Young children on antiretroviral therapy (ART) exhibit superior immune reconstitution to adults due partly to high frequencies of naïve T cells and preserved thymic function [1]. We report on immunological investigations in a child commenced on ART during the neonatal period by selecting markers previously associated with viraemic control in adults [2–5].
In 2013, a male infant was diagnosed with presumably intrauterine-acquired HIV [6] (confirmed integrated HIV DNA 1750 copies/106 peripheral blood mononuclear cells [PBMCs] at age 8 days). The infant was initiated on zidovudine/lamivudine/ritonavir-boosted lopinavir at age 2 days, and raltegravir (RAL) was added at age 5 days due to the high HIV RNA load (>10,000,000 copies/mL). His HIV DNA at that time was 1750 copies/106 PBMCs [6]. The infant's viral load dropped to <200 copies/mL after 6 months and to <50 copies/mL after 12 months of ART. At 18 months, while on RAL, lamivudine and abacavir, he lost maternal HIV antibodies (non-reactive fourth generation HIV-1/2 assay, Vironostika HIVAg/Ab, BioMérieux, and negative Western blot), indicating low HIV RNA viral burden [7,8]. At that time, he had protective antibody titres to hepatitis B virus, tetanus, poliomyelitis, diphtheria and pertussis vaccines and normal total immunoglobulin levels. His CD4 T lymphocyte count was 2030 cells/mm3 (26%), CD4:CD8 ratio was 1.5, HIV RNA was <50 copies/mL and HIV DNA was 123 copies/106 PBMCs.
We investigated innate and adaptive immune markers reported to be associated with viraemic controllers including elite controllers (ECs) and post-treatment controllers (PTCs) in the Visconti cohort of adults treated during primary HIV infection and subsequently able to control viraemia after treatment interruption [9].
HLA class I and II typing did not show the HLA-B35 and HLA-B07 alleles seen in PTCs or the HLA-B27 and B57 alleles associated with ECs.
There were high frequencies (35%) of activated (HLA-DR+) natural killer (NK) cells. Inducibility was low for NKp30 but high for NKp44 (1.09 and 103.8, respectively and reported as fold change in the proportion of activated NK cells expressing NKp30/NKp44 after 4 days of in vitro activation with rh-IL2). NK cells are activated in adults with untreated HIV [10], and can be persistent following successful ART and CD4 immune reconstitution [11,12]. Persistent NK cell activation is also reported in EC adults [5]. The reasons are unclear but could be due to virus replication or production in secondary lymphoid tissues with insufficient antiretroviral drug concentrations [13].
We analysed the proliferative activity and cytokine profile (percentage of antigen-specific IL-2 and IFN-γ producing cells) by flow cytometry of CD4 and CD8 T lymphocytes after in vitro stimulation with recall antigens: tetanus toxoid (TT), Candida albicans (Ca), influenza virus (FLU) and deca-pools of 20mer overlapping peptides of HIV retrotranscriptase (RT). There was a positive response to recall stimuli in both T cell subsets, and a weak response to some peptide deca-pools of RT by CD8 and CD4 T lymphocytes. A high frequency of CD4+ IL-2+ cells specific for RT peptides was observed in comparison with those for recall antigens. There were also low frequencies of CD4+ IFN-γ+ cells except for Ca antigens. There were higher frequencies of CD8+ IL-2+ cells against pool 1 of RT peptides compared to those observed for recall antigens and other RT peptide pools, and low levels of CD8+ IFN-γ+ cells, except for Ca antigens. These data indicate maintenance of circulating memory CD4+ and CD8+ T cells specific for HIV antigens [5,8,14,15].
