striatum and cortex of different groups. (C and D) Quantifications of above markers for A and B (n = 4 per group). In both brain regions, pretreatment with Ginseng or DMF significantly increase the expression levels of NQO1, HO1, Gpx1 and SOD2 proteins in WT mice, but not in Nrf2−/− mice, when compared with corresponding controls. In addition, Nrf2 deficiency significantly decreased the expression levels of NQO1 and HO1 in both brain regions and the expression level of SOD2 in striatum when compared to WT controls. *P < 0.05, **P < 0.01, #P < 0.05.
To examine the influence of pretreatment of Ginseng or DMF on HI induced inflammation and whether it is associated with Nrf2 mechanism, we examined the expression levels of inflammatory mediators at mRNA level by qPCR in peri-infarct striatum and cortex at 24 h after HI (Fig. 4). In both brain regions, ischemic insult led to obvious alternations in inflammatory mediators in WT and Nrf2−/− mice. Pretreatment with Ginseng or DMF significantly suppressed the expressions of pro-inflammatory mediators iNOS and IL-1β and promoted the expression of anti-inflammatory IL-10 in WT mice, but not in Nrf2−/− mice. In addition, consistent with previous reports [10, 22], Nrf2 deficiency appeared to increase the iNOS and IL-1β and decrease IL-10 at mRNA levels in both brain regions. No significant difference was detected in these measures between Ginseng and DMF pretreated groups at indicated dose. These results confirmed the important role of Nrf2 in anti-neuroinflammation; and pretreatment with Ginseng or DMF suppressed ischemia triggered inflammation in an Nrf2-dependent manner.
Figure 4. Pretreatment with Ginseng or DMF protects against neuroinflammation, but not in Nrf2−/− mice.
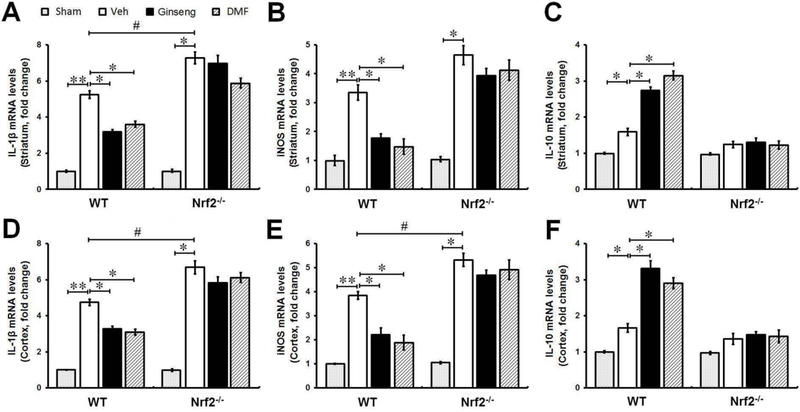
The effect of pretreatment with Ginseng or DMF on neuroinflammation following HI was examined by measuring the mRNA levels of inflammatory mediators iNOS, IL-1β and IL-10 in peri-infarct regions of striatum and cortex at 24 h after HI (n = 4 per group). Compared to sham controls, ischemic injury evoked significant increase in above markers. Pretreatment with Ginseng or DMF significantly suppressed the expression of pro-inflammatory mediator iNOS and IL-1β and promoted anti-inflammatory IL-10 expression following HI in WT, but not Nrf2−/− mice. In contrast, Nrf2 deficiency exacerbated the neuroinflammation induced by HI indicated by the increase of iNOS and IL-1β and decreased IL-10 at mRNA levels in both brain regions. *P < 0.05, **P < 0.01, #P < 0.05.
3.4. Reactive gliosis triggered by acute ischemic insult is attenuated by pretreatment with Ginseng or DMF in WT, but not Nrf2−/−, mice
Reactive gliosis, mainly refers to astrocytes and microglia, undergoes varying molecular, morphological, proliferating, gene expressional and biochemical changes, having diverse and vital functions in the ischemic injury onset, progression and functional recovery [24]. It is well known that Nrf2 downstream target genes are particularly enriched in glia cells [43], implying the potential crucial role of Nrf2 signals in reactive gliosis in response to ischemic injury. To examine the contribution of glia cells in the neuroprotective effects of pretreatment with Ginseng or DMF and Nrf2 induction, morphological changes caused by HI in both astrocytes and microglia were analyzed in the peri-infarct areas of striatum and cortex at 24 h after HI by immunohistochemical staining. The Fig. 5A, C and D showed the changes of astrocytes in response to acute ischemic insult with the anti-GFAP antibody. In the shams, astrocytes tiled the whole striatum and cortex in a regular distribution pattern in both genotypes, mostly displaying a nonreactive state with small soma and fine processes. In either WT or Nrf2−/−, acute ischemic insult evoked activation and proliferation of astrocytes indicated by the increased number of astrocytes at the peri-infarct area of both regions, with a larger proportion of reactive astrocytes that feature hypertrophic somas and highly stained processes. Meanwhile, the extent of this increase was significantly lower in Nrf2−/− mice than that in WT mice, and much more degenerated astrocytes with breakdown somas were observed in Nrf2−/− mice than that in WT mice, indicating the more severe deteriorative progression triggered by loss of Nrf2. In both regions, pretreatment with Ginseng or DMF significantly increased the total number of GFAP-positive cells in WT mice, but not in Nrf2−/− mice. No significant difference was detected between Ginseng and DMF pretreated groups. These findings indicated that Nrf2 activation plays a substantial role in astrocytic activation and proliferation that impact tissue preservation and functional outcome, supporting that such proliferative reactive astrogliosis in an Nrf2-dependent manner may contribute to the neuroprotection of pretreatment with Ginseng or DMF on HI damage.
Figure 5. The reactive gliosis in astrocyte and microglia in the acute phase of ischemic injury following HI is attenuated by pretreatment with Ginseng or DMF in an Nrf2-dependent manner.
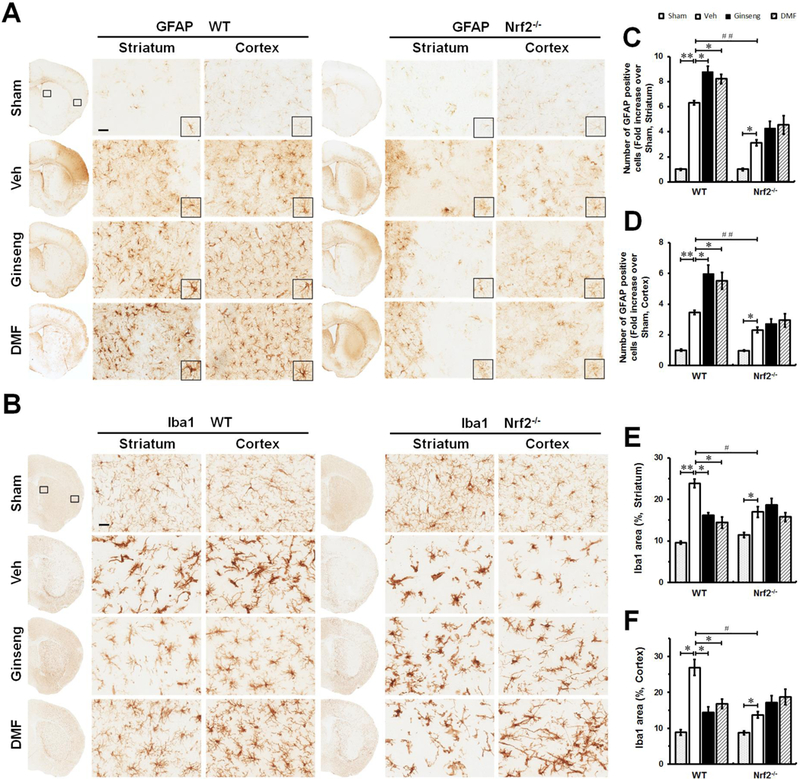
Representative images of GFAP positive astrocyte (A, scare bar: 100 μm) and Iba1 positive microglia (B, scare bar: 50 μm) in the ipsilateral cortex and striatum of mice at 24 h after HI; and open squares in top left image indicate the peri-infarct areas of striatum and cortex used for micrographic examination. (B and C) Quantifications of the total number of GFAP positive cells of A. (E and F) Quantifications of Iba1 signals area of B. In both genotypes (A, C and D), acute ischemic insult evoked astrocytic activation and proliferation, indicated by the increased number of reactive astrocytes with hypertrophic somas and highly stained processes. Apparently, much less degenerated reactive astrocytes with breakdown cell bodies were detected in Ginseng- or DMF- pretreated ischemic WT, but not Nrf2−/−, mice compared to corresponding controls. Meanwhile, pretreatment with Ginseng or DMF significantly protected against the decline in the total number of GFAP positive cells in WT mice, but not Nrf2−/− mice. In contrast, Nrf2 deficiency exacerbated the decline of reactive astrocytes compared to WT controls. In both WT and Nrf2−/− mice (B, E and F), acute ischemic insult triggered significant activation of microglia characterized by hypertrophic soma with thickened and retracted processes, while, interestingly, the Iba1 expression level displayed a tendency of decline in Nrf2−/− mice compared to WT controls. Pretreatment with Ginseng or DMF significantly reduced the Iba1positive signals in WT mice, but not Nrf2−/− mice. These results support that the pretreatment with Ginseng or DMF attenuated the deteriorative progression of reactive gliosis in astrocytes and microglia in an Nrf2-dependent fashion. n = 4–6 per group. *P < 0.05, **P < 0.01, #P < 0.05.
