Abstract
Objectives
The abuse of benzodiazepines and Z-drugs reduces the quality of life of millions of addicted people worldwide. They cannot be discontinued abruptly due to harmful withdrawal symptoms. Detoxification is usually based on replacement of short/middle acting benzodiazepines or Z-drugs by diazepam and tapering the dose over time. In order to enhance patient adherence to an individual withdrawal plan, suitable diazepam dosage forms have to be available. Hard capsules containing an exact and uniform dose could be used for the relief of symptoms caused by altering the plasma level and overcoming psychogenic stress from the dose reduction.
Methods
This work demonstrates that capsules with a content of diazepam ranging from 2.125mg to 0.492 mg (dose decreasing always by 15%) cannot be easily prepared by standard mortar technology in a pharmacy. To meet mass and content uniformity European Pharmacopoeia criteria, capsules were prepared by improved technology based on the preparation of binary blends of calcium phosphate anhydrous and diazepam in descending concentrations in a high-speed mixer (time 30 s) and densification of about 10% during filling of the capsules.
Results
All batches (n=20) prepared by improved technology met the requirement for content uniformity compared with only nine batches prepared by standard mortar blender technology. Based on the process capability index, none of the samples prepared by standard technology fitted pharmacopeia limits at the statistically acceptable level. On the other hand, all batches prepared by improved technology exhibited acceptable process capability index.
Conclusions
We have shown that at least 99.73% of batches prepared by our improved technology would meet the pharmacopoeia limits for content uniformity and are suitable for treatment of this type of addiction.
Keywords: Benzodiazepines, Detoxification, Dose Tapering, Withdrawal, Capsules, Weight Uniformity, Content Uniformity
Introduction
Addiction to benzodiazepines (BZD) or Z-drugs (ATC group N05CF) is one of the most frequent drug abuses. Before their addictiveness was known, they replaced highly hazardous barbiturates1 for the treatment of conditions such as anxiety, generalised anxiety, panic disorders, insomnia, alcohol addiction and epilepsy seizure stages, particularly status epilepticus.2 The development of drug tolerance during treatment also contributes to the addiction.
According to many physicians, addiction to these drugs overcomes opiate addiction.3 Chronic use leads to heavy and somatic addiction which reduces the quality of life, impairs social functioning and mental health and reduces labour intensity.4 They are widely prescribed and used by millions of people worldwide, afflicting up to a third of the current population.5 In adolescents, the lifetime prevalence of their use without prescription ranged from 2% to 15% in 24 EU member states in 2011.6
BZD and Z-drugs can rarely be discontinued abruptly (cold turkey). Withdrawal symptoms include restlessness, insomnia, tachycardia, tremor, dysphoria, anger or aggression, paranoid ideas, agoraphobia, panic attacks, deterioration of spatial vision, memory and attention disorders and may even result in death.7
The first step in detoxification is usually the replacement of short or middle acting BZD or Z-drugs by diazepam in the equivalent dose8 and the creation of a gradually decreasing withdrawal schedule (tapering). The blood concentrations are then more balanced and the nervous system can be stabilised. Diazepam is then slowly eliminated even when administered only once or twice a day.9
Adjuvant therapy is recommended during detoxification. To prevent epileptic seizures, carbamazepine or valproate may be used.10 It is also possible to use suitable non-BZD anxiolytics (eg, buspirone)10 and sedative antidepressants (eg, trazodone or mirtazapine) in sleep disorders.11 12 Antipsychotics (eg, cyamemazine) may sometimes be administered.13 For tachycardia adjustment, beta-blockers are recommended (eg, propranolol).14
For enhancing patient adherence to an individual withdrawal plan, the availability of dosage forms with an accurate dose of diazepam is essential. A relatively exact dose of diazepam could be obtained from an individually prepared or commercial solution. However, precise dosing by classic oral drops is difficult and ambulant treatment depends on the accuracy and self-control of the patient.15 Furthermore, in the case of sometimes recommended self-made suspensions from diazepam tablets, inadequate dosing may result in kinetic instability.16 Even more controversial is the use of marketed oral tablets; tablet breaking is not an effective method for obtaining an exact dose. Halving and quartering of tablets will not allow for adequate uniform dose tapering (eg, by 10–25%). Moreover, the patient is distressed by the visible reduction of the drug dose.17
These disadvantages could be overcome by individually prepared hard capsules containing the exact diazepam dose individually decreasing over time. Adjuvant drugs can also be included in these capsules. The patient would get professionally prepared medicine with good content uniformity (UC) according to an individual tapering plan. Moreover, he/she would not be additionally stressed by the obvious reduction in drug dose because identical looking capsules are administered until complete withdrawal.
