Abstract
Background
There are limited data describing the care outcome of youth living with HIV in Asia. We assessed attrition and treatment outcomes among youths with behaviourly acquired HIV (BIY) and adolescents with perinatally acquired HIV (PIY) who initiated antiretroviral treatment (ART) through the National AIDS Program (NAP) in Thailand.
Methods
People living with HIV aged 10–24 years who initiated antiretroviral therapy (ART) from 2008 to 2013 through the Thai NAP and who were followed up until 2014 were included in the analysis. We assessed youths initiating ART: BIY aged 15–19 years (BIY1) and BIY aged 20–24 (BIY2) compared against PIY aged 10–14 years. Attrition rates (mortality and loss to follow-up [LTFU]) were calculated and potential associations were assessed using Cox regression. Logistic regression was used to assess associations with treatment failure.
Results
Of 11,954 individuals, 9909 (83%) were BIY with a median follow-up of 2.1 years and 17% were PIY with 4.2 years of follow-up. The median baseline CD4 cell count in BIY was higher (190 vs 154 cells/mm3) compared to PIY. Mortality rates were not significantly different among PIY (2.5 per 100 person years [PY], BIY1 3.1/100 PY and BIY2 2.9/100 PY, P=0.46). Compared to PIY with a crude LTFU rate of 2.9/100 PY, LTFU was higher in BIY1 (13.9/100 PY) and BIY2 (9.5/100 PY), P<0.001 and P<0.001, respectively. At 1 year after initiating ART, 16% experienced virological failure (viral load above 1000 copies/mL). Combined treatment failure and LTFU rates at 1 year after ART were higher among BIY1 (45.0%) and BIY2 (34.4%) compared to PIY (29.9%), P<0.001 and 0.001, respectively.
Conclusion
Youth with behaviourally acquired HIV aged 15–19 years had poorer retention rates than older BIY and PIY. Targeted interventions for youth are urgently needed to improve overall treatment outcomes.
Keywords: youth, attrition, National AIDS program, Thailand
Introduction
In 2016, 4 million young people (youths) aged 15–24 years were living with HIV, and those between 15 and 19 years of age made up 53% of this group globally [1]. In that year, approximately 9700 young people aged 10–19 years were living with HIV in Thailand, and 3100 adolescents aged 15–24 had newly acquired HIV [1]. The numbers of newly diagnosed children with perinatally acquired HIV have declined in recent years owing to effective prevention of vertical transmission programmes [2]. Most children with perinatally acquired HIV are on treatment and ageing into adolescence. A minority, however, have slow disease progression, and were in need of ART initiation only after 10 years of age. Overall, most youth living with HIV in the world are behaviourally infected with high incidence rates observed amongst men who have sex with men [3-5],[6] and females [7-9].
Poor treatment retention is common among youths with behaviourally acquired HIV [10]. Recent studies from Africa reported that youth aged 15–24 years had higher loss to follow-up rates (LTFU) compared to those aged >24 years [11,12]. In addition, from a total of 924 youth living with HIV aged 15–21 years accessing outpatient care and treatment clinics in Kisumu, Kenya, more than half were documented as LTFU, and 139 (26%) were LTFU immediately after enrolment in care. The overall incidence rate of LTFU was 52.9 per 100 person years [9], but mechanisms to document death was lacking. This leads to an overestimation of LTFU rates and an underestimation of mortality rates [13]. To date, there is limited information on treatment outcomes of Asian youth that can inform healthcare policy and service delivery within national programmes.
Thailand has the National HIV/AIDS Treatment Program (NAP) that is part of the Universal Coverage Program (UC), and administered by the National Health Security Office (NHSO) since late 2007. This programme offers free-of-charge first- and second-line antiretroviral therapy (ART) per the national guidelines [14]. Demographic, HIV clinical and ART dispensing data are prospectively collected at routine clinic visits. Regions in the country are recorded to monitor service delivery in Thailand. Importantly, the vital status of patients is ascertained by linkage with the national death registry.
The objective of this study was to describe overall attrition including LTFU and mortality rates, and treatment failure at 1 year after ART initiation among youths diagnosed with HIV who initiated ART through the Thai NAP.
Methods
Study population
We enrolled adolescents living with HIV and youths who initiated ART between 1 January 2008 and 5 November 2013 and who were followed until 5 November 2014 in the NAP database. Criteria to initiate ART per the Thai national guidelines during the period 2008–2010 were symptomatic HIV or having a CD4 cell count <200 cell/mm3 [14]. The latter was modified to CD4 cell counts <350 cell/mm3 between 2011 and 2013. Patients were included if they initiated ART aged 10–24 years, with a regimen comprising at least three drugs, including a non-nucleoside reverse transcriptase inhibitor (NNRTI) or protease inhibitor (PI), plus 2–3 nucleoside reverse-transcriptase inhibitors (NRTI) in line with the Thai national guidelines [14]. We classified patients aged 10–24 years into two groups of HIV exposure based on age at ART initiation by making an assumption that youth with perinatally acquired HIV (PIY) were younger than youths with behaviourally acquired HIV (BIY). We defined PIY as youth who acquired HIV perinatally and started ART aged 10–14 years, and BIY as youth who acquired HIV behaviourally and started ART at aged 15–24 years. We further categorised the BIY group into two subgroups: those aged 15–19 years (BIY1) and those aged 20–24 years (BIY2). These youth were identified through HIV routine testing at antenatal care services, tuberculosis clinics, sexually transmitted infection clinics or owing to having opportunistic infections. We excluded patients who started ART before enrolment into the NAP, as baseline information was not available.