Microglia, the main cell type in the initiation and regulation of ischemia-induced neuroinflammation, respond rapidly to brain insults by morphologically changing, proliferating, and enhanced release of various inflammatory mediators. To address whether microglia activation contribute to the Nrf2-dependent anti-neuroinflammatory effect of pretreatment with Ginseng or DMF, we also examined microglia activation by Iba1 immunostaining (Fig. 5B, E and F). Here, we found that, compared to sham controls, ischemic injury evoked significant activation of microglia in both brain regions of WT and Nrf2−/− mice, characterized by hypertrophic soma with thickened and retracted processes. Similar as our observation on reactive astrocytes, Iba1 positive microglia exhibited significant lower expression level in Nrf2−/− mice than that in WT mice, implying the potential decline of activated microglia in response to severe ischemic insult when Nrf2 is absent. In both brain regions, ischemia triggered microglia activation was significantly reduced in WT mice pretreated with Ginseng or DMF, but not in Nrf2−/− mice. No significant difference was detected between Ginseng and DMF pretreated groups. These data supported the suppression role of pretreatment with Ginseng or DMF on ischemia triggered microglia activation, consistent well with the Nrf2-dependent anti-inflammatory effects.
3.5. Astrocytic dysfunction in glutamate metabolism and water homeostasis triggered by acute ischemic insult is affected by pretreatment with Ginseng or DMF in WT, but not in Nrf2−/−, mice
We next asked whether astrocytic functions on glutamate clearance and water transport were affected in peri-infarct regions of striatum and cortex at 24 h after HI, which are crucial for the formation and extent of ischemic lesion and brain edema. Accordingly, we examined the expression levels of glutamine synthetase (GS) and membrane protein aquaporin-4 (AQP4), two key markers that are predominantly expressed in astrocytes (Fig. 6). GS immunostaining signals were detected at both somas and processes of astrocytes in both striatum and cortex. GS expression level after HI was significantly increased in WT mice pretreated with Ginseng or DMF, which was absent in Nrf2 mice, while Nrf2 deficiency significantly reduced the sharp growth of GS. Pretreatment with Ginseng or DMF significantly protected against the sharp decline of AQP4 expression level triggered by HI in WT mice, but not in Nrf2−/− mice. No significant difference was detected between Ginseng and DMF pretreated groups. These results supported the important role of Nrf2 pathway in the functional regulation of glutamate metabolism and water homeostasis in the context of ischemia following HI, which may contribute to the neuroprotection of pretreatment with Ginseng or DMF.
Figure 6. The dysfunction of glutamate metabolism and water homeostasis triggered by HI is affected by pretreatment with Ginseng or DMF in an Nrf2-dependent manner.
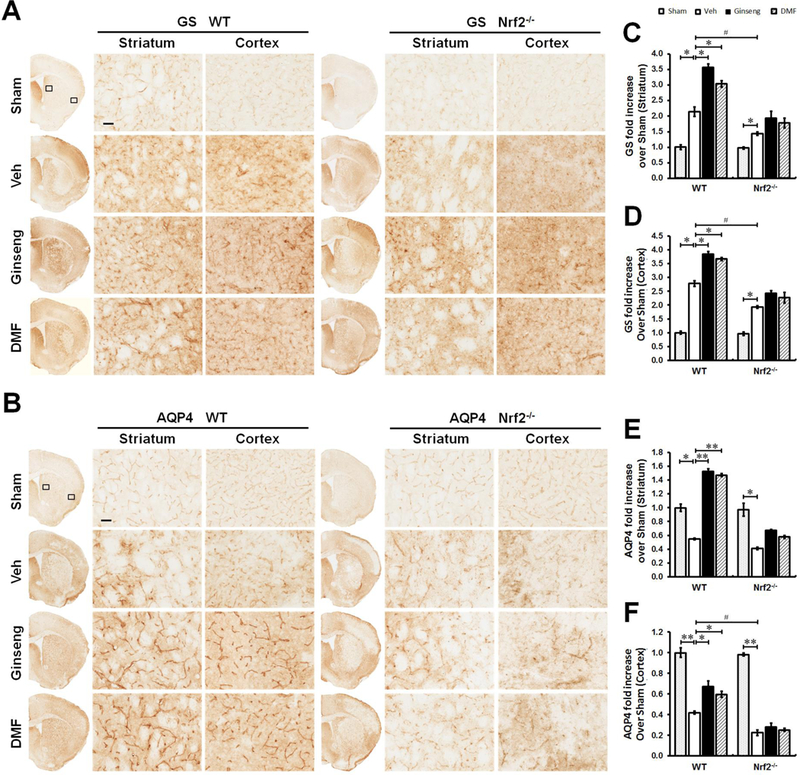
The representative images of GS (A) and AQP4 (B) in the ipsilateral striatum and cortex of mice at 24 h after HI; and open squares in top left image indicate the peri-infarct areas of striatum and cortex used for micrographic examination. Scare bar: 50 μm. Quantifications of GS signals (C and D) of A and AQP4 signals (C and D) of B. In both brain regions (A, C and D), GS immunostaining signals were detected at both somas and processes of astrocytes. In response to acute ischemic insult, GS expression level was significantly increased in WT, but not in Nrf2 mice pretreated with Ginseng or DMF, while Nr2 deficiency significantly reduced the sharp grow of GS expression. In both genotypes (B, E and F), acute ischemic insult triggered significant reduction in AQP4 expression, which is more severe in Nrf2−/− mice. In ischemic WT, but not Nrf2−/−, mice pretreated with Ginseng or DMF, AQP4 expression level was significantly higher than that of corresponding controls. n = 4–6 per group. *P < 0.05, **P < 0.01, #P < 0.05.
3.6. Pretreatment with Ginseng or DMF identically confers significant neuroprotection against the early phase of ischemic damage, whereas loss of Nrf2 reduces such protection
To further determine whether Nrf2 pathway plays crucial role in ischemia onset following HI and whether pretreatment with Ginseng or DMF could confer neuroprotection at the early phase of ischemic damage, we investigated the stroke outcome at 6 h after HI (Fig. 7). Compared to WT controls, ischemia-induced neurological deficits score were significantly ameliorated in WT mice pretreated with Ginseng or DMF, while significantly exacerbated in Nrf2−/− mice. Pretreatment with Ginseng or DMF significantly reduced infarct volume (infarct volume: 4.23 ± 0.39% vs 0.67 ± 0.05% or 0.74 ± 0.07%, P < 0.001) and brain edema (brain edema: 5.83 ± 0.41% vs 0.63 ± 0.11% or 0.86 ± 0.06%, P < 0.001), indicated by cresyl violet staining, in WT mice, but not in Nrf2−/− mice (infarct volume: 14.77 ± 1.05% vs 11.57 ± 1.33% or 10.34 ± 1.61%, P < 0.01; brain edema: 12.34 ± 1.29% vs 9.67 ± 0.95% or 10.39 ± 0.82%, P < 0.05). In contrast, Nrf2 deficiency exacerbated above ischemic outcomes. To confirm the findings in brain lesion, we further quantified the intensity of cresyl violet positive cells in ischemic core area. As shown in Fig. 7B, at the initial phase of ischemic injury, the intensity of residual cresyl violet positive cells at indicated site appeared to be significantly higher in WT mice pretreated with Ginseng or DMF. However, in contrast, these neuroprotective effects of pretreatment with Ginseng or DMF were not detected in Nrf2−/− mice. No significant difference was detected between Ginseng and DMF pretreated groups. These findings supported the important role of Nrf2 activation in the ischemia pathogenesis that might contribute to the identical neuroprotections of pretreatment with Ginseng or DMF at ischemia onset under HI.