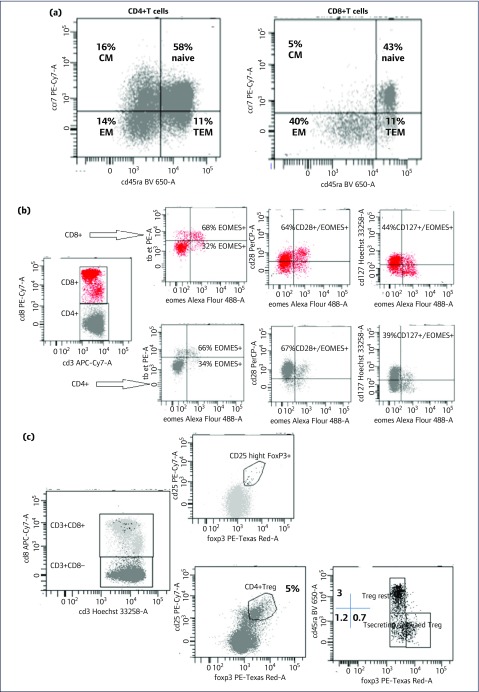
The frequencies of CD45RA+CCR7+ naïve cells were high on both CD4+ and CD8+ T lymphocytes (58% and 43%). The distribution of antigen-experienced T cells showed: (a) higher percentage of CD45RA-CCR7- effector memory (EM) (40%) cells compared to CD45RA-CCR7+ central memory (CM) (5%) and CD45RA+CCR7- terminally effector memory (TEM) (11%) cells on CD8 T cells; (b) similar percentages of CM, EM, TEM populations on CD4+ (16%, 14% and 11%, respectively) and CD3+CD8- T cell subsets (Figure 1a).
Figure 1.
Immunological status at age 18 months in a child who initiated antiretroviral therapy from age 2 days. (a) Evaluation of naïve and central memory (CM), effector memory (EM) and terminal effector memory (TEM) populations on CD4+ and CD8+ T cell subsets. (b) Expression of Eomes, T-bet transcription factors and CD28, CD127 markers on circulating CD8+ and CD4+ T lymphocytes. (c) Analysis of circulating CD4+ Treg population. Treg: regulatory T cells.
Eomes transcription factor expression was evaluated by flow cytometry on total CD4+ and CD8+ T lymphocytes: 25% of Eomes+ CD8+ T cells (32% T-betlowEomes+, 68% T-bethighEomes+ respectively); 64% of Eomes+ cells were CD28+ and 44% were CD127+. The data on CD4+ T cells showed: 8% Eomes+ (34% T-betlowEomes+ and 66% T-bethighEomes+); 67% of Eomes+ cells were CD28+ and 39% were CD127+ (Figure 1b). We observed PD-1 expression on 4.4% CD4+ and 8.1% CD8+ T cells. Adults with HIV were reported to have a subpopulation of HIV-restricted CD8+T-betloEomeshi exhausted T lymphocytes [2]. Our case had a profile similar to healthy individuals with a high proportion of Eomeshi cells including Eomes+ cells expressing CD127+ and CD28+ [4].
The analysis of regulatory T cells (Treg) revealed a normal frequency of CD25highFoxP3+ cells on CD4+ Treg population (5%), and no CD8+Treg circulating cells, consistently with an undetectable HIV RNA load [16]. The frequency of resting or naïve Treg (3% CD45RA+FoxP3low) was higher than those of secreting T cells (1.2% CD45RA-FoxP3low) and activated Treg (0.7% CD45RA-FoxP3high, Figure 1c).
We therefore concluded that early and suppressive ART initiated in infancy in this child led to immune preservation of CD4+ and CD8+ T cells with high frequencies of naïve cells and low expression of activation and exhaustion markers. HIV DNA continued to be detected. The RAL used in this case may have contributed to the rapid plasma viral load decline and immunological recovery. This case is an example of an early-treated child who might benefit from future immunotherapies to boost HIV-specific immunity.
Acknowledgements
TL, DC and TP wrote the manuscript with the support of MF, DMA, FD and DBA; TL, DC, FD and DBA contributed to conception and design of the article; FD, MF and BB performed all the immunological and virological laboratory tests; TL, DC, FD, DMA, FG and DBA contributed to laboratory test interpretation and application to clinical setting; BB, GC and CE contributed to the data acquisition and participated in drafting the article; DMA, FG and VC revised it critically for important intellectual content; all authors gave final approval for submission.