NAP test procedures
Testing procedures within the NAP follow the Thai national treatment guidelines and have previously been described [15]. Briefly, NAP patients have clinical visits for antiretroviral (ARV) refills and evaluation every 3 months. The first-line ART was an NNRTI-based regimen. CD4 cell testing and safety laboratory testing are performed every 6 months. Plasma HIV-RNA viral load (VL) is performed 6 months after ART initiation and yearly thereafter. The NAP database is linked to the national death registry. If a patient dies, the information is updated to the NAP database in real time. For women who initiated ART during pregnancy at the time of data collection, there was an option to discontinue ART after delivery when the CD4 cell count during pregnancy was >350 cells/mm3. ART could be restarted when CD4 cell counts fell to <350 cell/mm3. The recommended ART regimens used during pregnancy according to the Thai national guidelines during the period 2008–2010 were nevirapine based, and lopinavir/ritonavir based during the period 2010–2013.
Study outcomes
We studied three outcomes contributing to failures along the treatment cascade, namely LTFU, mortality and treatment failure at the first year of ART initiation.
The primary endpoints were LTFU or death. LTFU was defined as no ART prescription or no CD4 cell count performed for >12 months, irrespective of whether patients later returned to care. Patients who were LTFU and subsequently confirmed dead in the national death registry were also counted as LTFU. Mortality rate was confirmed by death date, through database linkage with the national death registry regardless of whether the patient was still in care or not.
The secondary endpoints were treatment failure during the first year of ART initiation,which was defined as virological failure (VF) with VL ≥1000 copies/mL within 12 months of ART initiation. In those who had no viral load test within a year, we further defined treatment failure if patients had either: (a) antiretroviral drug switch from NNRTI to PI or vice versa; or (b) LTFU or death in a 12-month window after starting ART.
Statistical analysis
Patient baseline characteristics were summarised by study group, and formal comparisons between groups (PIY, BIY1 and BIY2) were made with a Kruskal–Wallis test for continuous characteristics, or a Chi-squared test for categorical covariates. The crude LTFU and mortality rates and their 95% confidence intervals (CIs) were calculated using Poisson analysis. Person years (PY) of follow-up were calculated from baseline to endpoints (LTFU or death); patients who did not experience one of these outcomes were censored at their most recent clinic visit. The Kaplan–Meier method was used to calculate the probability of LTFU by study groups. Cox proportional hazards models were used to assess the relative risk of LTFU and death in BIY1 and BIY2 versus PIY as a reference. We assessed the influence of baseline age, sex, year of ART initiation, baseline CD4 cell count, region in the country and first ART regimen on LTFU and death; LTFU was included as a time-updated covariate in mortality models.
The composite endpoint of treatment failure at 1 year after ART initiation was expressed as a proportion, and logistic regression models were used to assess factors associated with the outcome, including baseline age, sex, calendar year, baseline CD4 cell count, region in the country and first ART regimen. Variables with P-values <0.10 in univariate Cox and logistic models were adjusted for multivariate models. Statistical significance was defined as two-sided P-value of 0.05. Statistical analysis was performed by using SAS version 9.4 (SAS Institute Inc, Cary, NC, USA) and with Stata version 14 (Stata Corp, College Station, TX, USA).
Results
Baseline characteristics
A total of 11,954 patients were included in the analysis. There were 2045 (17%) in the PIY, 3118 (26%) in the BIY1 and 6791 (57%) in the BIY2 groups. Baseline characteristics are shown in Table 1. Median baseline age was 21 years for BIY and 12 years for PIY. The majority were from the northeastern region of Thailand. About two-thirds had CD4 cell count measurements prior to ART initiation. The median baseline CD4 cell count of BIY was 190 cells/mm3 and 154 cells/mm3 for PIY.
Table 1.
Characteristics of youths living with HIV in the Thai National AIDS Program at time of ART initiation
| Characteristics
Total participants=11,954 |
PIY | BIY | P-value* | ||
|---|---|---|---|---|---|
| 10–14 years | 15–19 years
(BIY1) |
20–24 years
(BIY2) |
15–24 years
(BIY) |
||
| Participants, n (%) | 2045 (17) | 3118 (26) | 6791 (57) | 9909 (83) | |
| Median (IQR) age at ART initiation (years) | 12 (11–14) | 18 (17–19) | 22 (21–23) | 21 (19–23) | <0.001 |
| Female, n (%) | 1194 (58) | 2159 (69) | 3155 (46) | 5314 (54) | <0.001 |
| Year of ART initiation, n (%) | <0.001 | ||||
| 2008 | 631 (31) | 203 (7) | 523 (8) | 726 (7) | |
| 2009 | 404 (20) | 274 (9) | 742 (11) | 1016 (10) | |
| 2010 | 306 (15) | 495 (16) | 1065 (16) | 1560 (16) | |
| 2011 | 273 (13) | 700 (22) | 1377 (20) | 2077 (21) | |
| 2012 | 237 (12) | 731 (23) | 1536 (22) | 2267 (23) | |
| 2013 | 194 (9) | 715 (23) | 1548 (23) | 2263 (23) | |
| Geographical regions, n (%) | <0.001 | ||||
| Northeastern | 635 (31) | 884 (28) | 1816 (27) | 2700 (27) | |
| Northern | 526 (26) | 604 (19) | 1151 (17) | 1755 (18) | |
| Central | 196 (10) | 469 (15) | 1037 (15) | 1506 (15) | |
| Bangkok | 146 (7) | 357 (11) | 1108 (16) | 1465 (15) | |
| Southern | 244 (12) | 313 (10) | 770 (11) | 1083 (11) | |
| Eastern | 217 (11) | 335 (11) | 688 (10) | 1023 (10) | |
| Western | 81 (3) | 156 (6) | 221 (4) | 377 (4) | |
| n (%) with baseline CD4 cell count performed | 1417 (69) | 2088 (67) | 4222 (62) | 6310 (64) | |
| Median (IQR) baseline CD4 cell count, (cells/mm3) | 154 (39–307) | 241 (74–412) | 172 (45–303) | 190 (53–330) | <0.001 |
| Antiretroviral regimens, n (%) | <0.001 | ||||
| NNRTI based ART | 1908 (93) | 1850 (59) | 5282 (78) | 7132 (72) | |
| Efavirenz-based regimen | 623 (30) | 566 (18) | 1933 (28) | 2499 (25) | |
| Nevirapine-based regimen | 1285 (63) | 1284 (41) | 3349 (49) | 4633 (47) | |
| Boosted PI-based ART | 137 (7) | 1268 (41) | 1509 (22) | 2777 (28) | |
P-value between PIY, BIY1 and BIY2.