The aim of this study was to optimise manual filling of tapering diazepam concentrations into hard capsules meeting the uniformity of mass (UM) and UC criteria set by the European Pharmacopoeia (Ph Eur). The experimental study was performed in three phases. First, a suitable indifferent filler was selected based on the results of mass uniformity of drug-free capsules. Second, the appropriate mixing conditions (high-speed mixer) were found according to the results of UC of the diazepam mixture. Finally, hard capsules were filled with diazepam blend prepared by a technique based on the results obtained in the previous parts. Final capsules were tested for UM and UC and the results were compared with hard capsules prepared in the pharmacy by traditional mortar technology without any special mixer (mortar blender) and under any special conditions. The verification was performed using Cpk, the process capability index.
Materials and methods
Materials
Lactose monohydrate, milled (Pharmatose 200M, particle size 35 µm; DFE Pharma, Goch, Germany), spray-dried lactose (Pharmatose DCL 11, particle size 59 µm; DMW International GmbH, Veghel, Netherlands) and calcium phosphate anhydrous (Di-Cafos A60, particle size 61 µm; Chemische Fabrik Budenheim, Budenheim, Germany) were used as indifferent fillers. Diazepam (Dr Kulich Pharma, Hradec Králové, Czech Republic) was used as the active substance. All materials were of Ph Eur quality. Drug-free fillers or their mixtures with diazepam were filled into size 0 hard gelatine capsules (Dr Kulich Pharma).
Evaluation of flow properties of fillers
Fillers in amount of 100 g were evaluated according to Ph Eur 8: flowability (Medipo, Brno, Czech Republic, diameter of outflow opening 25.0±0.01 mm), bulk and tapped density, Hausner ratio and Carr’s index (SVM 102, Erweka, Heusenstammen, Germany) and angle of repose (fixed glass funnel) were measured.
Fillers in amount of 100% (100% means a volume in mL corresponding to the declared internal volume of the hard gelatine capsule multiplied by the number of capsules, 0.67 mL x 30 cps, i.e. 20.1 mL), 107.5% (21.6 mL) and 110% or 115% (21.1 mL or 23.1 mL) were manually filled into 30 hard gelatine capsules (manual 30 aperture capsule filling machine Heros, Olomouc, Czech Republic). Each capsule was weighed and the average value and relative standard deviation (RSD) were calculated.
Content uniformity (UC) of blends
The mixture (200 g) of selected filler amount (110%/115%) and diazepam (in amount corresponding to 2.125 mg per capsule) was mixed (Tefal Kaleo 676210, France) for 30 s, 60 s and 180 s. Tefal Kaleo is a high-speed mixer with a bottom four-blade impeller and a product bowl volume of 2 L. The mixing speed was 400 rpm. The samples (around 1 g precisely weighted) were taken from 10 different sites of powder mixture and the diazepam content was determined. A YL 9100 Young Lin high-performance liquid chromatography (HPLC) instrument with quarternary pump, autosampler (100 µL loop volume) and DAD detector set at 254 nm was used for analysis. A Venusil XBP C18 (3 µm; 4.6×150 mm) column kept at a temperature of 35°C was used. The mobile phase consisted of 0.02 mol/L phosphoric acid-methanol-acetonitrile in a proportion of 30:35:35 (v/v) with a flow rate of 1 mL/min.