Declaration of interests
The authors declare no conflict of interest.
Funding
No significant financial support was received.
References
- 1. Gibb DM, Newberry A, Klein N et al. . Immune repopulation after HAART in previously untreated HIV-1-infected children. Paediatric European Network for Treatment of AIDS (PENTA) Steering Committee. Lancet 2000; 355: 1331– 1332. [DOI] [PubMed] [Google Scholar]
- 2. Buggert M, Tauriainen J, Yamamoto T et al. . T-bet and Eomes are differentially linked to the exhausted phenotype of CD8+ T cells in HIV infection. PLoS Pathog 2014; 10: e1004251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Freguja R, Gianesin K, Mosconi I et al. . Regulatory T cells and chronic immune activation in human immunodeficiency virus 1 (HIV-1)-infected children. Clin Exp Immunol 2011; 164: 373– 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hasley RB, Hong C, Li W et al. . HIV immune activation drives increased Eomes expression in memory CD8 T cells in association with transcriptional downregulation of CD127. AIDS 2013; 27: 1867– 1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marras F, Nicco E, Bozzano F et al. . Natural killer cells in HIV controller patients express an activated effector phenotype and do not up-regulate NKp44 on IL-2 stimulation. Proc Natl Acad Sci U S A 2013; 110: 11970– 11975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ripamonti D, Tatarelli P, Mangili G et al. . Potential role of raltegravir-based therapy to induce rapid viral decay in highly viraemic HIV-infected neonates. J Chemother 2016; 28: 337– 340. [DOI] [PubMed] [Google Scholar]
- 7. Payne H, Mkhize N, Otwombe K et al. . Reactivity of routine HIV antibody tests in children who initiated antiretroviral therapy in early infancy as part of the Children with HIV Early Antiretroviral Therapy (CHER) trial: a retrospective analysis. Lancet Infect Dis 2015; 15: 803– 809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Persaud D, Gay H, Ziemniak C et al. . Absence of detectable HIV-1 viremia after treatment cessation in an infant. N Engl J Med 2013; 369: 1828– 1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saez-Cirion A, Bacchus C, Hocqueloux L et al. . Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog 2013; 9: e1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fogli M, Costa P, Murdaca G et al. . Significant NK cell activation associated with decreased cytolytic function in peripheral blood of HIV-1-infected patients. Eur J Immunol 2004; 34: 2313– 2321. [DOI] [PubMed] [Google Scholar]
- 11. Bisio F, Bozzano F, Marras F et al. . Successfully treated HIV-infected patients have differential expression of NK cell receptors (NKp46 and NKp30) according to AIDS status at presentation. Immunol Lett 2013; 152: 16– 24. [DOI] [PubMed] [Google Scholar]
- 12. Lichtfuss GF, Cheng WJ, Farsakoglu Y et al. . Virologically suppressed HIV patients show activation of NK cells and persistent innate immune activation. J Immunol 2012; 189: 1491– 1499. [DOI] [PubMed] [Google Scholar]
- 13. Fletcher CV, Staskus K, Wietgrefe SW et al. . Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc Natl Acad Sci U S A 2014; 111: 2307– 2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bitnun A, Samson L, Chun TW et al. . Early initiation of combination antiretroviral therapy in HIV-1-infected newborns can achieve sustained virologic suppression with low frequency of CD4+ T cells carrying HIV in peripheral blood. Clin Infect Dis 2014; 59: 1012– 1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Luzuriaga K, Tabak B, Garber M et al. . HIV type 1 (HIV-1) proviral reservoirs decay continuously under sustained virologic control in HIV-1-infected children who received early treatment. J Infect Dis 2014; 210: 1529– 1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fenoglio D, Dentone C, Signori A et al. . CD8(+)CD28(-)CD127(lo)CD39(+) regulatory T-cell expansion: A new possible pathogenic mechanism for HIV infection? J Allergy Clin Immunol 2018; 141: 2220– 2233. [DOI] [PubMed] [Google Scholar]