Abstract
Objectives:
The objective of this study was to investigate the dimensions of the osseous lumbar intervertebral foramen (IVF) regarding a sample without any clinical indication of spine pathology and, additionally, survey possible correlations of these measurements with clinical characteristics of the individuals.
Materials and Methods:
CT images of spine-related asymptomatic individuals were examined on parasagittal and oblique projections for the evaluation of cranial foramen width (CrFW), caudal foramen width (CaFW), vertebral height (VH) and foraminal height (FH) in accordance with gender, age, height, weight, body mass index (BMI) and vertebral level.
Results:
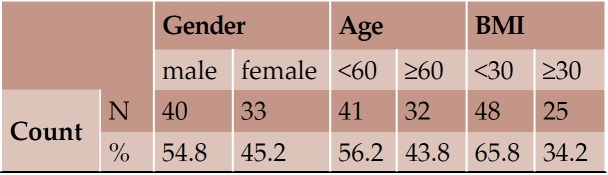
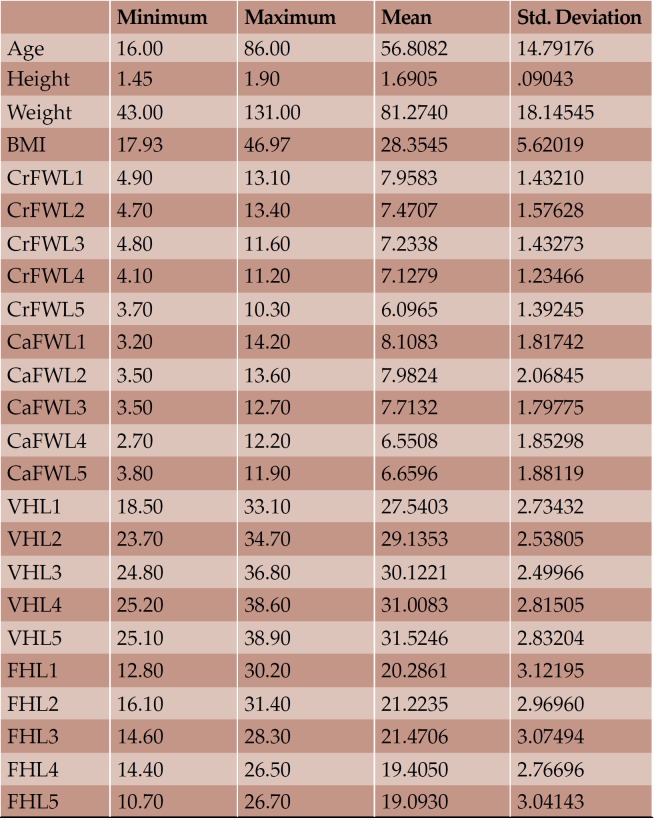
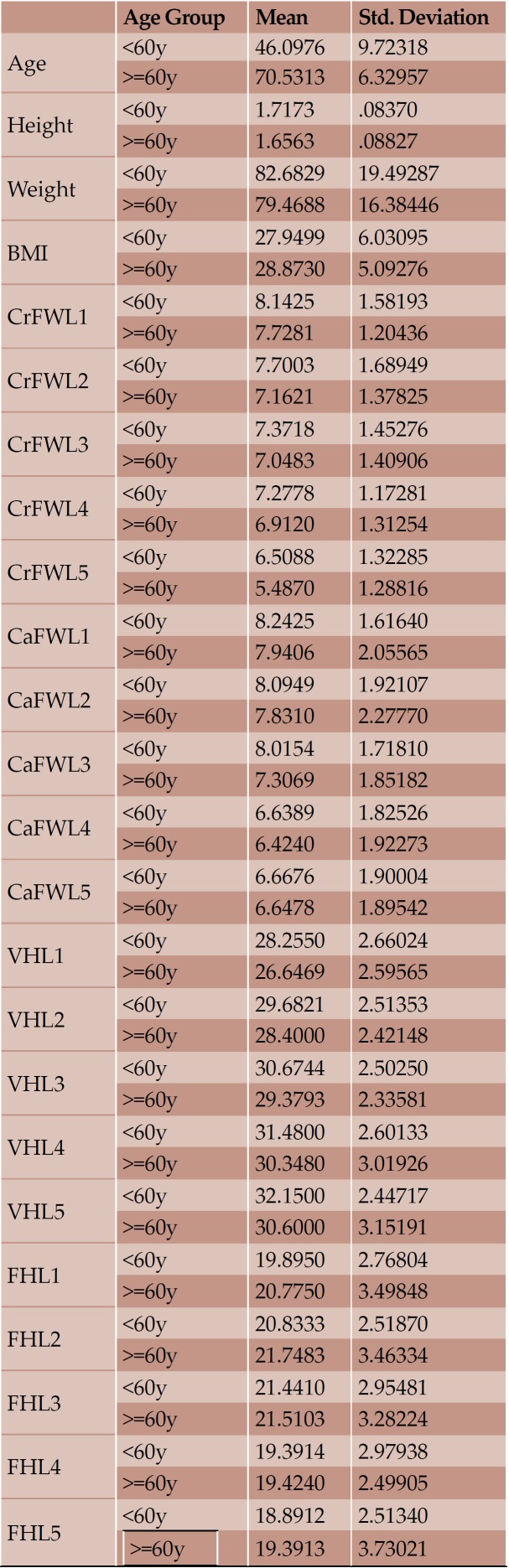
Overall, CT images of 73 individuals, 40 men and 33 women, with mean age 56.81 (± 14.79) years, mean height 1.69 (± 0.09) meters, mean weight 81.27 (± 18.14) kilograms and mean BMI 28.35 (± 5.62) were included. The maximum mean FW was the CaFWL1 and the minimum the CrFWL5, with values of 8.11 and 6.01 mm, respectively. Height and weight were presented as significantly bigger in men than women; however, women had bigger lumbar IVF values and no significant width measurement for IVF was morphoobserved at any level for either sex. Age showed a negative impact on the elderly by reducing height and the majority of FW measurements. Statistically important differences in accordance with BMI were not seen.
Conclusions:
Data comparison with previous studies is ambiguous due to methodological differences and possible populational variations and they reveal just a glimpse of the in vivo lumbar IVF. Our data could have clinical application on lumbar spine interventions.
Keywords:intervertebral foramen, lumbar, osseous, in vivo, radiologic, neurosurgery, spine surgery, foraminoplasty.
INTRODUCTION
It is known that the lumbar spine consists of five moveable vertebrae and it is characterized by a combination of these strong vertebrae linked by joint capsules, flexible ligaments, large muscles, and highly sensitive nerves. Lumbar spine is designed to be strong, to protect the spinal cord and the spinal nerve roots, and simultaneously flexible, providing mobility in many different planes of motion. A significant entity of the lumbar spine is the lumbar intervertebral foramen (IVF). It is the pathway between the spinal canal and the periphery. This foramen is special due to its boundaries consisting of two movable joints: ventral intervertebral joint and dorsal zygapophysial joint. Generally, it is formed by the inferior part of the pedicle, the posteroinferior part of the vertebral body, the posterior articular lamina and the posterolateral aspect of the intervertebral disc (1).