Figure 7. Pretreatment with Ginseng or DMF apparently prevents the early deterioration of brain damage following HI onset which is reduced under Nrf2 deficiency.
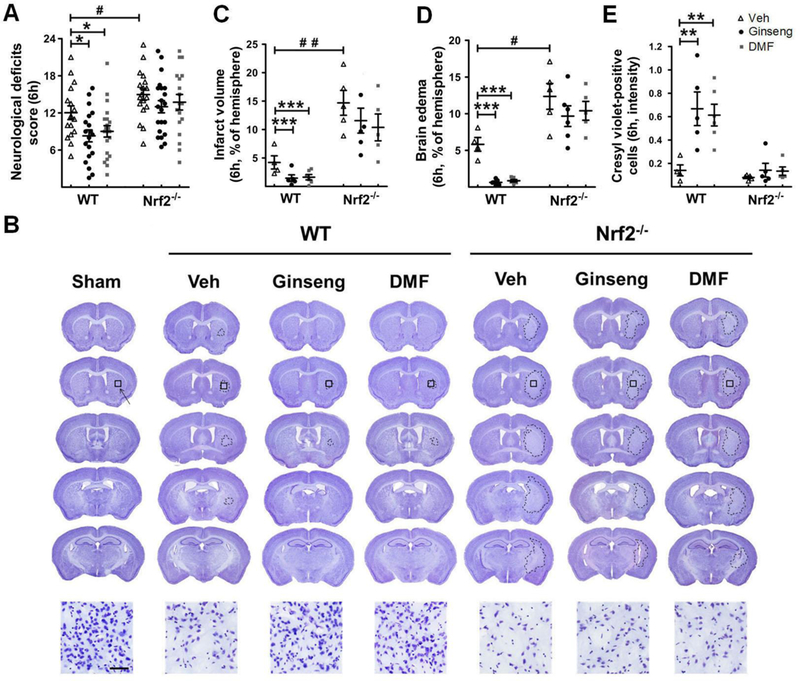
Following HI onset, quantitative analyses of neurological deficits score (A), infarct volume (B and C), brain edema (D) and the intensity of cresyl violet positive cells (E) were performed in mice at 6 h after injury. (A) Neurological deficits scoring, where a lower value indicates a better function (n = 16–21 per group). (B) Representative images of cresyl violet stained brain slices (n = 4–6 per group). (C, D and E) Quantitative analyses of infarct volume, brain edema and the intensity of cresyl violet positive cells (n = 4–6 per group). The neurological deficits score, infarct volume, brain edema, and the intensity of residual brain cells in ischemic core indicated by cresyl violet staining were significantly reduced in post-ischemia WT, but not Nrf2−/−, mice pretreated with Ginseng or DMF, while Nrf2 deficiency exacerbated these stroke outcomes compared to WT controls. *P < 0.05, **P < 0.01, ***P < 0.001, #P < 0.05.
3.7. The early phase of reactive gliosis following HI onset is remarkably attenuated by pretreatment with either Ginseng or DMF in an Nrf2-dependent manner
To determine whether the neuroprotective roles of Nrf2 pathway and pretreatment with Ginseng or DMF in the deterioration of acute ischemic damage are correlated with the temporal reactive gliosis progression, we also examined the early phase of reactive gliosis in peri-infarct regions of striatum and cortex by GFAP and Iba1 immunostainings at 6 h after HI (Fig. 8). Initial ischemic insult rapidly evoked astrocytic activation and proliferation in both genotypes, indicated by the increases of overall GFAP signals and the total number of reactive astrocytes, which were more obvious in WT mice. Pretreatment with Ginseng or DMF significantly attenuated the extent of astrocytic activation in WT mice, indicated by reduced GFAP signals without affecting the total number of astrocytes, but not in Nrf2−/− mice. In both genotypes, initial ischemic insult quickly evoked microglia activation, which was more evident in Nrf2−/− mice. Pretreatment with Ginseng or DMF significantly attenuated microglial activation process in WT mice, indicated by reduced Iba1 signals, but not in Nrf2−/− mice. No significant difference was detected between Ginseng and DMF pretreated groups. These data confirmed the key role of Nrf2 pathway and the neuroprotective effects of pretreatment with either Ginseng or DMF on reactive gliosis in astrocytes and microglia in the early phase of ischemia following HI. This temporal pattern of reactive gliosis process is consistent well with the aggravation of brain damage in the acute phase of ischemia following HI.
Figure 8. The early phase of reactive gliosis following HI onset is attenuated by pretreatment with Ginseng or DMF in an Nrf2-dependent manner.
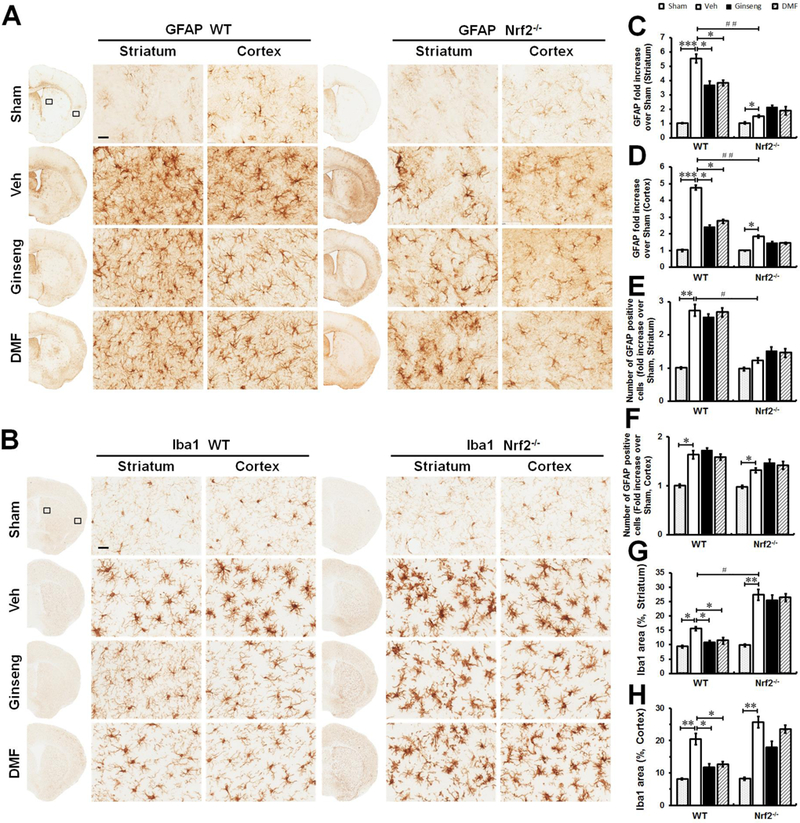
Representative images of GFAP positive astrocyte (A) and Iba1 positive microglia (B) in the ipsilateral cortex and striatum of mice at 6 h after HI; and open squares in top left image indicate the peri-infarct areas of striatum and cortex used for micrographic examination. Scare bar: 50 μm. Quantifications of the GFAP signals (C and D) and the total number of GFAP positive cells in (E and F) of A. Quantifications of Iba1 signals area (G and H) of B. In both genotypes (A, C, D, E and F), initial ischemic insult evoked astrocytic activation and proliferation, indicated by the increases of overall GFAP signals and the total number of reactive astrocytes, which was more obvious in WT mice. Pretreatment with Ginseng or DMF significantly attenuated the extent of astrocytic activation in WT mice, indicated by reduced GFAP signals without influencing the total number of astrocytes, but not in Nrf2−/− mice. Pretreatment with Ginseng or DMF significantly attenuated microglial activation in WT mice, indicated by reduced Iba1 signals, but not in Nrf2−/− mice. In contrast, Nrf2−/− mice exhibited significant higher level of Iba1 signals in ischemic striatum but not cortex compared to WT controls. n = 4–6 per group. *P < 0.05, **P < 0.01, #P < 0.05.