IQR: interquartile range; LTFU: loss to follow-up; PIY: youth with perinatally acquired HIV; BIY: youth with behaviourally acquired HIV; NNRTI: non-nucleoside reverse transcriptase inhibitor; PI: protease inhibitor; ART: antiretroviral therapy.
NNRTI-based ART was most commonly used. However, 28% of BIY and 7% of PIY initiated with a boosted PI-based regimen (P<0.001); the reasons for which were undocumented. Among the BIY group, 51% of women initiated a boosted PI regimen, compared to only 2% of men. Women with a baseline CD4 cell count >350 cell/mm3 were more likely to use a boosted PI regimen (88%) compared to those with lower baseline CD4 cell count (50% in women with CD4 cell counts of 200–350 cell/mm3and 14% in women with CD4 <200 cell/mm3).
Study outcomes
Attrition after ART initiation
Overall LTFU and mortality rates are shown in Table 2. PIY had a longer median duration on ART of 4.2 years (total of 8096 PY) versus 2.1 years (total of 24,485 PY) for BIY.
Table 2.
Attrition outcomes of youths living with HIV in the Thai National AIDS Program
| Characteristics | PIY | BIY | ||
|---|---|---|---|---|
| 10–14 years | 15–19 years
(BIY1) |
20–24 years
(BIY2) |
15–24 years
(BIY) |
|
| Participants, n (%) | 2045 (14) | 3118 (26) | 6791 (57) | 9909 (83) |
| Median (IQR) duration on ART, (years) | 4.2 (2.3–5.7) | 2 (1.1–3.3) | 2.2 (1.2–3.7) | 2.1 (1.1–3.6) |
| Follow-up (PY) | 8096 | 7140 | 17345 | 24485 |
| Loss to follow-up | ||||
| LTFU rate per 100 PY, (95% CI) | 2.9 (2.4–3.3) | 13.9 (12.9–14.8) | 9.5 (9.0–9.9) | 10.8 (10.3–11.2) |
| LTFU, n (%) | 224 (11) | 871 (28) | 1459 (21) | 2330 (24) |
| Female | 135 (11) | 737 (34) | 979 (31) | 1716 (32) |
| Male | 89 (10) | 134 (14) | 480 (13) | 614 (17) |
| CD4 cell count at LTFU, n (%) | 49 (22) | 270 (31) | 348 (24) | 618 (27) |
| Median (IQR) CD4 cell count at LTFU (cells/mm3) | 415 (260–601) | 550 (417–706) | 496 (321–671) | 520 (360–686) |
| Patients returning after loss to follow up, n (%) | 117 (52%) | 267 (31%) | 571 (39%) | 838 (36%) |
| Median (IQR) duration of return to care (months) | 20 (17–25) | 24 (19–32) | 23 (19–31) | 24 (19–31) |
| Mortality rate | ||||
| Mortality rate per 100 PY, (95% CI) | 2.5 (2.2–2.9) | 3.1 (2.7–3.6) | 2.9 (2.7–3.2) | 2.9 (2.8–3.2) |
| Deaths, n (%) | 206 (10) | 223 (7) | 504 (7) | 727 (7) |
| Characteristics of patients who died | ||||
| Median (IQR) time from ART initiation to death (months) | 27 (5–49) | 18 (6–35) | 14 (4–32) | 15 (4–33) |
| Median (IQR) age at death, years | 14.8 (13.0–16.8) | 19.7 (18.2–20.9) | 23.7 (22.7–25.1) | 23.0 (20.9–24.3) |
| Participants with available CD4 cell count prior to death n (%) | 192 (93) | 197 (88) | 444 (88) | 641 (88) |
| Median (IQR) latest CD4 cell count prior to death (cells/mm3) | 31 (12–119) | 41 (18–129) | 43 (12–141) | 42 (14–140) |
IQR: interquartile range; LTFU: loss to follow-up; PIY: youth with perinatally acquired HIV; BIY: youth with behaviourally acquired HIV; PY: person years.
Loss to follow-up
There were 2554 patients who were LTFU. The crude LTFU rates for PIY were 2.9 (95% CI 2.4–3.3) per 100 PY and 10.8 (95% CI 10.3–11.2) per 100 PY for BIY. BIY1 had higher LTFU rate than BIY2 (P<0.001). Among LTFU patients, 1445 (57%) never returned to care while 955 (37%) did return to care. Overall, 154 (6%) LTFU patients died. Rates of return-to-care were lower among BIY1 (31%) and BIY2 (39%) compared to PIY (52%) (Table 2).
