Abstract
Objectives
To assess the safety and tolerability as well as antiretroviral impact of ABX464, an oral investigational drug with a novel mechanism of HIV-1 inhibition (ClinicalTrials.gov NCT02735863).
Methods
Randomised, double-blind, placebo-controlled, Phase IIa study in individuals living with HIV-1 on antiretroviral therapy at six clinical centres in Spain, France and Belgium. ABX464 was administered once a day to 22 fully controlled HIV-1-positive participants at two doses (50 mg, n=6 and 150 mg, n=16) versus placebo, which was given to eight participants for 28 days in combination with a boosted protease inhibitor (darunavir/ritonavir or darunavir/cobicistat). The primary objective of the study was to assess ABX464 safety and tolerability when used in combination with darunavir boosted therapy. The secondary objective was to study antiretroviral efficacy on viral reservoirs using time to viral rebound following treatment interruption. The impact of ABX464 on HIV-1 reservoirs was further assessed by measuring levels of total HIV-1 in peripheral blood mononuclear cells (PBMCs) in the intervention arm versus placebo. A positive response was defined as an absolute reduction in HIV-1 DNA of at least 50 copies/106 PBMCs and a relative decrease >25% of HIV-1 DNA level.
Results
Twenty-six of the 30 randomly allocated participants completed the study according to the study protocol. ABX464 was found to be safe and well tolerated with the majority of adverse events (AEs) being mild or moderate. Of the participants, 22 (73.3%) experienced treatment-associated AEs (93.8%, 66.7%, 37.5% in the ABX464 150-mg, 50-mg dose and placebo arms, respectively). Percentages for combined grade 3/4 AEs for the three arms were 6.3%, 0% and 12.5%, respectively. Median time (Kaplan–Meier estimates) to viral rebound for ABX464 150-mg, 50-mg and placebo arms were 12.0 (95% confidence interval [CI]: 10–15), 15.5 (95% CI 14–22) and 15.5 (95% CI 1–22) days, respectively with no significant difference between the 150-mg treatment arm and placebo. Median changes in total HIV-1 DNA copies/106 PBMCs for ABX464 150-mg, 50-mg and placebo arms after 28 days of treatment were −40 (range −434 to +194), −115 (range −116 to −114) and 25 (range −35 to +218), respectively, showing a decrease in the intervention arms. There were 6/14, 2/2, and 0/4 responders for ABX464 150 mg, 50 mg and placebo, respectively. No significant difference was seen between treatment arms and placebo with respect to these virological parameters.
Conclusions
This small controlled study confirmed the good safety and tolerability of ABX464 and provides some evidence of a potential reduction of the HIV-1 reservoir in terms of HIV-1 DNA levels in PBMCs when it was added to an HIV-1 protease inhibitor-based regimen. These results will need to be confirmed in a larger study.
Keywords: ABX464, safety, tolerability, HIV-1, Phase IIa study, HIV-1 reservoirs, viral rebound
Introduction
Despite the major therapeutic success of antiretroviral therapy (ART) over the past decades, a functional (long-term control of HIV-1 in the absence of ART) or sterilising cure (elimination of all HIV-1-infected cells) still remains an elusive goal for the majority of patients [1]. This is due to the persistence of reservoirs in some long-lived cell populations that harbour integrated latent HIV-1 provirus [2, 3]. Although ART effectively suppresses HIV-1-replication to undetectable levels in plasma, it cannot target integrated latent HIV-1 provirus in the absence of active replication. Therefore, ART, including HIV-1 protease, integrase and reverse transcriptase inhibitors, will require additional agents in order to achieve a cure [4].
ABX464 is a compound with a novel mechanism of viral inhibition as it promotes HIV-1 RNA splicing events and interferes with the production of essential viral regulatory proteins such as rev and tat and inhibits viral mRNA export required for gag, pol, env production and generation of genomic RNA [5]. Both RNA export and splicing are controlled by the cap-binding complex (CBC) that interacts directly with either rev or the transcription/export (TREX) complex, a multiprotein complex, required for transcription and export of bulk mRNAs [6]. ABX464, by binding directly to CBC, specifically prevents rev-mediated splicing and export of viral RNA without interfering with CBC binding or cellular transcript export.
ABX464 has shown potent anti-HIV-1 activity in in vitro and in vivo preclinical studies [5]. It was highly effective in inhibiting replication of different HIV-1 subtypes in PBMCs and macrophages and also substantially reduced virus replication in two humanised mouse models infected with HIV-1. Most significantly, there was a sustained reduction of viral load (VL) levels in these mice after ABX464 treatment as compared to ART alone following treatment interruption [5].
A number of previous clinical studies that had enrolled a total of 72 healthy volunteers without HIV-1 and 50 individuals with HIV-1 have already confirmed ABX464 safety when given orally as monotherapy at doses up to 150 mg once a day (o.d.) [7–9]. In addition, an efficacy signal in terms of HIV-1 VL reduction was described when ABX464 was given at higher doses, i.e. 100 and 150 mg o.d. [9].
The present study was designed to further investigate ABX464 safety, tolerability and antiretroviral efficacy when used in combination with ART such as boosted darunavir monotherapy (darunavir/ritonavir [DRV/RTV] or darunavir/cobicistat [DRV/COBI]).
Materials and methods
Study design and participants
This was a placebo-controlled study aimed to assess the safety of ABX464, a novel antiretroviral agent, administered orally at the dose of 50 mg and 150 mg o.d. versus placebo in patients with HIV-1 on ART consisting of an HIV-1 boosted protease inhibitor such as 800 mg DRV with 100 mg RTV or 800 mg DRV with 150 mg COBI, for at least 8 weeks prior to baseline.
Participants needed to be fully suppressed (<50 HIV-1 copies/mL) for at least 6 months prior to enrolment. At Day 0 of enrolment into the study, ABX464 at either 50 mg or 150 mg o.d. or its matching placebo was added to this background therapy for the next 28 days.
A 3:1 randomisation ratio was applied per treatment block, i.e. three patients were to receive ABX464 on top of DRV/RTV or DRV/COBI and one patient placebo on top of DRV/RTV or DRV/COBI.
At Day 29, all treatment was to be discontinued. The HIV-1 viral load was monitored twice a week during the first 3 weeks of follow-up and weekly thereafter. In case of viral rebound (VR) as defined below, ART was resumed.
Dose limiting toxicity (DLT) was defined as a grade 3 or higher adverse event using the ‘Division of AIDS Table for Grading the Severity of Adult and Paediatric Adverse Events’ (version 2.0, 2014) (including signs/symptoms, laboratory toxicities and/or clinical events) considered by the Data Safety Monitoring Board as probably or definitely related to study treatment.
If more than two DLTs occurred during the treatment period of the first four treated participants, then the enrolment of additional patients was to be stopped. In addition, in case of a life-threatening (grade 4) adverse reaction, enrolment and treatment of ongoing patients was to be immediately discontinued. In both cases, enrolment would only be resumed upon the decision of the sponsor if the Data Safety Monitoring Board could conclude that the causality of the event was unrelated or unlikely to be related to the study treatment.
The primary outcome measures included the frequency of adverse reactions graded according to the ‘Division of AIDS table for Grading the Severity of Adult and Paediatric Adverse Events’ (version 2.0 November 2014) (time frame: up to 4 months).
The secondary outcome measures included the time to VR (time frame: up to 3 months). The time to VR was defined as the time between treatment stop (i.e. Day 29) and VR detection. We have further analysed HIV-1 reservoirs using total HIV-1 DNA/106 PBMCs.
The main objective of the pharmacokinetic (PK) analysis was to evaluate the impact of concomitant administration of ABX464 with boosted DRV (RTV/COBI) and on ABX464 and its main metabolite NGlc-ABX464 PK. Considering that boosted DRV (RTV or COBI) is a chronic treatment, it was deemed more relevant to evaluate the possible drug–drug interactions (DDI) at steady state and, as such, PK blood sampling was limited to the higher dosing interval.