3.8. The early phase of abnormal glutamate metabolism and disturbed water homeostasis following HI onset are affected by pretreatment with Ginseng or DMF in an Nrf2-dependent manner
Likewise, we examined the expression levels of GS and AQP4 by immunostainings homeostasis in peri-infarct regions of striatum and cortex at 6 h after HI (Fig. 9). In both genotypes, the initial ischemic insult rapidly led to significant increases of GS and AQP4 expression levels in both brain regions except AQP4 in ischemic striatum. Interestingly, compared with WT controls, Nrf2 deficiency led to significant decline of AQP4 in striatum, but not in cortex, highlighting the crucial role of Nrf2 on the dynamic regulation of water homeostasis in HI context. Both GS and AQP4 immunostaining signals were significantly attenuated in WT, but not Nrf2−/−, mice pretreated with Ginseng or DMF. No significant difference was detected between Ginseng and DMF pretreated groups. These results further supported the role of Nrf2 activation on astrocytic dysfunctions in affecting glutamate metabolism and water homeostasis in the initial phase of ischemia following HI, and this Nrf2-dependent neuroprotection might contribute to the beneficial effect of either Ginseng or DMF.
Figure 9. The abnormality of glutamate metabolism and disturbed water homeostasis in the early phase of ischemic injury following HI onset are affected by pretreatment with Ginseng or DMF in an Nrf2-dependent manner.
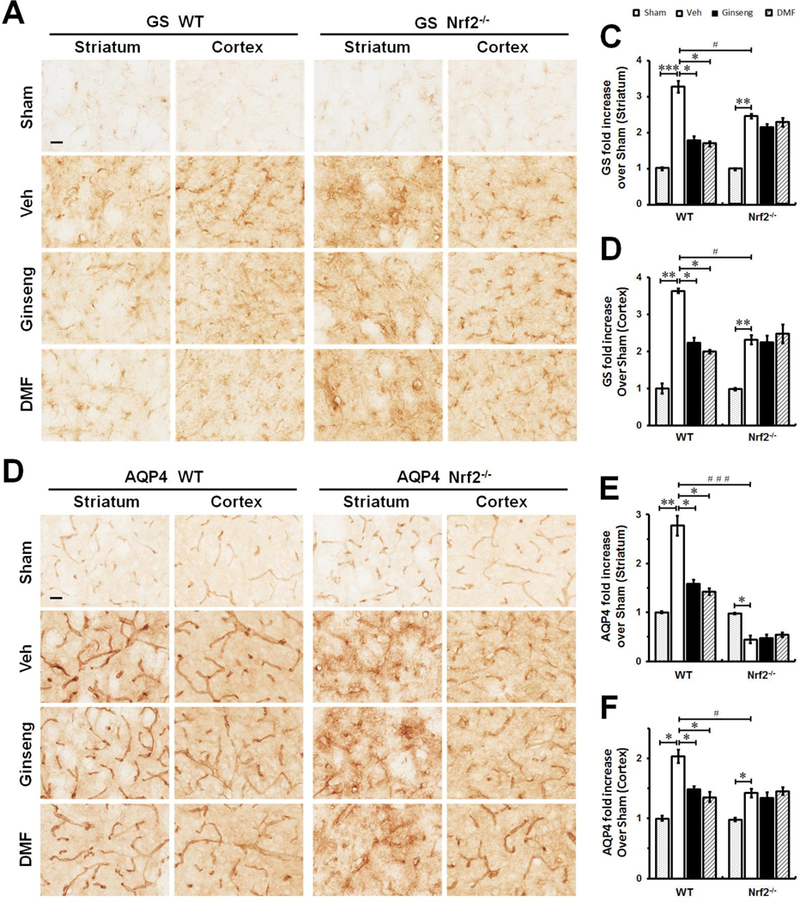
The representative images of GS (A) and AQP4 (B) in the ipsilateral striatum and cortex of mice at 24 h after HI; and open squares in top left image indicate the peri-infarct areas of striatum and cortex used for micrographic examination. Scare bar: 50 μm. Quantifications of GS signals (C and D) of A and AQP4 signals (E and F) of B. In the initial phase of ischemic injury, pretreatment with Ginseng or DMF significantly attenuated the rapid increase of GS and AQP4 expressions in both brain regions in WT mice, but not in Nrf2−/− mice. In contrast, Nrf2 deficiency displayed significant lower expression levels in GS and AQP4 signals compared to WT controls. Interestingly, in response to initial ischemic insult, Nrf2−/− mice underwent quick decline in AQP4 expression in striatum but not cortex compared to WT controls. n = 4–6 per group. *P < 0.05, **P < 0.01, #P < 0.05, ###P < 0.001.
3.9. Pretreatment with Ginseng or DMF identically displays long-lasting neuroprotective efficacy against ischemic injury, but failed under Nrf2 deficiency
Next, we asked whether such Nrf2-dependent neuroprotective effect by pretreatment with Ginseng or DMF could extend to late phase of cerebral ischemia over 7 days after HI (Fig. 10 and 11). Long-term functional assessment is a key component for testing the efficacy of potential therapeutics in translational stroke researches. As shown in Fig. 10, no significant difference in the locomotor activity was detected among groups on day 3 and 7, except that pretreatment with Ginseng significantly improved the locomotor activity in post-ischemia WT mice. On both day 3 and 7, pretreatment with Ginseng or DMF protected against the neurological deficits score in WT mice, but failed in Nrf2−/− mice. The sensorimotor deficit on day 7, revealed by the percentages of total left forelimb use and the first contact events with both forelimbs in cylinder test, was significantly recovered in WT, but not Nrf2−/−, mice pretreated with Ginseng or DMF. Additionally, on day 3 after HI, most post-ischemia mice could not behave well to carry out the cylinder test. Meanwhile, as shown in Fig. 11, compared to WT control, infarct volume and brain edema were dramatically reduced 7 days after HI in WT mice pretreated with Ginseng or DMF, but not in Nrf2−/− mice, while Nrf2 deficiency led to significant worse condition. No significant difference was detected between Ginseng and DMF pretreated groups, except that Ginseng but not DMF improvemed post-ischemia locomotor activity on day 3 after HI. These findings indicated the long-lasting Nrf2-dependent neuroprotective efficacy by pretreatment with either Ginseng or DMF against ischemic injury under HI and supported the critical role of Nrf2 in long-term neuroprotection in the context of HI.
Figure 10. Pretreatment with Ginseng or DMF improves the neurobehavioral outcomes over 7 days after HI, but not under Nrf2 deficiency.
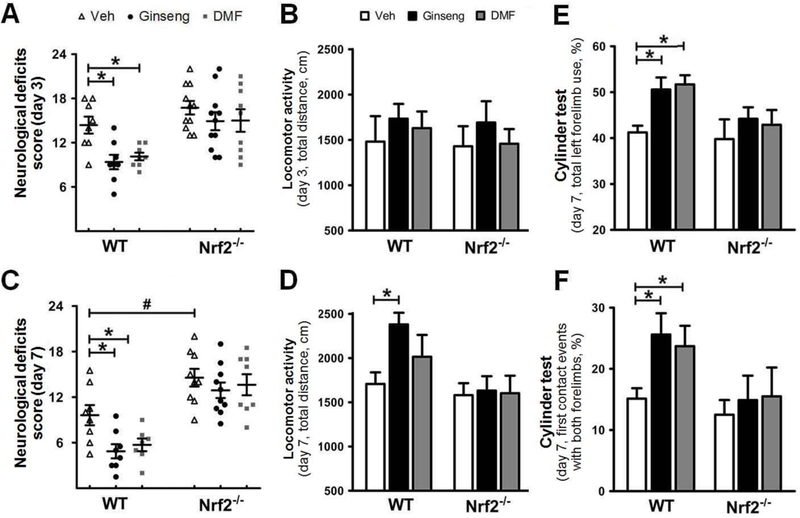
Neurological deficits score (A and C) and locomotor activity (B and D) were performed at 3 and 7 days after HI). Pretreatment with Ginseng or DMF ameliorated neurological deficits score at 3 and 7 days after HI in WT mice, but not in Nrf2−/− mice, whereas Nrf2 deficiency exacerbated neurological deficits score at 7 days after HI. There was no significance in locomotor activity among groups except that pretreatment with Ginseng significantly improved the locomotor activity in WT mice at 7 days after HI. Pretreatment with Ginseng or DMF ameliorated sensorimotor deficits at 3 and 7 days after HI in WT mice, but not in Nrf2−/− mice, revealed by cylinder test. n = 7–11 per group for 3 days, n = 7–10 per group for 7 days. *P < 0.05, #P < 0.05.
Figure 11. Pretreatment with Ginseng or DMF improves late phase of ischemic brain damage after HI, but not under Nrf2 deficiency.