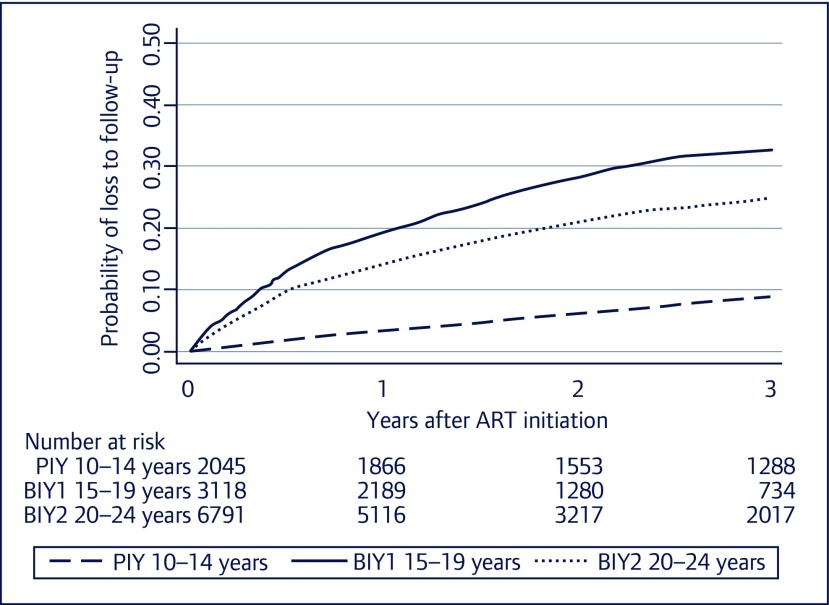
The probability of LTFU within 3 years after ART initiation is shown in Figure 1, with BIY having the highest probability of LTFU. In the multivariate Cox model, after adjusting for years of ART initiation and regions of the country, both BIY1 and BIY2 had a significantly higher risk of LTFU compared to PIY (P<0.001) (Table 3). Female sex, PI-based regimen and higher CD4 cell counts were also associated with an increased LTFU rate. A baseline CD4 cell count of 200– <350 cells/mm3 and CD4 cell count ≥350 cells/mm3 were associated with LTFU compared to a baseline CD4 cell count of <200 cells/mm3 (P=<0.001) (Table 3).
Figure 1.
Probability of loss to follow-up after ART initiation among youths living with HIV stratified by baseline age groups. PIY: youth with perinatally acquired HIV; BIY: youth with behaviourally acquired HIV.
Table 3.
Univariate and multivariate model showing associations with loss to follow-up and mortality among youths living with HIV in the Thai National AIDS program
| Characteristics
Total participants=11,954 |
LTFU
Total=2554 n (%) |
LTFU
Multivariate |
Death
Total=933 n (%) |
Mortality
multivariate |
||
|---|---|---|---|---|---|---|
| aHR (95% CI) | P-value | aHR (95% CI) | P-value | |||
| Sex | <0.001 | 0.88 | ||||
| Male | 703 (28) | ref | 491 (53) | ref | ||
| Female | 1851 (72) | 1.47 (1.32–1.62) | 442 (47) | 0.99 (0.86–1.13) | ||
| Age at ART initiation, years | <0.001 | |||||
| PIY age 10–14 | 224 (9) | ref | 206 (22) | |||
| BIY age 15–19 | 871 (34) | 2.95 (2.52–3.45) | 223 (24) | |||
| BIY age 20–24 | 1459 (57) | 2.72 (2.35–3.16) | 504 (54) | |||
| Year of ART initiation | <0.001 | 0.44 | ||||
| 2008 | 323 (13) | ref | 218 (23) | ref | ||
| 2009 | 389 (15) | 1.09 (0.94–1.26) | 187 (20) | 0.93 (0.76–1.14) | ||
| 2010 | 579 (23) | 0.94 (0.82–1.09) | 193 (21) | 1.09 (0.89–1.34) | ||
| 2011 | 698 (27) | 0.78 (0.67–0.90) | 156 (17) | 1.03 (0.82–1.29) | ||
| 2012 | 466 (18) | 0.56 (0.48–0.65) | 107 (11) | 0.88 (0.68–1.14) | ||
| 2013 | 99 (4) | 0.16 (0.13–0.21) | 72 (8) | 0.89 (0.66–1.19) | ||
| First regimen | <0.001 | <0.001 | ||||
| NNRTI-based ART | 1433 (56) | ref | 872 (93) | ref | ||
| PI-based ART | 1121 (44) | 2.42 (2.17–2.7) | 61 (7) | 0.55 (0.41–0.73) | ||
| Baseline CD4 cell count (cells/mm3) | <0.001 | <0.001 | ||||
| <200 | 531 (21) | ref | 517 (55) | ref | ||
| 200–350 | 374 (15) | 1.39 (1.22–1.60) | 66 (7) | 0.36 (0.28–0.47) | ||
| ≥350 | 611 (24) | 1.99 (1.74–2.27) | 16 (2) | 0.11 (0.07–0.19) | ||
| Region | <0.001 | 0.05 | ||||
| Bangkok | 377 (15) | ref | 103 (11) | ref | ||
| Central | 406 (16) | 0.91 (0.79–1.05) | 134 (14) | 1.21 (0.94–1.57) | ||
| Northern | 394 (15) | 0.66 (0.58–0.77) | 178 (19) | 1.11 (0.87–1.42) | ||
| Southern | 277 (11) | 0.68 (0.58–0.80) | 120 (13) | 1.40 (1.07–1.82) | ||
| Eastern | 303 (12) | 0.84 (0.72–0.98) | 74 (8) | 0.90 (0.66–1.21) | ||
| Western | 84 (3) | 0.66 (0.52–0.83) | 38 (4) | 1.17 (0.80–1.71) | ||
| North eastern | 713 (28) | 0.81 (0.72–0.93) | 286 (31) | 1.22 (0.97–1.53) | ||
| Loss to follow-up | NA | NA | 43 (5) | 2.89 (2.11–3.95) | <0.001 | |
LTFU: loss to follow-up; PIY: youth with perinatally acquired HIV; BIY: youth with behaviourally acquired HIV; aHR, adjusted hazard ratio; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Mortality
A total of 933 patients died; 779 died while taking ART and 154 died after LTFU (Table 2). Ten per cent of the PIY died after a median duration on ART of 27 months. Among the BIY group, 7% died after a median duration on ART of 15 months. The crude mortality rates were similar between PIY and BIY (P=0.46). The last CD4 cell count prior to death ranged from 31 to 141 cells/mm3.