The study was performed in one centre in France, two in Spain and three in Belgium and conducted from May 2016 to August 2017. The clinical study (protocol no. ABX464-004), informed consent documents, and any other appropriate study-related documents were reviewed and approved by each clinical centre's Independent Ethics Committee/Institutional Review Board.
Inclusion and exclusion criteria for enrolment and randomisation into the study are described in Appendix 1.
Randomisation and masking
Participants were randomly allocated at a 3:1 ratio to either an ABX464 or placebo arm at Day 0 if they fulfilled all inclusion criteria and none of the exclusion criteria. Randomisation was centrally managed in blocks of four individuals (three ABX464, one placebo) and performed via the electronic case report form (eCRF). Vial numbers to be used for a specific participant were assigned according to a predefined randomisation list.
Procedures
All participants were treated with DRV/RTV or DRV/COBI given as a monotherapy for at least 8 weeks prior to baseline. Boosted protease inhibitor treatment was given at the following doses: DRV 800 mg and RTV 100 mg o.d. with food or DRV 800 mg and COBI 150 mg o.d. with food in the morning. The original fixed dose (i.e. ABX464 50 mg o.d.) of the study drug was selected based on the first safety data generated with this dose and of ABX464-N-glucuronide concentration, its active metabolite [7, 8]. However, a first Phase IIa study result conducted in treatment-naïve individuals living with HIV-1 with high VL upon enrolment demonstrated ABX464 antiretroviral activity at higher doses. A VL reduction was observed in 3/12 participants in the ABX464 75- and 100-mg cohorts and in 4/6 participants in the ABX464 150-mg cohort, demonstrating a dose relationship effect of the drug [9]. Based on these findings it was decided to treat the first six participants with the 50 mg o.d. dose. After 28 days of treatment the safety data would be reviewed by the Data Safety Monitoring Board (DSMB) and in the event of no participants demonstrating a DLT, the study would enrol another 16 participants on ABX464 150-mg o.d. dose. The DSMB was convened after every four participants on ABX464 150 mg o.d. were recruited in order to review safety and to recommend, if appropriate, the continuation of the study.
Following participant screening, eligible individuals continued DRV/RTV or DRV/COBI o.d. with food in the morning. At Day 0, the study drug, i.e ABX464 or its matching placebo was added to background therapy for the next 28 days, at the fixed dose at breakfast of 50 mg (1×50-mg capsule) or 150 mg (3×50-mg capsules) o.d.
The initial study screening visit was carried out 21 ± 7 days prior to first dosing and individuals who met all the inclusion criteria and none of the exclusion criteria were eligible for inclusion into the study and a randomisation visit on the day of first study drug distribution (Day 0). During this visit, samples were drawn for serology (HIV, HBV and HCV), HIV-1 viral load and CD4/CD8 count. The randomisation visit was carried out on Day 0 with blood sampling. Subsequent visits were carried out on Day 7, 14, 21 and 28 ± 2 days and at Day 25 ± 4 (for PK measurements). The schedule and full list of laboratory tests and safety evaluations carried out for each visit are described in Appendix 2.
The treatment phase was terminated at the Day-28 visit with all treatment being interrupted. In this open-ended study, study visits were then performed initially twice weekly and then once weekly during the treatment interruption period until VR and reintroduction of ART. Viral rebound was defined as a single measurement of HIV-1 VL >1000 copies/mL plasma. ART reintroduction was performed according to the participant's medical and ART history with the aim of achieving undetectable VL. The participant with VR was withdrawn from the study and follow-up visits were carried out every 14 days until the VL had returned to undetectable levels. The last follow up was considered the end of study visits. In the event of an absence of VR, then the end of the study was designated to be 3 months after treatment interruption.
Prior to the statistical analyses, adverse events (AEs) were coded using the MedDRA dictionary in order to be tabulated by system organ class (SOC) and preferred term (PT). The number of participants (n[%]) with at least one treatment-emergent AE (TEAE) was tabulated by SOC and PT for each group and dose. The number of TEAEs was also analysed in the same way, by SOC and PT for each group and dose, by grade of severity for each group and dose, and by level of causality to the study drug for each group and dose.
Total HIV-1 DNA was quantified in participants on Day 0 and Day 28 of study drug plus background therapy by droplet digital PCR (ddPCR). Briefly, blood was drawn and PBMCs were isolated. Total genomic HIV-1 DNA was isolated from 107 cells and prior to PCR amplification, an enzyme restriction digestion with EcoRI (Promega, Leiden, the Netherlands) was performed. The ddPCR reaction mix consisted of 10 μl 2×ddPCR supermix for probes (Bio-Rad), 500 nM of primers and 300 nM of probe in a final volume of 20 μl (sequences of assay described in Schvachsa et al, [10]). Following amplification, droplets were read by the QX200 droplet reader (Bio-Rad) and data were analysed with the ddpcRquant analysis software [11]. Participants with <5 droplets in three replicates at the two time-points (days 0, 28) were excluded from the analysis, as it was considered that measurements of such low DNA content could be inaccurate.
Since PK analysis was performed at steady-state, PK blood sampling was limited to the higher dosing interval. A first blood sample (reference) for boosted DRV (RTV or COBI) was collected on the first day of treatment before any drug administration. Blood samples were then collected for PK purpose at Days 0, 7, 14 and 21, before the boosted DRV (RTV or COBI) morning dose and at Day 25, before the boosted DRV (RTV or COBI) morning dose and at 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 6, 8, 12, and 24 h post-dose. The following PK parameters were derived for ABX464, ABX464-NGlc, boosted DRV (RTV or COBI) on Day 25 for each patient: Cmax and the time taken to reach Cmax (tmax) were obtained directly from the concentration-time data; AUC0-t (24 h post-dose for ABX464 and ABX464-NGlc, and 12 h post-dose for DRV (RTV and COBI). If no concentration could be measured at this time-point, area under the plasma concentration-versus-time curve from time zero to the time of the last quantifiable concentration (AUC0-last) was calculated. Plasma samples were analysed using validated bioanalytical methods by Atlanbio (Saint Nazaire, France) according to good laboratory practice. ABX464 and its metabolite ABX464-NGlc, DRV, RTV and COBI plasma pharmacokinetic parameters were assessed using a non-compartmental method by WinNonlin (version 6.4) software.
Outcomes
The primary objective of the study was to evaluate the safety and tolerability of ABX464 versus placebo when administered in combination with DRV/RTV or DRV/COBI. The secondary objectives were: (i) to evaluate the longer-lasting effect of ABX464 versus placebo on VL after treatment interruption on Day 29 using time to VR; (ii) to compare the effect of ABX464 versus placebo on VL from Day 0 to the time of VR; (iii) to compare the effect of ABX464 versus placebo on the CD4 T cell count from Day 0 to the time of VR; (iv) to compare the effect of ABX464 versus placebo on the CD4/CD8 T cell ratio from Day 0 to the time of VR; (v) to evaluate the effect of ABX464 versus placebo on the HIV-1 reservoir (total HIV-1 DNA in PBMCss) from Day 0 to VR; and (vi) to evaluate the impact of concomitant administration of boosted DRV on ABX464 pharmacokinetics.
Statistical analysis
The sample size selection was based on a virological efficacy endpoint although safety was the primary endpoint. The endpoint chosen for the sample size calculation was the time to VR defined as the time between treatment stop (i.e. Day 29) and VR detection. According to published studies [12], the expected median time to VR (calculated from treatment stop) was expected to be 7 days in the DRV/RTV or COBI and placebo arm and at least 28 days in the DRV/RTV or COBI and ABX464 arm. The power to detect a difference between the intervention arms and placebo was calculated using the PROC POWER (SAS software, version 9.4). Thus, the enrolment of 28 evaluable participants (21 in the ABX464 arm at 50 mg or 150 mg o.d. and seven in the placebo arm) would allow an 80% chance to detect a significant difference at the P=0.05 level, between arms.
All efficacy analyses were conducted on both the full-analysis dataset population and the per-protocol dataset population, except if otherwise specified. Descriptive statistics are presented by treatment arm and include quantitative variables: mean, standard deviation, minimum and maximum, 95% confidence intervals.