A basic standard solution for the calibration curve of diazepam in methanol-water (20:80, v/v) was prepared at a concentration of 250 µg/mL. Calibration standards were prepared by transferring corresponding amounts of indifferent filler to a 100 mL volumetric flask with the appropriate volume of basic standard solution and filled with methanol-water mixture. After 10 min of sonication, 2 mL of the sample were transferred to an Eppendorf tube and centrifuged for 5 min at 5000 rpm. 50 µL of supernatant was assessed by HPLC. A calibration curve was produced with standards of the final concentrations set between 1 and 50 µg/mL.
Preparation and evaluation of capsules with diazepam
Based on previous results, 30 hard gelatine capsules (size 0) were prepared at each level of diazepam content (2.125–0.492 mg diazepam per capsule, regular 15% decrease through 10 dose levels) by improved technology (mixer blend, IT) as follows. The selected filler in amount of filling 110%/115% was mixed with diazepam in the Tefal Kaleo 676210 high-speed mixer for 30 s. Every sample was prepared twice (batches 1 and 2). All capsules had an exactly defined position in the filling machine (figure 1) and were analysed for UM and UC. The assay of diazepam content was performed by the HPLC method described above. The content of the capsule was transferred to a 100 mL flask and filled up with a methanol:water (20:80, v/v) mixture. After 10 min of sonication, 2 mL of the sample was centrifuged at 5000 rpm for 5 min. 50 µL of supernatant was assayed by HPLC.
Figure 1.
Sampling pattern.
In parallel, capsules with the same concentrations of diazepam were prepared in a pharmacy using a mortar blender (pharmacy technology, PT) as follows. Weighed diazepam was put into the cylinder and the selected filler was added to obtain the required volume of 20.1 mL. The non-homogenous mixture was placed in the mortar, usually homogenised and filled on the same device into 30 capsules (size 0).
UM and UC evaluation was performed according to Ph Eur 2.9.5 and 2.9.6, respectively. To evaluate the reproducibility of these parameters the process capability index Cpk was used, which statistically evaluates the capability of a process to produce output (ie, capsule mass or diazepam content) within specified limits. It can be expressed as:
where USL/LSL is the upper or lower specification limit, is the estimated mean and is the SD. It is clear that, by considering only the minimum value, Cpk evaluates the variation as well as centring of the process. If the result is not less than 1.0, at least 99.73% of batches produced by this process will pass the applied limits.18
Results
Evaluation of flow properties of fillers and UM of drug-free capsules
Table 1 shows the comparison of the flow properties of the fillers used (Pharmatose 200M, Pharmatose DCL11 and Di-Cafos A60). As expected, the results obtained confirmed that Di-Cafos A60 had better flow properties. UM (n=30) of hard capsules (size 0) with different amount of fillers (100%, 107.5% and 115%) was expressed by the RSD value.
Table 1.
Evaluation of flow properties of fillers, uniformity of mass of drug-free capsules
| Filler | F (s) | Α (°) | CI (%) | HR | BD (g/cm3) | TD (g/cm3) | Amount of filling (%) | RSD (%) of UM | |
| I | II | ||||||||
| Pharmatose 200M | N/A | N/A | 28.57 | 1.40 | 0.50 | 0.70 | 100.0 107.5 115.0 |
1.40 1.76 0.99 |
1.71 1.52 1.28 |
| Pharmatose DCL11 |
3.6 | 30 | 13.33 | 1.15 | 0.65 | 0.75 | 100.0 107.5 115 |
4.65 1.43 2.35 |
2.41 4.68 2.00 |
| DiCafos A60 |
3.3 | 28 | 12.34 | 1.14 | 1.35 | 1.54 | 100.0 107.5 110.0* |
4.10 1.43 1.21 |
1.94 2.84 1.04 |
A, angle of repose; BD, bulk density; CI, Carr’s index; F, flowability; HR, Hausner ratio; N/A, not applicable; NS, normal saline; TD, tapped density; UM, mass uniformity; I and II, batches.
*Lower amount of filling was used because of better flow properties of Dicafos.
Content uniformity (UC) of blends
Figure 2 shows the range of diazepam content (n=10) and respective RSD in mixtures containing either Pharmatose 200M (in 115% amount) or Di-Cafos A60 (in 110% amount) and the diazepam in an amount corresponding to 2.125 mg per capsule (size 0) during high-speed mixing at 30, 60 and 180 s time points.