In the univariate analysis, there was no significant difference in mortality rates between BIY1, BIY2 and PIY. In the multivariate Cox model, after adjusting for sex, years of ART initiation and regions of the country, there was a reduced risk of mortality for patients starting treatment with a PI-based regimen (P<0.001), whereas the risk was higher among patients who were LTFU (P<0.001). Baseline CD4 cell counts of 200–<350 cells/mm3 and ≥350 cells/mm3 were associated with a lower risk of mortality than baseline CD4 <200 cells/mm3 (P<0.001) (Table 3).
Treatment outcomes at the first year after ART initiation
Of 11,954 patients, 7160 (60%) patients had plasma HIV RNA measurements at 1 year after starting ART and 16% of them had VF (Table 4). BIY2 had the highest proportion of patients with VL <400 copies/mL (86%), followed by BIY1 (80%), and PIY (76%), P-value <0.001, respectively.
Table 4.
Treatment outcomes at 1 year after initiation of antiretroviral therapy among youths living with HIV in Thai National AIDS Program
| Characteristics | PIY | BIY | ||
|---|---|---|---|---|
| 10–14 years | 15–19 years
(BIY1) |
20–24 years
(BIY2) |
15–24 years
(BIY) |
|
| Participants, n (%) | 2045 (14) | 3118 (26) | 6791 (57) | 9909 (83) |
| Plasma HIV RNA measurements, n (%) | 1537 (75) | 1638 (53) | 3985 (59) | 5623 (57) |
| Median (IQR) time after ART initiation of plasma HIV RNA test (months) | 8 (6–12) | 7 (6–11) | 7 (6–11) | 7 (6–11) |
| Virological outcomes among patients with plasma HIV RNA measurement in the first year of ART | ||||
| Plasma HIV RNA <400 copies/mL, n (%) | 1164 (76) | 1308 (80) | 3419 (86) | 4727 (84) |
| Plasma HIV RNA 400–1000 copies/mL, n (%) | 37 (2) | 39 (2) | 77 (2) | 116 (2) |
| Plasma HIV RNA ≥1000 copies/mL, n (%) | 336 (22) | 291 (18) | 489 (12) | 780 (14) |
| Treatment outcome among patients without plasma HIV RNA measurement in the first year of ART | ||||
| Treatment switch between NNRTI and boosted PI and vice versa, n (%) | 13 (1) | 70 (2) | 114 (2) | 184 (2) |
| LTFU at 1 year after ART initiation, n (%) | 102 (5) | 652 (21) | 1031 (15) | 1683 (17) |
| Death at 1 year after ART initiation, n (%) | 81 (4) | 117 (4) | 320 (5) | 437 (4) |
| Censored /no failure at 1 year, n (%) | 312 (15) | 641 (21) | 1341 (20) | 1982 (20) |
| Composite endpoint of treatment failure: HIV RNA >1000 copies/mL, major class switch or LTFU or death | ||||
| Treatment failure* rate per 100 person-years (95% CI) | 29.9 (27.4–32.5) | 45.0 (42.5–47.7) | 34.4 (32.9–36.0) | 37.7 (36.4–39.0) |
Treatment failure was defined as a composite endpoint comprising virological failure (VF), or in those who had no HIV RNA tested, switching the major regimen, LTFU or death.
PIY: youth with perinatally acquired HIV; BIY: youth with behaviourally acquired HIV; LTFU: loss to follow-up; ART: antiretroviral therapy.
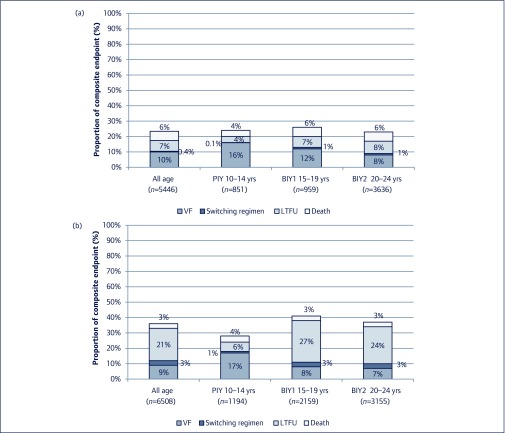
Among 4794 patients who had no plasma HIV RNA results in the 12-month period following ART initiation, 2500 met treatment failure criteria due to the following reasons: switching of a major drug class in the regimen (2%, n=197); LTFU (15%, n=1,785); and death (4%, n=518) (Figure 2). Data from the remaining 2294 (19%) patients were censored at 1 year. Among 197 individuals who had a major drug class switch, 61 (31%) switched from NNRTI to boosted PI and 136 (69%) switched from boosted PI to NNRTI. The latter was likely to be due to a regimen change during the postpartum period. Women had a higher LTFU rate compared to men (1397 [29%] vs 388 [8%], P<0.001).