The statistical study plan was finalised before study unblinding and analysis. It defined the following criteria: (i) only participants with >50 copies of total HIV-1 DNA/106 PBMCs at baseline were considered evaluable (this was related to the lower limit of quantification of the PCR assay as a decrease from a baseline of ≤50 copies/106 PBMCs would have been inappropriate for interpretation); and (ii) participants who showed a relative reduction of total HIV-1 DNA/106 PBMCs between Day 0 and Day 28 of at least 25% and an absolute reduction of at least 50 HIV-1 copies/106 PBMCs were defined as responders.
Results
Baseline demographic characteristics and participant disposition
The study was conducted in Belgium, Spain and France. A total of 35 participants were enrolled into the study, of whom 30 were randomly allocated to one of three arms. Their demographic characteristics are described in Table 1. These varied slightly across treatment arms; however, they were not considered clinically relevant. The study population had a median age of 38, 45.5 and 50 years and a CD4 T cell count of 784, 982 and 681 cells/mm3 in the 150-mg, 50-mg and placebo arms, respectively.
Table 1.
Demographic and baseline participant characteristics
| 50 mg ABX464
(n=6) |
150 mg ABX464
(n=16) |
Placebo
(n=8) |
||
|---|---|---|---|---|
| Age (years) | Mean (SD)
Median [range] |
45.2 (3.5)
45.5 [41–49] |
38.4 (10.4)
38.0 [21–60] |
48.3 (8.5)
50.0 [34–56] |
| Sex | Male
Female |
6 (100.0%)
0 |
15 (93.8%)
1 (6.3%) |
8 (100.0%)
0 |
| Ethnicity | White
Asian Black Other |
6 (100.0%)
0 0 0 |
14 (87.5%)
0 2 (12.5%) 0 |
7 (87.5%)
1 (12.5%) 0 0 |
| Height (cm) | Mean SD
Median [range] |
170.5 (6.7)
170.5 [160–180] |
175.9 (7.6)
176.0 [164–190] |
174.3 (8.4)
175.0 [165–184] |
| Weight (kg) | Mean SD
Median [range] |
73.00 (10.64)
73.50 [59.0–89.0] |
79.88 (18.92)
71.00 [58.0–115.0] |
76.25 (12.34)
73.00 [61.0–94.0] |
| BMI (kg/m2) | Mean SD
Median [range] |
25.10 (3.23)
25.10 [19.9–29.4] |
25.80 (5.95)
23.55 [20.4–41.5] |
24.98 (2.22)
25.20 [21.5–28.1] |
| CD4 cell count (cells/mm3) | Mean (SD)
Median [range] |
1004 (201)
982 [800–1340] |
926 (394)
784 [426–1792] |
698 (179)
681 [448–998] |
| Plasma HIV-1 viral load (copies/mL) | Mean (SD)
Median [range] |
29.2 (11.1)
29.0 [19–40] |
96.1 (275.9)
19.0 [19–1130] |
29.1 (10.8)
29.0 [19–40] |
n: number of participants; SD: standard deviation; BMI: body mass index.
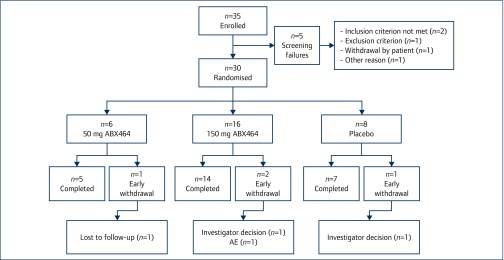
From among the 30 participants, six were randomly allocated to the ABX464 50-mg, 16 to the ABX464 150-mg dose and eight to the placebo (Figure 1). Twenty-six participants completed the study according to the study protocol: five from the ABX464 50-mg arm, 14 from the ABX464 150-mg arm and seven from the placebo arm. Four participants withdrew early from the study (one from the ABX464 50-mg arm, two from the ABX464 150-mg arm and one from the placebo arm). The reasons for withdrawal were investigators’ decision (two participants), AE (one participant), lost to follow-up (one participant).
Figure 1.
Study participant disposition. n: number of participants
Safety and tolerability
Overall, a total of 128 adverse events (AEs) were experienced by 27/30 participants (90%). Among these, 81 were classified as treatment emergent (TEAEs) (22/30 participants; 73·3%), 36 as post-TEAEs (14/30 participants; 46·7%). Eleven pretreatment events were reported. Of the 22 participants who experienced TEAEs, the highest frequency was observed in the ABX464 150-mg arm (15/16 participants experienced 58 AEs), followed by the ABX464 50-mg arm (4/6 participants experienced 10 AEs) and placebo arm (3/8 participants experienced 13 events). The frequency and severity of the most frequent TEAEs, by system order class (SOC), regardless of their relationship with the study treatment, are presented in Table 2. A full listing of all TEAEs by preferred term is provided in Appendix 3. The majority were graded as mild (grade 1) or moderate (grade 2); one TEAE was graded as severe (grade 3), i.e. a participant in the ABX464 150-mg arm suffered from severe fatigue. One serious AE (vagal faintness) was reported for a participant allocated to the placebo arm.
Table 2.
Summary of treatment-emergent adverse events, by system organ class and severity according to treatment allocation
| System organ class | Severity | ABX464 50 mg
Total=6 |
ABX464 150 mg
Total=16 |
Placebo
Total=8 |
|||
|---|---|---|---|---|---|---|---|
| TEAEs
n |
Participants
na (%) |
TEAEs
n |
Participants
na (%) |
TEAEs
n |
Participants
na (%) |
||
| Any treatment-emergent adverse events | All | 10 | 4 (66.7) | 58 | 15 (93.8) | 13 | 3 (37.5) |
| Mild | 9 | 3 (50.0) | 38 | 6 (37.5) | 11 | 2 (25.0) | |
| Moderate | 1 | 1 (16.7) | 19 | 8 (50.0) | 2 | 1 (12.5) | |
| Severe | 0 | 0 (0.0) | 1 | 1 (6.3) | 0 | 0 (0.0) | |
| Blood and lymphatic system disorders | All | 2 | 1 (16.7) | 0 | 0 (0.0) | 0 | 0 (0.0) |
| Mild | 2 | 1 (16.7) | 0 | 0 (0.0) | 0 | 0 (0.0) | |
| Moderate | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | |
| Severe | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | |
| Gastrointestinal disorders | All | 2 | 1 (16.7) | 23 | 13 (81.3) | 1 | 1 (12.5) |
| Mild | 2 | 1 (16.7) | 16 | 7 (43.8) | 1 | 1 (12.5) | |
| Moderate | 0 | 0 (0.0) | 7 | 6 (37.5) | 0 | 0 (0.0) | |
| Severe | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | |
| General disorders and administration site conditions | All | 2 | 1 (16.7) | 10 | 6 (37.5) | 1 | 1 (12.5) |
| Mild | 1 | 0 (0.0) | 4 | 3 (18.8) | 1 | 1 (12.5) | |
| Moderate | 1 | 1 (16.7) | 5 | 2 (12.5) | 0 | 0 (0.0) | |
| Severe | 0 | 0 (0.0) | 1 | 1 (6.3) | 0 | 0 (0.0) | |
| Infections and infestations | All | 0 | 0 (0.0) | 3 | 3 (18.8) | 7 | 3 (37.5) |
| Mild | 0 | 0 (0.0) | 2 | 2 (12.5) | 5 | 2 (25.0) | |
| Moderate | 0 | 0 (0.0) | 1 | 1 (6.3) | 2 | 1 (12.5) | |
| Severe | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | |
| Injury, poisoning and procedural complications | All | 2 | 2 (33.3) | 0 | 0 (0.0) | 0 | 0 (0.0) |
| Mild | 2 | 2 (33.3) | 0 | 0 (0.0) | 0 | 0 (0.0) | |
| Moderate | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | |
| Severe | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | |
| Investigations | All | 0 | 0 (0.0) | 2 | 2 (12.5) | 1 | 1 (12.5) |
| Mild | 0 | 0 (0.0) | 1 | 1 (6.3) | 1 | 1 (12.5) | |
| Moderate | 0 | 0 (0.0) | 1 | 1 (6.3) | 0 | 0 (0.0) | |
| Severe | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | |
| Metabolism and nutrition disorders | All | 0 | 0 (0.0) | 1 | 1 (6.3) | 0 | 0 (0.0) |
| Mild | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | |
| Moderate | 0 | 0 (0.0) | 1 | 1 (6.3) | 0 | 0 (0.0) | |
| Severe | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | |
| Musculoskeletal and connective tissue disorders | All | 0 | 0 (0.0) | 10 | 8 (50.0) | 2 | 2 (25.0) |
| Mild | 0 | 0 (0.0) | 7 | 5 (31.3) | 2 | 2 (25.0) | |
| Moderate | 0 | 0 (0.0) | 3 | 3 (18.8) | 0 | 0 (0.0) | |
| Severe | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | |
| Nervous system disorders | All | 2 | 2 (33.3) | 7 | 5 (31.3) | 0 | 0 (0.0) |
| Mild | 2 | 2 (33.3) | 6 | 4 (25.0) | 0 | 0 (0.0) | |
| Moderate | 0 | 0 (0.0) | 1 | 1 (6.3) | 0 | 0 (0.0) | |
| Severe | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | |
| Psychiatric disorders | All | 0 | 0 (0.0) | 1 | 1 (6.3) | 0 | 0 (0.0) |
| Mild | 0 | 0 (0.0) | 1 | 1 (6.3) | 0 | 0 (0.0) | |
| Moderate | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | |
| Severe | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | |
| Renal and urinary disorders | All | 0 | 0 (0.0) | 1 | 1 (6.3) | 0 | 0 (0.0) |
| Mild | 0 | 0 (0.0) | 1 | 1 (6.3) | 0 | 0 (0.0) | |
| Moderate | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | |
| Severe | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | |
| Skin and subcutaneous tissue disorders | All | 0 | 0 (0.0) | 0 | 0 (0.0) | 1 | 1 (12.5) |
| Mild | 0 | 0 (0.0) | 0 | 0 (0.0) | 1 | 1 (12.5) | |
| Moderate | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | |
| Severe | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | |
If a subject experienced multiple adverse events categorised under the same system organ class, this subject is shown only once within the highest severity grading. n: number of participants
The majority of TEAEs were considered by the investigators as not related to the ABX464 study drug. Of 81 TEAEs reported, 18 were considered ABX464 drug–related and were present in 8/16 participants in the ABX464 150-mg arm with none being present in the 50-mg arm. Two TEAEs led to the investigational study drug discontinuation in one participant in the ABX464 150-mg arm. The individual presented with abdominal pain (grade 2; unrelated to ABX464) and upper abdominal pain (grade 1; unrelated to ABX464), both of which started on study Day 1 and resolved 16 days later.
The most frequently reported TEAEs by SOC were gastrointestinal (50%), musculoskeletal and connective tissue disorders (33.3%), general disorders and administration site conditions (26.7%) and central nervous system disorders (23.3%) (Table 2). The TEAEs by PT that occurred in over 10% of participants across treatment arms included headache, back pain, upper abdominal pain, diarrhoea, abdominal pain, nausea, fatigue and arthralgia (Appendix 3).
CD4 T cell count and CD4/CD8 T cell ratio analysis
The CD4 T cell count and CD4/CD8 T cell ratio were assessed at all visits during the treatment period (Days 0, 7, 14, 21, 25 and 28), then, twice a week during the first 3 weeks after treatment interruption, and once a week thereafter until VR detection. No significant difference was seen between the ABX464 150-mg and placebo arms for these markers. The CD4 T cell count and CD4/CD8 T cell ratio are summarised in Appendices 4 and 5, respectively.
Efficacy
The main efficacy endpoint was the time (days) to VR defined as the time between the end of treatment (Day 29) and VR detection (viral load >1000 copies/mL). Results for the full analysis set for the three arms are summarised in Table 3.
Table 3.
Time to HIV-1 viral load rebound (full data analysis set)
| 50 mg ABX464
(n=6) |
150 mg ABX464
(n=16) |
Placebo
(n=8) |
P value | ||
|---|---|---|---|---|---|
| Time to viral load rebound (days) | Mean (SD)
Median [range] |
17.2 (3.4)
15.5 [14–22] |
14.4 (7.4)
11.5 [8–39] |
14.4 (7.3)
15.5 [1–23] |
|
| Kaplan–Meier estimate (days) | Median (95% CI) | 15.5 (14.0–22.0) | 12.0 (10.0–15.0) | 15.5 (1.0–22.0) | |
| Log-rank test | 0.5352 |
n: number of participants; SD: standard deviation; CI: confidence interval.
The log-rank test analysis was conducted for ABX464 150 mg and placebo only.
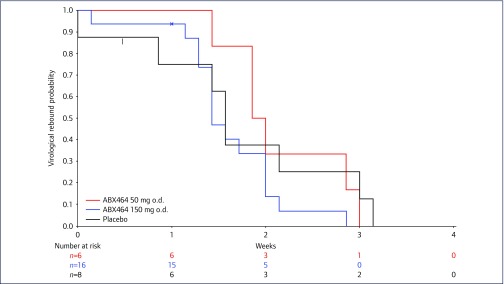
Median time to VR (Kaplan–Meier estimate) was 15.5 days (95% CI 14.0–22.0) for the ABX464 50-mg arm, 12.0 days (95% CI 10·0–15·0) for the ABX464 150-mg arms and 15.5 days (95% CI 1·0–22·0) for the placebo arm. Kaplan–Meier plots for the three treatment arms for time to VR are shown in Appendix 6. Log-rank test analysis showed no significant difference between the ABX464 150-mg and placebo arms (P=0·5352).
In addition, the impact of ABIVAX464 on HIV-1 reservoirs was investigated by assessing total HIV-1 DNA (copies/106 PBMCs) at Day 0 and 28 post-randomisation and were descriptively summarised by ABX464 dose and pooled doses (50 mg and 150 mg). The mean change in absolute total HIV-1 DNA levels from Day 0 to Day 28 is also summarised for the per-protocol set, by dose and pooled doses (Table 4). A number of individuals were excluded from the per-protocol analysis set because of consistently low HIV-1 reservoirs (<5 droplets) at both time-points (days 0 and 28), which prevented reliable conclusions on reservoir changes (see Methods). The data showed a potential impact of ABX464 on reservoirs with a median reduction in total HIV-1 DNA from Day 0 to Day 28 at the 50-mg dose (-115·00 copies/106 PBMCs; range −116 to −114) and at the 150-mg dose (–40.00 copies/106 PBMCs; range −434 to +194), while an increased level was noted in the placebo arm (+25.00 copies/106 PBMCs; range −35 to +218). The number (%) of responders for the per-protocol set who had a decrease in total HIV-1 DNA after ABX464 treatment are summarised in Table 5. This analysis confirmed the antiviral signal seen in terms of decrease in HIV-1 DNA copy number in PBMCs in ABX464-treated participants with a higher proportion of responders in the ABX464 150-mg (6/14 participants; 42·9%) and ABX464 50-mg arms (2/2 participants, 100%) in comparison to the placebo arm (0/4 participants, 0%) at Day 28. However, the difference in percentage of responders between the ABX464 150-mg and placebo arms was not significant (Fisher's exact test) (P=0.2451).
Table 4.
Changes in total HIV-1 DNA
| 50 mg ABX464
(n=2) |
150 mg ABX464
(n=14) |
Pooled ABX464 arms
(n=16) |
Placebo
(n=4) |
||
|---|---|---|---|---|---|
| Day 0 (copies/million PBMCs) | Mean (SD)
Median [range] |
300.0 (21.2)
300.0 [285–315] |
444.6 (289.5)
393.0 [72–961] |
426.6 (274.1)
352.5 [72–961] |
183.0 (85.4)
163.5 [112–293] |
| Day 28 (copies/million PBMCs) | Mean (SD)
Median [range] |
185.0 (22.6)
185.0 [169–201] |
398.9 (226.6)
388.5 [77–734] |
372.1 (223.3)
325.0 [77–734] |
241.3 (204.2)
188.5 [77–511] |
| Change between day 0 and day 28 (copies/million PBMCs) | Mean (SD)
Median [range] |
-115.00 (1.41)
-115.00 [-116.0–114.0] |
-45.79 (181.75)
-40.00 [-434.0–194.0] |
-54.44 (170.84)
-84.50 [-434.0–194.0] |
58.25 (118.99)
25.00 [-35.0–218.0] |
n: number of participants; SD: standard deviation.