Alterations of the dimensions of the lumbar IVF can hypothetically play a significant role in the pathophysiology of low back pain, a leading cause of disability in modern societies (2, 3). Additionally, the form of the lumbar IVF has been correlated with intervertebral disc pathology (4). Apart from disc pathology, the osseous borders of the foramen undergo ongoing remodeling and degenerative changes, which may lead to foraminal stenosis. The anatomy of the lumbar IVF has been well described, including recent reviews of the literature (5-8). Measurements of the osseous lumbar IVF are widely published and they mostly include cadaveric specimens (9). A few radiological studies referring to osteometric data of the osseous IVF have been conducted (10-15). However, so far, no radiologic study has fully investigated the static dimensions of the human osseous lumbar IVF of spine-related healthy population in vivo.
Thus, the main purpose of this study was to investigate the anatomic measurements of the osseous lumbar IVF referring to individuals without symptoms of spine-related disease and examine possible correlations of these measurements with clinical features including gender, age, height, weight, vertebral height and level, and body mass index (BMI). As an extension, the results of this study could be used for better application of the Efficacy of Transforaminal Endoscopic Spine System (TESSYS) Technique in treating lumbar disc herniation, especially during the foraminoplasty.
MATERIALS AND METHOD
Seventy three individuals (40 men and 33 women) with mean age 56.81 (± 14.79) years, mean height 1.69 (± 0.09) meters, mean weight 81.27 (± 18.14) kilograms and mean BMI 28.35 (± 5.62) were included in our study (Tables 1 and 2).
The selected individuals were patients who underwent CT scanning of their chest and/or abdomen. All of them were of Caucasian origin. The chosen individuals had a CT scanning of their lumbar spine for reasons concerning pathological entities of the gastrointestinal and urinary tract, which did not affect in any morphological way the lumbar vertebrae. The exclusion criteria involved individuals with any indication of spine-related disease or spine-related pain, which was retrieved from their medical records. Furthermore, at the beginning of the study, two individuals were excluded due to gross morphological abnormalities which were visible on their CT images.
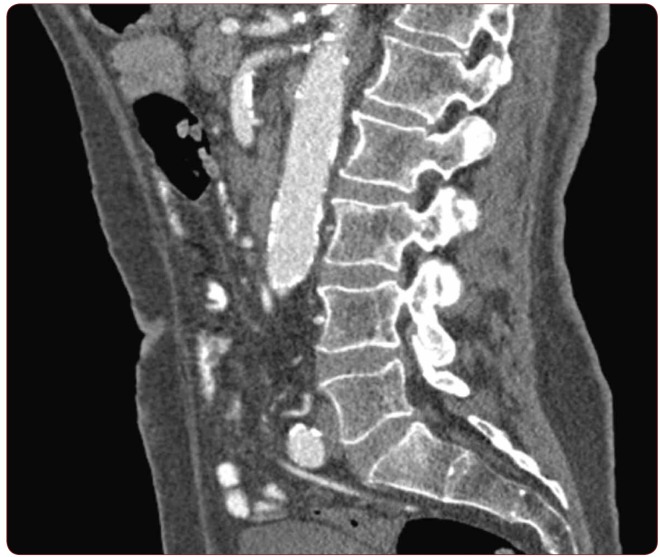
From the CT images of the chosen individuals, only the lumbar segments were used for data referring to the levels L1-L5. Four parameters were examined on the CT images, including cranial foramen width (CrFW), caudal foramen width (CaFW), vertebral height (VH) and foraminal height (FH) in accordance with gender, age, height, weight, BMI and vertebral level. The lumbar spine segments were imaged on parasagittal and oblique projections (Figure 1). CT scan was done in all the patients with 128 slice MDCT Siemens scanner. The routine protocol for scanning abdomen and/or pelvis was typically done with 120 kVp and an average of 250 mAs. The slice thickness for the all acquisitions was 6 mm with pitch equal to 0.6. After data acquisition, reconstruction series were typically being created with slice thickness of 1 mm and the B20f smooth kernel.
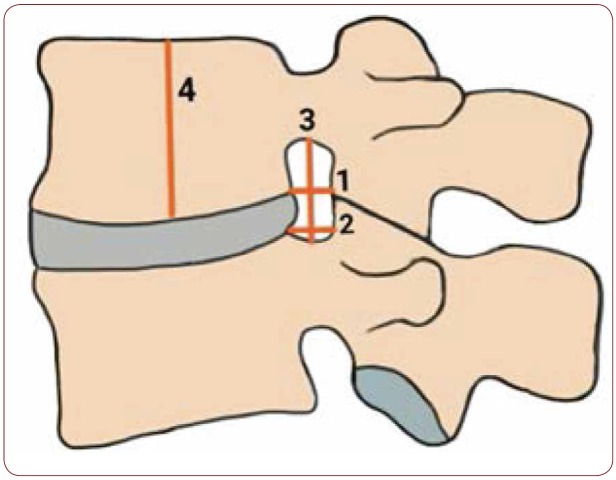
Concerning the data extraction, the measurements were electronically estimated using the radiologic software installed for processing and displaying medical images. The CrFW was defined as the shortest horizontal distance between superior posterior edges of vertebral body to corresponding superior articular process at level of cranial surface of vertebral body; the CaFW was defined as the shortest horizontal distance between inferior posterior edges of vertebral body to corresponding inferior articular process at level of caudal surface of vertebral body; the VH was defined as the height of each vertebra measured in the middle of the vertebral body on the parasagittal and oblique plane; the FH was defined as the distance between the superior and inferior vertebral notch of each IVF on the parasagittal and oblique plane (Figure 2). All measurements were made independently by two investigators; they were repeated twice, on both sides of the spine, and the average was used.
Regarding the data analysis, it was performed with the statistical package SPSS, version 23.00 (SPSS Inc, Chicago, IL). The p-value <0.05 was determined as statistically significant difference level. Continuous variables are expressed as mean ± standard deviation. We used Student’s-t test for equality of means for quantitive- continuous variables of independent samples when comparing the subgroups and Wilcoxon signed-rank test for repeated measurements within the whole sample. The correlation between the variables was studied with the Pearson correlation coefficient.