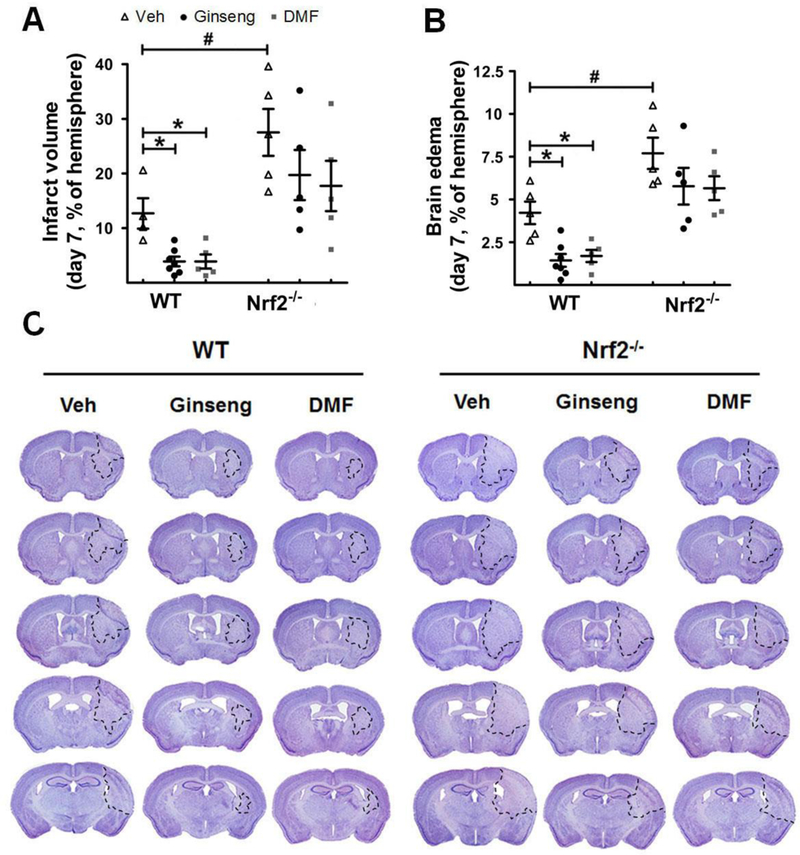
Quantitative analysis of infarct volume (A) and brain edema (B) by cresyl violet staining was performed in mice 7 days after HI (n = 5–7 per group). (C) Representative images of cresyl violet stained brain slices (n = 6–9 per group). Pretreatment with Ginseng or DMF significantly improved the late phase of ischemic brain damage in WT mice, but not in Nrf2−/− mice. Compared to WT controls, Nrf2 deficiency significantly exacerbated the ischemic brain damage. *P < 0.05, #P < 0.05.
4. Discussion
Endogenous neuroprotective mechanism by which the brain prevents itself from noxious stimuli and promotes recovery from severe ischemic damage is the focus of stroke research, ultimately facilitating functional recovery [3]. In the present study, we observed upregulated expression of Nrf2 signal profile at mRNA or protein levels in ischemic brain regions of mice after HI, indicating the involvement of Nrf2 pathway in ischemic injury. Our study is the first to demonstrate that pretreatment with Ginseng or DMF confers robust and prolonged neuroprotective efficacy against ischemia outcome indicated by significantly reduced neurological deficits score including sensorimotor dysfunction, infarct volume and brain edema at both early and late phases of ischemia after HI in WT mice, but not Nrf2−/− mice; suggesting the potential involvement of Nrf2 pathway during the neuroprotection process. Pretreatment with Ginseng or DMF significantly reduced ischemia triggered oxidative and pro-inflammatory markers after HI and improved Nrf2 targets expression levels and anti-inflammation marker, but not under Nrf2 deficiency, strongly supporting the crucial role of Nrf2 activation in this protection. The deteriorative progression of spatiotemporal reactive gliosis in astrocytes and microglia, including astrocytic dysfunction for affecting glutamate metabolism and water homeostasis, was significantly attenuated by pretreatment with Ginseng or DMF in WT mice, but not in Nrf2−/− mice, indicating the contribution of reactive gliosis in the neuroprotection by pretreatment with Ginseng or DMF in an Nrf2-dependent fashion. No significant difference was detected in above measures between Ginseng and DMF pretreated groups, indicating their identical efficacy on HI brain damage at indicated dose. In addition, compared to vehicle pretreated WT mice, Nrf2 deficiency exacerbated the ischemia outcome, oxidative and inflammatory damage, and the deteriorative progression of reactive gliosis after HI, further highlighting the crucial roles of Nrf2 in neuroprotection and reactive gliosis process in HI context. Taken together, these findings suggested that Nrf2 activation may contribute to the neuroprotection of pretreatment with Ginseng or DMF against ischemia injury after HI through attenuating reactive gliosis progression.
4.1. Pretreatment with Ginseng or DMF elicits robust and prolonged neuroprotective efficacy against ischemic damage in an Nrf2-dependent manner
Compared with the irreversible tissues damage in the ischemic core, the penumbral tissues are viable and salvageable, including ischemic areas that could spontaneously recover or progress to irreversible alternation without effective intervention [44, 45]. As such, the salvageable tissues are one primary target for preventive treatment. Currently, the neuroprotective effects of pretreatment with Ginseng or DMF against ischemic brain injury following HI have not been reported and compared. Recently, more attention has been focused on the pharmacological properties of Ginseng that can not only scavenge excessive free radicals but also mobilize endogenous protective mechanism, like cellular antioxidant capacity by inducing de novo synthesis of antioxidant enzymes, thus potentially preventing from subsequent severe damage. Our recent report showed the potent neuroprotection of Ginseng on permanent ischemic stroke model mice, and this motivated us to test its neuroprotective property in another ischemic stroke mouse model [38]. DMF has been reported to be a most successful Nrf2 inducer by up-regulating the transcriptional Nrf2 downstream genes in brain, indicating its potentials for future therapeutic intervention [46]. Brain injury and functional impairment caused by cerebral ischemia is the end result of complex pathophysiological events, interacting between endogenous protective mechanisms and ischemic noxious cascade [47]. Most studies have shown that beneficial effects of Ginseng were achieved by pretreatment. Pretreatment with Ginseng or DMF is likely to mobilize antioxidant constituents, alleviate the intracellular concentration of bioactive compounds and elicit a cascade involving boosting intrinsic endogenous neuroprotection, ultimately improve stroke outcome as preventive treatment against a subsequent longer period of ischemic damage. We have opted for pretreatment for numerous reasons, principally to reduce possible confounding factors of Ginseng or DMF’s direct antioxidant properties. Our goal is to demonstrate that whether pretreatment with Ginseng or DMF can strengthen the resistance to ischemic brain injury and whether they could display any difference in putative neuroprotection. Accordingly, we expanded our study in both acute and late phases of ischemia, assessed multiple stroke outcomes at 6 h, 24 h, 3 days and 7 days after HI. Our data most consistently demonstrated that oral administration of either Ginseng or DMF, at clinically relevant dose, for 7 days before ischemia displayed identical neuroprotective efficacy. Such pretreatments similarly prevented brain from the very early-phase of neuronal loss and brain edema, reduced deteriorative progression and promoted gradual recovery, along with the sustained improvement of neurological function including sensorimotor function. Further investigations are needed to test their dose-dependent efficacy. Demonstration of the neuroprotection in early and late phases of ischemic damage is critical for translating our finding to human studies. It is also noted here that oral intake of Ginseng or DMF is quite safe, uncostly and convenient. In particular, above neuroprotection appeared to be Nrf2-dependent, as the post-stroke outcomes up to 7 days after HI did not differ significantly among Nrf2−/− mice groups.