Table 5.
Total HIV-1 DNA analysis
| 50 mg ABX464 | 150 mg ABX464 | Placebo | Difference in proportion*
(95% CI) |
Fisher's exact test
P value |
||
|---|---|---|---|---|---|---|
| All evaluable participants at day 28 | n=2 | n=14 | n=4 | |||
| Responder | 2 (100.0%) | 6 (42.9%) | 0 | |||
| Non-responder | 0 | 8 (57.1%) | 4 (100.0%) | |||
| 42.9% (-10.6–67.4) | 0.2451 |
n: number of participants; CI: confidence interval.
*Difference in proportion is calculated for ABX464 150 mg versus placebo
Pharmacokinetics
Data showed that in most subjects, regardless of the dose, no ABX464 plasma concentrations could be detected after 8–12 h post-dose. Cmax was generally observed within the first 2 h post-dose. After repeated oral administration of 50 and 150 mg ABX464, the drug was extensively biotransformed into its N-glucuronide metabolite, ABX464-NGlc. The metabolite plasma concentrations were higher than those of the parent drug with mean Cmax about 40–54-fold higher than ABX464 Cmax; Cmax was observed around 4 h post-dose. For COBI, mean Cmax and mean AUClast (obtained at mean Tlast around 18 hours, 24 hours and 22 hours, respectively) were similar after repeated administration of placebo or after 50 or 150 mg ABX464 repeated administration. Mean Cmax and mean AUClast (obtained at mean Tlast around 24 hours) was similar for DRV after repeated administration of placebo or after the 50 or 150 mg ABX464 repeated administration. However for RTV, after 50 mg ABX464 repeated administration, mean Cmax and mean AUClast (obtained at mean Tlast around 24 hours) were about two times lower than after placebo or after 150 mg ABX464 repeated administration.
Discussion
This study confirms previous findings from published trials showing that ABX464, a novel antiretroviral investigational drug, has a good safety and tolerability profile in individuals living with HIV-1 and is the first to use ABX464 in combination with ART (i.e. boosted DRV monotherapy). Treatment simplification with DRV-boosted monotherapy is reported to be safe and effective in routine clinical practice and was deemed relevant with respect to the study design and objectives [13].
A comparison of the AEs in this study with previous studies, where ABX464 was used as monotherapy, did not show an inferior safety or tolerability profile when combined with a boosted HIV protease inhibitor.
Unlike in previous studies, vomiting was not recorded whereas up to 67% of individuals treated with the ABX464 150-mg dose suffered mild or moderate vomiting in a previous ABX464 monotherapy study [9]. In addition, the incidence of headache was lower in this study with 5/16 participants (31.3%) in the 150-mg group reporting mild or moderate events compared to 66.7–100% in the monotherapy study. However, fatigue and back pain, which had not been reported in previous studies, were present in a number of participants receiving the ABX464 150-mg dose.
The analysis carried out in this study also demonstrated that key PK parameters such as ABX464 mean Cmax and mean AUClast were similar in individuals after ABX464 repeated administration combined with boosted DRV (RTV or COBI) to those obtained in previously ART-naïve HIV-1-positive individuals [9]. However, the main ABX464 metabolite NGlc-ABX464 mean Cmax and AUClast were approximately 5- and 17-times lower, respectively after ABX464 repeated administration in participants receiving combined therapy as compared to previous results in untreated HIV-1-positive individuals [9]. Therefore, the impact of ART on ABX464 pharmacokinetics will need to be fully investigated in future studies. The co-administration of ABX464 with DRV/RTV or DRV/COBI did not appear to impact the PK characteristics of these drugs. Mean Cmax and mean AUClast were similar after repeated administration of placebo or after ABX464 repeated administration and the measured PK characteristics were in agreement with published data [14,15]. The only difference was seen for RTV: mean Cmax and mean AUClast were similar after repeated administration of placebo or after 150 mg ABX464 repeated administration and were in agreement with published data [14]. However; after 50 mg ABX464 repeated administration, mean Cmax and mean AUClast were approximately two times lower than after placebo or after 150 mg ABX464 repeated administration. These results have to be viewed with caution due to the low number of subjects and the fact that no differences were seen with the higher 150-mg ABX464 dose.
Previous data in treatment-naïve HIV-1-positive individuals had detected a dose-dependent efficacy signal with respect to plasma VL reduction following a 14-day ABX464 treatment period [9]. The present study was therefore designed to potentially enhance this effect by combining ABX464 with a boosted HIV-1 protease inhibitor, and to evaluate VR after treatment interruption. The primary efficacy variable for statistical comparison between treatment arms was the time to VR (days). The present data demonstrated no significant difference by Kaplan–Meier estimates between the ABX464 150-mg and placebo arms.
Although total HIV-1 DNA overestimates the size of the replication-competent HIV-1 reservoir [16], it has been shown to be more predictive of disease progression than plasma VL and, of time to VR post-treatment interruption [17]. A reduction in the reservoir size may prolong time to VR [18, 19].
An ABX464-mediated antiviral signal was demonstrated with a median decrease in total HIV-1 DNA of 40 copies per 106 PBMCs in the ABX464 150-mg treatment arm as compared to an increase of 25 copies in the placebo group. In addition, there were no responders (0/4) in the placebo arm, 2/2 in the ABX464 50-mg and 6/14 (42.9%) in the ABX464 150-mg treatment arms when using our study criteria for responders. These results are encouraging in view of the failure of multiple interventions to achieve a measurable reduction of the HIV-1 reservoir size in other randomised studies. A novel antiviral agent that blocks HIV Vpu ion channel activity [20] had a 63% reduction in the mean total HIV-1 DNA copy number in monocytes of treated individuals.
Our study failed to demonstrate a significant difference in time to VR in ABX464-treated individuals following treatment interruption. This was, however, in contrast to data obtained in preclinical humanised mouse studies, where HIV-1-infected NOG hu mice were treated with ABX464 for 4 weeks [5]. These studies had shown a substantial reduction in VR over the 52-day observation period after ABX464 treatment interruption. The reasons for the discrepancy are unclear. The duration of 28 days of ABX464 may have been insufficient to sufficiently reduce the reservoir load and consequently no delay in VR was observed. A longer treatment period with ABX464 may be needed in order to evaluate its potential full impact on the HIV-1 reservoir as well as a larger number of participants.
In conclusion, as in previous studies with ABX464 monotherapy [7–9], we have shown that ABX464 was safe and well tolerated in fully suppressed HIV-1-positive individuals on boosted DRV. An efficacy signal was observed in the ABX464-treated group with respect to a reduction of total HIV-1 DNA in PBMCs. but this was not associated with a delay in the time to VR after therapy interruption.
A second Phase IIa study is currently ongoing using ABX464 for a longer treatment period (84 days) in addition to triple therapy and will include a more extensive analysis of integrated HIV-1 DNA performed to evaluate the impact of ABX464 on the HIV-1 reservoir in blood and gut tissue.
Acknowledgements
The work was funded by ABIVAX.
ABIVAX employees were involved in the study design, data collection, analysis and interpretation, writing of the report and the decision to submit for publication.
The study design was provided by JMS, PG, JT, DS, HJE and LV. Study analyses and manuscript writing were provided by PNB, PG, JMS, JT, SR and LV. Nucleic acid analyses were carried out by SR, BC and SK.
JMS, PG, DS and HJE are ABIVAX employees and hold ABIVAX stock options. PNB performs consultancy services for ABIVAX. LV was investigator of the study and received consultancy fees. JT is a member of a collaborative laboratory that has received financial support from ABIVAX.
The authors thank all the study participants for their commitment and all personnel at the different clinical centres who took part in this study.