RESULTS
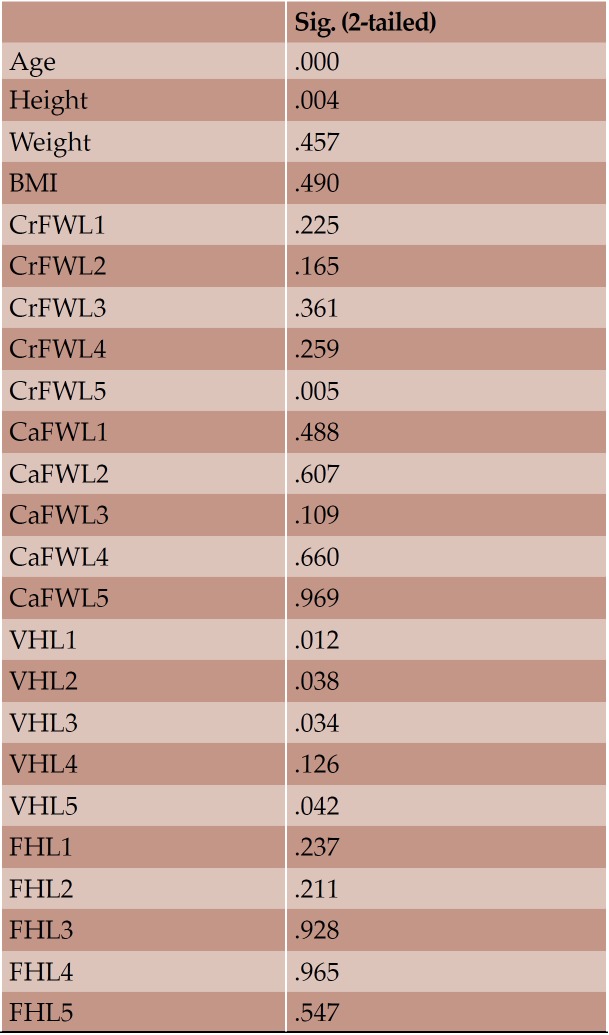
Overall, 73 lumbar spine segments were analyzed using the CT images of the individuals. Referring to the whole sample, the following statistically significant correlations between the variables are observed (see Table 3 in the online version of this article). A positive correlation between height and weight and a negative correlation between height and age is seen. All CrFWs, all VHs and the CaFWL1, CaFWL2, CaFWL3, CaFWL4 present a negative correlation with age. The CrFWs and the CaFWs present a significant positive correlation between and amongst them, except for the correlation of CrFWL1 with CaFWL5, which is not significant. All VHs and FHL1, FHL2 present a positive correlation with height. All VHs, apart from VHL3, exhibit positive correlation with CrFWL4. VHL1, VHL2, VHL3, VHL4 seem to have positive correlation amongst them and with FHL1. The VHL5 