Figure 2.
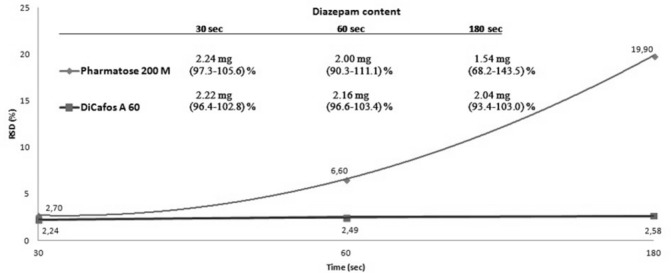
Content uniformity of diazepam/filler blends at different mixing times.
Preparation and evaluation of capsules with diazepam
The average weight (±RSD, n=30) and average diazepam content (±RSD, n=10 samples according to the sampling pattern) for hard capsules (filler Di-Cafos A60) with decreasing diazepam content (2.125–0.492 mg per capsule; regular 15% decrease) together with the Cpk19 determined for both pharmacopoeia limits pursuant to articles 2.9.5 and 2.9.6 (ie, UM −±7.5% and ±15%, UC −±15% and ±25%) were found.
Average masses of capsules prepared by PT were in the range 97.85–99.28% with RSD values between 1.07% and 6.26% while, for capsules prepared by IT, averages were in the range 94.03–102.22% with RSDs between 1.00% and 3.10%.
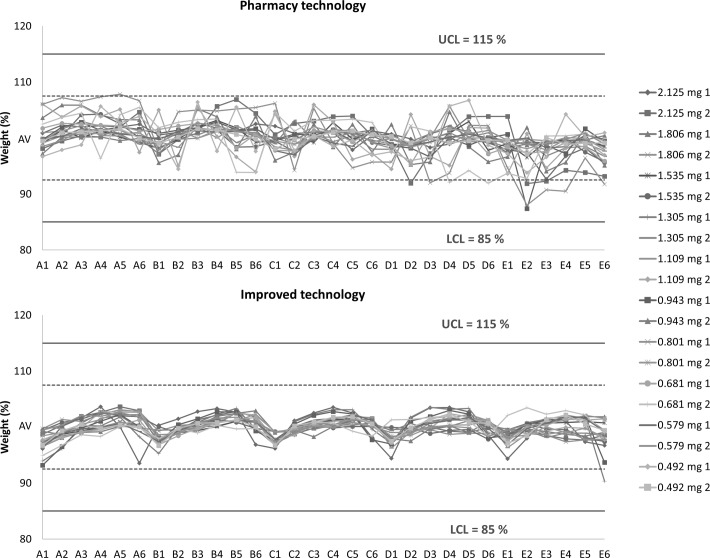
Graphical expression of the individual values of UM in the form of a correlation diagram is shown in figure 3 for all prepared batches. Both the 85–115% limits (solid line) and the 92.5–107.5% limits (dotted line) are displayed. The limits are derived from the average weight of samples in accordance with Ph Eur 2.9.5. Capsules prepared by PT and those prepared by IT (amount of filling 110%, time of mixing 30 s) are compared.
Figure 3.
Correlation diagrams of individual values of uniformity of mass comparing capsules filled by standard mortar technology and capsules prepared by improved technology. LCL, lower control limit; UCL, upper control limit; AV, average weight.