Appendix 1. Study inclusion and exclusion criteria
Inclusion criteria
A participant was eligible for inclusion into this study only if ALL of the following criteria applied:
-
•
Participants living with HIV-1;
-
•
Participants with HIV plasma viral load ≤50 copies/mL during the 6 months prior to screening with a maximum of two blips (increases above 50 copies/mL) during this period;
-
•
Participants treated with darunavir/ritonavir (DRV/r) or darunavir/cobicistat (DRV/c) as a monotherapy for at least 8 weeks prior to baseline;
-
•
Participants’ HIV plasma viral load ≤100,000 copies/mL at any time (apart from primary infection if recorded);
-
•
Participants’ CD4 T cell count ≥250 cells/mm3 at any time since diagnosis;
-
•
Participants with CD4 T cells count ≥600 cells/mm3 at screening;
-
•
Man or woman aged 18–65 years;
-
•Haematological and biochemical laboratory parameters as follows and within 7 days of baseline:
-
○Haemoglobin >9·0 g/100 mL
-
○Absolute neutrophil count ≥750/mm3;
-
○Platelets ≥100,000/μL
-
○Total serum creatinine ≤1·3×upper limit of normal (ULN);
-
○Creatinine clearance >50 mL/per min using the Cockcroft-Gault equation within 60 days of entry;
-
○Total serum bilirubin <1.5×ULN;
-
○Alkaline phosphatase, alanine aminotransferase (AST), serum glutamic pyruvate transaminase (SGOT) and alanine aminotransferase (ALT), serum glutamic pyruvate transaminase (SGPT) <1·5×ULN;
-
○Serum lipase ≤2·0×ULN;
-
○
-
•
Participants should have been able and willing to comply with study visits and procedures as per protocol;
-
•
Participants should have understood, signed and dated the written informed consent form at the screening visit prior to any protocol-specific procedures being performed;
-
•
Participants should have been affiliated to a social security regimen (for French sites only); and
-
•
Females and males receiving the study treatment and their partners must have agreed to use a highly effective contraceptive method during the study period and for 3 months after the end of the study period or early termination. Contraception should have been in place at least 2 weeks prior to study participation. Women must be surgically sterile or, if of childbearing potential, must have used a highly effective contraceptive method. Women of childbearing potential entered the study after confirmed menstrual periods and a negative pregnancy test. Highly effective methods of contraception included true abstinence, intrauterine device (IUD) or hormonal contraception associated with inhibition of ovulation, intrauterine hormone releasing system, bilateral tubal occlusion, vasectomised partner. True abstinence was defined when this was in line with the preferred and usual lifestyle of the participant. In each case of delayed menstrual period (over 1 month between menstruations) confirmation of absence of pregnancy was required. This recommendation also applied to women of childbearing potential with infrequent or irregular menstrual cycle.
Exclusion criteria
The following criteria were checked at the time of screening. If ANY exclusion criterion applied, the participant could not be included into the study:
-
•
Participant displaying any HIV protease inhibitor resistance mutation as listed in the current version of the HIV drug resistance database (Stanford University);
-
•
Participant having had previously an HIV viral load ≥500 copies/mL confirmed by a second measure since the initiation of the current antiretroviral therapies (ART);
-
•
History of an acquired immune deficiency syndrome (AIDS)-defining clinical illness;
-
•
Concomitant AIDS-related opportunistic disease;
-
•
History of allergic disease, anaphylaxis or reactions likely to have been triggered or exacerbated by any component of the study drug;
-
•
Acute or chronic infectious disease other than HIV infection (included but not limited to viral hepatitis such as hepatitis B, active tuberculosis, active syphilis [i.e. currently treated], human T-cell lymphotropic virus [HTLV-1, HTLV-2]). Of note co-infection with hepatitis C was allowed as long as liver function parameters were within the following ranges: platelet >150.000/mm3; gamma-glutamyl transferase (γGT) ≤2·5 ULN; albumin >40 g/L and provided that participants were not receiving specific treatment during the study that could have interfered with the study objectives;
-
•
Acute, chronic or history of clinically relevant pulmonary, cardiovascular, gastrointestinal, hepatic, pancreatic or renal functional abnormality, encephalopathy, neuropathy or unstable central nervous system (CNS) pathology, angina or cardiac arrhythmias, or any other clinically significant medical problems as determined by physical examination and/or laboratory screening tests and/or medical history;
-
•
Uncontrolled dyslipidaemia;
-
•
Acute, chronic or history of immunodeficiency or autoimmune disease other than HIV infection; unstable asthma (defined as sudden acute attacks occurring for less than 3 hours without an obvious trigger, hospitalisation for asthma in the last 2 years); food or wine induced asthma;
-
•
History of malignancy unless there had been surgical excision that was considered to have achieved cure;
-
•
Active malignancy that may have required chemotherapy or radiation therapy;
-
•
Seizure disorder or any history of prior seizure;
-
•
Serious illness requiring systemic treatment and/or hospitalisation within 7 days prior to baseline;
-
•
Pregnant or breastfeeding woman;
-
•
Active drug or alcohol abuse or dependence;
-
•
Use of any investigational or non-registered product within 3 months preceding baseline; and
-
•
Any condition, which in the opinion of the investigator, could have compromised the participant's safety or adherence to the study protocol.
Appendix 2. Flow chart of study visits
| Screening | Days | Treatment interruption – follow-up | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Time Window | ± 7 days | ± 2 days (except D25 ± 4) | ± 2 days | ||||||||
| Days (D) | D-21 | D0 | D7 | D14 | D21 | D25 | D28 | Twice weekly for 3 weeks | Every week till VR | ART reintroduction visit | FU visit(s)*** |
| Informed consent | X | ||||||||||
| Check of IN/EX criteria | X | X | |||||||||
| Physical examination | X | X | X | X | X | X | X | X | X | X | X |
| Body weight (kg) | X | X | X | X | X | X | X | X | X | X | X |
| Height measurement (cm) | X | ||||||||||
| Medical history | X | ||||||||||
| Telephone calls to participants | Day 3 and 5 | ||||||||||
| Serology for HBV, HCV, HIV | X | ||||||||||
| Haematology and biochemistry | X | X | X | X | X | X | X | X | X | X***** | |
| CD4 and CD8 count | X | X | X | X | X | X | X | X | X | X | X |
| Urinalysis | X | X | X | X | X | X | X | X**** | X | X | |
| Blood pregnancy test | X | ||||||||||
| Urine pregnancy test | X | X | X | X | X | X | X | X | X | ||
| Vital signs | X | X | X | X | X | X | X | X | X | ||
| ECG (12 lead) | X | X | X | X | X | ||||||
| DRV/RTV or COBI prescription | X | X | X | X | X | X | X | X | X | ||
| ABX464/placebo treatment dispensation and patient diary review | X | X | X | X | X | X | |||||
| Blood samples–drug PK | X* | X* | X* | X* | X** | ||||||
| Blood samples for viral load and miRNA | X | X | X | X | X | X | X | X | X | X | X |
| HIV-1 genotyping | X | ||||||||||
| Leukapheresis (optional) | X | X | |||||||||
Blood samples for reservoir assessment
|
X | X | X | X
X |
|||||||
| Adverse events recording | X | X | X | X | X | X | X | X | X | ||
DRV: darunavir; RTV: ritonavir; COBI: cobiscistat; FU: follow-up; VR: viral rebound.