presents positive correlation with the rest VHs but not with the FHL1. Additionally, the VHL5 presents positive correlation with the weight, CrFWL5, CaFWL3, CaFWL5. All VHs and FHL1, FHL2, FHL3 have a positive correlation with FHL5. All FHs present positive correlation amongst them. FHL5 presents positive correlation with CrFWL4. FHL4 with CrFWL3 positive correlation is observed. FHL4 presents positive correlation with CrFWL3. FHL3 presents positive correlation with CrFWL2, CrFWL3, CrFWL4. FHL2 shows positive correlation with CrFWL3. FHL1 presents positive correlation with CrFWL1, CrFWL2, CaFWL1. VHL1 presents a positive correlation with CaFWL5. A positive correlation between CrFWL4 and VHL1, VHL2, VHL4, VHL5 is seen.
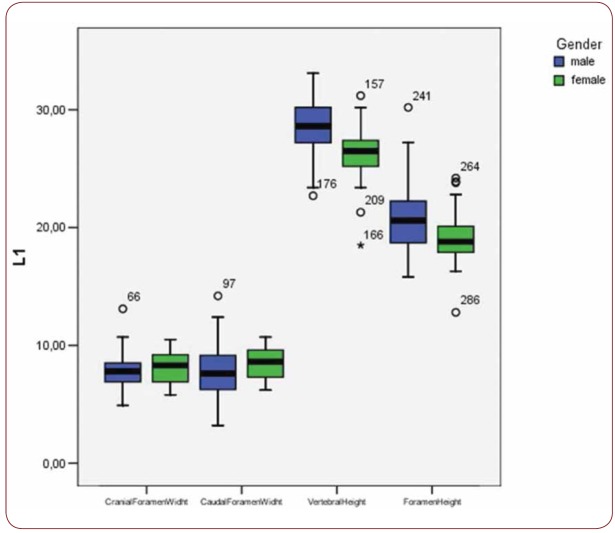
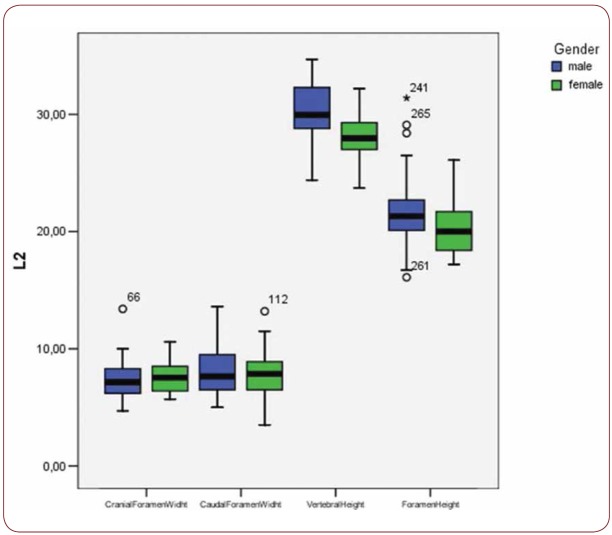
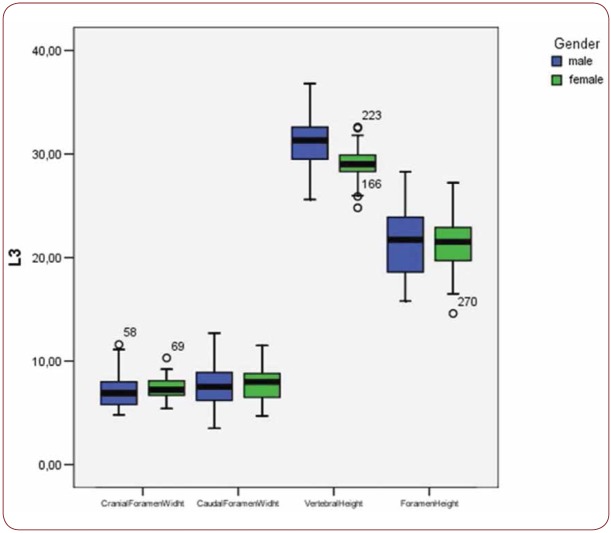
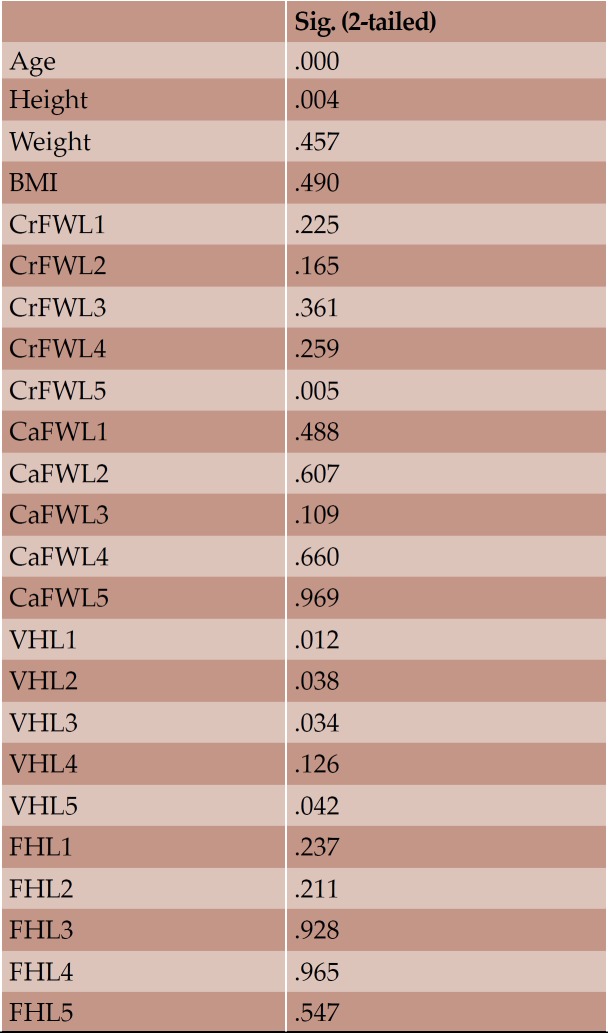
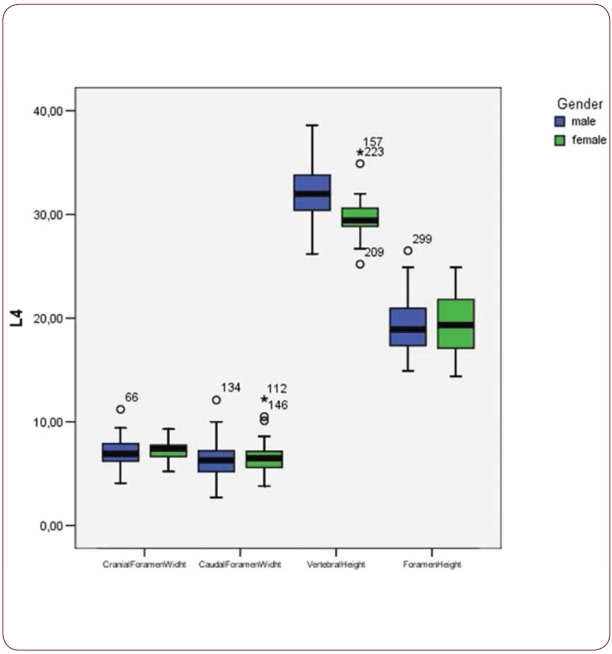