Figure 2.
Proportion of composite endpoints of treatment failure at the first year of ART initiation among youths living with HIV stratified by age and sex. (a) Male; (b) female. PIY: youth with perinatally acquired HIV; BIY: youth with behaviourally acquired HIV; LTFU: loss to follow-up; VF: virological failure.
The overall 1-year treatment failure rate in PIY was 29.9 (95% CI 27.4–32.5) per 100 PY compared to 37.7 (95% CI 36.4–39.0) per 100 PY in BIY groups. In BIY1 the composite rate of failure was 45.0 (95% CI 42.5–47.7) per 100 PY and 34.4 (95% CI 32.9–36.0) per 100 PY for BIY2 (Table 4).
In the multivariate model, BIY1 and BIY2 had a higher risk of treatment failure compared with PIY (P<0.001). Female sex and use of PI-based ART were associated with treatment failure. Lower baseline CD4 cell counts of <200 cells/mm3 and ≥350 cells/mm3 compared to <200 cells/mm3 were also associated with treatment failure. Patients treated in more recent years and those from the northern, southern and western regions were less likely to have treatment failure (Appendix 1).
Discussion
This is the first study to evaluate the attrition and treatment outcomes of youth living with HIV who initiated ART aged 10–24 years in the Thai National AIDS Program. Most were older than 14 years and were likely to have acquired HIV through behavioural transmission. These BIY had significantly higher LTFU rates than PIY. BIY had a lower risk of VF compared to PIY, but a higher chance of composite treatment failure endpoint that included VF, LTFU and switching of regimens. These findings underscore the need for programmes to support adherence to follow-up and ART in youth.
Overall, the rates of LTFU in this study are consistent with findings from previous studies that ranged from 14 to 20 per 100 person years. The higher LTFU rates in BIY compared to PIY are also consistent with reports from sub-Saharan Africa (Kenya, Mozambique, Rwanda, South Africa, Tanzania and Zimbabwe ) [11-13,16-18]. Our data also showed that females were at greater risk of LTFU than males. It is possible that this is due to postpartum ART interruption as it is linked to having a higher CD4 cell count and using PI-based regimens. At that time, the national programme had not yet been implemented for the life-long ART post-delivery of Option B+. This is similar with reports from Kenya [9,12] in which most young females were enrolled through prevention of vertical transmission services, and factors associated with LTFU were being pregnant and having a CD4 cell count >350 cells/mm3.
The mortality rates in our study were low, with no differences among age groups that defined our PIY and BIY. Amongst comparable populations in Zimbabwe [16], Uganda, Kenya [12] and South Africa [13], mortality rates ranged from 3 to 6 per 100 person years. Our rates were slightly lower at 2.5–3.1 per 100 patient years, reflecting the increase in CD4 cell count thresholds for initiating ART in Thailand in recent years [19]. Lower baseline CD4 cell counts were significantly associated with mortality in our study, and in studies from Kenya [12] and Haiti [20]. Even though these older PIY may have been slow progressors with survivor bias, they initiated ART at a lower median CD4 cell count of 154 cell/mm3 compared to 190 cell/mm3 in BIY. This might partially explain the similar mortality rates among PIY and BIY.
The higher proportion of VF in PIY and BIY in our study is similar to reports from South Africa [21] and another study from Thailand [22], with VF rates ranging from 5 to 11 per 100 person years. Most BIY in our study had treatment failure because they were LTFU. Studies in sub-Saharan Africa [11,13] also showed LTFU rates in youth living with HIV to be highest in the first year following ART. A recent study from Myanmar also supports our findings that adolescents, particularly those aged 15–19 years, were at a high risk of treatment failure and LTFU [23].
The strengths of this study include the nationwide prospective data collection of attrition and treatment outcomes among youths living with HIV in Thailand across 6 calendar years. The real-time linkage of the NAP database to the national death registry is unique and allows for the vital status of patients in care and LTFU to be ascertained.
Nevertheless, there are limitations. First, information on HIV exposure was not documented in this national database dataset. Our assumptions about transmission risk based on age of ART initiation may have caused youth to be misclassified [24]. Second, there were no data on pregnancy in the NAP and we could not determine whether the higher risk of LTFU in women was due to postpartum interruption of ART and follow-up. Third, approximately one-third of the participants did not have CD4 cell counts at the time of ART initiation, and are likely to have started ART based on advanced clinical disease. Additionally, 40% did not have VL after the first year of ART initiation, affecting the treatment monitoring in this programme. Fourth, there were no data on the reasons for switching ART regimen in the database; therefore, our categorisation of a major ARV class switch as treatment failure may have overestimated failure rates. A major class switch may have been due to drug toxicities or a change from boosted PI to NNRTI during the postpartum period. However, the effect size of this bias is small since there were <2% of individuals in the total cohort who had treatment switch. Finally, difficulties surrounding HIV disclosure, stigma and discrimination in youth living with HIV are also important factors that impact on attrition and treatment outcomes, but we could not evaluate these in our study.
In summary, our findings highlight that BIY were at higher risk of LTFU compared to PIY, which might reflect a service delivery problem for youth. Thai youths living with HIV are in a transition stage between paediatric and adult care and additional support is needed to prevent LTFU [25,26].Our study supports the urgent need for developing a health policy to encourage youths living with HIV to remain in care and provide targeted support for PIY entering adolescence.