*: pre DRV/RTV or DRV/COBI morning dose
**: pre DRV/RTV or DRV/COBI morning dose, 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 6, 8, 12 and 24 h post-dose (hospitalisation was not required)
***: every 14 days till undetectable viral load
****: urinalysis performed once a week
*****: only biochemistry required and for at least 28 days after treatment interruption
Appendix 3. Any treatment-emergent adverse events by system organ class and preferred term
| 50 mg ABX-464
(n=6) |
150 mg ABX-464
(n=16) |
Placebo
(n=8) |
||||
|---|---|---|---|---|---|---|
| E | n (%) | E | n(%) | E | n (%) | |
| Any treatment-emergent adverse events | 10 | 4 (66.7%) | 58 | 15 (93.8%) | 13 | 3 (37.5%) |
| Blood and lymphatic system disorders | 2 | 1 (16.7%) | 0 | 0 | 0 | 0 |
| Lymphadenopathy | 2 | 1 (16.7%) | 0 | 0 | 0 | 0 |
| Gastrointestinal disorders | 2 | 1 (16.7%) | 23 | 13 (81.3%) | 1 | 1 (12.5%) |
| Abdominal pain | 0 | 0 | 4 | 3 (18.8%) | 0 | 0 |
| Abdominal pain upper | 0 | 0 | 5 | 4 (25.0%) | 0 | 0 |
| Diarrhoea | 2 | 1 (16.7%) | 5 | 3 (18.8%) | 0 | 0 |
| Epigastric discomfort | 0 | 0 | 1 | 1 (6.3%) | 0 | 0 |
| Gastric disorder | 0 | 0 | 1 | 1 (6.3%) | 0 | 0 |
| Gastro-oesophageal reflux disease | 0 | 0 | 1 | 1 (6.3%) | 0 | 0 |
| Nausea | 0 | 0 | 2 | 2 (12.5%) | 1 | 1 (12.5%) |
| Rectal haemorrhage | 0 | 0 | 1 | 1 (6.3%) | 0 | 0 |
| Salivary gland disorder | 0 | 0 | 1 | 1 (6.3%) | 0 | 0 |
| Tooth infection | 0 | 0 | 1 | 1 (6.3%) | 0 | 0 |
| Toothache | 0 | 0 | 1 | 1 (6.3%) | 0 | 0 |
| General disorders and administration site conditions | 2 | 1 (16.7%) | 10 | 6 (37.5%) | 1 | 1 (12.5%) |
| Fatigue | 0 | 0 | 5 | 3 (18.8%) | 0 | 0 |
| Inflammation | 1 | 1 (16.7%) | 0 | 0 | 0 | 0 |
| Influenza like illness | 1 | 1 (16.7%) | 1 | 1 (6.3%) | 0 | 0 |
| Malaise | 0 | 0 | 1 | 1 (6.3%) | 0 | 0 |
| Oedema peripheral | 0 | 0 | 2 | 1 (6.3%) | 0 | 0 |
| Peripheral swelling | 0 | 0 | 1 | 1 (6.3%) | 0 | 0 |
| Pyrexia | 0 | 0 | 0 | 0 | 1 | 1 (12.5%) |
| Infections | 0 | 0 | 3 | 3 (18.8%) | 7 | 3 (37.5%) |
| Bronchitis | 0 | 0 | 0 | 0 | 1 | 1 (12.5%) |
| Eye infection | 0 | 0 | 0 | 0 | 1 | 1 (12.5%) |
| Nasopharyngitis | 0 | 0 | 0 | 0 | 2 | 2 (25.0%) |
| Pharyngitis | 0 | 0 | 0 | 0 | 1 | 1 (12.5%) |
| Rhinitis | 0 | 0 | 1 | 1 (6.3%) | 0 | 0 |
| Sinusitis | 0 | 0 | 1 | 1 (6.3%) | 1 | 1 (12.5%) |
| Syphilis | 0 | 0 | 0 | 0 | 1 | 1 (12.5%) |
| Upper respiratory tract infection | 0 | 0 | 1 | 1 (6.3%) | 0 | 0 |
| Injury, poisoning and procedural complications | 2 | 2 (33.3%) | 0 | 0 | 0 | 0 |
| Contusion | 1 | 1 (16.7%) | 0 | 0 | 0 | 0 |
| Scar | 1 | 1 (16.7%) | 0 | 0 | 0 | 0 |
| Investigations | 0 | 0 | 2 | 2 (12.5%) | 1 | 1 (12.5%) |
| Activated partial thromboplastin time prolonged | 0 | 0 | 1 | 1 (6.3%) | 0 | 0 |
| C-reactive protein increased | 0 | 0 | 1 | 1 (6.3%) | 1 | 1 (12.5%) |
| Metabolism and nutrition disorders | 0 | 0 | 1 | 1 (6.3%) | 0 | 0 |
| Gout | 0 | 0 | 1 | 1 (6.3%) | 0 | 0 |
| Musculoskeletal and connective tissue disorders | 0 | 0 | 10 | 8 (50.0%) | 2 | 2 (25.0%) |
| Arthralgia | 0 | 0 | 3 | 2 (12.5%) | 1 | 1 (12.5%) |
| Back pain | 0 | 0 | 6 | 5 (31.3%) | 0 | 0 |
| Myalgia | 0 | 0 | 0 | 0 | 1 | 1 (12.5%) |
| Pain in extremity | 0 | 0 | 1 | 1 (6.3%) | 0 | 0 |
| Nervous system disorders | 2 | 2 (33.3%) | 7 | 5 (31.3%) | 0 | 0 |
| Headache | 2 | 2 (33.3%) | 7 | 5 (31.3%) | 0 | 0 |
| Psychiatric disorders | 0 | 0 | 1 | 1 (6.3%) | 0 | 0 |
| Insomnia | 0 | 0 | 1 | 1 (6.3%) | 0 | 0 |
| Renal and urinary disorders | 0 | 0 | 1 | 1 (6.3%) | 0 | 0 |
| Dysuria | 0 | 0 | 1 | 1 (6.3%) | 0 | 0 |
| Skin and subcutaneous tissue disorders | 0 | 0 | 0 | 0 | 1 | 1 (12.5%) |
| Rash | 0 | 0 | 0 | 0 | 1 | 1 (12.5%) |
E: events; n: number of participants
Appendix 4. CD4 T cell count analysis (full analysis set)
| CD4+ (cells/mm3) | 50 mg ABX464
(total=6) |
150 mg ABX464
(total=16) |
Placebo
(total=8) |
Difference in LS means: 150 mg vs placebo (95% CI) | P value | |
|---|---|---|---|---|---|---|
| Day 0 | n | 6 | 16 | 8 | ||
| Mean (SD) | 1004.8 (201.7) | 926.4 (394.6) | 698.0 (179.8) | |||
| Median [Range] | 982.0 [800–1340] | 784.5 [426–1792] | 681.5 [448–998] | |||
| LS mean | 926.4 | 698.0 | 228.4 (-78.1–534.8) | 0.1365 | ||
| Day 7 | n | 6 | 16 | 7 | ||
| Mean (SD) | 964.8 (258.8) | 795.4 (268.8) | 815.9 (387.8) | |||
| Median [Range] | 949.0 [630–1377] | 758.0 [392–1290] | 616.0 [527–1591] | |||
| LS mean | 795.4 | 815.9 | -20.4 (-310.3–269.4) | 0.8849 | ||
| Day 14 | n | 5 | 15 | 7 | ||
| Mean (SD) | 1119.0 (350.4) | 846.6 (289.2) | 786.4 (406.8) | |||
| Median [Range] | 970.0 [780–1571] | 800.0 [506–1419] | 575.0 [511–1628] | |||
| LS mean | 846.6 | 786.4 | 60.2 (-253.9–374.2) | 0.6937 | ||
| Day 21 | n | 6 | 15 | 8 | ||
| Mean (SD) | 999.7 (358.0) | 786.5 (293.5) | 834.6 (368.8) | |||
| Median [Range] | 973.0 [520–1602] | 740.0 [415–1337] | 663.5 [547–1665] | |||
| LS mean | 786.5 | 834.6 | -48.2 (-340.0–243.7) | 0.7349 | ||
| Day 25 | n | 3 | 9 | 6 | ||
| Mean (SD) | 1089.7 (174.0) | 942.1 (284.2) | 816.7 (306.7) | |||
| Median [Range] | 1089.0 [916–1264] | 926.0 [518–1487] | 756.0 [401–1282] | |||
| LS mean | 942.1 | 816.7 | 125.4 (-208.2–459.1) | 0.4313 | ||
| Day 28 | n | 6 | 13 | 6 | ||
| Mean (SD) | 987.2 (304.1) | 853.4 (291.2) | 838.2 (339.3) | |||
| Median [Range] | 864.5 [728–1516] | 770.0 [494–1480] | 688.0 [612–1491] | |||
| LS mean | 853.4 | 838.2 | 15.2 (-303.5, 334.0) | 0.9209 | ||
n: number of participants; SD: standard deviation; LS mean: least squares mean; CI: confidence interval.