4.2. The Nrf2 pathway constitutes the neuroprotective mechanisms by pretreatment with either Ginseng or DMF
With regard to the molecular mechanisms underlying the beneficial effect of pretreatment with Ginseng or DMF, our study supported induction of Nrf2 downstream antioxidant target genes as mediators for its neuroprotective effects and functional benefits. Nrf2 is a pleiotropic transcription factor and a master regulator of redox homeostasis and inflammation by inducing the expression of various cytoprotective and detoxification genes encoding for phase II defense enzymes and antioxidant stress proteins. Given the central role of Nrf2-mediated gene regulation in response to damage of cellular components in basal and pathological conditions, we and others [5, 10, 38] have presented that Nrf2 may represent an excellent therapeutic and inventive target for neurological disorders including cerebral ischemia. In accordance with this assumption, we observed the high expression of Nrf2 signals in acute phase of ischemia under HI. To confirm the functional importance of Nrf2 activation in such protection, we further revealed that pretreatment with Ginseng or DMF prevented the brain from oxidative damage with strong enhancement of Nrf2 and its target genes NQO1, HO1, SOD2 and Gpx1 at mRNA or protein levels. Likely the upregulation of these Nrf2 target antioxidant genes benefited the stroke outcome. As the target genes of Nrf2, NQO1, HO1, SOD2 and Gpx1 have demonstrated cytoprotective capacities in various neurological conditions [8]. For example, HO1 is particularly important in the neuroprotection against cerebral ischemia, by the fact that HO1 overexpression mice exhibited reduced infarcts [48], and by the finding that HO1−/− mice displayed exacerbated infarcts [49]. In addition, pretreatment reduced expression of pro-inflammatory factor iNOS and IL-1β and increased the expression of anti-inflammatory IL-10. More importantly, the changes of above Nrf2 downstream markers and inflammatory factors by pretreatment with Ginseng or DMF could not be detected under Nrf2 deficiency. In addition, either Ginseng or DMF pretreatment showed similar effect on above marker at indicated dose. All of these features strongly support the substantial role of Nrf2 pathway in such neuroprotective mechanisms. Further investigation will be needed to precisely define their roles in this process. For instance, it remains to be determined whether the interaction of Nrf2 with Keap1 in the cytoplasm, Nrf2 nuclear translocation and DNA binding will be changed by pretreatment with Ginseng or DMF after HI. Additionally, the measurement of pro- and anti-inflammatory markers like TNF-α, IL-6 will be valuable for further confirmation.
4.3. Reactive gliosis is implicated in the Nrf2-dependent neuroprotection by pretreatment with Ginseng or DMF
Although studies have reported the crucial roles of glia cells especially astrocytes and microglia in the pathogenesis of CNS injury and neuroprotection, little is known about their functional importance, associated with Nrf2 pathway, in HI context and whether this process contributes to the neuroprotection by pretreatment with Ginseng or DMF. Glia cells play crucial roles in maintaining brain homeostasis, such as synaptic transmission regulation, energy metabolism, extracellular glutamate level control, potassium buffering, interstitial volume regulation, and the intrinsic protection, in many CNS disorders including cerebral ischemia [50]. Cerebral ischemia evokes acute-phase cellular injury to brain damage within minutes to hours (like neuronal death) and late phase brain damage that progresses in days after ischemic insult [44]. In response to multiple CNS injuries or diseases, they act as reactive gliosis and display cellular hypertrophy, proliferation and migration. Nrf2 target genes were reported to be preferentially activated in glia cells that consequently have higher level of antioxidant defense and detoxification than neurons [43]. As such, we examined whether the spatiotemporal pattern of reactive gliosis progression could be correlated with the Nrf2-mediated neuroprotection of pretreatment with Ginseng or DMF. Nrf2-mediated neuroprotection by pretreatment with Ginseng or DMF attenuated, during the initial ischemic neuronal loss and the formation of infarct process, the deterioration progression of reactive gliosis in astrocytes and microglia in the peri-infarct regions that conferred protective efficacy against ischemic insult. These benefits exhibited neuroprotective roles against immediate and long-term neurological deficits score after HI. Recent studies indicated that the increase in microglia density in response to CNS injury correlated with both the extension of endogenous resident microglia and the active recruitment of microglia progenitors from the blood [23, 51, 52]. Notably, at very early phase of ischemia onset like 6 h, the neuroprotection drove microgliosis (microglia activation), exhibited by the expansion of microglia mainly resulted from the local existing resident microglia [53]. Along with microglia, astrocytes border functional barrier that restrict inflammatory cells entering into CNS parenchyma in health and diseases [21, 54, 55].
Astrocytes transport more than vast majority of extracellular glutamates by glutamate-glutamine shuttle, a major effect that causes exudative stress, and account for most of the excitatory synaptic activity [2, 17]. ATP-dependent enzyme GS, exclusively distributed in astrocytes and maintaining the glutamate-glutamine cycle, is particularly sensitive to the free radicals generated during ischemic stroke [56]. AQP4, the most abundant water channel in the brain, is enriched in the perisynaptic and perivascular astrocytic endfeet, demonstrating key roles in water homeostasis in CNS [57–59]. AQP4 is up-regulated in the peri-infarct border of cerebral ischemia; and AQP4−/− mice exhibit significantly reduced brain edema and improved stroke outcome, revealing the conserved AQP4 level may attenuate edema formation that tightly contributes to infarct formation and recovery. The initiation of astrocyte swelling possibly results from the osmotic overload triggered by enhanced levels of glutamate and potassium ion. Consequently, activation of water influx through AQP4 causes a dramatic astrocytic swelling that eventually leads to a variety of second injury [57, 60–62]. Our study showed that pretreatment with Ginseng or DMF delayed the rapid increase at earlier phase and protected against the sharp decline at the later phase of acute ischemia after HI in GS and AQP4 expressions. The coordinated regulation of AQP4 in neuroprotection may compensate to reduce the intracellular hyperosmotic pressure initiated by impaired astrocytic function of glutamate clearance. Importantly, above findings were absent under Nrf2 deficiency. Interestingly, according to the time frame of measurements, we observed a comparably faster deteriorative progression in striatum than that in cortex, which might be related to the distance to the ischemic core. Further study is needed to provide more details of Nrf2 mechanism on astrocytic dysfunction under HI and intrinsic neuroprotection. Consistent with previous reports, these findings revealed that pretreatment with Ginseng or DMF attenuated the deteriorative disruption of microglia activation, astrocytic structural integrity and astrocytic dysfunctions including glutamate clearance and water transport [63, 64]. Either Ginseng or DMF showed similar effect on reactive gliosis. Together, our data suggested that attenuated spatiotemporal reactive gliosis plays a pivotal role in the neuroprotection of pretreatment with either Ginseng or DMF at indicated dose in an Nrf2-dependent fashion.
4.4. The Nrf2-mediated neuroprotection during cerebral ischemia initiation, progression and repair under HI is well revealed
Meanwhile, although Nrf2 has implied cytoprotective roles in multiple neurological diseases, supporting in vivo evidence is still lack in the context of ischemia under HI. To further address the question on the functional importance of Nrf2 activation on ischemia, we examined the stroke outcomes in WT and Nrf2−/− mice. Nrf2−/− mice provided a great transgenic loss-of-function model that display greater susceptibility to ischemic injury at both early and late phases after HI. Consistent with above findings, loss of Nrf2 exacerbated ischemia-induced neurological deficits score, infarct volume and brain edema at different phases of ischemia, as well as the reduction of antioxidant and anti-inflammation capacity. Reactive gliosis appeared to essentially participate in this Nrf2-dependent process. Considering the prominent preferential distribution and critical protective roles of Nrf2 target cytoprotective genes in glia cells, the data highlighted the plausible importance of reactive gliosis in Nrf2-mediated neuroprotection against ischemia. These findings enrich our understanding of the action mechanism of Nrf2 in neuroprotection. The proof-of-principle experiments with Nrf2 deficiency mice revealed the crucial neuroprotective role of Nrf2 mechanism during cerebral ischemia initiation, progression and repair. The contribution of reactive gliosis to this Nrf2-dependent neuroprotection may provide a key to the deeper understanding of the cellular basis of Nrf2-mediated endogenous neuroprotective system, further supporting our hypothesis. It should be pointed out that investigation of glial Nrf2 signals is needed to further determine whether glial Nrf2 predominately contributes to Nrf2-mediated protection.
One limitation of our study that should be mentioned is that we did not complete a detailed time curve looking at the specific nuclear Nrf2 translocation. This could have also helped to clarify which specific brain cells are most responsive to Ginseng/DMF treatment. Being aware of the selectivity/specificity issues with the commercially available mouse Nrf2 antibodies [40, 41], we have opted to look at the downstream Phase II proteins. We are working to develop more adequate anti-Nrf2 antibodies and the results are pending. Furthermore, future plans are to perform brain cell specific purification that would also further assist us in the quest to identify the unique Nrf2-dependent activated cellular pathway following Ginseng/Nrf2 treatment.