Acknowledgements
The dataset was provided by the Thai NHSO and the Ministry of Public Health of Thailand. The content of this publication is solely the responsibility of the authors, and does not necessarily reflect the views of the Australian Government, HIV-NAT, Thai Red Cross AIDS Research Centre, Bangkok, Thailand.
This data was presented as an oral abstract at the 9th International Workshop on HIV Pediatrics, Paris, France, 21–22 July 2017.
Declaration of interests
KR has received the Senior Research Scholar from Thailand Research Fund (TRF). He also received honoraria or consultation fees from Merck, Roche, Jensen-Cilag, Tibotec, Mylan and GPO (Governmental Pharmaceutical Organization, Thailand). He also has participated in a company sponsored speaker's bureau from Abbott, Gilead, Bristol-Myers Squibb, Merck, Roche, Jensen-Cilag, GlaxoSmithKline, and GPO. ML has received unrestricted research grants from Boehringer Ingelhiem, Gilead Sciences, Merck Sharp & Dohme, Bristol-Myers Squibb, Janssen-Cilag, ViiV HealthCare, DSMB sitting fees from Sirtex Pty Ltd, and consultancy and presentation fees from Gilead Sciences.
All other authors declare no conflicts of interest.
Funding
ST was funded as a CIPHER grantee from the International AIDS Society, 2018–2020, to conduct a study focusing on treatment outcomes among youth living with HIV in Thailand.
Appendix 1. Characteristics associated with composite treatment failure at 1 year after ART initiation for youths living with HIV in the Thai National AIDS program
| Characteristics | No treatment failure, n (%) | Treatment failure, n (%) | Univariate | Multivariate | ||
|---|---|---|---|---|---|---|
| N=11,954 | Total=8338 (70%) | Total=3616 (30%) | OR (95% CI) | P-value | aOR (95% CI) | P-value |
| Sex | 0.02 | <0.001 | ||||
| Male | 4200 (50) | 1246 (34) | ref | ref | ||
| Female | 4138 (50) | 2370 (66) | 1.93 (1.78–2.09) | 1.34 (1.22–1.48) | ||
| Age at ART initiation | 0.005 | <0.001 | ||||
| PIY age 10–14 years | 1513 (18) | 532 (15) | ref | ref | ||
| BIY age 15– 19 years | 1988 (24) | 1130 (31) | 1.62 (1.43–1.83) | 1.45 (1.26–1.66) | ||
| BIY age 20–24 years | 4837 (58) | 1954 (54) | 1.15 (1.03–1.28) | 1.22 (1.08–1.38) | ||
| Calendar year | 0.029 | <0.001 | ||||
| 2008 | 180 (2) | 114 (3) | ref | ref | ||
| 2009 | 792 (10) | 315 (9) | 0.63 (0.48–0.82) | 0.61 (0.47–0.81) | ||
| 2010 | 1022 (12) | 489 (14) | 0.76 (0.58–0.98) | 0.68 (0.52–0.88) | ||
| 2011 | 1226 (15) | 808 (22) | 1.04 (0.81–1.34) | 0.80 (0.62–1.04) | ||
| 2012 | 1533 (18) | 911 (25) | 0.94 (0.73–1.20) | 0.64 (0.50–0.83) | ||
| 2013 | 2164 (26) | 751 (21) | 0.55 (0.43–0.70) | 0.37 (0.29–0.48) | ||
| 2014 | 1421 (17) | 228 (6) | 0.25 (0.19–0.33) | 0.18 (0.13–0.24) | ||
| First regimen | 0.025 | <0.001 | ||||
| NNRTI-based ART | 6724 (81) | 2316 (64) | ref | ref | ||
| PI-based ART | 1614 (19) | 1300 (36) | 2.34 (2.14–2.55) | 2.28 (2.02–2.58) | ||
| Baseline CD4 cell counts (cells/mm3) | 0.006 | <0.001 | ||||
| <200 | 2940 (35) | 1161 (32) | 1.21 (1.07–1.38) | 1.42 (1.25–1.63) | ||
| 200–350 | 1446 (17) | 470 (13) | ref | ref | ||
| ≥350 | 1070 (13) | 640 (18) | 1.84 (1.60–2.12) | 1.21 (1.03–1.41) | ||
| Region | 0.003 | <0.001 | ||||
| Bangkok | 1155 (14) | 456 (13) | 1.13 (0.98–1.31) | 1.23 (1.06–1.43) | ||
| Central | 1125 (13) | 577 (16) | 1.47 (1.28–1.69) | 1.43 (1.24–1.65) | ||
| Northern | 1691 (20) | 590 (16) | ref | ref | ||
| Southern | 922 (11) | 405 (11) | 1.26 (1.08–1.46) | 1.14 (0.97–1.33) | ||
| Eastern | 830 (10) | 410 (11) | 1.42 (1.22–1.65) | 1.29 (1.10–1.51) | ||
| Western | 321 (4) | 137 (4) | 1.22 (0.98–1.53) | 1.13 (0.90–1.42) | ||
| Northeastern | 2294 (28) | 1041 (29) | 1.30 (1.15–1.46) | 1.27 (1.12–1.43) |
PIY: youth with perinatally acquired HIV; BIY: youth with behaviourally acquired HIV; aOR: adjusted odds ratio; NNRTI: non-nucleoside reverse transcriptase inhibitor; PI: protease inhibitor.