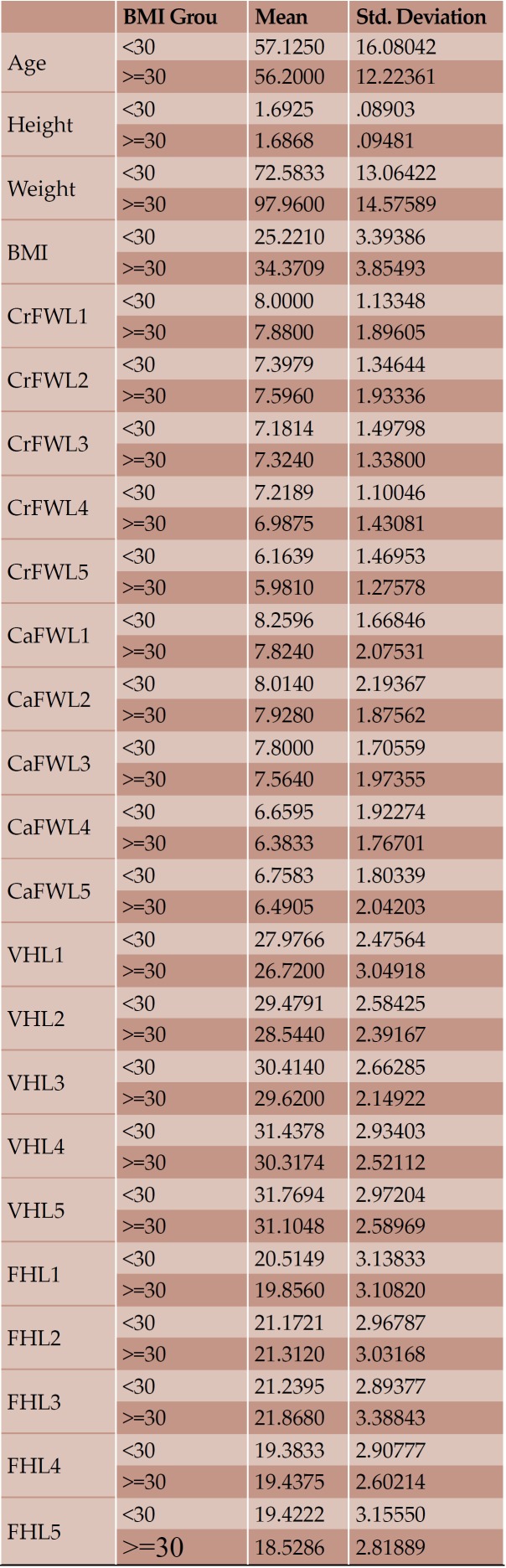
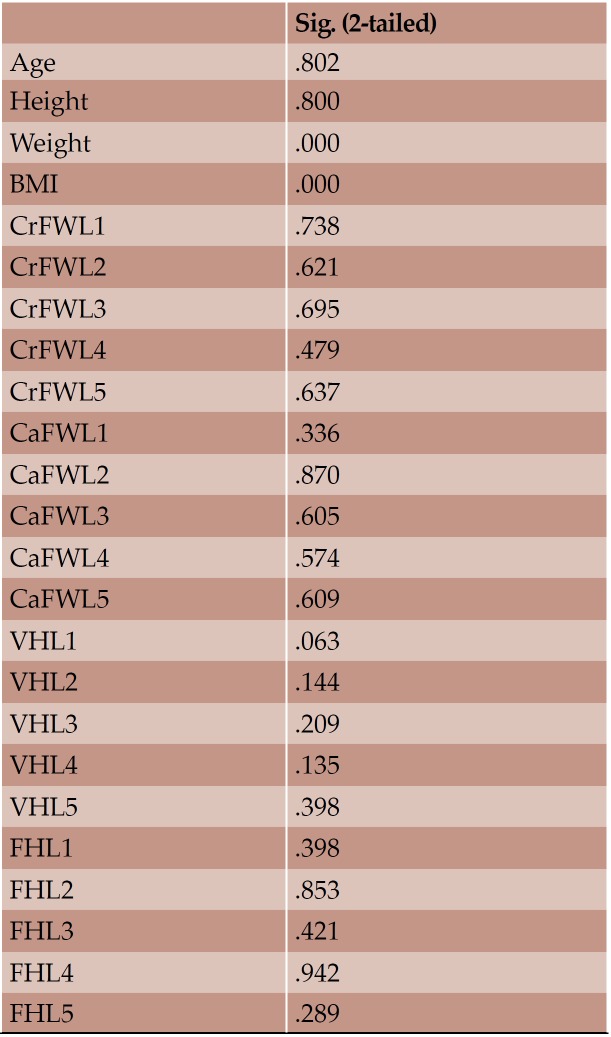
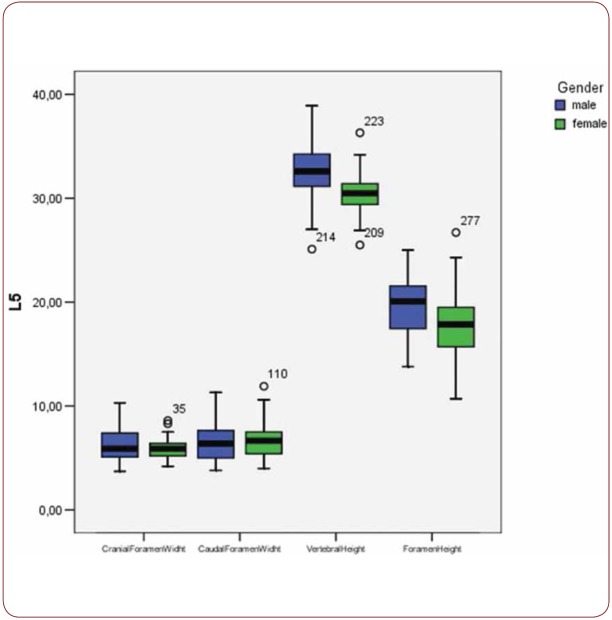
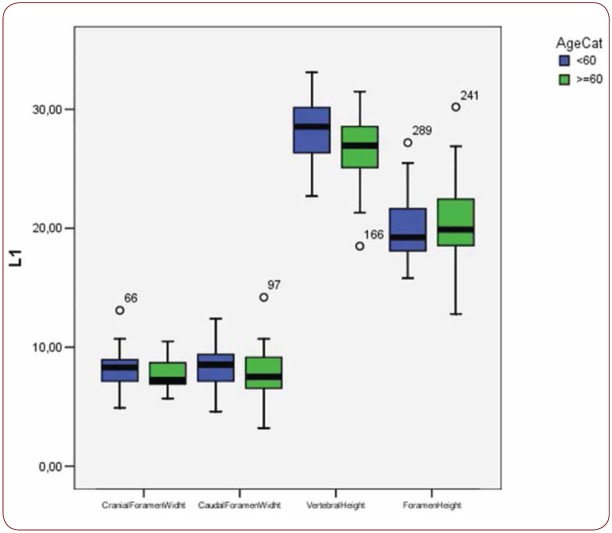
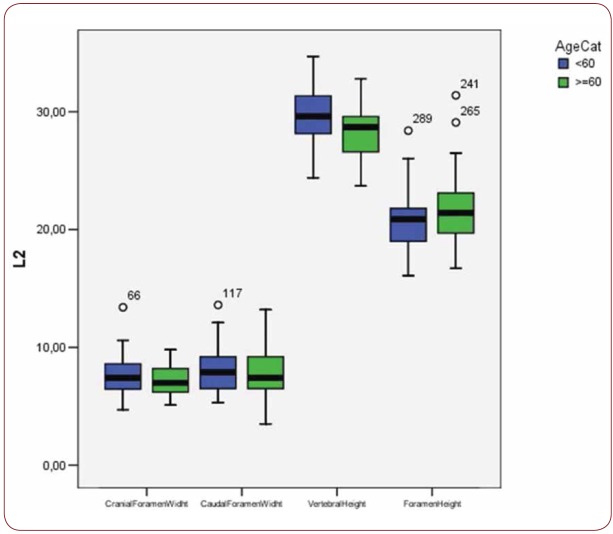
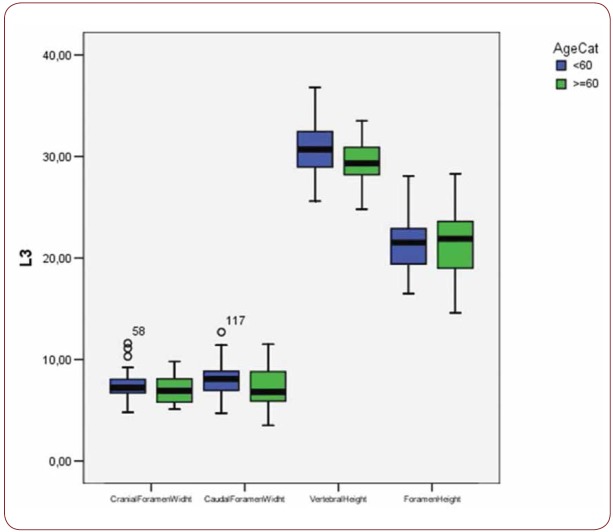
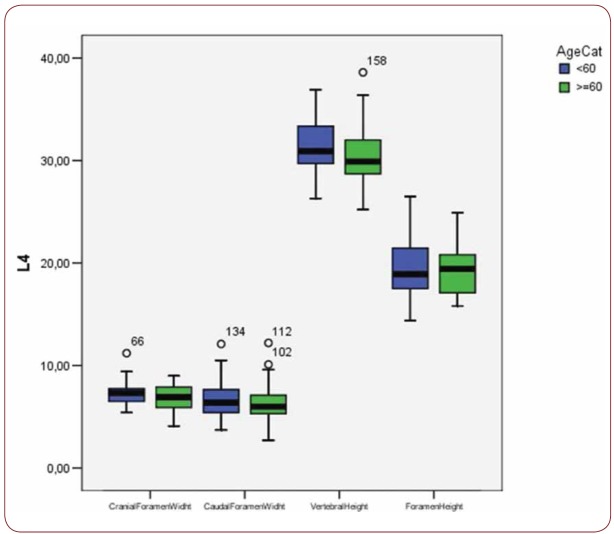
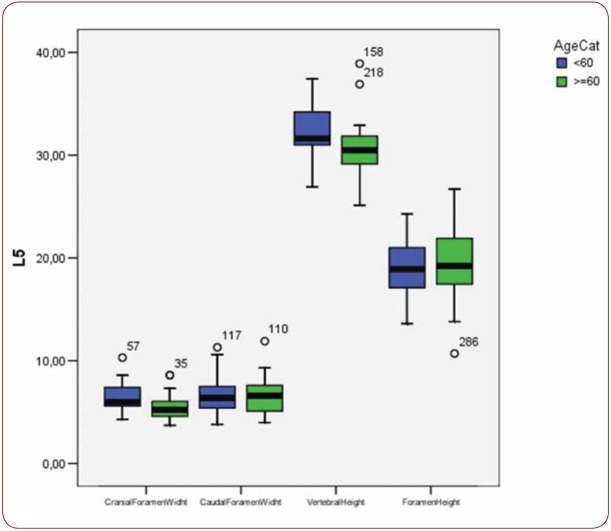
With regard to the comparison between groups, using the t-test for equality of mean for independent samples, we observe that the height and weight is presented as significantly bigger in men than women. Furthermore, the VHL1, VHL2, VHL3, VHL4, VHL5, FHL1, FHL2, FHL5 are bigger in men as compared to women (Tables 4, 5 and Figures 3-7). Height seems to be bigger in subjects aged under 60. In this age group, CrFWL5 is wider and VHL1, VHL2, VHL3, VHL5 is also bigger (Tables 6, 7 and Figures 8-12). The BMI groups do not present major differences in the means (Tables 8 and 9).