In summary, we presented that pretreatment with Ginseng or DMF could confer robust and long-lasting endogenous cellular neuroprotective efficacy in the context of cerebral ischemia after HI by Nrf2 mechanisms involving anti-oxidative and anti-inflammatory response and the attenuation of reactive gliosis in astrocyte and microglia. Either Ginseng or DMF implied identical preventive efficacy and Nrf2–dependent mechanisms at the given dose. Using transgenic Nrf2−/− mouse model, we also provided evidences highlighting the critical role of Nrf2 pathway in ischemia under HI, enriching our acknowledgement of the natural properties of Nrf2 in endogenous protective system in healthy and neurological conditions that may open a new window to utilize these endogenous neuroprotection mechanisms in neurological diseases including ischemia. These findings indicated that oral consumption with Ginseng or DMF potentially have similar benefit in high risk population or recurrent cerebral ischemia patients. Further understanding of the roles of respective cellular Nrf2 in cerebral ischemia may contribute to the future development of effective therapeutic and preventive approaches in ischemia-associated brain diseases.
Supplementary Material
Supplemental Figure 1. Body weight change over 7 days after HI. Pretreatment with Ginseng or DMF did not affect the body weight change compared to vehicle-pretreated controls of both genotypes. In addition, no significant difference was detected between vehicle-pretreated WT and Nrf2−/− groups. n = 7–11 per group.
Supplemental Figure 2. Survival rate over 7 days after HI. No obvious difference was observed among different treatments or between both genotypes. n = 21–26 for 6 h and 24 h, n = 9–13 for day 7.
Highlights:
Ginseng/DMF reduces the exacerbation in infarction, edema and neurological deficits
Ginseng/DMF attenuates Nrf2-dependent reactive gliosis in microglia and astrocyte
Ginseng/DMF likely alleviates Nrf2-dependent glutamate clearance and water transport
Ginseng/DMF decreases the oxidative and inflammatory insults by an Nrf2 mechanism
Ginseng/DMF displays a long-lasting efficacy, but lacks under Nrf2 deficiency
Acknowledgements
This study was supported by the National Institutes of Health grants (R01AT007429 and R01NS046400, S.D.) and the American Heart Association Postdoctoral Fellowship (16POST31220032, L.L.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosure Statement
The authors declare no conflicts of interests.
References
- 1.Xing C, Arai K, Lo EH, and Hommel M, Pathophysiologic cascades in ischemic stroke. Int J Stroke, 2012. 7(5): p. 378–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dirnagl U, Simon RP, and Hallenbeck JM, Ischemic tolerance and endogenous neuroprotection. Trends Neurosci, 2003. 26(5): p. 248–54. [DOI] [PubMed] [Google Scholar]
- 3.Bosetti F, Koenig JI, Ayata C, Back SA, Becker K, Broderick JP, et al. , Translational Stroke Research: Vision and Opportunities. Stroke, 2017. 48(9): p. 2632–2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar H, Kim IS, More SV, Kim BW, and Choi DK, Natural product-derived pharmacological modulators of Nrf2/ARE pathway for chronic diseases. Nat Prod Rep, 2014. 31(1): p. 109–39. [DOI] [PubMed] [Google Scholar]
- 5.Wang B, Cao W, Biswal S, and Doré S, Carbon monoxide-activated Nrf2 pathway leads to protection against permanent focal cerebral ischemia. Stroke, 2011. 42(9): p. 2605–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leonardo CC and Doré S, Dietary flavonoids are neuroprotective through Nrf2-coordinated induction of endogenous cytoprotective proteins. Nutr Neurosci, 2011. 14(5): p. 226–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keleku-Lukwete N, Suzuki M, and Yamamoto M, An Overview of the Advantages of KEAP1-NRF2 System Activation During Inflammatory Disease Treatment. Antioxid Redox Signal, 2017. [DOI] [PubMed]
- 8.Tonelli C, Chio IIC, and Tuveson DA, Transcriptional Regulation by Nrf2. Antioxid Redox Signal, 2017. [DOI] [PMC free article] [PubMed]
- 9.Kerins MJ and Ooi A, The Roles of NRF2 in Modulating Cellular Iron Homeostasis. Antioxid Redox Signal, 2017. [DOI] [PMC free article] [PubMed]
- 10.Zhao X, Sun G, Zhang J, Ting SM, Gonzales N, and Aronowski J, Dimethyl Fumarate Protects Brain From Damage Produced by Intracerebral Hemorrhage by Mechanism Involving Nrf2. Stroke, 2015. 46(7): p. 1923–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brennan MS, Patel H, Allaire N, Thai A, Cullen P, Ryan S, et al. , Pharmacodynamics of Dimethyl Fumarate Are Tissue Specific and Involve NRF2-Dependent and -Independent Mechanisms. Antioxid Redox Signal, 2016. 24(18): p. 1058–71. [DOI] [PubMed] [Google Scholar]
- 12.Rastogi V, Santiago-Moreno J, and Doré S, Ginseng: a promising neuroprotective strategy in stroke. Front Cell Neurosci, 2014. 8: p. 457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee SM, Bae BS, Park HW, Ahn NG, Cho BG, Cho YL, et al. , Characterization of Korean Red Ginseng (Panax ginseng Meyer): History, preparation method, and chemical composition. J Ginseng Res, 2015. 39(4): p. 384–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen H, Assmann JC, Krenz A, Rahman M, Grimm M, Karsten CM, et al. , Hydroxycarboxylic acid receptor 2 mediates dimethyl fumarate’s protective effect in EAE. J Clin Invest, 2014. 124(5): p. 2188–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.European Medicines Agency. Summary of product characteristics: Skilarence 30 mg gastro-resistant tablets, Skilarence 120 mg gastro-resistant tablets p. 2017.
- 16.Li S, Hafeez A, Noorulla F, Geng X, Shao G, Ren C, et al. , Preconditioning in neuroprotection: From hypoxia to ischemia. Prog Neurobiol, 2017. 157: p. 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dirnagl U, Becker K, and Meisel A, Preconditioning and tolerance against cerebral ischaemia: from experimental strategies to clinical use. Lancet Neurol, 2009. 8(4): p. 398–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gidday JM, Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci, 2006. 7(6): p. 437–48. [DOI] [PubMed] [Google Scholar]
- 19.Kuzuya T, Hoshida S, Yamashita N, Fuji H, Oe H, Hori M, et al. , Delayed effects of sublethal ischemia on the acquisition of tolerance to ischemia. Circ Res, 1993. 72(6): p. 1293–9. [DOI] [PubMed] [Google Scholar]
- 20.Hausenloy DJ and Yellon DM, Ischaemic conditioning and reperfusion injury. Nat Rev Cardiol, 2016. 13(4): p. 193–209. [DOI] [PubMed] [Google Scholar]
- 21.Sofroniew MV, Astrocyte barriers to neurotoxic inflammation. Nat Rev Neurosci, 2015. 16(5): p. 249–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambertsen KL, Biber K, and Finsen B, Inflammatory cytokines in experimental and human stroke. J Cereb Blood Flow Metab, 2012. 32(9): p. 1677–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hambardzumyan D, Gutmann DH, and Kettenmann H, The role of microglia and macrophages in glioma maintenance and progression. Nat Neurosci, 2016. 19(1): p. 20–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burda JE and Sofroniew MV, Reactive gliosis and the multicellular response to CNS damage and disease. Neuron, 2014. 81(2): p. 229–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sofroniew MV and Vinters HV, Astrocytes: biology and pathology. Acta Neuropathol, 2010. 119(1): p. 7–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pekny M, Pekna M, Messing A, Steinhauser C, Lee JM, Parpura V, et al. , Astrocytes: a central element in neurological diseases. Acta Neuropathol, 2016. 131(3): p. 323–45. [DOI] [PubMed] [Google Scholar]
- 27.Koh HS, Chang CY, Jeon SB, Yoon HJ, Ahn YH, Kim HS, et al. , The HIF-1/glial TIM-3 axis controls inflammation-associated brain damage under hypoxia. Nat Commun, 2015. 6: p. 6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baranova O, Miranda LF, Pichiule P, Dragatsis I, Johnson RS, and Chavez JC, Neuron-specific inactivation of the hypoxia inducible factor 1 alpha increases brain injury in a mouse model of transient focal cerebral ischemia. J Neurosci, 2007. 27(23): p. 6320–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim J, Chung SY, Park S, Park JH, Byun S, Hwang M, et al. , Enhancing effect of HT008–1 on cognitive function and quality of life in cognitively declined healthy adults: a randomized, double-blind, placebo-controlled, trial. Pharmacol Biochem Behav, 2008. 90(4): p. 517–24. [DOI] [PubMed] [Google Scholar]