Viral load analysed using an analysis of variance (ANOVA) model with treatment as a fixed effect.
Appendix 5. CD4+/CD8+ T cell count ratio analysis (full analysis set)
| CD4+/CD8+ ratio | 50 mg ABX-464
(total=6) |
150 mg ABX-464
(total=16) |
Placebo
(total=8) |
Difference in LS means: 150 mg vs placebo (95% CI) | P value | |
|---|---|---|---|---|---|---|
| Day 0 | n | 6 | 16 | 8 | ||
| Mean (SD) | 1.36 (0.64) | 1.05 (0.38) | 1.05 (0.270) | |||
| Median [Range] | 1.21 [0.7–2.2] | 1.08 [0.5–1.8] | 0.97 [0.7–1.6] | |||
| LS mean | 1.05 | 1.05 | 0.00 (-0.31, 0.32) | 0.9836 | ||
| Day 7 | n | 6 | 16 | 7 | ||
| Mean (SD) | 1.29 (0.63) | 0.94 (0.28) | 1.03 (0.24) | |||
| Median [Range] | 1.23 [0.6–2.2] | 0.92 [0.5–1.5] | 1.03 [0.6–1.4] | |||
| LS mean | 0.94 | 1.03 | -0.09 (-0.34, 0.16) | 0.4723 | ||
| Day 14 | n | 5 | 15 | 7 | ||
| Mean (SD) | 1.18 (0.56) | 1.08 (0.39) | 1.16 (0.30) | |||
| Median [Range] | 1.03 [0.6–1.9] | 1.10 [0.5–1.6] | 0.99 [0.9–1.6] | |||
| LS mean | 1.08 | 1.16 | -0.08 (-0.42, 0.27) | 0.6478 | ||
| Day 21 | n | 6 | 15 | 8 | ||
| Mean (SD) | 1.30 (0.60) | 1.04 (0.38) | 1.20 (0.44) | |||
| Median [Range] | 1.27 [0.6–2.0] | 1.10 [0.6–1.8] | 1.08 [0.7–2.0] | |||
| LS mean | 1.04 | 1.20 | -0.16 (-0.52, 0.21) | 0.3767 | ||
| Day 25 | n | 3 | 9 | 6 | ||
| Mean (SD) | 1.53 (0.73) | 1.11 (0.28) | 1.15 (0.27) | |||
| Median [Range] | 1.88 [0.7–2.0] | 1.09 [0.7–1.6] | 1.10 [0.9–1.6] | |||
| LS mean | 1.11 | 1.15 | -0.04 (-0.36, 0.27) | 0.7628 | ||
| Day 28 | n | 6 | 13 | 7 | ||
| Mean (SD) | 1.39 (0.47) | 1.06 (0.30) | 1.08 (0.34) | |||
| Median [Range] | 1.48 [0.8–1.9] | 1.05 [0.6–1.6] | 0.97 [0.7–1.7] | |||
| LS mean | 1.06 | 1.08 | -0.03 (-0.33, 0.28) | 0.8581 | ||
n: number of participants; SD: standard deviation; LS mean: least squares mean; CI: confidence interval.
Viral load analysed using an analysis of variance (ANOVA) model with treatment as a fixed effect.
Appendix 6. Kaplan-Meier plot of time to virological rebound
References
- 1. Margolis DM. Towards an HIV Cure: a view of a developing field. J Infect Dis 2017; 215 Suppl 3: S109– S110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lee GQ, Lichterfeld M. Diversity of HIV-1 reservoirs in CD4+ T-cell subpopulations. Curr Opin HIV AIDS 2016; 11: 383– 387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wong JK, Yukl SA. Tissue reservoirs of HIV. Curr Opin HIV AIDS 2016; 11: 362– 370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Margolis DM, Garcia JV, Hazuda DJ, Haynes BF. Latency reversal and viral clearance to cure HIV-1. Science 2016; 353: aaf6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Campos N, Myburgh R, Garcel A et al. . Long lasting control of viral rebound with a new drug ABX464 targeting Rev-mediated viral RNA biogenesis. Retrovirology 2015; 12: 1– 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tazi J, Bakkour N, Marchand V et al. . Alternative splicing: regulation of HIV-1 multiplication as a target for therapeutic action. FEBS J 2010; 277: 867– 876. [DOI] [PubMed] [Google Scholar]
- 7. Scherrer D, Rouzier R, Barrett PN, et al. . Pharmacokinetics and tolerability of ABX464, a novel first-in-class compound to treat HIV infection, in healthy HIV-uninfected subjects. J Antimicrob Chemother 2017; 72: 820– 828. [DOI] [PubMed] [Google Scholar]
- 8. Scherrer D, Rouzier R, Cardona M et al. . Randomized trial of food effect on pharmacokinetic parameters of ABX464 administered orally to healthy male subjects. Antimicrob Agents Chemother 2017; 61: e01288– 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Steens JM, Scherrer D, Gineste P et al. . Safety, pharmacokinetics and antiviral activity of a novel HIV antiviral, ABX464, in treatment-naïve HIV infected subjects: a Phase II randomized, controlled study. Antimicrob Agents Chemother 2017; 61: e00545– 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schvachsa N, Turk G, Burgard M et al. . Examination of real-time PCR for HIV-1 RNA and DNA quantitation in patients infected with HIV-1 BF intersubtype recombinant variants. J Virol Methods 2007; 140: 222– 227. [DOI] [PubMed] [Google Scholar]
- 11. Trypsteen W, Vynck M, De Neve J et al. . ddpcRquant: threshold determination for single channel droplet digital PCR experiments. Anal Bioanal Chem 2015; 407: 5827– 5834. [DOI] [PubMed] [Google Scholar]
- 12. El Bouzidi K, Collier D, Nastouli E et al. . Virological efficacy of PI monotherapy for HIV-1 in clinical practice. J Antimicrob Chemother 2016; 71: 3228– 3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arribas JR, Girard PM, Paton N et al. . Efficacy of protease inhibitor monotherapy vs. triple therapy: meta-analysis of data from 2303 patients in 13 randomized trials. HIV Med 2016; 17: 358– 367. [DOI] [PubMed] [Google Scholar]
- 14. King JR, Khatri A, Trinh R et al. . Pharmacokinetics of darunavir, ombitasvir, paritaprevir, ritonavir, dasabuvir and ribavirin in adults infected with hepatitis C virus genotype 1 and human immunodeficiency virus (HIV). 17th International Workshop on Clinical Pharmacology of HIV and Hepatitis Therapy. Washington DC, USA June 2016. Abstract O_20.
- 15. Kakuda TN, Van De Casteele T, Petrovic R et al. . Bioequivalence of a darunavir/cobicistat fixed-dose combination tablet versus single agents and food effect in healthy volunteers. Antivir Ther 2014; 19: 597– 606. [DOI] [PubMed] [Google Scholar]
- 16. Rouzioux C, Avettand-Fenoël V. Total HIV DNA: a global marker of HIV persistence. Retrovirology 2018; 15: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Williams JP, Hurst J, Stöhr W et al. ; SPARTAC Trial Investigators HIV-1 DNA predicts disease progression and post-treatment virological control. eLife 2014; 3: e03821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pinkevych M, Cromer D, Tolstrup M et al. . HIV reactivation from latency after treatment interruption occurs on average every 5–8 days. Implications for HIV remission. PLoS Pathog 2015; 11: e1005000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barton K, Winckelmann A, Palmer S. HIV-1 reservoirs during suppressive therapy. Trends Microbiol 2016; 24: 345– 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wilkinson J, Ewart G, Luscombe C et al. . A Phase 1b/2a study of the safety, pharmacokinetics and antiviral activity of BIT225 in patients with HIV-1 infection. J Antimicrob Chemother 2016; 71: 731– 738. [DOI] [PubMed] [Google Scholar]