DISCUSSION
The borders of each lumbar IVF contain spinal nerve roots, sinuvertebral nerves, spinal arteries, internal and external venous plexuses, lymphatic vessels, and fatty areolar network which fills the foramen (26). In particular, these borders are formed, anteriorly: from a posterolateral part of the inferior vertebra, the posterolateral part of the intervertebral disc, and the posterolateral aspect of the superior vertebra; superiorly: from the inferior vertebral notch of the superior vertebra; posteriorly: from the superior articular process of the inferior vertebra, a part of the facet synovial joint, and the inferior articular process of the superior vertebra; inferiorly: from the superior vertebral notch of the inferior vertebra (6, 1).
In our study, generally, the examined variables seem to present a positive correlation between and amongst them, resulting in the harmonic architecture of the non-pathologic spine. Few correlations with minor clinical significance are observed. The data presented here suggest that age has a negative impact on the height of the elderly due to age-related degenerations and ongoing remodeling. This is also expressed by the majority of CrFWs, CaFWs and VHs of the lumbar vertebrae, which tend to present a smaller value in the individuals aged over sixty years old. CrFWL5 is smaller in those over 60 years old. Height and weight are presented as significantly bigger in men than women. Also, the majority of VHs and FHs are bigger in men. The BMI subgroups do not show major differences in the means because the lumbar spine is a dynamic structure composed of bones, muscles and ligaments, which retains the normal anatomy even in increased axial load. CrFWL1 with CaFWL5 do not appear to have a significant correlation between them due to the fact that they are at the extremities of the lumbar spine and differentiate enough in terms of analogy and allometry. Similarly, the VHL5 presents positive correlation with the rest VHs but not with the FHL1.
Although few radiologic reports on the dimensions of the IVF exist, they do not estimate the in vivo size of the foramen. The majority of the studies examining the IVF dimensions are cadaveric studies. Contrary to Ruhli et al., in a similar study which used cadaveric sample, our data seems to present a correlation between age and height and osseous lumbar IVF size (8). We confirmed similarly that the CrFW was the largest at the L1 level for both sexes, and the CaFW at the L1 level only for women. Also, in the majority of measurements, women showed larger lumbar IVF values than men and no significant width measurement for IVF was observed at any level for either sex. This finding is in accordance with earlier reports on the sex-dependent size of spinal dimensions (17, 18). Humphreys et al. found that cervical IFWs in particular are larger in healthy individuals than in symptomatic persons (19). Furthermore, the size of the lumbar IVF was described by Stephens et al. to be biggest at L5/S1 (4). In the present study, the values of the osseous IFW at level L5 were smaller than at L1. In another recent cadaveric study, no statistically important differences referring to the descriptive data of both sexes were found (6). Nevertheless, statistically, important positive correlation between the vertebral height and the foraminal width was observed, especially for men. Additionally, Hong et al. mentioned a significant correlation between disc degeneration, nerve root compression, disc height and foraminal width (14). Cinotti et al. reported that narrowing of the disc space significantly reduces the vertical diameter of the foramen but has no significant effects on its sagittal dimensions. In contrast, the sagittal dimensions of the foramen are strictly related to the sagittal diameter of the spinal canal and the pedicle length (20).
Comparative studies using cadaveric specimens have shown the 3DCT to be an unreliable tool to quantify the dimensions of the lumbar foramen (21). Direct osteometric measurements and fresh cadaver studies are still considered the best method to determine spinal dimensions (22, 23). However, due to the dissimilar methods, it is difficult to compare directly the IFW values of the present study with the values published earlier. Furthermore, it is important to mention that the size of the IVF varies between populations and depending on axial loading, time of day and dynamic changes of the spine (24, 15). The measurement of two different dimensions of IFW in the same vertebra is reliable for assessing the overall dimension, and therefore, indirectly the functional capacity, of that particular IVF (20). We used both cranial and caudal foraminal width and also instead of the intervertebral disc height, as it has been measured in various studies, we estimated the foraminal height which including the previous. The intraobserver error of the measurement of IFW has been determined in a previous cadaveric study for the lumbar region as being up to 0.3–0.4 mm (24). The present study could be a case control study using CT or MRI, with an additional group of spine-related symptomatic individuals. However, we wanted to emphasize on the normal osseous dimensions and as a result we estimated CT images of asymptomatic sample. Furthermore, the sample may seem to have heterogenicity as regards to gender, age and BMI, however we wanted to include a broad spectrum of values in our sample while maintaining a minimum statistically sufficient number of individuals per subgroup. The increasing availability of three-dimensional digitizers and morphometry analysis tools will also enable more universal and eligible assessments of the IVF.
One of the major symptoms referred to the lumbar spine and the lumbar IVF is low back pain. The pathophysiology of low back pain is influenced by a wide range of etiologies (3). It has been linked with lumbar disc herniation and spinal stenosis. The narrowing of the maximum FW seems to be the only cause of radiculopathy or spinal stenosis and in a similar way the decrease of the FH is considered insignificant (25). The endoscopic lumbar discectomy is growing in popularity for treating lumbar disc herniation. Among minimally invasive techniques, the transforaminal endoscopic spine system (TESSYS) has become a prevalent therapy for lumbar disc herniation, presenting less trauma and quicker postoperative recovery in comparison with fenestration discectomy (26). With this technique it is possible to use foraminoplasty to operate inside the spinal canal and widen the foramen with different size reamers. Most recent percutaneous endoscopic discectomy techniques are based on the Kambin’s transforaminal approach and especially on the trapezoidal perspective of the working zone. A needle is initially placed through the Kambin’s triangle under fluoroscopic technique and after verification of the level, administration of mild sedation and analgesia, the passage of dilators and the cannula with the endoscope is the following step. Subsequently, the discectomy is performed with graspers. Crucial step of the surgery is the sequential passage of three different size reamers 5.5, 6.5, and 7.5 mm (joimax) (27). Data suggest that radiological measurements including the spinal root and foraminal areas should be obtained before endoscopic discectomy surgery for a safer procedure (28). In the study of Tamrong et al., the average dimension of the calculated largest ellipsoidal cannula that could be placed was 5.83×11.02 mm at L1–2, 6.97×10.78 mm at L2–3, 9.30×10.67 mm at L3–4, 8.84×13.15 mm at L4–5, and 6.61×14.07 mm at L5–S1 (29). Additionally, anatomic considerations for percutaneous lumbar interbody fusion were made by Wimmer et al. and they reported that the maximum safe cannula diameter was 8 mm from L1–L4 and 7 mm from L4–S1 (30). Therefore, the dimensions of the lumbar IVF should be taken into serious consideration when using the TESSYS technique in order to prevent possible tissue damages during foraminoplasty.