- 30.Kim YT, Yi YJ, Kim MY, Bu Y, Jin ZH, Choi H, et al. , Neuroprotection and enhancement of spatial memory by herbal mixture HT008–1 in rat global brain ischemia model. Am J Chin Med, 2008. 36(2): p. 287–99. [DOI] [PubMed] [Google Scholar]
- 31.Lastres-Becker I, Garcia-Yague AJ, Scannevin RH, Casarejos MJ, Kugler S, Rabano A, et al. , Repurposing the NRF2 Activator Dimethyl Fumarate as Therapy Against Synucleinopathy in Parkinson’s Disease. Antioxid Redox Signal, 2016. 25(2): p. 61–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parodi B, Rossi S, Morando S, Cordano C, Bragoni A, Motta C, et al. , Fumarates modulate microglia activation through a novel HCAR2 signaling pathway and rescue synaptic dysregulation in inflamed CNS. Acta Neuropathol, 2015. 130(2): p. 279–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang J, Li Y, Tang Y, Tang G, Yang GY, and Wang Y, CXCR4 antagonist AMD3100 protects blood-brain barrier integrity and reduces inflammatory response after focal ischemia in mice. Stroke, 2013. 44(1): p. 190–7. [DOI] [PubMed] [Google Scholar]
- 34.Munakata M, Shirakawa H, Nagayasu K, Miyanohara J, Miyake T, Nakagawa T, et al. , Transient receptor potential canonical 3 inhibitor Pyr3 improves outcomes and attenuates astrogliosis after intracerebral hemorrhage in mice. Stroke, 2013. 44(7): p. 1981–7. [DOI] [PubMed] [Google Scholar]
- 35.Glushakov AV, Robbins SW, Bracy CL, Narumiya S, and Doré S, Prostaglandin F2alpha FP receptor antagonist improves outcomes after experimental traumatic brain injury. J Neuroinflammation, 2013. 10: p. 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clark W, Gunion-Rinker L, Lessov N, and Hazel K, Citicoline treatment for experimental intracerebral hemorrhage in mice. Stroke, 1998. 29(10): p. 2136–40. [DOI] [PubMed] [Google Scholar]
- 37.Singh N, Ma B, Leonardo CC, Ahmad AS, Narumiya S, and Doré S, Role of PGE(2) EP1 receptor in intracerebral hemorrhage-induced brain injury. Neurotox Res, 2013. 24(4): p. 549–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu L, Vollmer MK, Fernandez VM, Dweik Y, Kim H, and Doré S, Korean Red Ginseng Pretreatment Protects Against Long-Term Sensorimotor Deficits After Ischemic Stroke Likely Through Nrf2. Front Cell Neurosci, 2018. 12: p. 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehta SL, Pandi G, and Vemuganti R, Circular RNA Expression Profiles Alter Significantly in Mouse Brain After Transient Focal Ischemia. Stroke, 2017. 48(9): p. 2541–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kemmerer ZA, Ader NR, Mulroy SS, and Eggler AL, Comparison of human Nrf2 antibodies: A tale of two proteins. Toxicol Lett, 2015. 238(2): p. 83–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lau A, Tian W, Whitman SA, and Zhang DD, The predicted molecular weight of Nrf2: it is what it is not. Antioxid Redox Signal, 2013. 18(1): p. 91–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tu J, Zhang X, Zhu Y, Dai Y, Li N, Yang F, et al. , Cell-Permeable Peptide Targeting the Nrf2-Keap1 Interaction: A Potential Novel Therapy for Global Cerebral Ischemia. J Neurosci, 2015. 35(44): p. 14727–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vargas MR and Johnson JA, The Nrf2-ARE cytoprotective pathway in astrocytes. Expert Rev Mol Med, 2009. 11: p. e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moskowitz MA, Lo EH, and Iadecola C, The science of stroke: mechanisms in search of treatments. Neuron, 2010. 67(2): p. 181–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bandera E, Botteri M, Minelli C, Sutton A, Abrams KR, and Latronico N, Cerebral blood flow threshold of ischemic penumbra and infarct core in acute ischemic stroke: a systematic review. Stroke, 2006. 37(5): p. 1334–9. [DOI] [PubMed] [Google Scholar]
- 46.Brennan MS, Matos MF, Li B, Hronowski X, Gao B, Juhasz P, et al. , Dimethyl fumarate and monoethyl fumarate exhibit differential effects on KEAP1, NRF2 activation, and glutathione depletion in vitro. PLoS One, 2015. 10(3): p. e0120254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Palace J, Neuroprotection and repair. J Neurol Sci, 2008. 265(1–2): p. 21–5. [DOI] [PubMed] [Google Scholar]
- 48.Zhang F, Wang S, Zhang M, Weng Z, Li P, Gan Y, et al. , Pharmacological induction of heme oxygenase-1 by a triterpenoid protects neurons against ischemic injury. Stroke, 2012. 43(5): p. 1390–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shah ZA, Nada SE, and Doré S, Heme oxygenase 1, beneficial role in permanent ischemic stroke and in Gingko biloba (EGb 761) neuroprotection. Neuroscience, 2011. 180: p. 248–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao Z, Zhu Q, Zhang Y, Zhao Y, Cai L, Shields CB, et al. , Reciprocal modulation between microglia and astrocyte in reactive gliosis following the CNS injury. Mol Neurobiol, 2013. 48(3): p. 690–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Priller J, Flugel A, Wehner T, Boentert M, Haas CA, Prinz M, et al. , Targeting gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. Nat Med, 2001. 7(12): p. 1356–61. [DOI] [PubMed] [Google Scholar]
- 52.Hickey WF and Kimura H, Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science, 1988. 239(4837): p. 290–2. [DOI] [PubMed] [Google Scholar]
- 53.Ajami B, Bennett JL, Krieger C, Tetzlaff W, and Rossi FM, Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci, 2007. 10(12): p. 1538–43. [DOI] [PubMed] [Google Scholar]
- 54.Iadecola C and Anrather J, Stroke research at a crossroad: asking the brain for directions. Nat Neurosci, 2011. 14(11): p. 1363–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rossi DJ, Brady JD, and Mohr C, Astrocyte metabolism and signaling during brain ischemia. Nat Neurosci, 2007. 10(11): p. 1377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jeitner TM, Battaile K, and Cooper AJ, Critical Evaluation of the Changes in Glutamine Synthetase Activity in Models of Cerebral Stroke. Neurochem Res, 2015. 40(12): p. 2544–56. [DOI] [PubMed] [Google Scholar]
- 57.Vella J, Zammit C, Di Giovanni G, Muscat R, and Valentino M, The central role of aquaporins in the pathophysiology of ischemic stroke. Front Cell Neurosci, 2015. 9: p. 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zador Z, Stiver S, Wang V, and Manley GT, Role of aquaporin-4 in cerebral edema and stroke. Handb Exp Pharmacol, 2009(190): p. 159–70. [DOI] [PMC free article] [PubMed]
- 59.Shin JA, Choi JH, Choi YH, and Park EM, Conserved aquaporin 4 levels associated with reduction of brain edema are mediated by estrogen in the ischemic brain after experimental stroke. Biochim Biophys Acta, 2011. 1812(9): p. 1154–63. [DOI] [PubMed] [Google Scholar]
- 60.Papadopoulos MC and Verkman AS, Aquaporin water channels in the nervous system. Nat Rev Neurosci, 2013. 14(4): p. 265–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Verkman AS, Anderson MO, and Papadopoulos MC, Aquaporins: important but elusive drug targets. Nat Rev Drug Discov, 2014. 13(4): p. 259–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nagelhus EA and Ottersen OP, Physiological roles of aquaporin-4 in brain. Physiol Rev, 2013. 93(4): p. 154362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hu X, Liou AK, Leak RK, Xu M, An C, Suenaga J, et al. , Neurobiology of microglial action in CNS injuries: receptor-mediated signaling mechanisms and functional roles. Prog Neurobiol, 2014. 119–120: p. 60–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim JY, Kim N, and Yenari MA, Mechanisms and potential therapeutic applications of microglial activation after brain injury. CNS Neurosci Ther, 2015. 21(4): p. 309–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Body weight change over 7 days after HI. Pretreatment with Ginseng or DMF did not affect the body weight change compared to vehicle-pretreated controls of both genotypes. In addition, no significant difference was detected between vehicle-pretreated WT and Nrf2−/− groups. n = 7–11 per group.
Supplemental Figure 2. Survival rate over 7 days after HI. No obvious difference was observed among different treatments or between both genotypes. n = 21–26 for 6 h and 24 h, n = 9–13 for day 7.