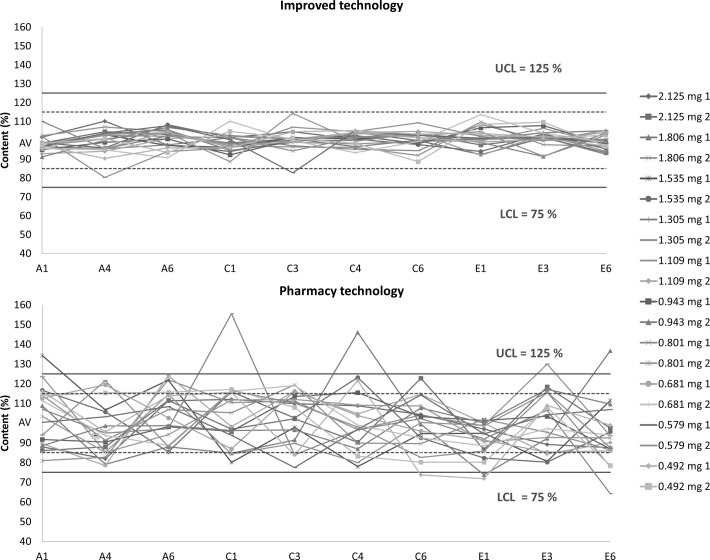
The average content of capsules prepared by PT was in the range 66.72–106.02% with RSDs between 9.30% and 23.99% and the average content of capsules prepared by IT was in the range 89.48–106.17% with RSDs between 2.53% and 8.37%. Graphical expression of individual values of UC in the form of a correlation diagram is shown in figure 4. Limits from 75% to 125% (solid line) and 85% to 115% (dotted line) are shown. The limits are derived from the average diazepam content in samples according to Ph Eur 2.9.6. Hard capsules prepared by PT and by IT are compared.
Figure 4.
Correlation diagrams of individual values of content uniformity comparing capsules filled by standard mortar technology and capsules prepared by improved technology. LCL, lower control limit; UCL, upper control limit; AV, average content.
Discussion
The aim of this study was to optimise the manual filling of different concentrations of diazepam into hard capsules so they would meet UM and UC criteria set by Ph Eur. To pass UM, only two out of 20 samples can deviate from the average mass by more than 7.5% and not more than 15%. To pass the UC criteria, only one of 10 samples can deviate from the average content by more than 15% but less than 25%. If two or three samples fall outside the 15% deviation range, another 20 samples are tested. To pass, only three out of 30 samples can deviate by more than 15% but still less than 25%. The tapering diazepam concentration (2.125–0.492 mg per capsule, 10 strengths, stepped down by 15%) could be used in the final phase of detoxification until complete withdrawal. These very low doses of diazepam cannot be precisely obtained by multiple breaking of commercially available tablets.
Selection of suitable filler
From RSD values (table 1), it can be concluded that the UM of drug-free capsules improved with increasing volume of filling (surplus 10% or 15%) in the case of all tested fillers. This can be explained by complete expelling of air from the filling mixture resulting in more uniform filling of capsules, as proved by the decrease in RSD.20 The lowest RSD values were found with Pharmatose 200M (0.99% and 1.28%) and Di-Cafos A60 (1.21% and 1.04%) used in 15% and 10% surplus, respectively whereas, for Pharmatose DC11, the RSD values were approximately two times higher. The flow properties of the fillers did not influence the results of UM.
UC of blends
For determination of optimal mixing time, Pharmatose 200M and Di-Cafos were selected due to the lowest RSD values for UM. It is obvious (figure 2) that a mixing time of 30 s was optimal for homogeneity of both blends. RSD values, obtained after 30 s of mixing, slightly favoured Di-Cafos A60 over Pharmatose DC11 (RSD 2.24% and 2.70%, respectively). Moreover, a time-dependent increase in RSD confirmed a strong over-blending phenomenon in the case of Pharmatose DC11. The diazepam content in the samples reduced with the mixing time. The phenomenon of mixing-induced reduction of drug content and its uniformity in low strength blend has also been reported in the literature.20 The best results for UC were obtained with Di-Cafos A60 and a mixing time of 30 s, so these conditions were selected as optimal for further experiments.
Evaluation of capsules with diazepam: UM
All batches of prepared capsules met the requirements set by Ph Eur 2.9.5. The Cpk for the 92.5–107.5% limit found seven samples of capsules prepared by PT (total Cpk range 0.40–2.43) and three samples prepared by IT (total Cpk range 0.79–2.51) to be unfit. On the other hand, for the 85–115% limit, all samples except one prepared by PT were found to be acceptable. Cpk values ranged between 0.80–4.86 (PT) and 1.59–5.02 (IT). Generally, IT samples exhibited Cpk values superior to PT samples for both limits. This proves that using a filler in surplus positively affected UM.