CONCLUSION
Morphometric data of the spine can have clinical significance and application considering spine surgery, physical anthropology and even forensics. Spine surgeons and interventional radiologists have to estimate and understand the anatomy of the osseous lumbar IVF and its dimensions. When using the TESSYS technique for the treatment of lumbar disc herniation, it is crucial to know the anatomy of the foramen and especially during foraminoplasty. Cadaveric measurements seem to be abundant in the relative literature. Radiologic studies provide an estimation of the in vivo size, which depends on soft tissue and dynamic components and tends to depict the age related alterations. An improved knowledge of human spinal dimensions could further enlighten our knowledge of the continuous evolution of the spine.
Conflicts of interest: Dr. Kapetanakis is a reference doctor for joimax® GmbH and receives payment for teaching.
Ethical approval: For this type of study, formal consent is not required.
TABLE 1.
Baseline data of the sample
TABLE 2.
Descriptive statistics of the sample
FIGURE 1.
Parasagittal oblique plane CT images of the lumbar spine
FIGURE 2.
Illustration depicting the sagittal parameters of measurement. 1) caudal foraminal width, defined as the distance between inferior posterior edges of vertebral body of the superior vertebra to the corresponding inferior articular process; 2) cranial foraminal width defined as the distance between superior posterior edges of vertebral body of the inferior vertebra to the corresponding superior articular process; 3) foraminal height defined as the distance between the superior and inferior vertebral notch; 4) vertebral height defined as the height of each vertebra measured in the middle of the vertebral body.
FIGURE 3.
Box plot of CrFW, CaFW, VH and FW at L1 level based on gender
FIGURE 4.
Box plot of CrFW, CaFW, VH and FW at L2 level based on gender
FIGURE 5.
Box plot of CrFW, CaFW, VH and FW at L3 level based on gender
TABLE 4.
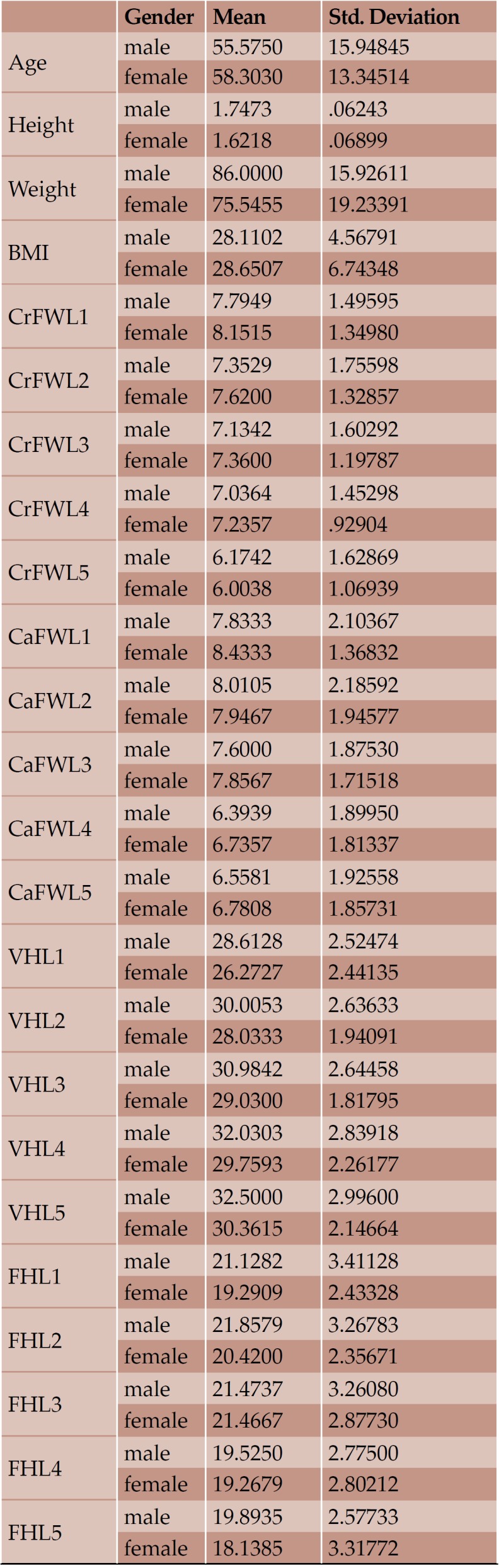
Mean value and standard deviation of each variable according to gender
TABLE 5.
Significance of means based on gender
TABLE 6.
Mean value and standard deviation of the each variable according to age
TABLE 7.
Significance of means based on age
FIGURE 6.
Box plot of CrFW, CaFW, VH and FW at L4 level based on gender
TABLE 8.
Mean value and standard deviation of the each variable according to age
TABLE 9.
Significance of means based on body mass index
FIGURE 7.
Box plot of CrFW, CaFW, VH and FW at L5 level based on gender
FIGURE 8.
Box plot of CrFW, CaFW, VH and FW at L1 level based on age
FIGURE 9.
Box plot of CrFW, CaFW, VH and FW at L2 level based on age
FIGURE 10.
Box plot of CrFW, CaFW, VH and FW at L3 level based on age
FIGURE 11.
Box plot of CrFW, CaFW, VH and FW at L4 level based on age
FIGURE 12.
Box plot of CrFW, CaFW, VH and FW at L5 level based on age
Contributor Information
Grigorios GKASDARIS, Papanikolaou Hospital and Interbalkan Medical Center, Thessaloniki, Greece.
Danai HOURMOUZI, Radiology Department, Interbalkan European Medical Center, Thessaloniki, Greece.
Constantinos CHANIOTAKIS, Spine Department and Deformities, Interbalkan European Medical Center, Thessaloniki, Greece.
Georgios HARITOUDIS, Spine Department and Deformities, Interbalkan European Medical Center, Thessaloniki, Greece.
Mohammad Moein ASHRAFI, Young Researchers and Elites Club, Faculty of Medicine, Islamic Azad University, Yazd Branch, Yazd, Iran.
Dimitrios MOUSELIMIS, School of Medicine, Aristotle University of Thessaloniki, Greece.
Stylianos KAPETANAKIS, Spine Department and Deformities, Interbalkan European Medical Center, Thessaloniki, Greece.