References
- 1. UNAIDS HIV indicators. 2017. Available at: aidsinfo.unaids.org/ ( accessed December 2018). [Google Scholar]
- 2. Sidibe M, Singh PK.. Thailand eliminates mother-to-child transmission of HIV and syphilis. Lancet 2016; 387: 2488– 2489. [DOI] [PubMed] [Google Scholar]
- 3. Rangsin R, Kana K, Chuenchitra T et al. Risk factors for HIV Infection among young Thai men during 2005–2009. PLoS One 2015; 10: e0136555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Griensven F, Varangrat A, Wimonsate W et al. Trends in HIV prevalence, estimated hiv incidence, and risk behavior among men who have sex with men in Bangkok, Thailand, 2003–2007. J Acquir Immune Defic Syndr 2010; 53: 234– 239. [DOI] [PubMed] [Google Scholar]
- 5. Centers for Disease C, Prevention HIV and syphilis infection among men who have sex with men–Bangkok, Thailand, 2005-2011. MMWR Morb Mortal Wkly Rep 2013; 62: 518– 520. [PMC free article] [PubMed] [Google Scholar]
- 6. Thongnopakun S, Maharachpong N, Abdullakasim P. Factors related to the sexual behaviors among youth in universities located in the eastern region of Thailand. J Med Assoc Thai 2016; 99 Suppl 1: S43– 50. [PubMed] [Google Scholar]
- 7. UNAIDS HIV prevention among adolescent girls and young women. 2016. Available at: www.unaids.org/sites/default/files/media_asset/UNAIDS_HIV_prevention_among_adolescent_girls_and_young_women.pdf ( accessed December 2018).
- 8. Dellar RC, Dlamini S, Karim QA.. Adolescent girls and young women: key populations for HIV epidemic control. J Int AIDS Soc 2015; 18: 19408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ojwang VO, Penner J, Blat C et al. Loss to follow-up among youth accessing outpatient HIV care and treatment services in Kisumu, Kenya. AIDS Care 2016; 28: 500– 507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Murray KR, Dulli LS, Ridgeway K et al. Improving retention in HIV care among adolescents and adults in low- and middle-income countries: a systematic review of the literature. PLoS One 2017; 12: e0184879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lamb MR, Fayorsey R, Nuwagaba-Biribonwoha H et al. High attrition before and after ART initiation among youth (15–24 years of age) enrolled in HIV care. AIDS 2014; 28: 559– 568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koech E, Teasdale CA, Wang C et al. Characteristics and outcomes of HIV-infected youth and young adolescents enrolled in HIV care in Kenya. AIDS 2014; 28: 2729– 2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Evans D, Menezes C, Mahomed K et al. Treatment outcomes of HIV-infected adolescents attending public-sector HIV clinics across Gauteng and Mpumalanga, South Africa. AIDS Res Hum Retroviruses 2013; 29: 892– 900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Puthanakit T, Tangsathapornpong A, Ananworanich J et al. Thai national guidelines for the use of antiretroviral therapy in pediatric HIV infection in 2010. Asian Biomedicine 2010; 4: 505– 513. [Google Scholar]
- 15. Chaivooth S, Bhakeecheep S, Ruxrungtham K et al. The challenges of ending AIDS in Asia: outcomes of the Thai National AIDS Universal Coverage Programme, 2000–2014. J Virus Erad 2017; 3: 192– 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bygrave H, Mtangirwa J, Ncube K et al. Antiretroviral therapy outcomes among adolescents and youth in rural Zimbabwe. PLoS One 2012; 7: e52856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Auld AF, Agolory SG, Shiraishi RW et al. Antiretroviral therapy enrollment characteristics and outcomes among HIV-infected adolescents and young adults compared with older adults–seven African countries, 2004-2013. MMWR Morb Mortal Wkly Rep 2014; 63: 1097– 1103. [PMC free article] [PubMed] [Google Scholar]
- 18. Teasdale CA, Alwar T, Chege D et al. Impact of Youth and adolescent friendly services on retention of 10–24-year-olds in HIV care and treatment programs in Nyanza, Kenya. J Acquir Immune Defic Syndr 2016; 71: e56– 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Manosuthi W, Ongwandee S, Bhakeecheep S et al. Guidelines for antiretroviral therapy in HIV-1 infected adults and adolescents 2014, Thailand. AIDS Res Ther 2015; 12: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reif LK, Bertrand R, Benedict C et al. Impact of a youth-friendly HIV clinic: 10 years of adolescent outcomes in Port-au-Prince, Haiti. J Int AIDS Soc 2016; 19: 20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nglazi MD, Kranzer K, Holele P et al. Treatment outcomes in HIV-infected adolescents attending a community-based antiretroviral therapy clinic in South Africa. BMC Infect Dis 2012; 12: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bunupuradah T, Sricharoenchai S, Hansudewechakul R et al. Risk of first-line antiretroviral therapy failure in HIV-infected Thai children and adolescents. Pediatr Infect Dis J 2015; 34: e58– 62. [DOI] [PubMed] [Google Scholar]
- 23. Kyaw NT, Harries AD, Kumar AM et al. High rate of virological failure and low rate of switching to second-line treatment among adolescents and adults living with HIV on first-line ART in Myanmar, 2005–2015. PLoS One 2017; 12: e0171780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ferrand RA, Corbett EL, Wood R et al. AIDS among older children and adolescents in Southern Africa: projecting the time course and magnitude of the epidemic. AIDS 2009; 23: 2039– 2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dahourou DL, Gautier-Lafaye C, Teasdale CA et al. Transition from paediatric to adult care of adolescents living with HIV in sub-Saharan Africa: challenges, youth-friendly models, and outcomes. J Int AIDS Soc 2017; 20: 21528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ruria EC, Masaba R, Kose J et al. Optimizing linkage to care and initiation and retention on treatment of adolescents with newly diagnosed HIV infection. AIDS 2017; 31 Suppl 3: S253– S260. [DOI] [PMC free article] [PubMed] [Google Scholar]