Figure 3 clearly shows that the IT samples always exhibited significantly lower deviation of individual weights. For both technologies, but more significantly for IT, the trend of lower weights for capsules located laterally on the filling plate (indexed 1 and 6) was observed. On the other hand, higher weights were typical for capsules situated in the medial positions (indexed 3 and 4). These results confirmed the presence of a typical human error (ie, the tendency to overfill capsules in the middle of a plate in comparison with capsules at the edges).
Evaluation of capsules with diazepam: UC
All samples from both batches prepared by IT fulfilled the requirement according to Ph Eur 2.9.6 for UC compared with only nine samples prepared by PT. Furthermore, based on calculated Cpk, none of the PT samples met the Ph Eur 85–115% limit (Cpk range 0.21–0.54) or even the 75–125% limit (Cpk range 0.35–0.90). On the other hand, all samples prepared by IT had acceptable Cpk values between 1.00 and 3.29 for a wider limit, proving that at least 99.73% of batches prepared by such technology would not exceed it. Cpk values for the 85–115% limit were between 0.60 and 1.97. The second smallest Cpk (0.69; diazepam dose 0.801 mg and 0.681 g per capsule) guarantees with high probability that no more than three out of 10 samples would exceed this limit. Only the smallest Cpk value (0.6; diazepam dose 0.579 mg per capsule) cannot fulfil the requirement for this limit at a statistically significant level. Generally, these low values were found in batches with a low diazepam content where achieving a good UC is usually very demanding.
As can be seen from figure 4, the IT samples always exhibited significantly lower deviation in individual content. There is no visible trend in the content of diazepam in capsules obtained from medial (indexed 1 and 6) and lateral (indexed 3 and 4) positions due to the fact that RSD values of UC were much higher than those of UM.
Conclusion
This work shows that hard capsules with a low diazepam content cannot be prepared in a pharmacy by standard mortar blender technology. However, capsules meeting pharmacopoeia criteria at a statistically significant level can be prepared by IT using a suitable filler, more intense blending, optimal blending time and densification of the filling material during filling. However, if this technology is to be employed, the subsequent doses of diazepam in capsules intended for withdrawal treatment cannot be reduced by less than 15%, which is the lowest reduction achievable at a statistically significant level.
Key messages.
What is already known on this subject
The long-term use of benzodiazepines and Z2 hypnotics causes a strong addiction and immediate withdrawal is not safe.
For its long half time, diazepam in gradually decreasing doses is used as a substitute until full withdrawal
Suitable dosage forms with a very low diazepam concentration for enhancing patient adherence to an individual withdrawal plan are not available.
What this study adds
In the pharmacy it is possible to prepare hard capsules with a wide range and accurate diazepam content covering long-term detoxification.
Using a suitable technology ensures the preparationof highly homogeneous capsules with a precise diazepam content.
Footnotes
Contributors: AF: coordinator of investigation, summarisation of obtained data, preparing of manuscript; KK: preparing of the hard capsules by improved technology, preparing of manuscript; JE: statistical evaluation; JM: evaluation of hard capsules prepared by improved technology; DV: evaluation of hard capsules prepared by pharmacy technology; JS: preparing of the hard capsules by pharmacy technology; RO: development of analytical HPLC method
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Correction notice: This paper has been amended since it was published Online First. The author first names and last names were transposed. This has now been corrected and we would like to apologise to the authors for this error.