REFERENCES
- 1.Drake R, Vogl W, Mitchell AVM, et al. Gray’s Anatomy for Medical Students. 2nd ed., Churchill Livingstone, New York. 2009; [Google Scholar]
- 2.Gaskill MF, Lukin R, Wiot JG. Lumbar disc disease and stenosis. Radiol Clin North Am. 1991;29:753–764. [PubMed] [Google Scholar]
- 3.Speed C. Low back pain. Br Med J. 2004;328:1119–1121. doi: 10.1136/bmj.328.7448.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stephens MM, Evans JH, O’Brien JP. Lumbar intervertebral foramens. An in vitro study of their shape in relation to intervertebral disc pathology. Spine. 1991;16:525–529. [PubMed] [Google Scholar]
- 5.Gilchrist RV, Slipman CW, Bhagia SM. Anatomy of the intervertebral foramen. Pain Physician. 2002;5:372–378. [PubMed] [Google Scholar]
- 6.Gkasdaris G, Tripsianis G, Kotopoulos K, Kapetanakis S. Clinical anatomy and significance of the thoracic intervertebral foramen: A cadaveric study and review of the literature. J Craniovertebr Junction Spine. 2016;7:228–235. doi: 10.4103/0974-8237.193266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silav G, Arslan M, Comert A, Acar HI, Kahilogullari G, Dolgun H, et al. Relationship of dorsal root ganglion to intervertebral foramen in lumbar region: an anatomical study and review of literature. J Neurosurg Sci. 2016;3:339–344. [PubMed] [Google Scholar]
- 8.Sioutas G, Kapetanakis S. Clinical anatomy and clinical significance of the cervical intervertebral foramen: A review. Folia Morphol (Warsz) 2016;75:143–148. doi: 10.5603/FM.a2015.0096. [DOI] [PubMed] [Google Scholar]
- 9.Rühli FJ, Müntener M, Henneberg M. Human osseous intervertebral foramen width. Am J Phys Anthropol. 2006;129:177–188. doi: 10.1002/ajpa.20263. [DOI] [PubMed] [Google Scholar]
- 10.Kaneko Y, Matsumoto M, Takaishi H, Nishiwaki Y, Momoshima S, Toyama Y. Morphometric analysis of the lumbar intervertebral foramen in patients with degenerative lumbar scoliosis by multidetector-row computed tomography. Eur Spine J. 2012;12:2594–2602. doi: 10.1007/s00586-012-2408-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loch-Wilkinson TJ, Izatt MT, Labrom RD, Askin GN, Pearcy MJ, Adam CJ. Morphometric Analysis of the Thoracic Intervertebral Foramen Osseous Anatomy in Adolescent Idiopathic Scoliosis Using Low-Dose Computed Tomography. Spine Deform. 2016;3:182–192. doi: 10.1016/j.jspd.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Schubiger O, Valavanis A, Hollmann J. Computed tomography of the intervertebral foramen. Neuroradiology. 1984;6:439–444. doi: 10.1007/BF00342678. [DOI] [PubMed] [Google Scholar]
- 13.Schubiger O, Valavanis A. X-ray computed tomography of the intervertebral foramen. Neurochirurgie. 1986;1:58–61. [PubMed] [Google Scholar]
- 14.Sohn HM, You JW, Lee JY. The relationship between disc degeneration and morphologic changes in the intervertebral foramen of the cervical spine: a cadaveric MRI and CT study. J Korean Med Sci. 2004;1:101–106. doi: 10.3346/jkms.2004.19.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhong W, Driscoll SJ, Tsai TY, Wang S, Mao H, Cha TD, et al. In vivo dynamic changes of dimensions in the lumbar intervertebral foramen. Spine J. 2015;7:1653–1659. doi: 10.1016/j.spinee.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Standring S. Gray’s Anatomy: The Anatomical Basis of Clinical Practice. 40th ed., Elsevier, London. 2008; [Google Scholar]
- 7.Hasue M, Kunogi J, Konno S, Kikuchi S. Classification by position of dorsal root ganglia in the lumbosacral region. Spine. 1989;14:1261–1264. doi: 10.1097/00007632-198911000-00021. [DOI] [PubMed] [Google Scholar]
- 18.Kikuchi S, Hasue M, Nishiyama K, Ito T. Anatomic and clinical studies of radicular symptoms. Spine. 1984;9:23–30. doi: 10.1097/00007632-198401000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Humphreys SC, Hodges SD, Patwardhan A, Eck JC, Covington LA, Sartori M. The natural history of the cervical foramen in symptomatic and asymptomatic individuals aged 20–60 years as measured by magnetic resonance imaging. A descriptive approach. Spine. 1998;23:2180–2184. doi: 10.1097/00007632-199810150-00007. [DOI] [PubMed] [Google Scholar]
- 20.Cinotti G, De Santis P, MD, Nofroni I, Postacchini F. Stenosis of Lumbar Intervertebral Foramen. Spine. 2002;27:223–229. doi: 10.1097/00007632-200202010-00002. [DOI] [PubMed] [Google Scholar]
- 21.Smith GA, Aspden RM, Porter RW. Measurement of Vertebral foraminal dimensions using threedimensional computerized tomography. Spine. 1993;18:629–636. doi: 10.1097/00007632-199304000-00016. [DOI] [PubMed] [Google Scholar]
- 22.Krag MH, Weaver DL, Beynnon BD, Haugh LD. Morphometry of the thoracic and lumbar spine related to transpedicular screw placement for surgical spinal fixation. Spine. 1988;13:27–32. doi: 10.1097/00007632-198801000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Scoles PV, Linton AE, Latimer B, Levy ME, Digiovanni BF. Vertebral body and posterior element morphology: the normal spine in middle life. Spine. 1988;13:1082–1086. doi: 10.1097/00007632-198810000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Fujiwara A, An HS, Lim TH, Haughton VM. Morphologic changes in the lumbar intervertebral foramen due to flexionextension, lateral bending, and axial rotation: an in vitro anatomic and biomechanical study. Spine. 2001;26:876–882. doi: 10.1097/00007632-200104150-00010. [DOI] [PubMed] [Google Scholar]
- 25.Rühli FJ, Henneberg M. Clinical perspectives on secular trends of intervertebral foramen diameters in an industrialized European society. Eur Spine J. 2004;13:733–739. doi: 10.1007/s00586-004-0682-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan Z, Ha Y, Yi S, Cao K. Efficacy of Transforaminal Endoscopic Spine System (TESSYS) Technique in Treating Lumbar Disc Herniation. Med Sci Monit. 2016;22:530–539. doi: 10.12659/MSM.894870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kapetanakis S, Charitoudis G, Thomaidis T, Theodosiadis P, Papathanasiou J, Giatroudakis K. Health-related quality of life after transforaminal percutaneous endoscopic discectomy: An analysis according to the level of operation. J Craniovertebr Junction Spine. 2017;1:44–49. doi: 10.4103/0974-8237.199872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guan X, Gu X, Zhang L. Morphometric analysis of the working zone for posterolateral endoscopic lumbar discectomy based on magnetic resonance neurography. J Spinal Disord Tech. 2015;28:78–84. doi: 10.1097/BSD.0000000000000145. [DOI] [PubMed] [Google Scholar]
- 29.Lertudomphonwanit T, Keorochana G, Kraiwattanapong C, Chanplakorn P, Leelapattana P, Wajanavisit W. Anatomic Considerations of Intervertebral Disc Perspective in Lumbar Posterolateral Approach via Kambin’s Triangle: Cadaveric Study. Asian Spine J. 2016;5:821–827. doi: 10.4184/asj.2016.10.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wimmer C, Maurer H. Anatomic consideration for lumbar percutaneous interbody fusion. Clin Orthop Relat Res. 2000;379:236–241. doi: 10.1097/00003086-200010000-00028. [DOI] [PubMed] [Google Scholar]