References
- 1. Ashton H. The diagnosis and management of benzodiazepine dependence. Curr Opin Psychiatry 2005;18:249–55. 10.1097/01.yco.0000165594.60434.84 [DOI] [PubMed] [Google Scholar]
- 2. Smith DE, Wesson DR. The benzodiazepines: current standards for medicalpractice. Dordrecht: Springer Netherlands, 1985:159–63. [Google Scholar]
- 3. Tyrer P. Benzodiazepine substitution for dependent patients—going with the flow. Addiction 2010;105:1875–6. 10.1111/j.1360-0443.2010.03067.x [DOI] [PubMed] [Google Scholar]
- 4. Lugoboni F, Mirijello A, Faccini M, et al. . Quality of life in a cohort of high-dose benzodiazepine dependent patients. Drug Alcohol Depend 2014;142:105–9. 10.1016/j.drugalcdep.2014.06.020 [DOI] [PubMed] [Google Scholar]
- 5. Szelenberger W, Soldatos C. Sleep disorders in psychiatric practice. World Psychiatry 2005;4:186–90. [PMC free article] [PubMed] [Google Scholar]
- 6. Hibell B, Guttormsson U, Ahlström S, et al. ; Summary 2011 ESPAD report: substance use among students in 36 European countries. European Monitoring Centre for Drugs and Drug Addiction. Luxembourg: Publications Office of the European Union, 2012. [Google Scholar]
- 7. Salzman C. Benzodiazepine dependence, toxicity, and abuse: a task force report of theAmerican Psychiatric Association. Am J Psychiatry 1991;148:151–2. [DOI] [PubMed] [Google Scholar]
- 8. Brett J, Murnion B. Management of benzodiazepine misuse and dependence. Aust Prescr 2015;38:152–5. 10.18773/austprescr.2015.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ashton H. How to withdraw from benzodiazepines after long-term use. http://www.benzo.org.uk/manual/bzcha02.htm
- 10. Rickels K, Case WG, Schweizer E, et al. . Benzodiazepine dependence: management of discontinuation. Psychopharmacol Bull 1990;26:63–8. [PubMed] [Google Scholar]
- 11. Ansseau M, De Roeck J. Trazodone in benzodiazepine dependence. J Clin Psychiatry 1993;54:189–91. [PubMed] [Google Scholar]
- 12. Lader M. Benzodiazepine harm: how can it be reduced? Br J Clin Pharmacol 2014;77:295–301. 10.1111/j.1365-2125.2012.04418.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Benyamina A, Naassila M, Bourin M. Potential role of cortical 5-HT(2A) receptors in the anxiolytic action of cyamemazine in benzodiazepine withdrawal. Psychiatry Res 2012;198:307–12. 10.1016/j.psychres.2012.01.009 [DOI] [PubMed] [Google Scholar]
- 14. Cantopher T, Olivieri S, Cleave N, et al. . Chronic benzodiazepine dependence. A comparative study of abrupt withdrawal under propranolol cover versus gradual withdrawal. Br J Psychiatry 1990;156:406–11. 10.1192/bjp.156.3.406 [DOI] [PubMed] [Google Scholar]
- 15. Wening K, Breitkreutz J. Oral drug delivery in personalized medicine: unmet needs and novel approaches. Int J Pharm 2011;404:1–9. 10.1016/j.ijpharm.2010.11.001 [DOI] [PubMed] [Google Scholar]
- 16. Newton DW, Schulman SG, Becker CH. Limitations of compounding diazepam suspensions from tablets. Am J Hosp Pharm 1976;33:450–2. [PubMed] [Google Scholar]
- 17. Franc A. Možnosti individualizované přípravy léků v oblasti léčby lékových závislostí. dny farmaceutické péče V – individualizovaná péče o pacienta. (In Czech). Czechia: VFU Brno, 25-26 September 2015. https://faf.vfu.cz/FaF/informace-o-fakulte/aktuality/dny-farmaceuticke-pece-2015.pdf [Google Scholar]
- 18. Kornchankul W, Hamed E, Parikh NH, et al. . Effect of drug proportion and mixing time on the content uniformity of a low dose drug in a high shear mixer. Pharmazie 2002;57:49–53. [PubMed] [Google Scholar]
- 19. Muselík J, Franc A, Doležel P, et al. . Influence of process parameters on content uniformity of a low dose active pharmaceutical ingredient in a tablet formulation according to GMP. Acta Pharm 2014;64:355–67. 10.2478/acph-2014-0022 [DOI] [PubMed] [Google Scholar]
- 20. Jales STL, Soares-Sobrinho JL, Nunes LCC, et al. . Formulation technology of a probiotic (Zymomonas mobilis) in gelatinous capsules. Lat Am J Pharm 2007;26:553–7. [Google Scholar]